Graphical abstract
Keywords: Coronavirus, SARS-CoV2, Spike Protein, Virus Entry, Vaccine
Abstract
SARS-CoV2 is a member of human coronaviruses and is the causative agent of the present pandemic COVID-19 virus. In order to control COVID-19, studies on viral structure and mechanism of infectivity and pathogenicity are sorely needed. The spike (S) protein is comprised of S1 & S2 subunits. These spike protein subunits enable viral attachment by binding to the host cell via ACE-2 (angiotensin converting enzyme-2) receptor, thus facilitating the infection. During viral entry, one of the key steps is the cleavage of the S1-S2 spike protein subunits via surface TMPRSS2 (transmembrane protease serine 2) and results in viral infection. Hence, the S-protein is critical for the viral attachment and penetration into the host. The rapid advancement of our knowledge on the structural and functional aspects of the spike protein could lead to development of numerous candidate vaccines against SARS-CoV2. Here the authors discuss about the structure of spike protein and explore its related functions. Our aim is to provide a better understanding that may aid in fighting against CoVID-19 and its treatment.
1. Introduction
Currently, the world is in the middle of a pandemic due to the newly emerged coronavirus also called as SARS-CoV2 which resulted in coronavirus disease 2019 (COVID-19). In the recent past, few pandemics have occurred around the world due to coronaviruses, such as SARS and MERS. The WHO confirmed COVID-19 a “pandemic” on March 11, 2020 (WHO, 2020). COVID-19 behaves differently from previous pandemics, which has led to a state of general panic due to the lack of appropriate medications/vaccination. Since vaccination and immediate medical solutions are currently lacking, all countries have widely agreed to practice social distancing as a strategy to prevent the spreading of disease and perform simultaneous screening confirmation tests. Recent studies revealed that the hyper-inflammation caused by COVID-19 leads to tissue damage, releases tissue factor, and induces thrombosis and vascular constriction. This is considered one of the main factors responsible for the increased mortality in patients with hypertension. Despite all the measures undertaken, the COVID-19 crisis is still causing devastation around the world, with mortality and morbidity increasing every day (Li et al., 2021). Worldwide, 218.94 million cases and 4.53 million deaths were reported as on September 05, 2021(https://www.who.int/covid-19). Thus, the current dramatic situation prompts us to generate appropriate vaccines and anti-COVID-19 drugs. SARS-CoV2 has an identical infrastructure to previous pandemic viruses, especially SARS. However, due to certain mutations in the genome, it behaves differently.
Symptoms of COVID-19 may develop in 2 days to 2 weeks after exposure and could be mild to moderate. The symptoms include pneumonia, cough, and difficulty in breathing, loss of smell and taste, diarrhea (Zhang et al., 2020a). On the other hand, in susceptible people such as immune-compromised, diabetic patients or the elderly, SARS-CoV2 may cause severe fever, followed by death due to multi-organ failure (Xian et al., 2020). Hence, a novel approach to combat COVID-19 is urgently needed.
Coronaviruses are a large member of respiratory viruses under the Coronaviridae family of Nidovirales order (Weiss and Leibowitz, 2011). They are enveloped viruses containing a positive-sense single-stranded RNA genome [(+)ssRNA virus] and causing mild to severe respiratory syndromes. Based on genotypic and serological characterization, coronaviruses are traditionally classified into 4 genera: 1) α coronavirus, 2) β coronavirus, 3) γ coronavirus, and 4) δ coronavirus (Yang and Leibowitz, 2015). Previously, six groups of human coronaviruses (H-CoVs) had been reported: i) H-CoV 229-E, ii) H-CoV OC-43, iii) H-CoV HKU-1, iv) H-CoV NL-63, v) SARS, and vi) MERS (Zaki et al., 2012). The ongoing SARS-CoV2, which is causing the COVID-19 pandemic outbreak, is a novel member in the Coronaviridae family.
COVID-19 is now grouped as the seventh member of H-CoV diseases, after OC43, 229E, NL63, HKU1, MERS, and SARS (Huang et al., 2020a, Huang et al., 2020b). SARS-CoV2 has been identified as a zoonotic virus that resides in bats and may infect intermediate hosts like palm civets before human transmission (Zhou et al., 2020). SARS-CoV2 was assumed to have originated from bats because of its high genetic matching to bat-coronaviruses (Lu et al., 2020). To date, no evidence is available regarding the intermediate reservoir linking SARS-CoV2 infection to humans. COVID-19 emerged from China and within a short time span spread to other countries.
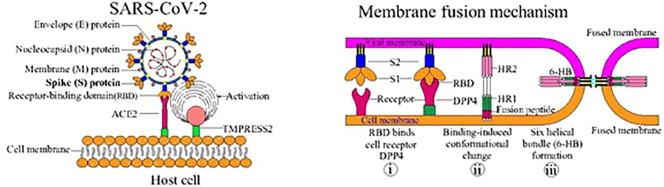
Coronaviruses harbor a special characteristic spike (S) protein that projects from the virion surface like a crown so the name coronavirus (Lu et al., 2020). The spike protein protrudes ∼ 150 Å out of the 500–2000 Å diameter viral envelope, is making it to be one of the most prominent players for host cell interaction and subsequent viral entry followed by a severe infection (Pal, 2021). The S-protein comprises of two subunits – S1 & S2. . The S1 subunit has four domains of which the receptor binding domain (RBD) is crucial as it binds to angiotensin converting enzyme-2 (ACE-2) receptor of the host cells. The S2 subunit of the spike protein is important for fusion of viral envelope and the host cell membrane (Shang et al., 2020). The priming of the S-protein is initiated by the enzyme transmembrane protease serine 2 (TMPRSS2), which is vital for the entry of SARS-CoV2 into the host (Hoffmann et al., 2020, Lan et al., 2020, Wrapp et al., 2020). Once the virion attaches to the host cell, TMPRSS2 cleaves the S-protein, thereby exposing the S2 subunit, and allowing viral fusion and viral RNA release into the host cell. The RNA is then replicated, disseminated to neighboring cells thus resulting in the spread of infection within the host (Du et al., 2009). Thus, the S-protein is essential in the infectivity of SARS-CoV2. This review describes what is currently known about the structural interactions of the S-protein and the host response to SARS-CoV2 entry, with a focus on make COVID-19 vaccine research and development.
2. Structural feature and life-cycle of SARS-CoV2
SARS-CoV2 is spherical shaped, enveloped particle with a diameter of 65–125 nm (Liu et al., 2020). It has a (+)ssRNA molecule bound to nucleoprotein in a capsid consisting of matrix-protein. Structurally, SARS-CoV2 is composed of 4 structural proteins: i) membrane(M)-protein, ii) nucleocapsid(N)-protein, iii) spike(S)-protein, and iv) envelope(E)-protein, (Lu et al., 2020). Spike protein, also called the S-protein, which has been viewed at the atomic level using electron microscopy, is responsible for viral entry into the host through a fusion process (Wrapp et al., 2020).
During infection, the heptad-repeats (HR1 & HR2) and the putative fusion-peptide (FP) of the S2 subunit of S-protein involve with a major role in fusion of the virion and the host cell (Xia et al., 2020). The infection of SARS-CoV2 is initiated with the binding of the S1 subunit (RBD domain) from the S1 subunit to the ACE-2 receptor on host cells (Fig. 1 ). Recent studies have shown that proteases of the host cell are crucial for the entry of SARS-CoV2 into the host (Belen-Apak and Sarialioglu, 2020). Recent corona viral pathogenesis studies with mouse Hepatitis-virus (MHV-A59) showed that the fusion loop of S-protein is essential for the viral fusion and infection (Pal, 2021). Further analysis of the spike protein revealed that the fusion loop brought 3 fusion peptides in contiguous manner resulting in synergistic trigger for the fusion with host cell (Pal, 2021).
Fig. 1.
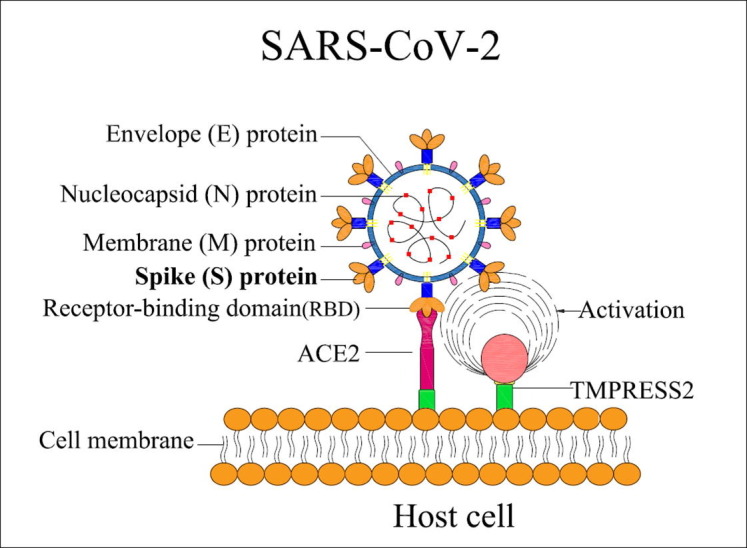
Structural features of SARS-CoV2 S-protein and its functions. Interaction of the S-protein with host cells: The host cell ACE-2 receptor anchors the virus via the RBD of the S-protein.
Although the first step is the binding of the virus to the host cell receptor, the cleavage of subunits S1-S2 to expose the S2 subunit is crucial for the proper fusion of the viral coat protein with host cells and thus, for infection (Zhou et al., 2020). Upon binding to ACE-2, TMPRSS2 exerts S-protein priming (Hoffmann et al., 2020). The efficiency of ACE-2 binding is crucial for efficient virus transmission as evidenced by the structural analysis of SARS-CoV2 S/ACE2 interface at the atomic level (Lan et al., 2020). Among the spikes of human coronaviruses, the SARS-CoV2 spike trimer is unique as it has the higher hydrophobicity, more rigid fusion protein due to the occurrence of consecutive prolines, aromatic/hydrophobic amino acids (Pal, 2021).
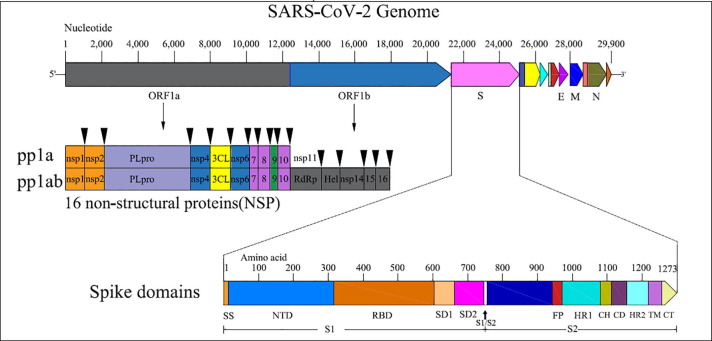
SARS-CoV2 predominantly enters the host through the respiratory airways by endosomal membrane fusion in the cells of the respiratory tract (Tang et al., 2020). Interaction of the RBD and cell receptor results in a series of conformational changes leading to a transient pre hairpin-intermediate of S-2 that exposes the hydrophobic region (HR1), allowing the FP to insert into the host cells (Lan et al., 2020). After fusion is established, the 6-helix bundle structure (6-HB) is stabilized by refolding of the S2 intermediate, with HR2 bringing the virus and host cell closer (Xia et al., 2020), thus completing the fusion and beginning the viral life cycle (Fig. 2 ). Recent reports on MERS-CoV and other coronaviruses have shown that the S2 domain is crucial for viral attachment and a major target to develop the coronavirus vaccines (Xu et al., 2019). The S2 domain of MERS-CoV has the following features: membrane fusion function, presence of a hydrophobic FP region at the N terminus, and HR1 and HR2 regions (Xu et al., 2019). Raj et al. (2013) showed in a co-purification experiment that the cellular receptor of MERS-CoV is CD26 [also known as di-peptidyl-peptidase-4 (DPP4)]. Further work on peptides that block 6-HB formation in MERS-CoV have shown the inhibition of viral replication and spike mediated virus-cell fusion, thus confirming the potential of the S-protein as a candidate for the prevention of viral entry and multiplication (Xia et al., 2020, Wang et al., 2019).
Fig. 2.
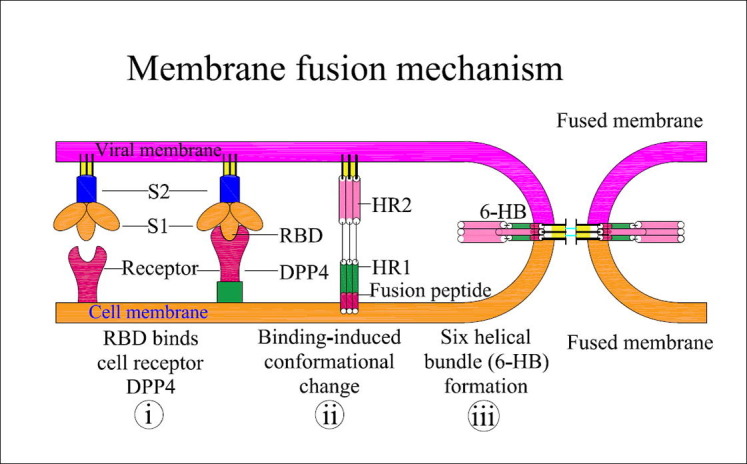
Graphic representation of S-protein assisted membrane fusion mechanism of SARS-CoV2: Interaction of the RBD from the S1 subunit peptide with the DPP4 receptor results in (i) the formation of a pre-hairpin structure, thus exposing HR1 and inserting the fusion peptide into the host cell membrane, (ii) formation of fusion intermediates by FP interlinked with the host cell membrane, and (iii) 6-HB formation by refolding of HR2 into stabilized trimer hairpins, thus bringing the virus and host cell closer for fusion. Additional conformational changes may occur during the fusion process.
3. Genome organization and targetdomains of the S-protein
SARS-CoV2 harbors a (+)ssRNA genome with 29.9 Kb in length and encoding 13 open reading frames (ORFs) and codes for 27 proteins (Wrapp et al., 2020, Li et al., 2021). The major ORF (ORF 1a and 1b) comprises 2/3 of the viral genome and codes for 2 polypeptides (PP1a and PP1b) and sixteen non-structural proteins (NSP). The remaining ORFs code for i) structural proteins like small envelope (E) protein and spike (S) glycoprotein, which are necessary for viral infection and multiplication; and ii) accessory proteins like nucleocapsid (N) protein and matrix (M) protein, which interfere with the host immune response (Fig. 3 ). SARS-CoV and MERS-CoV have large genomes of 27.9 Kb & 30.1 Kb in length, respectively (Wu et al., 2020); the SARS-CoV2 genome obtained from a COVID-19 patient from Wuhan (WHCV, Wuhan Hu1) was 29.9-Kb in length (Huang et al., 2020a, Huang et al., 2020b). Recently, 16 NSP (non-structural proteins) were discovered in the WHCV genome upon deep meta-transcriptomic sequencing analysis (Magrone et al., 2020).
Fig. 3.
Genomic organization of SARS-CoV2 and structural features of the S-protein: The genome of SARS-CoV2 exhibits of 2 major ORFs (1a and 1b), coding for sixteen-non-structural proteins (16 NSPs) and other structural proteins like the S-protein, E-protein, M−protein, N-protein. The spike protein contains S1 and S2 subunits; each carrying domains like the NTD, RBD, FP, etc. Arrowheads indicate protease-cleavage sites.
S-protein of SARS-CoV2 is known as a type-I-transmembrane glycoprotein derived from a precursor glycoprotein of 1273 amino acids in length, and majority of the protein including the amino terminus was found on the outer surface of the virus. Structural analysis of the S-protein revealed the presence of 4 domains: i) a single peptide domain (amino acid residues 1 ∼ 2), ii) an extracellular domain (13 ∼ 1195 amino acid residues, iii) a transmembrane domain (1196 ∼ 1215 amino acid residues), and iv) intracellular domain (1216 ∼ 1273 amino acid residues) (Wrapp et al., 2020). The host enzymes like trypsin, cathepsin I and Factor Xa have cleaved subunits, S1 and S2 of SARS-CoV S-protein (Belen-Apak and Sarialioglu, 2020). Appropriate proteases cleave the S-protein into S1 and S2 at furin and S20 sites (Andersen et al., 2020, Li et al., 2021). The S1 subunit contains an RBD domain (amino acids 319–510) that produces a concave surface upon interaction with the host cell receptor ACE-2 (Yan et al., 2020). For complete contact with ACE-2, the RBM (receptor binding motif) consisting of 424–494 amino acids of the RBD is essential, and hence is also known as receptor-binding loop (Weiss and Navas-Martin, 2005). In the SARS-CoV, two amino-acid residues at positions 480 and 490 of the RBM are crucial for disease progression and tropism (Xu et al., 2019). Studies using mice, rats, and civets have shown that any alteration in these amino acids results in drastic changes in the efficiency of animal ∼ human (or) human ∼ human spread (Xu et al., 2004, Zaki et al., 2012). The subunit, S2 of SARS-CoV is essential for fusion of the virus and the host cells, has been shown to consist of two domains (HR1 and HR2), and the HR1 domain was found to be longer than HR2 (Xu et al., 2019).
In MERS-CoV infection, the S-protein is cleaved in a similar fashion, resulting in S1 subunit and S2 subunit (Du et al., 2009). Like SARS-CoV, the S1 subunit possesses an RBD that assists in attachment of the virus to host cells via the receptor DPP4 instead of the ACE-2 receptor (Chen et al., 2013, Wan et al., 2020). The SARS-CoV2 S-protein has a strong structural match with SARS-CoV, as both viruses prefer ACE-2 for attachment (Chen et al., 2020, Du et al., 2009). During SARS-CoV infection, other cellular receptors such as C-type lectin CD209L and dendritic cell specific intercellular adhesion molecule three grabbing non-integrin(DC-SIGN) act as secondary receptors for viral attachment. Ultimately, the SARS-CoV2 S-protein is crucial for anchoring into host cells (Chen et al., 2020), and viral replication (Du et al., 2009). Several mutations have been reported with the SARS CoV2 genome and the resulting S-protein is with higher infectivity, spread and severity of the disease (Guruprasad, 2021, Huang et al., 2020a, Huang et al., 2020b, Ou et al., 2021, Korber et al., 2020). Two crucial mutations V367F of RBD and D614G in S glycoprotein were found to be important for the increased spreading of the disease (Huang et al., 2020a, Huang et al., 2020b, Korber et al., 2020, Ou et al., 2021).
4. Functions of SARS-CoV2 spike protein
The SARS-CoV2 S-protein plays pivotal functions for COVID-19 and subsequent pathogenesis, with subunits S1 and S2 playing major roles in: (i) identification and binding to host receptors (RBD domain of S1 subunit), and (ii) fusion in-between the membrane of host cell (S2 Subunit) and the viral envelope (Chen et al., 2020). The RBD (S1 subunit) was found to be crucial for attachment of the virus to the host cell receptors in SARS-CoV2 infection. The ACE-2 receptor binds to 14 of the 18 amino acids of the RBD, with positions R453 of RBD & K341 of ACE-2 necessary for complex configuration (Xiao et al., 2003, Yang et al., 2007). Recently, cryo-electron microscopic analysis on the interaction of N501Y mutant S-protein revealed the extra interaction between Y501 of S-protein and Y41 of ACE-2 receptor. This interaction has been predicted to increase the infectivity and affinity of the SARS-CoV2 UK variant in humans (Zhu et al., 2021). Simultaneously, FB is built up on the target host cell membrane as HR1 and HR2 domains make the fusion core 6HB structure thus, allowing the viral envelope and host cell membrane to come close for membrane fusion (Yang et al., 2007). A similar infection pattern was found in MERS-CoV infection, with HR1 and HR2 of the S2 subunit playing complementary roles (Chen et al., 2013). In SARS infection, secondary receptors like DC-SIGN and Liver SIGN lymph node (L-SIGN) have been found to anchor the virus (Han et al., 2007). The asparagine-related glycosylation of the amino acids at site of 109, 118, 119, 158, 227, 589, and 699 in spike was found to be vital for DC-SIGN/L-SIGN intermediate viral entry into the host. The residue functions independently of the ACE-2 receptor in SARS-CoV (Coleman et al., 2014). Finally, for SARS-CoV2 to enter the host cells, the activation of the S-protein by TMPRSS2 is essential, and once inside the host cell, the virus multiplies and spreads infection (Hoffmann et al., 2020). Hence the spike protein could be a potential candidate for vaccine development against COVID-19.
5. COVID-19 vaccines: Mediated by spike protein
To manage COVID-19, vaccines are one of the most effective and economical approaches. Currently, DNA vaccines (adenoviral based - Astrazeneca, Johnson & Johnson, Gam-COVID-vac - Sputnik) and mRNA-based vaccines (Pfizer and Moderna) with lipid nanoparticle delivery system have been approved by FDA for the emergency use due to lack of immediate antiviral drugs (Teijaro and Farber, 2021, Banerji et al., 2021). Though these have been approved for emergency, most of them are undergoing clinical trials (III phase) and safety issues like antibody dependent enhancement (ADE), pro-inflammation, blood clotting and cytokine storm needs addressing (Li et al., 2021, Vabret et al., 2020). Hence the development of an efficient vaccine is requiredurgently, and several researchers have been actively working on different types of vaccines against COVID-19. Studies from MERS and SARS have revealed that the S-protein is a main antigenic molecule that can induce antibodies blocking viral entry. In addition, such antibodies help to stimulate host-immune responses and neutralizing antibody production, protecting the host immune system against viral infection (Amanat and Krammer, 2020, Xu et al., 2019). Studies are reporting the S-protein as a major target to develop the vaccine development and study against SARS and MERS (Table 1 ).
Table 1.
Summary of potential vaccines for SARS-CoV & MER-CoV.
| Type of Vaccines | Target | Procs | Cons | Reference |
|---|---|---|---|---|
| Deoxyribonucleic acid (DNA) | Spike protein | Avoids handling of infectious virus, high thermal stability, rapid scale up possible, cheap production, human test conducted (SARS-CoV). | Delivery vehicles needed to obtain high immunogenicity. | (Zhao et al., 2004) |
| Ribonucleic acid (RNA) | Spike protein | Avoids handling of infectious virus, highly immunogenic, rapid scale up possible. | High reactivity of vaccine. | (Du et al., 2009) |
| S-protein (Full length) | Spike protein | Produces good T-cell response, neutralizing antibodies and protective immunity. | May cause liver damage and enhance infection. | (Czub et al., 2005) |
| Viral-vector | Spike protein | Produces good quality neutralizing antibodies, protective immunity and/ or T-cell responses. | ADE effect could be induced in susceptible cases. | (Bisht et al., 2004) |
| Recombinant S-protein | Spike protein | Avoids handling of infectious virus, adjuvants could increase immunogenicity. | Avoids handling of infectious virus, adjuvants could increase immunogenicity. | (Wang et al., 2014) |
| Receptor-Binding-Domain (RBD) | Entire virion | Produces protective immunity, neutralizing antibodies and T-cell response | Unknown | (Du et al., 2008) |
| Deoxyribonucleic acid (DNA) | Entire virion | Produces good quality neutralizing antibodies, protective immunity and/ or T-cell responses. | Limited response; mutants could not be neutralized. | (Liu et al., 2007) |
| Recombinant Receptor-Binding-Domain (rRBD) | Entire virion | Results in cross protection, provides protective immunity, produces neutralizing antibodies and T-cell response; More safe and effective vaccine than other RBD based vaccines. | More doses are required as adjuvants | (Zakhartchouk et al., 2007) |
| Killed vaccine (An inactivated) | Entire virion | Existing production process for licensed vaccines could be utilized; No need for additional infrastructure; immunogenicity could be improved with the use of adjuvants. | Utlization of high concentration of virus (attenuated virus could solve this issue); Integrity of epitope/ antigen is an issue. | (Bolles et al., 2011) |
| An attenuated vaccine (Live) | Entire virion | Existing production process for licensed vaccines could be utilized; No need for additional infrastructure. | Longer duration to obtain attenuated coronavirus vaccine seeds; extensive testing required for safety issues. | (Lamirande et al., 2008) |
The spike protein is indeed an excellent for vaccine research against SARS-CoV2 due to its imperative features (Zhang et al., 2020b). Firstly, the S-protein could be noticed by the host immune system easily as it is found on the virus surface. Secondly, it is essential for anchoring and interaction with ACE-2 for the viral entry. Finally, the S-proteins from SARS-CoV and MERS-CoV were used to develop the effective vaccines, which are closely related to the SARS-CoV2 S-protein (Zhang et al., 2020b). The S-protein (140 kDa) consists of 1270 amino acids and assembles into a homo-trimer commonly found in first class membrane fusion proteins. Clover Biopharmaceuticals has produced a vaccine (S-Trimer) based on the S-protein from SARS-CoV2 using a patented technology (Trimer Tag © technology). The vaccine was produced using a mammalian cell culture system and the preclinical safety trails will be completed soon (Padron-Regalado, 2020). Recently, two preventive approaches using gold nanoparticles based conjugation (AuNP-S461-493) and Biovacc-19 peptide vaccine (Farfán-Castro et al., 2021, Sorensen et al., 2020) have been reported and are in different phases of clinical trials. Another vaccine developmental study is involved in the production of recombinant RBD of SARS CoV2 in plants and further analysis in mice revealed the occurrence of induce potent neutralizing response (Mamedov et al., 2021).
Based on previous studies,S-protein was found to contain 2 domains: i) N-terminal domain (NTD) and ii) C-terminal domain (CTD), which contains the RBD. The S2-subunit was found to consist of elements essential for viral fusion, including the internal membrane protein, 7 peptide repeats (2 No’s), one membrane proximal external region (MPER), and one trans-member domain (TM) (Li, 2016). Very recently, the structure of two peptides from the SARS-CoV2 S-protein were studied in detail: i) S-trimer of SARS-CoV2 in perfusion conformation, and ii) RBD in association with ACE-2, which could potentially result in better vaccine design (Lan et al., 2020, Wrapp et al., 2020). Thus far, among the different subunits and domains of SARS-CoV2, the S-protein (full length), NTD, RBD, FP, and S1 subunit have emerged as prime candidates for vaccine development.
The SARS-CoV2 S-protein would be preferable over other subunits or domains, as it would have a proper conformation, more epitopes, and higher immunogenicity. The recombinant pre-fusion S-protein of MERS-CoV induces production of high neutralizing antibody titers upon vaccination of BALB/c mice, thereby conferring protection (Pallesen et al., 2017). Amanat and Krammer (2020) showed that immunization of mice with an S-protein produced in baculoviral insect cells assembled into nanoparticles and with alum as an adjuvant resulted in high neutralizing antibody titers. Muthumani et al. (2015) utilized a DNA vaccine strategy against MERS-CoV S-protein and demonstrated immunity in camels, Rhesus macaques, and mice, thus confirming the potential of the coronavirus S-protein in vaccine production.
6. Conclusions
In conclusion, the S-protein possesses rich unique features that are absent in other structural proteins of SARS-CoV2. The S-protein of SARS-CoV2 interacts with the host cell receptors like ACE-2 and results in viral attachment. The subsequent interaction of the different S subunits and domains assists entry of virus into the host cells, thus resulting in pathogenesis. Further studies on the mechanism of affinity between ACE-2 and SARS-CoV2, and the interaction of the viral envelope with host cell membrane would be helpful to develop the potential vaccines towards COVID-19. Vaccine development is the effective and economical strategies for preventing and controlling SARS-CoV2. At present, there is an immediate necessity to obtain strong and safe vaccines to prevent COVID19 infections. Many researchers are attempting to utilize the spike protein for vaccine design because they are safe and most effective in eliciting an immune response in the host as shown for other coronaviruses. However, concerns have been elevated about the utilization of the full-length S-protein, as it consists of non-structural peptides and non-neutralizing/immune-dominant epitopes that could result in the enhancement of SARS-CoV2 infection (or) cause an inflammatory response in immunized hosts. Thus, it is imperative to select a key functional region/subunit of the S-protein of SARS-CoV2 that could neutralize the whole virus. Alternatively, T-cell epitomes could be employed for vaccine production.
In the present review, we have discussed the latest information on pathogenesis of SARS-CoV2 with reference to the S-protein, including its structural features and functions in viral infection. We have also discussed the gaps in the field that need to be addressed to increase our knowledge regarding the role of the S-protein in pathogenicity. We further outlined the research areas that would allow the elucidation of the mechanism of viral infection, the creation of reproducible animal models, as well as the counter measures to overcome not only SARS-CoV2, but also emerging and re-emerging coronaviral diseases.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Amanat F., Krammer F. SARS-CoV-2 vaccines: Status report. Immunity. 2020;52(4):583–589. doi: 10.1016/j.immuni.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020;26(4):450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji A., Wickner P.G., Saff R., Stone C.A., Robinson L.B., Long A.A., et al. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: Current evidence and suggested approach. Allergy Clin. Immunol. Pract. 2021;9:1423–1437. doi: 10.1016/j.jaip.2020.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belen-Apak F.B., Sarialioglu F. The old but new: Can unfractioned heparin and low molecular weight heparins inhibit proteolytic activation and cellular internalization of SARS-CoV2 by inhibition of host cell proteases. Med. Hypotheses. 2020;142 doi: 10.1016/j.mehy.2020.109743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisht H., Roberts A., Vogel L., Bukreyev A., Collins P.L., Murphy B.R., Subbarao K., Moss B. Severe acute respiratory syndrome coronavirus spike protein expressed by attenuated vaccinia virus protectively immunizes mice. Proc. Natl. Acad. Sci. USA. 2004;101(17):6641–6646. doi: 10.1073/pnas.0401939101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolles M., Deming D., Long K., Agnihothram S., Whitmore A., Ferris M., Funkhouser W., Gralinski L., Totura A., Heise M., Baric R.S. A double-inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilicproinflammatory pulmonary response upon challenge. J. Virol. 2011;85(23):12201–12215. doi: 10.1128/JVI.06048-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Rajashankar K.R., Yang Y., Agnihothram S.S., Liu C., Lin Y.-L., Baric R.S., Li F. Crystal structure of the receptor-binding domain from newly emerged Middle East respiratory syndrome coronavirus. J. Virol. 2013;87(19):10777–10783. doi: 10.1128/JVI.01756-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Guo Y., Pan Y., Zhao Z.J. Structure analysis of the receptor binding of 2019-nCoV. Biochem. Biophys. Res. Commun. 2020;525(1):135–140. doi: 10.1016/j.bbrc.2020.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman C.M., Liu Y.V., Mu H., Taylor J.K., Massare M., Flyer D.C., Glenn G.M., Smith G.E., Frieman M.B. Purified coronavirus spike protein nanoparticles induce coronavirus neutralizing antibodies in mice. Vaccine. 2014;32(26):3169–3174. doi: 10.1016/j.vaccine.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czub M., Weingartl H., Czub S., He R., Cao J. Evaluation of modified vaccinia virus Ankara based recombinant SARS vaccine in ferrets. Vaccine. 2005;23(17-18):2273–2279. doi: 10.1016/j.vaccine.2005.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L., Zhao G., Lin Y., Chan C., He Y., Jiang S., Wu C., Jin D.-Y., Yuen K.-Y., Zhou Y., Zheng B.-J. Priming with rAAV encoding RBD of SARS-CoV S protein and boosting with RBD-specific peptides for T cell epitopes elevated humoral and cellular immune responses against SARS-CoV infection. Vaccine. 2008;26(13):1644–1651. doi: 10.1016/j.vaccine.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L., He Y., Zhou Y., Liu S., Zheng B.-J., Jiang S. The spike protein of SARS-CoV– a target for vaccine and therapeutic development. Nature Rev. Microbiol. 2009;7(3):226–236. doi: 10.1038/nrmicro2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farfán-Castro S., García-Soto M.J., Comas-García M., Arévalo-Villalobos J.I., Palestino G., González-Ortega O., Rosales-Mendoza S. Synthesis and immunogenicity assessment of a gold nanoparticle conjugate for the delivery of a peptide from SARS-CoV-2. Nanomed. Nanotechnol. Biol. Med. 2021;34:102372. doi: 10.1016/j.nano.2021.102372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guruprasad L. Human SARS CoV-2 spike protein mutations. Proteins. 2021;89:569–576. doi: 10.1002/prot.26042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D.P., Lohani M., Cho M.W. Specific asparagine-linked glycosylation sites are critical for DC-SIGN- and L-SIGN-mediated severe acute respiratory syndrome coronavirus entry. J. Virol. 2007;81(21):12029–12039. doi: 10.1128/JVI.00315-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y.i., Zhang L.i., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L.i., Xie J., Wang G., Jiang R., Gao Z., Jin Q.i., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S.-W., Miller S.O., Yen C.-H., Wang S.-F. Impact of genetic variability in ACE2 expression on the evolutionary dynamics of SARS-CoV-2 spike D614G mutation. Genes (Basel). 2020;12(1):16. doi: 10.3390/genes12010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korber B., Fischer W.M., Gnanakaran S., Yoon H., Theiler J., Abfalterer W., et al. Tracking changes in SARS-CoV-2 spike: Evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182:812–827. doi: 10.1016/j.cell.2020.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamirande E.W., DeDiego M.L., Roberts A., Jackson J.P., Alvarez E., Sheahan T., Shieh W.-J., Zaki S.R., Baric R., Enjuanes L., Subbarao K. A live attenuated severe acute respiratory syndrome coronavirus is immunogenic and efficacious in golden Syrian hamsters. J. Virol. 2008;82(15):7721–7724. doi: 10.1128/JVI.00304-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., Zhang Q.i., Shi X., Wang Q., Zhang L., Wang X. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581(7807):215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- Li F. Structure, function, and evolution of coronavirus spike proteins. Ann. Rev. Virol. 2016;3(1):237–261. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Ma M.L., Lei Q., Wang F., Hong W., Lai D.Y., et al. Linear epitope landscape of the SARS-CoV-2 spike protein constructed from 1051 Covid-19 patients. Cell Rep. 2021;34 doi: 10.1016/j.celrep.2021.108915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L.i., Fang Q., Deng F., Wang H., Yi C.E., Ba L., Yu W., Lin R.D., Li T., Hu Z., Ho D.D., Zhang L., Chen Z. Natural mutations in the receptor binding domain of spike glycoprotein determine the reactivity of cross-neutralization between palm civet coronavirus and severe acute respiratory syndrome coronavirus. J. Virol. 2007;81(9):4694–4700. doi: 10.1128/JVI.02389-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, T., Hu, J., Kang, M., Lin, L., Zhong, H., Xiao, J., et al., (2020). Transmission dynamics of 2019 novel coronavirus (2019-nCoV). bioRxiv.doi:10.1101/2020.01.25.919787.
- Lu R., Zhao X., Li J., Niu P., Yang B.o., Wu H., Wang W., Song H., Huang B., Zhu N.a., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L.i., Chen J., Meng Y., Wang J.i., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrone T., Magrone M., Jirillo E. Focus on receptors for coronaviruses with special reference to angiotensin-converting enzyme 2 as a potential drug target - a perspective. Endocr. Metab. Immune Disord. Drug Targets. 2020;20(6):807–811. doi: 10.2174/1871530320666200427112902. [DOI] [PubMed] [Google Scholar]
- Mamedov T., Yuksel D., Ilgın M., Gurbuzaslan I., Gulec B., Yetiskin H., Uygut M.A., Islam Pavel S.T., Ozdarendeli A., Mammadova G., Say D., Hasanova G. Plant-produced glycosylated and in vivo deglycosylated receptor binding domain proteins of SARS-CoV-2 induce potent neutralizing responses in mice. Viruses. 2021;13(8):1595. doi: 10.3390/v13081595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthumani K., Falzarano D., Reuschel E.L., Tingey C., Flingai S., Villarreal D.O., Wise M., Patel A., Izmirly A., Aljuaid A., Seliga A.M., Soule G., Morrow M., Kraynyak K.A., Khan A.S., Scott D.P., Feldmann F., LaCasse R., Meade-White K., Okumura A., Ugen K.E., Sardesai N.Y., Kim J.J., Kobinger G., Feldmann H., Weiner D.B. A synthetic consensus anti-spike protein DNA vaccine induces protective immunity against Middle East respiratory syndrome coronavirus in nonhuman primates. Sci. Transl. Med. 2015;7(301) doi: 10.1126/scitranslmed.aac7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou J., Zhou Z., Dai R., Zhang J., Zhao S., Wu X., Lan W., Ren Y.i., Cui L., Lan Q., Lu L.u., Seto D., Chodosh J., Wu J., Zhang G., Zhang Q., Gallagher T. V367F mutation in SARS-CoV-2 spike RBD emerging during the early transmission phase enhances viral infectivity through increased human ACE2 receptor binding affinity. J. Virol. 2021;95(16) doi: 10.1128/JVI.00617-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padron-Regalado E. Vaccines for SARS-CoV-2: Lessons from other coronavirus strains. Infect. Dis. Ther. 2020;9(2):255–274. doi: 10.1007/s40121-020-00300-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal D. Spike protein fusion loop controls SARS-CoV-2 fusogenicity and infectivity. J. Struc. Biol. 2021;213(2):107713. doi: 10.1016/j.jsb:2021.107713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallesen J., Wang N., Corbett K.S., Wrapp D., Kirchdoerfer R.N., Turner H.L., Cottrell C.A., Becker M.M., Wang L., Shi W., Kong W.-P., Andres E.L., Kettenbach A.N., Denison M.R., Chappell J.D., Graham B.S., Ward A.B., McLellan J.S. Immunogenicity and structures of a rationally designed prefusion MERS-CoV spike antigen. Proc. Natl. Acad. Sci. USA. 2017;114(35):E7348–E7357. doi: 10.1073/pnas.1707304114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj V.S., Mou H., Smits S.L., Dekkers D.H.W., Müller M.A., Dijkman R., Muth D., Demmers J.A.A., Zaki A., Fouchier R.A.M., Thiel V., Drosten C., Rottier P.J.M., Osterhaus A.D.M.E., Bosch B.J., Haagmans B.L. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495(7440):251–254. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang J., Ye G., Shi K.e., Wan Y., Luo C., Aihara H., Geng Q., Auerbach A., Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581(7807):221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen B., Susrud A., Dalgleish A.G. Biovacc-19: A candidate vaccine for Covid-19 (SARS-CoV-2) developed from analysis of its general method of action for infectivity. QRB Discovery. 2020;1(e6):1–11. doi: 10.1017/qrd.2020.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang T., Bidon M., Jaimes J.A., Whittaker G.R., Daniel S. Coronavirus membrane fusion mechanism offers a potential target for antiviral development. Antiviral Res. 2020;178:104792. doi: 10.1016/j.antiviral.2020.104792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teijaro J.R., Farber D.L. COVID-19 vaccines: modes of immune activation and future challenges. Nat. Rev. Immunol. 2021;21(4):195–197. doi: 10.1038/s41577-021-00526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabret N., Britton G.J., Gruber C., Hegde S., Kim J., Kuksin M., Levantovsky R., Malle L., Moreira A., Park M.D., Pia L., Risson E., Saffern M., Salomé B., Esai Selvan M., Spindler M.P., Tan J., van der Heide V., Gregory J.K., Alexandropoulos K., Bhardwaj N., Brown B.D., Greenbaum B., Gümüş Z.H., Homann D., Horowitz A., Kamphorst A.O., Curotto de Lafaille M.A., Mehandru S., Merad M., Samstein R.M., Agrawal M., Aleynick M., Belabed M., Brown M., Casanova-Acebes M., Catalan J., Centa M., Charap A., Chan A., Chen S.T., Chung J., Bozkus C.C., Cody E., Cossarini F., Dalla E., Fernandez N., Grout J., Ruan D.F., Hamon P., Humblin E., Jha D., Kodysh J., Leader A., Lin M., Lindblad K., Lozano-Ojalvo D., Lubitz G., Magen A., Mahmood Z., Martinez-Delgado G., Mateus-Tique J., Meritt E., Moon C., Noel J., O’Donnell T., Ota M., Plitt T., Pothula V., Redes J., Reyes Torres I., Roberto M., Sanchez-Paulete A.R., Shang J., Schanoski A.S., Suprun M., Tran M., Vaninov N., Wilk C.M., Aguirre-Ghiso J., Bogunovic D., Cho J., Faith J., Grasset E., Heeger P., Kenigsberg E., Krammer F., Laserson U. Immunology of COVID-19: current state of the science. Immunity. 2020;52(6):910–941. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y., Shang J., Graham R., Baric R.S., Li F., Gallagher T. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020;94(7) doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.-F., Tseng S.-P., Yen C.-H., Yang J.-Y., Tsao C.-H., Shen C.-W., Chen K.-H., Liu F.-T., Liu W.-T., Chen Y.-M., Huang J.C. Antibody-dependent SARS coronavirus infection is mediated by antibodies against spike proteins. Biochem. Biophys. Res. Commun. 2014;451(2):208–214. doi: 10.1016/j.bbrc.2014.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Hua C., Xia S., Li W., Lu L.u., Jiang S. Combining a fusion inhibitory peptide targeting the MERS-CoV S2 protein HR1 domain and a neutralizing antibody specific for the S1 protein receptor-binding domain (RBD) showed potent Synergism against pseudotyped MERS-CoV with or without mutations in RBD. Viruses. 2019;11(1):31. doi: 10.3390/v11010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S.R., Navas-Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol. Mol. Biol. Rev. 2005;69(4):635–664. doi: 10.1128/MMBR.69.4.635-664.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S.R., Leibowitz J.L. Coronavirus pathogenesis. Adv. Virus Res. 2011;81:85–164. doi: 10.1016/B978-0-12-385885-6.00009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . Switzerland; Geneva: 2020. World Health Organization clinical management of severe acute respiratory infection when novel coronavirus infection is suspected: Interim guidance. [Google Scholar]
- Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.-L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Zhao S.u., Yu B., Chen Y.-M., Wang W., Song Z.-G., Hu Y.i., Tao Z.-W., Tian J.-H., Pei Y.-Y., Yuan M.-L., Zhang Y.-L., Dai F.-H., Liu Y.i., Wang Q.-M., Zheng J.-J., Xu L., Holmes E.C., Zhang Y.-Z. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S., Liu M., Wang C., Xu W., Lan Q., Feng S., et al. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020;30:343–355. doi: 10.1038/s41422-020-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian Y., Zhang J., Bian Z., Zhou H., Zhang Z., Lin Z., Xu H. Bioactive natural compounds against human coronaviruses: a review and perspective. Acta Pharmceut. Sinica B. 2020;10(7):1163–1174. doi: 10.1016/j.apsb.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X., Chakraborti S., Dimitrov A.S., Gramatikoff K., Dimitrov D.S. The SARS-CoV S glycoprotein: expression and functional characterization. Biochem. Biophys. Res. Commun. 2003;312(4):1159–1164. doi: 10.1016/j.bbrc.2003.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Lou Z., Liu Y., Pang H., Tien P.o., Gao G.F., Rao Z. Crystal structure of severe acute respiratory syndrome coronavirus spike protein fusion core. J. Biol. Chem. 2004;279(47):49414–49419. doi: 10.1074/jbc.M408782200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Jia W., Wang P., Zhang S., Shi X., Wang X., Zhang L. Antibodies and vaccines against Middle East respiratory syndrome coronavirus. Emerg. Microbes Infect. 2019;8(1):841–856. doi: 10.1080/22221751.2019.1624482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z.Y., Huang Y., Ganesh L., Leung K., Kong W.P., Schwartz O., Subbarao K., Nabel G.J. pH-dependent entry of severe acute respiratory syndrome coronavirus is mediated by the spike glycoprotein and enhanced by dendritic cell transfer through DC-SIGN. J. Virol. 2007;78:5642–5650. doi: 10.1128/JVI.78.11.5642-5650.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D., Leibowitz J.L. The structure and functions of coronavirus genomic 3’ and 5’ ends. Virus Res. 2015;206:120–133. doi: 10.1016/j.virusres.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R., Zhang Y., Li Y., Xia L.u., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakhartchouk A.N., Sharon C., Satkunarajah M., Auperin T., Viswanathan S., Mutwiri G., Petric M., See R.H., Brunham R.C., Finlay B.B., Cameron C., Kelvin D.J., Cochrane A., Rini J.M., Babiuk L.A. Immunogenicity of a receptor-binding domain of SARS coronavirus spike protein in mice: implications for a subunit vaccine. Vaccine. 2007;25(1):136–143. doi: 10.1016/j.vaccine.2006.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D.M.E., Fouchier R.A.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. NewEngl. J. Med. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- Zhang Z.J., Yu X.J., Fu T., Liu Y., Jiang Y., Yang B.X., Bi Y. Novel coronavirus infection in newborn babies under 28 days in China. Eur. Respir J. 2020;55:2000697. doi: 10.1183/13993003.00697-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Zeng H., Gu J., Li H., Zheng L., Zou Q. Progress and prospects on vaccine development against SARS-CoV-2. Vaccines. 2020;8(2):153. doi: 10.3390/vaccines8020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P., Ke J.S., Qin Z.L., Ren H., Zhao L.J., Yu J.G., et al. DNA vaccine of SARS-CoV S gene induces antibody response in mice. Acta Biochim. Biophys. Sin. 2004;36:37–41. doi: 10.1093/abbs/36.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., Chen H.-D., Chen J., Luo Y., Guo H., Jiang R.-D., Liu M.-Q., Chen Y., Shen X.-R., Wang X.i., Zheng X.-S., Zhao K., Chen Q.-J., Deng F., Liu L.-L., Yan B., Zhan F.-X., Wang Y.-Y., Xiao G.-F., Shi Z.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Mannar D., Srivastava S.S., Berezuk A.M., Demers J.-P., Saville J.W., Leopold K., Li W., Dimitrov D.S., Tuttle K.S., Zhou S., Chittori S., Subramaniam S., Bhella D. Cryo-electron microscopy structures of the N501Y SARS-CoV-2 spike protein in complex with ACE2 and 2 potent neutralizing antibodies. PLoS Biol. 2021;19(4):e3001237. doi: 10.1371/journal.pbio.3001237. [DOI] [PMC free article] [PubMed] [Google Scholar]