Abstract
Stimulation of the cortex can modulate the connectivity between brain regions. Although targeted neuroplasticity has been demonstrated in-vitro, in-vivo models have been inconsistent in their response to stimulation. In this paper, we tested various stimulation protocols to characterize the effect of stimulation on connectivity in the non-human primate cortex in-vivo. We found that the stimulation latency, the state of the cortex during stimulation, and the stimulation site all affected the modulation of cortical connectivity. We further investigated features of a resting-state network that could predict how a connection is likely to change with stimulation.
I. INTRODUCTION
Neurological disorders affect millions of people worldwide. Many of these disorders, such as epilepsy, schizophrenia, and post-stroke cognitive impairment are linked to aberrant connectivity in the brain [1]. One possible approach to combat these disorders is targeted reorganization of neural connections through neural stimulation. As the brain has neuroplastic mechanisms, stimulation can leverage neuroplasticity to result in changed neuronal network dynamics.
Neuroplasticity has been shown to follow the framework of spike-timing dependent plasticity (STDP), which holds that when neuron A activates immediately before neuron B, the connection from A to B is strengthened (long-term potentiation), while when B fires before A then the connection from A to B is weakened (long-term depression) [2]. The degree of connection change is modulated by the latency between the two activations. In this paper we test different stimulation protocols with varying relations to the STDP latency window. For quantifying connection strength, we use the coherence metric, which has shown to be more robust than other commonly used neural connection metrics [3].
Previous studies have explored the effects of stimulation for changing cortical dynamics. Such studies have varied from electrical to optogenetic stimulation and have investigated the immediate and long-term effects of such stimulation between stimulation pairs, with varying degrees of success [4, 5, 6, 7]. In this study, we electrically stimulate cortical regions and characterize how stimulation affects the coherence over the whole network.
II. METHODS
A. Animal Model
One adult male rhesus macaque was used in this study. All experiments were performed under approval of the University of California, San Francisco Institutional Animal Care and Use Committee and were compliant with the Guide for the Care and Use of Laboratory Animals.
B. Data Acquisition
A macaque monkey was implanted with a 96-electrode Utah array in its primary somatosensory cortex (S1). We used a Tucker-Davis Technologies System (FL, USA) to control the recording and stimulation of the electrodes. For the duration of an experiment, the monkey was awake and headfixed, sitting in a primate chair. We monitored the animal during the experiment to ensure it remained awake.
The local field potentials were sampled at a rate of 24 kHz, and then downsampled to 3.051 kHz. We recorded neural activity before and after stimulation. Additionally, we interleaved 10 minutes of stimulation with 2-5 minute periods of neural recording to obtain data corresponding to stimulation blocks. This interleaving was necessary as stimulation results in saturation of the recording electrodes, preventing us from recording during stimulation. We repeated this stimulation-recording protocol 3 to 5 times per experiment.
C. Stimulation Protocols
Stimulation of an electrode consisted of a 5 ms burst of 1 kHz stimulation at 120 μA. In this study we used four different stimulation protocols: in-STDP, out-STDP, random, and single-site. In-STDP, out-STDP, and random correspond to protocols of paired stimulations (alternating stimulations of 2 channels) of different time intervals between the stimulations (Fig. 1). In-STDP stimulation consisted of two bursts, one in the first stimulated channel and one in the second, with a separation between the burst onsets of 10 ms. Out-STDP had a burst onset separation of 100 ms, while random had a burst onset separation randomly sampled from the uniform distribution [−100 ms, 100 ms]. Single-site corresponded to stimulation of a single channel. For each protocol the stimulations were repeated every 200 ms for the 10 minute period.
Fig. 1.
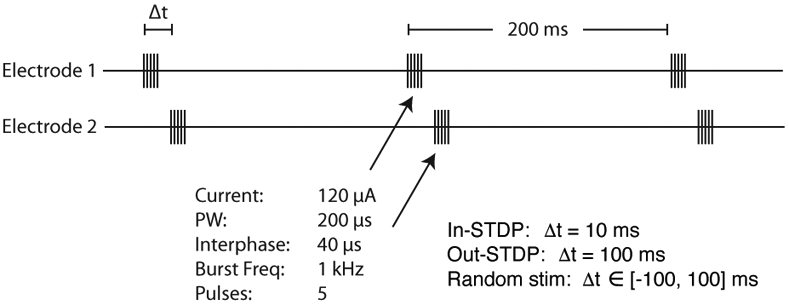
Paired stimulation protocol. A 5 ms 1 kHz burst is delivered to electrode 1, and then a 5 ms 1 kHz burst is delivered to electrode 2. The time delay, Δt, between onsets of the bursts is detailed in the lower-right of the figure. This protocol is applied in 10 minute blocks interleaved with recordings.
The in-STDP and out-STDP stimulation protocols correspond to different relationships to the STDP window. We set the lag between electrode 1 and 2 bursts of in-STDP stimulation at 10 ms in order to fall within the STDP long-term potentiation window [2]. In order to test a control stimulation of identical stimulation power but that would not evoke STDP-driven changes, we set the out-STDP lag to 100 ms, which fell outside the STDP long-term potentiation window.
D. Coherence
We divided the raw signals into 1-minute long segments. Within each segment, pairwise coherences were calculated with a Hamming window of 10 seconds and 50% overlap. Coherence is defined as:
| (1) |
Where Gxx and Gyy correspond to power spectral density of channels x and y, respectively, and Gxy corresponds to the cross-spectral power density of the two channels. Coherence values were binned into beta (12-30 Hz), gamma (30-55 Hz), and high-gamma (65-200 Hz) frequency ranges. Significant changes between different recording blocks were detected using paired 2-sided t-tests.
III. Results
A. Electric Stimulation Alters Connectivity Dynamics Between Stimulated Sites
We obtained results characterizing the effects of the various stimulation protocols on the coherence between a single pair of electrodes (channels A and B), as shown in Fig. 2. For the “Single” stimulation protocol, we stimulated only channel B, but still measured the coherence between channels A and B. The coherence during all stimulation protocols except out-STDP were significantly increased from the before-stimulation values (p-values<0.01). We repeated the recordings after stimulation and found the coherence during this period to still be significantly increased from before-stimulation (p-values<0.001). These after-stimulation values reflect a significant decrease from during-stimulation coherence for the majority of stimulation protocols, indicating a trend towards pre-stimulation baseline. We additionally performed a sham trial, in which no stimulation was applied, as a control and found no significance change in coherence.
Fig. 2.
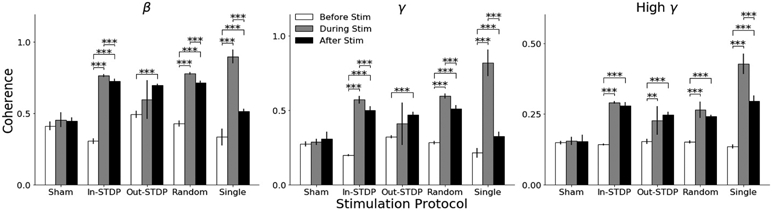
Coherence between stimulation electrodes before stimulation, during the stimulation recording block, and after stimulation ended. Bar heights indicate mean values, while error bars indicate 95% confidence interval. Asterisks indicate statistical significance, with ‘*’ indicating p<0.05, ‘**’ indicating p<0.01, and ‘***’ indicating p<0.001.
B. Stimulation-Induced Change Depends on the State of the Network
We stimulated multiple pairs of electrodes in the same experiment session, with later stimulation sessions performed before coherence values returned to baseline. On one day, we performed in-STDP stimulation, then an extended recording session, and then out-STDP stimulation. On another day, we reversed the order and performed out-STDP stimulation, an extended recording session, and then in-STDP stimulation. This reversal allowed us to compare differences in the evoked coherence change of a recently stimulated brain vs. a brain in a resting unstimulated state.
Our results, displayed in Fig. 3, demonstrated that stimulating the brain when already in a recently stimulated state can affect the stimulation-induced coherence. The induced coherences differed significantly (p-values <0.05) between the two approaches for all trials except in-STDP for beta band and gamma band.
Fig. 3.
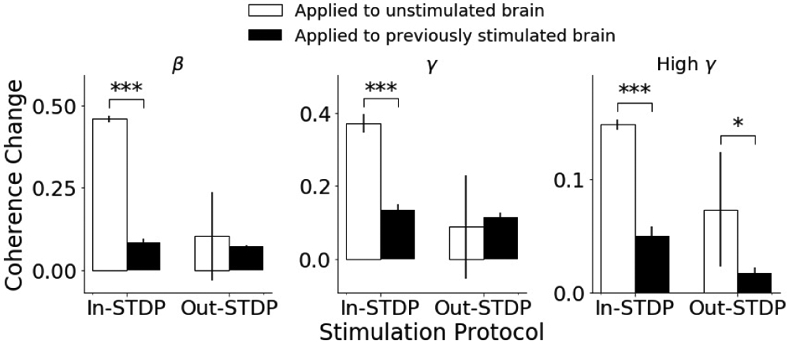
Comparison of coherence change for two types of paired stimulation, when applied as first stimulation of experimental session (white bars) vs. when applied to a brain that had already been stimulated in that experimental session (black bars).
C. Paired Stimulation Can Increase or Decrease Coherence Between Stimulation Sites
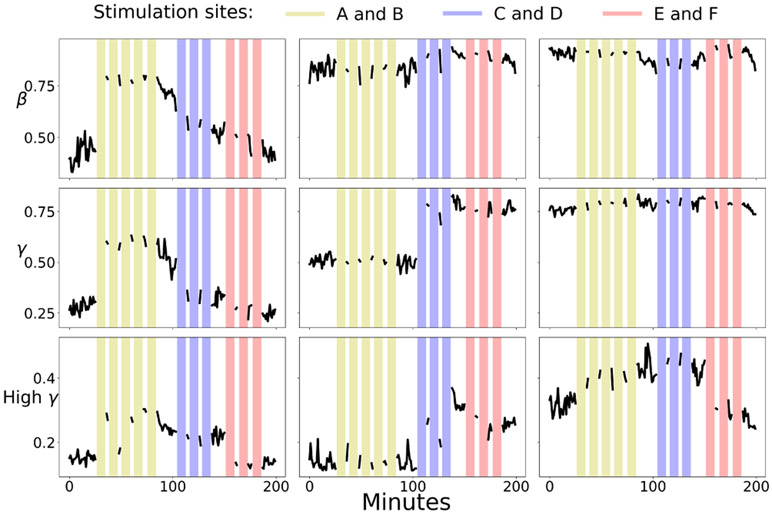
All stimulation-driven coherence changes between electrodes A and B corresponded to increases in coherence. We wanted to test whether coherence always increases following stimulation, or whether the measured increase was a facet of the specific neuronal location of electrodes A and B. We thus tested two other stimulation pairs. One of the pairs (C and D) was located far from our original stimulation electrode pair (A and B), while the other pair (E and F) was spatially close to A and B. The intra-pair distance was consistent between all pairs.
The trials for these channels were completed when the coherence had not yet returned to baseline following stimulation of pair A-B (Fig. 4). The data in this section are tested for significance with respect to the post A-B stimulation excited baselines. Therefore we must qualify the results of this section by stating that they are valid within the context of recent previous paired stimulation of sites A and B.
Fig. 4.
A time series of coherence values for three electrode channel pairs. Colored vertical bars indicate stimulation sessions. The left, center, and right plots indicate coherence between channels A and B, C and D, and E and F respectively. Yellow, light blue, and pink lines indicate 10 minute periods of paired stimulation between channels A and B, C and D, and E and F respectively.
For each stimulation electrode pair, we compared their coherence immediately preceding their stimulation, with their coherence during their respective stimulation block, and with their post-stimulation coherence. C-D pair demonstrated significant increase for all comparisons while E-F pair demonstrated significant decrease for all comparisons (p-values<0.01) except the beta coherence changes of pair E-F.
D. Features of Baseline Recording Are Related to Stimulation Induced Coherence Change Across Entire Network
We obtained coherence values for all electrode pair combinations of the 96 Utah array electrodes in response to stimulation of channels A and B. We quantified the change in coherence of each pair relative to baseline and performed least squares regression to quantify how baseline coherence, baseline coherence stability, and electrode pair distance are related to coherence change during stimulation. In order to quantify baseline coherence stability, we calculated the standard deviation of the coherences of each electrode pair during baseline recording. As change in coherence includes a term of the baseline coherence, the regression is biased. In order to unbias the regression, we split the coherences up into odd and even minutes, and calculated the baseline with the even minutes, while calculating the change in coherence with the odd minutes. A similar approach has been used in [4] in order to combat this same regression bias.
Our analysis showed that the baseline coherence, standard deviation of baseline coherence, and electrode pair distance were each significantly (p-value<0.001) linearly related to the change in coherence (Fig. 5). Baseline coherence yielded the highest r2 for beta and gamma band coherence change, while baseline standard deviation yielded the highest r2 for high gamma. All baseline coherence slopes were negative, and all baseline standard deviation slopes were positive.
Fig. 5.
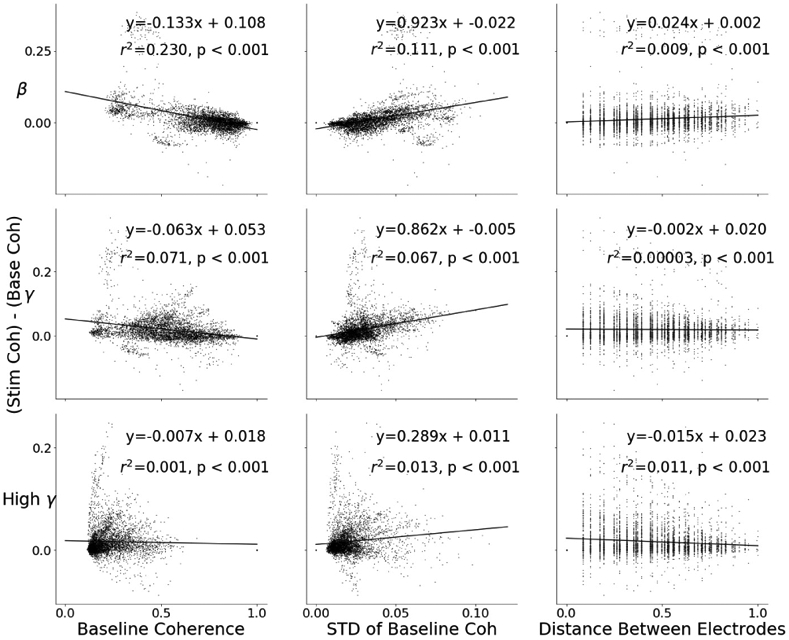
Scatter plots of stimulation-induced coherence change with respect to (from left to right) mean baseline coherence, standard deviation of baseline coherence, and distance between electrode pairs. The top plots are for beta frequency band, the middle for gamma, and the bottom for high-gamma. The data points represent all 96x96 coherence changes all possible electrode pairs. Each feature is statistically significantly related to the change in baseline coherence.
We then constructed a multiple regression model of these three features in order to evaluate the degree to which each feature could increase the explained variance ratio (adjusted r2) of the regression model. The multiple regression model yielded adjusted r2 values of 0.239, 0.123, and 0.028 for beta, gamma, and high gamma, respectively. These improved adjusted r2 values are appreciably higher than the highest r2 values of the single regression models for gamma (0.071, from baseline coherence) and high-gamma band (0.013, from baseline stability), but not for beta (0.23, from baseline coherence). Each feature continued to be statistically significant (p-values<0.001) in the multiple regression models.
IV. Discussion
In this study we investigated cortical network dynamics in response to electrical stimulation. We compared stimulation protocols, electrode pairs, and predictive baseline features. Our results indicate that coherence between electrodes can be modulated through stimulation, and that the protocol of stimulation affects the coherence modulation. Stimulation latency, site of stimulation, and the state of the network before stimulation all affect coherence during stimulation.
A hypothesis constructed from a naïve extrapolation of STDP excitation rules would be that the paired stimulation within the long-term potentiation window would result in unilateral increase in coherence between two stimulation sites. This hypothesis would explain the more significant coherence change of in-STDP and random stimulation compared to out-STDP (Fig. 2). However, although we did see increases in coherence for pairs A-B and C-D, pair E-F decreased in coherence when stimulated. STDP rules delineate that when A fires before B, the connection from A to B is strengthened but the connection from B to A is weakened. As magnitude of coherence measures the overall connectivity between two sites, and does not confer any information of directionality, it is possible that there was a larger decrease in connectivity from F to E than there was an increase from E to F, which yielded a net decrease in connectivity. Although this explanation might fit within the STDP framework, it does not take into account how the single-site stimulation of channel B resulted in increased coherence with channel A.
An alternative hypothesis is that connections between neuronal sites are largely unchanged, and that the chief mechanism of electrical stimulation induced coherence modulation is modulation of cortical oscillation rhythms. Since coherence is a measure of the degree to which two signals keep consistent phase with one another, modulation of cortical oscillation synchrony would result in changed coherence. However, similar studies have investigated the change in connection between two sites by using other connectivity metrics such as evoked response amplitudes or Granger Causality [4, 5, 6, 7] and have reported similar connectivity modulation with these metrics. In addition, coherence change has been directly compared to evoked response change and the two were shown to be significantly correlated [4].
It is clear from Fig. 4 that there are dynamics determined by the underlying cortical network that dictate the response of the network to stimulation. Fig. 5 is a first attempt at predicting this response from the network, and although the regressors are statistically significant it is still for the simple case of stimulation of just one electrode pair. The r squared values and p-values of the regression models indicate that simple features such as inter-electrode distance, baseline coherence, and baseline coherence stability may successfully predict the degree of coherence change during stimulation, but that as the frequency of the coherence rises, so does the difficulty in explaining the change in coherence. This is consistent with past analyses of local-field potential frequencies, in which higher frequencies are hypothesized to underlie more complex local connectivity [8]. If more data is made available experimentally, more complex modeling tools can be leveraged to gain an understanding of the cortical network structure that underlies connectivity modulation.
V. Conclusion
As a means to combat neural disorders stemming from aberrant connectivity, cortical stimulation is a powerful method. The inherent complexity of the brain, however, makes targeted reorganization of brain connectivity using stimulation a complex task. As connections between neural areas are mediated by many intermediary connections, the dynamics of paired-site connectivity modulation can only be understood by gaining an understanding of the connectivity dynamics of the entire network. In this paper we evaluated the effect of different stimulation protocols on coherence between brain regions, both between stimulation sites and of the network. This work can offer direction to researchers choosing a methodology of stimulation in order to affect cortical connections, for either rehabilitative or purely scientific purposes.
VI. Acknowledgments
We thank Kate Derosier and Julien Rechenmann for help with data collection, Dhanush Bekal Kannangola for help with data analysis and Lindsey Presson for animal care.
This project was supported by the Eunice Kennedy Shiver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number K12HD073945 (AY), the Washington National Primate Research Center (WaNPCR, P51 OD010425), the American Heart Association post-doctoral fellowship (AY), National Science Foundation Graduate Fellowship Research Program (DBS), Paralyzed Veterans of America Research Foundation Fellowship (JEO), and the Center for Neurotechnology (CNT, a National Science Foundation Engineering Research Center under Grant EEC-1028725).
Contributor Information
Julien A. Bloch, University of Washington, Seattle, WA 98105 USA.
Karam Khateeb, University of Washington, Seattle, WA 98105 USA..
Daniel B. Silversmith, University of California, San Francisco, CA 94143 USA.
Joseph E. O’Doherty, University of California, San Francisco, CA 94143 USA; Neuralink, San Francisco, CA 94110.
Philip N. Sabes, University of California, San Francisco, CA 94143 USA; Neuralink, San Francisco, CA 94110.
Azadeh Yazdan-Shahmorad, University of Washington, Seattle, WA 98105 USA..
References
- [1].Stam CJ, “Modern network science of neurological disorders,” Nature Reviews Neuroscience, pp. 683–695, 2014. [DOI] [PubMed] [Google Scholar]
- [2].Bi G and Poo M “Synaptic Modifications in Cultured Hippocampal Neurons: Dependence on Spike Timing, Synaptic Strength, and Postsynaptic Cell Type,” Journal of Neuroscience, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bastos AM, “A Tutorial Review of Functional Connectivity Analysis Methods and Their Interpretational Pitfalls,” Frontiers in Systems Neuroscience, January 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yazdan-Shahmorad A, “Targeted cortical reorganization using optogenetics in non-human primates,” ELife, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lucas T and Fetz EE, “Myo-cortical crossed feedback reorganizes primate motor cortex output,” 33(12), pp. 5261–5274, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rebesco JM, and Miller LE, “Enhanced detection threshold for in vivo cortical stimulation produced by Hebbian conditioning,” Journal of Neural Engineering, 8(1), 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Seeman SC, Mogen BJ, Fetz EE, and Perlmutter SI, “Paired Stimulation for Spike-Timing-Dependent Plasticity in Primate Sensorimotor Cortex,” The Journal of Neuroscience, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Buzsáki G, and Schomburg EW, “What does gamma coherence tell us about inter-regional neural communication?” Nature Neuroscience, pp. 484–489, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]