Abstract
Arachidonic acid-derived lipid mediators play crucial roles in the development and progression of cardiovascular diseases. Eicosanoid metabolites generated by lipoxygenases and cytochrome P450 enzymes produce several classes of molecules, including the epoxyeicosatrienoic acid (EET) and hydroxyeicosatetraenoic acids (HETE) family of bioactive lipids. In general, the cardioprotective effects of EETs have been documented across a number of cardiac diseases. In contrast, members of the HETE family have been shown to contribute to the pathogenesis of ischemic cardiac disease, maladaptive cardiac hypertrophy and heart failure. The net effect of 12(S)- and 20-HETE depends upon the relative amounts generated, ratio of HETEs/EETs produced, timing of synthesis, as well as cellular and subcellular mechanisms activated by each respective metabolite. HETEs are synthesized by and affect multiple cell types within the myocardium. Moreover, cytochrome P450- (CYP) and lipoxygenase- (LOX) derived metabolites have been shown to directly influence cardiac myocyte growth and the regulation of cardiac fibroblasts. The mechanistic data uncovered thus far has employed the use of enzyme inhibitors, HETE antagonists and the genetic manipulation of lipid-producing enzymes and their respective receptors, all of which influence a complex network of outcomes that complicate data interpretation. This review will summarize and integrate recent findings on the role of 12(S)-/20-HETE in cardiac diseases.
Introduction
Heart Failure (HF) remains as a leading cause of morbidity and mortality worldwide. A 2019 report from the American Heart Association estimates a 40–46% increase in global HF prevalence in the next decade. The overall annual cost of HF continues to rise in the US and is projected to reach $69.8 billion1 by 2030.
HF results from a combination of myocyte death, adverse structural remodeling and progressive contractile dysfunction. Coronary artery disease, hypertension and diabetes all contribute to HF development and progression. Despite recent advances in diagnosis, therapeutic intervention and pharmacotherapy, the prevalence of HF continues to rise, in part due to the aging population and increased incidence of type 2 diabetes, ischemic heart disease and metabolic syndrome. A common underlying etiology of these conditions is a pro-inflammatory environment that initiates progressive, maladaptive structural remodeling and systolic/diastolic dysfunction. Recent evidence indicates a causative role of arachidonic acid-derived hydroxyeicosatetraenoic acid (HETE) metabolites in cardiovascular disease progression. This review will focus on their role in cardiac/cardiovascular function and the cellular pathways influenced by these bioactive lipids that culminate in disease activation and progression.
Overview of Arachidonic Acid Metabolism
The metabolic pathways of arachidonic acid (5Z,8Z,11Z,14Z-eicosatetraenoic acid, AA) are well characterized within the cardiovascular system. AA is cleaved from membrane phospholipids by members of the Phospholipase A2 family. Three major enzymatic pathways metabolize AA to form a variety of biologically active metabolites. Cyclooxygenases (COX-1 and COX-2) produce prostaglandins, thromboxanes and prostacyclins, which have multiple functions including immune surveillance, inflammation, and the regulation of vascular tone and permeability2. Lipoxygenases catalyze the formation of hydroperoxide intermediates that are reduced by glutathione peroxidase to form mid-chain hydroxyeicosatetraenoic acid (HETE), lipoxins and leukotrienes3. Finally, the cytochrome P450 (CYP) family of enzymes convert AA to a number of cardiovascular mediators. CYPs are a superfamily of NADPH-dependent, heme-containing monooxygenases that metabolize a number of endogenous compounds, steroids and drugs. The CYP450 epoxygenases (e.g. CYP2J2, CYP2C9) convert AA to epoxyeicosatrienoic acids (EETs) while CYP450 hydroxylases (CYP4A, CYP4B and CYP4F) produce HETEs through hydroxylation (e.g. 20-hydroxyeicosatetraenoic acid (20-HETE)) or allylic oxidation (e.g. 12S-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid (12(S)-HETE)).
Hydroxyeicosatetraenoic Acid Synthesis in the Heart
Multiple cell types in the heart synthesize and respond to HETEs, including cardiac myocytes and fibroblasts, infiltrating immune cells (neutrophils and macrophages), coronary artery endothelial and vascular smooth muscle cells, and autonomic nerves4–6. This heterogeneity dictates the spatiotemporal pattern of eicosanoid production and the types and amounts of eicosanoids that are generated, which often complicates the interpretation of each mediator as to whether it acts independently or in concert with other members of arachidonic acid metabolites. For example, while several EETs are considered to be anti-inflammatory and cardioprotective, various HETEs are considered to be pro-inflammatory and cardiotoxic.
12-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid (12-HETE),12-HETE Synthesis Inhibitors and the Heart.
There are several enzymes implicated in cardiac 12-HETE production. 12-HETE is produced primarily by the12-lipoxygenase enzyme. The 12-lipoxygenase is encoded by the alox12 gene and catalyzes the stereoselective dioxygenation of arachidonic acid into 12(S)-hydroperoxyicosa-5,8,10,14-tetraenoic acid (12-HpETE), which is quickly reduced by glutathione peroxidase in the cell to form 12(S)-HETE3. Originally identified as a platelet lipoxygenase, 12-LOX is also expressed in cardiac myocytes7 and cardiac fibroblasts8, 9. Aside from platelets, cardiac myocytes and fibroblasts, 12-LOX can also be found in human islets, adipose tissue, kidneys, macrophages, neutrophils, skin and smooth muscle10. Many of the early inhibitors of 12-LOX, including nordihydroguaiaretic acid (NDGA), and 5,6,7-trihydroxyflavone (baicalein) acted as redox inhibitors that blocked oxidation of the non-heme iron in the catalytic site3. However, since all lipoxygenases require non-heme oxidation, these inhibitors are non-selective. Fatty acid analogs, such as 5,8,11,14-eicosatetraynoic acid (ETYA), potently block the generation of12(S)-HETE but also lack selectivity3. Work by the Holman group11 sought to discover potent and selective 12-LOX inhibitors, identifying several compounds and key attributes to these inhibitors. Additional studies in this field uncovered several selective and potent 12-LOX inhibitors including, ML35512, 13 and the 12/15-LOX inhibitors ML35114, 9908915, and LOXblock-116.
The 12/15-lipoxygenase (ALOX15) also produces 12(S)- and 15(S)-HETE, but the relative proportion of HETEs generated is species specific. In humans, 12/15-LOX produces 10% 12(S)-HETE vs 90% 15(S)-HETE while in mice the enzyme produces 90% 12(S)-HETE vs 10% 15(S)-HETE17. Due to these species differences, results of studies in mice may overestimate the causative role of 12/15-LOX-derived 12(S)-HETE production in cardiac disease as 12/15-LOX is expressed in the heart and is upregulated in HF18. 12/15-LOX is also expressed in adipose tissue19 and across several infiltrating cells including macrophages and neutrophils20, In fact, the observed changes in 12-HETE across tissues under pathological conditions may be elevated by the mobilization and presence of these infiltrating cells. The benzothiopyranoindole compound (PD-146176) is a potent inhibitor of human 15-LOX21 and has been shown to inhibit atherosclerosis in hypercholesterolemic rabbits22.
12-HETE is also produced in the heart by cytochrome P450-dependent mechanisms. CYP1A1 and CYP1B1, both capable of forming mid-chain HETEs (12, 13 or 15-HETE) via lipoxygenase like activity23, also produce EETs and ω-terminal HETEs24. Within the myocardium, CYP1B1 is expressed across coronary blood vessels and cardiac myocytes. CYP1B1 exhibits low cardiac basal expression but is rapidly induced by pressure overload and hypertrophic agonists25–28 and by hypoxia29. CYP1B1 is also involved in the metabolism of sex steroids. Within the cardiovascular system, CYP1B1 protects against angiotensin II (Ang II)-induced hypertension and associated cardiovascular changes in female mice, most likely due to the metabolism of 17-β estradiol into hydroxyestradiols (OHE), especially 2-OHE30. Conversely, 6β-hydroxytestosterone, a CYP1B1 metabolite of testosterone, contributes to angiotensin II-induced hypertension in male mice31.
Although there are at least 4 classes of CYP1B1 inhibitors, the stilbene 2,4,3′,5′-Tetramethoxystilbene (TMS) is the most often used inhibitor across cardiac studies30, 32–34. Given the multiple byproducts of CYP1B1 enzymatic activity, one must use caution in interpreting these data with regards to establishing the direct involvement of 12-HETE. Genetic manipulation of CYP1B1 with siRNA or targeted gene deletion have been used to validate a role for the CYP1B1–12-HETE pathway in cardiovascular disease but these strategies also cannot delineate between steroid metabolism, ROS production and 12-HETE formation31, 33, 35, 36.
12(S)-HETE-mediated Signaling and Receptors.
Recently, a role for G-protein coupled receptors in 12(S)-HETE and 20-HETE signaling has been reported. 12-HETE appears to mediate its effects through several receptors, including the low-affinity leukotriene B4 (BLT2) receptor, since it was shown to compete for radiolabeled leukotriene B4 binding37. In addition, 12(S)- and 12(R)-HETE have also been shown to influence the thromboxane A2 (TP) receptor as competitive inhibitors that promote the relaxation of mouse mesenteric arteries38. Studies by Guo et al.39 uncovered the orphan receptor GPR31 as a 12(S)-HETE receptor (12(S)-HETER1) with high binding affinity using the PC3 human prostate cancer cell line39, 40. GPR31 belongs to the rhodopsin-like group A subfamily of receptors, coupling to Gα(i) in the Gi/Go family. In PC3 cells 12-S-HETE binding was shown to activate a MEK, ERK1/2 and NF-kB signaling cascade (Figure 1)39. Interestingly, GPR31 is also activated by protons41 and lactic acid42, further increasing the complex nature of GPR31-mediated receptor signaling and activation. Secondly 12(S)-HETE activates a vast array of signaling programs across various cell types with conflicting results. In particular, when looking at 12(S)-HETE’s effects across the vasculature there are several differences. Prior to the identification of GPR31 as a 12(S)-HETER1, 12(S)-HETE was shown to be a competitive antagonist of the PGH2/TXA2 thromboxane receptor43. While the 12(S)-HETER1 was discovered in the context of cancer cell biology, within the vasculature, 12(S)-HETE has been observed to mediate increases in endothelial-dependent vasodilation in porcine and human coronary vessels as well as in rat resistant vessels44–48. This effect primarily occurs through the activation of large conductance K channels (BKCa) located across smooth muscle cells44 as well as through the inhibition of thromboxane receptor-mediated signaling38. Conflicting reports suggest that 12(S)-HETE serves as a potent vasoconstrictor as exposure to 12(S)-HETE constricts isolated dog arcuate arteries49. Another study suggests that 12(S)-HETE modulates intracellular actions of Ang II in cultured rat vascular smooth muscle cells (VSMC)50, enhancing Ang II signaling. There are currently no recent reports to suggest the precise mechanisms activated by the interaction of 12(S)-HETE and GPR31 on VSMC or the vasodilatory/constrictive effects of 12(S)-HETE/GPR31 occurring across the vasculature. In fact, one report suggests that the aorta and mesenteric arteries of mice are devoid of GPR3138.
Figure 1: 12(S)-HETE/GPR31 Signaling Pathways.
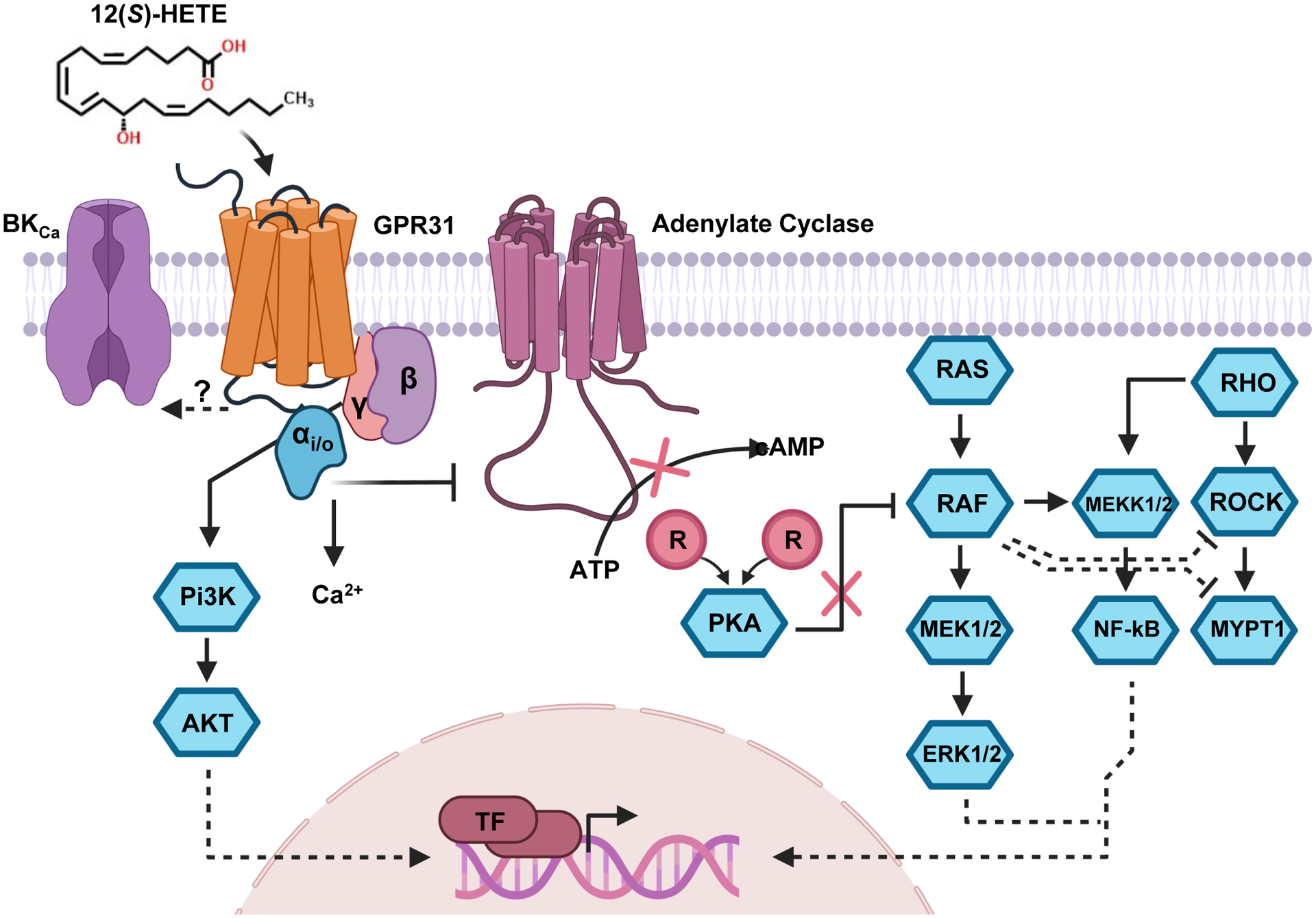
The 12(S)-HETE receptor (GPR31) is Gαi/o coupled and inhibits adenylate cyclase from converting ATP to cAMP. This action causes the reassociation of the regulatory subunits of PKA to its catalytic subunits, inhibiting the kinase activity of PKA. RAF, a member of the MAPK pathway, is capable of being inhibited through phosphorylation by PKA. RAF has been shown to phosphorylate MEK which subsequently activates ERK1/2 which can translocate to the nucleus and promotes gene expression through the activation of nuclear transcription factors. Additionally, RAF has also been shown to induce NF-кB through MEKK1 which also promotes gene expression. Additionally, through alterations of RAF activity, 12(S)-HETE acting on GPR31 may also exert an inhibitory effect on ROCK and MYPT1 related signaling. Lastly, the Gβγ effects of GPCRs like GPR31 have been demonstrated to influence intracellular calcium levels as well as drive PI3K/AKT signaling pathways with downstream consequences to alter gene expression. It is still unknown how the pairing of 12(S)-HETE and GPR31 alters the activation of the large conductance K channels (BKCa).
Subsequent studies following the identification of the 12(S)-HETER1 have identified 12(S)-HETE as a mediator of various other signaling cascades including several observations in lymph-endothelial cells, including the release of calcium that occurs in concert with the activation of a RHO-ROCK-MYPT driven signaling cascade that activates myosin light chain-2 (MLC2) (Figure 1)51, 52. This particular pathway has been shown to be important for fibroblasts mechanosignaling and in fibroblast transformation to myofibroblasts53. The overexpression of 12-LOX is illustrated to be mitogenic for cardiac fibroblasts9. Moreover, in lymph-endothelial cells, 12(S)-HETE induces the expression of SRY-related HMG-box 18 (SOX18) and prospero homeobox protein 1 (PROX1), two potent influencers of endothelial cells development54. In pulmonary artery endothelial cells (PAEC), 12(S)-HETE promotes PAEC survival through the activation of a phosphatidylinositol 3-kinase (PI3K)/Akt-dependent pathway55. Interestingly, under levels of high glucose, endothelial cells exposed to 12(S)-HETE have dramatic elevations in P‐Iκbα, P‐P65, ICAM‐1 and VCAM‐1 alongside impairments in vascular endothelial permeability56. Recent reports place the 12-LOX/ 12(S)-HETE/GPR31 signaling pathway at the forefront of thrombosis, demonstrating that the 12(S)-HETE/GPR31 pairing enhances the activation of human platelets and thrombosis observed in mouse carotid artery injury models57.
12-HETE: Antagonists
Currently there are no clearly defined biological or synthetic 12-HETE antagonists. DUP 654 (2-benzyl-1-naphthol), is a potent 5-lipoxygenase inhibitor and has been evaluated as an anti-inflammatory agent58, 59. At one point it was characterized and referred to as a 12(S)-HETE receptor antagonist in a human epidermal cell line when the 12(S)-HETER1 had yet to be identified60. Further understanding and characterization of the 12(S)-HETE/GPR31 interaction and its relationship with other receptors may serve beneficial for the development of new 12(S)-HETE antagonist and novel pharmacological tools to disrupt or augment 12(S)-HETE’s bioactions.
20- hydroxyeicosatetraenoic acid (20-HETE) Synthesis in the Heart
The major pathway for 20-HETE production is the ω-hydroxylation of arachidonic acid by the CYP4A and CYP4F sub-families. In mice, CYP4a12a is the primary 20-HETE synthase while CYP4A1,2,3 and CYP4F1 and CYP4F2 produce 20-HETEs in rodents (see Waldman et al.61 for a comprehensive review). In humans, CYP4A11 and CYP4F2 are the predominant 20-HETE synthases although CYP4F11 and CYP4F3 have limited capacity to produce 20-HETE. The CYP4 enzymes share significant sequence homology and catalytic properties but exhibit distinct tissue distribution and regulation. These enzymes are expressed within the coronary vasculature and cardiac myocytes but not in cardiac fibroblasts61–63. Additionally, CYP4A is subject to transcriptional regulation by nuclear receptors, including peroxisomal proliferator-activated receptor α (PPARα) and the androgen receptor (AR)64–67, and post-translational regulation by microRNAs63, 68. Angiotensin II (Ang II) increases CYP4A isoform expression in the kidney69, 70 and the vasculature71.
20-HETE-mediated Signaling and Receptors.
20-HETE is a potent vasoactive eicosanoid and elevations in 20-HETE are associated with the onset and progression of various pathologies including myocardial infarction, stroke and hypertension. Most of our current understanding of 20-HETE actions in the cardiovascular system stems from mechanistic studies in endothelial cells and vascular smooth muscle cells. Moreover, 20-HETE activates a wide array of signaling pathways that promote pro-inflammatory and pro-hypertensive signaling programs. In this section we will highlight key findings from these cell types.
Work by Garcia et al. identified the orphan G-protein coupled receptor (GPCR), GPR75, to be a high affinity 20-HETE receptor (20HR)72. The characterization of the 20HR included the use of click chemistry compounds, ligand binding assays, proteomics, bioinformatics, immunoprecipitation and gene silencing in both in vitro human endothelial cell experiments and in vivo mouse models knocking down GPR7572, 73. Previous reports examining 20-HETE’s bioactions across the vasculature have clearly identified 20-HETE to be a potent uncoupler of endothelial nitric oxide synthase (eNOS), reducing nitric oxide (NO) bioavailability and a strong inducer of endothelial angiotensin converting enzyme (ACE), increasing circulating angiotensin II levels74, 75. These 20-HETE mediated effects rely on an EGFR-MAPK-IKKβ-NFκB-dependent signaling pathway that requires the presence and activation of the 20HR (GPR75) (Figure 2)72, 76, 77. More recently GPR40 (also known as (free fatty acid receptor-1 (FFAR-1)) was reported to bind 20-HETE with low affinity and promote glucose-stimulated insulin secretion78. It is noteworthy to mention that GPR40 also binds to epoxyeicosatrienoic acids with a similar low affinity and lacks ligand selectivity79.
Figure 2: 20-HETE/GPR75 Signaling Pathways.
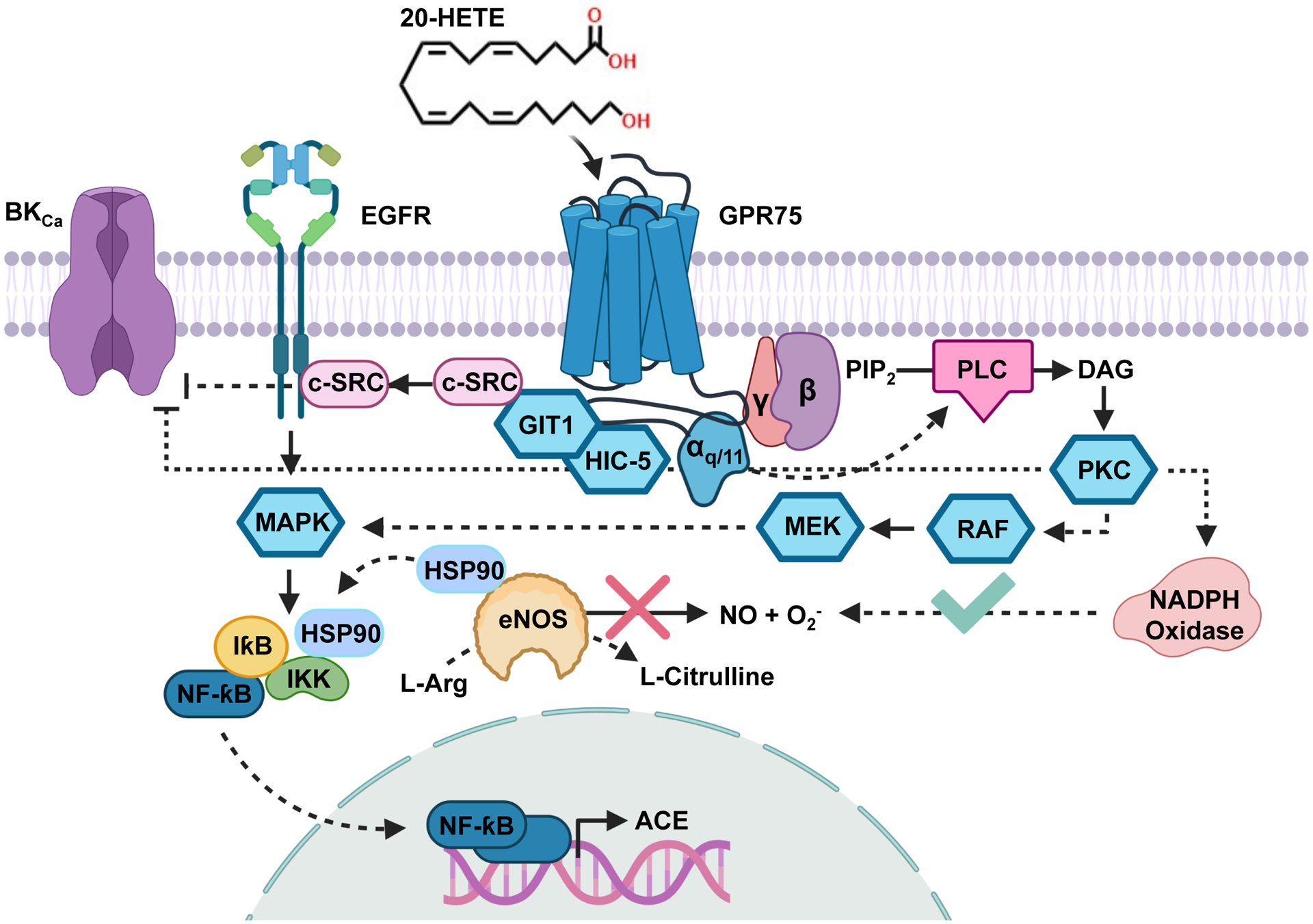
The 20-HETE receptor (20HR) (GPR75) is Gαq/11 coupled and promotes changes in intracellular calcium. In endothelial cells, the activation of GPR75 via 20-HETE promotes the transactivation of the epidermal growth factor receptor (EGFR) through a GIT1-/c-SRC-dependent mechanism. Activation of the EGFR results in sequential activation of a MAPK/IƙB/IKK and the translocation of NF-ƙB to stimulate the promoter regions of angiotensin converting enzyme (ACE). Simultaneously, the activation of IKK promotes the recruitment of the chaperone protein HSP90 towards IKK and away from endothelial nitric oxide synthase (eNOS), resulting in the uncoupling of eNOS and a reduction in NO production/bioavailability. Additionally, phospholipase C (PLC) stimulation driven by GPR75 Gαq/11 results in the activation of PKC and subsequent increases in NADPH oxidase-derived reactive oxygen species (ROS) generation. The phosphorylation of the large conductance K channels (BKCa) by PKC also promotes a vasoconstrictor stimuli. These changes promote endothelial dysfunction and set into motion a pro-inflammatory signaling program that elevate various mediators including the synthesis of the chemokine IL-6.
Interestingly, other reports have identified an interaction between the chemokine CCL5/RANTES and GPR75, suggesting that CCL5 drives calcium influx80, 81 and can stimulate insulin secretion from pancreatic islets82 through GPR75. However, these studies did not provide evidence of direct binding experiments and CCL5/RANTES interaction failed to show receptor activation in repeated β-arrestin recruitment assays83, 84. Further studies are necessary to more fully characterize CCL5/RANTES binding to GPR75 and whether it acts as an antagonist for 20-HETE binding.
20-HETE is intimately associated with the promotion of inflammatory signals including the production and release of superoxide/reactive oxygen species (ROS), adhesion molecules and cytokines. Studies by Guo et al. illustrated 20-HETE’s potential to stimulate superoxide formation in endothelial cells, an effect that stimulates vascular endothelial growth factor (VEGF) synthesis and promotes cell proliferation85, 86. In addition to driving the production of ROS, 20-HETE promotes the expression of adhesion molecules including intracellular cell adhesion molecule 1 (ICAM-1)87. In fact, during the identification of this finding, it was also observed that increases in the cytokine IL-6 were also 20-HETE mediated, a finding that was corroborated in transgenic mice expressing CYP4F2 specifically in endothelial cells (Tie2-CYP4F2-Tr)87, 88.
In vascular smooth muscle cells (VSMC), 20-HETE activates constrictor stimuli and is also involved in the increased sensitization and enhanced responsiveness of vessels to constrictor stimuli63. This is accomplished through a coordinated interplay between kinases, channels and changes in calcium. Previous reports identified the activation of protein kinase C (PKC), mitogen-activated protein kinases (MAPK), tyrosine kinase and Rho kinase as the signal cascades that culminate in the phosphorylation and inhibition of the Ca2+-activated K+ channels (BKca)63, 89–91. This results in VSMC depolarization and elevation in cytosolic [Ca2+] through increased Ca2+ entry via the L-type Ca2+ channels92, 93. The 20-HETE-GPR75 pairing initiates a signaling cascade that involves conventional G protein (Gαq/11) mediated changes alongside the promotion of a GIT1-mediated PKC-stimulated phosphorylation of MaxiKβ (BKca channel β subunit) that facilitates vasoconstriction72. Moreover, studies show 20-HETE to be a mediator of the transient receptor potential (TRP), activating TRPC6 (a non-voltage-gated Ca2+ entry/depolarization channel)94 and transient receptor potential vanilloid 1 (TRPV1) (a nonselective cation channel)95, increasing Ca2+ mobilization and furthering 20-HETE’s pro-vasocontrictor effects96. In addition to influencing channels, 20-HETE increases the Ca2+ sensitivity of the contractile apparatus through the activation of Rho kinase and subsequent phosphorylation of myosin light chain (MLC20)63, 97. Aside from influencing signals that promote vasoconstriction, 20-HETE has also been shown to elicit increases in VSMC mitochondrial superoxide production and may promote a secretory phenotype through the activation of a MAPK1-Elk-1-dependent pathway98.
Several of these observed signaling pathways are also involved in cardiac myocyte hypertrophy/apoptosis and cardiac-fibroblast dependent extracellular matrix remodeling. Further studies are necessary to elucidate the role of 20-HETE and the 20HR in this context.
20-HETE: Synthesis Inhibitors and Antagonists
One aspect that has truly benefited investigators exploring 20-HETE’s role across various pathologies has been the consistent development and characterization of 20-HETE synthesis inhibitors and 20-HETE antagonists. N-hydroxy-N’-(4-butyl-2-methylphenyl)-formamidine (HET0016)99 and dibromo-dodecenyl-methylsulfimide (DDMS)100 have served as potent 20-HETE synthesis inhibitors across various studies101, 102. 20-HETE antagonist compounds such as 20-hydroxyeicosa-6(Z),15(Z)-dienoic acid (20-HEDE) and N-[20-hydroxyeicosa-6(Z),15(Z)-dienoyl]glycine (20-HEDGE)77 have served as critical tools in the study of 20-HETE biology, preventing many of 20-HETE’s bioactions across the vasculature, including helping to elucidate several of 20-HETE’s blood pressure-independent effects72, 77. Recent developments by Falck and colleagues103–106 have provided investigators with new 20-HETE antagonists with increased water solubility. [2,5,8,11,14,17-hexaoxanonadecan-19-yl 20-hydroxyicosa-6(Z),15(Z)-dienoate] (20-SOLA), was the first water soluble 20-HETE antagonist to be synthesized, showing great efficacy at ameliorating the hypertension and renal injury associated with a diabetic mouse model that display dramatic elevations in circulating 20-HETE levels103.
HETEs and Cardiac Pathophysiology
Heart disease encompasses a group of disorders involving the myocardium and coronary vasculature. Coronary artery disease, hypertension and diabetes are leading causes of heart disease that contribute to both ischemic (myocardial infarction) and non-ischemic (hypertrophy, fibrosis) heart disease resulting in myocardial infarction, contractile dysfunction and adverse structural remodeling107. Based on the cells activated by, and signaling pathways involved, 12(S)-HETE and 20-HETE exert their cardiac effects through direct and indirect mechanisms influencing the coronary circulation, inflammation, cardiac myocytes and cardiac fibroblasts.
Myocardial Infarction and Ischemia/reperfusion injury.
Progressive coronary artery disease involves chronic inflammation that often results in unstable atherosclerotic plaques that undergo abrupt rupture and thrombus formation. This results in coronary arterial obstruction, diminished blood flow and resultant ischemia and myocyte necrosis108. Cardiomyocyte death triggers the activation of innate immune system and a surge in inflammatory responses, which can be sub-divided into an acute phase (1–3 days), a resolution phase (3–14 days) and a remodeling phase. Multiple lipid mediators have been implicated in these stages and inflammatory responses. Human cardiac ischemia and MI studies examining changes in eicosanoid levels identified elevations in 12-HETE and 20-HETE in diseased patients compared to patients without cardiovascular events109, 110. Their effects are often accentuated or modified in the presence of chronic hypertension and or Type II diabetes. For example, 12(S)-HETE has been implicated in the pathogenesis of atherosclerosis111 and diabetes112, with studies showing strong correlations between 12(S)-HETE and Type 2 diabetic patients with and without coronary artery disease (CAD)113. A direct link between 12(S)-HETE and Ang II has also been made in the context of diabetes wherein hyperglycemia increases renal Ang II levels that promote subsequent 12(S)-HETE production through the activation of the AT1 receptor114. This interplay between 12(S)-HETE and the components of RAS may prove vital for identifying 12(S)-HETE’s full contribution to cardiovascular related pathologies. Further evidence is necessary to better dissect these relationships and also determine how the 12(S)-HETER1 (GRP31) plays a role under these conditions.
Similarly, 20-HETE levels have been correlated with MI, and a recent study looking at a male cohort of patients undergoing carotid endarterectomy revealed 20-HETE to be a significant metabolite associated with carotid atheroma plaque when compared to healthy subjects115. This study also identified positive correlations between 20-HETE, body mass index and diastolic blood pressure in these patients115. This human study suggests a potential role for 20-HETE in the development and progression of ischemic heart disease, although the spatiotemporal patterns for 20-HETE expression have not been fully defined. Such studies would necessitate continuous sampling of plasma 20-HETE levels that could be linked to cardiac performance, additional biomarkers or structural indicators.
12(S)-HETE, Coronary Artery Disease, Myocardial infarction, and post-infarct remodeling.
12(S)-HETE has context-specific effects within the myocardium. For example, 12(S)-HETE exerts a cardioprotective effect during hypoxia-induced preconditioning via TRPVI-dependent vasodilation116, but is deleterious during long-term remodeling following myocardial infarction117. 12-HETE contributes to sustained inflammation and increased cardiomyocyte death, elevating pro-inflammatory markers such as MCP-1 and IL-6, resulting in monocyte and neutrophil recruitment during the acute phase post MI118, 119. Elevations in 12-HETE have been observed in patients 24–40 hours post-MI120. 12/15-LOX appears to be the major source of 12(S)-HETE since both inhibition and genetic manipulation of 12/15-LOX has been shown to alter cardiac function and inflammation post MI. For example, Kayama et al.18 reported that 12/15-LOX overexpression in mice resulted in increased 12(S)-HETE levels, macrophage infiltration and systolic dysfunction compared to wild-type controls. Recent work from Kain et al.117 indicated that genetic deletion of 12/15-LOX improved post-MI survival and LV function following permanent coronary artery ligation117. Less is known about the role of CYP1B1 derived 12-HETE in myocardial ischemic injury.
Mechanistically, 12-HETE induces cardiac apoptosis during the acute and chronic phases of myocardial infarction and HF. Early reports by Nazarewicz et al.121 indicated that 12-HETE stimulates mitochondrial NO synthase activity, resulting in dissipation of the mitochondrial transmembrane potential, cytochrome c release and apoptosis. In a series of studies, Gross and colleagues demonstrated that distinct phospholipase A2 isoforms are localized to mitochondria and provide AA that is subsequently metabolized to produce local eicosanoids (12-HETE, 20-HETE, 14,15-EET)122. Under non-pathological conditions, cPLA2ζ is the predominant isoform, and AA is shunted into a CYP-dependent pathway that favors the generation of cardioprotective EETS. However, in mouse models of cardiac ischemia/reperfusion (I/R) injury or in cardiac tissue from HF patients, iPLA2γ is the predominant isoform and AA are shunted into the CYP- and 12-LOX pathways to favor the release of cardiotoxic 12-HETE and 20-HETE, resulting in pathologic mPTP opening, mitochondrial swelling, cytochrome c release and apoptosis122. These studies underscore the contribution of subcellular compartmentalization and disease-induced changes in synthetic enzymes in determining the role of HETEs in heart failure progression following ischemia/reperfusion injury.
Conversely, several studies demonstrated a potential cardioprotective role for 12-LOX-derived 12(S)-HETE in myocardial ischemia reperfusion injury. Gabel et al.123 used isolated heart preparations from 12-LOX deficient mice to examine the effects of 12-HETE on preconditioning-induced cardioprotection. Their results suggested that preconditioning decreases cardiac necrosis and improves post-ischemic recovery via a 12-HETE-dependent mechanism123. These effects are likely mediated by transient receptor potential vanilloid 1 (TRPV1) activation in a PKC-dependent manner116. A potential caveat to both studies is that isolated hearts were used and these preparations do not measure the impact of infiltrating 12-HETE releasing immune cells that occurs in vivo upon reperfusion of an ischemic myocardium. It is possible that the amount of 12-HETE differs between invading immune cells and cells of the cardiovascular system.
20-HETE: Coronary Artery Disease, Myocardial infarction, and post-infarct remodeling
Extensive data implicate 20-HETEs as a potent mediator in the development and progression of hypertension, coronary artery disease and ischemic cardiomyocyte injury. The role of 20-HETE in coronary artery disease and hypertension was briefly touched upon in previous sections, but the reader is directed to several excellent review articles on these topics63, 91. This section will focus on 20-HETE and myocardial injury.
In humans, 20-HETE has been associated with increased incidences of myocardial infarction (MI)124, an observation that has been dissected further through various studies. In animal models, the heart contains the CYP4A and 4F isoforms necessary for 20-HETE synthesis63, 106, 125, 126. Gross and colleagues reported that 60 min of ischemia followed by a 60 min reperfusion period resulted in a significant increase in 20-HETE levels in coronary venous plasma127. 20-HETE synthesis inhibitors 17-ODYA and DMMS reduced 20-HETE levels that correlated with a marked reduction in myocardial infarct size126, 128. Studies using the 20-HETE antagonist 20-HEDE confirmed that 20-HETE exacerbates cardiac injury129. Moreover, studies in rodents have implicated a role for CYP4A-20-HETE in ischemia (40 min)/reperfusion (30 min) in diabetic rats. Interestingly, pretreatment with HET0016 resulted in significant improvement in cardiac function in the hearts obtained from diabetic but not in control rats, while an inhibitor of soluble epoxide hydrolase, improved cardiac functional recovery in both control and diabetic animals130. This was one of the first studies to suggest that the overall ratio of EET/HETE dictates the degree of ischemia-reperfusion injury.
The site of 20-HETE synthesis and release during ischemia/reperfusion may also account for the observed deleterious effects. For example, rats subjected to a repetitive ischemia protocol revealed marked increases in 20-HETE production across ischemic collateral-dependent zones (CZ) of the heart as opposed to nonischemic zones (NZ)106. Interestingly these elevations in 20-HETE across the CZ were exacerbated in a rat model of metabolic syndrome (JCR:LA-cp, JCR), corroborating studies that correlate 20-HETE with metabolic syndrome parameters such hypertension72, insulin signaling/resistance131 and obesity131–133. Further studies in JCR rats demonstrated that elevations in 20-HETE promote large artery stiffness and decreased arterial compliance as a consequence of increased elastin degradation, MMP12 activation and pronounced systolic hypertension134. 20-SOLA has also proven to be effective with regards to the heart as it can promote the restoration of coronary collateral growth (CCG) after ischemic injury, preventing endothelial dysfunction and apoptosis106.
At the cellular level, 20-HETE is involved in mediating cardiac myocyte apoptosis. Early reports indicate that the addition of 20-HETE to neonatal cardiac myocytes resulted in dissipation of the mitochondrial membrane potential, increased the expression of pro-apoptotic Bax and caspase 3 activity135. Additional studies by this group136 reported that 20-HETE mediated Ang II-induced apoptosis occurs via the regulation of the mitochondrial permeability transition pore and increased production of reactive oxygen species (ROS). 20-HETE also participates in β-adrenergic induced cardiac myocyte apoptosis through a calmodulin dependent kinase pathway137. Moreover, increased advanced glycation end products in diabetes induced- HF promotes cardiac myocyte apoptosis through a 20-HETE-dependent activation of the NADPH oxidase-2 isoform138.
Heart Failure (HF).
Chronic responses to I/R injury and hemodynamic overload involves a complex interplay of inflammation, mechanical stress and hormone that result in pathological hypertrophy and remodeling of the extracellular matrix139. HETEs have both indirect and direct actions on HF pathogenesis. The indirect actions are mediated by their roles in the promotion of hypertension and coronary artery disease140–142. Myocardial changes in expression of CYP and LOX enzymes and subsequent changes in metabolite generation are associated with the pathogenesis of cardiac hypertrophy, fibrosis and HF via direct effects on cardiac myocyte growth and the regulation of cardiac fibroblast phenotypes143, 144.
The role of 12-HETE in cardiac remodeling has been well established. Increased 12-HETE-levels were observed in response to pressure overload hypertrophy26 and in animal models of HF145, 146. 12-HETE has been shown to play an important role in Ang II and isoproterenol (ISO) induced cardiac myocyte hypertrophy. Several studies using the human cardiomyocyte RL-14 cells from El-Kadi’s group demonstrated that pharmacological inhibition of CYP1B1 with TMS or genetic knockdown with CYP1B1-siRNA prevented ISO-induced increases in cell volume and pro-hypertrophic gene expression147. In this model, 12-HETE increased superoxide production and ERK1/2 and NF-kB signaling33. The same pathways appear to be regulated by 12-HETE in response to Ang II-induced hypertrophy148, 149. Interestingly, resveratrol has been shown to prevent the Ang II-mediated increases in cardiac myocyte hypertrophy, which correlated with a reduction in CYP1B1 protein expression149. 12-HETE derived from 12/15-LOX regulate cardiac fibroblast growth transformation to a myofibroblast phenotype and resultant fibrosis143, 150. Thus, 12-HETE plays a significant role in extracellular matrix remodeling and myocyte hypertrophy that drives HF progression.
Little is known about the role of 20-HETE in mediating pathological remodeling mechanisms of the heart. Elevated cardiac 20-HETE levels have been observed during Ang II-induced cardiac hypertrophy151. 20-HETE’s ability to increase ACE expression76 may likely be involved in the amplification of hypertrophic and fibrotic signaling within the myocardium as previously mentioned. Support for this notion comes from a recent study showing that N-disodium succinate-20-hydroxyeicosa-6(Z),15(Z)-diencarboxamide (AAA), another water soluble 20-HETE antagonist, attenuated the development of cardiac hypertrophy in Cyp1a1-Ren-2 transgenic rats, a model of ANG II-dependent malignant hypertension104. Consequently, future transgenic and pharmacological approaches targeting GPR75 are warranted to address the role of 20-HETE in pathological remodeling of the heart.
Future Directions
As the eicosanoid field continues to explore and expand its understanding of the etiology of HF and concurrent cardiovascular diseases, it is highly likely that it will begin to uncover new signaling mediators and novel therapeutic targets. Presently, data suggest that both 12(S)- and 20-HETE are key mediators influencing various signaling mechanisms that promote and contribute to HF progression. These studies rely heavily upon pharmacological inhibitors and transgenic deletion/overexpression of enzymes and receptors. Although these approaches have served as invaluable tools in the assessment and characterization of HETE’s bioactions across a multitude of biological conditions and scenarios, they are not without limitations. Pharmacological inhibitors of biosynthetic enzymes often result in changes across several eicosanoids and could impact the overall ratios of EET/HETEs. As discussed throughout the review, there are several exciting observations with regards to newfound lipid-receptor interactions. It will be vital to understand whether or not these lipids exert conventional “unbiased” or “biased” ligand signals and the ramifications of these cascades. This concept is particularly important as the preservation of unique signaling mechanisms may provide a benefit to the myocardium. The exploration of biased agonism has yielded a host of new therapeutically relevant compounds including the AT1R-β-arrestin biased ligand TRV120027 which targets the AT1R and favors reductions in blood pressure and increases in cardiac performance152.
The chemical and functional diversity of cardiac eicosanoids requires new systems-based biological approaches, from analytical methods to sophisticated computational approaches to model lipid metabolism and lipid-receptor interactions. The identification of signaling nodes as well as multivariate data analytics of lipidomic profiles will allow investigators to capture metabolic snapshots at different stages of heart disease development and progression153. Likewise, mechanistic characterization of lipid metabolite-GPCR interactions could lead to the generation of novel therapeutics that target specific receptors to treat cardiovascular diseases.
Acknowledgements
This work was supported by the diversity supplement grant from the National Institutes of Health to Victor Garcia HL139793-1S. All figures were created with BioRender.com.
Non-standard Abbreviations and Acronyms:
- EET
Epoxyeicosatrienoic acid
- HETE
Hydroxyeicosatetraenoic acids
- CYP
Cytochrome P450
- LOX
Lipoxygenase
- HF
Heart Failure
- AA
Arachidonic Acid
- COX
Cyclooxygenases
- 12(S)-HETE
12S-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid
- 20-HETE
20-hydroxy-5,8,11,14-eicosatetraenoic acid
- GPCR
G-protein Coupled Receptor
- NO
Nitric Oxide
- ACE
Angiotensin Converting Enzyme
- AAA
N-disodium succinate-20-hydroxyeicosa-6(Z),15(Z)-diencarboxamide
- MI
Myocardial infarction
Footnotes
Conflict of Interest Statement: The authors have declared that no conflict of interest exists.
REFERENCES
- 1.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS and Subcommittee AHACoEaPSCaSS. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139:e56–e52. [DOI] [PubMed] [Google Scholar]
- 2.Sonnweber T, Pizzini A, Nairz M, Weiss G and Tancevski I. Arachidonic acid metabolites in cardiovascular and metabolic diseases. Int J Mol Sci. 2018;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tourdot BE and Holinstat M. Targeting 12-Lipoxygenase as a Potential Novel Antiplatelet Therapy. Trends Pharmacol Sci. 2017;38:1006–1015. [DOI] [PubMed] [Google Scholar]
- 4.Zordoky BN, Anwar-Mohamed A, Aboutabl ME and El-Kadi AO. Acute doxorubicin cardiotoxicity alters cardiac cytochrome P450 expression and arachidonic acid metabolism in rats. Toxicol Appl Pharmacol. 2010;242:38–46.. [DOI] [PubMed] [Google Scholar]
- 5.Jenkins CM, Cedars A, Gross RW. Eicosanoid signalling pathways in the heart. Cardiovas Res. 2009;82:240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edin ML, Lih FB, Hammock BD, Thomson S, Zeldin DC and Bishop-Bailey D. Vascular Lipidomic profiling of potential endogenous Fatty Acid PPAR ligands reveals the coronary artery as major producer of CYP450-Derived epoxy fatty acids. Cells. 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breitbart E, Sofer Y, Shainberg A, Grossman S. Lipoxygenase activity in heart cells. FEBS Lett. 1996;395:148–52. [DOI] [PubMed] [Google Scholar]
- 8.Gu J, Liu Y, Wen Y, Natarajan R, Lanting L, Nadler JL. Evidence that increased 12-lipoxygenase activity induces apoptosis in fibroblasts. J Cell Physiol. 2001;186:357–6. [DOI] [PubMed] [Google Scholar]
- 9.Wen Y, Gu J, Liu Y, Wang PH, Sun Y, Nadler JL. Overexpression of 12-lipoxygenase causes cardiac fibroblast cell growth. Circ Res. 2001;88:70–6. [DOI] [PubMed] [Google Scholar]
- 10.Zheng Z, Li Y, Jin G, Huang T, Zou M, Duan S. The biological role of arachidonic acid 12-lipoxygenase (ALOX12) in various human diseases. The biological role of arachidonic acid 12-lipoxygenase (ALOX12) in various human diseases. Biomed Pharmacother. 2020;129:110354. [DOI] [PubMed] [Google Scholar]
- 11.Kenyon V, Rai G, Jadhav A, Schultz L, Armstrong M, Jameson JB 2nd, Perry S, Joshi N, Bougie JM, Leister W, Taylor-Fishwick DA, Nadler JL, Holinstat M, Simeonov A, Maloney DJ, Holman TR. Discovery of potent and selective inhibitors of human platelet-type 12- lipoxygenase. J Med Chem. 2011;54:5485–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adili R, Tourdot BE, Mast K, Yeung J, Freedman JC, Green A, Luci DK, Jadhav A, Simeonov A, Maloney DJ, Holman TR, Holinstat M. First Selective 12-LOX Inhibitor, ML355, impairs thrombus formation and vessel occlusion in vivo with minimal effects on hemostasis. Arterioscler Thromb Vasc Biol. 2017;37:1828–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luci D, Jameson JB II, Yasgar A, Diaz G, Joshi N, Kantz A, Markham K, Perry S, Kuhn N, Yeung J, Schultz L, Holinstat M, Nadler J, Taylor-Fishwick DA, Jadhav A, Simeonov A, Holman TR, Maloney DJ. Discovery of ML355, a Potent and Selective Inhibitor of Human 12-Lipoxygenase In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010. –. [PubMed] [Google Scholar]
- 14.Rai G, Joshi N, Perry S, Yasgar A, Schultz L, Jung JE, Liu Y, Terasaki Y, Diaz G, Kenyon V, Jadhav A, Simeonov A, van Leyen K, Holman TR, Maloney DJ. Discovery of ML351, a Potent and Selective Inhibitor of Human 15-Lipoxygenase-1. In Probe Reports from the NIH Molecular Libraries Program. Bethesda (MD), 2010. [PubMed] [Google Scholar]
- 15.Armstrong MM, Freedman CJ, Jung JE, Zheng Y, Kalyanaraman C, Jacobson MP, Simeonov A, Maloney DJ, van Leyen K, Jadhav A, Holman TR. A potent and selective inhibitor targeting human and murine 12/15-LOX. Bioorg Med Chem. 2016;24:1183–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kar F, Hacioglu C, Senturk H, Donmez DB, Kanbak G, Uslu S. Curcumin and LOX block-1 ameliorates ischemia-reperfusion induced inflammation and acute kidney injury by suppressing the semaphorin-plexin pathway. Life Sci. 2020;256:118016. [DOI] [PubMed] [Google Scholar]
- 17.Dobrian AD, Morris MA, Taylor-Fishwick DA, Holman TR, Imai Y, Mirmira RG, Nadler JL. Role of the 12-lipoxygenase pathway in diabetes pathogenesis and complications. Pharmacol Ther. 2019;195:100–110.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kayama Y, Minamino T, Toko H, Sakamoto M, Shimizu I, Takahashi H, Okada S, Tateno K, Moriya J, Yokoyama M, Nojima A, Yoshimura M, Egashira K, Aburatani H, Komuro I. Cardiac 12/15 lipoxygenase-induced inflammation is involved in heart failure. J Exp Med. 2009;206:1565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lieb DC, Brotman JJ, Hatcher MA, Aye MS, Cole BK, Haynes BA, Wohlgemuth SD, Fontana MA, Beydoun H, Nadler JL and Dobrian AD. Adipose tissue 12/15 lipoxygenase pathway in human obesity and diabetes. J Clin Endocrinol Metab. 2014;99:E1713–20.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snodgrass RG, Brune B. Regulation and Functions of 15-Lipoxygenases in Human Macrophages. Fron Pharmocol 2019;10:719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orafaie A, Mousavian M, Orafai H, Sadeghian H. An overview of lipoxygenase inhibitors with approach of in vivo studies. Prostaglandins Other Lipid Mediat. 2020;148:106411. [DOI] [PubMed] [Google Scholar]
- 22.Bocan TM, Rosebury WS, Mueller SB, Kuchera S, Welch K, Daugherty A, Cornicelli JA. A specific 15-lipoxygenase inhibitor limits the progression and monocyte-macrophage enrichment of hypercholesterolemia-induced atherosclerosis in the rabbit. Atherosclerosis. 1998;136:203–16. [DOI] [PubMed] [Google Scholar]
- 23.Choudhary D, Jansson I, Stoilov I, Sarfarazi M and Schenkman JB. Metabolism of retinoids and arachidonic acid by human and mouse cytochrome P450 1b1. Drug Metab Dispos. 2004;32:840–7. [DOI] [PubMed] [Google Scholar]
- 24.Falero-Perez J, Song YS, Sorenson CM, Sheibani N. CYP1B1: A key regulator of redox homeostasis. Trends Cell Mol Biol. 2018;13:27–45. [PMC free article] [PubMed] [Google Scholar]
- 25.Alsaad AMS. Dasatinib induces gene expression of CYP1A1, CYP1B1, and cardiac hypertrophy markers (BNP, β-MHC) in rat cardiomyocyte H9c2 cells. Toxicol Mech Methods. 2018;28:678–684. [DOI] [PubMed] [Google Scholar]
- 26.El-Sherbeni AA, El-Kadi AO. Alterations in cytochrome P450-derived arachidonic acid metabolism during pressure overload-induced cardiac hypertrophy. Biochem Pharmacol. 2014;87:456–66. [DOI] [PubMed] [Google Scholar]
- 27.Messina A, Puccinelli E, Gervasi PG, Longo V. Expression and inducibility of CYP1A1, 1A2, 1B1 by β-naphthoflavone and CYP2B22, CYP3As by rifampicin in heart regions and coronary arteries of pig. Res Vet Sci. 2013;94:77–83. [DOI] [PubMed] [Google Scholar]
- 28.Zordoky BN, Aboutabl ME, El-Kadi AO. Modulation of cytochrome P450 gene expression and arachidonic acid metabolism during isoproterenol-induced cardiac hypertrophy in rats. Drug Metab Dispos. 2008;36:2277–86. [DOI] [PubMed] [Google Scholar]
- 29.Mastyugin V, Aversa E, Bonazzi A, Vafaes C, Mieyal P, Schwartzman ML. Hypoxia-induced production of 12-hydroxyeicosanoids in the corneal epithelium: involvement of a cytochrome P-4504B1 isoform. J Pharmacol Exp Ther. 1999;289:1611–9. [PubMed] [Google Scholar]
- 30.Jennings BL, George LW, Pingili AK, Khan NS, Estes AM, Fang XR, Gonzalez FJ, Malik KU. Estrogen metabolism by cytochrome P450 1B1 modulates the hypertensive effect of angiotensin II in female mice. Hypertension. 2014;64:134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pingili AK, Kara M, Khan NS, Estes AM, Lin Z, Li W, Gonzalez FJ, Malik KU. 6β-hydroxytestosterone, a cytochrome P450 1B1 metabolite of testosterone, contributes to angiotensin II-induced hypertension and its pathogenesis in male mice. Hypertension. 2015;65:1279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jennings BL, Montanez DE, May ME, Estes AM, Fang XR, Yaghini FA, Kanu A, Malik KU. Cytochrome P450 1B1 contributes to increased blood pressure and cardiovascular and renal dysfunction in spontaneously hypertensive rats. Cardiovasc Drugs Ther. 2014;28:145–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maayah ZH, Althurwi HN, El-Sherbeni AA, Abdelhamid G, Siraki AG and El-Kadi AO. The role of cytochrome P450 1B1 and its associated mid-chain hydroxyeicosatetraenoic acid metabolites in the development of cardiac hypertrophy induced by isoproterenol. Mol Cell Biochem. 2017;429:151–165. [DOI] [PubMed] [Google Scholar]
- 34.Sahan-Firat S, Jennings BL, Yaghini FA, Song CY, Estes AM, Fang XR, Farjana N, Khan AI, Malik KU. 2,3’,4,5’-Tetramethoxystilbene prevents deoxycorticosterone-salt-induced hypertension: contribution of cytochrome P-450 1B1. Am J Physiol Heart Circ Physiol. 2010;299:H1891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conway DE, Sakurai Y, Weiss D, Vega JD, Taylor WR, Jo H, Eskin SG, Marcus CB, McIntire LV. Expression of CYP1A1 and CYP1B1 in human endothelial cells: regulation by fluid shear stress. Cardiovasc Res. 2009;81:669–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jennings BL, Sahan-Firat S, Estes AM, Das K, Farjana N, Fang XR, Gonzalez FJ, Malik KU. Cytochrome P450 1B1 contributes to angiotensin II-induced hypertension and associated pathophysiology. Hypertension. 2010;56:667–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yokomizo T, Kato K, Hagiya H, Izumi T, Shimizu T. Hydroxyeicosanoids bind to and activate the low affinity leukotriene B4 receptor, BLT2. J Biol Chem. 2001;276:12454–9. [DOI] [PubMed] [Google Scholar]
- 38.Siangjong L, Gauthier KM, Pfister SL, Smyth EM, Campbell WB. Endothelial 12(S)-HETE vasorelaxation is mediated by thromboxane receptor inhibition in mouse mesenteric arteries. Am J Physiol Heart Circ Physiol. 2013;304:H382–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo Y, Zhang W, Giroux C, Cai Y, Ekambaram P, Dilly AK, Hsu A, Zhou S, Maddipati KR, Liu J, Joshi S, Tucker SC, Lee MJ, Honn KV. Identification of the orphan G protein-coupled receptor GPR31 as a receptor for 12-(S)-hydroxyeicosatetraenoic acid. J Biol Chem. 2011;286:33832–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Honn KV, Guo Y, Cai Y, Lee MJ, Dyson G, Zhang W, Tucker SC. 12-HETER1/GPR31, a high-affinity 12(S)-hydroxyeicosatetraenoic acid receptor, is significantly up-regulated in prostate cancer and plays a critical role in prostate cancer progression. FASEB J. 2016;30:2360–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mashiko M, Kurosawa A, Tani Y, Tsuji T, Takeda S. GPR31 and GPR151 are activated under acidic conditions. J Bioichem. 2019. 23:mvz04. [DOI] [PubMed] [Google Scholar]
- 42.Morita N, Umemoto E, Fujita S, Hayashi A, Kikuta J, Kimura I, Haneda T, Imai T, Inoue A, Mimuro H, Maeda Y, Kayama H, Okumura R, Aoki J, Okada N, Kida T, Ishii M, Nabeshima R, Takeda K. GPR31-dependent dendrite protrusion of intestinal CX3CR1(+) cells by bacterial metabolites. Nature. 2019;566:110–114. [DOI] [PubMed] [Google Scholar]
- 43.Fonlupt P, Croset M and Lagarde M. 12-HETE inhibits the binding of PGH2/TXA2 receptor ligands in human platelets. Thromb Res. 1991;63:239–48. [DOI] [PubMed] [Google Scholar]
- 44.Zink MH, Oltman CL, Lu T, Katakam PV, Kaduce TL, Lee H, Dellsperger KC, Spector AA, Myers PR, Weintraub NL. 12-lipoxygenase in porcine coronary microcirculation: implications for coronary vasoregulation. Am J Physiol Heart Circ Physiol. 2001;280:H693–704. [DOI] [PubMed] [Google Scholar]
- 45.Larsen BT, Miura H, Hatoum OA, Campbell WB, Hammock BD, Zeldin DC, Falck JR, Gutterman DD. Epoxyeicosatrienoic and dihydroxyeicosatrienoic acids dilate human coronary arterioles via BK(Ca) channels: implications for soluble epoxide hydrolase inhibition. Am J Physiol Heart Circ Physiol. 2006;290:H491–9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller AW, Katakam PV, Lee HC, Tulbert CD, Busija DW, Weintraub NL. Arachidonic acid-induced vasodilation of rat small mesenteric arteries is lipoxygenase-dependent. J Pharm Exp Ther. 2003;304:139–44. [DOI] [PubMed] [Google Scholar]
- 47.Faraci FM, Sobey CG, Chrissobolis S, Lund DD, Heistad DD, Weintraub NL. Arachidonate dilates basilar artery by lipoxygenase-dependent mechanism and activation of K(+) channels Am J Physiol Regul Integr Comp Physiol. 2001;281:R246–53.. [DOI] [PubMed] [Google Scholar]
- 48.Dobrian AD, Lieb DC, Cole BK, Taylor-Fishwick DA, Chakrabarti SK and Nadler JL. Functional and pathological roles of the 12- and 15-lipoxygenases. Prog Lipid Res. 2011;50:115–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ma YH, Harder DR, Clark JE Roman RJ. Effects of 12-HETE on isolated dog renal arcuate arteries. Am J Physiol Heart Circ Physiol. 1991;261:H451–6. [DOI] [PubMed] [Google Scholar]
- 50.Saito F, Hori MT, Ideguchi Y, Berger M, Golub M, Stern N, Tuck ML. 12-Lipoxygenase products modulate calcium signals in vascular smooth muscle cells. Hypertension. 1992;20:138–43. [DOI] [PubMed] [Google Scholar]
- 51.Nguyen CH, Brenner S, Huttary N, Li Y, Atanasov AG, Dirsch VM, Holzner S, Stadler S, Riha J, Krieger S, Milovanovic D, Fristiohardy A, Simonitsch-Klupp I, Dolznig H, Saiko P, Szekeres T, Giessrigl B, Jager W, and Krupitza G 12(S)-HETE increases intracellular Ca(2+) in lymph-endothelial cells disrupting their barrier function in vitro; stabilization by clinical drugs impairing calcium supply. Cancer Lett. 2016. 380.174–183. [DOI] [PubMed] [Google Scholar]
- 52.Nguyen CH, Stadler S, Brenner S, Huttary N, Krieger S, Jager W, Dolznig H, Krupitza G. Cancer cell-derived 12(S)-HETE signals via 12-HETE receptor, RHO, ROCK and MLC2 to induce lymph endothelial barrier breaching. Br J Cancer. 2016;115:364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stadler S, Nguyen CH, Schachner H, Milovanovic D, Holzner S, Brenner S, Eichsteininger J, Stadler M, Senfter D, Krenn L, Schmidt WM, Huttary N, Krieger S, Koperek O, Bago-Horvath Z, Brendel KA, Marian B, de Wever O, Mader RM, Giessrigl B, Jager W, Dolznig H, Krupitza G. Colon cancer cell-derived 12(S)-HETE induces the retraction of cancer-associated fibroblast via MLC2, RHO/ROCK and Ca(2+) signalling. Cell Mol Life Sci. 2017;74:1907–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fristiohady A, Milovanovic D, Krieger S, Huttary N, Nguyen CH, Basilio J, Jager W, De Martin R, Krupitza G. 12(S)-HETE induces lymph endothelial cell retraction in vitro by upregulation of SOX18. Int J Oncol. 2018;53:307–316. [DOI] [PubMed] [Google Scholar]
- 55.Zhang C, Ma C, Yao H, Zhang L, Yu X, Liu Y, Shen T, Zhang F, Chen X, Zhu D. 12-Lipoxygenase and 12-hydroxyeicosatetraenoic acid regulate hypoxic angiogenesis and survival of pulmonary artery endothelial cells via PI3K/Akt pathway. Am J Physiol Lung Cell Mol Physiol. 2018;314:L606–L616. [DOI] [PubMed] [Google Scholar]
- 56.Wang X, Gao L, Xiao L, Yang L, Li W, Liu G, Chen L and Zhang J. 12(S)-hydroxyeicosatetraenoic acid impairs vascular endothelial permeability by altering adherens junction phosphorylation levels and affecting the binding and dissociation of its components in high glucose-induced vascular injury. J Diabetes Invest. 2019;10:639–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van Doren L, Nguyen N, Garzia C, Fletcher EK, Stevenson R, Jaramillo D, Kuliopulos A and Covic L. Lipid Receptor GPR31 (G-Protein-Coupled Receptor 31) regulates platelet reactivity and thrombosis without affecting hemostasis. Arterioscler Thromb Vasc Biol. 2021;41:e33–e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kerr JS, Batt DG, Pinto DJ and Stampfli HF. An evaluation of 2-benzyl-1-naphthol (DuP 654) analogs as systemic anti-inflammatory agents. Res Commun Chem Pathol Pharmacol. 1992;77:77–86. [PubMed] [Google Scholar]
- 59.Harris RR, Batt DG, Galbraith W and Ackerman NR. Topical anti-inflammatory activity of DuP 654, a 2-substituted 1-naphthol. Agents actions. 1989;27:297–9. [DOI] [PubMed] [Google Scholar]
- 60.Arenberger P, Raap A, Armah B, Kemeny L and Ruzicka T. The lipoxygenase inhibitor 2-phenylmethyl-1-naphthol (DuP 654) is a 12(S)-hydroxyeicosatetraenoic acid receptor antagonist in the human epidermal cell line SCL-II. Skin Pharmacol. 1993;6:148–51. [DOI] [PubMed] [Google Scholar]
- 61.Waldman M, Peterson SJ, Arad M, Hochhauser E. The role of 20-HETE in cardiovascular diseases and its risk factors. Prostaglandins Other Lipid Mediat. 2016;125:108–17. [DOI] [PubMed] [Google Scholar]
- 62.Levick SP, Loch DC, Taylor SM, Janicki JS. Arachidonic acid metabolism as a potential mediator of cardiac fibrosis associated with inflammation. J Immunol. 2007;178:641–6. [DOI] [PubMed] [Google Scholar]
- 63.Rocic P and Schwartzman ML. 20-HETE in the regulation of vascular and cardiac function. Pharmacol Ther. 2018;192:74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hiratsuka M, Matsuura T, Watanabe E, Sato M, Suzuki Y. Sex and strain differences in constitutive expression of fatty acid omega-hydroxylase (CYP4A-related proteins) in mice. J Biochem. 1996;119:340–5. [DOI] [PubMed] [Google Scholar]
- 65.Holla VR, Adas F, Imig JD, Zhao X, Price E, Olsen N, Kovacs WJ, Magnuson MA, Keeney DS, Breyer MD, Falck JR, Waterman MR, Capdevila JH. Alterations in the regulation of androgen-sensitive Cyp 4a monooxygenases cause hypertension. Proc Natl Acad Sci USA. 2001;98:5211–5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vasudevan H, Yuen VG and McNeill JH. Testosterone-dependent increase in blood pressure is mediated by elevated Cyp4A expression in fructose-fed rats. Mol Cell Biochem. 2012;359:409–18.. [DOI] [PubMed] [Google Scholar]
- 67.Thomas M, Winter S, Klumpp B, Turpeinen M, Klein K, Schwab M, Zanger UM. Peroxisome proliferator-activated receptor alpha, PPARalpha, directly regulates transcription of cytochrome P450 CYP2C8. Front Pharm. 2015;6:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hutcheson R, Terry R, Chaplin J, Smith E, Musiyenko A, Russell JC, Lincoln T, Rocic P. MicroRNA-145 restores contractile vascular smooth muscle phenotype and coronary collateral growth in the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2013;33:727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rong R, Hu G, Wang W, Muroya Y, Miura T, Ogawa Y, Kohzuki M, Ito O. Angiotensin II upregulates CYP4A isoform expression in the rat kidney through angiotensin II type 1 receptor. Prostaglandins Other Lipid Mediat. 2018;139:80–86. [DOI] [PubMed] [Google Scholar]
- 70.Zhou Y, Yu J, Liu J, Cao R, Su W, Li S, Ye S, Zhu C, Zhang X, Xu H, Chen H and Guan Y. Induction of cytochrome P450 4A14 contributes to angiotensin II-induced renal fibrosis in mice. Biochim Biophys Acta Mol Basis Dis. 2018;1864:860–870. [DOI] [PubMed] [Google Scholar]
- 71.Ito O, Omata K, Ito S, Hoagland KM and Roman RJ. Effects of converting enzyme inhibitors on renal P-450 metabolism of arachidonic acid. Am J Physiol Regul Integr Comp Physiol. 2001;280:R822–30. [DOI] [PubMed] [Google Scholar]
- 72.Garcia V, Gilani A, Shkolnik B, Pandey V, Zhang FF, Dakarapu R, Gandham SK, Reddy NR, Graves JP, Gruzdev A, Zeldin DC, Capdevila JH, Falck JR and Schwartzman ML. 20-HETE signals through G-Protein-Coupled Receptor GPR75 (Gq) to affect vascular function and trigger hypertension. Circ Res. 2017;120:1776–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fan F, Roman RJ. GPR75 Identified as the first 20-HETE receptor: a chemokine receptor adopted by a new family. Circ Res. 2017;120:1696–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cheng J, Ou JS, Singh H, Falck JR, Narsimhaswamy D, Pritchard KA Jr. and Schwartzman ML. 20-hydroxyeicosatetraenoic acid causes endothelial dysfunction via eNOS uncoupling. Am J Physiol Heart Circ Physiol. 2008;294:H1018–26. [DOI] [PubMed] [Google Scholar]
- 75.Cheng J, Garcia V, Ding Y, Wu CC, Thakar K, Falck JR, Ramu E, Schwartzman ML. Induction of angiotensin-converting enzyme and activation of the renin-angiotensin system contribute to 20-hydroxyeicosatetraenoic acid-mediated endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2012;32:1917–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Garcia V, Shkolnik B, Milhau L, Falck JR, Schwartzman ML. 20-HETE activates the transcription of angiotensin-converting enzyme via Nuclear Factor-kappa translocation and promoter binding. J Pharmacol Exp Ther. 2016. 356.525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Garcia V, Joseph G, Shkolnik B, Ding Y, Zhang FF, Gotlinger K, Falck JR, Dakarapu R, Capdevila JH, Bernstein KE, Schwartzman ML. Angiotensin II receptor blockade or deletion of vascular endothelial ACE does not prevent vascular dysfunction and remodeling in 20-HETE-dependent hypertension. Am J Physiol Regul Integr Comp Physiol. 2015;309:R71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tunaru S, Bonnavion R, Brandenburger I, Preussner J, Thomas D, Scholich K, Offermanns S. 20-HETE promotes glucose-stimulated insulin secretion in an autocrine manner through FFAR1. Nat Comm. 2018;9:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Park SK, Herrnreiter A, Pfister SL, Gauthier KM, Falck BA, Falck JR and Campbell WB. GPR40 is a low-affinity epoxyeicosatrienoic acid receptor in vascular cells. J Biol Chem. 2018;293:10675–10691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ignatov A, Robert J, Gregory-Evans C, Schaller HC. RANTES stimulates Ca2+ mobilization and inositol trisphosphate (IP3) formation in cells transfected with G protein-coupled receptor 75. Br J Pharmacol. 2006;149:490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dedoni S, Campbell LA, Harvey BK, Avdoshina V, Mocchetti I. The orphan G-protein-coupled receptor 75 signaling is activated by the chemokine CCL5. J Neurochem. 2018;146:526–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu B, Hassan Z, Amisten S, King AJ, Bowe JE, Huang GC, Jones PM, Persaud SJ. The novel chemokine receptor, G-protein-coupled receptor 75, is expressed by islets and is coupled to stimulation of insulin secretion and improved glucose homeostasis. Diabetologia. 2013;56:2467–76. [DOI] [PubMed] [Google Scholar]
- 83.Southern C, Cook JM, Neetoo-Isseljee Z, Taylor DL, Kettleborough CA, Merritt A, Bassoni DL, Raab WJ, Quinn E, Wehrman TS, Davenport AP, Brown AJ, Green A, Wigglesworth MJ, Rees S. Screening beta-arrestin recruitment for the identification of natural ligands for orphan G-protein-coupled receptors. J Biomol Screen. 2013;18:599–609. [DOI] [PubMed] [Google Scholar]
- 84.Davenport AP, Alexander SP, Sharman JL, Pawson AJ, Benson HE, Monaghan AE, Liew WC, Mpamhanga CP, Bonner TI, Neubig RR, Pin JP, Spedding M and Harmar AJ. International Union of Basic and Clinical Pharmacology. LXXXVIII. G protein-coupled receptor list: recommendations for new pairings with cognate ligands. Pharmacol Rev. 2013;65:967–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guo AM, Arbab AS, Falck JR, Chen P, Edwards PA, Roman RJ and Scicli AG. Activation of vascular endothelial growth factor through reactive oxygen species mediates 20-hydroxyeicosatetraenoic acid-induced endothelial cell proliferation. J Pharm Exp Ther. 2007;321:18–27. [DOI] [PubMed] [Google Scholar]
- 86.Guo AM, Scicli G, Sheng J, Falck JC, Edwards PA, Scicli AG. 20-HETE can act as a nonhypoxic regulator of HIF-1alpha in human microvascular endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H602–13. [DOI] [PubMed] [Google Scholar]
- 87.Ishizuka T, Cheng J, Singh H, Vitto MD, Manthati VL, Falck JR, Laniado-Schwartzman M. 20-Hydroxyeicosatetraenoic acid stimulates nuclear factor-kappaB activation and the production of inflammatory cytokines in human endothelial cells. J Pharm Exp Therap. 2008;324:103–10. [DOI] [PubMed] [Google Scholar]
- 88.Cheng J, Edin ML, Hoopes SL, Li H, Bradbury JA, Graves JP, DeGraff LM, Lih FB, Garcia V, Shaik JS, Tomer KB, Flake GP, Falck JR, Lee CR, Poloyac SM, Schwartzman ML, Zeldin DC. Vascular characterization of mice with endothelial expression of cytochrome P450 4F2. FASEB J. 2014;28:2915–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fan F, Sun CW, Maier KG, Williams JM, Pabbidi MR, Didion SP, Falck JR, Zhuo J and Roman RJ. 20-Hydroxyeicosatetraenoic acid contributes to the inhibition of K+ channel activity and vasoconstrictor response to angiotensin II in rat renal microvessels. PloS one. 2013;8:e82482.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zou AP, Fleming JT, Falck JR, Jacobs ER, Gebremedhin D, Harder DR, Roman RJ. 20-HETE is an endogenous inhibitor of the large-conductance Ca(2+)-activated K+ channel in renal arterioles. Am J Physiol Regul Integr Comp. 1996;270:R228–37. [DOI] [PubMed] [Google Scholar]
- 91.Fan F, Ge Y, Lv W, Elliott MR, Muroya Y, Hirata T, Booz GW and Roman RJ. Molecular mechanisms and cell signaling of 20-hydroxyeicosatetraenoic acid in vascular pathophysiology. Front Biosci. 2016;21:1427–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gebremedhin D, Lange AR, Narayanan J, Aebly MR, Jacobs ER, Harder DR. Cat cerebral arterial smooth muscle cells express cytochrome P450 4A2 enzyme and produce the vasoconstrictor 20-HETE which enhances L-type Ca2+ current. J Physiol. 1998;507:771–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zeng Q, Han Y, Bao Y, Li W, Li X, Shen X, Wang X, Yao F, O’Rourke ST, Sun C. 20-HETE increases NADPH oxidase-derived ROS production and stimulates the L-type Ca2+ channel via a PKC-dependent mechanism in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2010;299:H1109–17.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Basora N, Boulay G, Bilodeau L, Rousseau E and Payet MD. 20-hydroxyeicosatetraenoic acid (20-HETE) activates mouse TRPC6 channels expressed in HEK293 cells. J Biol Chem. 2003;278:31709–16. [DOI] [PubMed] [Google Scholar]
- 95.Wen H, Ostman J, Bubb KJ, Panayiotou C, Priestley JV, Baker MD, Ahluwalia A. 20-Hydroxyeicosatetraenoic acid (20-HETE) is a novel activator of transient receptor potential vanilloid 1 (TRPV1) channel. J Biol Chem. 2012;287:13868–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Inoue K, Sodhi K, Puri N, Gotlinger KH, Cao J, Rezzani R, Falck JR, Abraham NG, Laniado-Schwartzman M. Endothelial-specific CYP4A2 overexpression leads to renal injury and hypertension via increased production of 20-HETE. Am J Physiol Renal Physiol. 2009;297:F875–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Randriamboavonjy V, Busse R, Fleming I. 20-HETE-induced contraction of small coronary arteries depends on the activation of Rho-kinase. Hypertension. 2003;41:801–6. [DOI] [PubMed] [Google Scholar]
- 98.Lakhkar A, Dhagia V, Joshi SR, Gotlinger K, Patel D, Sun D, Wolin MS, Schwartzman ML Gupte SA. 20-HETE-induced mitochondrial superoxide production and inflammatory phenotype in vascular smooth muscle is prevented by glucose-6-phosphate dehydrogenase inhibition. Am J Physiol Heart Circ Physiol. 2016;310:H1107–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Miyata N, Taniguchi K, Seki T, Ishimoto T, Sato-Watanabe M, Yasuda Y, Doi M, Kametani S, Tomishima Y, Ueki T, Sato M and Kameo K. HET0016, a potent and selective inhibitor of 20-HETE synthesizing enzyme. Br J Pharm. 2001;133:325–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Alonso-Galicia M, Drummond HA, Reddy KK, Falck JR and Roman RJ. Inhibition of 20-HETE production contributes to the vascular responses to nitric oxide. Hypertension. 1997;29:320–5. [DOI] [PubMed] [Google Scholar]
- 101.Jain M, Gamage NH, Alsulami M, Shankar A, Achyut BR, Angara K, Rashid MH, Iskander A, Borin TF, Wenbo Z, Ara R, Ali MM, Lebedyeva I, Chwang WB, Guo A, Bagher-Ebadian H, Arbab AS. Intravenous formulation of HET0016 decreased human glioblastoma growth and implicated survivalbenefit in rat xenograft models. Sci Rep. 2017;7:41809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen L, Tang S, Zhang FF, Garcia V, Falck JR, Schwartzman ML, Arbab AS, Guo AM. CYP4A/20-HETE regulates ischemia-induced neovascularization via its actions on endothelial progenitor and preexisting endothelial cells. Am J Physiol Heart Circ Physiol. 2019;316:H1468–H1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gangadhariah MH, Luther JM, Garcia V, Paueksakon P, Zhang MZ, Hayward SW, Love HD, Falck JR, Manthati VL, Imig JD, Schwartzman ML, Zent R, Capdevila JH,Pozzi A. Hypertension is a major contributor to 20-hydroxyeicosatetraenoic acid-mediated kidney injury in diabetic nephropathy. J Amer Soc Nephrol. 2015;26:597–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sedlakova L, Kikerlova S, Huskova Z, Cervenkova L, Chabova VC, Zicha J, Falck JR, Imig JD, Kompanowska-Jezierska E, Sadowski J, Kratky V, Cervenka L and Kopkan L. 20-Hydroxyeicosatetraenoic acid antagonist attenuates the development of malignant hypertension and reverses it once established: a study in Cyp1a1-Ren-2 transgenic rats. Biosci Rep. 2018;38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Savas U, Wei S, Hsu MH, Falck JR, Guengerich FP, Capdevila JH and Johnson EF. 20-Hydroxyeicosatetraenoic Acid (HETE)-dependent hypertension in human hytochrome P450 (CYP) 4A11 transgenic mice: normalization of blood pressure by sodium restriction, hydrochlorothiazide, or blockade of the Type 1 Angiotensin II Receptor. J Biol Chem. 2016;291:16904–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Joseph G, Soler A, Hutcheson R, Hunter I, Bradford C, Hutcheson B, Gotlinger KH, Jiang H, Falck JR, Proctor S, Schwartzman ML and Rocic P. Elevated 20-HETE impairs coronary collateral growth in metabolic syndrome via endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2017. 312.H528–H540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Azevedo PS, Polegato BF, Minicucci MF, Paiva SA and Zornoff LA. Cardiac remodeling: concepts, clinical impact, pathophysiological mechanisms and pharmacologic treatment Arq Bras Cardiol. 2016. 106.62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Malakar AK, Choudhury D, Halder B, Paul P, Uddin A and Chakraborty S. A review on coronary artery disease, its risk factors, and therapeutics. J Cell Physiol. 2019. 234.16812–16823. [DOI] [PubMed] [Google Scholar]
- 109.Huang CC, Chang MT, Leu HB, Yin WH, Tseng WK, Wu YW, Lin TH, Yeh HI, Chang KC, Wang JH, Wu CC, Shyur LF, Chen JW. Association of arachidonic acid-derived lipid mediators with subsequent onset of acute myocardial infarction in patients with coronary artery disease. Sci Rep. 2020;10:8105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Issan Y, Hochhauser E, Guo A, Gotlinger KH, Kornowski R, Leshem-Lev D, Lev E, Porat E, Snir E, Thompson CI, Abraham NG, Laniado-Schwartzman M. Elevated level of pro-inflammatory eicosanoids and EPC dysfunction in diabetic patients with cardiac ischemia. Prost Lipid Med. 2013;100–101:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Natarajan R, Gerrity RG, Gu JL, Lanting L, Thomas L and Nadler JL. Role of 12-lipoxygenase and oxidant stress in hyperglycaemia-induced acceleration of atherosclerosis in a diabetic pig model. Diabetologia. 2002;45:125–33. [DOI] [PubMed] [Google Scholar]
- 112.Antonipillai I, Nadler J, Vu EJ, Bughi S, Natarajan R, Horton R. A 12-lipoxygenase product, 12-hydroxyeicosatetraenoic acid, is increased in diabetics with incipient and early renal disease. J Clin Endo Metabol. 1996;81:1940–5. [DOI] [PubMed] [Google Scholar]
- 113.Zhang HJ, Sun CH, Kuang HY, Jiang XY, Liu HL, Hua WF, Liu ZJ, Zhou H, Sui H, Qi R. 12S-hydroxyeicosatetraenoic acid levels link to coronary artery disease in Type 2 diabetic patients. J Endo Invest. 2013;36:385–9. [DOI] [PubMed] [Google Scholar]
- 114.Abdel-Rahman EM, Abadir PM, Siragy HM. Regulation of renal 12(S)-hydroxyeicosatetraenoic acid in diabetes by angiotensin AT1 and AT2 receptors. Amer J Physiol: Reg Integ Comp Physiol. 2008;295:R1473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Auguet T, Aragones G, Colom M, Aguilar C, Martin-Paredero V, Canela N, Ruyra X, Richart C. Targeted metabolomic approach in men with carotid plaque. PloS one. 2018;13:e0200547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lu MJ, Chen YS, Huang HS, Ma MC. Hypoxic preconditioning protects rat hearts against ischemia-reperfusion injury via the arachidonate12-lipoxygenase/transient receptor potential vanilloid 1 pathway. Basic Res Cardiol. 2014;109:414. [DOI] [PubMed] [Google Scholar]
- 117.Kain V, Ingle KA, Kabarowski J, Barnes S, Limdi NA, Prabhu SD and Halade GV. Genetic deletion of 12/15 lipoxygenase promotes effective resolution of inflammation following myocardial infarction. J Mol Cell Cardiol. 2018;118:70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kuzuya T, Hoshida S, Nishida M, Kim Y, Kamada T, Tada M. Increased production of arachidonate metabolites in an occlusion-reperfusion model of canine myocardial infarction. Cardiovasc Res. 1987;21:551–8. [DOI] [PubMed] [Google Scholar]
- 119.Shibata N, Akagami H, Sanma H, Goshima K. Augmentation of eicosanoids in ischemic heart muscle in dogs: its role in the deterioration of the ischemic lesion. Jpn Circ J. 1988;52:673–83. [DOI] [PubMed] [Google Scholar]
- 120.Halade GV, Kain V, Dillion C, Beasley M, Dudenbostel T, Oparil S, Limdi NA. Race-based and sex-based differences in bioactive lipid mediators after myocardial infarction. ESC Heart Failure. 2020;7:1700–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nazarewicz RR, Zenebe WJ, Parihar A, Parihar MS, Vaccaro M, Rink C, Sen CK, Ghafourifar P. 12(S)-hydroperoxyeicosatetraenoic acid (12-HETE) increases mitochondrial nitric oxide by increasing intramitochondrial calcium. Arch Biochem Biophys. 2007;468:114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Moon SH, Liu X, Cedars AM, Yang K, Kiebish MA, Joseph SM, Kelley J, Jenkins CM and Gross RW. Heart failure-induced activation of phospholipase iPLA2γ generates hydroxyeicosatetraenoic acids opening the mitochondrial permeability transition pore. J Biol Chem. 2018;293:115–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gabel SA, London RE, Funk CD, Steenbergen C and Murphy E. Leukocyte-type 12-lipoxygenase-deficient mice show impaired ischemic preconditioning-induced cardioprotection. Am J Physiol Heart Circ Physiol. 2001;280:H1963–9. [DOI] [PubMed] [Google Scholar]
- 124.Fu Z, Ma Y, Xie X, Huang D, Yang H, Nakayama T, Sato N. A novel polymorphism of the CYP4A11 gene is associated with coronary artery disease.Clin Appl Thromb Hemost. 2013. 19.60–5. [DOI] [PubMed] [Google Scholar]
- 125.Gross ER, Nithipatikom K, Hsu AK, Peart JN, Falck JR, Campbell WB and Gross GJ. Cytochrome P450 omega-hydroxylase inhibition reduces infarct size during reperfusion via the sarcolemmal KATP channel. J Mol Cell Cardiol. 2004;37:1245–9. [DOI] [PubMed] [Google Scholar]
- 126.Nithipatikom K, Gross ER, Endsley MP, Moore JM, Isbell MA, Falck JR, Campbell WB, Gross GJ. Inhibition of cytochrome P450 ω-hydroxylases: a novel endogenous cardioprotective pathway. Circ Res. 2004;95:e65–71. [DOI] [PubMed] [Google Scholar]
- 127.Nithipatikom K, DiCamelli RF, Kohler S, Gumina RJ, Falck JR, Campbell WB, Gross GJ. Determination of cytochrome P450 metabolites of arachidonic acid in coronary venous plasma during ischemia and reperfusion in dogs. Anal Biochem. 2001;292:115–24.. [DOI] [PubMed] [Google Scholar]
- 128.Gross GJ, Falck JR, Gross ER, Isbell M, Moore J, Nithipatikom K. Cytochrome P450 and arachidonic acid metabolites: role in myocardial ischemia/reperfusion injury revisited. Cardiovasc Res. 2005;68:18–25. [DOI] [PubMed] [Google Scholar]
- 129.Nithipatikom K, Endsley MP, Moore JM, Isbell MA, Falck JR, Campbell WB, Gross GJ. Effects of selective inhibition of cytochrome P-450 ω-hydroxylases and ischemic preconditioning in myocardial protection. Am J Physiol Heart Circ Physiol. 2006;290:H500–5. [DOI] [PubMed] [Google Scholar]
- 130.Yousif MH, Benter IF and Roman RJ. Cytochrome P450 metabolites of arachidonic acid play a role in the enhanced cardiac dysfunction in diabetic rats following ischaemic reperfusion injury. Auton Autacoid Pharmacol. 2009;29:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gilani A, Pandey V, Garcia V, Agostinucci K, Singh SP, Schragenheim J, Bellner L, Falck JR, Paudyal MP, Capdevila JH, Abraham NG and Laniado Schwartzman M. High-fat diet-induced obesity and insulin resistance in CYP4a14(−/−) mice is mediated by 20-HETE. Am J Physiol Regul Integr Comp Physiol. 2018. 315.R934–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Peterson SJ, Vanella L, Gotlinger K, Jiang H, Singh SP, Sodhi K, Maher E, O’Hanlon K, Shapiro JI, Abraham NG. Oxidized HDL is a potent inducer of adipogenesis and causes activation of the Ang-II and 20-HETE systems in human obese females. Prostaglandins Other Lipid Mediat. 2016;123:68–77. [DOI] [PubMed] [Google Scholar]
- 133.Laffer CL, Laniado-Schwartzman M, Nasjletti A, Elijovich F. 20-HETE and circulating insulin in essential hypertension with obesity. Hypertension. 2004;43:388–92. [DOI] [PubMed] [Google Scholar]
- 134.Soler A, Hunter I, Joseph G, Hutcheson R, Hutcheson B, Yang J, Zhang FF, Joshi SR, Bradford C, Gotlinger KH, Maniyar R, Falck JR, Proctor S, Schwartzman ML, Gupte SA, Rocic P. Elevated 20-HETE in metabolic syndrome regulates arterial stiffness and systolic hypertension via MMP12 activation. J Mol Cell Cardiol. 2018;117:88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bao Y, Wang X, Li W, Huo D, Shen X, Han Y, Tan J, Zeng Q, Sun C. 20-Hydroxyeicosatetraenoic acid induces apoptosis in neonatal rat cardiomyocytes through mitochondrial-dependent pathways. J Cardiovasc Pharmacol. 2011;57:294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhao H, Qi G, Han Y, Shen X, Yao F, Xuan C, Gu Y, Qian SY, Zeng Q, O’Rourke ST and Sun C. 20-Hydroxyeicosatetraenoic Acid Is a key mediator of angiotensin II-induced apoptosis in cardiac myocytes. J Cardiovasc Pharmacol. 2015;66:86–95. [DOI] [PubMed] [Google Scholar]
- 137.Jiang S, Huo D, Wang X, Zhao H, Tan J, Zeng Q, O’Rourke ST and Sun C. Beta-adrenergic receptor-stimulated cardiac myocyte apoptosis: role of Cytochrome P450 ω-hydroxylase. J Cardiovasc Pharmacol. 2017;70:94–101. [DOI] [PubMed] [Google Scholar]
- 138.Wang R, Wang L, He J, Li S, Yang X, Sun P, Yuan Y, Peng J, Yan J, Du J and Li H. Specific inhibition of CYP4A alleviates myocardial oxidative stress and apoptosis induced by advanced glycation end-products. Fron Pharmacol. 2019;10:876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kalogeris T, Baines CP, Krenz M and Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int Rev Cell Molec Biol. 2012;298:229–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wu CC, Gupta T, Garcia V, Ding Y, Schwartzman ML. 20-HETE and blood pressure regulation: clinical implications. Cardiol Rev. 2014;22:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jamieson KL, Endo T, Darwesh AM, Samokhvalov V and Seubert JM. Cytochrome P450-derived eicosanoids and heart function. Pharm Therap. 2017;179:47–83. [DOI] [PubMed] [Google Scholar]
- 142.Roman RJ and Fan F. 20-HETE: hypertension and beyond. Hypertension. 2018;72:12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wen Y, Gu J, Peng X, Zhang G. Nadler JL. Overexpression of 12-lipoxygenase and cardiac fibroblast hypertrophy. Trends Cardiovas Med. 2003;13:129–36 [DOI] [PubMed] [Google Scholar]
- 144.Zordoky BN and El-Kadi AO. Modulation of cardiac and hepatic cytochrome P450 enzymes during heart failure. Curr Drug Metabol. 2008;9:122–8. [DOI] [PubMed] [Google Scholar]
- 145.Matsumura N, Takahara S, Maayah ZH, Parajuli N, Byrne NJ, Shoieb SM, Soltys CM, Beker DL, Masson G, El-Kadi AOS, Dyck JRB. Resveratrol improves cardiac function and exercise performance in MI-induced heart failure through the inhibition of cardiotoxic HETE metabolites. J Mol Cell Cardiol. 2018;125:162–173. [DOI] [PubMed] [Google Scholar]
- 146.Berkowicz P, Kij A, Walczak M and Chlopicki S. Eicosanoid profiling in effluent of isolated perfused heart of Tgαq*44 mice with advanced heart failure. J Physiol Pharm: 2019;70:135–14. [DOI] [PubMed] [Google Scholar]
- 147.Maayah ZH and El-Kadi AO. 5-, 12- and 15-Hydroxyeicosatetraenoic acids induce cellular hypertrophy in the human ventricular cardiomyocyte, RL-14 cell line, through MAPK- and NF-(B-dependent mechanism. Arch Toxicol. 2016;90:359–73. [DOI] [PubMed] [Google Scholar]
- 148.E Elkhatali S, Maayah ZH, El-Sherbeni AA, Elshenawy OH, Abdelhamid G, Shoieb SM and El-Kadi AOS. Inhibition of mid-chain HETEs protects against angiotensin II-induced cardiachypertrophy. J Cardiovasc Pharmacol. 2017;70:16–24. [DOI] [PubMed] [Google Scholar]
- 149.Shoieb SM, El-Kadi AOS. Resveratrol attenuates angiotensin II-induced cellular hypertrophy through the inhibition of CYP1B1 and the cardiotoxic mid-chain HETE metabolites. Molec Cell Biochem. 2020;471:165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kain V, Halade GV. Immune responsive resolvin D1 programs peritoneal macrophages and cardiac fibroblast phenotypes in diversified metabolic microenvironment. J Cell Physiol. 2019;234:3910–3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Elkhatali S, El-Sherbeni AA, Elshenawy OH, Abdelhamid G, El-Kadi AO. 19-Hydroxyeicosatetraenoic acid and isoniazid protect against angiotensin II-induced cardiac hypertrophy. Toxicol App Pharmacol. 2015;289:550–9. [DOI] [PubMed] [Google Scholar]
- 152.Boerrigter G, Soergel DG, Violin JD, Lark MW and Burnett JC Jr TRV120027, a novel β-arrestin biased ligand at the angiotensin II type I receptor, unloads the heart and maintains renal function when added to furosemide in experimental heart failure. Circ: Heart Failure. 2012;5:627–34. [DOI] [PubMed] [Google Scholar]
- 153.Alves MA, Lamichhane S, Dickens A, McGlinchey A, Ribeiro HC, Sen P, Wei F, Hyotylainen T, Oresic M. Systems biology approaches to study lipidomes in health and disease. Biochim Biophys Acta: Molec Cell Biol Lipids. 2021;1866:158857. [DOI] [PubMed] [Google Scholar]


