Abstract
Cell culture is one of the most core and fundamental techniques employed in the fields of biology and medicine. At present, although the two-dimensional cell culture method is commonly used in vitro, it is quite different from the cell growth microenvironment in vivo. In recent years, the limitations of two-dimensional culture and the advantages of three-dimensional culture have increasingly attracted more and more attentions. Compared to two-dimensional culture, three-dimensional culture system is better to realistically simulate the local microenvironment of cells, promote the exchange of information among cells and the extracellular matrix (ECM), and retain the original biological characteristics of stem cells. In this review, we first present three-dimensional cell culture methods from two aspects: a scaffold-free culture system and a scaffold-based culture system. The culture method and cell characterizations will be summarized. Then the application of three-dimensional cell culture system is further explored, such as in the fields of drug screening, organoids and assembloids. Finally, the directions for future research of three-dimensional cell culture are stated briefly.
1. Introduction
Cell culture is one of the most important and fundamental techniques in the fields of biology and medicine. It involves extracting cells from biological tissues, simulating the survival environment in vivo to ensure their growth and reproduction, and maintaining their main structures and functions under sterile conditions with suitable temperature, pH, and adequate nutrient conditions.
At present, cell culture methods in vitro include two-dimensional (2D) adherent culture and three-dimensional (3D) spherical culture [1], with the former being most typically used. In this method, a glass or polystyrene dish provides mechanical support for the cells, and the supplies of exogenous nutrients and the removal of metabolites are kept under the same conditions. The conditions are well controlled, and the cells are easy to be observed and collected. However, the 2D method has its drawbacks, as it fails to mimic complex cell microenvironment.
In vivo, most cells interact with neighbouring cells and the extracellular matrix (ECM) to form a complex communication network of biochemical and mechanical signals, which is the basis for maintaining cell normal functions [2]. Oxygen, hormones, and nutrients can be transported between cells, metabolic waste can be removed from cells, and cells can move in response to mechanical or chemical stimuli [3]. With 2D culture, cells grow in a limited space and thus being subject to contact inhibition. Thus, cell proliferation is slower, and cell morphology and cell function are also changed [4]. For example, stem cells are prone to lose self-renewal ability, become senescence, or differentiate into bone cells or adipocytes spontaneously [5]. Long-term cultures will gradually lead to tissue specificity lost [6], observed differences between the results of in vitro cell culture and in vivo animal experiments. Therefore, understanding how to better simulate the physiological environment under in vitro conditions is very important for medical research [7].
Continuous advancements of technology have yielded higher requirements for cell culture models, leading to the development of 3D cell culture. Compared to 2D culture, 3D culture is closer to the organism in structure and function and more accurately simulates the microenvironment of cells in vivo [1, 3, 8]. This 3D cell culture can affect cell growth and proliferation, promote the self-renewal of stem cells, and inhibit their differentiation. In addition, like cells in vivo, 3D culture is better for the transfer of molecules between cells and between cells and the ECM, nutrients uptake, gas exchange, and metabolic waste discharge in a balanced way [9, 10]. Therefore, to maintain the original characteristics of cells and better realistically simulate the state of the cells in vivo [11], researchers have developed a variety of 3D culture systems. For drug development, 3D culture has become a bridge between 2D culture and animal experiments [12]. In this review, we will first summarize the research states of 3D culture methods, with or without scaffold support (Table 1). Then, the applications of 3D culture will be assessed. In particular, we lay emphasis on the cutting-edge applications, for example, organoids and assembloids.
Table 1.
A comprehensive summary of 3D cell culture methods.
| Method | Culture cell types | Viability | Proliferation | Differentiation | Time length | Spheroid diameter (μm) | Advantages | Disadvantages | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| Liquid overlay | (1) MDPSCs; (2) ADSCs | Well-preserved | Osteogenic | -1 day | 30-100 | (1) Simple; (2) economical | Size of the spheroids cannot be controlled | [15–21] | |
| Hanging drop culture | WJ-MSCs | Cell death rate was below 40% | Higher than 2D |
Early osteogenic | Depend on cell types | Controllable | Uniformity | Volume cannot more than 30 μL | [2, 22–27] |
| Rotating bioreactor | BMSCs | High level of viability | Osteoblastic | 1 day | 100–200 | (1) Simple; (2) efficient | (1) Shear stress; (2) equipment | [18, 28] | |
| Magnetic suspension | NIH3T3 | 99% cell viability | Exponential growth | 5 minutes | (1) Repeatable ability; (2) size stability. | Potential impact of nanoparticles is uncertain | [24, 29–34] | ||
| Chemical reagents culture | ADSCs | High level of viability | Suppressed | Osteogenesis | 3-4 days | 50-200 | (1) Simple; (2) practical; (3) special equipment unnecessary | Potential impacts of reagent are uncertain | [6, 35] |
| Alginate-PEG gels | mMSCs | Improves the viability of cell | Reducing cellular apoptosis | Osteogenic | Biocompatibility high mechanical strength | Early complicate | [36–57] |
2. Three-Dimensional Stem Cell Culture Systems
3D cell culture technology, which refers to the cocultivation of carriers with 3D structures of different materials and various types of cells in vitro, so that the cells can migrate and grow in the 3D structure of the carrier to form a 3D cell-carrier complex, can be divided into scaffold-free and scaffold-based culture systems [13], with both types having their own applications in different studies (Figure 1).
Figure 1.
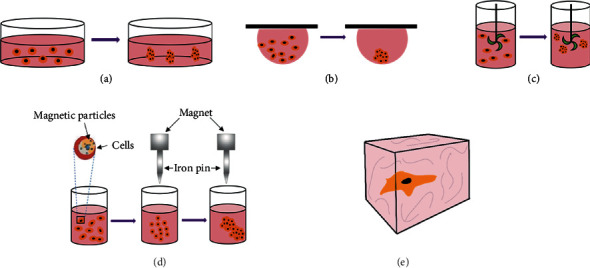
Scheme of 3D cell culture systems: (a) liquid overlay culture; (b) hanging drop culture; (c) rotating bioreactor culture; (d) magnetic suspension culture; (e) scaffold-based culture (hydrogel).
2.1. Scaffold-Free Culture System
This culture system does not have a supporting structure for cell adhesion, growth, and diffusion, leading to cells in the culture media to aggregate into tissue-like spheres called spheroids. These spheroids form their own ECM, without the need for exogenous scaffold or matrix. The ECM includes glycosaminoglycans, proteoglycans, structural proteins, adhesion proteins, and other macromolecular substances, which affect a series of activities such as cell shape, metabolism, function, migration, proliferation, and differentiation [14].
2.1.1. Liquid Overlay Culture
The liquid overlay technique is one of the simplest and most economical 3D cell culture methods [15]. This method relies on materials that inhibit cell adhesion to the bottom of the cell culture vessel, such as agar and agarose, HEMA, or ultralow attachment plates [16]. The intercellular force is greater than between cells and the material surface [17], and this force between cells can spontaneously aggregate to form cell spheres within 24–72 hours [18–20]. Corning ultralow attachment microplates are commonly used, which are characterized by a covalently bonded hydrogel layer that minimizes cell adhesion, protein adhesion, and cell activation, with spontaneous cell assembly relying on self-secreted ECM [21].
2.1.2. Hanging Drop Culture
The hanging drop culture is one of the most widely used methods of 3D cell culture and relies on the cell's own gravity to make individual cells aggregate to form 3D spheres [22, 23]. To perform this method, one drops the cell suspension droplets on the inner lid of the tissue culture dish, with the droplet volume being 10–20 μL [24] and containing approximately 50–500 cells. Following this, the lid is turned upside down, and the droplets are fixed in place by surface tension. The microgravity environment of each droplet condenses the cells, forming a single sphere at the tip of the droplet and proliferating [25]. The size of the cell sphere can be controlled by adjusting the cell density of the cell suspension [26]. The obtained cell spheres are densely packed and uniform in morphology, forming spherical cells of the same size [27]. Additional advantages of this method include its low cost, convenient operation, high production efficiency, and coculturing of different types of cells. However, the disadvantages are that the volume of the cell suspension cannot be more than 30 μL; otherwise, the droplets may fall. Additionally, workload is intensive and mass production is therefore difficult [2]. Since the cell suspension is small and can evaporate easily, changing cell culture medium is too difficult to maintain long-term cell culture. The 384-hanging drop array provides improvements to the hanging drop culture method. The design of the reservoir structure effectively reduces the evaporation of small-volume hanging drops. Part of the culture medium can be changed, so that the cell spheroids can be cultured for a longer time. Mass production of 3D spheres can be used for basic biological research [26].
2.1.3. Rotating Bioreactor Culture
In this method, the high-density cell suspension is placed in the bioreactor, and the cell suspension is kept in motion by rotating and agitate so that the cells cannot settle and adhere to the substrate, therefore maximizing the contact between cells to form 3D spheres [18]. The system includes a container for cell culture and a continuously stirred impeller to ensure cell suspension and medium mixing. The flow of liquid not only prevents the adhesion of cells but also ensures the uniform distribution of various nutrients and oxygen, which is conducive to the formation and metabolism of 3D cell spheres. This method is relatively simple and can produce a large number of spheres in a short time. Cell culture using this method is simple and easy to mass production, the dynamic culture aids in nutrient transportation, and the spheroids are easy to obtain. However, the disadvantages are obvious in this system. The foam and shear stress of the fluid generated during the stirring process may cause damage to the cells, 3D cell spheres vary in size, and the special equipment is indispensable. Studies have shown that rotating cell culture can induce osteogenic differentiation of human bone marrow mesenchymal stem cells [28]. The possible reason is rotating culture which is more similar to the in vivo cell environment, is more conducive to bone formation, and promotes earlier osteocalcin synthesis and calcium deposition.
2.1.4. Magnetic Suspension Culture
Magnetic suspension culture is a method that uses magnetic nanoparticles (such as iron oxide or gold nanoparticles) and magnetism to gather single cells into 3D spheres [29]. Cells are incubated with magnetic nanoparticles overnight to make them magnetic; then, the magnetic cells are extracted and recultured [30]. The magnetic field is applied during the cell culture process, and the cells gather into 3D spheres at a height whereby the magnetic force and gravity balance [31]. Spheroids can be formed rapidly within five minutes, have repeatability and size stability, and can be extracted and transferred by magnetic tools [24]. This method allows for coculturing of a variety of cells [32, 33]; however, the potential impact of nanoparticles on cell signaling and function is a limitation of this approach [34].
2.1.5. Chemical Reagent Culture
Chemical reagent culture is a culture method that uses special chemical reagents to make cells self-assemble to form 3D spheres. Chen et al. found that due to difference in human or bovine serum albumin batches, the experimental results were inconsistent before and after culturing stem cells with TeSR medium. Subsequently, the components in TeSR media were studied. After sequentially screening, they finally developed a practical, determined, and albumin-free TeSR-E8 media, containing eight ingredients, namely, DMEM/F12, insulin, selenium, transferrin, L-ascorbic acid, FGF2, TGFβ, and NaHCO3 [35], which is suitable for stem cell culture. Zhao et al. have verified that human mesenchymal stem cells with a chemically defined serum-free TeSR-E8 medium can spontaneously assemble into 3D spheres. And the research found compared to 2D, the stemness of 3D cells is enhanced, which increases the treatment efficiency of endotoxemia mice and reduces mortality [6].
2.2. Scaffold-Based Culture System
Natural ECM has poor mechanical properties and high sensitivity to enzymes, which limits its application potential [36]. In recent years, with the advancement of biomaterial technology, scaffolds composed of artificial ECM are the most commonly used material and can simulate the complex 3D structure and main characteristics of living tissues. The function of the scaffold is to provide a spatial living environment for cells and enhance their adhesion, proliferation, and secretion of cytokines. Furthermore, this scaffold can promote interactions between cells and between cell and the ECM and further affect the shape of cells, metabolism, function, migration, proliferation, and differentiation [37]. Moreover, it also serves as a medium for the diffusion of soluble factors.
3D cell culture scaffolds can be divided into two types according to the source of materials: natural material scaffolds and synthetic material scaffolds [38], including hyaluronic acid, collagen, polylactic acid, and polyethylene glycol. Among them, hydrogel is one of the most widely used materials for 3D culture [39]. There are natural polymer hydrogels and synthetic polymer hydrogels [40]. Hydrogel has a network structure with a large number of hydrophilic groups, which can hold a large amount of water. The network structure of the hydrogel allows nutrients and oxygen to flow in and out freely, and the cells in it can be adequately nourished [41]. At the same time, it can also cross-link bioactive factors to regulate cell growth and differentiation, making it an excellent substitute for ECM.
2.2.1. Natural Polymer Hydrogel
Natural polymer hydrogels are mainly natural materials supplemented by other biological materials or molecules [42]. Natural materials are obtained from animal, plant, or human tissues or cells, including hyaluronic acid [43], collagen [44], fibrin [45], silk fibroin [46], alginate [47], chondroitin sulfate [48], gelatin [49], and agarose and chitosan sugar [50]. They individually or mutually aggregate to form a 3D network structure similarly to the organism under certain conditions.
Natural polymer materials have limited mechanical properties; the composition of ECM of human or animal is uncertain. Therefore, there may be pathogen risks and inconsistencies between batches [13, 51]. Natural polymers usually show good biocompatibility, sensitivity to the environment, low toxicity, and cell adhesion sites. Besides, wide source and low price are also its outstanding advantages. Furthermore, these natural materials have their own advantages, and their combination shows excellent performance. For example, some researchers have combined gelatin and polysaccharides to form gel scaffold, which takes advantage of the therapeutic and regenerative properties of gelatin and the mechanical properties of polysaccharides. Composite applications provide a promising method for the development of superior biomaterials [42]. The chitosan-alginate-gelatin composite hydrogel can promote the chondrogenic differentiation of hMSCs and contribute to cartilage regeneration in patients with related cartilage diseases [52]. Some researchers put hepatocyte-like cells derived from human pluripotent stem cells into the widely used animal-derived hydrogel Matrigel, which is a plant-derived nanocellulose hydrogel in agarose microporous 3D culture plates. These cells can all form 3D spheres and accelerate the liver maturation of hepatocyte-like cells, which further shows that the hydrogels of plant origin and animal origin have the same functions, but the former can avoid disadvantages such as endotoxin and batch-to-batch differences [53].
2.2.2. Synthetic Polymer Hydrogel
Synthetic polymer scaffold materials include polylactic acid (PLA) [54], polyethylene glycol (PEG) [55–57], polycaprolactone (PCL) [58], polylactic acid glycolic acid (PLGA) [59], poly L-lactic acid (PLLA) [60], and polyglycolic acid (PGA) [61]. These polymers are cross-linked to form a hydrogel, which can be used as a 3D cell culture platform. This inert gel has a clear chemical composition, high reproducibility, high mechanical strength [62], simple processing and manufacturing, and greater predictability of results and higher versatility. Thus, the hydrogel possesses broad application prospects in tissue engineering scaffold materials [51]. Unfortunately, synthetic hydrogels usually do not have cell adhesion sites [13], integrin-binding peptides, or growth factor binding sites. And ECM degradation protease domains that promote cell-ECM cross-linking are required [7], making the construction process relatively complicated. Additionally, the shortcomings of poor biocompatibility, poor toughness, and slow water absorption limit their direct application in the field of cell culture scaffolds and therefore require continuous research and improvement.
Generally speaking, it is difficult for a single type of material to meet the requirements of cell culture scaffold materials. As such, combining several single materials through a suitable method and comprehensively considering the advantages and disadvantages of each of the materials to form a composite material can achieve good effects [63]. Due to natural material excellent water absorption performance, strong biocompatibility, low cost, and abundance, the inclusion of cell adhesion sites comprising natural materials and the adjustable mechanical strength of synthetic materials makes for an ideal combination for preparing 3D cultured hydrogel. Researchers have developed a collagen-bioceramic composite hydrogel that can promote the osteogenic differentiation of hADSCs, offering a new approach for the treatment of bone defects [64]. Some researchers have also used gelatin-methacryloyl hydrogel, as the fusion of biological, and biological manufacturing methods can accelerate the clinical transformation of tissue repair [65]. However, the mechanism of materials in regulating 3D culture cell functions and behaviours needs further investigation.
3. Applications
3.1. Drug Screening
The preclinical screening process of therapeutic drugs usually includes the process from 2D cells, evolving from animal models to clinical trials [66], with only a few drugs ultimately passing clinical trials for approval by regulatory agencies to enter the market [67]. One possible reason is that there are differences in tissue structure between cells and organs, as well as differences in cell growth patterns [68], with inherent differences between humans and animals. Additionally, animal models are expensive, time-consuming, and raise ethical issues [24]. At the same time, the increase in drug compounds and the requirements for high-throughput screening have slowed the progress of drug research.
In view of the above, it is necessary to develop more effective preclinical screening methods to accelerate the process of determining the failure of new drug research and development, as timely termination will cause a reduction in waste. 3D cell culture may be the best candidate, as the emergence of 3D culture models has greatly improved cell-based drug screening through the identification of toxic and ineffective substances in the early stages of drug discovery [69], bridging the gap between 2D cell analysis and animal experiment results and reducing the drawbacks caused by 2D culture [68]. The experimental uncertainty reduces the cost of drug development and achieves more effective drug screening [70]. Although the accepted standard for in vitro drug screening and in vivo toxicity studies is still the 2D cell culture model [70], the 3D culture is expected to become an effective tool in the drug development process.
In the screening of cancer drugs, the 3D culture method possesses special significance. On the one hand, it provides an in vitro model similar to in vivo tumors [71]. On the other hand, normal cells around the tumor can affect the sensitivity of tumor cells to drugs. Researchers have confirmed that using a 3D coculture model can accelerate the screening of insulin-resistant diabetes drugs, and spherical coculture can be used for tumor drug screening [72].
3.2. Organoids
Organoids are the cell-derived in vitro 3D-culture organ models, with pluripotent stem cells or progenitor cells of specific tissues forming similar tissues of corresponding organs [73]. They have the ability of self-renew and self-organization and maintain the characteristics of physiological structure and function of the tissues from which they originate [74]. The development of the 3D organoid culture system has presently been acknowledged as a major technological advancement in the field of stem cells [75].
Although 2D cells are widely used in biomedical researches for a long time, they are generally regarded as pure physical contact between cells, lacking in tissue structure and complexity [76]. Compared with 2D cell culture, organoids have more abundant cell types [77], closer behaviours to physiological cells [78], more stable genomes [73], and more suitable for biological transfection [79]. At present, a variety of 3D organoid models have been successfully constructed, such as optic cup/retinal organoids [80], brain organoids [81], intestinal organoids [82], kidney organoids [83], gastric organoids [84], liver organoids [85], pancreatic organoids [86], lung organs [87], vascular organoids [88], heart organoids [89], and bladder organoids [90].
As a 3D culture system that simulates the structure and function of organs in vitro, with the recognition, organoids are widely used in disease modeling, biobanking, precision medicine, and regenerative medicine [91] (Figure 2).
Figure 2.
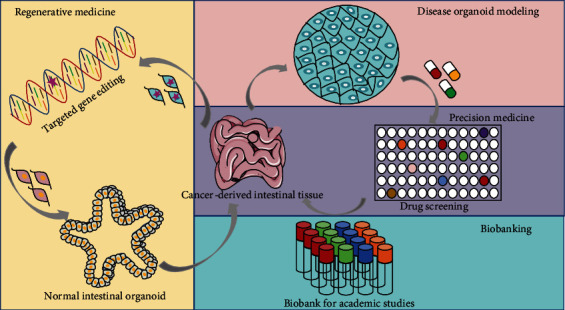
Multiple applications of organoid technology: (1) regenerative medicine; (2) disease modeling; (3) precision medicine; (4) biobanking.
3.2.1. Disease Modeling
Mounting achievements have provided convincing evidence for organoid application on disease models, such as tumors, developmental diseases, and infectious diseases [77]. Compared with the 2D culture system, 3D organoids help to clarify the development, homeostasis, and pathogenesis of diseases and provide possible new methods for the diagnosis and treatment [92].
Tumors are recognized as the primary cause of human death globally [93]. Composed of a variety of different cells, tumors develop as a result of complex intercellular interactions between cells and between cells and the ECM in a 3D environment [94]. Accordingly, 3D spheroids simulate tumor behaviour more effectively than conventional 2D cell cultures because spheroids are very similar to tumors [95]. These spheroids contain surface-exposed and deeply buried cells, proliferating and nonproliferating cells, and well-oxygenated and hypoxic cells [96], rendering them superior to 2D cells in terms of hypoxia [13], dormancy, antiapoptotic characteristics, and drug resistance [97]. Due to the complexity of tumor development, traditional 2D cultures cannot simulate the 3D microenvironment in which tumor cells reside and [98], as such, may provide misleading results on the predicted response of tumor cells to antitumor drugs [67]. Therefore, the tumor organoid model is acknowledged in cancer research.
Some characteristics of tumor organoids prove that it is suitable as a model for tumor research. Tumor organoids, which can be formed by using 3D culture technology, are the cultivation of tumor stem cells in vitro, maintaining the functions of the original stem cells and continuously dividing and differentiating to form microtumor tissues that are similar in space and structure to the source organ tissues, genes, structures, and functions [77, 99]. These organoids reproduce the in vivo characteristics and heterogeneity of the primary tumor and require a short time for formation and stable passage. They can be used for the study of tumorigenesis and development matrix, drug screening, individualized treatment, etc. [74]. 3D culture allows coculturing of multiple types of cells [69]. Some researchers have proposed that the existing cell culture methods are not enough to study fibroblasts and their interaction with cancer stem cells. They have found that fibroblasts promote the stemness of cancer stem cells under 3D environment [100] and their interaction affected cell invasion and metastasis [101]. The impact of tumor ECM on tumor progression has always been a hot issue for researchers [102]. 3D organoids provide tumor cells with a microenvironment consistent with in vivo, which is expected to find new tumor treatments.
Organoid models are used to study developmental diseases, especially brain organoids. Some researchers have developed a brain organoid model derived from human pluripotent stem cells, which can summarize the characteristics of human cerebral cortex development and can even be used to simulate microcephaly that is difficult to reproduce in mice. It also proved that the premature differentiation of neurons in the patient's organoids can be the cause of microcephaly [103]. Brain organoids have also been used to study Zika virus, which can preferentially infect neural progenitor cells and reduce their proliferation and viability. This may be an important cause of head deformities caused by Zika virus [104].
Organoids can also be used to simulate host-microbe interactions. With the emergence of various types of organoid models, the study of microbial infections will help to better understand the pathogenic mechanism and then find the best treatment strategy. Schlaermann et al. have established a powerful and quasi-immortal 3D organoid model, which is believed to be useful for future research aimed at understanding the underlying mechanisms of human gastric infections, mucosal immunity, and cancer [105]. Some organoid models have been applied to the study of microbial pathogenesis, for example, researchers used a kidney organoid model to study the mechanism of Shiga Toxin Type 2's renal cell toxicity [106]. Following the COVID-19 outbreak, Bing Zhao and Xinhua Lin's team used human organoids to study the molecular mechanisms of SARS-CoV-2 infection and liver damage, providing important tools for the study of new coronavirus cell tropism, pathogenic mechanisms, and subsequent drug development [107].
3.2.2. Biobanking and Precision Medicine
For cancer research in the past, 2D culture is the most commonly used model in vitro for high-throughput drug sensitivity tests and correlating them with changes in the genome [108]. However, 2D culture often causes lost genomic characteristics of the original donor and does not maintain individual heterogeneity, which makes it difficult to accurately predict the sensitivity of specific patients to specific drugs. The human-derived tumor xenograft models can solve this problem well. The orthotopic tumor from patients can be transplanted into an immunodeficient animal, which can maximize the preservation of the heterogeneity of the donor [109]. However, the establishment of the 3D model is time-consuming and requires high costs, which is not conducive to high-throughput drug screening. Besides, organoids can be expanded indefinitely and cryopreserved [110]. Due to the above advantages, the creation of organoid biobanks becomes possible.
In 2015, Van De Wetering's team established a colorectal cancer organoid biobank for the first time [111]. It can be used for the study of the genome and its functions at the individual level of colorectal cancer patients and has the characteristics of short time-consuming and high-throughput, which cannot be achieved by traditional cell line models and human-derived tumor xenograft models. And because organoids can well retain the heterogeneity of donor tissues, they can be used for high-throughput and high-sensitivity drug sensitivity testing and make personalized treatment plans for colorectal cancer patients, which have high application value.
Subsequently, many different tumor organoid biobanks were established, including stomach, liver, pancreas, breast, prostate cancer, lung cancer, glioblastoma, and bladder cancer. As researcher's interest in the use of organoids for disease modeling grows, biobanks will soon expand beyond cancer, such as intestinal and lung organoids for cystic fibrosis patients [112] and for liver organoids of patients suffering from various metabolic diseases [113]. Scientists strive to create a biobank of organs of healthy and diseased patients as a renewable resource that can be used by researchers around the world.
Organoids through drug screening and drug safety testing provide a unique opportunity for precision medicine. A number of drug development failures in clinical trials are partly due to insufficient evaluation of drug toxicity in the preclinical testing phase. The emerging 3D organoid technology can correctly assess the toxicity of drugs, and it is possible to determine the best and most effective dose to kill tumor cells with minimal damage to normal tissues [114]. For example, liver and kidney organoids will be an excellent platform for evaluating potential drug-related liver and kidney toxicity [115, 116]. Before treatment, patient-derived organoids are used to screen drug responses in vitro, which have been proved to be valuable diagnostic tools [117].
3.2.3. Regenerative Medicine
Organ transplantation is the most commonly used treatment for tissue and organ defects. However, this method has several shortcomings, such as insufficient donor tissue and a cumbersome process of donor selection, a risk of infection, and immune rejection [10]. In recent years, due to the favorable biological characteristics of stem cells, such as high proliferative capacity, self-renewal ability, multidirectional differentiation potential, abundancy, easy selection of materials, and absence of ethical issues, stem cells have received increasing attention from researchers in the field of tissue engineering and regenerative medicine, which has great clinical application value [118]. The stem cell transplantation process includes stem cell isolation, culture, targeted induction, and gene modification, with a large number of stem cells expanded in vitro or constructed into tissues and organs before being implanted in the body for treatment of clinical diseases [119].
As an in vitro model of tissue, organoids have attracted great attention in the field of regenerative medicine [120]. Providing appropriate 3D scaffolds and biochemical factors, cells derived from pluripotent stem cells can self-organize to form tissue-specific organoids, including optic cup [121], brain [103], intestine [122], liver [123], kidney [124], and pancreas [125].
After establishing the mouse intestinal organoids for the first time, Yui et al. injected the mouse colon organoids in the form of fragments into the mouse colitis model induced by sodium dextran sulfate. Later, the transplanted cells were observed to adhere to the injured intestinal area. Histological examination found that the graft formed a crypt-like structure in the colon of the recipient mouse. The functional test results show that the graft can maintain the intestinal epithelial barrier function [126]. Retinal tissue derived from mouse pluripotent stem cells (iPSC) has been transplanted by researchers into a mouse model of end-stage retinal degeneration, which showcased improvement of the vision of mice with end-stage retinal degeneration [127]. Additionally, researchers have used magnetic levitation to gather salivary secreting epithelial cells into 3D spheres to form salivary organoids, which can replace damaged salivary glands to secrete saliva under cholinergic stimulation [128]. Studies have also shown that, by rotating a bioreactor, bone marrow mesenchymal stem cells (BMSCs) form 3D spheres, which improves the osteogenic differentiation of (BMSCs) when implanted into a rat skull defect model to promote bone repair [28]. Moreover, researchers have made dental pulp stem cells (DPSCs) into 3D spheres without scaffolds through the action of heat-responsive hydrogel, and these spheres were introduced into human root canals as well as implanted under the skin of immune-deficient mice. After 6 weeks, a vascularized pulp-like tissue formed in the root canal [129].
These studies demonstrate that stem cells are induced to differentiate into specific tissues and organs, which can replace or repair damaged organs, and thus have broad prospects in the field of regenerative medicine.
The widespread application of organoid technology in the research community is still in its infancy, but as a tool, organoid technology has great potential, including developmental biology, disease pathology, cell biology, regeneration mechanisms, precision medicine, drug toxicity, and drug efficacy test. Organoid technology has unique and powerful characteristics that can completely change the traditional in vitro research tools used to simulate human development and diseases. However, the current organoid technology has a fundamental limitation, that is, it cannot simulate the mature structure of the organ and lacks the microenvironment within the tissue. In addition, there is a shortage of critical interactions between various cells in human tissues. These limitations have been regarded as the main problem for accurately simulating various refractory diseases including cancer.
3.3. Assembloids
The complexity of human organs has been difficult to study due to the dynamic interaction between numerous cell types and specific spaces. Organoids can be manipulated as a tool for studying development or disease; however, they reflect particular cell characters of the whole organ with inhomogeneity. In order to reflect the complicacy human tissues, the assembling of organoids is constantly increasing. Assembloids are organoids with spatial tissue structure produced by a variety of cell types [130]. This new type of microorgans can surpass organoids and is closer to real human tissue in structure and function [131].
Kim et al. constructed assembloids that simulate tissue regeneration and cancer for the first time [90]. They created multilayer bladder assembloids combining bladder stem cells with stromal, these assembloids were comprised of three compartments well organized to form bladder-like architectures. It contained multilayered urothelium and thick connective stroma, surrounded by a muscle layer. These researchers found that the cell composition and gene expression of these assembloids at the single-cell level are exactly the same as those of mature adult organs, and they mimic the in vivo regeneration response of normal tissues to injury. They also developed patient-specific tumor assembloids, which perfectly mimic the pathological characteristics of tumors in the body.
In a new study, researchers from the Stanford University School of Medicine have assembled a working model of the human neural circuit responsible for autonomous movement for the first time in the scientific community [132]. They used human pluripotent stem cells to generate the three components of the neural circuit, including human cortical spheroids, human spinal spheroid, and human skeletal muscle spheroids, and let them assemble together in a dish. Glutamate uncaging or optogenetic stimulation of cortical spheroids triggers robust contraction of skeletal muscle spheroids, and assembloids are morphologically and functionally intact for up to ten weeks postfusion (Figure 3). This progress is expected to accelerate research on various neurological diseases.
Figure 3.

The assembly of 3D cultures derived from hiPS cells resembling the cerebral cortex, hindbrain/spinal cord, and skeletal muscle forms neural circuits that can be readily manipulated to model cortical control of muscle contraction in vitro. Reprinted with permission from [132], Copyright 2020 Elsevier.
Assembloids are powerful tools to unveil inaccessible aspects of neurobiology. To study how the human cortex-striatum pathway and its dysfunction can lead to neuropsychiatric diseases, Miura's team assembled the striatal organoids and cortical organoids to form cortical striatal assembloids [133]. The striatum is a brain structure that is the center of pleasant feelings and motivational behaviours. The assembloids may prove useful for studying the causes of schizophrenia, depression, and addiction. Martins et al. used spinal cord neurons and skeletal muscle cells derived from human pluripotent stem cells [134]. These cells self-organized to generate human neuromuscular assembloids. They successfully used neuromuscular assembloids to summarize the key aspects of the pathology of myasthenia gravis. This highlights the great potential of neuromuscular assembloids in simulating neuromuscular diseases in the future.
With the joint efforts of researchers around the world, it is believed that more and more assembloids will appear to provide more realistic models for the study of human tissues and organs, as well as pathological processes.
4. Conclusions
In recent years, 3D cell culture has emerged as a prominent culture technique. In comparison to 2D cell culture, the advantages are that it provides a 3D microenvironment in which cells complete proliferation, differentiation, movement, apoptosis, etc. 3D cell culture more accurately simulates the cell state in the human microenvironment to a large extent, so it has great developability. As such, it has potential applications in tissue engineering, regenerative medicine, drug development, toxicity testing, and organoid and assembloid formation. However, 3D culture technology is still in its infancy; its cost is still high, and because the culture conditions are not yet in the most optimal state, there is still a gap between the culture real situations in the body. Because the viability and differentiation of cells are limited, the present target of research is how to continue to improve the technology to make the 3D culture system closer to the actual environment of the human body, how to achieve an efficient and automated culture system while reducing the cost, how to better utilize the advantages of various materials in the design as well as the use of composite materials, etc. We believe that with the development and progress of tissue engineering technology, these problems, such as the interdisciplinary development of life sciences, engineering and materials science, and the unremitting efforts of scientific researchers, will be increasingly discussed in depth and, thus, will gradually be resolved.
Acknowledgments
The authors acknowledge the financial support from the projects of the Peking University Clinical Medicine Plus X–Young Scholars Project No. PKU2021LCXQ003 (NJ), the National Natural Science Foundations of China No. 81970901 (NJ), the Beijing Nova Program No. Z201100006820080 (NJ), and the Health Commission of Shanxi Province (2017117).
Contributor Information
Nan Jiang, Email: nanjiang@bjmu.edu.cn.
Xuejun Ge, Email: gxj19722003@163.com.
Data Availability
No data were used, available upon request, or included within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Authors' Contributions
X.W. conceived and wrote this review; J.S. created the table; J.W. cured the bibliographical references; N.J. and X.G. revised critically the review.
References
- 1.Duval K., Grover H., Han L. H., et al. Modeling physiological events in 2D vs. 3D cell culture. Physiology (Bethesda, Md.) . 2017;32(4):266–277. doi: 10.1152/physiol.00036.2016. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 2.Seo J., Lee J. S., Lee K., et al. Switchable water-adhesive, superhydrophobic palladium-layered silicon nanowires potentiate the angiogenic efficacy of human stem cell spheroids. Advanced materials (Deerfield Beach, Fla.) . 2014;26(41):7043–7050. doi: 10.1002/adma.201402273. [DOI] [PubMed] [Google Scholar]
- 3.Linand R. Z., Chang H. Y. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnology Journal . 2008;3(9-10):1172–1184. doi: 10.1002/biot.200700228. [DOI] [PubMed] [Google Scholar]
- 4.Chaicharoenaudomrung N., Kunhorm P., Noisa P. Three-dimensional cell culture systems as anin vitroplatform for cancer and stem cell modeling. Journal of Stem Cells . 2019;11(12):1065–1083. doi: 10.4252/wjsc.v11.i12.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim J., Kang J. W., Park J. H., et al. Biological characterization of long-term cultured human mesenchymal stem cells. Archives of Pharmacal Research . 2009;32(1):117–126. doi: 10.1007/s12272-009-1125-1. [DOI] [PubMed] [Google Scholar]
- 6.Zhao Y., Xiao E., Lv W., et al. A chemically defined serum-free culture system for spontaneous human mesenchymal stem cell spheroid formation. Stem Cells International . 2020;2020 doi: 10.1155/2020/1031985.1031985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lutolfand M. P., Hubbell J. A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nature Biotechnology . 2005;23(1):47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 8.Su G., Zhao Y., Wei J., et al. The effect of forced growth of cells into 3D spheres using low attachment surfaces on the acquisition of stemness properties. Biomaterials . 2013;34(13):3215–3222. doi: 10.1016/j.biomaterials.2013.01.044. [DOI] [PubMed] [Google Scholar]
- 9.Ylostalo J. H. 3D stem cell culture. Cell . 2020;9(10):p. 2178. doi: 10.3390/cells9102178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryu N. E., Lee S. H., Park H. Spheroid culture system methods and applications for mesenchymal stem cells. Cell . 2019;8(12):p. 1620. doi: 10.3390/cells8121620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koledova Z. 3D cell culture: an introduction. Methods in Molecular Biology . 2017;1612:1–11. doi: 10.1007/978-1-4939-7021-6_1. [DOI] [PubMed] [Google Scholar]
- 12.Yamada K. M., Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell . 2007;130(4):601–610. doi: 10.1016/j.cell.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Knight E., Przyborski S. Advances in 3D cell culture technologies enabling tissue-like structures to be created in vitro. Journal of Anatomy . 2015;227(6):746–756. doi: 10.1111/joa.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lou J., Stowers R., Nam S., Xia Y., Chaudhuri O. Stress relaxing hyaluronic acid-collagen hydrogels promote cell spreading, fiber remodeling, and focal adhesion formation in 3D cell culture. Biomaterials . 2018;154:213–222. doi: 10.1016/j.biomaterials.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Costa E. C., Gaspar V. M., Coutinho P., Correia I. J. Optimization of liquid overlay technique to formulate heterogenic 3D co-cultures models. Biotechnology and Bioengineering . 2014;111(8):1672–1685. doi: 10.1002/bit.25210. [DOI] [PubMed] [Google Scholar]
- 16.Hickman J. A., Graeser R., de Hoogt R., et al. Three-dimensional models of cancer for pharmacology and cancer cell biology: capturing tumor complexity in vitro/ex vivo. Biotechnology Journal . 2014;9(9):1115–1128. doi: 10.1002/biot.201300492. [DOI] [PubMed] [Google Scholar]
- 17.Carvalho M. P., Costa E. C., Miguel S. P., Correia I. J. Tumor spheroid assembly on hyaluronic acid-based structures: a review. Carbohydrate Polymers . 2016;150:139–148. doi: 10.1016/j.carbpol.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Cesarz Z., Tamama K. Spheroid culture of mesenchymal stem cells. Stem Cells International . 2016;2016 doi: 10.1155/2016/9176357.9176357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Achilli T. M., Meyer J., Morgan J. R. Advances in the formation, use and understanding of multi-cellular spheroids. Expert Opinion on Biological Therapy . 2012;12(10):1347–1360. doi: 10.1517/14712598.2012.707181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.do Amaral J. B., Rezende-Teixeira P., Freitas V. M., Machado-Santelli G. M. MCF-7 cells as a three-dimensional model for the study of human breast Cancer. Tissue Engineering. Part C, Methods . 2011;17(11):1097–1107. doi: 10.1089/ten.tec.2011.0260. [DOI] [PubMed] [Google Scholar]
- 21.Chen H., Fu H., Wu X., et al. Regeneration of pulpo-dentinal-like complex by a group of unique multipotent CD24a(+) stem cells. Science Advances . 2020;6(15):p. 1514. doi: 10.1126/sciadv.aay1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jørgensen A., Young J., Nielsen J. E., et al. Hanging drop cultures of human testis and testis cancer samples: a model used to investigate activin treatment effects in a preserved niche. British Journal of Cancer . 2014;110(10):2604–2614. doi: 10.1038/bjc.2014.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foty R. A simple hanging drop cell culture protocol for generation of 3D spheroids. Journal of visualized experiments: JoVE . 2011;51(51) doi: 10.3791/2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lv D., Hu Z., Lu L., Lu H., Xu X. Three-dimensional cell culture: a powerful tool in tumor research and drug discovery. Oncology Letters . 2017;14(6):6999–7010. doi: 10.3892/ol.2017.7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breslinand S., O'driscoll L. Three-dimensional cell culture: the missing link in drug discovery. Drug Discovery Today . 2013;18(5-6):240–249. doi: 10.1016/j.drudis.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Tung Y. C., Hsiao A. Y., Allen S. G., Torisawa Y. S., Ho M., Takayama S. High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. The Analyst . 2011;136(3):473–478. doi: 10.1039/C0AN00609B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang S. W., Tzeng S. C., Chen J. K., Sun J. S., Lin F. H. A dynamic hanging-drop system for mesenchymal stem cell culture. International Journal of Molecular Sciences . 2020;21(12):p. 4298. doi: 10.3390/ijms21124298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suenaga H., Furukawa K. S., Suzuki Y., Takato T., Ushida T. Bone regeneration in calvarial defects in a rat model by implantation of human bone marrow-derived mesenchymal stromal cell spheroids. Materials in medicine . 2015;26(11):p. 254. doi: 10.1007/s10856-015-5591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Desai P. K., Tseng H., Souza G. R. Assembly of hepatocyte spheroids using magnetic 3D cell culture for CYP450 inhibition/induction. International Journal of Molecular Sciences . 2017;18(5):p. 1085. doi: 10.3390/ijms18051085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adine C., Ng K. K., Rungarunlert S., Souza G. R., Ferreira J. N. Engineering innervated secretory epithelial organoids by magnetic three- dimensional bioprinting for stimulating epithelial growth in salivary glands. Biomaterials . 2018;180:52–66. doi: 10.1016/j.biomaterials.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 31.Türker E., Demirçak N., Arslan-Yildiz A. Scaffold-free three-dimensional cell culturing using magnetic levitation. Biomaterials Science . 2018;6(7):1745–1753. doi: 10.1039/C8BM00122G. [DOI] [PubMed] [Google Scholar]
- 32.Kim J. A., Choi J. H., Kim M., et al. High-throughput generation of spheroids using magnetic nanoparticles for three-dimensional cell culture. Biomaterials . 2013;34(34):8555–8563. doi: 10.1016/j.biomaterials.2013.07.056. [DOI] [PubMed] [Google Scholar]
- 33.Lewis N. S., Lewis E. E., Mullin M., Wheadon H., Dalby M. J., Berry C. C. Magnetically levitated mesenchymal stem cell spheroids cultured with a collagen gel maintain phenotype and quiescence. Journal of tissue engineering . 2017;8 doi: 10.1177/2041731417704428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yaman S., Anil-Inevi M., Ozcivici E., Tekin H. C. Magnetic force-based microfluidic techniques for cellular and tissue bioengineering. Frontiers in Bioengineering and Biotechnology . 2018;6:p. 192. doi: 10.3389/fbioe.2018.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen G., Gulbranson D. R., Hou Z., et al. Chemically defined conditions for human iPSC derivation and culture. Nature Methods . 2011;8(5):424–429. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang Q., Zou Y., Arno M. C., et al. Hydrogel scaffolds for differentiation of adipose-derived stem cells. Chemical Society Reviews . 2017;46(20):6255–6275. doi: 10.1039/C6CS00052E. [DOI] [PubMed] [Google Scholar]
- 37.Ivanovska J., Zehnder T., Lennert P., et al. Biofabrication of 3D alginate-based hydrogel for cancer research: comparison of cell spreading, viability, and adhesion characteristics of colorectal HCT116 tumor Cells. Tissue Engineering. Part C, Methods . 2016;22(7):708–715. doi: 10.1089/ten.tec.2015.0452. [DOI] [PubMed] [Google Scholar]
- 38.Liu Z., Tang M., Zhao J., Chai R., Kang J. Looking into the future: toward advanced 3D biomaterials for stem-cell-based regenerative medicine. Advanced materials (Deerfield Beach, Fla.) . 2018;30(17, article e1705388) doi: 10.1002/adma.201705388. [DOI] [PubMed] [Google Scholar]
- 39.Nam S., Stowers R., Lou J., Xia Y., Chaudhuri O. Varying PEG density to control stress relaxation in alginate-PEG hydrogels for 3D cell culture studies. Biomaterials . 2019;200:15–24. doi: 10.1016/j.biomaterials.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaudhuri O. Viscoelastic hydrogels for 3D cell culture. Biomaterials Science . 2017;5(8):1480–1490. doi: 10.1039/C7BM00261K. [DOI] [PubMed] [Google Scholar]
- 41.Lu L., Yuan S., Wang J., et al. The formation mechanism of hydrogels. Current Stem Cell Research & Therapy . 2018;13(7):490–496. doi: 10.2174/1574888X12666170612102706. [DOI] [PubMed] [Google Scholar]
- 42.Afewerki S., Sheikhi A., Kannan S., Ahadian S., Khademhosseini A. Gelatin-polysaccharide composite scaffolds for 3D cell culture and tissue engineering: towards natural therapeutics. Bioengineering & translational medicine . 2019;4(1):96–115. doi: 10.1002/btm2.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu X., Gurski L. A., Zhang C., Harrington D. A., Farach-Carson M. C., Jia X. Recreating the tumor microenvironment in a bilayer, hyaluronic acid hydrogel construct for the growth of prostate cancer spheroids. Biomaterials . 2012;33(35):9049–9060. doi: 10.1016/j.biomaterials.2012.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elenjord R., Allen J. B., Johansen H. T., et al. Collagen I regulates matrix metalloproteinase-2 activation in osteosarcoma cells independent of S100A4. The FEBS Journal . 2009;276(18):5275–5286. doi: 10.1111/j.1742-4658.2009.07223.x. [DOI] [PubMed] [Google Scholar]
- 45.Noori A., Ashrafi S. J., Vaez-Ghaemi R., Hatamian-Zaremi A., Webster T. J. A review of fibrin and fibrin composites for bone tissue engineering. International Journal of Nanomedicine . 2017;Volume 12:4937–49611. doi: 10.2147/IJN.S124671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mao Z., Bi X., Ye F., et al. The relationship between crosslinking structure and silk fibroin scaffold performance for soft tissue engineering. International Journal of Biological Macromolecules . 2021;182:1268–1277. doi: 10.1016/j.ijbiomac.2021.05.058. [DOI] [PubMed] [Google Scholar]
- 47.Akeda K., Nishimura A., Satonaka H., et al. Three-dimensional alginate spheroid culture system of murine osteosarcoma. Oncology Reports . 2009;22(5):997–1003. doi: 10.3892/or_00000527. [DOI] [PubMed] [Google Scholar]
- 48.Suriand S., Schmidt C. E. Ccell-laden hydrogel constructs of hyaluronic acid, collagen, and laminin for neural tissue engineering. Tissue Engineering. Part A . 2010;16(5):1703–1716. doi: 10.1089/ten.tea.2009.0381. [DOI] [PubMed] [Google Scholar]
- 49.Tytgat L., van Damme L., van Hoorick J., et al. Additive manufacturing of photo-crosslinked gelatin scaffolds for adipose tissue engineering. Acta Biomaterialia . 2019;94:340–350. doi: 10.1016/j.actbio.2019.05.062. [DOI] [PubMed] [Google Scholar]
- 50.Saeedi Garakani S., Khanmohammadi M., Atoufi Z., et al. Fabrication of chitosan/agarose scaffolds containing extracellular matrix for tissue engineering applications. International Journal of Biological Macromolecules . 2020;143:533–545. doi: 10.1016/j.ijbiomac.2019.12.040. [DOI] [PubMed] [Google Scholar]
- 51.Carletti E., Motta A., Migliaresi C. Scaffolds for tissue engineering and 3D cell culture. Methods in Molecular Biology . 2011;695:17–39. doi: 10.1007/978-1-60761-984-0_2. [DOI] [PubMed] [Google Scholar]
- 52.Sahai N., Gogoi M., Tewari R. P. 3D printed chitosan composite scaffold for chondrocytes differentiation . Current medical imaging; 2021. [DOI] [PubMed] [Google Scholar]
- 53.Toivonen S., Malinen M. M., Küblbeck J., et al. Regulation of human pluripotent stem cell-derived hepatic cell phenotype by three-dimensional hydrogel models. Tissue Engineering. Part A . 2016;22(13-14):971–984. doi: 10.1089/ten.tea.2016.0127. [DOI] [PubMed] [Google Scholar]
- 54.Polonio-Alcalá E., Rabionet M., Guerra A. J., Yeste M., Ciurana J., Puig T. Screening of additive manufactured scaffolds designs for triple negative breast cancer 3D cell culture and stem-like expansion. International Journal of Molecular Sciences . 2018;19(10):p. 3148. doi: 10.3390/ijms19103148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jabbari E., Sarvestani S. K., Daneshian L., Moeinzadeh S. Optimum 3D matrix stiffness for maintenance of cancer stem cells is dependent on tissue origin of cancer cells. Plo S one . 2015;10(7, article e0132377) doi: 10.1371/journal.pone.0132377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fu Y., Xu K., Zheng X., Giacomin A. J., Mix A. W., Kao W. J. 3D cell entrapment in crosslinked thiolated gelatin-poly(ethylene glycol) diacrylate hydrogels. Biomaterials . 2012;33(1):48–58. doi: 10.1016/j.biomaterials.2011.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mendez U., Zhou H., Shikanov A. Synthetic PEG hydrogel for engineering the environment of ovarian follicles. Methods in Molecular Biology . 2018;1758:115–128. doi: 10.1007/978-1-4939-7741-3_9. [DOI] [PubMed] [Google Scholar]
- 58.Jung J. W., Lee H., Hong J. M., et al. A new method of fabricating a blend scaffold using an indirect three-dimensional printing technique. Biofabrication . 2015;7(4, article 045003) doi: 10.1088/1758-5090/7/4/045003. [DOI] [PubMed] [Google Scholar]
- 59.Zhao X., Sun X., Yildirimer L., et al. Cell infiltrative hydrogel fibrous scaffolds for accelerated wound healing. Acta Biomaterialia . 2017;49:66–77. doi: 10.1016/j.actbio.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Song K., Ji L., Zhang J., et al. Fabrication and cell responsive behavior of macroporous PLLA/gelatin composite scaffold with hierarchical micro-nano pore structure. Nanomaterials (Basel, Switzerland) . 2015;5(2):415–424. doi: 10.3390/nano5020415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kemmochi A., Tamura T., Shimizu Y., et al. A novel hydrogel sheet prevents postoperative pancreatic fistula in a rat model. Journal of Hepato-Biliary-Pancreatic Sciences . 2021;28(2):192–201. doi: 10.1002/jhbp.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li G., Zhang T., Li M., et al. Electrospun fibers for dental and craniofacial applications. Current Stem Cell Research & Therapy . 2014;9(3):187–195. doi: 10.2174/1574888X09666140213151717. [DOI] [PubMed] [Google Scholar]
- 63.Zhu Y., Zhang Q., Shi X., Han D. Hierarchical hydrogel composite interfaces with robust mechanical properties for biomedical applications. Advanced materials (Deerfield Beach, Fla.) . 2019;31(45, article e1804950) doi: 10.1002/adma.201804950. [DOI] [PubMed] [Google Scholar]
- 64.Kimand W., Kim G. Collagen/bioceramic-based composite bioink to fabricate a porous 3D hASCs-laden structure for bone tissue regeneration. Biofabrication . 2019;12(1, article 015007) doi: 10.1088/1758-5090/ab436d. [DOI] [PubMed] [Google Scholar]
- 65.Klotz B. J., Gawlitta D., Rosenberg A., Malda J., Melchels F. P. W. Gelatin-methacryloyl hydrogels: towards biofabrication-based tissue repair. Trends in Biotechnology . 2016;34(5):394–407. doi: 10.1016/j.tibtech.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hughes J. P., Rees S., Kalindjian S. B., Philpott K. L. Principles of early drug discovery. British Journal of Pharmacology . 2011;162(6):1239–1249. doi: 10.1111/j.1476-5381.2010.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Westhouse R. A. Safety assessment considerations and strategies for targeted small molecule cancer therapeutics in drug discovery. Toxicologic Pathology . 2010;38(1):165–168. doi: 10.1177/0192623309354341. [DOI] [PubMed] [Google Scholar]
- 68.He B., Chen G., Zeng Y. Three-dimensional cell culture models for investigating human viruses. Virologica Sinica . 2016;31(5):363–379. doi: 10.1007/s12250-016-3889-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ravi M., Paramesh V., Kaviya S. R., Anuradha E., Solomon F. D. P. 3D cell culture systems: advantages and applications. Journal of Cellular Physiology . 2015;230(1):16–26. doi: 10.1002/jcp.24683. [DOI] [PubMed] [Google Scholar]
- 70.Amelian A., Wasilewska K., Megias D., Winnicka K. Application of standard cell cultures and 3D _in vitro_ tissue models as an effective tool in drug design and development. Pharmacological reports: PR . 2017;69(5):861–870. doi: 10.1016/j.pharep.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 71.Shehzad A., Ravinayagam V., AlRumaih H., et al. Application of three-dimensional (3D) tumor cell culture systems and mechanism of drug resistance. Current Pharmaceutical Design . 2019;25(34):3599–3607. doi: 10.2174/1381612825666191014163923. [DOI] [PubMed] [Google Scholar]
- 72.Park S. B., Koh B., Jung W. H., et al. Development of a three‐dimensionalin vitroco‐culture model to increase drug selectivity for humans. Diabetes, Obesity & Metabolism . 2020;22(8):1302–1315. doi: 10.1111/dom.14033. [DOI] [PubMed] [Google Scholar]
- 73.Schutgens F., Clevers H. Human organoids: tools for understanding biology and treating diseases. Annual Review of Pathology . 2020;15(1):211–234. doi: 10.1146/annurev-pathmechdis-012419-032611. [DOI] [PubMed] [Google Scholar]
- 74.Clevers H. Modeling development and disease with organoids. Cell . 2016;165(7):1586–1597. doi: 10.1016/j.cell.2016.05.082. [DOI] [PubMed] [Google Scholar]
- 75.He J., Zhang X., Xia X., et al. Organoid technology for tissue engineering. Molecular and Cellular Biology . 2020;12(8):569–579. doi: 10.1093/jmcb/mjaa012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Koo B., Choi B., Park H., Yoon K. J. Past, present, and future of brain organoid technology. Molecules and Cells . 2019;42(9):617–627. doi: 10.14348/molcells.2019.0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fatehullah A., Tan S. H., Barker N. Organoids as an _in vitro_ model of human development and disease. Nature Cell Biology . 2016;18(3):246–254. doi: 10.1038/ncb3312. [DOI] [PubMed] [Google Scholar]
- 78.Li M., Belmonte J. C. I. Organoids-preclinical models of human disease. The New England Journal of Medicine . 2019;380(6):569–579. doi: 10.1056/NEJMra1806175. [DOI] [PubMed] [Google Scholar]
- 79.Rajendra C., Wald T., Barber K., Spence J. R., Fattahi F., Klein O. D. Generation of knockout gene-edited human intestinal organoids. Methods in Molecular Biology . 2020;2171:215–230. doi: 10.1007/978-1-0716-0747-3_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xie H., Zhang W., Zhang M., et al. Chromatin accessibility analysis reveals regulatory dynamics of developing human retina and hiPSC-derived retinal organoids. Science Advances . 2020;6(6):p. eaay5247. doi: 10.1126/sciadv.aay5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Trujillo C. A., Gao R., Negraes P. D., et al. Complex oscillatory waves emerging from cortical organoids model early human brain network development. Cell Stem Cell . 2019;25(4):558–569.e7. doi: 10.1016/j.stem.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Spence J. R., Mayhew C. N., Rankin S. A., et al. Directed differentiation of human pluripotent stem cells into intestinal tissue _in vitro_. Nature . 2011;470(7332):105–109. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Takasato M., Er P. X., Chiu H. S., et al. Erratum: Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature . 2016;536(7615):238–238. doi: 10.1038/nature17982. [DOI] [PubMed] [Google Scholar]
- 84.Bartfeld S., Bayram T., van de Wetering M., et al. In Vitro Expansion of Human Gastric Epithelial Stem Cells and Their Responses to Bacterial Infection. Gastroenterology . 2015;148(1):126–136.e6. doi: 10.1053/j.gastro.2014.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang S. Y., Wang X., Tan Z. L., et al. Human ESC-derived expandable hepatic organoids enable therapeutic liver repopulation and pathophysiological modeling of alcoholic liver injury. Cell Research . 2019;29(12):1009–1026. doi: 10.1038/s41422-019-0242-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang D. S., Wang J. Q., Bai L. Y., et al. Long-Term Expansion of Pancreatic Islet Organoids from Resident Procr+ Progenitors. Cell . 2020;180(6):1198–1211.e19. doi: 10.1016/j.cell.2020.02.048. [DOI] [PubMed] [Google Scholar]
- 87.Dye B. R., Dedhia P. H., Miller A. J., et al. A bioengineered niche promotes in vivo engraftment and maturation of pluripotent stem cell derived human lung organoids. eLife . 2016;5 doi: 10.7554/eLife.19732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wimmer R. A., Leopoldi A., Aichinger M., et al. Human blood vessel organoids as a model of diabetic vasculopathy. Nature . 2019;565(7740):505–510. doi: 10.1038/s41586-018-0858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Drakhlis L., Biswanath S., Farr C. M., et al. Human heart-forming organoids recapitulate early heart and foregut development. Nature Biotechnology . 2021;39(6):775–775. doi: 10.1038/s41587-021-00960-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim E., Choi S., Kang B., et al. Creation of bladder assembloids mimicking tissue regeneration and cancer. Nature . 2020;588(7839):664–669. doi: 10.1038/s41586-020-3034-x. [DOI] [PubMed] [Google Scholar]
- 91.Rossi G., Manfrin A., Lutolf M. P. Progress and potential in organoid research. Genetics . 2018;19(11):671–687. doi: 10.1038/s41576-018-0051-9. [DOI] [PubMed] [Google Scholar]
- 92.Qian X., Song H., Ming G. L. Brain organoids: advances, applications and challenges. Development (Cambridge, England) . 2019;146(8) doi: 10.1242/dev.166074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wu C., Li M., Meng H., et al. Analysis of status and countermeasures of cancer incidence and mortality in China. Life Sciences . 2019;62(5):640–647. doi: 10.1007/s11427-018-9461-5. [DOI] [PubMed] [Google Scholar]
- 94.Candini O., Grisendi G., Foppiani E. M., et al. A Novel 3D _In Vitro_ Platform for Pre-Clinical Investigations in Drug Testing, Gene Therapy, and Immuno-oncology. Scientific Reports . 2019;9(1):p. 7154. doi: 10.1038/s41598-019-43613-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Koehler K. R., Mikosz A. M., Molosh A. I., Patel D., Hashino E. Generation of inner ear sensory epithelia from pluripotent stem cells in 3D culture. Nature . 2013;500(7461):217–221. doi: 10.1038/nature12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chvetsov A. V., Stewart R. D., Kim M., Meyer J., Rengan R. Volume effects in the TCP for hypoxic and oxygenated tumors. Medical Physics . 2020;47(9):4626–4633. doi: 10.1002/mp.14257. [DOI] [PubMed] [Google Scholar]
- 97.IMAMURA Y., MUKOHARA T., SHIMONO Y., et al. Comparison of 2D- and 3D-culture models as drug-testing platforms in breast cancer. Oncology Reports . 2015;33(4):1837–1843. doi: 10.3892/or.2015.3767. [DOI] [PubMed] [Google Scholar]
- 98.Lovitt C. J., Shelper T. B., Avery V. M. Advanced cell culture techniques for cancer drug discovery. Biology . 2014;3(2):345–367. doi: 10.3390/biology3020345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lancasterand M. A., Knoblich J. A. Organogenesis in a dish: modeling development and disease using organoid technologies. Science (New York, N.Y.) . 2014;345:p. 6194. doi: 10.1126/science.1247125. [DOI] [PubMed] [Google Scholar]
- 100.Zhao H., Jiang E., Shang Z. 3D co-culture of cancer-associated fibroblast with oral cancer organoids. Journal of Dental Research . 2021;100(2):201–208. doi: 10.1177/0022034520956614. [DOI] [PubMed] [Google Scholar]
- 101.Erdoganand B., Webb D. J. Cancer-associated fibroblasts modulate growth factor signaling and extracellular matrix remodeling to regulate tumor metastasis. Biochemical Society Transactions . 2017;45(1):229–236. doi: 10.1042/BST20160387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yuzhalin A. E., Lim S. Y., Kutikhin A. G., Gordon-Weeks A. N. Dynamic matrisome: ECM remodeling factors licensing cancer progression and metastasis. Reviews on cancer . 2018;1870(2):207–228. doi: 10.1016/j.bbcan.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 103.Lancaster M. A., Renner M., Martin C. A., et al. Cerebral organoids model human brain development and microcephaly. Nature . 2013;501(7467):373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Trujilloand C. A., Muotri A. R. Brain organoids and the study of neurodevelopment. Trends in Molecular Medicine . 2018;24(12):982–990. doi: 10.1016/j.molmed.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schlaermann P., Toelle B., Berger H., et al. A novel human gastric primary cell culture system for modelling Helicobacter pylori infection In Vitro. Gut . 2016;65(2):202–213. doi: 10.1136/gutjnl-2014-307949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.DesRochers T. M., Kimmerling E. P., Jandhyala D. M., et al. Effects of Shiga toxin type 2 on a bioengineered three-dimensional model of human renal tissue. Infection and Immunity . 2015;83(1):28–38. doi: 10.1128/IAI.02143-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhao B., Ni C., Gao R., et al. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein & Cell . 2020;11(10):771–775. doi: 10.1007/s13238-020-00718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Barretina J., Caponigro G., Stransky N., et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature . 2012;483(7391):603–607. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jin K., Teng L., Shen Y., He K., Xu Z., Li G. Patient-derived human tumour tissue xenografts in immunodeficient mice: a systematic review. Clinical & Translational Oncology . 2010;12(7):473–480. doi: 10.1007/s12094-010-0540-6. [DOI] [PubMed] [Google Scholar]
- 110.Boj S. F., Hwang C. I., Baker L. A., et al. Organoid models of human and mouse ductal pancreatic cancer. Cell . 2015;160(1-2):324–338. doi: 10.1016/j.cell.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.van de Wetering M., Francies H. E., Francis J. M., et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell . 2015;161(4):933–945. doi: 10.1016/j.cell.2015.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sachs N., Papaspyropoulos A., Zomer-van Ommen D. D., et al. Long-term expanding human airway organoids for disease modeling. The EMBO Journal . 2019;38(4) doi: 10.15252/embj.2018100300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nuciforoand S., Heim M. H. Organoids to model liver disease. JHEP Rep . 2021;3(1):p. 100198. doi: 10.1016/j.jhepr.2020.100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Weeber F., Ooft S. N., Dijkstra K. K., Voest E. E. Tumor organoids as a pre-clinical cancer model for drug discovery. Cell Chemical Biology . 2017;24(9):1092–1100. doi: 10.1016/j.chembiol.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 115.Olgasi C., Cucci A., Follenzi A. iPSC-derived liver organoids: a journey from drug screening, to disease modeling, arriving to regenerative medicine. International Journal of Molecular Sciences . 2020;21(17):p. 6215. doi: 10.3390/ijms21176215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yousef Yengej F. A., Jansen J., Rookmaaker M. B., Verhaar M. C., Clevers H. Kidney organoids and tubuloids. Cell . 2020;9(6):p. 1326. doi: 10.3390/cells9061326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Driehuis E., Kretzschmar K., Clevers H. Establishment of patient-derived cancer organoids for drug-screening applications. Nature Protocols . 2020;15(10):3380–3409. doi: 10.1038/s41596-020-0379-4. [DOI] [PubMed] [Google Scholar]
- 118.Zakrzewski W., Dobrzyński M., Szymonowicz M., Rybak Z. Stem cells: past, present, and future. Stem Cell Research & Therapy . 2019;10(1):p. 68. doi: 10.1186/s13287-019-1165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jin J. Stem cell treatments. JAMA . 2017;317(3):p. 330. doi: 10.1001/jama.2016.17822. [DOI] [PubMed] [Google Scholar]
- 120.Kawaguchi N., Hatta K., Nakanishi T. 3D-culture system for heart regeneration and cardiac medicine. Bio Med research international . 2013;2013, article 895967 doi: 10.1155/2013/895967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Eiraku M., Takata N., Ishibashi H., et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature . 2011;472(7341):51–56. doi: 10.1038/nature09941. [DOI] [PubMed] [Google Scholar]
- 122.Serra D., Mayr U., Boni A., et al. Self-organization and symmetry breaking in intestinal organoid development. Nature . 2019;569(7754):66–72. doi: 10.1038/s41586-019-1146-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Takebe T., Sekine K., Enomura M., et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature . 2013;499(7459):481–484. doi: 10.1038/nature12271. [DOI] [PubMed] [Google Scholar]
- 124.Takasato M., Er P. X., Becroft M., et al. Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nature Cell Biology . 2014;16(1):118–126. doi: 10.1038/ncb2894. [DOI] [PubMed] [Google Scholar]
- 125.Huch M., Bonfanti P., Boj S. F., et al. Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr 5/R-spondin axis. The EMBO Journal . 2013;32(20):2708–2721. doi: 10.1038/emboj.2013.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yui S., Nakamura T., Sato T., et al. Functional engraftment of colon epithelium expanded _in vitro_ from a single adult Lgr5+ stem cell. Nature Medicine . 2012;18(4):618–623. doi: 10.1038/nm.2695. [DOI] [PubMed] [Google Scholar]
- 127.Mandai M., Fujii M., Hashiguchi T., et al. iPSC-Derived Retina Transplants Improve Vision in _rd1_ End-Stage Retinal- Degeneration Mice. Stem Cell Reports . 2017;8(1):69–83. doi: 10.1016/j.stemcr.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ferreira J. N., Hasan R., Urkasemsin G., et al. A magnetic three-dimensional levitated primary cell culture system for the development of secretory salivary gland-like organoids. Journal of Tissue Engineering and Regenerative Medicine . 2019;13(3):495–508. doi: 10.1002/term.2809. [DOI] [PubMed] [Google Scholar]
- 129.Itoh Y., Sasaki J. I., Hashimoto M., Katata C., Hayashi M., Imazato S. Pulp regeneration by 3-dimensional dental pulp stem cell constructs. Journal of Dental Research . 2018;97(10):1137–1143. doi: 10.1177/0022034518772260. [DOI] [PubMed] [Google Scholar]
- 130.Vogt N. Assembloids. Nature Methods . 2021;18(1):p. 27. doi: 10.1038/s41592-020-01026-x. [DOI] [PubMed] [Google Scholar]
- 131.Martonand R. M., Pasca S. P. Organoid and assembloid technologies for investigating cellular crosstalk in human brain development and disease. Trends in Cell Biology . 2020;30(2):133–143. doi: 10.1016/j.tcb.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 132.Andersen J., Revah O., Miura Y., et al. Generation of functional human 3D cortico-motor assembloids. Cell . 2020;183(7):1913–1929.e26. doi: 10.1016/j.cell.2020.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Miura Y., Li M. Y., Birey F., et al. Generation of human striatal organoids and cortico-striatal assembloids from human pluripotent stem cells. Nature Biotechnology . 2020;38(12):1421–1430. doi: 10.1038/s41587-020-00763-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Faustino Martins J. M., Fischer C., Urzi A., et al. Self-organizing 3D human trunk neuromuscular organoids. Cell Stem Cell . 2020;26(2):172–186.e6. doi: 10.1016/j.stem.2019.12.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were used, available upon request, or included within the article.


