Abstract
STUDY QUESTION
What is the impact of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection on the outcome of a pregnancy after medically assisted reproduction (MAR)?
SUMMARY ANSWER
Our results suggest that MAR pregnancies are not differentially affected by SARS-CoV-2 infection compared to spontaneous pregnancies.
WHAT IS KNOWN ALREADY
Information on the effects of coronavirus disease 2019 (COVID-19) on pregnancy after MAR is scarce when women get infected during MAR or early pregnancy, even though such information is vital for informing women seeking pregnancy.
STUDY DESIGN, SIZE, DURATION
Data from SARS-CoV-2 affected MAR pregnancies were collected between May 2020 and June 2021 through a voluntary data collection, organised by the European Society of Human Reproduction and Embryology (ESHRE).
PARTICIPANTS/MATERIALS, SETTING, METHODS
All ESHRE members were invited to participate to an online data collection for SARS-CoV-2-infected MAR pregnancies.
MAIN RESULTS AND THE ROLE OF CHANCE
The dataset includes 80 cases from 32 countries, including 67 live births, 10 miscarriages, 2 stillbirths and 1 maternal death. An additional 25pregnancies were ongoing at the time of writing.
LIMITATIONS, REASONS FOR CAUTION
An international data registry based on voluntary contribution can be subject to selective reporting with possible risks of over- or under-estimation.
WIDER IMPLICATIONS OF THE FINDINGS
The current data can be used to guide clinical decisions in the care of women pregnant after MAR, in the context of the COVID-19 pandemic.
STUDY FUNDING/COMPETING INTEREST(S)
The authors acknowledge the support of ESHRE for the data registry and meetings. J.S.T. reports grants or contracts from Sigrid Juselius Foundation, EU and Helsinki University Hospital Funds, outside the scope of the current work. The other authors declare that they have no conflict of interest.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: SARS-CoV-2, COVID-19, medically assisted reproduction, ESHRE, pregnancy, IVF
Introduction
In March 2020, at the outset of the coronavirus disease 2019 (COVID-19) pandemic, potential implications of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection during the preconception period, medically assisted reproduction (MAR) treatments or early pregnancy were among the concerns of MAR professionals and international societies. This resulted in a discontinuation of reproductive care, except for urgent fertility preservation in oncology patients (Veiga et al., 2020). The emergence of data and application of strict mitigation measures allowed gradual resumption of reproductive care (Gianaroli et al., 2021).
Knowledge of prior coronavirus infections, i.e. SARS and Middle East respiratory syndrome (MERS), in pregnancy was limited by small numbers of reported cases not permitting any conclusion on the impact on pregnancy outcomes (Peiris et al., 2003; Zumla et al., 2015). Data on the outcomes of SARS-CoV-2 infected pregnant patients emerged, but initially were related to women who were infected and diagnosed in the third and late second trimesters (Allotey et al., 2020). Data collections for SARS-CoV-2 and pregnancy were started, but they focused on all pregnancies, not allowing conclusions for MAR pregnancies.
There are consistent data suggesting systematic differences between MAR pregnancies and spontaneous pregnancies (Berntsen et al., 2019). MAR pregnancies have been associated with significantly higher rates of preterm birth (adjusted risk between 1.41 and 2.04), being born small for gestational age (adjusted risk around 1.5) and perinatal mortality (adjusted risk between 1.7 and 2.0). Additionally, MAR pregnancies have been found to be associated with increased risks of obstetric complications, such as hypertensive disorders in pregnancy, placental complications (placenta previa, abruption and third trimester bleeding), gestational diabetes, interventions (e.g. caesarean section and medical induction of labour) and preterm prelabour rupture of the membranes (Berntsen et al., 2019). Thus, it is plausible to anticipate that COVID-19 could affect spontaneous pregnancies and MAR pregnancies differently.
While it was expected that more information on SARS-CoV-2 infection during early pregnancy would become available over time, the European Society of Human Reproduction and Embryology (ESHRE) COVID-19 working group considered that it is important to assess whether MAR pregnancies can be differentially affected by COVID-19. To address the above-mentioned gap in knowledge and to gain more information, particularly on the course and outcome of pregnancies resulting from MAR, ESHRE started an international registry to collect data on SARS-CoV-2 infected MAR pregnancies. Herein, we report the currently available data that have been accumulated in the registry between May 2020 and June 2021.
Materials and methods
The ESHRE COVID-19 working group started an international registry in May 2020 to collect data on SARS-CoV-2 infected MAR pregnancies and their outcomes. Data were collected through an online registry developed through the SurveyMonkey software. The registry included 21 questions divided into four sections: (i) background information, including contact information of the person submitting the case, (ii) information on the COVID-19 infection, (iii) information on the pregnancy and (iv) information on obstetric and neonatal outcomes (Zegers-Hochschild et al., 2017) (Supplementary Data File S1).
As of May 2020, invitations to contribute were distributed at least monthly to all ESHRE members (n > 10 000) through different ESHRE communication channels, including mass mailings and social media.
The data registry focused on MAR pregnancies impacted by SARS-CoV-2 infection, and cases were excluded if basic information on the data submitter was not available, if no pregnancy was established, or if the patients did not have a COVID-19 infection during pregnancy. ‘A strong suspicion of COVID-19 infection’ at earlier timepoints with insufficient testing availability was considered acceptable. For all cases fitting the inclusion criteria, data submitted through the online registry were confirmed by contacting the data submitter with a summary of the reported case and requesting confirmation of the case. In case no contact details were available, or the data submitter did not confirm the case (after a minimum of three emails and an attempted phone call), the case was excluded from the final dataset.
Upon confirmation, further details were requested including maternal age, gravidity, parity and BMI. For pregnancies that were ongoing at the time of data submission, the data submitters were again contacted near the estimated delivery date to provide further information on the obstetric and neonatal outcomes.
The current report is limited to cases reported up to 1 June 2021; the data registry remains open and further updates of this report will be published on the ESHRE website. The data submitters for all cases included in this report are listed as contributors to this publication (Supplementary Data File S2).
Results
We obtained voluntarily provided and fully anonymised data from 214 cases, of which 163 fitted the predefined inclusion criteria. Excluded entries had incorrect/duplicate entry (n = 17), no information on the data submitter (n = 28), no COVID-19 infection (n = 3) or no pregnancy (n = 3). After further exclusion of cases which could not (yet) be confirmed (n = 52) or for which data on delivery could not be obtained (n = 6), as well as pregnancies that were still ongoing at the time of publication (n = 25), the final dataset included data from 80 cases from 32 countries (Figs 1 and 2).
Figure 1.
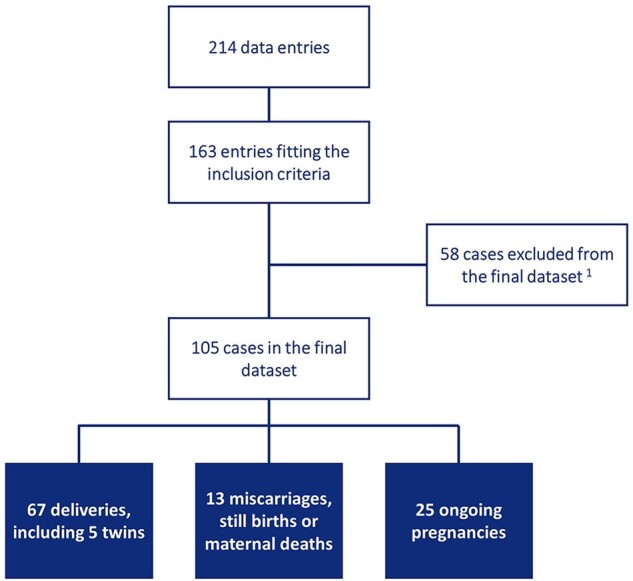
Overview of data entries to final dataset. 1cases excluded from the final dataset included 52 cases that could not yet be validated, and 6 cases with incomplete data on delivery.
Figure 2.
Countries (in blue) represented within the final dataset (80 cases and 25 ongoing pregnancies).
SARS-CoV-2 infection and symptoms
Most of the infections were diagnosed using real-time PCR (RT-PCR). Of the infected women, 68.6% were symptomatic, with fever being the most frequent symptom (40.0%) followed by cough (35.2%) and anosmia (28.6%). Of the infected women, 31.4% were hospitalised for COVID-19 including 2.9% who received respiratory support and 1.9% who were treated in intensive care units. Of all COVID-19 infections, 10.5% were detected during the MAR treatment (including 15 days after embryo transfer), 30.5% were in the first trimester, 20.0% were in the second trimester and the remaining 39.0% were during the third trimester or at delivery (Table I).
Table I.
Overview of the characteristics of the COVID-19 infection and medically assisted reproduction techniques used for the 105 cases reported in total and subdivided according to the outcome of the pregnancy.
| COVID-19 affected MAR pregnancies (n = 105) | Characteristics according to outcome |
||||
|---|---|---|---|---|---|
| Live births (n = 67) | Miscarriage (n = 10) | Stillbirth (n = 2) | Maternal death (n = 1) | ||
| Diagnosis COVID-19 | |||||
| RT-PCR | 97 (92.4%) | 60 (89.5%) | 9 (90.0%) | 2 (100%) | 1 (100%) |
| Antibody | 3 (2.9%) | 3 (4.5%) | 0 | 0 | 0 |
| Other | 5 (4.8%) | 4 (6.0%) | 1 (10.0%) | 0 | 0 |
| Time of diagnosis | |||||
| During the MAR treatment (including 15 days after embryo transfer) | 11 (10.5%) | 2 (3.0%) | 3 (30.0%) | 0 | 0 |
| First trimester | 32 (30.5%) | 11 (16.4%) | 7 (70.0%) | 0 | 1 (100%) |
| Second trimester | 21 (20.0%) | 14 (20.9%) | 0 | 2 (100%) | 0 |
| Third trimester (including delivery) | 41 (39.0%) | 40 (59.7%) | 0 | 0 | 0 |
| Symptoms | |||||
| Asymptomatic | 33 (31.4%) | 24 (35.8%) | 3 (30.0%) | 0 | 0 |
| Fever | 42 (40.0%) | 28 (41.8%) | 4 (40.0%) | 1 (50.0%) | 1 (100%) |
| Cough | 37 (35.2%) | 23 (34.3%) | 3 (30.0%) | 2 (100%) | 1 (100%) |
| Anosmia | 30 (28.6%) | 18 (26.9%) | 3 (30.0%) | 1 (50.0%) | 0 |
| Ageusia | 15 (14.3%) | 9 (13.4%) | 4 (40.0%) | 0 | 0 |
| Gastrointestinal symptoms | 12 (11.4%) | 8 (11.9%) | 0 | 0 | 0 |
| Other | 19 (17.3%) | 15 (22.4%) | 2 (20.0%) | 0 | 0 |
| Hospitalization | |||||
| None | 72 (68.6%) | 38 (56.7%) | 10 (100%) | 1 (50.0%) | 0 |
| General ward | 28 (26.7%) | 25 (37.3%) | 0 | 1 (50.0%) | 0 |
| ICU | 2 (1.9%) | 2 (3.0%) | 0 | 0 | 0 |
| Respiratory support | 3 (2.9%) | 2 (3.0%) | 0 | 0 | 1 (100%) |
| MAR technique | |||||
| ART | 67 (63.8%) | 38 (56.7%) | 8 (80.0%) | 0 | 1 (100%) |
| Ovulation induction, with or without IUI | 38 (36.2%) | 29 (43.3%) | 2 (20.0%) | 2 (100%) | 0 |
| Maternal age | |||||
| Mean ± SD age (years)1 | 33.7 ± 6.1a | 33.1 ± 1.7b | 38.0 ± 2.6c | 34.0d | 25.0e |
Data are represented as numbers and percentages.
COVID-19, coronavirus disease 2019; ICU, intensive care unit; MAR, medically assisted reproduction; RT-PCR, real-time PCR.
Data available for 81a, 58b, 7c, 1d and 1e cases.
Pregnancy outcomes
Of all pregnancies, 63.8% were achieved through ART (i.e. IVF/ICSI), and 36.2% were achieved through ovulation induction with or without IUI. There were 25 pregnancies still ongoing at the time of writing. Of the remaining 80 pregnancies, 83.75% resulted in live births, 12.5% ended in miscarriage, 2.5% were stillbirths, while there was 1 (1.25%) maternal mortality. Other pregnancy complications amongst the 80 pregnancies were as follows: 16.25% preterm birth, 2.5% intrauterine growth restriction, 1.25% pre-eclampsia, 1.25% hypertension and 5.0% other pregnancy complications. For 57.5% of the pregnancies, no complications were reported. A caesarean section was performed for 61.2% of the deliveries (Table II).
Table II.
Overview of the deliveries and neonatal outcomes for 80 MAR pregnancy deliveries, resulting in 72 neonates.
| Pregnancies (n = 80) | ||
|---|---|---|
| Pregnancy complications | ||
| None | 46 (57.5%) | |
| Maternal death | 1 (1.25%) | |
| Stillbirth | 2 (2.5%) | |
| Miscarriage | 10 (12.5%) | |
| Ectopic pregnancy | 0 | |
| Excessive bleeding | 0 | |
| Pre-eclampsia | 1 (1.25%) | |
| Intrauterine growth restriction (IUGR) | 2 (2.5%) | |
| Preterm birth (<37 weeks) | 11 (13.75%) | With vasa previa (1), pre-eclampsia (4), IUGR (1) |
| Very preterm birth (<32 weeks) | 1 (1.25%) | With pre-eclampsia (1) |
| Extremely preterm birth (<28 weeks) | 1 (1.25%) | |
| Hypertension | 1 (1.25%) | |
| Other | 4 (5.0%) | Uterine hyperstimulation (1), thrombophilia (1), haemorrhage (1), hyperemesis and polyhydramnios 25 weeks (1) |
| Live births (n = 67) | ||
| Mode of delivery | ||
| Vaginal delivery | 25 (37.3%) | |
| Caesarean section | 41 (61.2%) | |
| Unknown | 1 (1.5%) | |
| Neonates (n = 72) | ||
| Neonatal complications | ||
| None | 62 (86.1%) | |
| Respiratory symptoms | 5 (6.9%) | |
| Fever | 0 | |
| Other complications | 5 (6.9%) | Complications related to prematurity (3), hypoxia (1), weight loss (1) |
| Birth weight | ||
| Normal (>2500 g) | 59 (81.9%) | |
| Low (<2500 g) | 10 (13.9%) | |
| Very low (<1500 g) | 2 (2.8%) | |
| Extremely low (<1000 g) | 1 (1.4%) | |
| COVID-19 | ||
| Not tested | 38 (52.8%) | |
| SARS-CoV-2 positive (RT-PCR) | 2 (2.8%) | |
| SARS-CoV-2 negative (RT-PCR) | 32 (44.4%) |
Data are represented as numbers and percentages.
COVID-19, coronavirus disease 2019; RT-PCR, real-time PCR; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Neonatal outcomes
From the 67 pregnancies resulting in live birth, 72 neonates (62 singletons, 5 twins) were born. Of all the neonates, 22.2% were born preterm, and 18.0% had low birth weight, including two and one neonates with very low and extremely low birth weight, respectively. Five neonates (6.9%) had respiratory symptoms at birth, one had signs of hypoxia and four others had complications related to prematurity. Fever was not reported in any of the neonates. Of all neonates, 47.2% were tested with RT-PCR. Two neonates were SARS-CoV-2 positive (2.8% of all neonates, 5.8% of all tested neonates), the remaining 32 were negative. There were no neonatal deaths (Table II).
Term versus preterm delivery
Of the 67 deliveries, 13 were preterm. Women who delivered preterm were more often diagnosed with SARS-CoV-2 infection in the third trimester or during delivery (53.7% of term deliveries versus 84.6% of preterm deliveries were diagnosed with SARS-CoV-2 in the third trimester or during delivery; P = 0.04). There were also more twin pregnancies in the preterm delivery group (2/54 versus 3/13; P = 0.02). Neonates born preterm more often had low birth weight (3/56 versus 10/16; P < 0.01) and respiratory symptoms at birth (1/56 versus 4/16; P < 0.01) (Table III).
Table III.
Characteristics of the COVID-19 infection and medically assisted reproduction techniques used for the 67 deliveries (72 neonates) subdivided between term and preterm deliveries.
| Term birth (≥37 weeks) | Preterm birth (<37 weeks) | |
|---|---|---|
| n = 54 | n = 13 | |
| Diagnosis COVID-19 | ||
| RT-PCR | 49 (90.7%) | 11 (84.6%) |
| Antibody | 3 (5.6%) | 0 |
| Other | 2 (3.7%) | 2 (15.4%) |
| Time of diagnosis | ||
|
During the MAR treatment (including 15 days after embryo transfer) |
2 (3.7%) | 0 |
| First trimester | 10 (18.5%) | 1 (7.7%) |
| Second trimester | 13 (24.1%) | 1 (7.7%) |
| Third trimester (including delivery) | 29 (53.7%) | 11 (84.6%) |
| Symptoms | ||
| Asymptomatic | 19 (35.2%) | 5 (38.5%) |
| Fever | 22 (40.7%) | 6 (46.2%) |
| Cough | 20 (37.0%) | 3 (23.1%) |
| Anosmia | 16 (29.6%) | 2 (15.4%) |
| Ageusia | 7 (13.0%) | 2 (15.4%) |
| Gastrointestinal symptoms | 6 (11.1%) | 2 (15.4%) |
| Other | 11 (20.4%) | 4 (30.8%) |
| Hospitalization | ||
| None | 33 (61.1%) | 5 (38.5%) |
| General ward | 17 (31.5%) | 8 (61.5%) |
| ICU | 2 (3.7%) | 0 |
| Respiratory support | 2 (3.7%) | 0 |
| MAR technique | ||
| ART | 30 (55.6%) | 8 (61.5%) |
| Ovulation induction, with or without IUI | 24 (44.4%) | 5 (38.5%) |
| Maternal age | ||
| (Mean ± SD) (years)1 | 33.5 ± 6.5a | 31.7 ± 6.4b |
| Twin pregnancies | 2 (3.7%) | 3 (23.1%) |
| Mode of delivery | ||
| Vaginal delivery | 20 (37.0%) | 5 (38.5%) |
| Caesarean section | 33 (61.1%) | 8 (61.5%) |
| Unknown | 1 (1.9%) | 0 |
| Neonates | Neonates | |
| n = 56 | n = 16 | |
| Neonatal complications | ||
| None | 53 (94.6%) | 9 (56.3%) |
| Respiratory symptoms | 1 (1.8%) | 4 (25.0%) |
| Fever | 0 | 0 |
| Other complications | 2 (3.6%) | 3 (18.8%) |
| Hypoxia (1), weight loss (1) | All related to prematurity | |
| Birth weight | ||
| Normal (>2500 g) | 53 (94.6%) | 6 (37.5%) |
| Low (<2500 g) | 3 (5.4%) | 7 (43.8%) |
| Very low (<1500 g) | 0 | 2 (12.5%) |
| Extremely low (<1000 g) | 0 | 1 (6.3%) |
| COVID-19 | ||
| Not tested | 31 (55.4%) | 7 (43.8%) |
| SARS-CoV-2 positive (RT-PCR) | 0 | 2 (12.5%) |
| SARS-CoV-2 negative (RT-PCR) |
Data are represented as numbers and percentages.
COVID-19, coronavirus disease 2019; ICU, intensive care unit; MAR, medically assisted reproduction; RT-PCR, real-time PCR; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Data available for 46a and 12b cases.
Discussion
We reported on 80 women who conceived with MAR and were affected by COVID-19 during their pregnancy. About 40% of the women were diagnosed with COVID-19 during MAR treatment or during the first trimester of pregnancy. The majority were diagnosed with RT-PCR.
Comparison with overall pregnancy data in COVID-19 infected mothers or non-COVID-19 ART pregnancies
Allotey et al. (2020) have conducted a living systematic review of clinical manifestations, maternal and perinatal outcomes of COVID-19 in pregnancy, which is regularly updated. They reported on 67 271 pregnant and recently pregnant women who had suspected or confirmed COVID-19. While pregnant women were less likely to be symptomatic than non-pregnant women (77% versus 89%), the most common symptom was fever and cough in 40% and 41%, respectively (Allotey et al., 2020). This is consistent with our observations; 68.6% of the women were symptomatic, with fever being the most frequent symptom reported for 40.0% followed by cough for 35.2% of women in our sample. ‘All-cause’ mortality rates in pregnant women with COVID-19 have varied between 0.02% and 0.8% (Allotey et al., 2020; Pineles et al., 2021). In our study, there was one maternal death (mortality rate 1.25%). Stillbirth has been reported in 0.8–1% of pregnant women with COVID-19 (Allotey et al., 2020; Mark et al., 2021), and in our study, the prevalence was 2.5% (two cases). While the rates of maternal mortality and stillbirth seem to be higher in our sample, there was only one case of maternal mortality and two cases of stillbirth, which may appear higher when expressed as percentages in a small sample. It is also possible that survey participants were more likely to report these rare negative outcomes rather than uncomplicated cases. It is noteworthy that both stillbirths were conceived with ovulation induction, which could be considered to resemble spontaneous conceptions more than ART pregnancies. Moreover, one of the two women had a history of previous pregnancy losses. In our opinion, these data would render it difficult to claim that ART pregnancies would be under higher risk of stillbirth with COVID-19. The only case of maternal mortality was reported from a public hospital in a lower middle-income country as noted by the data submitter. She was a young woman with no known comorbidities, normal BMI and no smoking or alcohol use. Therefore, we think, as did the data submitter, that her death can be a direct consequence of COVID-19.
In our dataset, 12.5% of reported pregnancies resulted in miscarriage. In the latest report from the European IVF-monitoring Consortium (EIM), the miscarriage rate after ART was 16.4% (The European IVF-monitoring Consortium for the European Society of Human Reproduction Embryology et al., 2020). In the Australian and New Zealand Assisted Reproduction Database (ANZARD) data collection, an early pregnancy loss rate of 19.5% was reported for 2018 (Newman et al., 2020). Despite the indirect nature of these comparisons, our data do not suggest an alarming increase in miscarriage rates in MAR pregnancies affected by COVID-19. Yet, with three miscarriages being reported out of 11 COVID-19 infections detected during (or shortly after) MAR treatment, an increased risk of miscarriage in women pregnant after MAR cannot be confidently excluded. It should be noted that data on COVID-19 affected spontaneous pregnancies are still dominated by third trimester infections, limiting firm conclusions on the incidence of miscarriage (Allotey et al., 2020; Mark et al., 2021).
A higher rate of preterm delivery and Caesarean section (C-section) have been reported in MAR pregnancies when compared with spontaneous pregnancies (Berntsen et al., 2019). Preterm birth was reported in 15.0–28% of deliveries after spontaneous pregnancy with SARS-CoV-2 infection (Allotey et al., 2020; Mark et al., 2021), and 14.1% of non-COVID-19 infected singleton MAR pregnancies (The European IVF-monitoring Consortium for the European Society of Human Reproduction Embryology et al., 2020), while in our dataset preterm birth rates of 19.4% and 16.1% were calculated for all MAR pregnancies and singleton MAR pregnancies, respectively. In our sample, the incidence of COVID-19 symptoms seemed similar between women who gave birth at term or earlier, thus a higher proportion of women with preterm delivery being diagnosed with SARS-CoV-2 infection during the third trimester or at time of delivery could be probably due to routine screening for the infection at time of admission to the hospital for delivery or an incidental finding, rather than the timing of infection affecting time of birth. With regards to the mode of delivery, a C-section rate of 38.7–57% was reported for COVID-19 affected spontaneous pregnancies (Allotey et al., 2020; Mark et al., 2021). In our dataset, C-section was reported for 61.1% of the deliveries. While these figures are higher than general Caesarean delivery rates, it is possible that concerns about transmission of the infection and potential deterioration of maternal status during labour have led to an increase in C-sections (Engels Calvo et al., 2021). Other possible explanations for the high rate of C-sections are physicians having a lower threshold for C-section for MAR pregnancies or random variation in this small sample.
The limitations of our study include its limited sample size, the use of external and historical controls, and the use of voluntary reporting which could be selective with possible risks of over- or under-estimation.
Despite these limitations, our findings offer reassurance that COVID-19 infection during pregnancy results in similar outcomes irrespective of whether the pregnancy is established after MAR or not.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
Supplementary Material
Acknowledgements
The authors acknowledge the ESHRE members and MAR professionals who contributed to the data collection. Without their time and effort, this attempt to gather information on COVID-19 affected MAR pregnancies would not have been feasible.
Authors’ roles
All authors contributed to the design of the data registry. S.M. and N.V. managed data collection and verified the data. B.A. prepared the manuscript, which was critically reviewed and approved by all authors.
Funding
The authors acknowledge the support of ESHRE for the data registry and meetings.
Conflict of interest
J.S.T. reports grants or contracts from Sigrid Juselius Foundation, EU and Helsinki University Hospital Funds, outside the scope of the current work. The other authors declare that they have no conflict of interest.
Contributor Information
The ESHRE COVID-19 Working Group:
Walid Abou Rjeily, Cengiz Alataş, Tamal Alkon, Martha Luna, Devleta Balic, Alberto Barros, Nicole Beckers, Rashida Begum, Frederico Boeykens, Ioan Boleac, Cosmina Bunescu, Carlos Calhaz-Jorge, Antonio Colicchia, Irida Dajti, Marjolein De Vreis, Samuel Dos Santos Ribeiro, Marija Dundovic, Victoria Antequera Duran, Mohamed Mamdouh Elhusien, Sandro Esteves, Eduarda Felgueira, Ewa Goncikowska, Enric Güell, Ernestine Gwet-Bell, Lara Heleno, Joana Mesquita Guimarães, Mitranovici Melinda Ildiko, Guvenc Karlikaya, Nalini Kaul-Mahajan, Kseniia Khahylenko, Peter Kovacs, Manja Krause, Aswathy Kumaran, Manu Lkshmi, Daniela Nogueira, Aylin Pelin Cil, Valeriia Pelvina, Fernanda Polisseni, Roxana Popovici, Mahadinata Putra, Kamal Eldin Rageh, Nazdar Raouf, Edo Rezaldy Edward, Ricardo Sertã, Ayse Seyhan, Sergio Soares, Barbara Sonntag, Eva Stastna, Anupama Suwal Gurung, Margarita Torres Vives, Mert Turgal, and Pedro Xavier
References
- Allotey J, Stallings E, Bonet M, Yap M, Chatterjee S, Kew T, Debenham L, Llavall AC, Dixit A, Zhou D. et al. ; for PregCOV-19 Living Systematic Review Consortium. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ 2020;370:m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntsen S, Söderström-Anttila V, Wennerholm UB, Laivuori H, Loft A, Oldereid NB, Romundstad LB, Bergh C, Pinborg A.. The health of children conceived by ART: ‘the chicken or the egg?’. Hum Reprod Update 2019;25:137–158. [DOI] [PubMed] [Google Scholar]
- Engels Calvo V, Cruz Melguizo S, Abascal-Saiz A, Forcén Acebal L, Sánchez-Migallón A, Pintado Recarte P, Cuenca Marín C, Marcos Puig B, Del Barrio Fernández PG, Nieto Velasco O. et al. ; Spanish Obstetric Emergency Group. Perinatal outcomes of pregnancies resulting from assisted reproduction technology in SARS-CoV-2-infected women: a prospective observational study. Fertil Steril 2021;116:731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaroli L, Ata B, Lundin K, Rautakallio-Hokkanen S, Tapanainen JS, Vermeulen N, Veiga A, Mocanu E; ESHRE COVID-19 Working Group. The calm after the storm: re-starting ART treatments safely in the wake of the COVID-19 pandemic. Hum Reprod 2021;36:275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark EG, McAleese S, Golden WC, Gilmore MM, Sick-Samuels A, Curless MS, Nogee LM, Milstone AM, Johnson J.. Coronavirus disease 2019 in pregnancy and outcomes among pregnant women and neonates: a literature review. Pediatr Infect Dis J 2021;40:473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JE, Paul RC, Chambers GM.. Assisted Reproductive Technology in Australia and New Zealand 2018. Sydney: National Perinatal Epidemiology and Statistics Unit, the University of New South Wales, Sydney, 2020. [Google Scholar]
- Peiris JS, Yuen KY, Osterhaus AD, Stohr K.. The severe acute respiratory syndrome. N Engl J Med 2003;349:2431–2441. [DOI] [PubMed] [Google Scholar]
- Pineles BL, Goodman KE, Pineles L, O'Hara LM, Nadimpalli G, Magder LS, Baghdadi JD, Parchem JG, Harris AD.. In-hospital mortality in a cohort of hospitalized pregnant and nonpregnant patients with COVID-19. Ann Intern Med 2021;174:1186–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The European IVF-monitoring Consortium for the European Society of Human Reproduction Embryology, Wyns C, Bergh C, Calhaz-Jorge C, De Geyter C, Kupka MS, Motrenko T, Rugescu I, Smeenk J, Tandler-Schneider A, et al. ART in Europe, 2016: results generated from European registries by ESHRE. Hum Reprod Open 2020;2020:hoaa032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga A, Gianaroli L, Ory S, Horton M, Feinberg E, Penzias A.. Assisted reproduction and COVID-19: a joint statement of ASRM, ESHRE and IFFS. Hum Reprod Open 2020;2020:hoaa033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, Rienzi L, Sunde A, Schmidt L, Cooke ID. et al. The international glossary on infertility and fertility care, 2017. Hum Reprod 2017;32:1786–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumla A, Hui DS, Perlman S.. Middle East respiratory syndrome. Lancet 2015;386:995–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.