To the Editor:
The recent article by Hagin et al1 reports that most patients with inborn errors of immunity (IEI) generate humoral and cellular immune responses to the Pfizer-BioNTech COVID-19 vaccine. Neutralizing anti–receptor-binding domain (RBD) antibodies, RBD-specific B cells of the IgG+ and IgA+ isotype, and T cells producing IL-2 and IFN-γ were detected in most vaccinated patients.
Hagin et al1 conclude that patients with IEI should be vaccinated because most of them are able to generate protective responses. Although we completely agree on the necessity of vaccinating patients with IEI, it is also indispensable to correctly evaluate the establishment and duration of protective immunity in this group of patients.
We conducted a similar study in a cohort of 33 patients with common variable immunodeficiency (CVID). We evaluated the level of SARS-CoV-2–specific serum antibodies and frequency of memory B cells (MBCs) following administration of the Pfizer-BioNTech vaccine. Only 33% of our patients with CVID showed an antibody response, compared with 85.7% of the patients (12 of 14) reported by Hagin et al (see Fig 2, A in Hagin et al1). Hagin et al1 also measured RBD-specific MBCs, which play a fundamental role in long-term protection when serum antibody levels decline.2 In Fig E2 of Hagin et al1 (available in the Online Repository at www.jacionline.org), the gating strategy to identify RBD-specific IgG+ and IgA+ B cells and the percentage thereof are shown. Hagin et al1 conclude that RBD-binding B cells are detected in healthy vaccinated donors and patients with CVID.
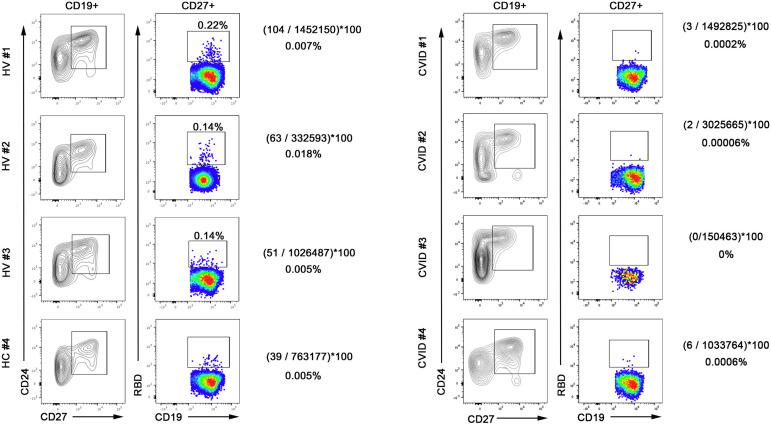
We have different results showing that healthy vaccinated donors generate RBD-specific MBCs after 2 vaccine doses, whereas patients with CVID are unable to do so (Fig 1 ).
Fig 1.
Detection of RBD-binding MBCs in healthy vaccinated donors (HVs) (left plots) and patients with CVID (right plots). MBCs, contained in the CD19 gate, were identified as CD24+CD27+. MBCs binding RBDs were detected by RBD-biotin labeled with streptavidin–fluorescein isothiocyanate. The numbers shown correspond to the RBD+ events divided by the total number of acquired live cells. HVs 1, 2, and 3 have the requisites to reach the limit of quantification (LOQ), and the percentages can be considered reliable. HV 4 is just under the limit of LOQ, but the number of events is sufficient for the limit of detection. None of the samples from patients with CVID had sufficient RBD+ events to reach either the limit of detection or the LOQ. Thus, frequencies are not reported.
Our different results might be explained by the difficulty of correctly quantifying cells present at low frequency in the sample analyzed. Flow cytometry can effectively and accurately manage extremely rare event analyses down to 10-5. In cases of rare event analysis, the nonspecific cell events can often outnumber the relevant cell frequency, making the count totally unreliable.3 Introduction of the concepts of limit of detection and limit of quantification in rare event analysis has been a remarkable advancement to ensure robust and reliable measurements of rare events. Only when the limit of quantification is achieved can the frequency be considered reliable.4 The numbers of relevant events (RBD-specific B cells in this case) should be a defined percentage of the total acquired events.
RBD+ cells are a fraction of the MBCs generated by vaccination. B cells acquire increased specificity and affinity thanks to the mechanisms of somatic mutation and selection in the germinal centers. These mechanisms are severely impaired in patients with CVID.5
Beyond the technicalities, an inaccurate evaluation of the number of specific MBCs may lead to the conclusion that patients are protected and will be able to react to a SARS-CoV-2 encounter thanks to their MBCs. In contrast, when serum titers decline, patients with CVID will be unable to produce new specific antibodies because they lack the right MBCs. Administration of mAbs may prevent severe disease and emergence of new viral variants in these cases.
Footnotes
Supported by the Italian Ministry of Health (grants RF2013-02358960 and COVID-2020-12371817).
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
References
- 1.Hagin D., Freund T., Navon M., Halperin T., Adir D., Marom R., et al. Immunogenicity of Pfizer-BioNTech COVID-19 vaccine in patients with inborn errors of immunity. J Allergy Clin Immunol. 2021;148:739–749. doi: 10.1016/j.jaci.2021.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pollard A.J., Bijker E.M. A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol. 2021;21:83–100. doi: 10.1038/s41577-020-00479-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hulspas R., O’Gorman M.R.G., Wood B.L., Gratama J.W., Sutherland D.R. Considerations for the control of background fluorescence in clinical flow cytometry. Cytometry B Clin Cytom. 2009;76:355–364. doi: 10.1002/cyto.b.20485. [DOI] [PubMed] [Google Scholar]
- 4.Illingworth A., Marinov I., Sutherland D.R., Wagner-Ballon O., DelVecchio L. ICCS/ESCCA consensus guidelines to detect GPI-deficient cells in paroxysmal nocturnal hemoglobinuria (PNH) and related disorders part 3 - data analysis, reporting and case studies. Cytometry B Clin Cytom. 2018;94:49–66. doi: 10.1002/cyto.b.21609. [DOI] [PubMed] [Google Scholar]
- 5.del Pino-Molina L., López-Granados E., Lecrevisse Q., Torres Canizales J., Pérez-Andrés M., Blanco E., et al. Dissection of the pre-germinal center B-cell maturation pathway in common variable immunodeficiency based on standardized flow cytometric euroflow tools. Front Immunol. 2021;11:3889. doi: 10.3389/fimmu.2020.603972. [DOI] [PMC free article] [PubMed] [Google Scholar]