Abstract
Objectives:
With the increasing recognition of gastrointestinal (GI) manifestation of coronavirus disease-19 (COVID-19), various abdominal imaging findings are increasingly being noted. We scoped the existing literature on the abdominal imaging findings in COVID-19.
Methods:
A systematic literature search was performed on PubMed, Embase, Google scholar and World Health Organization COVID-19 database.
Results:
35 studies were included in the final descriptive synthesis. Among the studies reporting positive abdominal imaging findings in patients with COVID-19, majority described imaging abnormalities of the GI tract (16 studies), of which bowel wall thickening was most frequently reported. Other findings noted were abdominal imaging manifestations of bowel ischemia with thrombosis of the splanchnic vasculature, and imaging features suggestive of pancreatitis. Imaging findings suggestive of solid organ infarction were reported in nine studies. An association between imaging evidence of hepatic steatosis and COVID-19 was noted in three studies. Incidental lung base findings on abdominal imaging were noted in 18 studies, where patients presented with predominant GI symptoms. The most common finding was bilateral ground glass opacities (90.7%) with predominant multilobar (91.1%) and peripheral (64.4%) distribution.
Conclusion:
This systematic review provides insight into the abdominal imaging findings in patients with COVID-19. Knowledge of these imaging manifestations will not only help in further research but also will aid in curtailing transmission of the SARS-CoV-2. Further prospective studies are needed to gain better insight into the pathophysiology of these imaging manifestations.
Advances in knowledge:
This review highlights the abdominal imaging findings in patients with COVID-19, to gain insight into the disease pathophysiology and gear the abdominal radiologist through the pandemic.
Introduction
The world has faced a great challenge in public health with the Coronavirus Disease-19 (COVID-19) pandemic. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is known to infect the respiratory epithelium and cause respiratory illness ranging from mild flu to severe interstitial pneumonia and acute lung injury. The virus targets the type II alveolar cells abundantly expressing the angiotensin converting enzyme II (ACE-II) receptors.1 Gastrointestinal (GI) involvement was first reported by Xiao et al2 where they demonstrated viral ribonucleic acid in the stool specimen of patients with COVID-19. Further studies showed that GI tract may be a target organ of SARS-CoV-2 as the ACE-II receptors are abundantly expressed on the epithelium of both small and large intestine.2,3 Despite the increasing recognition of the abdominal manifestations, the literature on abdominal imaging in COVID-19 is sparse and scattered. The abdominal or emergency radiologist may be the first to suspect and suggest a diagnosis of COVID-19 in a patient presenting with atypical symptoms. Knowledge of the abdominal imaging findings of patients infected with SARS-CoV-2 and presenting with GI symptoms may help in understanding the pathophysiology of the disease, and thus aid in better prognostication of this subset of patients. This review was conducted with the aim to scope the existing literature on the abdominal imaging findings in confirmed COVID-19 cases. Prior to the start of this review, we checked to make sure that there was no existing or ongoing review on this topic.
Methods and materials
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.4 The review was registered on PROSPERO (CRD42020193885). An initially planned meta-analysis could not be performed because of the inadequacy of published literature for quantitative synthesis of data.
Data source and literature search
Literature databases including PubMed (Medline), Embase (Ovid) and selected parts of the Cochrane library relating to COVID-19 were searched from inception of COVID-19 to August 2, 2020. An updated literature search was performed on September 5, 2020. Keywords that made up our search included (“coronavirus” OR “nCoV” OR “2019-nCoV” OR “COVID-19”) AND (“abdominal imaging” OR “abdominal CT” OR “abdominal ultrasound “OR “abdominal MRI”). The detailed search strategy is mentioned in the Supplementary Material 1, supplementary appendix. Furthermore, COVID-19 publications in the WHO publication database, The Lancet COVID-19 Resource Centre, JAMA, BMJ were screened for relevant publications. Additional articles were retrieved by screening the reference lists of the included studies and reviews. We hand-searched conference and meeting abstract lists and the relevant articles were cross-referenced to identify relevant studies. There was no language restriction in the searches. There were no restrictions in terms of country or publication date.
Research question
The following research questions guided this systematic review: What are the abdominal imaging findings in patients with COVID-19 infection? Do the abdominal imaging findings correlate with the severity of COVID-19 infection?
Eligibility criteria
All studies included in this systematic review were peer-reviewed, and included original research, case series and case reports, that described or provided an overview of abdominal imaging findings among confirmed COVID-19 cases. Only the articles which described abdominal imaging findings in confirmed COVID-19 cases were included in the review. No age-related exclusions were made.
Study selection
Study selection was performed by two independent investigators (LA, AA). In the final review, the studies or articles which comprised of patients with confirmed COVID-19 and had reported abdominal imaging (ultrasound, CT, or MRI) findings were included. Discrepancies between reviewers were resolved through consensus with a third reviewer (KSM).
Data extraction and synthesis
The following data categories were collected when available: study design, country, patient demographics, clinical presentation, imaging modality and findings on abdominal imaging, severity grading of COVID-19 infection (Supplementary Table 1). One of the reviewers performed the data extraction (AA) and the other reviewer assessed the accuracy of the extracted data (AC).
Risk of bias
Two reviewers (SA, VK) independently rated the quality of the included studies using the National Institutes of Health Quality Assessment Tools5 (Tables 1–3). Any difference was resolved by consensus, in the presence of the third reviewer (KSM). Studies rated as poor quality on the NIH quality assessment tool were excluded from the final qualitative analysis.
Table 1.
Quality assessment of the included observational cohort studies based on the National Institutes of Health (NIH) quality assessment tool
| First author (Reference no.) |
Question (NIH assessment tool)a | Overall rating | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | Reviewer#1 | Reviewer#2 | |
| Goldberg-Stein et al6 | Yes | Yes | Yes | NA | No | Yes | No | No | No | No | Yes | No | No | No | Fair | Fair |
| King et al7 | Yes | Yes | Yes | NA | No | Yes | No | No | No | No | Yes | No | No | No | Fair | Fair |
| Shea et al8 | Yes | Yes | Yes | NA | No | Yes | No | No | No | No | Yes | No | No | No | Fair | Good |
| Norsa et al9 | Yes | Yes | Yes | No | No | Yes | No | No | No | No | Yes | No | No | No | Fair | Fair |
| Bhayana et al10 | Yes | Yes | Yes | Yes | No | Yes | No | No | No | No | Yes | No | No | No | Good | Fair |
| Palomar-Lever et al11 | Yes | Yes | Yes | No | No | Yes | No | No | No | No | Yes | No | No | No | Good | Fair |
| Uchida et al12 | Yes | Yes | Yes | No | No | Yes | No | No | No | No | Yes | No | No | No | Fair | Fair |
| Dane et al13 | Yes | Yes | Yes | CD | No | Yes | CD | CD | CD | No | Yes | CD | CD | No | Fair | Good |
| Shiralkar et al14 | Yes | Yes | Yes | No | No | Yes | No | No | No | No | Yes | No | No | No | Fair | Fair |
| Xiao et al.15 | Yes | Yes | Yes | NA | No | Yes | No | No | No | No | Yes | No | No | No | Fair | Fair |
| Sellevol et al.16 | Yes | No | Yes | CD | No | No | CD | CD | CD | No | No | CD | CD | No | Fair | Fair |
| Liu et al.17 | Yes | Yes | Yes | Yes | No | Yes | No | No | No | No | Yes | No | No | No | Fair | Good |
CD, Cannot determine; NA, Not applicable; NIH, National Institutes of Health; NR, Not reported.
Source: National Heart, Lung, and Blood Institute; National Institutes of Health; U.S. Department of Health and Human Services).
The NIH Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies5 includes 14 questions: 1 = Was the research question or objective in this paper clearly stated? 2 = Was the study population clearly specified and defined? 3 = Was the participation rate of eligible persons at least 50%? 4 = Were all the subjects selected or recruited from the same or similar populations (including the same time period)? Were inclusion and exclusion criteria for being in the study prespecified and applied uniformly to all participants? 5 = Was a sample size justification, power description, or variance and effect estimates provided? 6 = For the analyses in this paper, were the exposure(s) of interest measured prior to the outcome(s) being measured? 7 = Was the timeframe sufficient so that one could reasonably expect to see an association between exposure and outcome if it existed? 8 = For exposures that can vary in amount or level, did the study examine different levels of the exposure as related to the outcome (e.g., categories of exposure, or exposure measured as continuous variable)? 9 = Were the exposure measures (independent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? 10 = Was the exposure(s) assessed more than once over time? 11 = Were the outcome measures (dependent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? 12 = Were the outcome assessors blinded to the exposure status of participants? 13 = Was loss to follow-up after baseline 20% or less? 14 = Were key potential confounding variables measured and adjusted statistically for their impact on the relationship between exposure(s) and outcome(s)?
Table 2.
Quality assessment of the included case–control studies based on the National Institutes of Health (NIH) quality assessment tool
| First author (Reference no.) |
Question (NIH assessment tool)a | Overall rating | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | Reviewer#1 | Reviewer#2 | |
| Bari Dane et al18 | Yes | Yes | No | CD | Yes | Yes | No | Yes | Yes | No | No | No | Fair | Fair |
| Medeiros et al19 | Yes | Yes | No | No | Yes | Yes | No | Yes | Yes | No | No | No | Fair | Fair |
CD, Cannot determine; NA, Not applicable; NIH, National Institutes of Health; NR, Not reported.
Source: National Heart, Lung, and Blood Institute; National Institutes of Health; U.S. Department of Health and Human Services).
The NIH Quality Assessment Tool for Case–Control Studies5 includes 12 questions: 1 = Was the research question or objective in this paper clearly stated? 2 = Was the study population clearly specified and defined? 3 = Did the authors include a sample size justification? 4 = Were controls selected or recruited from the same or similar population that gave rise to the cases (including the same timeframe)? 5 = Were the definitions, inclusion and exclusion criteria, algorithms or processes used to identify or select cases and controls valid, reliable, and implemented consistently across all study participants? 6 = Were the cases clearly defined and differentiated from controls? 7 = If less than 100 percent of eligible cases and/or controls were selected for the study, were the cases and/or controls randomly selected from those eligible? 8 = Was there use of concurrent controls? 9 = Were the investigators able to confirm that the exposure/risk occurred prior to the development of the condition or event that defined a participant as a case? 10 = Were the measures of exposure/risk clearly defined, valid, reliable, and implemented consistently (including the same time period) across all study participants? 11 = Were the assessors of exposure/risk blinded to the case or control status of participants? 12 = Were key potential confounding variables measured and adjusted statistically in the analyses? If matching was used, did the investigators account for matching during study analysis?
Table 3.
Quality assessment of the included case series/reports studies based on the NIH quality assessment tool
| First author (Reference no.) |
Question (NIH assessment tool)a | Overall rating | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | Reviewer#1 | Reviewer#2 | |
| Ahmed et al20 | Yes | Yes | CD | CD | Yes | Yes | No | CD | Yes | Good | Good |
| Azouz et al21 | No | Yes | NA | NA | Yes | No | CD | NA | Yes | Fair | Fair |
| Bessuti et al22 | No | Yes | CD | CD | NA | Yes | NA | NA | Yes | Poor | Poor |
| Colino et al23 | Yes | Yes | NA | NA | NA | No | CD | NA | No | Fair | Fair |
| Vu et al24 | Yes | CD | CD | Yes | NA | NA | No | NA | Yes | Good | Good |
| Poggiali et al25 | Yes | Yes | CD | Yes | Yes | Yes | CD | NA | Yes | Good | Good |
| Gahide et al26 | Yes | No | CD | CD | No | Yes | No | NA | Yes | Fair | Fair |
| Ignat et al27 | Yes | No | CD | No | NR | CD | CD | NA | Yes | Fair | Fair |
| Kim et al28 | Yes | NA | NA | NA | CD | No | CD | NA | Yes | Fair | Fair |
| Mazrouei et al29 | Yes | No | NA | NA | No | Yes | CD | NA | Yes | Fair | Fair |
| Pessoa et al30 | Yes | Yes | CD | CD | NA | NA | NA | NA | Yes | Good | Good |
| Kumar et al31 | Yes | NA | NA | NA | NA | Yes | NA | NA | Yes | Fair | Fair |
| Sattar et al32 | Yes | Yes | CD | CD | Yes | Yes | CD | NA | Yes | Good | Good |
| Sendi et al33 | Yes | No | NA | NA | No | Yes | CD | NA | Yes | Fair | Fair |
| Tay et al34 | Yes | Yes | NA | NA | Yes | Yes | CD | NA | Yes | Good | Good |
| Siegel et al35 | Yes | Yes | NA | Yes | Yes | Yes | CD | NA | Yes | Good | Good |
| Jaijakul et al36 | Yes | Yes | NA | NA | Yes | Yes | No | NA | Yes | Good | Good |
| Voutsinas et al37 | Yes | Yes | CD | Yes | Yes | No | CD | NA | Yes | Good | Good |
| Yokoo et al38 | No | Yes | CD | CD | CD | Yes | NR | NA | Yes | Poor | Poor |
| Akin et al39 | Yes | Yes | NA | NA | Yes | Yes | Yes | NA | Yes | Fair | Fair |
| Faqeeh et al40 | Yes | Yes | NA | NA | Yes | Yes | Yes | NA | Yes | Fair | Fair |
| Jafari et al41 | Yes | Yes | NA | NA | Yes | Yes | Yes | NA | Yes | Fair | Fair |
| Bashari et al42 | Yes | Yes | NA | NA | Yes | Yes | Yes | NA | Yes | Fair | Fair |
| Beccara et al43 | Yes | Yes | NA | NA | Yes | Yes | No | NA | Yes | Fair | Fair |
CD, Cannot determine; NA, Not applicable; NIH, National Institutes of Health; NR, Not reported.
(Source: National Heart, Lung, and Blood Institute; National Institutes of Health; U.S. Department of Health and Human Services).
The NIH Quality Assessment Tool for Case Series Studies5 includes nine questions: 1 = Was the study question or objective clearly stated? 2 = Was the study population clearly and fully described, including a case definition? 3 = Were the cases consecutive? 4 = Were the subjects comparable? 5 = Was the intervention clearly described? 6 = Were the outcome measures clearly defined, valid, reliable, and implemented consistently across all study participants? 7 = Was the length of follow-up adequate? 8 = Were the statistical methods well-described? 9 = Were the results well-described?
Results
Overview of the included studies
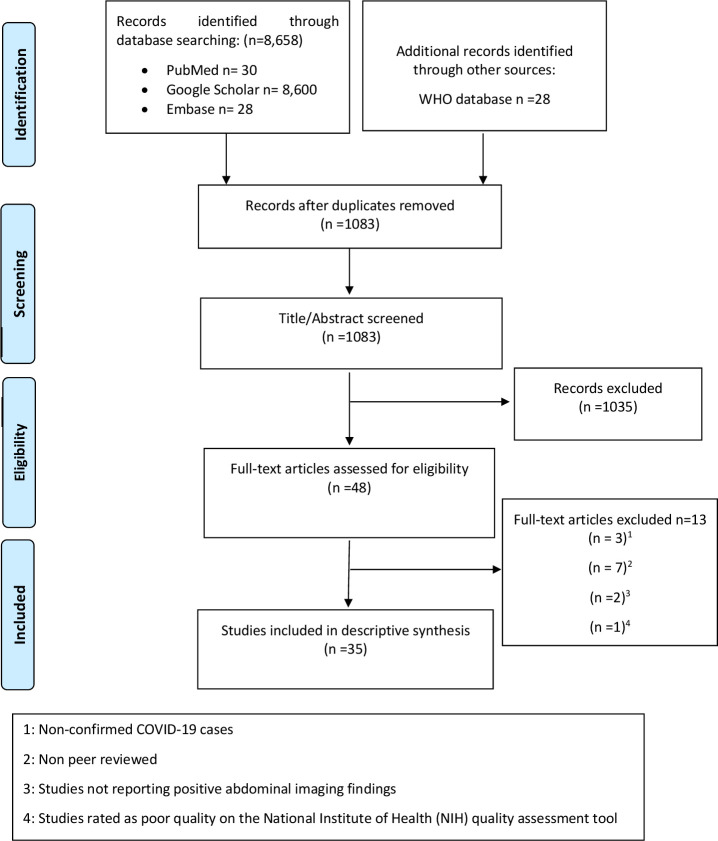
Only English language papers were identified. 35 studies consisting of 11 retrospective cohort studies (RCS), 2 retrospective case–control studies, 9 case series and 13 case reports, with a total of 801 COVID positive patients were included in the final review, as shown in PRISMA flow diagram (Figure 1). Characteristics of the included studies are presented in Supplementary Table 1. The number of the abdominal imaging modalities evaluated in the included studies was 721 CT scans.
Figure 1.
PRISMA flow diagram showing study selection process. PRISMA, Preferred reporting items for systematic reviews and meta-analyses; WHO, World Health Organization.
Abdominal imaging manifestations
A plethora of abdominal imaging findings in COVID-19 were reported in several studies (Table 4). These imaging manifestations have been grouped by the organ involved as shown below.
Table 4.
Abdominal imaging findings in COVID-19 patients noted across various studies
| First Author [Reference No.] | Imaging Changes in Bowel | Bowel dilatation/Fluid filled colon | Imaging changes in Pancreas | Solid Organ infarction | Hepatic Steatosis | Incidental lung base findings |
|---|---|---|---|---|---|---|
| Goldberg-Stein et al.6 | + | + | ||||
| King et al.7 | + | |||||
| Bari Dane et al.18 | + | |||||
| Shea et al.8 | + | + | ||||
| Norsa et al.9 | + | |||||
| Bhayana et al.10 | + | + | + | + | ||
| Medeiros et al.19 | + | |||||
| Palomar-Lever et al.11 | + | |||||
| Uchida et al.12 | + | |||||
| Dane et al.13 | + | |||||
| Shiralkar et al.14 | + | |||||
| Xiao et al.15 | + | |||||
| Sellevol et al.16 | + | |||||
| Liu et al.1717 | + | |||||
| Kumar et al.31 | + | |||||
| Sendi et al.33 | + | |||||
| Ignat et al.27 | + | |||||
| Tay et al.34 | + | |||||
| Vu et al.24 | + | |||||
| Colino et al.23 | + | |||||
| Sattar et al.32 | + | + | ||||
| Siegel et al.35 | + | |||||
| Gahide et al.26 | + | |||||
| Jaijakul et al.36 | + | |||||
| Voutsinas et al.37 | + | |||||
| Kim et al.28 | + | |||||
| Poggiali et al.25 | + | |||||
| Ahmed et al.20 | + | |||||
| Mazrouei et al.29 | + | |||||
| Pessoa et al.30 | + | |||||
| Azouz et al.21 | + | |||||
| Akin et al.39 | + | |||||
| Faqeeh et al.40 | + | |||||
| Bashari et al.42 | + | |||||
| Beccara et al.43 | + |
Imaging changes in bowel
15 studies reported bowel wall abnormalities on imaging among COVID-19 patients presenting with abdominal complaints.6,8,10,14,18,21,22,25,27,28,32,36,37,43,44 Among them, eight studies reported bowel wall thickening alone. Bhayana et al10 noted bowel wall thickening in 29% (12 out of 42 CT scans) of their abdominal CT scans in 40 patients, which involved the small bowel in 5 scans and large bowel and rectum in 7. They also found that bowel wall abnormalities were significantly associated with ICU admissions (Odds ratio: 15.56; p = 0.01). Goldberg-Stein et al6 also reported mural thickening in 15% (12 out of 80) patients with COVID-19. A few case reports also noted bowel wall thickening in patients with COVID-19.25,36 Sattar et al32 reported colonic findings on abdominal CT in three patients presenting with abdominal pain. Out of them, two showed wall thickening of large bowel, which involved the entire colon and rectum in one patient. Similar cases of colonic wall thickening on abdominal CT in patients with abdominal symptoms were also reported by Kim et al28 and Voutsinas et al37.
A total of six studies8,10,21,27,41,44 described imaging evidence of ischemic bowel changes. Besides bowel wall thickening, other findings reported were non-enhancing bowel wall, bowel wall pneumatosis, mesenteric venous gas, portal venous gas and mild fluid in the peritoneal cavity. Norsa et al44 in their retrospective cohort study, involving SARS-CoV-2 positive patients, noted six patients with features of intestinal ischemia in abdominal contrast-enhanced CT scan with two of these patients showing thromboembolic filling defects in IVC (Inferior vena cava) and superior mesenteric vein (SMV). The IVC thrombosis was probably related to the hypercoagulable state and not responsible for bowel ischemia. However, the prevalence of iliofemoral venous thrombosis or pulmonary emboli was not mentioned in their study. Hence, its relevance remains questionable. Shea et al8 noted CT features of bowel ischemia in four patients with COVID-19. Bhayana et al10, noted ischemic bowel changes in four patients. Few other authors also reported similar cases where abdominal pain led to cross-sectional imaging diagnosis of bowel ischemia.10,21,22,27 Ignat et al27 reported portal vein (PV) and SMV thrombosis with subsequent development of segmental ischemia of the small bowel.
Bowel dilatation/fluid-filled colon
In the patients presenting with diarrhea, fluid-filled colon was noted on CT scan. Bhayana et al10 noted this finding in 18/42 (43%) of abdominal CT scans and it was more common in patients who were in the intensive care unit. Sattar et al32 reported colonic ileus in a patient presenting with constipation and abdominal pain.
Imaging changes in pancreas
Four studies6,14,29,45 reported pancreatic abnormalities in patients with confirmed COVID-19. Goldberg-Stein et al6 noted pancreatic ductal dilatation in three patients with COVID-19. Liu et al17, in their retrospective observational study, noted imaging findings in the form of focal enlargement of the pancreas or dilatation of the main pancreatic duct, without any evidence of necrosis, in five patients with severe COVID-19. Mazrouei et al29 in their report of a 24-year-old male with COVID-19 and acute pancreatitis, showed mild edema of distal pancreas with peripancreatic fluid on abdominal CT.
Solid organ infarction
Nine studies reported imaging findings of solid organ infarction (spleen, kidney, adrenal, liver) in patients with COVID-19.6,8,10,18,22,30,31,39,40 Isolated splenic infarct was noted in 10 patients, isolated renal infarct in 11 patients and liver infarct in 1 patient. One patient had both splenic and renal infarcts and one had bilateral adrenal infarcts. Goldberg-Stein et al6 in their RCS of COVID-19 patients with abdominal imaging, noted splenic and renal infarcts in four patients each. Faqeeh et al40 reported hypoperfusion changes in bilateral kidneys on abdominal CT associated with SARS-CoV-2 infection in a 19-year-old boy.
Hepatic steatosis (HS)
Four studies,10–12,19 noted the presence of HS among COVID-19 positive patients. Medeiros et al19 noted a significantly higher prevalence of HS among patients with COVID-19 compared to non-COVID controls (31.9% vs 7.1%; p value < 0.001). Palomar-Lever et al11 in their retrospective study involving 213 COVID-19 patients, noted HS to be independently associated with severity of COVID-19 pneumonia. Uchida et al12 in their retrospective study involving 35 patients with mild-moderate COVID-19, noted reduced hepatic CT attenuation values to correlate with COVID-19 disease severity.10
Incidental COVID detection: lung base findings of COVID-19 on abdominal CT in patients with predominant GI symptoms
A total of 18 studies (5 RCS, 7 case reports and 6 case series), reported basal lung findings on abdominal CT scans performed for predominant abdominal complaints.6,7,10,13,15,16,20,23,24,26,28,31,33–35,37,38,42 All the patients showed findings suggestive of COVID-19 in the basal lung sections included in the abdominal scans. These patients did not have any significant upper and lower respiratory symptoms, making the imaging findings in basal lung segments critical in raising a suspicion of COVID-19. The nature of incidental basal lung findings was reported in 118 patients. The most common finding was ground glass opacities, seen in 107 patients (90.7%) and in most of these patients (n = 76; 64.4%), the distribution of the opacities was peripheral and subpleural. The reverse halo sign and mild pleural effusion were noted in only three patients. The laterality of lung involvement was reported in 101 patients and among them, 92 (91.1%) had bilateral distribution of the lung base findings.
Discussion
To our knowledge, this is the first review systematically scoping the various luminal and hepatopancreatobiliary imaging findings in patients with COVID-19. In our review, among studies reporting positive abdominal imaging findings in patients with COVID-19, majority reported imaging abnormalities of the GI tract, of which bowel wall thickening was the most frequent. It has been shown that intestinal abnormalities were associated with significantly worse outcomes in the form of requirement of ICU admissions. Imaging evidence of thromboembolism is being increasingly noted in patients with COVID-19. Imaging findings related to mesenteric ischemia and solid organ infarcts should be actively sought in COVID-19 patients presenting with pain abdomen. The higher occurrence of HS in patients with COVID-19 and its correlation with the severity of the disease has been noted in recent studies.11,12,19 However, further studies are needed to confirm or refute this association. The imaging features of involvement of other solid organs (liver, GB, pancreas) in COVID-19, is increasingly being recognized. . Lung base findings maybe incidentally noted on abdominopelvic CT of unsuspected patients, presenting with predominant abdominal complaints. Ground-glass opacities in the lung bases, usually multilobar and peripheral in distribution, are often the only pertinent findings observed.13 Hamilton et al46, in their retrospective study, noted a low overall diagnostic yield by performing an additional whole chest CT subsequent to the abdominal CT performed as part of their abdominal pain protocol. They noted that this lack of definitive benefit at the cost of increased radiation exposure makes it unjustified to add an extra chest CT scan. Similarly, Brennan et al47 did not find the benefit of addition of chest imaging to abdominal cross-sectional imaging, for the identification of patients with COVID-19.
The SARS-CoV-2 binds to the endothelial cells of the GI tract via ACE-2 receptors causing cytokine and chemokine release, responsible for acute intestinal inflammation.48 Findings on cross-sectional imaging of the affected patients support this inflammation theory with direct (ileocolitis) or indirect features (mesenteric lymphadenitis). The presence of bowel wall thickening should also merit consideration of intestinal ischemia due to vascular thrombosis. Hence, the two possibilities - presence of ACE-2 receptors in GI tract and procoagulant state - may be responsible for the bowel involvement in COVID 19. However, till this is completely proven, attribution of bowel wall thickening to COVID-19 should be a diagnosis of exclusion. Further, colonic dilatation and fluid in its lumen may also be due to direct or indirect effects of the virus. Since colonoscopy is an aerosol generating procedure, this procedure in patients with diarrhea or CT findings of colonic thickening, fluid or dilatation should prompt adequate precautions.
The association of COVID-19 with vascular thrombosis is increasingly being recognized and mesenteric vasculature remains no exception. Both small and large vessel thrombosis has been reported in patients with COVID-19.49 The resulting bowel ischemia has several direct and indirect radiological findings. Bhayana et al,10 in their preliminary observation, noted small bowel ischemic changes in 20% of the abdominal CT scans performed on patients with COVID-19 admitted to ICU. Several other reports have noted ischemic bowel changes on abdominal CT, among patients with COVID-19 presenting with abdominal pain. Although screening for pulmonary embolisms and deep vein thrombosis has been recommended in the clinical setting of COVID-19, little attention has been paid to thrombosis of the splanchnic venous system. Published reports have mentioned SMV and PV thrombosis as a result of the hypercoagulable state due COVID-19 per se. Visceral organ infarction should be considered in the differential diagnosis among COVID-19 patients presenting as unexplained abdominal pain. Clinicians need to be aware of the thrombotic manifestations of COVID-19 and radiologists should monitor patients for thrombosis to facilitate early diagnosis. Literature suggests that COVID-19 provokes arterial and venous thrombosis, although the mechanism remains largely unknown. Whether the thrombotic complications are a direct effect of SARS-CoV-2 or a consequence of ensuing cytokine storm remains contentious.
Pancreatic injury in patients with COVID-19 could be due to a direct cytopathic effect mediated by local SARS-CoV-2 replication or a result of the systemic response induced by SARS-CoV-2 infection.17 This could be inferred from the fact that, in one study, elevated pancreatic enzymes were noted in 13 patients, but the imaging changes suggestive of acute pancreatitis were seen in only 5.17 Although the imaging alterations suggested that pancreatitis was not severe, the problem should not be ignored, especially in patients with severe COVID-19. Further high-quality, prospective and well-reported research and larger series are warranted to evaluate whether this subset of patients have clinical pancreatitis as a presenting or concomitant disease entity. Studies are also needed to evaluate the management for this group and whether sequelae such as chronic pancreatitis may develop in the absence of early management.
Several studies have shown a higher occurrence of HS in patients with COVID-19 and have also reported a prognostic value.6,50,51 Presence of HS correlates with visceral adiposity, underlying metabolic disease, as well as overt and chronic inflammation.52 No causal association between HS and COVID-19 has been shown till date. Whether HS increases the predisposition for SARS-CoV-2 infection or is a manifestation of liver involvement, remains a conundrum.53 This needs to be conjectured from larger prospective studies.
Study limitations
There are several limitations of this systematic review. Though our review gives an idea about the spectrum of abdominal imaging abnormalities seen in COVID-19 patients, no conclusion can be made regarding the incidence of these findings, as most of the reported literature consisted of case series and case reports. Although we aimed to correlate the abdominal imaging findings with the severity of COVID-19, this was not possible due to the immaturity of the available literature. Due to the novelty of SARS-CoV-2 and the brief timeframe since the start of the pandemic, the validity of evidence is limited. Case reports and case series form the most of the currently available literature. Nevertheless, given the paucity of high-quality evidence, inferences from such reports can guide decision- making and further research.54 Due to the relative paucity of literature on this topic, we included varied study designs in our review. The heterogeneity in the reporting of radiological findings may partly be attributed to extraction of data from clinical reports in few studies and re-reviewing of CT images in others. It is possible that certain findings (mural thickening, fluid-filled colon) may be relatively under reported in the clinical setting as compared with the research setting. Secondly, as studies reporting HS in patients with COVID-19 are all retrospective, causality cannot be determined, and the reported results may be considered as mere statistical associations. With our limited understanding of the disease pathophysiology, it is imperative to evaluate manifestations of all the systems which may be attributed to this disease. We need to discuss whether the abdominal manifestations in patients with COVID-19 or even silent abdominal imaging findings are due to unrelated concomitant pathological process or due to COVID-19 or a result of COVID-19 modifying the pathophysiology of a concomitant disease. However, before we can attempt to answer this question, we need to have a holistic understanding of both the abdominal manifestations and imaging findings in the COVID-19 patients. In this review, we noted that most studies reported findings on CT imaging. This might be reflective of the fact that it is the most widely available, reliable and relevant imaging modality. Ultrasound is less reliable and is influenced by COVID-19 safety measures. Despite the above limitations, this review systematically scopes and provides a spectrum of abdominal imaging findings in patients with COVID-19. We will continue to monitor the literature, and this review shall be updated as and when new evidence emerges.
Conclusion
In conclusion, in this systematic review, we found a multitude of abdominal imaging findings among COVID-19 patients with predominant abdominal complaints. This review summarizes the abdominal imaging findings that have been described so far in COVID-19 patients and provides a platform for further research.
Contributor Information
Lokesh Agarwal, Email: devloksang@gmail.com.
Ayushi Agarwal, Email: ayushi.193@gmail.com.
Shailesh Advani, Email: sa1542@georgetown.edu.
Varidh Katiyar, Email: katiyar.varidh@gmail.com.
Aprajita Chaturvedi, Email: aprajitachaturvedi995@gmail.com.
Kumble Seetharama Madhusudhan, Email: drmadhuks@gmail.com.
REFERENCES
- 1.Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol 2020; 5: 562–9. doi: 10.1038/s41564-020-0688-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology 2020; 158: 1831–3. doi: 10.1053/j.gastro.2020.02.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang J, Wang S, Xue Y. Fecal specimen diagnosis 2019 novel coronavirus–infected pneumonia. J Med Virol 2020; 92: 680–2. doi: 10.1002/jmv.25742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339: b2700. doi: 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NHLBI, NIH. Study quality assessment tools. 2020. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
- 6.Goldberg-Stein S, Fink A, Paroder V, Kobi M, Yee J, Chernyak V. Abdominopelvic CT findings in patients with novel coronavirus disease 2019 (COVID-19. Abdom Radiol 2020; 45: 2613–23. doi: 10.1007/s00261-020-02669-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King MJ, Lewis S, El Homsi M, Hernandez Meza G, Bernheim A, Jacobi A, et al. Lung base CT findings in COVID-19 adult patients presenting with acute abdominal complaints: case series from a major new York City health system. Eur Radiol 2020; 30: 6685–93. doi: 10.1007/s00330-020-07040-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Shea A, Parakh A, Hedgire S, Lee SI. Multisystem assessment of the imaging manifestations of coagulopathy in hospitalized patients with coronavirus disease (COVID-19). AJR Am J Roentgenol 2021; 216: 1088–98. doi: 10.2214/AJR.20.24132 [DOI] [PubMed] [Google Scholar]
- 9.Norsa L, Valle C, Morotti D, Bonaffini PA, Indriolo A, Sonzogni A. Intestinal ischemia in the COVID-19 era. Dig Liver Dis 2020; 52: 1090–1. doi: 10.1016/j.dld.2020.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhayana R, Som A, Li MD, Carey DE, Anderson MA, Blake MA, et al. Abdominal imaging findings in COVID-19: preliminary observations. Radiology 2020; 297: E207–15. doi: 10.1148/radiol.2020201908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palomar‐Lever A, Barraza G, Galicia‐Alba J, Echeverri‐Bolaños M, Escarria‐Panesso R, Padua‐Barrios J, et al. Hepatic steatosis as an independent risk factor for severe disease in patients with COVID ‐19: A computed tomography study. JGH Open 2020; 4: 1102–7. doi: 10.1002/jgh3.12395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uchida Y, Uemura H, Yamaba S, Hamada D, Tarumoto N, Maesaki S, et al. Significance of liver dysfunction associated with decreased hepatic CT attenuation values in Japanese patients with severe COVID-19. J Gastroenterol 2020; 55: 1098–106. doi: 10.1007/s00535-020-01717-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dane B, Brusca-Augello G, Kim D, Katz DS. Unexpected findings of coronavirus disease (COVID-19) at the lung bases on abdominopelvic CT. AJR Am J Roentgenol 2020; 215: 603–6. doi: 10.2214/AJR.20.23240 [DOI] [PubMed] [Google Scholar]
- 14.Shiralkar K, Chinapuvvula N, Ocazionez D. Cross-Sectional abdominal imaging findings in patients with COVID-19. Cureus 2020; 12: e9538. doi: 10.7759/cureus.9538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao N, Abboud S, McCarthy DM, Parekh N. Incidentally discovered COVID-19 in low-suspicion patients-a threat to front line health care workers. Emerg Radiol 2020; 27: 589–95. doi: 10.1007/s10140-020-01792-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sellevoll HB, Saeed U, Young VS, Sandbæk G, Gundersen K, Mala T. Acute abdomen as an early symptom of COVID-19. Tidsskr Nor Laegeforen 2020; 14005 05 2020. doi: 10.4045/tidsskr.20.0262 [DOI] [PubMed] [Google Scholar]
- 17.Liu F, Long X, Zhang B, Zhang W, Chen X, Zhang Z. ACE2 expression in pancreas may cause pancreatic damage after SARS-CoV-2 infection. Clin Gastroenterol Hepatol 2020; 18: 2128–30. doi: 10.1016/j.cgh.2020.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dane B, Smereka P, Wain R, Kim D, S Katz D. Hypercoagulability in patients with coronavirus disease (COVID-19): identification of arterial and venous thromboembolism in the abdomen, pelvis, and lower extremities. AJR Am J Roentgenol 2021; 216: 104–5. doi: 10.2214/AJR.20.23617 [DOI] [PubMed] [Google Scholar]
- 19.Medeiros AK, Barbisan CC, Cruz IR, de Araújo EM, Libânio BB, Albuquerque KS, et al. Higher frequency of hepatic steatosis at CT among COVID-19-positive patients. Abdom Radiol 2020; 45: 2748–54. doi: 10.1007/s00261-020-02648-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmed AOE, Badawi M, Ahmed K, Mohamed MFH. Case report: COVID-19 masquerading as an acute surgical abdomen. Am J Trop Med Hyg 2020; 103: 841–3. doi: 10.4269/ajtmh.20-0559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azouz E, Yang S, Monnier-Cholley L, Arrivé L. Systemic arterial thrombosis and acute mesenteric ischemia in a patient with COVID-19. Intensive Care Med 2020; 46: 1464–5. doi: 10.1007/s00134-020-06079-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Besutti G, Bonacini R, Iotti V, Marini G, Riva N, Dolci G, et al. Abdominal visceral infarction in 3 patients with COVID-19. Emerg Infect Dis 2020; 26: 1926–8. doi: 10.3201/eid2608.201161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blanco-Colino R, Vilallonga R, Martín R, Petrola C, Armengol M. Suspected acute abdomen as an extrapulmonary manifestation of Covid-19 infection. Cirugía Española 2020; 98: 295–6. doi: 10.1016/j.cireng.2020.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vu D, Ruggiero M, Choi WS, Masri D, Flyer M, Shyknevsky I, et al. Three unsuspected CT diagnoses of COVID-19. Emerg Radiol 2020; 27: 229–32. doi: 10.1007/s10140-020-01775-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poggiali E, Mateo Ramos P, Bastoni D, Vercelli A, Magnacavallo A. Abdominal pain: a real challenge in novel COVID-19 infection. Eur J Case Rep Intern Med 2020; 7: 001632. doi: 10.12890/2020_001632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gahide G, Frandon J, Vendrell J-F. COVID-19 patients presenting with afebrile acute abdominal pain. Clin Med 2020; 20: e4–6. doi: 10.7861/clinmed.2020-0150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ignat M, Philouze G, Aussenac-Belle L, Faucher V, Collange O, Mutter D, et al. Small bowel ischemia and SARS-CoV-2 infection: an underdiagnosed distinct clinical entity. Surgery 2020; 168: 14–16. doi: 10.1016/j.surg.2020.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim J, Thomsen T, Sell N, Goldsmith AJ. Abdominal and testicular pain: an atypical presentation of COVID-19. Am J Emerg Med 2020; 38: 1542.e1–1542.e3. doi: 10.1016/j.ajem.2020.03.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazrouei SSA, Saeed GA, Al Helali AA, Hilali A. COVID-19-associated acute pancreatitis: a rare cause of acute abdomen. Radiology Case Reports 2020; 15: 1601–3. doi: 10.1016/j.radcr.2020.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pessoa MSL, Lima CFC, Pimentel ACF, Costa JCG, Holanda JLB. Multisystemic infarctions in COVID-19: focus on the spleen. Eur J Case Rep Intern Med 2017; 7: 001747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar R, Guruparan T, Siddiqi S, Sheth R, Jacyna M, Naghibi M, et al. A case of adrenal infarction in a patient with COVID 19 infection. BJR|case reports 2020; 6: 20200075. doi: 10.1259/bjrcr.20200075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sattar Y, Connerney M, Rauf H, Saini M, Ullah W, Mamtani S, et al. Three cases of COVID-19 disease with colonic manifestations. Am J Gastroenterol 2020; 115: 948–50. doi: 10.14309/ajg.0000000000000692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sendi AA, Saggat DF, Alzahrani SJ. Incidental typical COVID-19 appearance on the lung bases, visualized at abdominal CT for a patient that presented with abdominal pain and nausea. Radiol Case Rep 2020; 15: 1238–41. doi: 10.1016/j.radcr.2020.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tay HS, Harwood R. Atypical presentation of COVID-19 in a frail older person. Age Ageing. Published online 2020;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siegel A, Chang PJ, Jarou ZJ, Paushter DM, Harmath CB, Arevalo JB, et al. Lung base findings of coronavirus disease (COVID-19) on abdominal CT in patients with predominant gastrointestinal symptoms. AJR Am J Roentgenol 2020; 215: 1–3. doi: 10.2214/AJR.20.23232 [DOI] [PubMed] [Google Scholar]
- 36.Jaijakul S. Colitis as a sole presentation of SARS-CoV-2 infection: case report. SN Compr Clin Med 2020; June 11: 879–81. doi: 10.1007/s42399-020-00346-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Voutsinas N, Toussie D, Jacobi A, Bernheim A, Chung M. Incidental CT findings in the lungs in COVID-19 patients presenting with abdominal pain. Clin Imaging 2020; 67: 1–4. doi: 10.1016/j.clinimag.2020.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yokoo P., K. U. N F. E., Filho M. O., and Chate R. C., et al. Abdominal symptoms as an initial manifestation of COVID-19 infection: report of two cases. USA: Research Square; Available at:https://www.researchsquare.com/article/rs-28198/v1. 2020. doi: 10.21203/rs.3.rs-28198/v1 [DOI] [Google Scholar]
- 39.Basara Akin I, Altay C, Eren Kutsoylu O, Secil M. Possible radiologic renal signs of COVID-19. Abdom Radiol 2021; 46: 692–5. doi: 10.1007/s00261-020-02671-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Faqeeh S, Madkhali R. Kidney imaging findings in a patient with COVID-19. Radiology Case Reports 2020; 15: 2449–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jafari SH, Naseri R, Khalili N, Haseli S, Bahmani M. Portal vein thrombosis associated with COVID-19: points to consider. BJR|case reports 2020; 6: 20200089. doi: 10.1259/bjrcr.20200089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bashari DR, Peguero-Tejada JL, Shah JI. An atypical presentation of COVID-19 in a previously healthy young male with a rare cause of abdominal pain. J Clin Med Res 2020; 12: 624–8. doi: 10.14740/jocmr4285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.A Beccara L, Pacioni C, Ponton S, Francavilla S, Cuzzoli A. Arterial mesenteric thrombosis as a complication of SARS-CoV-2 infection. Eur J Case Rep Intern Med 2020; 7: 001690. doi: 10.12890/2020_001690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Norsa L, Bonaffini PA, Indriolo A, Valle C, Sonzogni A, Sironi S. Poor outcome of intestinal ischemic manifestations of COVID-19. Gastroenterology 2020; 159: 1595–7. doi: 10.1053/j.gastro.2020.06.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu F, Long X, Zhang B, Zhang W, Chen X, Zhang Z. ACE2 expression in pancreas may cause pancreatic damage after SARS-CoV-2 infection. Clin Gastroenterol Hepatol 2020; 18: 2128–30. doi: 10.1016/j.cgh.2020.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hamilton NE, Adam GH, Ifan DL, Lam SS, Johnson K, Vedwan KAG, et al. Diagnostic utility of additional whole-chest CT as part of an acute abdominal pain CT imaging pathway during the COVID-19 pandemic. Clin Radiol 2020; 75: 592–8. doi: 10.1016/j.crad.2020.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brennan C, Morrissey B, Dubois-Marshall S, McAteer D, Qadir A, Ramsay G. COVID-19: no benefit of chest inclusion in acute abdomen CT. Br J Surg 2020; 107: e474–5. doi: 10.1002/bjs.11934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qi F, Qian S, Zhang S, Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun 2020; 526: 135–40. doi: 10.1016/j.bbrc.2020.03.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pons S, Fodil S, Azoulay E, Zafrani L. The vascular endothelium: the cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit Care 2020; 24: 353. doi: 10.1186/s13054-020-03062-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, et al. COVID-19: abnormal liver function tests. J Hepatol 2020; 73: 566–74. doi: 10.1016/j.jhep.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, et al. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol 2020; 73: 807–16. doi: 10.1016/j.jhep.2020.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nassir F, Rector RS, Hammoud GM, Ibdah JA. Pathogenesis and prevention of hepatic steatosis. Gastroenterol Hepatol 2015; 11: 167–75. [PMC free article] [PubMed] [Google Scholar]
- 53.Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, et al. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J Hepatol 2020; 73: 451–3. doi: 10.1016/j.jhep.2020.03.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Djulbegovic B, Guyatt GH. Progress in evidence-based medicine: a quarter century on. The Lancet 2017; 390: 415–23. doi: 10.1016/S0140-6736(16)31592-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.