Abstract
Ischemic stroke is a major cause of disability among adults worldwide. Despite its prevalence, few effective treatment options exist to alleviate sensory and motor dysfunctions that result from stroke. In the past, rodent models of stroke have been the primary experimental models used to develop stroke therapies. However, positive results in these studies have failed to replicate in human clinical trials, highlighting the importance of nonhuman primate (NHP) models as a preclinical step. Although there are a few NHP models of stroke, the extent of tissue damage is highly variable and dependent on surgical skill. In this study, we employed the photothrombotic stroke model in NHPs to generate controlled, reproducible ischemic lesions. Originally developed in rodents, the photothrombotic technique consists of intravenous injection of a photosensitive dye such as Rose Bengal followed by illumination of an area of interest to induce endothelial damage resulting in the formation of thrombi in the illuminated vasculature. We developed a quantitative model to predict the extent of tissue damage based on the light scattering profile of light in the cortex of NHPs. We then employed this technique in the sensorimotor cortex of two adult male Rhesus Macaques. In vivo optical coherence tomography imaging of the cortical microvasculature and subsequent histology confirmed the formation of focal cortical infarcts and demonstrated its reproducibility and ability to control the sizes and locations of light-induced ischemic lesions in the cortex of NHPs. This model has the potential to enhance our understanding of perilesional neural dynamics and can be used to develop reliable neurorehabilitative therapeutic strategies to treat stroke.
I. Introduction
Stroke is the second leading cause of death globally and the primary cause of long-term disability in the United States [1, 2]. Accounting for 80% of strokes, ischemic stroke is caused by the occlusion of a major cerebral artery resulting in irreversible tissue damage. While technological advancements have made it possible to alleviate certain post-stroke ailments, few treatment options are available to treat sensory, speech, and motor dysfunctions. To investigate the underlying mechanisms of neuroplasticity and the pathophysiology of stroke, several experimental models have been generated.
Although numerous pre-clinical rodent stroke studies have yielded positive results, the outcomes of subsequent human trials have not mirrored these results [3, 4]. Furthermore, rodent stroke models are highly variable and are often dependent on the animal strain, size, and age [5, 6]. Despite these discrepancies, few nonhuman primate (NHP) stroke models exist. Current endovascular and surgical intervention methods of producing infarcts in NHPs such as occlusion via endothelin-1 can be technically challenging and often yield inconsistent infarct sizes [7], prompting the need for a highly reproducible pre-clinical NHP stroke model.
In this study, we explore the photothrombotic stroke model in the cortex of NHPs, a model first developed for rodents by Watson and colleagues [8]. Following intravenous infusion of a photosensitive dye such as Rose Bengal, an exposed region of the brain is irradiated with a cold light source. Photo-excitation of the dye yields reactive oxygen species, endothelial damage, platelet aggregation, vascular occlusion, and downstream neuronal cell death. Thus, focal irradiation of tissue such as the NHP cortex can lead to localized, highly reproducible, and modular ischemic infarcts. By controlling the sizes of the irradiating light beam, we can induce large strokes to mimic human strokes, or induce small strokes for minimal behavioral deficits. Advantageously, smaller lesions would allow for an increase in the number of lesion replicates within a single animal subject, reducing the number of animals needed for experiments.
II. Methods
A. Quantitative Modeling
To estimate the degree of damage induced photochemically, we developed a quantitative model based on McLean’s light beam spread function to predict the scattering profile of light in cortical tissue as described previously [9]. Briefly, the light beam spread function, used to predict the scattering of a pencil beam light source in cortical tissue, was integrated temporally and convolved with the geometry of a circular light beam.
B. Experimental Procedures
All animal procedures were approved by the University of Washington Institutional Animal Care and Use Committee.
1). Surgical Procedures
Using standard aseptic technique, two adult male rhesus macaques (monkey A: 14 years, 18.45 kg; monkey B: 16 years, 10.75 kg) were anesthetized with isoflurane and placed in a stereotaxic frame (KOPF) in which they remained throughout the procedure. The animal’s temperature, heart rate, oxygen saturation, electrocardiographic responses, and end-tidal partial pressure of CO2 were monitored throughout the procedure. A coronal incision was made about 1 cm anterior to the interaural line that extended about 4 cm on the right side for monkey A and 8 cm expanding both sides for monkey B. Following removal of the soft tissue from the skull, a 25 mm diameter trephine (GerMedUSA, Inc., SKU:GV70-42) was used to create a craniotomy in the right hemisphere of monkey A and in both hemispheres for monkey B covering primary somatosensory and motor cortices guided by stereotaxic coordinates from a macaque brain atlas [10]. To allow for optical access, the native dura was resected and replaced with a transparent artificial dura (10:1 silicone mixture of Shin-Etsu KE1300-T and CAT-1300) with a thickness of 0.5 mm and a diameter of 25 mm.
2). Photothrombotic Technique
To control the light beam diameters at various locations of the sensorimotor cortex, an opaque silicone mask with patterned apertures of diameters 0.5 mm, 1.0 mm, and 2.0 mm was placed on the artificial dura (Fig. 1A, 1B). A 3D printed ring was inserted into the cranial window and positioned over the mask to guide and position the light source with a flange extending over the skull to prevent ambient light exposure (Fig. 1C). We injected 20 mg/kg of Rose Bengal (Sigma Aldrich, Inc.) dye (40 mg/mL) intravenously over the course of 5 minutes. Illumination by a cold light source (Schott Inc., KL 2500 LCD) with a 3200K temperature setting was initiated at the start of Rose Bengal infusion and sustained for 30 minutes.
Figure 1. Photothrombotic procedure.
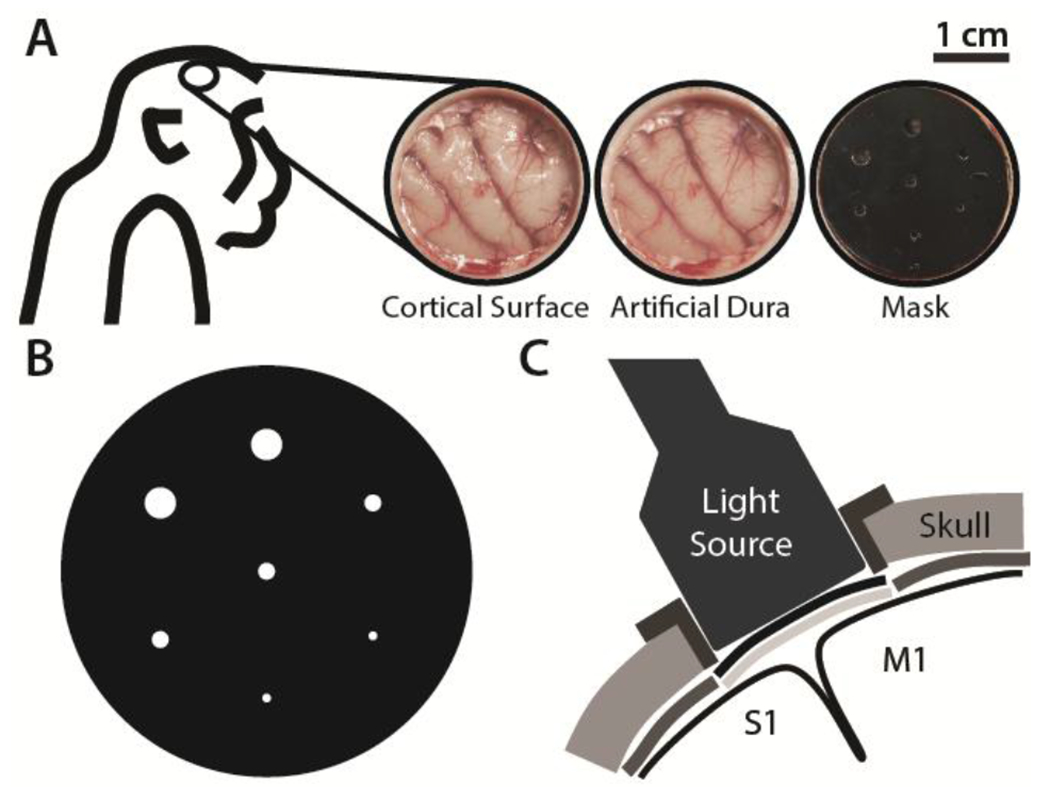
A) Craniotomy over the sensorimotor cortex exposing the cortical surface (left). Transparent artificial dura provides optical access (middle). Opaque mask with apertures for controlled light beam diameters is placed over artificial dura (right). B) Opaque mask schematic showing apertures of diameters 0.5, 1.0, and 2.0 mm. C) Schematic of the light source illuminating primary motor (M1) and somatosensory (S1) cortices through the mask apertures and the transparent artificial dura after intravenous injection of Rose Bengal dye.
3). Optical Coherence Tomography
Optical coherence tomography (OCT) images were acquired for monkey B prior to light-induced thrombosis and 3 hours post-stroke, using a custom-built swept-source OCT (SS-OCT) system [11] with a lateral field of view of the OCT volume of 9 × 9 mm2. OCT angiography (OCTA) images were acquired by processing the volumetric C-scans with OMAG algorithm [12], and 2D maximum intensity projection images are produced to visualize the blood vessel network.
B. Histological Analysis
Deeply sedated animals were perfused transcardially four hours after stroke with heparinized phosphate buffered saline (PBS) followed by cold 4% paraformaldehyde (PFA) in phosphate buffer. The brain was extracted and post-fixed in 4% PFA for 48 hours at 4°C and then sectioned into three 25 mm-thick coronal blocks using a custom matrix. After 7-10 days of incubation in 30% sucrose, blocks were frozen and cut into 50 µm coronal slices on a cryostat (Microm). Coronal slices of the brain were stained with cresyl violet and NeuN using standard techniques to evaluate areas of ischemic damage.
III. Results
Based on the projected spatial distribution of light, our quantitative model estimated that 1 and 2 mm diameter light beams of uniform intensity would result in roughly 1 and 2 mm diameter lesions, respectively, extending to depths of about 1 mm within cortical tissue (Fig. 2).
Figure 2. Predictive quantitative model.
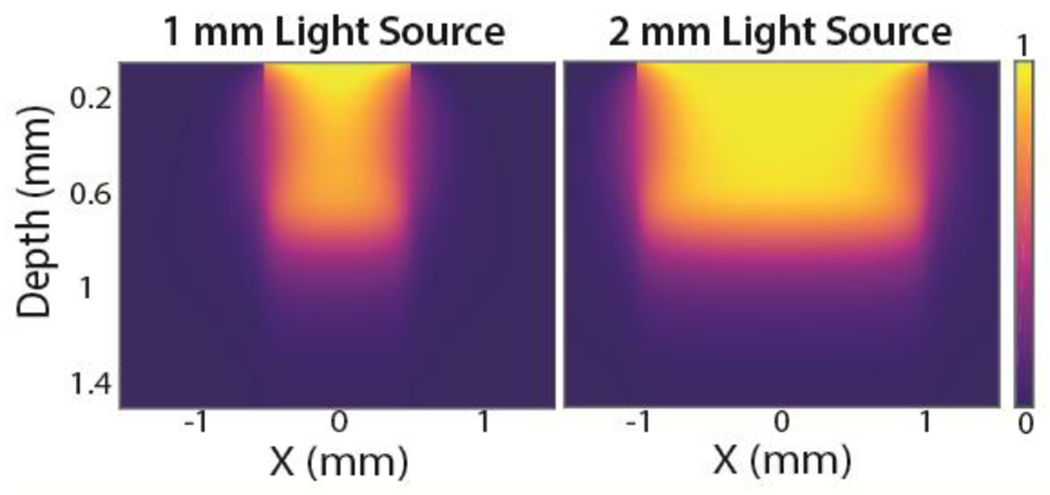
Predicted spatial distribution of light intensity of light beams (1 and 2 mm diameters) penetrating the cortical surface.
To evaluate these estimates and establish that we could create focal strokes using the photothrombotic technique in the right hemisphere of monkey A, coronal sections of the brain fixed four hours post-stroke were analyzed by histology. Without any histological staining, lesions were evident in bright red areas where the formation of thrombi entrapped the Rose Bengal in the local microvasculature (Fig. 3A). Cresyl violet and NeuN staining revealed lesions marked by cell death extending across all cortical layers with diameters of approximately 2-3 mm (Fig. 3B,C). The diameters of lesions correlated with the diameters of the incident light beams wherein beam diameters of 1 mm yielded lesion sizes of roughly 2 mm, and 2 mm beam diameters resulted in 4 mm diameter lesions.
Figure 3. Histology analysis.
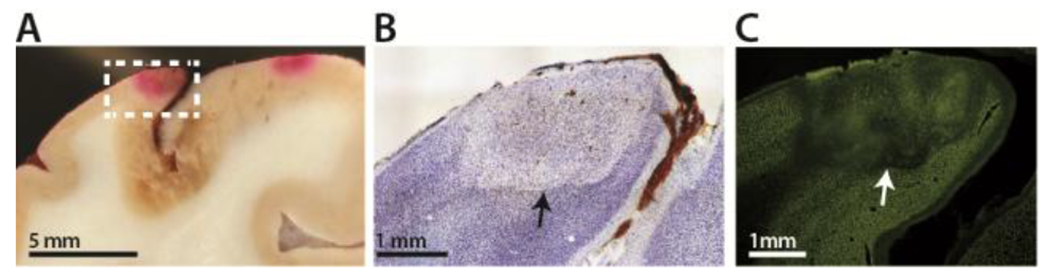
A) Brain slice with two lesions as indicated by red areas of entrapped Rose Bengal. B) Cresyl violet staining. Lesion is approximately 2 mm in diameter. C) NeuN staining of the same lesion.
These results demonstrated that focal ischemic lesions can be reliably formed in the cortex of NHPs. However, the extent of damage caused by the photothrombotic lesions in the cortex would ideally be measured in live animals soon after the lesions are created to ensure the efficacy of the technique. As such, we employed in vivo OCTA imaging of the cortical microvasculature before and 3 hours after stroke to evaluate the sizes of the induced lesions in monkey B. We first illuminated seven regions with beam diameters ranging from 0.5 mm to 2.0 mm in the sensorimotor cortices of both hemispheres of monkey B. Following the reconstruction of OCTA images, we were able to resolve vascular structures up to 1.2 mm deep into the cortical tissue. Acquired OCTA images 3 hours after the induction of photothrombotic lesions revealed that out of seven potential lesions, we identified three distinct lesions in the left hemisphere and 5 lesions in the right hemisphere with diameters ranging between 0.5 mm and 4.0 mm (Fig. 4). The remaining unidentified lesions were located in regions for which OCTA images were not acquired. We then compared the sizes of these lesions with those observed by cresyl violet staining of the brain fixed 4 hours post-stroke. The lesion sizes estimated by OCTA were similar to those estimated by cresyl violet staining (Fig. 3, 4C).
Figure 4. In vivo optical coherence tomography.
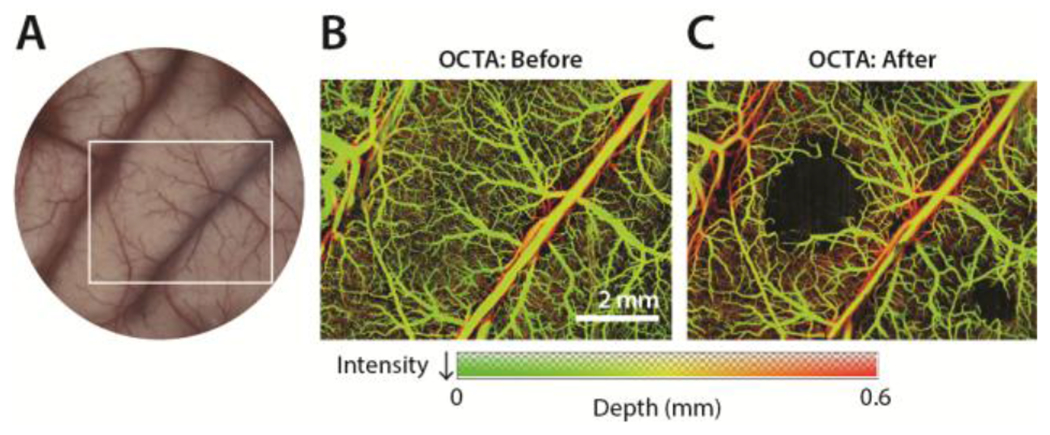
A) Cranial window. White box indicates sample region imaged by OCTA. B) Maximum intensity projection OCTA image prior to stroke. C) 3 hours post-stroke OCTA image acquisition reveals the presence of lesions.
IV. Discussion
In this study, we developed a reproducible method of photochemically inducing focal infarcts in the cortex of NHPs. By controlling the sizes and locations of the irradiating light beams, we were able to control the sizes and locations of the cortical lesions. While our quantitative model predicted cortical lesions would be approximately the same diameter as the irradiating light beam, post-stroke histological analysis revealed lesion diameters greater than those of the corresponding light beams. However, the estimated depths of the lesions as determined by histology extended across all layers of the cortex in some instances, similar to those predicted by our model. These inconsistencies can be explained by the lack of uniformity of the irradiating light beams. While our model was developed based on collimated light beams of uniformly distributed intensity, the light source used in our experiments exhibited a gradual radial decay in intensity of uncollimated light. As such, the regions closer to the center of the cranial window received light of greater intensity, while more distal regions were illuminated by light of weaker intensity. Current efforts are directed towards refining our model to account for these discrepancies to better predict lesion sizes.
While histological analysis validated the presence of focal cortical lesions post-mortem, we acquired OCTA images of the cortical microvasculature a few hours after stroke to confirm the presence of lesions in vivo. The diameters of the induced lesions as estimated by OCTA were similar to those estimated by histology, rendering OCTA a valuable tool for in vivo imaging and validation following the induction of focal cortical strokes. Although OCTA could only resolve structures at sub-millimeter depths, the lack of variability in lesion depth achieved as estimated by cresyl violet staining may mitigate this limitation. Importantly, in vivo evaluation of induced lesions can be used to ensure the efficacy of the technique and enable the estimation of lesion sizes without the need for histology, extending the life of animal subjects.
The ability to reliably induce focal ischemic lesions in the cortex of NHPs has the potential to expand the field of neurorehabilitative research by offering a less ethically challenging alternative to NHP stroke models. It is important to note, however, that these focal ischemic lesions may not mimic the large-scale pathologies exhibited by naturally occurring strokes in humans, as exclusively cortical ischemic strokes are rare in incidence. Moreover, the dependence of this technique on optical access limits our ability to target deep structures. Alternatively, other methods capable of inducing focal lesions in these regions such as electrocautery do not generate ischemic lesions and can potentially trigger different signaling cascades within the lesional and perilesional areas. By producing small ischemic lesions, we can examine the perilesional dynamics of cortical ischemic infarcts while maintaining the quality of life of the animal subject. Similarly, the ability to target specific areas of the cortex enables us to further investigate the functional properties of cortical regions. This method can also be implemented in combination with previously developed neural interfaces that allow optical access through the use of an artificial dura for optogenetic stimulation along with electrical recording [13–17]. Future studies will be directed towards investigating the functional outcomes of such lesions and the dependence on lesion size in a more chronic context. As such, the reproducible nature of this technique will enhance our ability to dependably explore neurorehabilitative strategies for cortical strokes. Moreover, this model allows for the induction and evaluation of multiple focal lesions with minimal behavioral deficits, highlighting the practicality of this NHP ischemic stroke model.
Acknowledgment
We thank Toni Haun, Christopher English, and Sandy Lee for help with animal care and experimental preparation.
This project was supported by the Eunice Kennedy Shiver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number K12HD073945, the Washington National Primate Research Center (WaNPCR, P51 OD010425), and the Center for Neurotechnology (CNT, a National Science Foundation Engineering Research Center under Grant EEC-1028725).
Contributor Information
Karam Khateeb, University of Washington, Seattle, WA 98195 USA.
Zhaojie Yao, University of Washington, Seattle, WA 98195 USA.
Viktor N. Kharazia, University of California, San Francisco, CA
Evelena P. Burunova, University of Washington, Seattle, WA 98195 USA
Shaozhen Song, University of Washington, Seattle, WA 98195 USA.
Ruikang Wang, University of Washington, Seattle, WA 98195 USA.
Azadeh Yazdan-Shahmorad, University of Washington, Seattle, WA 98195 USA.
References
- [1].Norrving B and Kissela B, “The global burden of stroke and need for a continuum of care,” Neurology, vol. 80, no. 3 Suppl 2, pp. S5–12, January 15 2013. [DOI] [PubMed] [Google Scholar]
- [2].Go AS et al. , “Heart disease and stroke statistics--2013 update: a report from the American Heart Association,” Circulation, vol. 127, no. 1, pp. e6–e245, January 1 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Maeda M et al. , “Characterization of a novel thrombotic middle cerebral artery occlusion model in monkeys that exhibits progressive hypoperfusion and robust cortical infarction,” J Neurosci Methods, vol. 146, no. 1, pp. 106–15, July 15 2005. [DOI] [PubMed] [Google Scholar]
- [4].Cook DJ and Tymianski M, “Nonhuman primate models of stroke for translational neuroprotection research,” Neurotherapeutics, vol. 9, no. 2, pp. 371–9, April 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fluri F, Schuhmann MK, and Kleinschnitz C, “Animal models of ischemic stroke and their application in clinical research,” Drug Des Devel Ther, vol. 9, pp. 3445–54, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Carmichael ST, “Rodent models of focal stroke: size, mechanism, and purpose,” NeuroRx, vol. 2, no. 3, pp. 396–409, July 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fan J, Li Y, Fu X, Li L, Hao X, and Li S, “Nonhuman primate models of focal cerebral ischemia,” Neural Regen Res, vol. 12, no. 2, pp. 321–328, February 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Watson BD, Dietrich WD, Busto R, Wachtel MS, and Ginsberg MD, “Induction of reproducible brain infarction by photochemically initiated thrombosis,” Ann Neurol, vol. 17, no. 5, pp. 497–504, May 1985. [DOI] [PubMed] [Google Scholar]
- [9].Yao Z and Yazdan-Shahmorad A, “A Quantitative Model for Estimating the Scale of Photochemically Induced Ischemic Stroke,” Conf Proc IEEE Eng Med Biol Soc, vol. 2018, pp. 2744–2747, July 2018. [DOI] [PubMed] [Google Scholar]
- [10].Paxinos X.-F. H. George, Toga Arthur W., The Rhesus Monkey Brain in Stereotaxic Coordinates, 2nd ed. Toga, 2000. [Google Scholar]
- [11].Xu J, Song S, Wei W, and Wang RK, “Wide field and highly sensitive angiography based on optical coherence tomography with akinetic swept source,” Biomed Opt Express, vol. 8, no. 1, pp. 420–435, January 1 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].An L, Qin J, and Wang RK, “Ultrahigh sensitive optical microangiography for in vivo imaging of microcirculations within human skin tissue beds,” Opt Express, vol. 18, no. 8, pp. 8220–8, April 12 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ledochowitsch P et al. , “Strategies for optical control and simultaneous electrical readout of extended cortical circuits,” J Neurosci Methods, vol. 256, pp. 220–31, December 30 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yazdan-Shahmorad A, Silversmith DB, and Sabes PN, “Novel techniques for large-scale manipulations of cortical networks in non-human primates,” Conf Proc IEEE Eng Med Biol Soc, vol. 2018, pp. 5479–5482, July 2018. [DOI] [PubMed] [Google Scholar]
- [15].Yazdan-Shahmorad A et al. , “A Large-Scale Interface for Optogenetic Stimulation and Recording in Nonhuman Primates,” Neuron, vol. 89, no. 5, pp. 927–39, March 2 2016. [DOI] [PubMed] [Google Scholar]
- [16].Yazdan-Shahmorad A, Diaz-Botia C, Hanson T, Ledochowitsch P, Maharabiz MM, and Sabes PN, “Demonstration of a setup for chronic optogenetic stimulation and recording across cortical areas in non-human primates,” SPIE BiOS, 2015. [Google Scholar]
- [17].Yazdan-Shahmorad A, Silversmith DB, Kharazia V, and Sabes PN, “Targeted cortical reorganization using optogenetics in non-human primates,” Elife, vol. 7, May 29 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]


