Abstract
Background
Plant-derived medicines are widespread and continue to increase in traditional and modern medicine, especially in developing countries. Physalis peruviana L. is among the most used plants in conventional medication worldwide. This review aimed to highlight the ethnotherapeutic uses and phytochemical status of identified compounds in P. peruviana.
Methods
Data were collected from Google Scholar, PubMed/Medline, SciFinder, Science Direct, Scopus, the Wiley Online Library, Web of Science, and any other helpful search engine using Physalis peruviana as the primary keyword.
Results
Some countries, worldwide, use P. peruviana in their traditional medicine system to manage diverse ailments, mainly diseases and gastrointestinal tract disorders (25.33%). Leaf was the mostly used part (49.28%), prepared by decoction (31.58%) and overall administrated orally (53.57%) as the main route of admission. Around 502 phytoconstituents were identified in different plant parts, especially fruit (38.19%) ethanol/ethyl acetate extract. In most cases (36.17%), the solvent of the extract was not specified. Several phytochemical classes were found in the plant, especially terpenes (26.09%) and phenolic compounds (14.94%). Esters were also abundant (11.55%). In the terpenes category, carotenoids were the most abundant (11.15% followed by monoterpenes (8.76%) and diterpenes (3.18%). However, flavonoids (5.17%) followed by cinnamic acid derivatives (3.99%), monophenolic compounds (1.79%), and phenolic acids (1.33 M) are the most reported phenolic compounds. Hexadecanoic acid (palmitic acid) was the most cited (five times).
Conclusion
P. peruviana plays an essential role in managing diseases in some countries and is rich in chemical compounds, which need to be isolated and investigated pharmacologically before clinical trials.
1. Introduction
According to the World Health Organization (WHO), about 80% of the population in developing countries uses herbal medicine to meet their primary healthcare requirements [1]. Humans have used natural products since prehistoric times, which include animals, marine organisms, microorganisms, and plants, in medicines to prevent, diagnose, and treat diseases [2]. Plants still contribute primarily to health care, so many specific herbal extracts have been demonstrated to be productive for particular conditions [3]. More than 50,000 plants would possess therapeutic virtues globally. In Africa and Asia, it is estimated that more than 80 percent of the population uses traditional medicine for primary health care. This form of therapy remains prevalent in all world regions, and its use is rapidly spreading in developed countries [4].
Physalis peruviana (Solanaceae) is a native plant from the Andean region and a semiupright herbaceous shrub or perennial, producing a group of branched stems native to the Andean region. P. peruviana is adapted to a wide range of altitudes, soils, and climatic conditions. It is also the most widely distributed species from the Physalis. Physalis genus contains several species with a long history of ethnomedical use to treat diverse diseases, especially asthma, cancer, dermatitis, hepatitis, bacterial infections, kidney and liver disorders, and malaria and has immunomodulatory antipyretic properties [5]. It contains different types of compounds, including physalins and alkaloids, flavonoids, carotenoids, vitamins, and polysaccharides [6, 7]. The health benefits of the plant are related to the content of phytochemicals.
This report summarizes ethnomedicinal use and phytoconstituents identified in P. peruviana. Previous reviews have been focused on nutritional values, pharmacological evidence, and phytochemical profiling of isolated compounds from the plant [8].
This review aimed to highlight the ethnotherapeutic use and phytochemical status of identified compounds in P. peruviana L.
2. Literature Review Method
Different search databases, including Google Scholar, PubMed/Medline, Science Direct, Scopus, the Wiley Online Library, Web of Science, and any other helpful search engines using P. peruviana as the primary keyword, were used. Full articles in English or French languages were retrieved without time limit restriction.
3. Results and Discussion
3.1. Ethnopharmacological Data of P. peruviana L
The following Table 1 presents the uses of P. peruviana in traditional medicines in different countries.
Table 1.
Ethnomedicinal uses of P. peruviana L. in different countries.
| Countries | Vernacular names | Part(s) used | Traditional uses | Formulation/method of administration | Voucher number | References |
|---|---|---|---|---|---|---|
| Cameroon | — | Twigs | Cancer or disease relevance to cancer or cancer-like symptoms | — | Yes | [9] |
| Ajijieuh | Leaf and stem | Bile, swelling of legs and ankles for pregnant women | Maceration/oral | Yes | [10] | |
| Ma pe pie | Leaf and stem | Fungal infections | Maceration/oral | Yes | [11] | |
|
| ||||||
| Colombia | Uchuva | Fruit | Ear pain and diabetes | — | No | [12] |
| Uchuva | Fruit | Conjunctivitis and prevention of cataract | Juice/oral | No | [13] | |
|
| ||||||
| Equator | Uvilla | Flower | Disinfectant and healing of wounds | Decoction/bathe | Yes | [14] |
|
| ||||||
| Democratic Republic of the Congo | Mbuma | Leaf | Malaria, intestinal worms, and splenomegaly | Decoction and infusion/oral | Yes | [15] |
| Mbuma, Mbupuru, Umuhire | Aerial part | Diabetes mellitus, colic in children, spleen, malaria, and | ||||
|
| ||||||
| inflammation | Decoction/oral | No | [16] | |||
| Mpuhuhu | Whole plant | Helminthiasis | Maceration/oral | Yes | [17] | |
| India | Donam as | Fruit | Gastric | Mastication/oral | No | [18] |
| Fatki | Leaf and root | Leucorrhea and hydrocele | Decoction/oral | No | [19] | |
| Kitutu | Leaf | Induction of labor and ease childbirth | Decoction/oral | No | [20] | |
| Kopalphoota | Whole plant | Jaundice | Raw/oral | No | [21] | |
| Phakphake | Ripe fruit | Throat sore | Mastication/oral | Yes 0032 | [22] | |
| Pottipalam | Leaf and dried seed | Jaundice and glaucoma | — | No | [23] | |
| Rashbhari | Leaf | Abdominal disorder during pregnancy | Juice/oral | No | [24] | |
| Tsiibobopro | Leaf and fruit | Diarrhea and dysentery | Decoction and raw/oral | No | [25] | |
| Sodukku thakkali | Whole plant | Skin diseases | Extraction/- | No | [26] | |
| — | Leave | Jaundice | Decoction/oral | No | [27] | |
| — | Whole plant | Gout | — | No | [28] | |
| — | Leaf | Jaundice | Paste/- | Yes | [29] | |
|
| ||||||
| Indonesia | Depuk-depuk | Fruit and whole plant | Smallpox | Decoction/oral | No | [30] |
| Pultak-pulta | All parts of the plant | Stomach ache | Decoction/oral | No | [31] | |
|
| ||||||
| Java | Ciplukan | Leaf and fruit | Diabetes mellitus | — | No | [32] |
|
| ||||||
| Kenya | Embunwe, emiilwa (wanga) | Stem, root, fruit, and leaf | Inflammation and abdominal ailmentsΨ | Raw and infusion/poultices and enema | No | [33] |
| Mayengo | Leaf | Malaria | Decoction/steam inhalation | Yes | [34] | |
| Mŭnathi | Leaf | Postpartum pain | Decoction/oral | No | [35] | |
| Mŭnathi | Leaf | Anthelmintic, postpartum pains, and typhoid | — | No | [36] | |
| Mŭnathi | Seed, bulb, fruit, leaf, and root | Diarrhea | — | No | [37] | |
| — | Leaf | Diabetes, malaria, and pneumonia | Decoction/oral | No | [38] | |
| — | Leaf | Typhoid and pneumonia | Decoction/- | No | [39] | |
|
| ||||||
| Nepal | Gangathopa | Root | Jaundice | Maceration/oral | No | [40] |
| Ram bhutka, Jangali mewa | Root | Piles | — | No | [41] | |
| — | Leaf | Sore throat and abdominal pain | Juice/oral | No | [42] | |
|
| ||||||
| New Guinea | Mondon | Leaf | Boils and ulcers | Heating/topical application on to cuts and scratches∗ | No | [43] |
|
| ||||||
| Rwanda | Umuhuhu | Leaf | Facilitates the issuance of the placenta and abortifacient | — | No | [44] |
|
| ||||||
| South Africa | Igquzu | Leaf | Diarrhea and associated ailments. | As food/oral | No | [45] |
| Igquzu | Leaf | Diarrhea | Decoction/oral | Yes | [46] | |
| — | Whole plant and leaf | Cancer | Decoction/oral | DS00095 | [47] | |
| Tanzania | Kitutun kikubwa | Leaf | Malaria | Maceration/oral | Yes | [48] |
| Msupu | Leaf | Skin fungal infections | Juice/topical application∗ | Yes | [49] | |
| Ntuntunu | Fruit | Typhoid fever | Juice/oral | Yes | [50] | |
|
| ||||||
| Uganda | Entuutu | Leaf and fruit | Snakebite | Infusion/oral | No | [51] |
| Entuutu | Fresh leaf | Skin problems in babies | Decoction/bathe∗ | No | [52] | |
| Entuutu | — | Wounds (fresh) | — | No | [53] | |
| Kitutu | Leaf | Induce of labor during childbirth | Juice/oral | No | [54] | |
| Ntuntunu enene | Leaf | Vomiting | Smoked and infusion/bathe | Yes | [55] | |
| Ntuntunu | Leaf | Malaria | Decoction/oral∗ | Yes | [56] | |
| Decoction/oral∗ | ||||||
| Ntuntunu | Leaf | Infections (antibacterial) | Juice/oral | No | [57] | |
| Ntutunu enene | Fruit | Ear and eye infection | Chewing and swallowing/oral | Yes | [58] | |
| Ntutunu enene | Aerial part and leaf | HIV/AIDS | — | Yes | [59] | |
| — | Whole plant | Rash and ringworm | Juice/- | Yes | [60] | |
Veterinary use (Ψ); specific characteristics (∗); not specified (—).
According to this table (Table 1), fourteen countries worldwide use P. peruviana in their traditional medicinal system to treat several diseases. India represented the most cited country with twelve references, followed by Uganda (10), Kenya (7), Cameroon, Democratic Republic of Congo, Nepal, South Africa, and Tanzania, each with three references. Colombia and Indonesia were cited only twice.
Referring to the number of diseases treated by country, India is the most representative country (20.27%), followed by Uganda (16.22%), the Democratic Republic of Congo (12.16%), Cameroon (6.76%), Colombia, Nepal, and South Africa (5.41%). It is known that the plant (mainly le fruit) is produced predominantly in Colombia and South Africa but exported in Netherlands, Germany, Belgium, and Canada [61]. However, its use in traditional medicine is widespread in other countries, including India and Uganda.
The plant is widely known in various local names and used in Ayurvedic medicine for many human and animal purposes. The fruit is available from January to April [62]. In Uganda, the plant grows naturally in abandoned bush fallows, and it is helpful for income. It has been identified as a priority plant for commercialization (used popularly for its berries and associated derivative products such as juice, jam, and wine). It is also used as food and has medicinal applications [63].
Local names, parts used, traditional utilization, preparation, and administration modes were documented.
Figure 1 indicates that the leaves are the most used part (49.28%) followed by fruits (14.49%), whole plant (11.59%), roots (7.5%), stem (4.35%), aerial parts, and seeds (2.90%). However, bulbs, flowers, ripe fruits, and twigs were cited once (1.45%). In some cases, the used parts were not specified (1.45%). Leaves are the most used in the formulation of remedies, as indicated above. The frequent use of leaves is associated with ease of accessibility among the aboveground parts of plants in natural ecosystems [50].
Figure 1.
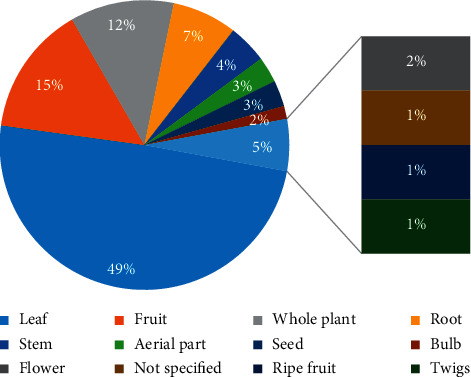
Frequencies of parts used.
Decoction has often been found as the effective formulation of herbal remedies as it is easy to prepare by mixing a drug with boiling water [64]. In this study (Figure 2), the decoction was used in almost 31.58% of all cases. However, other preparation modes have been found including juice (14.04%), maceration (8.77%), infusion (7.02%), extraction, and raw material (3.51%). In 19.30% of cases, the preparation mode was not reported.
Figure 2.
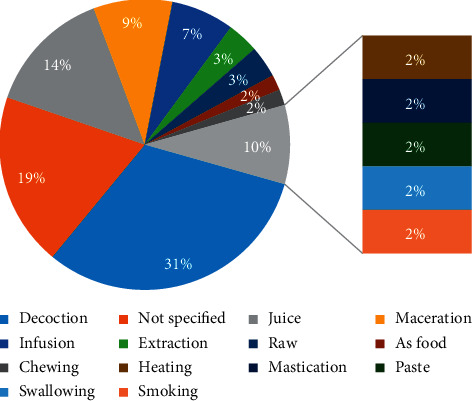
Frequencies of formulations.
P. peruviana is indicated to treat various diseases, mainly in humans. Rarely, it is used in the management of diseases in veterinary medicine. For example, in western Kenya, it is used for livestock tick prevention and control. The results in Figure 3 show that diseases and disorders of the gastrointestinal tract were the most treated by the plant (25.33%), followed by female genital tract and breast (13.33%), skin (9.33%), liver and biliary tract (8.01%), eye and ear (8.01%), immune system (5.33%), endocrine system (5.33%), respiratory system (2.67%), and metabolic disorders (2.67%). Diseases of bones, joints, skeletal muscle, and body fluid-related diseases and disorders represent 1.33%. Another category of diseases, including helminthiasis, inflammations, malaria, snake bite, fungal infections, bacterial infections, and smallpox, represents 17.33%. About 4000 species had ethnomedical data supporting the use of these plants to treat, and most of them were native to tropical countries due to the extraordinary biodiversity in these countries [65].
Figure 3.
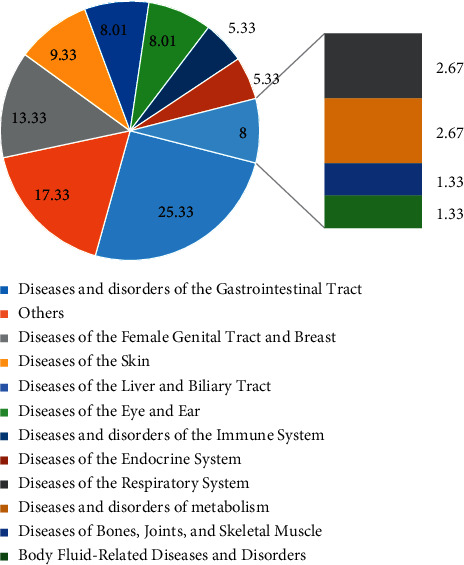
Frequencies of diseases and disorders treated.
Mostly, oral route is the way of drug administration based on different formulations. Because of safety, good patient compliance, ease of ingestion, pain avoidance, and versatility to accommodate various types of drugs, the oral administration route is preferred over the different other administration routes of drug delivery [66]. Nevertheless, the route of the administration is not specified in a few cases (20.41%). Secondarily, bathe, tropical application, scratches, and steam inhalation are reported (Figure 4).
Figure 4.
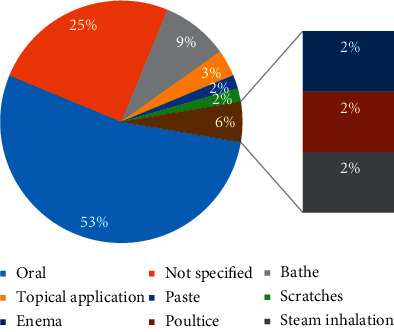
Frequencies of routes of administration.
There are some specific indications in formulations or modes of drug administration. For example, in India, the plant is associated with Impatiens roylei and Stephania hernandifolia to treat jaundice. In the same way, in Uganda, the plant is combined with Solanum esculentum and Solanum melongena to manage skin problems in babies and honey in treating malaria. It is possible that combining several plants can produce a more pronounced pharmacological response than using a single plant due to the synergy of action between different constituents. According to Sofowora et al. [67], the combined effects were much more effective than individual ones. Rarely, duration of treatment and posology were mentioned. However, those two factors depended on the type of diseases treated and the parts used. For example, in Uganda, treating malaria needs seven days by taking two teaspoons three times a day of a decoction or half a glass thrice a day. In Tanzania, an application of leaf juice on the affected area twice a day was indicated to treat skin fungal infections or heating/topical application on to cuts and scratches in New Guinea for boils and ulcers. In Nepal, the treatment of jaundice in children could take from four to ten days.
The voucher number of plant material was not specified in 63.46% against 36.54%. Overall, in research studies that involved plant or animal materials, providing voucher specimens is necessary for several reasons. The main reason is to keep a permanent record documenting the plant used in a specific study to trace the true identity and source of the plant material [68]. In most cases, the plant species look alike (morphologically and chemically), and it is quite possible to have a confusing error when harvesting. To be reassured of the real identity of the plant, it is crucial to have it authenticated with an expert, for example, a botanist. In the event of a future contestation, the voucher number recorded in the herbarium will always be essential to confirm the integrity of its identity. It is also vital for reproducibility, which is very critical in research.
3.2. Phytoconstituents Identified in Different Parts of P. peruviana L
Table 2 summarizes the chemical compounds identified and characterized from other parts and extracts of P. peruviana. Therefore, various classes of phytoconstituents have been found, including terpenes (monoterpenes, sesquiterpenes, diterpenes, triterpenes, and carotenoids), phenolic compounds (phenolic acids, phenolic esters, phenolic aldehydes, chalcones, coumarins, cinnamic acid derivatives, flavonoids, and glucosides), alcohols, aldehydes, ketones, carboxylic acids, lactones, steroids and withanolides, alkaloids, sucrose esters, glucosides, siloxanes, vitamins, phytoprostanes, phytol derivatives, enols, heterocycles, alkanes, alkenes, benzimidazoles, and diverse functional groups.
Table 2.
Chemical compounds identified from different parts and extracts of P. peruviana.
| Organs | Phytoconstituents | Source | References |
|---|---|---|---|
| Aerial parts | 3α-Tigloylnxytropane | Ethanol | [69] |
| 3β-Acetoxytropane | Ethanol | [69] | |
| Antheraxanthin | Hexane/acetone/ethanol | [70] | |
| Cuscohygrine | Ethanol | [69] | |
| Hygrine | Ethanol | [69] | |
| Lutein | Hexane/acetone/ethanol | [70] | |
| Neoxanthin | Hexane/acetone/ethanol | [70] | |
| N-Methylpyrrolidinylhygrine A | Ethanol | [69] | |
| N-Methylpyrrolidinylhygrine B | Ethanol | [69] | |
| Physoperuvine | Ethanol | [69] | |
| Phytofluene | Hexane/acetone/ethanol | [70] | |
| Tropine | Ethanol | [69] | |
| Violaxanthin | Hexane/acetone/ethanol | [70] | |
| Zeaxanthin | Hexane/acetone/ethanol | [70] | |
| γ-Carotene | Hexane/acetone/ethanol | [70] | |
|
| |||
| Body | (S)-4-Iodo-1,2-epoxybutane | — | [71] |
| 1,1,1,5,7,7,7-Heptamethyl-3,3 bis(trimethylsiloxy) tetrasiloxane | — | [71] | |
| 1,2,3-Tri(t-butyl) cyclopropenylium tribromide | — | [71] | |
| 1,2-Benzenedicarboxylic acid | — | [71] | |
| 3,3-Dimethyl-hexane | — | [71] | |
| 3,3-Dimethyl-octane | — | [71] | |
| Diethyl ester | — | [71] | |
| Docosane | — | [71] | |
| Eicosamethyl cyclodecasiloxane | — | [71] | |
| Eicosamethyl cyclodecasiloxane | — | [71] | |
|
| |||
| Calyces | (all-E)-Lutein | Hexane/acetone/ethanol | [72] |
| (all-E)-Lutein 3-O-myristate | Hexane/acetone/ethanol | [72] | |
| (all-E)-Neoxanthin | Hexane/acetone/ethanol | [72] | |
| (all-E)-Neoxanthin palmitate | Hexane/acetone/ethanol | [72] | |
| (all-E)-Taraxanthin | Hexane/acetone/ethanol | [72] | |
| (all-E)-Taraxanthin ester | Hexane/acetone/ethanol | [72] | |
| (all-E)-Violaxanthin | Hexane/acetone/ethanol | [72] | |
| (all-E)-Violaxanthin or (all-E)-neoxanthin ester | Hexane/acetone/ethanol | [72] | |
| (all-E)-α-Carotene | Hexane/acetone/ethanol | [72] | |
| (all-E)-α-Cryptoxanthin myristate | Hexane/acetone/ethanol | [72] | |
| (E)-Vanillic acid | Ethyl acetate | [73] | |
| (E)-α-Carotene | Hexane/acetone/ethanol | [72] | |
| (Z)-Lutein 1 | Hexane/acetone/ethanol | [72] | |
| (Z)-Lutein 2 | Hexane/acetone/ethanol | [72] | |
| (Z)-Lutein ester | Hexane/acetone/ethanol | [72] | |
| (Z)-Taraxanthin | Hexane/acetone/ethanol | [72] | |
| (Z)-Taraxanthin-⍺-linolenic acid | Hexane/acetone/ethanol | [72] | |
| (Z)-β-Carotene | Hexane/acetone/ethanol | [72] | |
| ⍺-Copaeneol | Ethanol/ethyl acetate | [74] | |
| 13-Epimanool | Ethanol/ethyl acetate | [74] | |
| 16-B1-PhytoP | Ethanol/ethyl acetate | [74] | |
| 16α-Methylpregnenolone | Ethanol/ethyl acetate | [74] | |
| 17,27-Dihydroxylated withaloid D isomer 1 | Ethanol/ethyl acetate | [74] | |
| 2,3-Dihydro-17,27-hydroxylated withanolide D derivative | Ethanol/ethyl acetate | [74] | |
| 2,3-Dihydro-27-hydroxylated withanolide D isomer 1 | Ethanol/ethyl acetate | [74] | |
| 2,3-Dihydro-27-hydroxylated withanolide D isomer 2 | Ethanol/ethyl acetate | [74] | |
| 2,3-Dihydro-27-hydroxy-4β- hydroxywithanolide E isomer | Ethanol/ethyl acetate | [74] | |
| 2,3-Dihydro-4β-hydroxywithanolide E | Ethanol/ethyl acetate | [74] | |
| 2,3-Dihydro-hydroxylated 4β-hydroxywithanolide E derivative | Ethanol/ethyl acetate | [74] | |
| 2′,4′-Dimethoxy-3-hydroxy-6-methylflavone | Methanol | [75] | |
| 2′,5′-Dimethoxyflavone | Methanol | [75] | |
| 27-Hydroxy-4β-hydroxywithanolide E isomer | Ethanol/ethyl acetate | [74] | |
| 2-Hydroxy-2′,4′,6′-trimethoxychalcone | Methanol | [75] | |
| 3-(3,4-Dimethoxyphenyl)-6-methyl-4-phenylcoumarin | Methanol | [75] | |
| 3-(3,4-Dimethoxyphenyl)-7- hydroxy-4-methylcoumarin | Methanol | [75] | |
| 3,2′,4′,5′,6-Pentamethoxyflavone | Methanol | [75] | |
| 3,4,5-Methoxy cinnamic | Ethyl acetate | [73] | |
| 3,5,3′,5′-Tetra-tert-butyldiphenoquinone | Methanol | [75] | |
| 3,6,2′,3′-Tetramethoxyflavone | Methanol | [75] | |
| 3,6,3′,4′-Tetramethoxyflavone | Methanol | [75] | |
| 3′-Benzyloxy-5,6,7,4′-tetramethoxyflavone | Methanol | [75] | |
| 3-Hydroxy-7,8,2′-trimethoxyflavone | Methanol | [75] | |
| 3-O-Caffeoylquinic acid | Methanol/water/formic acid | [76] | |
| 3-O-Feruloylquinic acid | Methanol/water/formic acid | [76] | |
| 3-O-p-Coumaroylquinic acid | Methanol/water/formic acid | [76] | |
| 4,4-Dimethyl-5-α-cholestane-3-one | Ethanol/ethyl acetate | [74] | |
| 4-Aminobenzoic acid | Ethyl acetate | [73] | |
| 4-Hydroxy chalcone | Methanol | [75] | |
| 4-O-Feruloylquinic acid | Methanol/water/formic acid | [76] | |
| 5-(7a-Isopropenyl-4,5-dimethyl-octahydroinden-4-yl)- 3-methyl-pent-2-en-1-ol | Ethanol/ethyl acetate | [74] | |
| 5,6-Epoxy-β-carotene | Hexane/acetone/ethanol | [72] | |
| 5-O-Caffeoylquinic acid (chlorogenic acid) | Methanol/water/formic acid | [76] | |
| 5-O-Feruloylquinic acid | Methanol/water/formic acid | [76] | |
| 7-Hydroxycoumarin-3- carboxylic acid | Methanol | [75] | |
| 7δ-Ergosterol | Ethanol/Ethyl acetate | [74] | |
| 9-D1t-PhytoP | Methanol | [76] | |
| 9-Epi-9-D1t-PhytoP | Methanol | [76] | |
| 9-Epi-9-F1t-PhytoP | Methanol | [76] | |
| 9-F1t-PhytoP | Methanol | [76] | |
| 9-L1-PhytoP | Methanol | [76] | |
| Acecetin | Ethyl acetate | [73] | |
| Ambrial | Ethanol/ethyl acetate | [74] | |
| Apg 6 arabinose 8 glucose | Ethyl acetate | [73] | |
| Apg 6 glucose 8 rhamnose | Ethyl acetate | [73] | |
| Apg 6 rhamnose 8 glucose | Ethyl acetate | [73] | |
| Apig-7-O-neohespiroside | Ethyl acetate | [73] | |
| Apigenin | Ethyl acetate | [73] | |
| Apigenin 7 glucose | Ethyl acetate | [73] | |
| Benzoic acid | Ethanol/Ethyl acetate | [74] | |
| Biotin | Methanol | [75] | |
| Caffeic acid | Ethanol/Ethyl acetate | [74] | |
| Caffeine | Ethyl acetate | [73] | |
| Catechol | Ethyl acetate | [73] | |
| Chlorogenic acid | Ethyl acetate | [73] | |
| Chlorophyll a | Hexane/acetone/ethanol | [72] | |
| Chlorophyll a derivative | Hexane/acetone/ethanol | [72] | |
| Chlorophyll b | Hexane/acetone/ethanol | [72] | |
| Chlorophyll b derivative 2 | Hexane/acetone/ethanol | [72] | |
| Cinnamic acid | Ethyl acetate | [73] | |
| Coniferol | Ethanol/ethyl acetate | [74] | |
| Copalol isomer 1 | Ethanol/ethyl acetate | [74] | |
| Copalol isomer 2 | Ethanol/ethyl acetate | [74] | |
| Copalol isomer 3 | Ethanol/ethyl acetate | [74] | |
| Coumarin | Ethyl acetate | [73] | |
| Cryptomeridiol | Ethanol/ethyl acetate | [74] | |
| Diepicedrene-1-oxide | Ethanol/ethyl acetate | [74] | |
| Dihydro-4β-hydroxywithanolide E | Ethanol/ethyl acetate | [74] | |
| Dihydromanoyl oxide 1 | Ethanol/ethyl acetate | [74] | |
| Dihydromanoyl oxide 2 | Ethanol/ethyl acetate | [74] | |
| Dihydromanoyl oxide 3 | Ethanol/ethyl acetate | [74] | |
| Dihydromanoyl oxide 4 | Ethanol/ethyl acetate | [74] | |
| Dihydromanoyloxide-7-carboxylic acid methyl ester | Ethanol/ethyl acetate | [74] | |
| Di-O-isobutanoyl-O-(2-methylbutanoyl)-O-pentenoylsucrose | Ethanol/ethyl acetate | [74] | |
| Di-O-isobutanoylsucrose | Ethanol/ethyl acetate | [74] | |
| Di-O-isobutanoyl-O-nonanoylsucrose | Ethanol/ethyl acetate | [74] | |
| Di-O-isobutanoyl-O-decanoylsucrose | Ethanol/ethyl acetate | [74] | |
| Di-O-isobutanoyl-O-octanoylsucrose | Ethanol/ethyl acetate | [74] | |
| Di-O-isobutanoyl-O-pentenoylsucrose | Ethanol/ethyl acetate | [74] | |
| Ellagic acid | Ethyl acetate | [73] | |
| Ent-16-B1-PhytoP | Methanol | [76] | |
| Ent-9-L1-PhytoP | Methanol | [76] | |
| Ent-16-epi-16-F1t-PhytoP | Methanol | [76] | |
| Ent-16-F1t-PhytoP | Methanol | [76] | |
| Epicatechin | Ethyl acetate | [73] | |
| Epimanoyl oxide | Ethanol/ethyl acetate | [74] | |
| Eudesmadienol | Ethanol/ethyl acetate | [74] | |
| Farnesol acetate | Ethanol/ethyl acetate | [74] | |
| Ferulic acid-hexoside | Methanol | [76] | |
| Feruloylquinic acid | Methanol | [76] | |
| Friedelan-3-one | Ethanol/ethyl acetate | [74] | |
| Ferulic acid | Ethanol/ethyl acetate | [74] | |
| Gallic acid | Ethanol/ethyl acetate | [74] | |
| Gardenin | Methanol | [75] | |
| Germacratrienol isomer 1 | Ethanol/ethyl acetate | [74] | |
| Germacratrienol isomer 2 | Ethanol/ethyl acetate | [74] | |
| Germacratrienol isomer 3 | Ethanol/ethyl acetate | [74] | |
| Hesperetin | Ethyl acetate | [73] | |
| Hydroxylated 4β-hydroxywithanolide E derivative | Ethanol/ethyl acetate | [74] | |
| Isoaromadendrene epoxide | Ethanol/ethyl acetate | [74] | |
| Isoferulic acid | Ethyl acetate | [73] | |
| Isorhamnetin | Ethanol/ethyl acetate | [74] | |
| Isovitexin | Methanol | [75] | |
| Kaempferol | Ethanol/ethyl acetate | [74] | |
| Kaempferol-3-O-rhamnosyl(1⟶6)glucoside | Methanol/water/formic acid | [76] | |
| Kaempferol-3-O-rhamnosyl(1⟶6)glucoside- 7-O-glucoside | Methanol/water/formic acid | [76] | |
| Kaempferol-hexoside | Ethanol/ethyl acetate | [74] | |
| Kaempferol-rutinoside | Ethanol/ethyl acetate | [74] | |
| Kamp3(2-p-manryl)glucose | Ethyl acetate | [73] | |
| Kamp3-7 di-rhamnoside | Ethyl acetate | [73] | |
| Khusiol | Ethanol/ethyl acetate | [74] | |
| Limonene | Ethanol/ethyl acetate | [74] | |
| Luteo 6 glucose 8 arabinose | Ethyl acetate | [73] | |
| Luteo 7 glucose | Ethyl acetate | [73] | |
| Maalialcohol | Ethanol/ethyl acetate | [74] | |
| Methyl-3,7-bis(acetyloxy)cholestan-26-oate | Ethanol/ethyl acetate | [74] | |
| Methylprednisolone succinate | Methanol | [75] | |
| Myricetin | Ethanol/ethyl acetate | [74] | |
| Naringin | Ethyl acetate | [73] | |
| Naringenin | Ethyl acetate | [73] | |
| O-Butanoyl-di-O-isobutanoylsucrose | Ethanol/ethyl acetate | [74] | |
| O-Decanoyl-O-isobutanoylsucrose | Ethanol/ethyl acetate | [74] | |
| O-Isobutanoyl-O-(2-methylbutanoyl)-O-octanoylsucrose | Ethanol/ethyl acetate | [74] | |
| O-Isobutanoyl-O-(2-methylbutanoyl)-O-pentenoylsucrose | Ethanol/ethyl acetate | [74] | |
| O-Isobutanoyl-O-(2-methylbutanoyl)sucrose | Ethanol/ethyl acetate | [74] | |
| O-Isobutanoyl-O-octenoylsucrose | Ethanol/ethyl acetate | [74] | |
| O-Isobutanoylsucrose | Ethanol/ethyl acetate | [74] | |
| p-Coumaric acid | Ethanol/ethyl acetate, ethyl acetate | [73, 74] | |
| Pheophytin a | Hexane/acetone/ethanol | [72] | |
| p-Hydroxy benzoic acid | Ethyl acetate | [73] | |
| Phytoene | Hexane/acetone/ethanol | [72] | |
| Phytol | Ethanol/ethyl acetate | [74] | |
| Protocatechuic acid | Ethanol/ethyl acetate, ethyl acetate | [73, 74] | |
| Pyrogallol | Ethyl acetate | [73] | |
| Quercetin | Methanol, ethanol/ethyl acetate, ethyl acetate | [74] | |
| Quercetin-3-O-glucoside | Methanol/water/formic acid | [76] | |
| Quercetin-3-O-rhamnosyl(1⟶6)glucoside-7-O-glucoside | Methanol/water/formic acid | [76] | |
| Quercetin-hexoside | Ethanol/ethyl acetate | [74] | |
| Quercetrin | Ethyl acetate | [73] | |
| Rhamncetin | Ethyl acetate | [73] | |
| Rosmarinic acid | Ethyl acetate | [73] | |
| Quercetin-3-O-rutinoside | Methanol/water/formic acid, ethanol/ethyl acetate | [74, 76] | |
| Salicylic acid | Ethyl acetate | [73] | |
| Sclareol | Ethanol/ethyl acetate | [74] | |
| Sclareol oxide | Ethanol/ethyl acetate | [74] | |
| Sesquichamene | Ethanol/ethyl acetate | [74] | |
| Sesquiterpeneol isomer | Ethanol/ethyl acetate | [74] | |
| Spironolactone | Methanol | [75] | |
| trans-Geranylgeraniol | Ethanol/ethyl acetate | [74] | |
| Tyrosol | Ethanol/ethyl acetate | [74] | |
| Vanillic acid | Ethanol/ethyl acetate | [74] | |
| Vanillin | Ethanol/ethyl acetate | [74] | |
| Vitexin | Methanol | [75] | |
| Withanolide D isomer | Ethanol/ethyl acetate | [74] | |
| Withanolide E isomer 1 | Ethanol/ethyl acetate | [74] | |
| Withanolide E isomer 2 | Ethanol/ethyl acetate | [74] | |
| Withanolide E isomer 3 | Ethanol/ethyl acetate | [74] | |
| Xanthine | Methanol | [75] | |
| α-13,13-Dimethylpodocarp-7-en-3⍺-ol | Ethanol/ethyl acetate | [74] | |
| ⍺-Coumaric acid | Ethyl acetate | [73] | |
| ⍺-Elemol | Ethanol/ethyl acetate | [74] | |
| α-Tocopherol | Ethanol/ethyl acetate | [74] | |
| α-Tocopherol-β-D-mannoside | Ethanol/ethyl acetate | [74] | |
| β-Sitosterol | Ethanol/ethyl acetate | [74] | |
| β-Tocopherol | Ethanol/ethyl acetate | [74] | |
| δ-Cadinol | Ethanol/ethyl acetate | [74] | |
| δ-Terpineol | Ethanol/ethyl acetate | [74] | |
| δ-Tocopherol | Ethanol/ethyl acetate | [74] | |
|
| |||
| Fruits | (-)-Caryophyllene oxide | — | [77] |
| (5á)-Pregnane-3,20á-diol | Juice | [78] | |
| (9Z)-β-Carotene | Hexane/acetone/ethanol | [72] | |
| (all-E)-Antheraxanthin myristate-palmitate | Hexane/acetone/ethanol | [72] | |
| (all-E)-Lutein | Hexane/acetone/ethanol | [72] | |
| (all-E)-Lutein 3′-O-palmitate | Hexane/acetone/ethanol | [72] | |
| (all-E)-Lutein 3-O-myristate | Hexane/acetone/ethanol | [72] | |
| (all-E)-Lutein 3-O-palmitate-3′-O-myristate | Hexane/acetone/ethanol | [72] | |
| (all-E)-Lutein dimyristate | Hexane/acetone/ethanol | [72] | |
| (all-E)-Lutein dipalmitate | Hexane/acetone/ethanol | [72] | |
| (all-E)-Neoxanthin | Hexane/acetone/ethanol | [72] | |
| (all-E)-Neoxanthin dipalmitate | Hexane/acetone/ethanol | [72] | |
| (all-E)-Neoxanthin myristate | Hexane/acetone/ethanol | [72] | |
| (all-E)-Neoxanthin palmitate | Hexane/acetone/ethanol | [72] | |
| (all-E)-Taraxanthin | Hexane/acetone/ethanol | [72] | |
| (all-E)-Taraxanthin ester | Hexane/acetone/ethanol | [72] | |
| (all-E)-Violaxanthin | Hexane/acetone/ethanol | [72] | |
| (all-E)-Violaxanthin dimyristate | Hexane/acetone/ethanol | [72] | |
| (all-E)-Violaxanthin dipalmitate | Hexane/acetone/ethanol | [72] | |
| (all-E)-Violaxanthin myristate-palmitate | Hexane/acetone/ethanol | [72] | |
| (all-E)-Zeaxanthin dimyristate | Hexane/acetone/ethanol | [72] | |
| (all-E)-Zeaxanthin dipalmitate | Hexane/acetone/ethanol | [72] | |
| (all-E)-Zeaxanthin myristate-palmitate | Hexane/acetone/ethanol | [72] | |
| (all-E)-Zeinoxanthin | Hexane/acetone/ethanol | [72] | |
| (all-E)-α-Carotene | Hexane/acetone/ethanol | [72] | |
| (all-E)-α-Cryptoxanthin | Hexane/acetone/ethanol | [72] | |
| (all-E)-α-Cryptoxanthin myristate | Hexane/acetone/ethanol | [72] | |
| (all-E)-α-Cryptoxanthin palmitate | |||
|
| |||
| Palmitate | Hexane/acetone/ethanol | [72] | |
| (E)-2-Hexenol | — | [79] | |
| (E)-Non-2-enal | Dichloromethane | [80] | |
| (E)-α-Carotene | Hexane/acetone/ethanol | [72] | |
| (E2, Z6)-Nona-2,6-dienal | Dichloromethane | [80] | |
| (S)-4-Iodo-1,2-epoxybutane | — | [71] | |
| (Z)-Lutein 1 | Hexane/acetone/ethanol | [72] | |
| (Z)-Lutein ester | Hexane/acetone/ethanol | [72] | |
| (Z)-Neoxanthin- or (Z)-violaxanthin ester | Hexane/acetone/ethanol | [72] | |
| (Z)-Stigmasta-5,24(28)-dien-3β-ol | Dichloromethane | [80] | |
| (Z)-Taraxanthin | Hexane/acetone/ethanol | [72] | |
| (Z)-β-Carotene | Hexane/acetone/ethanol | [72] | |
| (Z)-γ-Carotene | Hexane/acetone/ethanol | [72] | |
| ∆5-Avenasterol | Crude oil | [81, 82] | |
| ∆7-Avenasterol | Crude oil | [81, 82] | |
| 1,1,1,5,7,7,7-Heptamethyl-3,3 bis(trimethylsiloxy) tetrasiloxane | — | [71] | |
| 1,25-Dihydroxyvitamin D2 | Juice | [71] | |
| 1,2-Benzenedicarboxylic acid | — | [71] | |
| 1,8-Menthadien-4-ol | — | [77] | |
| 1-Phenyl-1,2-propanediol | — | [79] | |
| 2,3-Diethyl-5-methyl pyrazine | Hexane and ethanol | [83] | |
| 2,3-Dimethyl-1-butanol | — | [77] | |
| 2-Acetyl-1-pyrroline | Dichloromethane | [80] | |
| 2-Butanone | — | [77] | |
| 2-Heptanol | — | [79] | |
| 2-Heptanone | — | [77] | |
| 2-Methylbutanal | — | [77] | |
| 2-Methylbutanol | — | [77, 79] | |
| 2-Methylbutanoic acid | — | [79] | |
| 2-Methylbutyl acetate | — | [77] | |
| 2-Methylpropanol | — | [79] | |
| 2-Methylpropanoic acid | — | [79] | |
| 2-Methylpropanal | Dichloromethane | [80] | |
| 2-Methylpropenal | — | [77] | |
| 2-Nonadecanol | — | [77] | |
| 2-Norbornanone | — | [77] | |
| 2-Pentanone | — | [77] | |
| 2-Phenyl ethyl alcohol | Juice | [78] | |
| 2-Phenylacetaldehyde | Dichloromethane | [80] | |
| 2-Phenylethanol | Dichloromethane | [79, 80] | |
| 2-Propanone | — | [77] | |
| 2-Undecenal | Hexane and ethanol | [83] | |
| 3,3-Dimethyl-hexane | — | [71] | |
| 3,3-Dimethyl-octane | — | [71] | |
| 3,4-Dimethylbenzoic acid | — | [71] | |
| 3,5-Octadienone | Hexane and ethanol | [83] | |
| 3,7-Dimethyl-1-octene | — | [77] | |
| 3-Ethyl-4-heptanol | — | [77] | |
| 3-Hydroxy-2-butanone | — | [79] | |
| 3-Methyl-1-hexanol | — | [77] | |
| 3-Methyl-1-penten-3-ol | — | [84] | |
| 3-Methyl-3-vinyl-1-cyclopropene | — | [84] | |
| 3-Methyl butyl butanoate | — | [77] | |
| 3-Octenol | — | [77] | |
| 3-Oxo-7,8-dihydro-α-ionol | Dichloromethane | [80] | |
| 3-Phenyl propanol | — | [77] | |
| 4-Hydroxy butyl acrylate | Hexane and ethanol | [83] | |
| 4-Isopropyl-1-methyl-2-cyclohexen-1-ol | — | [77] | |
| 4-Methyl-1-pentanol | — | [77] | |
| 4-Nonanone | — | [77] | |
| 4-Octanol | — | [77] | |
| 4-Propyl guaiacol | Hexane and ethanol | [83] | |
| 4-Terpineol | — | [77] | |
| 4-Vinylguaiacol | — | [79] | |
| 4-Vinylphenol | — | [79] | |
| 4-Vinylsyringol | — | [79] | |
| 4β-Hydroxywithanolide E | Hexane and ethanol | [83] | |
| 5,6-Epoxy-β-carotene | — | [72] | |
| 5,8-Epoxy-α-carotene | — | [72] | |
| 6-Methyl-2-heptanone | — | [77] | |
| 6-Methyl-5-heptene-2-one | — | [84] | |
| 6-Methyl-hept-5-en-2-ol | — | [77] | |
| 9-(Z)-Octadecenoic acid | — | [79] | |
| Acetaldehyde | — | [77] | |
| Acetic acid | — | [79] | |
| Allyl caproate | Hexane and ethanol | [83] | |
| Apigenin | Ethanol or water | [85] | |
| Apigenin 7 glucose | Ethyl acetate | [73] | |
| Benzaldehyde | — | [77, 84] | |
| Benzoic acid | Ethanol/ethyl acetate, ethanol, or water | [72, 85] | |
| Benzyl acetate | Hexane and ethanol | [83] | |
| Benzyl alcohol | — | [77, 79, 84] | |
| Betulin | Juice | [78] | |
| Butanal | — | [77] | |
| Butane-2,3-dione | — | [77] | |
| Butanoic acid | — | [77, 79] | |
| Butanol | — | [77, 79] | |
| Butanol-2-methyl | Hexane and ethanol | [83] | |
| Butyl 3-hydroxybutyrate | — | [84] | |
| Butyl acetate | Crude oil | [77, 84] | |
| Butyl butanoate | — | [77] | |
| Butyl decanoate | — | [77] | |
| Butyl dodecanoate | — | [77] | |
| Butyl octanoate | — | [77] | |
| Butyl-3-hydroxybutanoate | — | [77, 79] | |
| Caffeic acid | Methanol, ethanol/ethyl acetate | [78] | |
| Caffeine | Ethanol or water | [85] | |
| Campesterol | Dichloromethane | [86] | |
| Camphene | — | [77] | |
| Capric acid, methyl ester | — | [84] | |
| Carvacrol | — | [77] | |
| Caryophyllene oxide | — | [84] | |
| Catechin | Ethanol and isopropanol | [87] | |
| Catechol | Ethanol or water | [85] | |
| Cedr-8-en-9-alpha-ol acetate | Hexane and ethanol | [83] | |
| Cedrenol | Hexane and ethanol | [83] | |
| Chlorophyll a | Hexane/acetone/ethanol | [72] | |
| Chlorophyll b | Hexane/acetone/ethanol | [72] | |
| Chlorophyll b derivative 1 | Hexane/acetone/ethanol | [72] | |
| Chlorophyll b derivative 2 | Hexane/acetone/ethanol | [72] | |
| Cinnamic acid | -/ethanol or water | [72, 85] | |
| cis-3-Hexenol | — | [77] | |
| cis-Myrtanol | — | [77] | |
| cis-Piperitone oxide | — | [77] | |
| cis-p-Mentha-1(7),8-dien-2-ol | — | [77] | |
| cis-Verbenol | — | [77] | |
| Citronellyl acetate | Hexane and ethanol | [83] | |
| Cyclooctatetraene | — | [77] | |
| Cyclosativene | Hexane and ethanol | [83] | |
| Cymenene | — | [77] | |
| Decanal | — | [77] | |
| Decanoic acid | Juice, crude oil | [77, 79, 81, 82] | |
| Dehydrosabinene | — | [77] | |
| Diethyl ester | — | [71] | |
| Diethylene glycol | Methanol | [88] | |
| Dihomo-γ-linolenic acid | Crude oil | [81] | |
| Dihydroactinidiolide | — | [77] | |
| Dihydrocarveol | Hexane and ethanol | [83] | |
| Dimethylvinylcarbinol | — | [77] | |
| Docosane | — | [77] | |
| Docosanoic acid | — | [89] | |
| Dodecane | — | [84] | |
| Dodecanoic acid, methyl ester | — | [84] | |
| Eicosamethylcyclodecasiloxane | — | [71] | |
| Eicosanoic acid | Crude oil | [81, 82] | |
| Eicosenoic acid | Crude oil | [81, 82] | |
| Endo-borneol | — | [77] | |
| Epicatechin | Ethanol and isopropanol | [87] | |
| Erucic acid | Crude oil | [81, 82] | |
| Ergosterol | Crude oil | [81, 82] | |
| Ethanol | — | [77] | |
| Ethyl 2-methyl propanoate | Dichloromethane | [80] | |
| Ethyl acetate | — | [77] | |
| Ethyl benzoate | Juice | [78] | |
| Ethyl butanoate | Dichloromethane | [77, 80] | |
| Ethyl caprate | — | [84] | |
| Ethyl caproate | — | [84] | |
| Ethyl decanoate | — | [77] | |
| Ethyl dodecanoate | — | [77, 84] | |
| Ethyl hexanoate | Dichloromethane | [77, 80] | |
| Ethyl hexanol | — | [77] | |
| Ethyl hydroxyl hexanoate | — | [84] | |
| Ethyl octanoate | Dichloromethane, hexane, and ethanol | [77, 80, 83] | |
| Ethyl pentanoate | — | [77] | |
| Ethyl-2-butenoate | — | [77] | |
| Ethyl-3-hydroxybutanoate | — | [79] | |
| Ethyl-3-hydroxyhexanoate | — | [79] | |
| Ethyl-3-hydroxyoctanoate | — | [79] | |
| Ethyl-5-hydroxyoctanoate | — | [79] | |
| Eucalyptol | Hexane and ethanol | [77, 83] | |
| Farnesol | — | [77] | |
| Fenchol | — | [77] | |
| Ferulic acid | Methanol, ethanol/ethyl acetate | [78, 88] | |
| Furaneol | Dichloromethane | [80] | |
| Gallic acid | Ethanol and isopropanol, ethanol, or water | [85, 87] | |
| Geranaldehyde | — | [77] | |
| Geraniol | — | [77] | |
| Geranoic acid | — | [79] | |
| Geranyl acetone | — | [77] | |
| Guaiacol | — | [79] | |
| Heptan-2-ol | — | [77] | |
| Heptanal | — | [77] | |
| Heptanol | — | [77] | |
| Hexadecanoic acid | Crude oil, dichloromethane | [72, 76, 77] | |
| Hexadecanoic acid ester | Hexane and ethanol | [83] | |
| Hexanal | Crude oil, dichloromethane | [77, 80, 84] | |
| Hexanoic acid | — | [77, 79] | |
| Hexanol | — | [77, 79] | |
| Hexyl butanoate | — | [77] | |
| Hexyl ethanoate | — | [77] | |
| Hexyl octanoate | — | [77] | |
| Homofuraneol | Hexane and ethanol | [83] | |
| Hydrocinnamic alcohol | — | [77] | |
| Isoamyl octanoate | — | [77] | |
| Isobutyl acetate | — | [77] | |
| Isobutyl alcohol | — | [77] | |
| Isobutyl butanoate | — | [77] | |
| Isobutyl decanoate | — | [77] | |
| Isobutyl dodecanoate | — | [77] | |
| Isobutyl octanoate | — | [77] | |
| Isoeugenol | Hexane and ethanol | [83] | |
| Isophorone | — | [77] | |
| Isopropenyl ethyl ketone | — | [77] | |
| Isopulegol | — | [77] | |
| Kaempferol | Ethanol/ethyl acetate, ethanol, or water | [78, 85] | |
| Kaempferol 3-O-rutinoside | Juice | [78] | |
| Lanosterol | Crude oil | [81, 82] | |
| Limonene | Ethanol/ethyl acetate | [74, 77] | |
| Linalool | — | [77, 84] | |
| Linalool oxide | — | [77] | |
| Linoleic acid | Crude oil | [81, 82] | |
| Lucenin-2 | Juice | [78] | |
| Lutein ester | [72] | ||
| Methional | Dichloromethane | [80] | |
| Methyl acetate | — | [77] | |
| Methyl benzoate | — | [84] | |
| Methyl butanoate | Hexane and ethanol | [77, 83] | |
| Methyl butene | Hexane and ethanol | [83] | |
| Methyl decanoate | — | [77] | |
| Methyl heptenone | — | [77] | |
| Methyl hexanoate | — | [77] | |
| Methyl octanoate | — | [77] | |
| Methyl salicylate | — | [77, 79] | |
| Methyl ß-methylcrotonate | — | [84] | |
| Methyl-11-cyclopentylundecanoate | — | [77] | |
| Methyl-2-methoxyoct-2-enoate | — | [84] | |
| Methyl-3-hydroxybutanoate | — | [79] | |
| Myrcenol | — | [77] | |
| Neric acid | — | [77] | |
| Naringenin | Ethanol or water | [85] | |
| Nervonic acid | Crude oil | [81, 82] | |
| Neryl acetate | Hexane and ethanol | [83] | |
| Nonanal | — | [77] | |
| Nonanoic acid | — | [77] | |
| Nonanol | Hexane and ethanol | [83] | |
| Nopol | — | [77] | |
| O-Coumaric acid | Ethanol or water | [85] | |
| Oct-1-en-3-ol | Dichloromethane | [80] | |
| Octadecanoic acid | Crude oil | [81, 82] | |
| Octanal | -/Dichloromethane | [77, 80] | |
| Octanoic acid | — | [77, 79] | |
| Octanoic acid, 3-methylbutyl ester | — | [84] | |
| Octanol | — | [77] | |
| Oleic acid | Crude oil | [81, 82] | |
| Palmitoleic acid | Crude oil | [81, 82] | |
| p-Anisaldehyde | Hexane and ethanol | [83] | |
| p-Cymen-8-ol | — | [77] | |
| p-Cymene | — | [77] | |
| Pentyl alcohol | — | [84] | |
| Phenethyl alcohol | — | [77] | |
| Phenol | — | [79] | |
| Phenyl ethyl benzoate | Hexane and ethanol | [83] | |
| Phenylethyl acetate | — | [77] | |
| Pheophytin b | Hexane/acetone/ethanol | [72] | |
| p-Hydroxy benzoic acid | Ethanol or water | [85] | |
| Phytoene | Hexane/acetone/ethanol | [72] | |
| Phytofluene | Hexane/acetone/ethanol | [72] | |
| p-Menth-4(8)-ene-1,2-diol | — | [79] | |
| Propyl decanoate | — | [77] | |
| Propyl hexanoate | Hexane and ethanol | [83] | |
| Propyl octanoate | — | [77] | |
| Quercetin 3,4′,7-trimethyl ether | Juice | [78] | |
| Rosoxide | — | [77] | |
| Salicylic acid | Ethanol or water | [85] | |
| sec-Butyl butyrate | — | [77] | |
| Stigmasterol | Dichloromethane | [86] | |
| Syringic acid | Ethanol or water | [85] | |
| Terpinen-4-ol | — | [84] | |
| Terpinolene | — | [84] | |
| Tetradecanoic acid | Crude oil | [81, 82] | |
| Tetracosanoic acid | Crude oil | [81, 82] | |
| trans-3-Hexenol | — | [77] | |
| trans-Citral | — | [77] | |
| Trimethyl phenyl butenone | Hexane and ethanol | [83] | |
| Vanillic acid | Ethanol/Ethyl acetate, ethanol, or water | [74, 85] | |
| Vanillin | Ethanol/Ethyl acetate, ethanol, or water | [74, 85] | |
| Verbenene | Hexane and ethanol | [77, 83] | |
| Verbenone | — | [77] | |
| Vitamin B9 (folic acid) | Juice | [78] | |
| Vitamin E | Crude oil, dichloromethane | [81, 86] | |
| Vitamin K1 | Crude oil | [81] | |
| α-Cubebene | Juice | [78] | |
| α-Linolenic acid | Crude oil | [76, 77] | |
| α-Pinene | Hexane and ethanol | [77, 83] | |
| α-Terpinene | — | [77] | |
| α-Terpineol | — | [77, 79, 84] | |
| α-Terpinolene | — | [77] | |
| α-Tocopherol | Crude oil, ethanol/ethyl acetate | [74, 81, 82] | |
| β-Bisabolol | Juice | [78] | |
| β-Carotene | Crude oil | [81, 82] | |
| β-Citronellol | — | [77] | |
| β-Cyclocitral | — | [77] | |
| β-Ionone | — | [77] | |
| β-Ionone-5,6-epoxide | — | [77] | |
| β-Linalool | Dichloromethane | [80] | |
| β-Myrcene | — | [77] | |
| β-Sitosterol | Crude oil | [81, 82] | |
| β-Tocopherol | Crude oil | [81, 82] | |
| β-trans-Ocimene | — | [77] | |
| γ-Butyl-γ-butyrolactone | — | [84] | |
| γ-Caprolactone | — | [84] | |
| γ-Ethylbutyrolactone | — | [77] | |
| γ-Linoleic acid | Crude oil | [81, 82] | |
| γ-Octalactone | Hexane and ethanol | [79, 83] | |
| γ-Terpinene | — | [77, 84] | |
| γ-Tocopherol | — | [82] | |
| γ-Undecalactone | — | [77] | |
| δ-Muurolene | Hexane and ethanol | [83] | |
| δ-Octalactone | — | [77, 79] | |
|
| |||
| Leaves | (S)-4-Iodo-1,2-epoxybutane | — | [71] |
| 1,1,1,5,7,7,7-Heptamethyl-3,3 | — | [71] | |
| 1,2-Benzenedicarboxylic acid | — | [71] | |
| 3,3-Dimethyl-hexane | — | [71] | |
| 3,3-Dimethyl-octane | — | [71] | |
| Campesterol | Dichloromethane | [86] | |
| Diethyl ester | — | [71] | |
| Docosane | — | [77] | |
| Eicosamethylcyclodecasiloxane | — | [71] | |
| Ethyl isoallocholate | Dichloromethane | [86] | |
| Hexadecanoic acid | Dichloromethane | [86] | |
| Hexahydrofarnesyl acetone | Dichloromethane | [86] | |
| Linoleic acid | Dichloromethane | [86] | |
| Perulactone B | — | [90] | |
| Physalin B | — | [90] | |
| Physalin D | — | [90] | |
| Physalin F | — | [90] | |
| Phytol | Methanol, dichloromethane | [81, 86] | |
| Stigmasterol | Dichloromethane | [86] | |
| Vitamin E | Dichloromethane | [86] | |
| Withanolide E | — | [90] | |
| Withanolide F | — | [90] | |
|
| |||
| Peel | (all-E)-Antheraxanthin myristate-palmitate | Hexane/acetone/ethanol | [72] |
| (all-E)-Lutein | Hexane/acetone/ethanol | [72] | |
| (all-E)-Lutein 3′-O-palmitate | Hexane/acetone/ethanol | [72] | |
| (all-E)-Lutein 3-O-myristate | Hexane/acetone/ethanol | [72] | |
| (all-E)-Lutein 3-O-palmitate-3′-O-myristate | Hexane/acetone/ethanol | [72] | |
| (all-E)-Lutein dimyristate | Hexane/acetone/ethanol | [72] | |
| (all-E)-Lutein dipalmitate | Hexane/acetone/ethanol | [72] | |
| (all-E)-Neoxanthin | Hexane/acetone/ethanol | [72] | |
| (all-E)-Neoxanthin dipalmitate | Hexane/acetone/ethanol | [72] | |
| (all-E)-Neoxanthin myristate | Hexane/acetone/ethanol | [72] | |
| (all-E)-Neoxanthin palmitate | Hexane/acetone/ethanol | [72] | |
| (all-E)-Taraxanthin | Hexane/acetone/ethanol | [72] | |
| (all-E)-Taraxanthin ester | Hexane/acetone/ethanol | [72] | |
| (all-E)-Violaxanthin | Hexane/acetone/ethanol | [72] | |
| (all-E)-Violaxanthin dimyristate | Hexane/acetone/ethanol | [72] | |
| (all-E)-Violaxanthin dipalmitate | Hexane/acetone/ethanol | [72] | |
| (all-E)-Violaxanthin myristate-palmitate | Hexane/acetone/ethanol | [72] | |
| (all-E)-Zeaxanthin dimyristate | Hexane/acetone/ethanol | [72] | |
| (all-E)-Zeaxanthin dipalmitate | Hexane/acetone/ethanol | [72] | |
| (all-E)-Zeaxanthin myristate-palmitate | Hexane/acetone/ethanol | [72] | |
| (all-E)-Zeinoxanthin | Hexane/acetone/ethanol | [72] | |
| (all-E)-α-Carotene | Hexane/acetone/ethanol | [72] | |
| (all-E)-α-Cryptoxanthin | Hexane/acetone/ethanol | [72] | |
| (all-E)-α-Cryptoxanthin myristate | Hexane/acetone/ethanol | [72] | |
| (all-E)-α-Cryptoxanthin palmitate | |||
|
| |||
| Hexane/acetone/ethanol | [72] | ||
| (E)-α-Carotene | Hexane/acetone/ethanol | [72] | |
| (Z)-Lutein 1 | Hexane/acetone/ethanol | [72] | |
| (Z)-Lutein ester | Hexane/acetone/ethanol | [72] | |
| (Z)-Neoxanthin- or (Z)-violaxanthin ester | Hexane/acetone/ethanol | [72] | |
| (Z)-Taraxanthin | Hexane/acetone/ethanol | [72] | |
| (Z)-β-Carotene | Hexane/acetone/ethanol | [72] | |
| (Z)-γ-Carotene | Hexane/acetone/ethanol | [72] | |
| 5,6-Epoxy-β-carotene | Hexane/acetone/ethanol | [72] | |
| 5,8-Epoxy-α-carotene | Hexane/acetone/ethanol | [72] | |
| Lutein ester | Hexane/acetone/ethanol | [72] | |
| Phytoene | Hexane/acetone/ethanol | [72] | |
| Phytofluene | Hexane/acetone/ethanol | [72] | |
|
| |||
| Pulp | (all-E)-Lutein | Hexane/acetone/ethanol | [72] |
| (all-E)-Lutein 3-O-myristate | Hexane/acetone/ethanol | [72] | |
| (all-E)-Lutein 3-O-palmitate-3′-O-myristate | Hexane/acetone/ethanol | [72] | |
| (all-E)-Lutein dimyristate | Hexane/acetone/ethanol | [72] | |
| (all-E)-Lutein dipalmitate | Hexane/acetone/ethanol | [72] | |
| (all-E)-Neoxanthin | Hexane/acetone/ethanol | [72] | |
| (all-E)-Neoxanthin dipalmitate | Hexane/acetone/ethanol | [72] | |
| (all-E)-Neoxanthin myristate | Hexane/acetone/ethanol | [72] | |
| (all-E)-Taraxanthin | Hexane/acetone/ethanol | [72] | |
| (all-E)-Taraxanthin ester | Hexane/acetone/ethanol | [72] | |
| (all-E)-Violaxanthin | Hexane/acetone/ethanol | [72] | |
| (all-E)-Violaxanthin dimyristate | Hexane/acetone/ethanol | [72] | |
| (all-E)-Violaxanthin dipalmitate | Hexane/acetone/ethanol | [72] | |
| (all-E)-Violaxanthin myristate-palmitate | Hexane/acetone/ethanol | [72] | |
| (all-E)-Zeinoxanthin | Hexane/acetone/ethanol | [72] | |
| (all-E)-α-Carotene | Hexane/acetone/ethanol | [72] | |
| (all-E)-α-Cryptoxanthin | Hexane/acetone/ethanol | [72] | |
| (all-E)-α-Cryptoxanthin myristate | Hexane/acetone/ethanol | [72] | |
| (E)-α-Carotene | Hexane/acetone/ethanol | [72] | |
| (Z)-Lutein 1 | Hexane/acetone/ethanol | [72] | |
| (Z)-Lutein ester | Hexane/acetone/ethanol | [72] | |
| (Z)-β-Carotene | Hexane/acetone/ethanol | [72] | |
| (Z)-γ-Carotene | Hexane/acetone/ethanol | [72] | |
| ∆5-Avenasterol | Crude oil | [81, 82] | |
| ∆7-Avenasterol | Crude oil | [81, 82] | |
| 5,6-Epoxy-β-carotene | Hexane/acetone/ethanol | [72] | |
| 5,8-Epoxy-α-carotene | Hexane/acetone/ethanol | [72] | |
| Campesterol | Crude oil | [81, 82] | |
| Decanoic acid | Crude oil | [81, 82] | |
| Eicosanoic acid | Crude oil | [81, 82] | |
| Eicosenoic acid | Crude oil | [81, 82] | |
| Erucic acid | Crude oil | [81, 82] | |
| Ergosterol | Crude oil | [81, 82] | |
| Hexadecanoic acid | Crude oil | [81, 82] | |
| Homo-γ-linolenic acid | — | [82] | |
| Lanosterol | Crude oil | [81, 82] | |
| Linoleic acid | Crude oil | [81, 82] | |
| Lutein ester | Hexane/acetone/ethanol | [72] | |
| Nervonic acid | Crude oil | [81, 82] | |
| Octadecanoic acid | Crude oil | [81, 82] | |
| Oleic acid | Crude oil | [81, 82] | |
| Palmitoleic acid | Crude oil | [81, 82] | |
| Phytoene | Crude oil | [81,82] | |
| Phytofluene | Crude oil | [81,82] | |
| Stigmasterol | Crude oil | [81, 82] | |
| Tetradecanoic acid | Crude oil | [81, 82] | |
| Tetracosanoic acid | Crude oil | [81, 82] | |
| α-Linolenic acid | Crude oil | [81, 82] | |
| α-Tocopherol | Crude oil | [81, 82] | |
| β-Carotene | Crude oil | [81, 82] | |
| β-Sitosterol | Crude oil | [81, 82] | |
| β-Tocopherol | Crude oil | [81, 82] | |
| γ-Linolenic acid | Crude oil | [81, 82] | |
| γ-Tocopherol | — | [82] | |
| δ-Tocopherol | Ethanol/ethyl acetate | [78] | |
|
| |||
| Roots | (S)-4-Iodo-1,2-epoxybutane | — | [71] |
| 1,1,1,5,7,7,7-Heptamethyl-3,3 bis(trimethylsiloxy) tetrasiloxane | — | [71] | |
| 1,2,3-Tri(t-butyl)cyclopropenylium tribromide | — | [71] | |
| 1,2-Benzenedicarboxylic acid | — | [71] | |
| 3,3-Dimethyl-hexane | — | [71] | |
| 3,3-Dimethyl-octane | — | [71] | |
| 3α-Tigloylnxytropane | Ethanol | [69] | |
| 3β-Acetoxytropane | Ethanol | [69] | |
| Cuscohygrine | Ethanol | [69] | |
| Diethyl ester | — | [71] | |
| Dimethyl-flubendazole | |||
| Docosane | — | [71] | |
| Eicosamethylcyclodecasiloxane | — | [71] | |
| Hygrine | Ethanol | [69] | |
| N-Methylpyrrolidinylhygrine A | Ethanol | [69] | |
| N-Methylpyrrolidinylhygrine B | Ethanol | [69] | |
| Physoperuvine | Ethanol | [69] | |
| Tropine | Ethanol | [69] | |
| Dimethyl-flubendazole | — | [71] | |
|
| |||
| Seeds | (S)-4-Iodo-1,2-epoxybutane | — | [71] |
| 1,1,1,5,7,7,7-Heptamethyl-3,3 bis(trimethylsiloxy)tetrasiloxane | — | [71] | |
| 1,2-Benzenedicarboxylic acid | — | [71] | |
| 3,3-Dimethyl-hexane | — | [71] | |
| 3,3-Dimethyl-octane | — | [71] | |
| 1,2,3-Tri(t-butyl) cyclopropenylium tribromide | Methanol | [88] | |
| Caffeic acid | Methanol | [88] | |
| Diethyl ester | — | [71] | |
| Diethylene glycol | Methanol | [88] | |
| Docosane | — | [77] | |
| Eicosamethyl cyclodecasiloxane | — | [71] | |
| Octadecanoic acid | Methanol | [88] | |
Different parts of P. peruviana contain terpenes, and polyphenols represent the main two classes of identified phytoconstituents. They represent 26.09% and 14.94%, respectively. In the terpenes category, carotenoids are the most representative (11.15%), followed by monoterpenes (8.76%), sesquiterpenes (5.57%), and diterpenes (3.18%). A considerable amount of sesquiterpenes (22.3%) and fatty acids (22.8%) has been found in P. angulata, a Physalis species close to P. peruviana, as volatile components of leaf essential oil [91]. However, phytol (17.88%) was the most diterpenes found in ethanolic extracts of leaves, roots, and fruits of P. minima, beyond other phytoconstituents, including fatty acids [92]. According to our results, phytol was identified right now, only in calyces and leaves of P. peruviana.
The presence of phytoene can justify the richness of the plant in carotenoids. Therefore, phytoene is an alkene hydrocarbon with 40 carbon atoms intermediate in the biosynthesis of carotenoids. The synthesis of phytoene is necessary for that of carotenoids in plants. The biosynthetic pathway from phytoene to violaxanthin is common to the genus Physalis [70]. Furthermore, carotenoid pigments from different species of the Physalis genus are primarily used in the food industry as food dyes for fats and oils. Their seeds can contain up to 30% fatty oil [93]. The presence of carotenoids in the Physalis genus has been confirmed by Ramadan [94]. All-trans-β-carotene, 9-cis-β-carotene, and all-trans-α-cryptoxanthin were the primary carotenoids found in the fruits.
Referring to phenolic compounds, flavonoids are the most phytoconstituents found (5.17%) in the plant than cinnamic acid derivatives (3.98%), monophenolic compounds (1.79%), phenolic acids (1.39%), coumarins (0.79%), phenolic esters (0.79%), chalcones (0.39%), phenolic aldehydes (0.39%), and stilbenes (0.19%). Similarly, phenolic, flavonoid, and phenolic acid contents were identified and quantified in different parts of five members of the Physalis genus including P. angulate, P. patula, P. subulata, P. solanacea, and P. hederifolia. However, quercetin, kaempferol, and phenolic acids were identified as the major phenolic phytoconstituents in those five plant species, in different concentrations according to organs [95]. Overall, monophenolic and polyphenolic compounds are synthesized and then accumulated in all plant tissues, but their concentration can be varied from different parts. Among phenolic compounds, phenolic acids and flavonoids are the most studied, mainly pharmacological properties exploited for medical purposes [96]. Gupta et al. [97] noted the strong influence of phenolic compounds and the carotenoid content with bioactivity.
The plant also contains fatty acids, which are the most cited in the literature. For example, hexadecanoic acid (palmitic acid) was the most cited, five times (0.82%), followed by decanoic acid, linoleic acid, and octadecanoic acid, which were mentioned four times (0.66%). Hexadecanoic acid (palmitic acid) is the most common saturated fatty acid in plants, animals, and microorganisms, and linoleic acid is central in plant lipids. It is essential for humans (animals) because it is derived mainly from dietary plant oils [98].
Beyond the sucrose esters identified in plants (2.58%), others such as peruvioses A, B, C, D, and F had already been isolated before in the dichloromethane extract of the sticky exudate that covers the fruit [99, 100]. Nicandroses, other sucrose esters, have been isolated in the Physalis genus. Their presence is confirmed in different species including P. nicandroides var. attenuata, P. solanaceus, P. sordida, and P. viscosa [5].
Steroids and withanolides (a group of naturally occurring polyoxygenated steroidal lactones) were also identified in the plant and represented 6.97%. Physalins (steroidal constituents) are the most active representatives of secondary metabolites of the genus [101]. Most withanolide compounds are produced by Solanaceae plants, in particular 19 genera of Solanaceae, including Acnistus, Datura, Deprea, Dunalis, Discopodium, Exodeconus, Hyoscyamus, Iochroma, Jaborosa, Larnax, Lycium, Nicandra, Physalis, Salpichroa, Trechonaetes, Tubocapsicum, Vassobia, Withania, and Witheringia [102, 103]. Nowadays, several withanolides have been isolated and characterized from different parts of P. peruviana, including dihydrowithaferins, physachenolides, physacoztolides, perulactones, withaperuvins, alkekenginins, withaferins, hydroxy-withanolides, physagulins, withaperuvins, physalolactones, withalongolide, physapubescins, withaphysanolides, viscosalactones, and phyperunolides [5, 8]. Almost 351 withanolides have been identified and isolated from the Physalis genus, mainly from P. peruviana and P. angulata [104].
Steroids such as ergosterol, campesterol, stigmasterol, lanosterol, ß-sitosterol, Δ5-avenasterol, and Δ7-avenasterol have been reported in P. peruviana pomace and fruit juice. A number of the vitamins have been identified primarily in pomace and fruits, including 1,25-dihydroxy vitamin D2 (derived from vitamin D), vitamin B9 (folic acid), vitamin K, vitamin E (α,β,γ,δ-tocopherols), and biotin. A study on the phytochemical composition of goldenberry pomace confirmed the presence of those vitamins. In addition to vitamins A, D, and K, niacin, riboflavin, thiamin, pyridoxine, vitamin B12, choline chloride, and p-aminobenzoic acid have been identified and quantified [105, 106].
Among ten alkaloids identified in the plant, cuscohygrine was subsequently isolated from the roots [107], and physoperuvine has already been isolated from P. peruviana roots [108]. The other alkaloids have been explicitly isolated in the aerial and roots. They are the only parts of plants where alkaloids were identified.
4. Conclusion
P. peruviana plays a significant role in managing various pathologies of different organ systems, but its ethnotherapeutic use is strongly limited to a few countries. The plant is very rich in compounds, considering the number of identified compounds. Regarding phytochemical profiling, effort must be directed towards isolating and characterizing more compounds, particularly those that can present a significant therapeutic interest via extensive pharmacological investigations.
5. Disclosure
This study is part of the Ph.D. training of FMK. The funding agent had no role in the study design, data collection, data analysis, and writing of the present manuscript.
Acknowledgments
The authors thank the Pharm-Biotechnology and Traditional Medicine Center of Excellence (PHARMBIOTRAC) and Mbarara University of Science and Technology (MUST) for their support during the work. The authors are also grateful to The World Academic of Sciences (TWAS) for providing research scholarship to FMK. The World Bank funds the training through Pharm-Bio Technology and Traditional Center (PHARMBIOTRAC)/Africa Centre of Excellence, Mbarara University of Science and Technology.
Data Availability
All relevant data are presented in the manuscript. However, any required further information can be provided by the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Tugume P., Nyakoojo C. Ethno-pharmacological survey of herbal remedies used in the treatment of paediatric diseases in Buhunga parish, Rukungiri District, Uganda. BMC Complementary and Alternative Medicine . 2019;19(1):p. 353. doi: 10.1186/s12906-019-2763-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuan H., Ma Q., Ye L., Piao G. The traditional medicine and modern medicine from natural products. Molecules . 2016;21(559) doi: 10.3390/molecules21050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghosh D. Quality issues of herbal medicines: internal and external factors. International Journal of Complementary and Alternative Medicine . 2018;11(1):67–69. doi: 10.15406/ijcam.2018.11.00350. [DOI] [Google Scholar]
- 4.Akinyemi O., Oyewole S., Jimoh K. Medicinal plants and sustainable human health: a review. Horticulture International Journal . 2018;2(4):194–195. [Google Scholar]
- 5.Zhang W.-N., Tong W.-Y. Chemical constituents and biological activities of plants from the Genus Physalis. Chemistry and Biodiversity . 2016;13(1):48–65. doi: 10.1002/cbdv.201400435. [DOI] [PubMed] [Google Scholar]
- 6.Singh N., Singh S., Maurya P., et al. An updated review on Physalis peruviana fruit: cultivational, nutraceutical and pharmaceutical aspects. Indian Journal of Natural Products and Resources . 2019;10(2):97–110. [Google Scholar]
- 7.Popova V., Stoyanova A., Mazova N. Phytochemical composition and biological activity of Physalis spp.: a mini-review. Food Science and Applied Biotechnology . 2020;3(1):56–70. doi: 10.30721/fsab2020.v3.i1.80. [DOI] [Google Scholar]
- 8.Kasali F. M., Tuyiringire N., Peter E. L., et al. Chemical constituents and evidence-based pharmacological properties of Physalis peruviana L .: an overview. Journal of Herbmed Pharmacology . 2021;10(4) [Google Scholar]
- 9.Mbaveng A. T., Manekeng H. T., Nguenang G. S., Dzotam J. K., Kuete V., Efferth T. Cytotoxicity of 18 Cameroonian medicinal plants against drug sensitive and multi-factorial drug resistant cancer cells. Journal of Ethnopharmacology . 2018;222:21–33. doi: 10.1016/j.jep.2018.04.036. [DOI] [PubMed] [Google Scholar]
- 10.Yemele M. D., Telefo P. B., Lienou L. L., et al. Ethnobotanical survey of medicinal plants used for pregnant women׳s health conditions in Menoua division-West Cameroon. Journal of Ethnopharmacology . 2015;160:14–31. doi: 10.1016/j.jep.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 11.Tchuenguem R. T., Kechia F. A., Kuiate J. R., Dzoyem J. P. Ethnopharmacological survey, antioxidant and antifungal activity of medicinal plants traditionally used in Baham locality (Cameroon) to treat fungal infections. Archives of Medical and Biomedical Research . 2017;3(2):91–103. doi: 10.4314/ambr.v3i2.5. [DOI] [Google Scholar]
- 12.González J. Y. T. Traditional use of medicinal plants in the Sidewalk San Isidro, municipality of San Jose de Pare-Boyacá: a preliminary study using. Acta Biológica Colombiana . 2006;11(2):137–146. [Google Scholar]
- 13.Lagos-López M. Estudio etnobotánico de especies vegetales con propiedades medicinales en seis municipios de Boyacá, Colombia. Actualidades Biológicas . 2007;29(86):87–96. [Google Scholar]
- 14.Tene V., Malagón O., Finzi P. V., Vidari G., Armijos C., Zaragoza T. An ethnobotanical survey of medicinal plants used in Loja and Zamora-Chinchipe, Ecuador. Journal of Ethnopharmacology . 2007;111(1):63–81. doi: 10.1016/j.jep.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 15.Manya M. H., Keymeulen F., Ngezahayo J., et al. Antimalarial herbal remedies of Bukavu and Uvira areas in DR Congo: an ethnobotanical survey. Journal of Ethnopharmacology . 2020;249 doi: 10.1016/j.jep.2019.112422.112422 [DOI] [PubMed] [Google Scholar]
- 16.Kasali M. F., Mahano A. O., Bwironde F. M., et al. Ethnopharmacological survey of plants used against diabetes in Bukavu city (D.R. Congo) 2013;119:538–546. [Google Scholar]
- 17.Chifundera K. Contribution to the inventory of medicinal plants from the bushi area, south kivu province, democratic republic of Congo. Fitoterapia . 2001;72(4):351–368. doi: 10.1016/s0367-326x(00)00294-x. [DOI] [PubMed] [Google Scholar]
- 18.Murtem G., Chaudhry P. An Ethnobotanical study of medicinal plants used by the tribes in upper Subansiri district of Arunachal Pradesh, India. American Journal of Ethnomedicine . 2016;3(3):35–49. doi: 10.21472/bjbs.030506. [DOI] [Google Scholar]
- 19.Mistry J. Traditional medicinal plants used by local people of Murshidabad district, West Bengal, India. World Journal of Pharmacy and Pharmaceutical Sciences . 2015;4(09):1225–1234. [Google Scholar]
- 20.Karemore M. N., Avari J. G. Herbal medicines used during pregnancy, childbirth and postpartum care. International Journal of Pharma Sciences and Research . 2017;8(12):5326–5335. [Google Scholar]
- 21.Barua I., Sonowal R. Indigenous herbal medicine among the Sonowal Kachari tribe : a study in a forest village in Dibrugarh, Assam, India. NeBIO . 2014;2(4):30–35. [Google Scholar]
- 22.Chettri D., Moktan S., Das A. Ethnobotanical studies on the tea garden workers. Pleione . 2014;8(1):124–132. [Google Scholar]
- 23.Sarvalingam A., Dhaarani V., Pavithra C., Sharmila S., Rajendran A. Inventory and ethnomedicinal plants used by rural people of eastern Ghats of Tamil Nadu, India. Journal of Ecobiotechnology . 2017;9:5–12. [Google Scholar]
- 24.Pandey K., Sharma N. K. Traditional meditional flora of the district Ghazipur (Uttar Pradesh, India) International Journal of Ayurveda and Traditional Medicine . 2012;2(2):307–321. [Google Scholar]
- 25.Gurumayum S., Soram J. S. Some anti-diarrhoeic and anti-dysenteric ethno-medicinal plants of Mao Naga tribe community of Mao, Senapati district, Manipur. Indian Journal of Pure & Applied Biosciences . 2014;2(1):147–155. [Google Scholar]
- 26.Anjalam A., Kalpana S., Vijai D., Premalatha S. Documentation of medicinal plants used by Malayali tribes in Kolli Hills. International Journal of Advanced Research in Biological Sciences . 2016;3(3):101–107. [Google Scholar]
- 27.Thomas B., Mathews R. P., Rajendran A., Kumar P. K. Ethnobotanical observations on tribe arnatans of nilambur forest, western ghats region of Kerala, India. Research in Plant Biology . 2013;3(2):12–17. [Google Scholar]
- 28.Kapoor B., Kaur G., Gupta M., Gupta R. Indian medicinal plants useful in treatment of gout: a review for current status and future prospective. Asian Journal of Pharmaceutical and Clinical Research . 2017;10(11):p. 407. doi: 10.22159/ajpcr.2017.v10i11.20170. [DOI] [Google Scholar]
- 29.Paulsamy S., Vijayakumar K. K., Murugesan M., Padmavathy S., Senthilkumar P. Ecological status of medicinal and other economically important plants in the shola understories of Nilgiris, the Western Ghats. Natural Product Radiance . 2007;6(1):55–61. [Google Scholar]
- 30.Silalahi M., Nisyawati The ethnobotanical study of edible and medicinal plants in the home garden of Batak Karo sub-ethnic in North Sumatra, Indonesia. Biodiversitas . 2018;19(1):2085–4722. doi: 10.13057/biodiv/d190131. [DOI] [Google Scholar]
- 31.Simbolon H. Ethnobotany of people around the dolok sibual-buali nature reserve area, north sumatra, Indonesia. Tropics . 1994;4(1):69–78. doi: 10.3759/tropics.4.69. [DOI] [Google Scholar]
- 32.Oktavia A. I., Indriyani S., Indriani S., Batoro J. Ethnobotanical study of toxic plants in ngadiwono village, tosari district, pasuruan regency, east java. Jurnal Pembangunan dan Alam Lestari . 2017;8(2):83–88. doi: 10.21776/ub.jpal.2017.008.02.04. [DOI] [Google Scholar]
- 33.Wanzala W., Takken W., Mukabana W. R., Pala A. O., Hassanali A. Ethnoknowledge of Bukusu community on livestock tick prevention and control in Bungoma district, Western Kenya. Journal of Ethnopharmacology . 2012;140(2):298–324. doi: 10.1016/j.jep.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 34.Mukungu N., Abuga K., Okalebo F., Ingwela R., Mwangi J. Medicinal plants used for management of malaria among the Luhya community of Kakamega East sub-County, Kenya. Journal of Ethnopharmacology . 2016;194:98–107. doi: 10.1016/j.jep.2016.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Njoroge G. N., Bussmann R. W. Ethnotherapeutic management of sexually transmitted diseases (STDs) and reproductive health conditions in central province of Kenya. Indian Journal of Traditional Knowledge . 2009;8(2):255–261. [Google Scholar]
- 36.Njoroge N. G., Bussmann W. R., Gemmill B., Newton L. E., Ngumi V. W. Utilisation of weed species as sources of traditional medicines in Central Kenya. Lyonia . 2004;7(2):71–87. [Google Scholar]
- 37.Njoroge G. N., Kibunga J. W. Herbal medicine acceptance, sources and utilization for diarrhoea management in a cosmopolitan urban area (Thika, Kenya) African Journal of Ecology . 2007;45(SUPPL. 1):65–70. [Google Scholar]
- 38.Maobe M. A. G., Gitu L., Gatebe E., et al. Antifungal activity of eight selected medicinal herbs used for the treatment of diabetes, malaria and pneumonia in Kisii Region, Southwest Kenya. World Journal of Medical Sciences . 2013;8(1):74–78. [Google Scholar]
- 39.Ndegwa F. K., Kondam C., Ghose D., et al. Kenyan traditional medicine : Exploring old solutions to the modern antibacterial crises through natural products chemistry. 2021. https://www.biorxiv.org/content/10.1101/2021.03.26.436821v1 .
- 40.Rai S. K. Medicinal plants used by Meche people of Jhapa district, Eastern Nepal. Our Nature . 2006;2:27–32. [Google Scholar]
- 41.Acharya (Siwakoti E., Pokhrel B. Ethno-medicinal plants used by bantar of bhaudaha, morang, Nepal. Our Nature . 1970;4(1):96–103. [Google Scholar]
- 42.Paudel N., Chandra D., Dev B. Some medicinal plants uses in ethnical group from Biratnagar, Eastern, Nepal. American Academic Scientific Research Journal for Engineering, Technology, and Sciences . 2018;41(1):233–239. [Google Scholar]
- 43.Holdsworth D. High altitude medicinal plants of Papua New Guinea. International Journal of Crude Drug Research . 1989;27(2):95–100. [Google Scholar]
- 44.Chagnon M. Inventaire pharmacologique general des plantes medicinales rwandaises. Journal of Ethnopharmacology . 1984;12(3):239–251. doi: 10.1016/0378-8741(84)90053-9. [DOI] [PubMed] [Google Scholar]
- 45.Bisi-Johnson M. A., Obi C. L., Kambizi L., Nkomo M. A survey of indigenous herbal diarrhoeal remedies of O.R . Tambo district, Eastern Cape province, South Africa. African Journal of Biotechnology . 2010;9(8):1245–1254. [Google Scholar]
- 46.Madikizela B., Ndhlala A. R., Finnie J. F., Van Staden J. Ethnopharmacological study of plants from Pondoland used against diarrhoea. Journal of Ethnopharmacology . 2012;141(1):61–71. doi: 10.1016/j.jep.2012.01.053. [DOI] [PubMed] [Google Scholar]
- 47.Fouche G., Cragg G. M., Pillay P., Kolesnikova N., Maharaj V. J., Senabe J. In vitro anticancer screening of South African plants. Journal of Ethnopharmacology . 2008;119(3):455–461. doi: 10.1016/j.jep.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 48.Nondo R. S. O., Zofou D., Moshi M. J., et al. Ethnobotanical survey and in vitro antiplasmodial activity of medicinal plants used to treat malaria in Kagera and Lindi regions, Tanzania. Journal of Medicinal Plants Research . 2015;9(6):179–192. [Google Scholar]
- 49.Runyoro D. K. B., Ngassapa O. D., Matee M. I. N., Joseph C. C., Moshi M. J. Medicinal plants used by Tanzanian traditional healers in the management of Candida infections. Journal of Ethnopharmacology . 2006;106(2):158–165. doi: 10.1016/j.jep.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 50.Moshi M. J., Otieno D. F., Weisheit A. Ethnomedicine of the Kagera Region, north western Tanzania. Part 3: plants used in traditional medicine in Kikuku village, Muleba District. Journal of Ethnobiology and Ethnomedicine . 2012;8(14):p. 14. doi: 10.1186/1746-4269-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Musinguzi E., Kikafunda J., Kiremire B. Utilization of indigenous food plants in Uganda - a case study of South - western Uganda. African Journal of Food, Agriculture, Nutrition and Development . 2006;6(2):1–21. [Google Scholar]
- 52.Namukobe J., Kasenene J. M., Kiremire B. T., et al. Traditional plants used for medicinal purposes by local communities around the Northern sector of Kibale National Park, Uganda. Journal of Ethnopharmacology . 2011;136(1):236–245. doi: 10.1016/j.jep.2011.04.044. [DOI] [PubMed] [Google Scholar]
- 53.Muwanguzi P. A. Plants and Wound Healing in Uganda: A Mixed Methods Study . Leeds, UK: The University of Leeds; 2012. [Google Scholar]
- 54.Kamatenesi-Mugisha M., Oryem-Origa H. Medicinal plants used to induce labour during childbirth in western Uganda. Journal of Ethnopharmacology . 2007;109(1):1–9. doi: 10.1016/j.jep.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 55.Tabuti J. R., Dhillion S. S., Lye K. A. Ethnoveterinary medicines for cattle (Bos indicus) in Bulamogi county, Uganda: plant species and mode of use. Journal of Ethnopharmacology . 2003;88(2–3):279–286. doi: 10.1016/s0378-8741(03)00265-4. [DOI] [PubMed] [Google Scholar]
- 56.Adia M. M., Anywar G., Byamukama R., et al. Medicinal plants used in malaria treatment by Prometra herbalists in Uganda. Journal of Ethnopharmacology . 2014;155(1):580–588. doi: 10.1016/j.jep.2014.05.060. [DOI] [PubMed] [Google Scholar]
- 57.Walugembe J., Iramiot J. S., Katuura E. Indigenous knowledge and antibacterial activity of selected herbs used locally to treat common cold in Central Uganda. Journal of Medicinal Plants Research . 2016;10(31):520–528. [Google Scholar]
- 58.Tugume P., Kakudidi E. K., Buyinza M., et al. Ethnobotanical survey of medicinal plant species used by communities around Mabira Central Forest Reserve, Uganda. Journal of Ethnobiology and Ethnomedicine . 2016;12(5):p. 5. doi: 10.1186/s13002-015-0077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nyamukuru A., Tabuti J. R. S., Lamorde M., Kato B., Sekagya Y., Aduma P. R. Medicinal plants and traditional treatment practices used in the management of HIV/AIDS clients in Mpigi District, Uganda. Journal of Herbal Medicine . 2017;7:51–58. doi: 10.1016/j.hermed.2016.10.001. [DOI] [Google Scholar]
- 60.Hamill F. A., Apio S., Mubiru N. K., et al. Traditional herbal drugs of southern Uganda, I. Journal of Ethnopharmacology . 2000;70(3):281–300. doi: 10.1016/s0378-8741(00)00180-x. [DOI] [PubMed] [Google Scholar]
- 61.García-arias F. L., Osorio-guarín J. A., Zarantes V. M. N. Association study reveals novel genes related to yield and quality of fruit in cape gooseberry (Physalis peruviana L.) Frontiers in Pharmacology . 2018;9:362. doi: 10.3389/fpls.2018.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bharthi V., Reddy P., Tr S., Venkateshwarlu G. Phytochemical evaluation and powder microscopy of medicinal and nutritional fruits of Physalis peruviana L. International Journal of Herbal Medicine . 2016;4(1):43–46. [Google Scholar]
- 63.Barirega A. Potential for value chain improvement and commercialization of cape gooseberry (Physalis peruviana L.) for livelihood improvement in Uganda. Ethnobotany Research and Applications . 2014;12(1):131–140. doi: 10.17348/era.12.0.525-533. [DOI] [Google Scholar]
- 64.Mahomoodally M. F., Mootoosamy A., Wambugu S. Traditional therapies used to manage diabetes and related complications in Mauritius: a comparative ethnoreligious study. Evidence-based Complementary and Alternative Medicine . 2016;2016:25. doi: 10.1155/2016/4523828.4523828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mahady G. Medicinal plants for the prevention and treatment of bacterial infections. Current Pharmaceutical Design . 2005;11(19):2405–2427. doi: 10.2174/1381612054367481. [DOI] [PubMed] [Google Scholar]
- 66.Zhang J., Xie Z., Zhang N., Zhong J. Nanosuspension Drug Delivery System: Preparation, Characterization, Postproduction Processing, Dosage Form, and Application . Amsterdam, Netherlands: Elsevier; 2017. [Google Scholar]
- 67.Sofowora A., Ogunbodede E., Onayade A. The role and place of medicinal plants in the strategies for disease prevention. African Journal of Traditional, Complementary, and Alternative Medicines : AJTCAM . 2013;10(5):210–229. doi: 10.4314/ajtcam.v10i5.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eisenman S. W., Tucker A. O., Struwe L. Voucher specimens are essential for documenting source material used in medicinal plant investigations. Journal of Medicinal Plants Research . 2012;1(1):30–43. [Google Scholar]
- 69.Kubwabo C., Rollmann B., Tilquin B. Analysis of alkaloids from Physalis peruviana by capillary GC, capillary GC-MS, and GC-FTIR. Planta Medica . 1993;59(2):161–163. doi: 10.1055/s-2006-959634. [DOI] [PubMed] [Google Scholar]
- 70.Yu Y., Chen X., Zheng Q. Metabolomic profiling of carotenoid constituents in Physalis peruviana during different growth stages by LC‐MS/MS Technology. Journal of Food Science . 2019;84(12):3608–3613. doi: 10.1111/1750-3841.14916. [DOI] [PubMed] [Google Scholar]
- 71.Ertürk Ö., Çol Ayvaz M., Can Z., Karaman Ü., Korkmaz K. Antioxidant, antimicrobial activities and phenolic and chemical contents of Physalis peruviana L. from Trabzon, Turkey. Indian Journal of Pharmaceutical Education and Research . 2017;51(3):S213–S216. doi: 10.5530/ijper.51.3s.15. [DOI] [Google Scholar]
- 72.Etzbach L., Pfeiffer A., Weber F., Schieber A. Characterization of carotenoid profiles in goldenberry (Physalis peruviana L.) fruits at various ripening stages and in different plant tissues by HPLC-DAD-APCI-MS. Food Chemistry . 2018;245:508–517. doi: 10.1016/j.foodchem.2017.10.120. [DOI] [PubMed] [Google Scholar]
- 73.Abou Baker D. H., Rady H. M. Bioassay-guided approach employed to isolate and identify anticancer compounds from Physalis peruviana calyces. Plant Archery . 2020;20(1):3285–3291. [Google Scholar]
- 74.Ballesteros-Vivas D., Álvarez-Rivera G., del Pilar Sánchez-Camargo A., Ibáñez E., Parada-Alfonso F., Cifuentes A. A multi-analytical platform based on pressurized-liquid extraction, in vitro assays and liquid chromatography/gas chromatography coupled to high resolution mass spectrometry for food by-products valorisation. Part 1: withanolides-rich fractions from goldenberry (Physalis peruviana L.) calyces obtained after extraction optimization as case study. Journal of Chromatography A . 2019;1584:155–164. doi: 10.1016/j.chroma.2018.11.055. [DOI] [PubMed] [Google Scholar]
- 75.Wahdan O. A., Badr S. E.-S. A., Abdelfattah M. S. Phytochemical analysis, antibacterial and anticancer activities of the Physalis peruviana calyces growing in Egypt. Food and Nutrition . 2017;4:197. [Google Scholar]
- 76.Medina S., Collado-González J., Ferreres F., et al. Potential of Physalis peruviana calyces as a low-cost valuable resource of phytoprostanes and phenolic compounds. Journal of the Science of Food and Agriculture . 2019;99(5):2194–2204. doi: 10.1002/jsfa.9413. [DOI] [PubMed] [Google Scholar]
- 77.Yilmaztekin M. Analysis of volatile components of cape gooseberry (Physalis peruviana L.) grown in Turkey by HS-SPME and GC-MS. The Scientific World JOURNAL . 2014;2014:8. doi: 10.1155/2014/796097.796097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Al-Olayan E. M., El-Khadragy M. F., Aref A. M., Othman M. S., Kassab R. B., Moneim A. E. A. The potential protective effect of Physalis peruviana L. against carbon tetrachloride-induced hepatotoxicity in rats is mediated by suppression of oxidative stress and downregulation of MMP-9 expression. Oxidative Medicine and Cellular Longevity . 2014;2014:12. doi: 10.1155/2014/381413.381413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mayorga H., Knapp H., Winterhalter P., Duque C. Glycosidically bound flavor compounds of cape gooseberry (Physalis peruviana L.) Journal of Agricultural and Food Chemistry . 2001;49(4):1904–1908. doi: 10.1021/jf0011743. [DOI] [PubMed] [Google Scholar]
- 80.Majcher M. A., Scheibe M., Jeleń H. H. Identification of odor active compounds in Physalis peruviana L. Molecules . 2020;25:245. doi: 10.3390/molecules25020245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ramadan M. F., Mörsel J.-T. Oil goldenberry (Physalis peruviana L.) Journal of Agricultural and Food Chemistry . 2003;51(4):969–974. doi: 10.1021/jf020778z. [DOI] [PubMed] [Google Scholar]
- 82.Ramadan M. F., Moersel J. T. Impact of enzymatic treatment on chemical composition, physicochemical properties and radical scavenging activity of goldenberry (Physalis peruviana L.) juice. Journal of the Science of Food and Agriculture . 2007;87(3):452–460. doi: 10.1002/jsfa.2728. [DOI] [Google Scholar]
- 83.Ramadan M., El-Ghorab A., Ghanem K. Volatile compounds, antioxidants, and anticancer activities of Cape gooseberry fruit (Physalis peruviana L.): an in-vitro study. Journal of The Arab Society for Medical Research . 2015;10(2):56–64. doi: 10.4103/1687-4293.175556. [DOI] [Google Scholar]
- 84.Dymerski T., Namieśnik J., Leontowicz H., et al. Chemistry and biological properties of berry volatiles by two-dimensional chromatography, fluorescence and Fourier transform infrared spectroscopy techniques. Food Research International . 2016;83:74–86. doi: 10.1016/j.foodres.2016.02.017. [DOI] [Google Scholar]
- 85.El-beltagi H. S., Mohamed H. I., Safwat G., Gamal M., Megahed B. M. H. Chemical Composition and Biological Activity of Physalis Peruviana L . Germany: Gesunde Pflanz.; 2019. [Google Scholar]
- 86.Peter K. K., Zipporah N., Francis M. N., John T. In vitro antiplasmodial, cytotoxicity assay and partial chemical characterization of Kenyan Physalis peruviana L. (Solanaceae family) extracts. Journal of Medicinal Plants Research . 2020;14(2):73–80. doi: 10.5897/jmpr2019.6882. [DOI] [Google Scholar]
- 87.Mier-Giraldo H., Díaz-Barrera L. E., Delgado-Murcia L. G., Valero-Valdivieso M. F., Cáez-Ramírez G. Cytotoxic and immunomodulatory potential activity of Physalis peruviana fruit extracts on cervical cancer (HeLa) and fibroblast (L929) cells. Journal of Evidence-Based Complementary & Alternative Medicine . 2017;22(4):777–787. doi: 10.1177/2156587217718751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Darwish A. G., Shaker E. S. Physalis peruviana juice and seeds methanolic extracts; Gas chromatography mass spectrometry; antioxidant and anticancer against human A549, HepG2. 2021;17(1–5) doi: 10.4103/pm.pm_262_20. [DOI] [Google Scholar]
- 89.Rodrigues E., Rockenbach I. I., Cataneo C., Gonzaga L. V., Chaves E. S., Fett R. Minerals and essential fatty acids of the exotic fruit Physalis peruviana L. Ciência e Tecnologia de Alimentos . 2009;29(3):642–645. doi: 10.1590/s0101-20612009000300029. [DOI] [Google Scholar]
- 90.Fukushima A., Nakamura M., Suzuki H., et al. Comparative characterization of the leaf tissue of Physalis alkekengi and Physalis peruviana using RNA-seq and metabolite profiling. Frontiers of Plant Science . 2016;7(1883) doi: 10.3389/fpls.2016.01883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ogundajo A. L., Akpome A. S., Tijani N. A., Ogunwande I. A. Chemical constituents of the leaf essential oil of Physalis angulata L. Asian Journal of Applied Sciences . 2015;03(04):652–655. [Google Scholar]
- 92.Usaizan N., Abdullah N. A. P., Ahmad S. H., Saleh G. Preliminary phytochemical screening and GC-MS analysis of ethanol extract of Physalis Minima L.(Solanaceae) Journal of Advanced Agricultural Technologies . 2014;1(2):100–103. doi: 10.12720/joaat.1.2.100-103. [DOI] [Google Scholar]
- 93.Asilbekova D. T., Ul’chenko N. T., Glushenkova A. I. Lipids from Physalis alkekengi. Chemistry of Natural Compounds . 2016;52(1):96–97. doi: 10.1007/s10600-016-1556-0. [DOI] [Google Scholar]
- 94.Ramadan M. F. Bioactive phytochemicals, nutritional value, and functional properties of cape gooseberry (Physalis peruviana): an overview. Food Research International . 2011;44(7):1830–1836. doi: 10.1016/j.foodres.2010.12.042. [DOI] [Google Scholar]
- 95.Medina-Medrano J. R., Almaraz-Abarca N., González-Elizondo M. S., Uribe-Soto J. N., González-Valdez L. S., Herrera-Arrieta Y. Phenolic constituents and antioxidant properties of five wild species of Physalis (Solanaceae) Botanische Studien . 2015;56(24) doi: 10.1186/s40529-015-0101-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang Q., Cui H. Simultaneous determination of quercetin, kaempferol, and isorhamnetin in phytopharma-ceuticals ofHippophae rhamnoides L. by high-performance liquid chromatography with chemiluminescence detection. Journal of Separation Science . 2005;28(11):1171–1178. doi: 10.1002/jssc.200500055. [DOI] [PubMed] [Google Scholar]
- 97.Gupta A. K., Rather M. A., Kumar Jha A., et al. Artocarpus lakoocha Roxb. and Artocarpus heterophyllus Lam. flowers: New sources of bioactive compounds. Plants . 2020;9(10):p. 1329. doi: 10.3390/plants9101329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rustan A. C., Drevon C. A. Fatty acids: structures and properties. “Encyclopedia of Life Sciences . 2005:1–7. doi: 10.1038/npg.els.0003894. [DOI] [Google Scholar]
- 99.Bernal C.-A., Castellanos L., Aragón D. M., et al. Peruvioses A to F, sucrose esters from the exudate of Physalis peruviana fruit as α-amylase inhibitors. Carbohydrate Research . 2018;461:4–10. doi: 10.1016/j.carres.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 100.Franco L., Ocampo Y., Gómez H., De la Puerta R., Espartero J., Ospina L. Sucrose esters from Physalis peruviana calyces with anti-inflammatory activity. Planta Medica . 2014;80(17):1605–1614. doi: 10.1055/s-0034-1383192. [DOI] [PubMed] [Google Scholar]
- 101.Laczkó-Zöld E., Forgó P., Zupkó I., Sigrid E., Hohmann J. Isolation and quantitative analysis of physalin D in the fruit and calyx of Physalis alkekengi L. Acta Biologica Hungarica . 2017;68(3):300–309. doi: 10.1556/018.68.2017.3.7. [DOI] [PubMed] [Google Scholar]
- 102.Veleiro A. S., Oberti J. C., Burton G. Chemistry and bioactivity of withanolides from south american Solanaceae. Bioactive Natural Products (Part L) . 2005;32:1019–1052. doi: 10.1016/s1572-5995(05)80072-9. [DOI] [Google Scholar]
- 103.Chen L.-X., He H., Qiu F. Natural withanolides: an overview. Natural Product Reports . 2011;28(4) doi: 10.1039/c0np00045k. [DOI] [PubMed] [Google Scholar]
- 104.Huang M., He J. X., Hu H. X., et al. Withanolides from the genus Physalis: a review on their phytochemical and pharmacological aspects. Journal of Pharmacy and Pharmacology . 2019;(–21):p. 1. doi: 10.1111/jphp.13209. [DOI] [PubMed] [Google Scholar]
- 105.Ramadan M. F. Physalis peruviana pomace suppresses highcholesterol diet-induced hypercholesterolemia in rats. Grasas Y Aceites . 2012;63(4):411–422. doi: 10.3989/gya.047412. [DOI] [Google Scholar]
- 106.Ramadan M. F., Hassan N. A., Elsanhoty R. M., Sitohy M. Z. Goldenberry (Physalis peruviana) juice rich in health-beneficial compounds suppresses high-cholesterol diet-induced hypercholesterolemia in Rats. Journal of Food Biochemistry . 2013;37(6):708–722. doi: 10.1111/j.1745-4514.2012.00669.x. [DOI] [Google Scholar]
- 107.El-Gengaihi S. E., Hassan E. E., Hamed M. A., Zahran H. G., Mohammed M. A. Chemical composition and biological evaluation ofPhysalis peruvianaRoot as hepato-renal protective agent. Journal of Dietary Supplements . 2013;10(1):39–53. doi: 10.3109/19390211.2012.760509. [DOI] [PubMed] [Google Scholar]
- 108.Sahai M., Ray A. B. Secotropane alkaloids of Physalis peruviana. Journal of Organic Chemistry . 1980;45(16):3265–3268. doi: 10.1021/jo01304a024. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are presented in the manuscript. However, any required further information can be provided by the corresponding author upon request.


