Background
Since their existence on earth, humans have used herbal medicine to meet their requirements for medication. The aim of the study: This work refers to a study conducted to carry out an ethnopharmacological survey of medicinal plants used for the treatment of cancer in Fez-Meknes region of Morocco. Material and Methods: To achieve this goal, 300 informants including 237 local people and 63 herbalists. They were requested to fill a survey related questionnaire aiming at the collection of data about the addressed objective. Informants were asked about the vernacular names, parts of medicinal plants used, mode of preparation, route of administration, reference area as well as the ecological distribution. The Relative Frequency of Citation (RFC) and Fidelity Level (FL) were calculated to identify the most effective plants recommended by informants for disease treatment. Results: The findings obtained in the present survey revealed that 94 species belonging to 47 families have been used for cancer treatment in the region of Fez-Meknes. Fruits, leaves, and seeds are the most commonly used plant parts, by the time powder and infusion arethe most common methods used fordrug preparations. Conclusion: This work may contribute towards the society as it provides interesting data on traditional medicinal knowledge of medicinal plantsused to fight cancer.
Keyword: Ethnopharmacology
1. Introduction
Morocco is a Mediterranean country located in the North of Africa. It’s geologically characterized by the presence of four large mountainous areas named the Rif, the Middle Atlas, the High Atlas, and the Anti-Atlas. Morocco is limited by the Mediterranean sea, the Atlantic Ocean on the north, and the west respectively as well as Sahara in the far south (El-Hilaly et al., 2003).
Morocco is known for its large variety of climatic conditions including both moderate humid and sub-humid climates (Born et al., 2009). The Moroccan flora is one of the richest and varied worldwide with about 4200 species. 22% of which are endemic to Moroccan soil. A large number of medicinal plants have been used by the Moroccan population to fight diseases and most of which got registered in Moroccan pharmacopeia (Rankou et al., 2013); (FENNANE and REJDALI, 2016).
Medicinal plants have widely been used as a natural source of remedies for curing multiple diseases including cancer. According to the World Health Organization (WHO), in developing countries, more than 80 % of people use herbal medicine in the treatment of different diseases (Benali et al., 2017).
Cancer remains one of the most common leading causes of death worldwide despite scientific advances in treatment options (World Health Organization, 2018). For this reason, researchers have a big interest in discovering new natural anticancer agents to mitigate this lethal disease. According to the World Health Organization, cancer was responsible for 9.6 million deaths in 2018. Moreover, about 70% of deaths from cancer occur in developing countries (World Health Organization, 2018).
Due to their accessibility and affordability, natural remedies are widely used in low-income countries to treat cancer (Kabbaj et al., 2012). Anticancer agents based herbal medicines play a crucial role in the daily routines of many urban and rural Moroccans lives. However, ethnopharmacological knowledge of some regions in Morocco, especially in Fez-Meknes region remains poorly known.
This survey aims at identifying the medicinal plants used for cancer treatment by the indigenous population of Fez-Meknes region of Morocco and to contribute towards society as it provides ethnopharmacological knowledge related to cancer treatment in the study area.
2. Materials and methods
2.1. Study area
The study was conducted in four sites within the Fez-Meknes region in Morocco (Fez, Moulay yacoub, Meknes, and Taounate) covering both rural and urban localities (Fig. 1) with the following coordinates; latitude 34 °022003 N□. Geographically, it approximately covers 40.075 Km2 making 5.7% of the total land area of the national territory and populated with 4.236.892 people. The climate of the region ranges from the Mediterranean to the continental type, precipitation levels vary between 300 mm/year and 800 mm/a (la Direction Générale des Collectivités Locales, 2015).
Fig. 1.
Map of the study area (Fez- Meknes region- Morocco) with geographic boundaries.
Fez-Meknes region- Morocco is among the most recognized region in the country for use of traditional herbal medicine against diseases and, therefore, was selected for being a study area (Jouad et al., 2001); (Ammor et al., 2020).
2.2. Data collection
An ethnopharmacological survey was conducted from October 2016 until February 2017 in the Fez-Meknes region (Morocco). During this period, 300 informants including 237 local people (sellers, purchasers, consumers, cultivators, workers) and 63 herbalists living in different urban and rural areas were selected to be as informants. Afterward, informants were invited to respond to a face-to-face interview about the vernacular name of plants used to treat cancer, parts of plants used, methods of preparation, route of administration, frequency of use, plant- type of cancer treated, prescription, unique use of traditional medicine or combined of modern. Further questions on criteria of the selected informants including age, gender, education, healthcare choices were addressed in the survey. Afterward, the collected data was analysed and discussed according to the earlier literature.
Patients diagnosed with cancer in the study area, they use herbal medicines as an alternative treatment to fight cancer according to methods reported in the culture and knowledge inherited from previous generations either verbally or written. Based on the medical diagnosis that leads to the identification of cancer type, traditional healers recommend treatment protocols including natural preparations for patients according to the type of cancer diagnosed.
2.3. Data analysis
Data analysis as well as graphs were performed using Excel 10. Ethnobotanical data were calculated using quantitative indices such as the relative frequency of citation (RFC) and fidelity level (FL).
2.3.1. Relative frequency of citation
The relative frequency of citation (RFC) refers to the most commonly cited plants in the study area for use against diseases (Tardío and Pardo de Santayana, 2008). RFC was calculated according to the following formula:
RFC (%) = (FC /N) × 100
FC: Number of informants who use plant species against any disease
N: Total number of informants.
2.3.2. Fidelity level
Fidelity level (FL) was calculated to determine the most frequently used plant species to treat a particular disease.e. cancer in the study area (Friedman et al., 1986). Fidelity level (FL) was calculated according to the following formula:
FL (%) = Np/Ni*100
Np: Number of informants who claim the use of species to treat a particular disease
Ni: Total number of informants who claim the use of plant against any given disease
Plants have high FL values are considered the most preferred and effective species for the treatment of ailment categories (Friedman et al., 1986).
3. Results and discussion
Collected data on medicinal plants used for the treatment of cancer in the Fez- Meknes region was analysed based on the relative frequency of citation (RCF) and fidelity level (FL). RCF and FL values obtained in this work ranged from 0.33 % to 17.67 % and 12.5 % to 89.83 % respectively.
The present survey was carried out in the Fez Meknes region in 4 different locations (Fez, Moulay Yacoub, Meknes, and Taounate) in which it was recorded that 94 plant species belonging to 47 families have been used to fight cancer. These findings were partially in agreement with the earlier data which showed that 63 medicinal plants are traditionally used against cancer (Samouh et al., 2019).
The following medicinal plants were the most reported species in traditional use against cancer in the study area; Apteranthes europaea Guss (17.67 %), Aristolochia longa L. (12 %), Nigella sativa L. (10 %), Allium sativum L. (9.33 %) and Marrubium vulgare L. (7.33 %). On the other hand, our findings were in accordance with the earlier literature which reported that Nigella sativa L. and Aristolochia longa L. have been used for the treatment of cancer in two Moroccan regions; Rabat and Greater Casablanca (Samouh et al., 2019) (Table 1Table 2).
Table 1.
Medicinal plants used for the treatment of cancer in the region of Fez-Meknes Morocco.
|
Family Scientific name |
Vernacular names |
RCF (%) |
FL % | Reference area(% of use) | Ecological distribution |
|---|---|---|---|---|---|
| Actinidiaceae | |||||
| Actinidia chinensis var. deliciosa (A.Chev.) A.Chev. | Kiwi | 0.67 | 50 | Fez (1) Meknes (1) |
Cul |
| Aloaceae | |||||
| Aloe vera (L.) Burm.f | Sabar | 2.67 | 57.14 | Fez (6) Meknes (2) |
Cul |
| Amaranthaceae | |||||
| Beta vulgaris L. | Barba | 0.33 | 25 | Meknes (1) | Cul |
| Annonaceae | |||||
| Annona cherimola Mill. | Chirimoya | 1 | 33.33 | Fez (3) | Imp |
| Apiaceae | |||||
| Petroselinum crispum (Mill.) Fuss | ma'adnūs | 2.67 | 61.53 | Meknes (2) Fez (5) Taounate (1) |
Cul |
| Cuminum cyminum L. | Kemmūn | 1 | 33.33 | Fez (3) | Cul |
| Apium graveolens L. | Krāfes | 1 | 60 | Fez (3) | Sp/ Cul |
| Ammi visnaga (L.) Lam. | bešnīha | 0.33 | 25 | Fez (1) | Sp |
| Foeniculum vulgare Mill. | nāfa' | 0.33 | 20 | Taounate (1) | Sp/ Cul |
|
Daucus carota L. |
hizzu | 1 | 37.5 | Fez (2) Meknes (1) |
Cul |
| Ammodaucus leucotrichus Coss. | kammūn eṣ-ṣōfi | 0.33 | 33.33 | Fez (1) | Sp |
| Pimpinella anisum L. | ḥabbat ḥalāwa | 0.33 | 33.33 | Taounate (1) | Cul |
| Thapsia garganica L. | Deryās | 0.33 | 33.33 | Fez (1) | Sp |
| Magydaris panacifolia (Vahl.) Lange | Frifra | 0.33 | 50 | Meknes (1) | Sp |
| Apocynaceae | |||||
| Apteranthes europaea (Guss.) Murb. | Daghmos | 17.67 | 89.83 | Taounate (3) Fez (40) Meknes (10) |
Sp |
| Arecaceae | |||||
| Phoenix dactylifera L. | nnhel ’Jmar |
0.33 | 20 | Fez (1) | Sp |
| Aristelochiaceae | |||||
| Aristolochia longa L. | berezṭom | 12 | 85.71 | Fez (31) Meknes (3) Taounate (2) |
Sp |
| Asteraceae | |||||
|
Artemisia herba-alba Asso. |
Chih | 4 | 60 | Fez (10) Meknes (1) Taounate (1) |
Sp |
| Dittrichia viscosa (L.) | māgrāmān terhalā |
1 | 60 | Fez (2) Meknes (1) |
Sp |
| Atractylis gummifera L. | Ddād | 0.33 | 50 | Fez (1) | Sp |
| Chamaemelum nobile (L.) All. | Bābnūj | 0.33 | 14.28 | Fez (1) | Cul |
| Cynara cardunculus L. | horšef | 0.33 | 25 | Fez (1) | Cul |
| Berberidaceae | |||||
| Berberis hispanica Boiss. & Reut. | Arġīs | 3 | 64.28 | Fez (6) Taounate (3) |
Sp |
| Boraginaceae | |||||
| Borago officinalis L. | lisān aṯ-ṯūr | 0.33 | 25 | Fez (1) | Sp |
| Brassicaceae | |||||
|
Lepidium sativum L. |
ḥabb er-ršād | 1.33 | 50 | Fez (2) Meknes (1) Taounate (1) |
Cul |
| Brassica oleracea L. | Suflur | 0.33 | 50 | Fez (1) | Cul |
| Brassica rapa L. | Left | 0.33 | 33.33 | Fez (1) | Cul |
| Raphanus raphanistrum subsp. sativus (L.) Domin | Lefjel | 0.33 | 25 | Taounate (1) | Cul |
| Sinapis alba L. | Khardal | 1 | 50 | Fez (1) | Sp /Cul |
| Cactaceae | |||||
| Opuntia ficus-indica (L.) Mill. | Hendi/zaaloul | 1.33 | 50 | Taounate (2) Meknes (2) |
Sp/ Cul |
| Camelliaceae | |||||
| Camellia sinensis (L.) Kuntze | Atāy | 1.67 | 41.66 | Fez (4) Taounate (1) |
Imp |
| Cannabaceae | |||||
| Cannabis sativa L. | l-kīf | 0.33 | 25 | Fez (1) | Cul |
| Capparidaceae | |||||
|
Capparis spinosa L. |
Kabār | 2 | 60 | Fez (2) Taounate (3) Meknes (1) |
Sp |
| Caryophyllaceae | |||||
| Herniaria hirsuta L. | herras leḥjar | 0.33 | 25 | Meknes (1) | Sp |
| Cucurbitaceae | |||||
| Citrullus lanatus(Thunb.) Matsum. & Nakai | Hadja | 0.33 | 25 | Fez (1) | Sp |
| Cucumis sativus L. | hiyār | 0.33 | 33.33 | Fez (1) | Sp |
| Ephedraceae | |||||
| Ephedra alata Decne | l-a'lenda | 0.67 | 40 | Meknes (1) Fez (1) |
Sp |
| Euphorbiaceae | |||||
| Euphorbia resinifera O.Berg | zakkūm /tikiūt ddaġmūs |
0.33 | 25 | Fez (1) | Sp |
| Fabaceae | |||||
| Vicia faba L. | Fūl | 0.33 | 25 | Fez (1) | Cul |
| Cicer arietinum L. | l-ḥommṣ | 0.33 | 25 | Fez (1) | Cul |
| Vicia ervilia (L.) Willd. | Kersenna | 0.33 | 33.33 | Fez (1) | Sp + cul |
|
Trigonella foenum-graecum L. |
l-ḥelba | 4.67 |
53.84 | Fez (12) Meknes(1) Taza(1) |
Cul |
| Glycyrrhiza glabra L. | 'arq as-sūs | 0.33 | 16.66 | Fez (1) | Sp |
| Fagaceae | |||||
|
Quercus ilex L. |
al-bellūṭ | 0.67 | 28.57 | Fez (1) Meknes (1) |
Sp |
| Geraniaceae | |||||
| Erodium guttatum (Desf.) Willd. | Wadmi | 1 | 13 | Fez (1) | Sp |
| Lamiaceae | |||||
|
Marrubium vulgare L. |
merrīwut, merrīwa īfzi |
7.33 | 62.85 | Fez (14) Meknes (5) Taounate (2) My Yacoub (1) |
Sp |
| Ajuga iva (L.) Schreb. | Sendgūra | 1.33 | 44.44 | Fez (3) Meknes (1) |
Sp |
| Rosmarinus officinalis L. | Azīr | 1.33 | 36.36 | Fez (3) Taounate (1) |
Sp + Cul |
| Origanum vulgare L. | Zaatar/Sahtar | 1.67 | 33.33 | Fez (3) Taounate (2) |
Sp + Cul |
| Satureja granatensis (Boiss. & Reut.) Sennen & Mauricio | z'ītra | 0.33 | 20 | Taounate (1) | Sp |
|
Salvia officinalis L. |
Sālmiya | 1.67 | 35.71 | Fez (4) My Yacoub (1) |
Cul |
| Origanum majorana L. | Merdedūš | 1 | 33.33 | Fez (3) | Cul |
| Lauraceae | |||||
| Cinnamomum camphora (L.) J.Presl | Kāfūr | 0.33 | 33.33 | Fez (1) | Imp |
| Liliaceae | |||||
|
Allium sativum L. |
ṯūm, ṯūma | 9.33 | 63.63 | Fez (19) Meknes (4) Taounate (5) |
Cul |
|
Allium cepa L. |
l-beṣla hamra | 3.33 | 55.55 | Fez (5) Meknes(5) |
Cul |
| Drimia maritima (L.) Stearn | Bsel l 'anṣal | 0.67 | 33.33 | Fez (2) | Sp |
| Allium ampeloprasum L. | Borrō | 0.67 | 13 | Fez (1) Meknes (1) |
Cul |
| Linaceae | |||||
| Linum usitatissimum L. | zerrī'at l-kettān | 2 | 60 | Fez (2) | Cul |
| Malvaceae | |||||
| Malva sylvestris L. | l-hubbeyza | 0.33 | 33.33 | Taounate (1) | Sp |
| Meliaceae | |||||
| Rubia cordifolia L. | L'acajou | 0.33 | 20 | Meknes (1) | Cul |
| Mimosaceae | |||||
| Acacia raddiana Savi | ṭalḥ | 0.33 | 20 | Fez (1) | Sp |
| Vachellia seyal (Delile) P.J.H.Hurter | l-’alk | 0.33 | 25 | Fez (1) | Sp |
| Moraceae | |||||
|
Ficus carica L. |
qormīš | 1.66 | 41.66 | Fez (2) Taounate (3) |
Cul |
| Myrtaceae | |||||
| Eugenia caryophyllata Thunb. | qoronfel | 0.67 | 20 | Fez (2) | Imp |
| Oleaceae | |||||
|
Olea europaea L. |
Zaytūn | 2.67 | 42.10 | Fez (7) Meknes (1) |
Sp + Cul |
| Pedaliaceae | |||||
| Sesamum indicum L. | Jeljlān | 0.67 | 25 | Fez (2) | Cul |
| Pinaceae | |||||
| Cedrus atlantica (Endl.) Manetti ex Carrière | l-ārz | 0.33 | 33.33 | Taounate (1) | Sp |
| Pinus halepensis Mill. | Taydā | 0.67 | 66.66 | Fez (1) | Sp |
| Piperaceae | |||||
| Piper nigrum L. | l-yebzār | 0.33 | 12.5 | Fez (1) | Imp |
| Poaceae | |||||
| Hordeum vulgare L. | ša’īr | 1 | 50 | Fez (1) | Cul |
| Triticum aestivum L. | l-gemḥ | 0.33 | 25 | Fez (1) | Cul |
| Portulacaceae | |||||
| Portulaca oleracea L. | Rejla | 0.33 | 50 | Taounate (1) | Sp |
| Punicaceae | |||||
|
Punica granatum L. |
er-rummān | 1.33 | 40 | Fez (1) Taounate (2) Meknes (1) |
Cul |
| Ranunculaceae | |||||
|
Nigella sativa L. |
Sānūj/ Haba Souda/ ḥabbet el baraka |
10 | 71.42 | Fez (18) Meknes (4) Taounate (4) My yacoub (4) |
Cul |
| Rhamnaceae | |||||
| Ziziphus jujube Mill. | Nbak/Sadra | 0.33 | 16.66 | Taounate (1) | Sp |
| Rosaceae | |||||
| Fragaria × ananassa (Duchesne ex Weston) Duchesne ex Rozier | l-frez | 0.33 | 50 | Taounate (1) | Cul |
| Rutaceae | |||||
|
Citrus aurantium L. |
ḥāmmed beldī | 1.33 | 40 | Taounate (1) Fez (1) |
Cul |
| Solanaceae | |||||
| Solanum lycopersicum L. | maṭīša | 0.33 | 33.33 | Fez (1) | Cul |
| Solanum tuberosum L. | bṭāṭa | 0.33 | 50 | Fez (1) | Cul |
| Urticaceae | |||||
| Urtica dioica L. | Horiga | 0.67 | 40 | Fez (2) | Sp |
| Vitaceae | |||||
| Vitis vinifera L. | Aanab | 1 | 37.5 | Taounate | Cul |
| Zingiberaceae | |||||
| Curcuma longa L. | harqum | 2.67 | 57.14 | Fez (8) | Imp |
|
Zingiber officinale Roscoe |
skenjbīr, skenjabīl | 3 | 56.25 | Fez (5) My Yacoub (1) Meknes (1) |
Imp |
| Elettaria cardamomum (L.) Maton | qa’qolla | 0.33 | 33.33 | Fez (1) | Imp |
| Zygophylaceae | |||||
|
Peganum harmala L. |
Harmal | 1.33 | 66.66 | My Yacoub (3) Fez (1) |
Sp |
Sp: Spontaneous; Cul: Cultivated; Imp: Imported.
Informants provided data on plants used for the treatment of cancer in the fez Meknes region including methods of preparation, plant parts used as well as the administration mode. The obtained results are summarized in.
Table 2.
Information about medicinal plants used alone for the treatment of cancer in the region of Fez-Meknes.
|
Scientific name Family |
Parts used |
Preparation Modes |
Solvant/ Excipient |
Administraion route |
Cancer type |
|---|---|---|---|---|---|
| Actinidiaceae | |||||
| Actinidia chinensis var. deliciosa (A.Chev.) A.Chev. | F | Raw form Juice |
_ Milk |
Oral | Colon |
| Aloaceae | |||||
| Aloe vera (L.) Burm.f | Ap | Powder Powder mixed with honey Vegetable oil Juice Raw form |
Water _ _ Water _ |
Oral | Leukemia/ Liver |
| Amaranthaceae | |||||
| Beta vulgaris L. | F | Raw form |
_ | Oral | Leukemia |
| Annonaceae | |||||
|
Annona cherimola Mill. |
F | Raw form |
_ | Oral | Breast |
| Apiaceae | |||||
| Petroselinum crispum (Mill.) Fuss | Ap L St + L |
Decoction Infusion Infusion |
Water Water Water |
Oral |
Uterus |
| Cuminum cyminum L. | Sd F |
Powder Powder mixed with honey Powder |
Water/ Oliveoil/ lemon juice _ Water |
Oral | Stomach/ Liver |
| Apium graveolens L. | L | Infusion | Water | Oral | Leukemia |
| Ammi visnaga (L.) Lam. | Fl | Decoction | Water | Oral | Colon |
| Foeniculum vulgare Mill. | Sd | Powder mixed with honey | _ | Oral | Colon/ Breast/ Leukaemia |
| Daucus carota L. | F | Juice | Water | Oral | Leukaemia/ Colon/Lung |
| Ammodaucus leucotrichus Coss. | L + Fl | Powder | Water | Oral | Lung/ Leukemia |
| Pimpinella anisum L. | Sd | Powder | Water | Oral | Colon |
| Thapsia garganica L. | R | Infusion Mixed with honey |
Water _ |
Oral | Breast/ Prostate/ Liver |
| Magydaris panacifolia (Vahl.) Lange | _ | Mixed with honey | _ | Oral | Breast |
| Apocynaceae | |||||
| Apteranthes europaea (Guss.) Murb. | Ap | Powder Powder mixed with honey Juice |
Rancid butter Milk/ Water _ Lemon juice |
Oral | Uterus/ Breast/ Lung/ Leukemia |
| Arecaceae | |||||
| Phoenix dactylifera L. | F | Raw form | _ | Oral | Liver |
| Aristelochiaceae | |||||
| Aristolochia longa L. | R | Powder Powder mixed with honey Raw form Decoction/ Infusion |
Rancid butter/ Olive oil / Water/ Milk _ _ Water |
Oral | Breast/ Uterus/ Colon/ Lung/ Bone/ Prostate/ Ovary/ Stomach |
| Asteraceae | |||||
| Artemisia herba-alba Asso. | L ; AP | Decoction/ Infusion Powder mixed with honey |
Water _ |
Oral Poultice/ Oral |
Lung |
| Dittrichia viscosa (L.) | L | Powder mixed with honey Infusion |
_ Water |
Poultice Oral |
Colorectal |
| Atractylis gummifera L. | R | Incense | _ | Inhalation | Lung |
| Chamaemelum nobile (L.) All. | F | Infusion | Water | Oral | Uterus |
| Berberidaceae | |||||
| Berberis hispanica Boiss. & Reut. | R/Ap/St | Powder Powder mixed with honey |
Milk _ |
Oral | Breast |
| Borraginacees | |||||
| Borago officinalis L. | AP /Sd |
Powder | Water | Oral | Breast |
| Brassicaceae | |||||
| Lepidium sativum L. | Sd | Decoction Mixed with honey |
Water _ |
Oral | Lung/ Bone/Uterus |
| Brassica oleracea L. | Ap | Raw form | _ | Oral | Breast |
| Raphanus raphanistrum subsp. sativus (L.) Domin | F | Raw forme | _ | Oral | Leukemia |
| Sinapis alba L. | F | Powder | _ | Oral | Colon |
| Cactaceae | |||||
| Opuntia ficus-indica (L.) Mill. | F C |
Raw form Raw form Juice |
_ _ Water |
Oral | Bone/ Prostate |
| Camelliaceae | |||||
| Camellia sinensis (L.) Kuntze | L | Decotion/Infusion | Water (Sugar free) |
Oral | Breast/ Bone |
| Cannabaceae | |||||
| Cannabis sativa L. | Sd | Powder mixed with honey | _ | Oral | Brain |
| Capparidaceae | |||||
| Capparis spinosa L. | F L |
Powder Powder mixed with honey |
Vegetable oil/ Water _ |
Oral | Breast/Uterus |
| Caryophyllaceae | |||||
| Herniaria hirsuta L. | Ap | Powder | Water | Oral | Leukemia |
| Cucurbitaceae | |||||
| Citrullus lanatus(Thunb.) Matsum. &Nakai | F | Juice | Water | Oral | Breast/ Leukemia |
| Cucumis sativus L. | F | Raw form | _ | Oral | Leukemia |
| Ephedraceae | |||||
| Ephedra alata Decne | Ap | Decoction | Water | Oral | Breast |
| Euphorbiaceae | |||||
| Euphorbia resinifera O.Berg | Ap/R | Powder mixed with honey Decoction + Maceration with Cicer arietinum |
_ Water/water |
Oral Oral |
Breast/ Lung/ Uterus/ Leukemia |
| Fabaceae | |||||
| Vicia faba L. | Sd | Powder mixed with honey | _ | Oral | Breast |
| Cicer arietinum L. | F | Raw form | Water | Oral | Leukemia |
| Vicia ervilia (L.) Willd. | Sd | Powder | Milk | Oral | Breast |
| Trigonella foenum-graecum L. | Sd |
Raw form Powder infusion Powder mixed with honey |
Water Water _ |
Oral Oral |
Colon/ Breast/ Uterus/ Colon/ Lung/ Leukemia/ Stomach |
| Glycyrrhiza glabra L. | Ap | Powder mixed with honey | _ | Oral | Breast/ Colon |
| Fagaceae | |||||
| Quercus ilex L. | F | Juice Steamed |
Milk |
Oral | Leukemia |
| Geraniaceae | |||||
| Erodium guttatum (Desf.) Willd. | R | Powder Powder mixed with honey |
Milk | Oral | Bone |
| Lamiaceae | |||||
| Marrubium vulgare L. | Ap | Decoction/infusion Powder Powder mixed with honey |
Water Water _ |
Oral | Colon/ Breast/ Uterus/ Leukaemia/ Stomach |
| Ajuga iva (L.) Schreb. | Fl L |
Powder mixed with honey Powder |
_ Water |
Oral | Breast |
| Rosmarinus officinalis L. | L | Decoction/infusion | Water | Oral | Colon/ Breast/ Uterus/ Stomach |
| Origanum vulgare L. | Ap | Infusion | Water | Oral | Stomach |
| Satureja granatensis (Boiss. & Reut.) Sennen & Mauricio | Ap | Infusion | Water | Oral | Lung |
| Salvia officinalis L. | L | Infusion Powder |
Water Water |
Oral | Lung/ Prostate |
| Origanum majorana L. | L | Powder Infusion |
Water Water |
Oral | Breast |
| Lauraceae | |||||
| Cinnamomum camphora (L.) J.Presl | Powder | _ | Oral | Uterus | |
| Liliaceae | |||||
| Allium sativum L. | B | Cooked with chicken Juice Maceration Raw form mixed with honey |
Olive oil Olive oil _ |
Oral | Breast/ Uterus/ Colon/ leukemia / Prostate |
| Allium cepa L. | B | Cooked with chicken Juice |
_ Water |
Oral | Leukemia/ Breast/ Uterus/ Colon/ Lung/ Prostate/ Ovary |
| Drimia maritima (L.) Stearn | R |
Maceration Infusion |
Apple cider vinegar Water |
Oral | Lung |
| Allium ampeloprasum L. | _ | Infusion | Water | Oral | Breast |
| Linaceae | |||||
| Linum usitatissimum L. | Sd |
Powder Powder/ Raw form mixed with honey Raw form |
Olive oil /Water _ Water |
Oral | Breast/ Prostate/ Colon/ Leukemia/ Ovary |
| Malvaceae | |||||
| Malva sylvestris L. | Ap | cooked (dish) | _ | Oral | Leukemia/ Breast |
| Meliaceae | |||||
| Rubia cordifolia L. | N | Raw form | _ | Oral | Leukemia |
| Mimosaceae | |||||
| Acacia raddiana Savi | Rs | Infusion | Water | Leukemia | |
| Vachellia seyal (Delile) P.J.H.Hurter | Rs | Infusion | Water | Oral | Leukemia |
| Moraceae | |||||
| Ficus carica L. | F |
Powder mixed with honey Maceration (One week) |
_ Olive oil |
Oral | Lung/ Prostate/ Breast/ Uterus |
| Myrtaceae | |||||
| Eugenia caryophyllata Thunb. | Sd | Powder | Water | Oral | Stomach |
| Oleaceae | |||||
| Olea europaea L. | F L |
vegetable oil Decoction |
_ Water |
Oral | Breast/ Colon/ Stomach/ Leukemia/ Uterus/ Lung/ |
| Pedaliaceae | |||||
| Sesamum indicum L. | Sd | Powder mixed with honey | _ | Oral | Lung/ Pancreatic |
| Pinaceae | |||||
| Cedrus atlantica (Endl.) Manetti ex Carrière | F | Powder mixed with honey | _ | Oral | Lung |
|
Pinus halepensis Mill. |
St + L | Decoction/Infusion | Water | Oral | Lung |
| Piperaceae | |||||
| Piper nigrum L. | Sd | Powder (spice) | _ | Oral | Breast/Colon |
| Poaceae | |||||
| Hordeum vulgare L. | Sd | Powder (Soup ; Bread) Infusion |
Water + Milk Water |
Oral | Colon |
| Triticum aestivum L. | Sd | Powder (Bread) Infusion |
Water | Oral | Colon/ Stomach |
| Portulacaceae | |||||
| Portulaca oleracea L. | Ap | cooked (dish) | _ | Oral | Stomach |
| Punicaceae | |||||
| Punica granatum L. | E M |
Decoction/Infusion Raw form |
Water _ |
Oral | Breast |
| Ranunculaceae | |||||
| Nigella sativa L. | Sd |
Powder Powder mixed with honey Infusion |
Milk _ Water |
Oral | Colon/ Breast/ Leukemia/ Uterus/ Lung |
| Rhamnaceae | |||||
| Ziziphus jujube Mill. | F | Powder mixed with honey | _ | Oral | Breast |
| Rosaceae | |||||
| Fragaria × ananassa (Duchesne ex Weston) Duchesne ex Rozier | F | Raw form | _ | Oral | Breast |
| Rutaceae | |||||
| Citrus aurantium L. | F | Juice | _ | Oral | Breast/ Colon/ Lung |
| Solanaceae | |||||
| Solanum lycopersicum L. | F | Raw form | _ | Oral | Prostate |
| Solanum tuberosum L. | _ | Juice | Water | Oral | Breast |
| Urticaceae | |||||
| Urtica dioica L. | L | Infusion | Water | Oral | Leukemia |
| Vitaceae | |||||
| Vitis vinifera L. | F | Raw form | _ | Oral | Breast/ Uterus/ Leukemia |
| Zingiberaceae | |||||
| Curcuma longa L. | R St |
Powder Decoction/ Mixed with honey |
tea/Milk/Water/ Spice Water/ Lemon juice |
Oral | Breast/ Leukemia/ Uterus/ Colon/ Stomach/ Lung/ Bone |
|
Zingiber officinale Roscoe |
Ap R |
Decoction Raw form Powder mixed with honey |
Water Water _ |
Oral |
Breast/Colon/ Prostate/ Uterus |
| Elettaria cardamomum (L.) Maton | Sd | Powder | Orange juice | Oral | Breast |
| Zygophylaceae | |||||
| Peganum harmala L. | Sd/St |
Powder/ mixed with honey |
Pomegra nate juice / Apple juice |
Oral | Lung / Breast |
Ap : Aerial part ; B : Bulb; C : Cladode; E : Exocarp ; F : fruits; Fl : Flowers; L : Leaves;
M : Mesocarp ; N : Nut; Rs: Resin; R : Roots ; Sd : Seeds ; Sh : Shoot ; St : Stem ; T : Tubers ; Wp : Whole plant.
Fidelity level parameter permits the identification of the most abundant species used to treat cancer in the study area. Among 94 inventoried plants 14 species were identified with FL equal or greater than 60%.
In the current research study, it was reported that Apteranthes europaea Guss. (89.83 %), Aristolochia longa L. (85.71 %), Nigella sativa L. (71.42 %), Peganum harmala L. (66.66 %), Pinus halepensis Mill. (66.66 %), Berberis hispanica Boiss. & Reut. (64.28 %), Allium sativum L. (63.63 %) and Marrubium vulgare L. (62.85 %) were the most common medicinal plants used for cancer treatment in the Fez-Meknes region. Our study agrees with the earlier literature which showed that plants higher in FL values are recommended for cancer treatment (Tadesse et al., 2018).
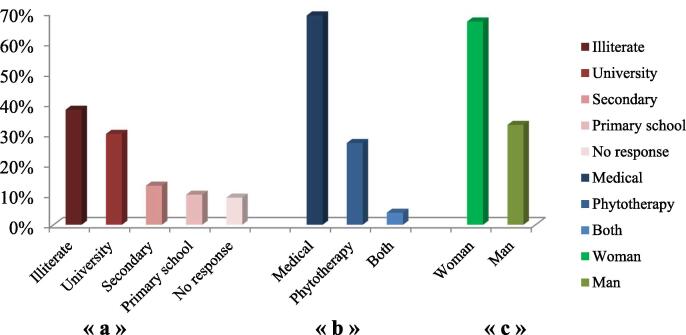
The use of medicinal plants is more among women rather than men (67% vs. 33%) according to our survey (Fig. 2c). Women are more knowledgeable about the uses of medicinal plants. Apart from this, women of the region studied also impart knowledge to their wards either verbally or written. These results were in accordance with the earlier data (Samouh et al., 2019). In the current survey, it was reported that several informants were illiterate (Fig. 2.a). This finding was in agreement with other ethnobotanical studies (Kabbaj et al., 2012);(Ammor et al., 2020) . 69% of respondents prefer the use of modern medicine against cancer vs. 27 % prefer the use of herbal medicine. However, only 4 % of informants prefer the combination of both (Fig. 2.b). Reasons that make many people based in the study area using herbal medicines for medication against cancer have already been reported in the previous literature (Bourhia et al., 2019). The latter reported the effectiveness of traditional preparations in the treatment of cancer, less side effects compared to conventional drugs, inaccessibility to modern medicines for those living in rural areas, the high and rising cost of medical care for people with low income. However, many informants (69 %) as reported in our survey are no longer interested in using traditional medicine for medication against cancer. This result can be explained by the fact that several informants are aware of side effects of the uncontrolled use of plants in the treatment, potential toxicities of plants, lack of scientific validity for plants randomly used in the treatment (González-Tejero et al., 2008).
Fig. 2.
General profile of informants (Fez-Meknes Region).
« a »: Educational Level; « b »: Healthcare choices; « c »: Gender of informants
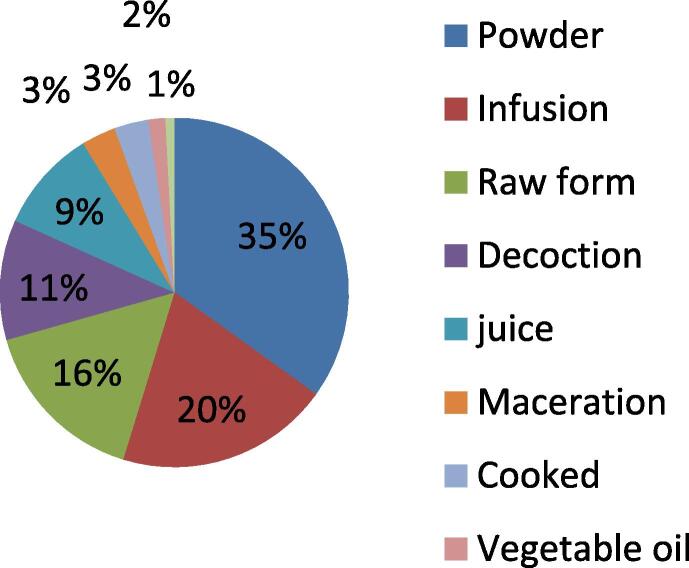
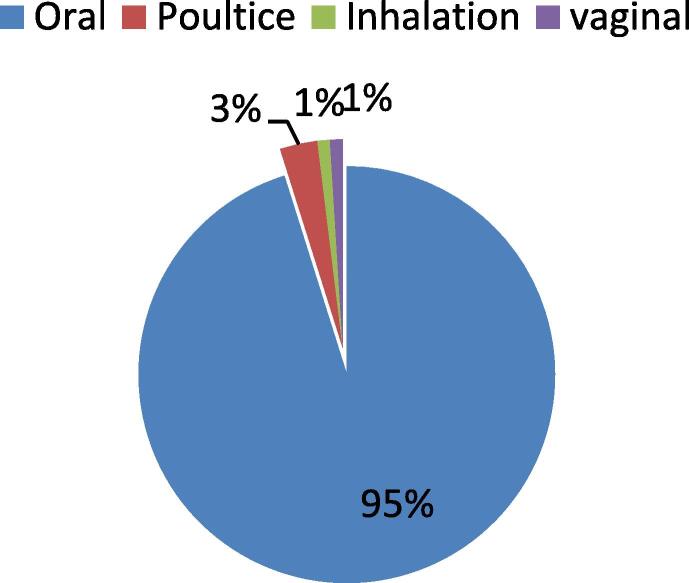
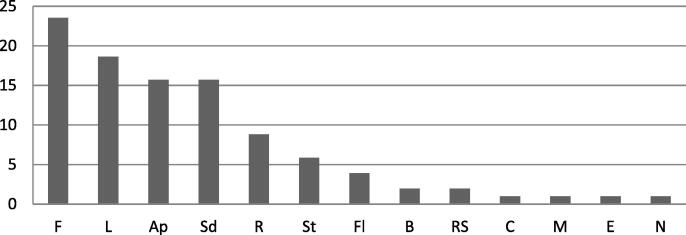
In the present research work, we found that the the mostely used plants for the treatment of cancer in the region are prepared into powder form 35 %, infusion 20 %, raw form 15 %, decoction 11 %, juice 6 %, maceration and cooking 3 %, vegetable oil 2 % and incense 1 % (Fig. 3). 95 % of remedies are taken orally (Fig. 4). Fruits are the mostely used plant parts with a percentage of 23.53 %, followed by leaves 18, 63 %, aerial parts, and seeds 15.69 % in each, roots 8.82 %, stems 5.88 %, flowers 3.92 %. However, Bulb, resin, cladode, mesocarp, exocarp, and nut are rarely used (Fig. 5).
Fig. 3.
Mode of preparation.
Fig. 4.
Mode of administration.
Fig. 5.
Percentage of plant parts used for cancer treatment in the study area.
F : fruits; L : Leaves; Ap : Aerial part ; Sd : Seeds; R : Roots ; St : Stem; Fl : Flowers; B : Bulb; Rs: Resin; C : Cladode; M : Mesocarp; E : Exocarp; N : Nut.
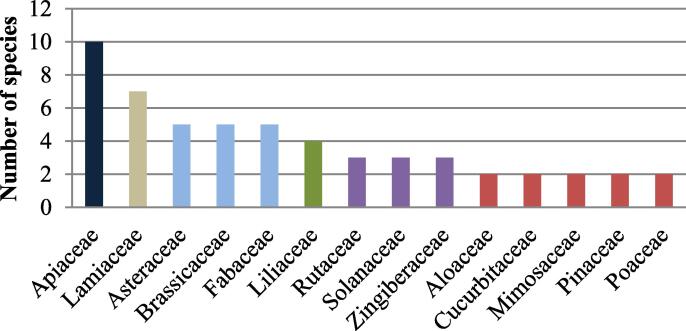
The most useful plant families for the treatment of cancer in the Fez-Meknes region are Apiaceae (10 species), followed by Lamiaceae (7 species), Asteraceae, Brassicaceae, Fabaceae (5 species in each), and Liliaceae (4 species) (Fig. 6). This ethnobotanical survey disagrees with another survey conducted in the Greater Casablanca region which reported that Aristolochiaceae is the most common plant families used in the traditional treatment of cancer (Bourhia et al., 2019).
Fig. 6.
Number of plant species per family.
The results of the present survey showed that 31.67 % of respondents were aged between 21 and 30 years, 23.33 % between 31 and 40 years, 17.33 % between 41 and 50 years, and 15.33 % between 51 and 60 years (Table 3). These results are in accordance with the earlier literature (Agyare et al., 2018).
Table 3.
Distribution of informants according to age group.
| Age range | Percentage % |
|---|---|
| [19–20] [21–30] [31–40] [41–50] [51–60] greater than 60 No answer |
2.33 31.67 23.33 17.33 15.33 73 |
The treatment of cancer with traditional medicines in the study area goes back to many decades ago according to the informant’s response. The results of the present survey summarize the old traditional use of herbal medicine in fighting cancer in the Fez Meknes region (Table 4).
Table 4.
Recipes used to treat cancer in the fez-Meknes region.
| Recipe | Scientific name | Preparation mode |
Administration route |
|---|---|---|---|
| Jeljlān Sanouj lkhal Sanouj lhmar |
Sesamum indicum L. Nigella sativa L. Lepidium sativum L. |
Powders mixed with olive oil | Oral |
| Habat lhalwa Fliyo Zaatar Z'ītra |
Pimpinella anisum L. Mentha pulegium L. Origanum vulgare L. Satureja granatensis (Boiss. & Reut.) Sennen & Mauricio |
Powder Powder Powder Powder |
Oral |
| Haba souda Tūma |
Nigella sativa L. Allium sativum L. |
Powder Raw form (Mixed with honey) |
Oral |
| Merrīwut Magraman |
Marrubium vulgare L. Dittrichia viscosa (L.) |
Powder Powder |
Vaginal inhalation |
|
Khizou Hommṣ Laft Kebbar Jeljlān |
Daucus carota L. Cicer arietinum L. Brassica rapa L. Capparis spinosa L. Sesamum indicum L. |
Maceration of the seeds in the water then mix with the banana juice |
Oral |
| Horiga Marrîwet Zit Zaytūn |
Urtica dioica L. Marrubium vulgare L. Olea europaea L. |
Raw form (Leaves) Raw form (Aerial part) (Mixed with olive oil) |
Oral |
| hiyār Kiwi Ma'adnūs |
Cucumis sativus L. Actinidia chinensis var. deliciosa (A.Chev.) A.Chev. Petroselinum crispum(Mill.) Fuss |
Juice | Oral |
| pBerraztem Lhaba souda |
Aristolochia longa L. Nigella sativa L. |
Powder Powder (Mixed with honey) |
Oral |
| Tūma Lhaba souda |
Allium sativum L. Nigella sativa L. |
Raw form Powder |
Oral |
| Bṭāṭa hizzu Barba Krāfes |
Solanum tuberosum L. Daucus carota L. Beta vulgaris L. Apium graveolens L. |
Boiled and plucked | oral |
| ṯūma Skenjbīr |
Allium sativum L. Zingiber officinale Roscoe |
Raw form Powder (Mixed with eggs) |
oral |
| ma'adnūs l-beṣla hamra |
Petroselinum crispum(Mill.) Fuss Allium cepa L. |
Raw form Raw form |
Oral |
| Khorchf sekoum |
Cynara cardunculus L. Euphorbia resinifera O.Berg |
Raw form (Steems) Powder (roots, steems) + Water |
Oral |
| borrō ma'adnūs |
Allium ampeloprasum L. Petroselinum crispum(Mill.) Fuss |
mixed with honey and lemon juice | Oral |
Anticancer activity of the main reported plants in our survey against breast, leukemic, cervical lymphoma, laryngeal, lung, liver, and kidney cancers has already been investigated in previous research works to have significant anticancer effects under both in vitro and in vivo conditions as shown in Table 5. Apteranthes europaea (Guss.), Nigella sativa L., Zingiber officinale Roscoe, Petroselinum crispum (Mill.) Fuss, and Capparis spinosa L. showed an antiproliferative activity on breast cancer cell lines (Amrati et al., 2020a); (Periasamy et al., 2016); (Rahman et al., 2011); (Farshori et al., 2013); (Aljaiyash et al., 2014), Artemisia herba-alba Asso, Allium cepa L., and Olea europaea L. on leukemic cell lines (Khlifi et al., 2013);(Votto et al., 2010);(Fares et al., 2011). Marrubium vulgare L. and Linum usitatissimum L. on cervical cancer cells (Zarai et al., 2011); (Joseph et al., 2020) by the time Aristolochia longa L.and Curcuma longa L. on lung cancer (Hinou et al., 1990); (Wu et al., 2010). Trigonella foenum-graecum L. found effective vs. T-cell lymphoma (Alsemari et al., 2014). Berberis hispanica Boiss. & Reut. vs. human laryngeal cancer cells Hep-2 (Boudjlida et al., 2019). In vivo tests showed an important effect of Allium sativum L. on the reduction of the frequency progression of colorectal adeno carcinoma and bladder cancers (Wargovich, 1987) (Table 5).
Table 5.
Literature about medicinal plants used to fight cancer in the Fez-Meknes region.
| Plants | The medical interest of the inventoried plants according to the literature | Ethnopharmacological use |
|---|---|---|
| Apteranthes europaea (Guss.) Murb. | Cytotoxic activity on MDA-MB-231 and MCF-7 lin cells of breast cancer (Amrati et al., 2020b). | Anti-inflammatory, ulcer, antidiabetic, and anti-bacterial (Adnan et al., 2014) |
| Aristolochia longa L. | Apoptogenic activity on Burkitt’s lymphoma BL41 cells (Benarba et al., 2012). |
Abortifacient, sedative, analgesic, anti-inflammatory, anti-feedant, muscle relaxant, antihistaminic, and anti-allergic (Benarba and Meddah, 2014). |
| Cytotoxic activity against lymphocytic leukemia (P-388) and bronchial epidermoid carcinoma of human origin (NSCLCN6) (Hinou et al., 1990). | ||
| Nigella sativa L. | Anticancer activity on human breast cancer cells: MCF-7 (Periasamy et al., 2016). |
Treatment of rheumatoid arthritis, diabetes, asthma, Obesity, and other metabolic disorders (Namazi et al., 2018). |
| Anticancer effect on human lung carcinoma (A549), larynx epidermoid carcinoma (HEp-2), colon adenocarcinoma (HT-29), and pancreas carcinoma (MIA PaCa-2) (Rooney and Ryan, 2005). | ||
| Allium sativum L. | Reduction of the frequency progression of colorectal adeno carcinoma (Wargovich, 1987) |
Prevention and treatment of atherosclerosis, cardiovascular, hyperlipidemia, thrombosis, hypertension, microbial infections, diabetes, and asthma (Lyantagaye, 2011). |
| Treatment of bladder cancer (Lamm and Riggs, 2000) | ||
| Marrubium vulgare L. | Cytotoxic activity against the cervical cancer lines: HeLa cells (Zarai et al., 2011). | Neurosedative and anti-inflammatory (Sahpaz et al., 2002). |
| Trigonella foenum-graecum L. | Cytotoxic effect against T-cell lymphoma (Alsemari et al., 2014). | Antidiabetic, carminative, tonic, and antinociceptive (Hamza et al., 2012); (Javan et al., 1997); (Zia et al., 2001). |
|
Artemisia herba-alba Asso. |
Antiproliferative activity against P815 and BSR kidney carcinoma cell lines (Tilaoui et al., 2015). |
Treatment of infectious diseases, inflammatory disorders (colds, coughing, bronchitis, diarrhea), diabetes, neuralgias, antiseptic and against skin diseases, scabies, syphilis, fever as well as menstrual and nervous disorders (Abu-Darwish et al., 2015). |
| Anticancer activity against human bladder carcinoma RT112, and human myelogenous leukemia K562 (Khlifi et al., 2013). | ||
| Allium cepa L. | Cytotoxic activity vs. multidrug resistant erythroleukemic cell lines: (Lucena and K562) (Votto et al., 2010). | Blood purifier, infectious diseases, digestive problems, skin diseases, metabolic disease, insect bites (Teshika et al., 2018). |
| Apoptosis and suppression of Bcl-2 through inhibition of PI3K/Akt Signaling Pathway in AGS human cancer cells (Lee et al., 2014). | ||
| Berberis hispanica Boiss. & Reut. | Apoptotic effect in human laryngeal cancer cells Hep-2 (Boudjlida et al., 2019). | Treatment of gastrointestinal affections, liver inflammation, and biliary disorders (el Hamsas el Youbi, 2011) |
|
Zingiber officinale Roscoe |
Ameliorate chemotherapy- induced nausea and vomiting (Jagetia et al., 2003). | Treatment of airway infections, nausea, and spasm (Moghaddasi and Haddad Kashani, 2012), (Chang et al., 2013). |
| Anticancer activity against human breast carcinoma cell lines (MCF-7 cancer cells and MDA-MB-231 (Rahman et al., 2011). | ||
| Effect on cancer cell growth of human Hela cancer cells (Cheng et al., 2011). | ||
| Petroselinum crispum (Mill.) Fuss | Induction cell death in MCF-7 cells (Farshori et al., 2013). | Treatment of gastrointestinal disorder, inflammation, halitosis, kidney stone, amenorrhoea, dermatitis, headcool, vision performance, hemorrhoid (Farzaei et al., 2013). |
| Reduction of the cell viability of HepG2 (Human Hepatocellular Carcinoma Cells) (Farshori et al., 2014). | ||
| Olea europaea L. | Anti-proliferative activity on human leukemic cell line (Jurkat) (Fares et al., 2011). | Hypertension, inflammatory diseases, diabetes, diarrhea, respiratory and urinary tract infections, stomach, intestinal diseases, asthma, and rheumatism (Scheffler et al., 2008), (Song et al., 2019). |
| Anti-cancer effect on human BRAF melanoma cells (Ruzzolini et al., 2018). | ||
| Curcuma longa L. | Cytotoxic activity against lymphocytes and Dalton's lymphoma cells (Kuttan et al., 1985). | Biliary disorders, anorexia, cough, diabetic wounds, hepatic disorders, rheumatism and sinusitis (Kumar and Sakhya, 2013). |
| Anti-tumor activity against Ehrlich ascites tumour (Ruby et al., 1995). | ||
| Induction of apoptosis in human cell lung cancer NCI-H460 cells 37 (Wu et al., 2010). | ||
| Linum usitatissimum L. | Anticancer effect against Human cervical cancer cell line: Hela cells (Joseph et al., 2020). |
Diarrhea, gastrointestinal infections, asthma, cough, bronchitis, pneumonia, renal colic, renal calculi, rheumatic swelling (Khan et al., 2017). |
| Inhibition of Jeg3 cell growth by the phytoestrogens isolated from Linum usitatissimum L. (Abarzua et al., 2007). | ||
|
Capparis spinosa L. |
Cytotoxic activity against both breast and colon cell lines: MCF-7 and HCT-116 (Aljaiyash et al., 2014). |
The treatment of rheumatism, gout and abdominal pains (Maresca et al., 2016). |
| Erodium guttatum (Desf.) Willd. | No data | Wound healing (Helmstädter, 2017). |
| Camellia sinensis (L.) Kuntze | Suppression of the growth of prostate cancer (HH870 and DU145) and epithelial ovarian cancer cell lines (HH450 and HH639) (Ravindranath et al., 2006). |
Stimulant, diuretic, astringent, heart health, flatulence, regulating body temperature and blood sugar, promoting digestion, and improving mental processes (Chopade et al., 2008). |
| Origanum vulgare L. | Anticancer activity against cervical cancer line: Hella cells (Koldaş et al., 2015). | Treatment of respiratory, cutaneous infections, igestive disorders, headaches, sore throats or colds (Vale-Silva et al., 2012), (de Torre et al., 2020). |
| Apoptosis in human colon cancer caco2 cells (Savini et al., 2009). | ||
| Salvia officinalis L. | Cytotoxic activity on colon cancer: HCT-116 cells (El Hadri et al., 2010). | Treatment of emmenagogue, cold, throat infections, skin diseases, ulcers, laryngitis, inflammation, bronchitis and Alzheimer’s disease (Sharma et al., 2019). |
| Peganum harmala L. | Antitumor effect on Lewis Lung, sarcoma180 and HepA tumor (Chen et al., 2005) | Antihypertensive, blood purifier, Antidiarrheal, intestinal pain, Antispasmodic in colic, Against nervosity, Emmenagogue, antalgic, Antidiabetic, Anti-pyretic (Moloudizargari et al., 2013). |
| Antiproliferative effect on leukemic cell line cells of Jurkat (Lamchouri et al., 2013) | ||
| Lepidium sativum L. | Apoptosis in human breast cancer cells: MCF-7 cell lines (Mahassni and Al-Reemi, 2013). | Treatment of chronic liver and spleen complaints, inflammatory rheumatic pains, skin diseases, asthma, diarrhea (Divanji et al., 2011). |
| Opuntia ficus-indica (L.) Mill. | Anticancer activity on Ehrlich ascites carcinoma cells (Abou-Elella and Ali, 2014). | Treatment of burns, wounds, edema, diabetes, obesity, indigestion, hyperlipidemy, viral and inflammatory infections (Kaur et al., 2012). |
| Apoptosis activity in human chronic myeloid leukemia Cell line-K562 (Sreekanth et al., 2007). | ||
| Rosmarinus officinalis L. | Antiproliferative effect on human ovarian cancer cells (A2780 and A2780CP70) (Tai et al., 2012). | Analgesic, anti-inflammatory, anti-rheumatic, spasmolytic, carminative and choleretic applications (Minaiyan et al., 2011). |
| Anticancer Effect on CaCo-2 colon cancer cells (Moore et al., 2016). | ||
| Ficus carica L. | Anti-angiogenic activity on human umbilical vein endothelial cells (Mostafaie et al., 2011) | Treatment of gastrointestinal disorders (colic, indigestion, loss of appetite, and diarrhea), respiratory (sore throats, cough, and bronchial problems), inflammatory, and cardiovascular disorders (Mawa et al., 2013). |
| di Punica granatum L. | Induction of apoptosis in prostate cancer cell line (Sineh Sepehr et al., 2012). | Treatment of atherosclerosis, diabetes,hypertension, hyperlipidemia, cancer, peptic ulcer and oral diseases (Ge et al., 2021). |
| Anticancer activity on human cancer cell lines melanoma (A375), colon cancer (HCT116), and hepatocellular carcinoma (HepG2) (Joseph et al., 2013). | ||
|
Cuminum cyminum L. |
Cytotoxic activity against colon cancer cells (502713, Colo-205, Hep-2, A-549, OVCAR-5, PC-5, SF-295) (Prakash and Gupta, 2014). | Treatment of asthma, bronchitis, rheumatism, hypolipidemia, cancer, diabetes and other inflammatory diseases (Mnif and Aifa, 2015), (Srinivasan, 2018). |
| Apium graveolens L. | Anticancer activity vs. rhabdomysarcom (RD) and murine (L20B) (AL-Jumaily, 2018). | Treatment of spasm, stomach problems, used also as diuretic, laxative, and sedative, and to lower the blood pressure (Al-Asmari et al., 2017). |
|
Daucus carota L. |
Antiproliferative effect on human Lymphoid Leukaemia Cells 67 (Zaini et al., 2012). | Treatment of gastric disorders, acidity and gastric ulcers (Nayeem et al., 2010) |
| Anticancer effect against human colon (HT‐29, Caco‐2) and breast (MCF‐7, MDA‐MB‐231) cancer cell (Shebaby et al., 2013). | ||
| Dittrichia viscosa (L.) | Antiproliferative and apoptosis effects on breast cancer cells (MCF-7) (Talib et al., 2012). | Wound healing, herniated disc, stomachache, anticancer, kidney pain, kidney stones, skin, hair, eye ailments and cancer (Sevgi et al., 2021). |
|
Sinapis alba L. |
Anticancer effect on colon cancer cell lines (SW480) (Yuan et al., 2011). | Anti-tumor, antiviral, and analgesic agent (Mitrović et al., 2020). |
| Suppression of colonic cancer (Zhu et al., 2012). | ||
| Origanum majorana L. | Apoptotic and anti-proliferative activity on human lymphoblastic leukemic cell line (Jurkat) (Abdel-Massih et al., 2010). | Treatment of insomnia, gastritis, asthma and nervousness (Singla and Vasudeva, 2015). |
| Origanum vulgare L. | Anticancer activity on human lung cancer cell line (A549) (Sankar et al., 2013). | Digestive and circulatory stimulant, antispasmodic, calmative, carminative, diaphoretic, expectorant, stomachic agent (de Torre et al., 2020). |
|
Hordeum vulgare L. |
Inhibition of oxidative DNA damage and apoptosis (J. B. Jeong et al., 2009). | Treatment of inflammatory, cardiovascular diseases, obesity, diabetes, circulatory disorders, arthritis, anemia, excessive cholesterol levels, renal difficulties, and cancer (Gul et al., 2014), (Thatiparthi et al., 2019). |
| Antitumor effect in mammary carcinogenesis (Kubatka et al., 2016). | ||
| Vitis vinifera L. | Anticancer effect on liver (HepG2) and cervical (HeLa) cancer cells (Apostolou et al., 2013). | Treatment of diarrhea, hepatitis and stomachaches (Aouey et al., 2016). |
|
Ephedra alata Decne |
Antiproliferative and pro-apoptotic potential against the MCF-7 breast cancer cell line (Danciu et al., 2018). | Treatment of allergies, asthma, colds, coughs, edema, fever, headaches, and nasal congestion (Mighri et al., 2019). |
| Drimia maritima (L.) Stearn | Cytotoxicity on lung cancer cell lines (A549) (Bozcuk et al., 2011). | No data |
| Pinus halepensis Mill. | Antiangiogenic activity on adenocarcinoma of human basal epithelial cells (A549), human colon adenocarcinoma (HCT15) and human myeloma (HL60) (Kadri et al., 2014). | Treatment of diarrhea, wounds, rheumatism, cough, gastrointestinal illnesses, hypertension, and hemorrhoids, used also as antiseptic, astringent, antifungal, and anti-tuberculosis (El Omari et al., 2021). |
|
Urtica dioica L. |
Antiproliferative activity on human prostate cancer cells (LNCaP, hPCPs) (Konrad et al., 2000). | Used as antihypertensive blood purifier, emmenagogue, diuretic, as well as to treat menstrual hemorrhage, rheumatism, and eczema (Testai et al., 2002), (Ilhan et al., 2019) |
| Citrus aurantium L. | Induction of G2/M phase arrest and apoptosis in human gastric cancer AGS Cells (Lee et al., 2012). | Treatment of influenza, insomnia, used also as tranquilizer, cardiovascular analeptic, and antispasmodic (Karthikeyan, 2014). |
| Induction of the cell cycle arrest and apoptosis in lung cancer cells (A549) (Park et al., 2012). | ||
| Cytotoxic effect on human colorectal carcinoma cell line (lim1863) (Odeh et al., 2012). |
Some plant species are used against cancer in the study area despite their toxicities such as Apteranthes europaea Guss (Issiki et al., 2017), Aristolochia longa L. (Benarba et al., 2017), Capparis spinosa L. (Fanoudi et al., 2017), Nigella sativa L. (Zaoui et al., 2002), Artemisia herba-alba Asso. (Bertella et al., 2018), Berberis hispanica Boiss. & Reut. (Kheir et al., 2010), Peganum harmala L. (Lamchouri et al., 2002), Origanum vulgare L. (Yazdani et al., 2014), Urginea maritima (L.) Baker (Tuncok et al., 1995), Citrus aurantium L. (Arbo et al., 2009), and Rosmarinus officinalis L. (Anadón et al., 2008) (Table 5).
In the present survey, many respondents have little data regarding plant toxicities due to illiteracy (Fig. 2.a) and the limited understanding of product toxicity. Collected data reported some contradiction in terms of the efficiency and toxicity of some reported plants .i.e. genus Aristolochia was highly recommended by the informants to treat cancer by contrast many scientific studies have demonstrated severe toxic effects with irreversible kidney tissue lesions induced by aristolochic acid contained in this genus. This phenomenon reflects some misunderstanding of traditional medicine based on traditions rather than sciences. It is thus researchers should work on creating awareness among people regarding the inconvenience of plant use without scientific validity.
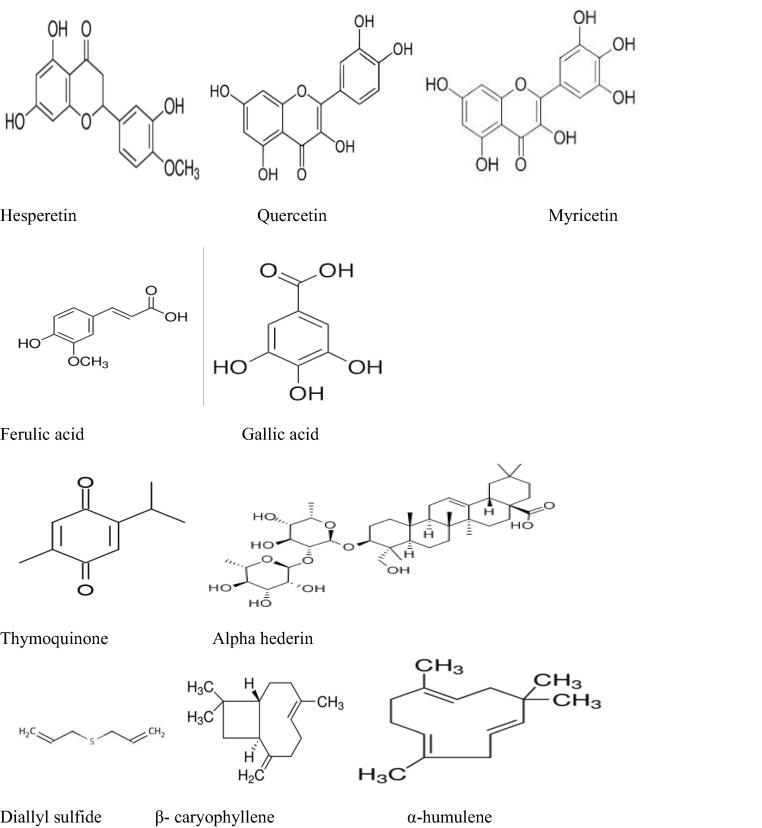
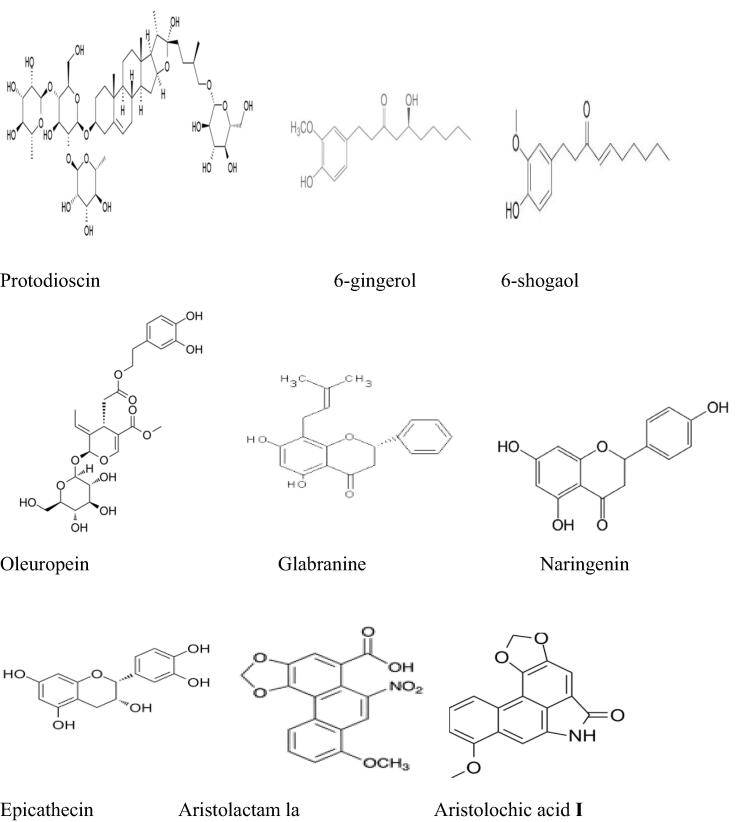
The antitumor effect of some bioactive compounds contained in the inventoried plants was also discussed in the previous works. Previously published literature investigated the chemical compounds related to potential anticancer effects of some inventoried plant species in the current research such as hesperetin (Choi, 2007), quercetin, myricetin (Lu et al., 2006), ferulic acid (Yang et al., 2015), and gallic acid (Chen et al., 2009) contained in Apteranthes europaea (Guss.) Murb (Amrati et al., 2020a), (Amrati et al., 2021), aristolochic acid I (AAsI) and aristolactam la (ALIa) in Aristolochia longa L. (Hinou et al., 1990), thymoquinone, and alpha-hederin in Nigella sativa L.(Rooney and Ryan, 2005); (Adamska et al., 2019), diallyl sulfide in Allium sativum L.(Wargovich, 1987), β-caryophyllene and α-humulene components in Marrubium vulgare L.(Fidyt et al., 2016); (El Hadri et al., 2010) ; protodioscin in Trigonella foenum-graecum L. [31; 100] (Ma et al., 2019) ; (Alsemari et al., 2014) flavonoids and tannins in Artemisia herba-alba Asso (Khlifi et al., 2013), quercetin in Allium cepa L. (Votto et al., 2010), 6-gingerol, and 6-shogaol in Zingiber officinale Roscoe (C.-H. Jeong et al., 2009) ; (Wu et al., 2015) ; (Cheng et al., 2011) ; Oleuropein in Olea europaea L. (Ruzzolini et al., 2018), curcuminoids in Curcuma longa L. (Anto et al., 1995) ; (Hsiao et al., 2018) ; glabranine and naringenin in Linum usitatissimum L. (Joseph et al., 2020), and Epicatechins in Camellia sinensis (L.) Kuntze (Ravindranath et al., 2006); (Bitu Pinto et al., 2015). The chemical structures of the mentioned compounds are presented in Fig. 7.
Fig 7.
Chemical structures of compounds involved in the anticancer effect of some inventoried plant species.
In the present survey, many respondents have little data regarding plant toxicities due to illiteracy and the limited understanding of product toxicity. Collected data reported some contradiction in terms of the efficiency and toxicity of some reported plants. i.e. genus Aristolochia was highly recommended by the informants to treat cancer by contrast many scientific studies have demonstrated severe toxic effects with irreversible kidney tissue lesions induced by Aristolochic acid contained in this genus. This phenomenon reflects some misunderstanding of traditional medicine based on traditions rather than sciences. For this reason, researchers should work on creating awareness among people regarding the inconvenience of plant use without scientific validity.
4. Conclusion
The present survey provides comprehensive data about the medicinal plant used in the Fez-Meknes region in Morocco for cancer treatment. The outcome of the present work showed that traditional medicines are still largely used among the rural and urban tribes of the region as a weapon to fight cancer. This study makes its way to contribute towards society as it provides a detailed study on the medicinal plants including plant parts used, mode of preparation, route of administration, as well as doses that find a place in cancer-fighting. This work hoping to constitute valuable data that can serve as medicines to develop natural anticancer compounds.
Author’s contribution
F.E.Z,. M.B., M.S.: writing the original Draft. A.M.S., A.A., R.U.: reviewing, and editing. A.B.,D.B.: Supervision and data validation
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group.
no (RG-1441-360).
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Fatima Ez-Zahra Amrati, Email: fatima.ezzahra.amrati@gmail.com.
Mohammed Bourhia, Email: bourhiamohammed@gmail.com.
References
- Abarzua S., Szewczyk M., Gailus S., Richter D., Ruth W., Briese V., Piechulla B. Effects of Phytoestrogen Extracts from Linum usitatissimum on the Jeg3 Human Trophoblast Tumour Cell Line. Anticancer Res. 2007;27:2053–2058. [PubMed] [Google Scholar]
- Abdel-Massih R., Fares R., Bazzi S., El-Chami N., Baydoun E. The apoptotic and anti-proliferative activity of Origanum majorana extracts on human leukemic cell line. Leuk. Res. 2010;34:1052–1056. doi: 10.1016/j.leukres.2009.09.018. [DOI] [PubMed] [Google Scholar]
- Abou-Elella F., Ali R. Antioxidant and Anticancer Activities of Different Constituents Extracted fromEgyptian Prickly Pear Cactus (Opuntia ficus-indica) Peel. Biochemistry & Analytical Biochemistry. 2014;3:158. doi: 10.4172/2161-1009.1000158. [DOI] [Google Scholar]
- Abu-Darwish M.S., Cabral C., Gonçalves M.J., Cavaleiro C., Cruz M.T., Efferth T., Salgueiro L. Artemisia herba-alba essential oil from Buseirah (South Jordan): Chemical characterization and assessment of safe antifungal and anti-inflammatory doses. J. Ethnopharmacol. 2015;174:153–160. doi: 10.1016/j.jep.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Adamska A., Stefanowicz-Hajduk J., Ochocka J.R. Alpha-Hederin, the Active Saponin of Nigella sativa, as an Anticancer Agent Inducing Apoptosis in the SKOV-3 Cell Line. Molecules. 2019;24:2958. doi: 10.3390/molecules24162958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adnan M., Jan S., Mussarat S., Tariq A., Begum S., Afroz A., Shinwari Z.K. A review on ethnobotany, phytochemistry and pharmacology of plant genus Caralluma R Br. J. Pharm. Pharmacol. 2014;66:1351–1368. doi: 10.1111/jphp.12265. [DOI] [PubMed] [Google Scholar]
- Agyare C., Spiegler V., Asase A., Scholz M., Hempel G., Hensel A. An ethnopharmacological survey of medicinal plants traditionally used for cancer treatment in the Ashanti region, Ghana. J. Ethnopharmacol. 2018;212:137–152. doi: 10.1016/j.jep.2017.10.019. [DOI] [PubMed] [Google Scholar]
- Al-Asmari A.K., Athar M.T., Kadasah S.G. An Updated Phytopharmacological Review on Medicinal Plant of Arab Region: Apium graveolens Linn. Pharmacogn Rev. 2017;11:13–18. doi: 10.4103/phrev.phrev_35_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aljaiyash A., Gonaid M., Islam M., Chaouch A. Antibacterial and cytotoxic activities of some Libyan medicinal plants. J. Nat. Product Plant Res. 2014;4:43–51. [Google Scholar]
- AL-Jumaily R. Evaluation of Anticancer Activities of Crude Extracts of Apium graveolens L. Seeds in Two Cell Lines, RD and L20B in vitro. Iraqi Journal of Cancer and Medical Genetics. 2018;3:18–23. [Google Scholar]
- Alsemari A., Alkhodairy F., Aldakan A., Al-Mohanna M., Bahoush E., Shinwari Z., Alaiya A. The selective cytotoxic anti-cancer properties and proteomic analysis of Trigonella Foenum-Graecum. BMC Complement Altern Med. 2014;14:114. doi: 10.1186/1472-6882-14-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammor K., Mahjoubi F., Bousta D., Chaqroune A. Ethnopharmacological survey of medicinal plants used in the traditional treatment of kidney stones realized in Fez-Morocco. Ethnobotany Research and Applications. 2020;19:1–12. [Google Scholar]
- Amrati, F. ez-zahra, Bourhia, M., Slighoua, M., Ibnemoussa, S., Bari, A., Ullah, R., Amaghnouje, A., Di Cristo, F., El Mzibri, M., Calarco, A., Benbacer, L., Bousta, D., 2020a. Phytochemical Study on Antioxidant and Antiproliferative Activities of Moroccan Caralluma europaea Extract and Its Bioactive Compound Classes. Evidence-Based Complementary and Alternative Medicine 2020. 10.1155/2020/8409718. [DOI] [PMC free article] [PubMed]
- Amrati, F. ez-zahra, Bourhia, M., Slighoua, M., Ibnemoussa, S., Bari, A., Ullah, R., Amaghnouje, A., Di Cristo, F., El Mzibri, M., Calarco, A., Benbacer, L., Bousta, D., 2020b. Phytochemical Study on Antioxidant and Antiproliferative Activities of Moroccan Caralluma europaea Extract and Its Bioactive Compound Classes [WWW Document]. Evidence-Based Complementary and Alternative Medicine. 10.1155/2020/8409718. [DOI] [PMC free article] [PubMed]
- Amrati F.E.-Z., Bourhia M., Slighoua M., Boukhira S., Ullah R., Ezzeldin E., Mostafa G.A.E., Grafov A., Bousta D. Protective Effect of Chemically Characterized Polyphenol-Rich Fraction from Apteranthes europaea (Guss.) Murb. subsp. maroccana (Hook.f.) Plowes on Carbon Tetrachloride-Induced Liver Injury in Mice. Appl. Sci. 2021;11:554. doi: 10.3390/app11020554. [DOI] [Google Scholar]
- Anadón, A., Martínez-Larrañaga, M.R., Martínez, M., Ares, I., García-Risco, M., Señoráns, F., REGLERO, G., 2008. Acute oral safety study of rosemary extracts in rats. J. Food Protect. 71, 790–795. 10.4315/0362-028X-71.4.790 [DOI] [PubMed]
- Anto, R., Kuttan, G., K V, D.B., Rajasekharan, K., Kuttan, R., 1995. Anti-tumour and antioxidant activity of natural curcuminoids. Cancer Lett. 94, 79–83. 10.1016/0304-3835(95)03827-J. [DOI] [PubMed]
- Aouey B., Samet A.M., Fetoui H., Simmonds M.S.J., Bouaziz M. Anti-oxidant, anti-inflammatory, analgesic and antipyretic activities of grapevine leaf extract (Vitis vinifera) in mice and identification of its active constituents by LC–MS/MS analyses. Biomed. Pharmacother. 2016;84:1088–1098. doi: 10.1016/j.biopha.2016.10.033. [DOI] [PubMed] [Google Scholar]
- Apostolou A., Stagos D., Galitsiou E., Spyrou A., Haroutounian S., Portesis N., Trizoglou I., Wallace Hayes A., Tsatsakis A.M., Kouretas D. Assessment of polyphenolic content, antioxidant activity, protection against ROS-induced DNA damage and anticancer activity of Vitis vinifera stem extracts. Food and Chemical Toxicology, Mechanisms involved in oxidative stress regulation. 2013;61:60–68. doi: 10.1016/j.fct.2013.01.029. [DOI] [PubMed] [Google Scholar]
- Arbo M.D., Schmitt G.C., Limberger M.F., Charão M., Moro A., Ribeiro G.L., Dallegrave E., Garcia S., Leal M.B., Limberger R.P. Subchronic toxicity of Citrus aurantium L. (Rutaceae) extract and p-synephrine in mice. Regul. Toxicol. Pharm. 2009;54:114–117. doi: 10.1016/j.yrtph.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Benali T., Khabbach A., Ennabili A., Hammani K. Ethnopharmacological prospecting of medicinal plants from the Province of Guercif (NE of Morocco) Moroccan J. Biol. 2017:1–14. [Google Scholar]
- Benarba B., Ambroise G., Aoues A., Meddah B., Vazquez A. Aristolochia longa aqueous extract triggers the mitochondrial pathway of apoptosis in BL41 Burkitt’s lymphoma cells. Int. J. Green Pharm. (IJGP) 2012;6 doi: 10.22377/ijgp.v6i1.237. [DOI] [Google Scholar]
- Benarba B., Meddah B. Ethnobotanical study, antifungal activity, phytochemical screening and total phenolic content of Algerian Aristolochia longa. J Intercult Ethnopharmacol. 2014;3:150–154. doi: 10.5455/jice.20140826030222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarba B., Pandiella A., Elmallah A. Anticancer activity, phytochemical screening and acute toxicity evaluation of an aqueous extract of Aristolochia longa L. International Journal of Pharmaceutical and Phytopharmacological Research. 2017;6:20–26. doi: 10.24896/eijppr.2016614. [DOI] [Google Scholar]
- Bertella A., Benlahcen K., Abouamama S., Pinto D., Maamar K., Kihal M., Silva A. Artemisia herba-alba Asso. essential oil antibacterial activity and acute toxicity. Ind. Crops Prod. 2018;116:137–143. doi: 10.1016/j.indcrop.2018.02.064. [DOI] [Google Scholar]
- Bitu Pinto N., da Silva Alexandre B., Neves K.R.T., Silva A.H., Leal L.K.A.M., Viana G.S.B. Neuroprotective Properties of the Standardized Extract from Camellia sinensis (Green Tea) and Its Main Bioactive Components, Epicatechin and Epigallocatechin Gallate, in the 6-OHDA Model of Parkinson’s Disease. Evid Based Complement Alternat Med. 2015;2015 doi: 10.1155/2015/161092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born, K., Christoph, M., H. Fink, A., Knippertz, P., Paeth, H., E. Speth, P., 2009. Moroccan Climate in the Present and Future: Combined View from Observational Data and Regional Climate Scenarios | Semantic Scholar. Climatic Changes and Water Resources in the Middle East and North Africa 29–45. 10.1007/978-3-540-85047-2_4.
- Boudjlida A., Kaci S., Karaki S., Benayad T., Rocchi P., Smati D., Bouguerra Aouichat S. Berberis hispanica alkaloids extract induced cell death and apoptosis in human laryngeal cancer cells Hep-2. S. Afr. J. Bot. 2019;125:134–141. doi: 10.1016/j.sajb.2019.04.006. [DOI] [Google Scholar]
- Bourhia, M., A Shahat, A., Almarfadi, O.M., Naser, F.A., Abdelmageed, W.M., Ait Haj Said, A., El Gueddari, F., Naamane, A., Benbacer, L., Khlil, N., 2019. Ethnopharmacological Survey of Herbal Remedies Used for the Treatment of Cancer in the Greater Casablanca-Morocco. Evidence-based Complementary and Alternative Medicine 1–9. 10.1155/2019/1613457. [DOI] [PMC free article] [PubMed]
- Bozcuk, H., Özdoğan, M., Aykurt, O., Topçuoğlu, F., Öztürk, H., Eki̇nci̇, D., Karadeni̇z, A., Mutlu, A., Burgucu, D., 2011. Urginea maritima (L.) Baker (Liliaceae) extract induces more cytotoxicity than standard chemotherapeutics in the A549 non-small cell lung cancer (NSCLC) cell line. Turk J Med Sci 41, 101–108.
- Chang J.S., Wang K.C., Yeh C.F., Shieh D.E., Chiang L.C. Fresh ginger (Zingiber officinale) has anti-viral activity against human respiratory syncytial virus in human respiratory tract cell lines. J. Ethnopharmacol. 2013;145:146–151. doi: 10.1016/j.jep.2012.10.043. [DOI] [PubMed] [Google Scholar]
- Chen H.-M., Wu Y.-C., Chia Y.-C., Chang F.-R., Hsu H.-K., Hsieh Y.-C., Chen C.-C., Yuan S.-S. Gallic acid, a major component of Toona sinensis leaf extracts, contains a ROS-mediated anti-cancer activity in human prostate cancer cells. Cancer Lett. 2009;286:161–171. doi: 10.1016/j.canlet.2009.05.040. [DOI] [PubMed] [Google Scholar]
- Chen Q., Chao R., Chen H., Hou X., Yan H., Zhou S., Peng W., Xu A. Antitumor and neurotoxic effects of novel harmine derivatives and structure-activity relationship analysis. Int. J. Cancer. 2005;114:675–682. doi: 10.1002/ijc.20703. [DOI] [PubMed] [Google Scholar]
- Cheng X.-L., Liu Q., Peng Y.-B., Qi L.-W., Li P. Steamed ginger (Zingiber officinale): Changed chemical profile and increased anticancer potential. Food Chem. 2011;129:1785–1792. doi: 10.1016/j.foodchem.2011.06.026. [DOI] [Google Scholar]
- Choi E.J. Hesperetin Induced G1-Phase Cell Cycle Arrest in Human Breast Cancer MCF-7 Cells: Involvement of CDK4 and p21. Nutr. Cancer. 2007;59:115–119. doi: 10.1080/01635580701419030. [DOI] [PubMed] [Google Scholar]
- Chopade V.V., Phatak A.A., Upaganlawar A.B., Tankar A.A. Green tea (Camellia sinensis): Chemistry, Traditional, Medicinal uses and its Pharmacological activities- A Review. Pharmacognosy Review. 2008;2:157–162. [Google Scholar]
- Danciu, C., Muntean, D., Alexa, E., Farcas, C., Oprean, C., Zupko, I., Bor, A., Minda, D., Proks, M., Buda, V., Hancianu, M., Cioanca, O., Soica, C., Popescu, S., Dehelean, C.A., 2018. Phytochemical Characterization and Evaluation of the Antimicrobial, Antiproliferative and Pro-Apoptotic Potential of Ephedra alata Decne. Hydroalcoholic Extract against the MCF-7 Breast Cancer Cell Line. Molecules 24. 10.3390/molecules24010013. [DOI] [PMC free article] [PubMed]
- de Torre M.P., Vizmanos J.L., Cavero R.Y., Calvo M.I. Improvement of antioxidant activity of oregano (Origanum vulgare L.) with an oral pharmaceutical form. Biomed. Pharmacother. 2020;129 doi: 10.1016/j.biopha.2020.110424. [DOI] [PubMed] [Google Scholar]
- Divanji, M., G.L., V. shastry, Nagesh, S., Jain, V., H N, S., 2011. Ethnopharmacology of Lepidium Sativum Linn (Brassicaceae): A Review. Int. J. Phytothear. Res. 2, 1–7.
- El Hadri, A., Gómez del Río, M.Á., Sanz, J., González Coloma, A., Idaomar, M., Ribas Ozonas, B., Benedí González, J., Sánchez Reus, M.I., 2010. Cytotoxic activity of Alfa-humulene and transcaryophyllene from Salvia officinalis in animal and human tumor cells. An. R. Acad. Farm 343–356.
- el Hamsas el Youbi A. In vitro immunomodulation effects of the aqueous and protein extracts of Berberis hispanica Boiss and Reut. (Family Berberidaceae) J. Med. Plants Res. 2011;6 doi: 10.5897/JMPR12.381. [DOI] [Google Scholar]
- El Omari N., Ezzahrae Guaouguaou F., El Menyiy N., Benali T., Aanniz T., Chamkhi I., Balahbib A., Taha D., Shariati M.A., Zengin G., El-Shazly M., Bouyahya A. Phytochemical and biological activities of Pinus halepensis mill., and their ethnomedicinal use. J. Ethnopharmacol. 2021;268 doi: 10.1016/j.jep.2020.113661. [DOI] [PubMed] [Google Scholar]
- El-Hilaly J., Hmammouchi M., Lyoussi B. Ethnobotanical studies and economic evaluation of medicinal plants in Taounate province (Northern Morocco) J. Ethnopharmacol. 2003;86:149–158. doi: 10.1016/s0378-8741(03)00012-6. [DOI] [PubMed] [Google Scholar]
- Fanoudi S., Rakhshandeh H., Afshari A.R., Mollazadeh H., Boroushaki M.T. Nephrotoxicity and Hepatotoxicity of Capparis Spinosahydro-Alcoholic Extract in Mice. JOJ Urology Nephrol. 2017;4 doi: 10.19080/JOJUN.2017.04.555638. [DOI] [Google Scholar]
- Fares R., Bazzi S., Baydoun S.E., Abdel-Massih R.M. The antioxidant and anti-proliferative activity of the Lebanese Olea europaea extract. Plant Foods Hum Nutr. 2011;66:58–63. doi: 10.1007/s11130-011-0213-9. [DOI] [PubMed] [Google Scholar]
- Farshori N., Al-Sheddi E., Al-Oqail M., Musarrat J., Al-Khedhairy A., Siddiqui M. Anticancer Activity of Petroselinum sativum Seed Extracts on MCF-7 Human Breast Cancer Cells. Asian Pacific J. Cancer Prev. APJCP. 2013;14:5719–5723. doi: 10.7314/APJCP.2013.14.10.5719. [DOI] [PubMed] [Google Scholar]
- Farshori N.N., Al-Sheddi E.S., Al-Oqail M.M., Musarrat J., Al-Khedhairy A.A., Siddiqui M.A. Cytotoxicity assessments of Portulaca oleracea and Petroselinum sativum seed extracts on human hepatocellular carcinoma cells (HepG2) Asian Pac. J. Cancer Prev. 2014;15:6633–6638. doi: 10.7314/apjcp.2014.15.16.6633. [DOI] [PubMed] [Google Scholar]
- Farzaei M.H., Abbasabadi Z., Ardekani M.R.S., Rahimi R., Farzaei F. Parsley: a review of ethnopharmacology, phytochemistry and biological activities. J. Tradit. Chin. Med. 2013;33:815–826. doi: 10.1016/S0254-6272(14)60018-2. [DOI] [PubMed] [Google Scholar]
- Fennane M., Rejdali M. Aromatic and medicinal plants of Morocco : Richness, diversity and threats. Bulletin de l’Institut Scientifique, Rabat, Section Sciences de la Vie. 2016:27–42. [Google Scholar]
- Fidyt K., Fiedorowicz A., Strządała L., Szumny A. β-caryophyllene and β-caryophyllene oxide—natural compounds of anticancer and analgesic properties. Cancer Med. 2016;5:3007–3017. doi: 10.1002/cam4.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J., Yaniv Z., Dafni A., Palewitch D. A preliminary classification of the healing potential of medicinal plants, based on a rational analysis of an ethnopharmacological field survey among Bedouins in the Negev desert, Israel. J Ethnopharmacol. 1986;16:275–287. doi: 10.1016/0378-8741(86)90094-2. [DOI] [PubMed] [Google Scholar]
- Ge, S., Duo, L., Wang, J., GegenZhula, Yang, J., Li, Z., Tu, Y., 2021. A unique understanding of traditional medicine of pomegranate, Punica granatum L. and its current research status. Journal of Ethnopharmacology 271, 113877. 10.1016/j.jep.2021.113877. [DOI] [PubMed]
- González-Tejero M.R., Casares-Porcel M., Sánchez-Rojas C.P., Ramiro-Gutiérrez J.M., Molero-Mesa J., Pieroni A., Giusti M.E., Censorii E., de Pasquale C., Della A., Paraskeva-Hadijchambi D., Hadjichambis A., Houmani Z., El-Demerdash M., El-Zayat M., Hmamouchi M., ElJohrig S. Medicinal plants in the Mediterranean area: Synthesis of the results of the project Rubia. J. Ethnopharmacol. 2008;116:341–357. doi: 10.1016/j.jep.2007.11.045. [DOI] [PubMed] [Google Scholar]
- Gul S., Ahmed S., Kifli N., Uddin Q.T., Batool Tahir N., Hussain A., Jaafar H.Z., Moga M., Zia-Ul-Haq M. Multiple pathways are responsible for Anti-inflammatory and Cardiovascular activities of Hordeum vulgare L. J. Translational Med. 2014;12:316. doi: 10.1186/s12967-014-0316-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamza N., Berke B., Cheze C., Le Garrec R., Umar A., Agli A.-N., Lassalle R., Jové J., Gin H., Moore N. Preventive and curative effect of Trigonella foenum-graecum L. seeds in C57BL/6J models of type 2 diabetes induced by high-fat diet. J. Ethnopharmacol. 2012;142:516–522. doi: 10.1016/j.jep.2012.05.028. [DOI] [PubMed] [Google Scholar]
- Helmstädter A. The botanical explorer’s legacy: a promising bioprospecting tool. Drug Discovery Today. 2017;22:757–760. doi: 10.1016/j.drudis.2016.11.011. [DOI] [PubMed] [Google Scholar]
- Hinou J., Demetzos C., Harvala C., Roussakis C. Cytotoxic and Antimicrobial Principles from the Roots of Aristolochia longa. Int. J. Crude Drug Res. 1990;28:149–151. doi: 10.3109/13880209009082801. [DOI] [Google Scholar]
- Hsiao Y.-T., Kuo C.-L., Chueh F.-S., Liu K.-C., Bau D.-T., Chung J.-G. Curcuminoids Induce Reactive Oxygen Species and Autophagy to Enhance Apoptosis in Human Oral Cancer Cells. Am. J. Chin. Med. 2018;46:1145–1168. doi: 10.1142/S0192415X1850060X. [DOI] [PubMed] [Google Scholar]
- Ilhan M., Ali Z., Khan I.A., Taştan H., Küpeli Akkol E. Bioactivity-guided isolation of flavonoids from Urtica dioica L. and their effect on endometriosis rat model. J. Ethnopharmacol. 2019;243 doi: 10.1016/j.jep.2019.112100. [DOI] [PubMed] [Google Scholar]
- Issiki Z., Moundir C., Marnissi F., Seddik N., Benjelloun N., Zaid Y., Oudghiri M. Toxicological Evaluation of the Aqueous Extract of Caralluma europaea and Its Immunomodulatory and Inflammatory Activities. Pharmacognosy Res. 2017;9:390–395. doi: 10.4103/pr.pr_24_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagetia G.C., Baliga M.S., Venkatesh P., Ulloor J. Influence of Ginger Rhizome (Zingiber officinale Rosc) on Survival, Glutathione and Lipid Peroxidation in Mice after Whole-Body Exposure to Gamma Radiation. Radiat Res. 2003;160:584–592. doi: 10.1667/RR3057. [DOI] [PubMed] [Google Scholar]
- Javan M., Ahmadiani A., Semnanian S., Kamalinejad M. Antinociceptive effects of Trigonella foenum-graecum leaves extract. J. Ethnopharmacol. 1997;58:125–129. doi: 10.1016/S0378-8741(97)00089-5. [DOI] [PubMed] [Google Scholar]
- Jeong C.-H., Bode A.M., Pugliese A., Cho Y.-Y., Kim H.-G., Shim J.-H., Jeon Y.-J., Li H., Jiang H., Dong Z. [6]-Gingerol Suppresses Colon Cancer Growth by Targeting Leukotriene A4 Hydrolase. Cancer Res. 2009;69:5584–5591. doi: 10.1158/0008-5472.CAN-09-0491. [DOI] [PubMed] [Google Scholar]
- Jeong J.B., Hong S.C., Jeong H.J. 3,4-dihydroxybenzaldehyde purified from the barley seeds (Hordeum vulgare) inhibits oxidative DNA damage and apoptosis via its antioxidant activity. Phytomedicine. 2009;16:85–94. doi: 10.1016/j.phymed.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Joseph A., Sridharan S., Palanisamy S., Ramalingam S., Saravanan R. Identification of anticancer compounds from Linum usitatissimum seed extract and their effect on HeLa cells. Pharmacognosy Magazine. 2020;16:221. doi: 10.4103/pm.pm_341_19. [DOI] [Google Scholar]
- Joseph M.M., Aravind S.R., George S.K., Varghese S., Sreelekha T.T. A galactomannan polysaccharide from Punica granatum imparts in vitro and in vivo anticancer activity. Carbohydr Polym. 2013;98:1466–1475. doi: 10.1016/j.carbpol.2013.07.023. [DOI] [PubMed] [Google Scholar]
- Jouad H., Haloui M., Rhiouani H., El Hilaly J., Eddouks M. Ethnobotanical survey of medicinal plants used for the treatment of diabetes, cardiac and renal diseases in the North centre region of Morocco (Fez–Boulemane) J. Ethnopharmacol. 2001;77:175–182. doi: 10.1016/S0378-8741(01)00289-6. [DOI] [PubMed] [Google Scholar]
- Kabbaj F.Z., Meddah B., Cherrah Y., Faouzi M.E.A. Ethnopharmacological profile of traditional plants used in Morocco by cancer patients as herbal therapeutics | Request PDF. Phytopharmacology. 2012;2:243–256. [Google Scholar]
- Kadri N., Khettal B., Adjebli A., Cresteil T., Yahiaoui-Zaidi R., Barragan-Montero V., Montero J.-L. Antiangiogenic activity of neutral lipids, glycolipids, and phospholipids fractions of Pinus halepensis Mill. seeds. Ind. Crops Prod. 2014;54:6–12. doi: 10.1016/j.indcrop.2013.12.051. [DOI] [Google Scholar]
- Karthikeyan V. Citrus aurantium (Bitter Orange): A Review of its Traditional Uses, Phytochemistry and Pharmacology. Int. J. Drug Discovery and Herb. Res. (ijddhr) 2014;4:766–772. [Google Scholar]
- Kaur M., Kaur A., Sharma R. Pharmacological actions of Opuntia ficus indica: a review. J. Appl. Pharm. Sci. 2012;2:15–18. [Google Scholar]
- Khan Z.J., Khan N., Naseem I., Nami S.A.A. Therapeutics, phytochemistry and pharmacology of Tukhm-e-Katan (Linum usitatissimum L.) Int J Adv Pharm Med Bioallied Sci. 2017;111:1–15. [Google Scholar]
- Kheir M.M., Wang Y., Hua L., Hu J., Li L., Lei F., Du L. Acute toxicity of berberine and its correlation with the blood concentration in mice. Food Chem. Toxicol. 2010;48:1105–1110. doi: 10.1016/j.fct.2010.01.033. [DOI] [PubMed] [Google Scholar]
- Khlifi D., Sghaier R.M., Amouri S., Laouini D., Hamdi M., Bouajila J. Composition and anti-oxidant, anti-cancer and anti-inflammatory activities of Artemisia herba-alba, Ruta chalpensis L. and Peganum harmala L. Food Chem. Toxicol. 2013;55:202–208. doi: 10.1016/j.fct.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Koldaş S., Demirtas I., Ozen T., Demirci M.A., Behçet L. Phytochemical screening, anticancer and antioxidant activities of Origanum vulgare L. ssp. viride (Boiss.) Hayek, a plant of traditional usage. J. Sci. Food Agric. 2015;95:786–798. doi: 10.1002/jsfa.6903. [DOI] [PubMed] [Google Scholar]
- Konrad L., Müller H.-H., Lenz C., Laubinger H., Aumüller G., Lichius J.J. Antiproliferative Effect on Human Prostate Cancer Cells by a Stinging Nettle Root (Urtica dioica) Extract. Planta Med. 2000;66:44–47. doi: 10.1055/s-2000-11117. [DOI] [PubMed] [Google Scholar]
- Kubatka P., Kello M., Kajo K., Kruzliak P., Výbohová D., Šmejkal K., Maršík P., Zulli A., Gönciová G., Mojžiš J., Kapinová A., Murin R., Péč M., Adamkov M., Przygodzki R.M. Young Barley Indicates Antitumor Effects in Experimental Breast Cancer In Vivo and In Vitro. Nutr Cancer. 2016;68:611–621. doi: 10.1080/01635581.2016.1154577. [DOI] [PubMed] [Google Scholar]
- Kumar N., Sakhya S.K. Ethnopharmacological Properties of Curcuma Longa: a Review. IJPSR. 2013;4:103–112. [Google Scholar]
- Kuttan R., Bhanumathy P., Nirmala K., George M.C. Potential anticancer activity of turmeric (Curcuma longa) Cancer Lett. 1985;29:197–202. doi: 10.1016/0304-3835(85)90159-4. [DOI] [PubMed] [Google Scholar]
- la Direction Générale des Collectivités Locales, 2015. Monographie de la région Fès-Meknès – Région Fès Meknès.
- Lamchouri F., Settaf A., Cherrah Y., Hamidi M.N. Experimental toxicity of Peganum harmala seeds. Annales Pharmaceutiques Françaises. 2002;60:123–129. [PubMed] [Google Scholar]
- Lamchouri F., Zemzami M., Jossang A., Abdellatif A., Israili Z., Badiaa L. Cytotoxicity of alkaloids isolated from Peganum harmala seeds. Pakistan J. Pharm. Sci. 2013;26:699–706. [PubMed] [Google Scholar]
- Lamm D., Riggs D. The potential application of Allium sativum (garlic) for the treatment of bladder cancer. Urologic Clinics. 2000;27:157–162. doi: 10.1016/S0094-0143(05)70243-3. [DOI] [PubMed] [Google Scholar]
- Lee, D.-H., Park, K.-I., Park, H.-S., Kang, S.-R., Nagappan, A., Kim, J.-A., Kim, E.-H., Lee, W.-S., Hah, Y.-S., Chung, H.-J., An, S.-J., Kim, G.-S., 2012. Flavonoids Isolated from Korea Citrus aurantium L. Induce G2/M Phase Arrest and Apoptosis in Human Gastric Cancer AGS Cells. Evidence-Based Complementary and Alternative Medicine. 10.1155/2012/515901. [DOI] [PMC free article] [PubMed]
- Lee W.S., Yi S.M., Yun J.W., Jung J.H., Kim D.H., Kim H.J., Chang S.-H., Kim G., Ryu C.H., Shin S.C., Hong S.C., Choi Y.H., Jung J.-M. Polyphenols Isolated from Allium cepa L. Induces Apoptosis by Induction of p53 and Suppression of Bcl-2 through Inhibiting PI3K/Akt Signaling Pathway in AGS Human Cancer Cells. J Cancer Prev. 2014;19:14–22. doi: 10.15430/jcp.2014.19.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Papp L.V., Fang J., Rodriguez-Nieto S., Zhivotovsky B., Holmgren A. Inhibition of Mammalian Thioredoxin Reductase by Some Flavonoids: Implications for Myricetin and Quercetin Anticancer Activity. Cancer Res. 2006;66:4410–4418. doi: 10.1158/0008-5472.CAN-05-3310. [DOI] [PubMed] [Google Scholar]
- Lyantagaye S.L. Ethnopharmacological and Phytochemical Review of Allium Species (Sweet Garlic) and Tulbaghia Species (Wild Garlic) from Southern Africa. Tanzania J. Sci. 2011;37 doi: 10.4314/tjs.v37i1. [DOI] [Google Scholar]
- Ma Y.-L., Zhang Y.-S., Zhang F., Zhang Y.-Y., Thakur K., Zhang J.-G., Wei Z.-J. Methyl protodioscin from Polygonatum sibiricum inhibits cervical cancer through cell cycle arrest and apoptosis induction. Food Chem. Toxicol. 2019;132 doi: 10.1016/j.fct.2019.110655. [DOI] [PubMed] [Google Scholar]
- Mahassni S.H., Al-Reemi R.M. Apoptosis and necrosis of human breast cancer cells by an aqueous extract of garden cress (Lepidium sativum) seeds. Saudi J Biol Sci. 2013;20:131–139. doi: 10.1016/j.sjbs.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresca M., Micheli L., Di Cesare Mannelli L., Tenci B., Innocenti M., Khatib M., Mulinacci N., Ghelardini C. Acute effect of Capparis spinosa root extracts on rat articular pain. J. Ethnopharmacol. 2016;193:456–465. doi: 10.1016/j.jep.2016.09.032. [DOI] [PubMed] [Google Scholar]
- Mawa, S., Husain, K., Jantan, I., 2013. Ficus carica L. (Moraceae): Phytochemistry, Traditional Uses and Biological Activities. Evidence-Based Complementary and Alternative Medicine 2013, e974256. 10.1155/2013/974256. [DOI] [PMC free article] [PubMed]
- Mighri H., Akrout A., Bennour N., Eljeni H., Zammouri T., Neffati M. LC/MS method development for the determination of the phenolic compounds of Tunisian Ephedra alata hydro-methanolic extract and its fractions and evaluation of their antioxidant activities. S. Afr. J. Bot. 2019;124:102–110. doi: 10.1016/j.sajb.2019.04.029. [DOI] [Google Scholar]
- Minaiyan M., Ghannadi A.R., Afsharipour M., Mahzouni P. Effects of extract and essential oil of Rosmarinus officinalis L. on TNBS-induced colitis in rats. Res Pharm Sci. 2011;6:13–21. [PMC free article] [PubMed] [Google Scholar]
- Mitrović P.M., Stamenković O.S., Banković-Ilić I., Djalović I.G., Nježić Z.B., Farooq M., Siddique K.H.M., Veljković V.B. White Mustard (Sinapis alba L.) Oil in Biodiesel Production: A Review. Front. Plant Sci. 2020;11:1–22. doi: 10.3389/fpls.2020.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mnif S., Aifa S. Cumin (Cuminum cyminum L.) from Traditional Uses to Potential Biomedical Applications. Chem. Biodivers. 2015;12:733–742. doi: 10.1002/cbdv.201400305. [DOI] [PubMed] [Google Scholar]
- Moghaddasi M., Haddad Kashani H. Ginger (Zingiber officinale): A review. J. Med. Plants Res. 2012;6 doi: 10.5897/JMPR011.787. [DOI] [Google Scholar]
- Moloudizargari M., Mikaili P., Aghajanshakeri S., Asghari M.H., Shayegh J. Pharmacological and therapeutic effects of Peganum harmala and its main alkaloids. Pharmacogn Rev. 2013;7:199–212. doi: 10.4103/0973-7847.120524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J., Yousef M., Tsiani E. Anticancer Effects of Rosemary (Rosmarinus officinalis L.) Extract and Rosemary Extract Polyphenols. Nutrients. 2016;8:731. doi: 10.3390/nu8110731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafaie A., Mansouri K., Norooznezhad A.H., Mohammadi-Motlagh H.-R. Anti-Angiogenic Activity of Ficus carica Latex Extract on Human Umbilical Vein Endothelial Cells. Cell. 2011;12:525–528. null. [Google Scholar]
- Namazi N., Larijani B., Ayati M.H., Abdollahi M. The effects of Nigella sativa L. on obesity: A systematic review and meta-analysis. J. Ethnopharmacol. 2018;219:173–181. doi: 10.1016/j.jep.2018.03.001. [DOI] [PubMed] [Google Scholar]
- Nayeem K., Godad A., Hashilkar N., Joshi R.K. Gastroprotective activity of the aqueous extract from the roots of Daucus carota L in rats. Int. J. Res. Ayurveda Pharm. (IJRAP) 2010;1:112–119. [Google Scholar]
- Odeh F., Rahmo A., Alnori A.S., Chaty M.E. THE CYTOTOXIC EFFECT OF ESSENTIAL OILS CITRUS AURANTIUM PEELS ON HUMAN COLORECTAL CARCINOMA CELL LINE (LIM1863) J. Microbiol. Biotechnol. Food Sci. 2012;1:1476–1487. [Google Scholar]
- Park K.I., Park H.S., Nagappan A., Hong G.E., Lee D.H., Kang S.R., Kim J.A., Zhang J., Kim E.H., Lee W.S., Shin S.C., Hah Y.S., Kim G.S. Induction of the cell cycle arrest and apoptosis by flavonoids isolated from Korean Citrus aurantium L. in non-small-cell lung cancer cells. Food Chem. 2012;135:2728–2735. doi: 10.1016/j.foodchem.2012.06.097. [DOI] [PubMed] [Google Scholar]
- Periasamy V.S., Athinarayanan J., Alshatwi A.A. Anticancer activity of an ultrasonic nanoemulsion formulation of Nigella sativa L. essential oil on human breast cancer cells. Ultrason. Sonochem. 2016;31:449–455. doi: 10.1016/j.ultsonch.2016.01.035. [DOI] [PubMed] [Google Scholar]
- Prakash, E., Gupta, D.K., 2014. Cytotoxic Activity of Ethanolic Extract of Cuminum cyminum Linn against Seven Human Cancer Cell Line. Universal J. Agricult. Res. 2, 27–30. 10.13189/ujar.2014.020104.
- Rahman S., Salehin F., Iqbal A. In vitro antioxidant and anticancer activity of young Zingiber officinale against human breast carcinoma cell lines. BMC Complement Altern Med. 2011;11:76. doi: 10.1186/1472-6882-11-76. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rankou H., Culham A., Jury S.L., Christenhusz M.J.M. The endemic flora of Morocco. Phytotaxa. 2013;78:1–69. doi: 10.11646/phytotaxa.78.1.1. [DOI] [Google Scholar]
- Ravindranath M.H., Saravanan T.S., Monteclaro C.C., Presser N., Ye X., Selvan S.R., Brosman S. Epicatechins Purified from Green Tea (Camellia sinensis) Differentially Suppress Growth of Gender-Dependent Human Cancer Cell Lines. Evid Based Complement Alternat Med. 2006;3:237–247. doi: 10.1093/ecam/nel003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney S., Ryan M.F. Effects of Alpha-hederin and Thymoquinone, constituents of Nigella sativa, on Human Cancer Cell Lines. Anticancer Res. 2005;25:2199–2204. [PubMed] [Google Scholar]
- Ruby A., Kuttan G., Babu D., Rajasekharan K., Kuttan R. Anti-tumour and antioxidant activity of natural curcuminoids. Cancer Lett. 1995;94:79–83. doi: 10.1016/0304-3835(95)03827-j. [DOI] [PubMed] [Google Scholar]
- Ruzzolini J., Peppicelli S., Andreucci E., Bianchini F., Scardigli A., Romani A., La Marca G., Nediani C., Calorini L. Oleuropein, the Main Polyphenol of Olea europaea Leaf Extract, Has an Anti-Cancer Effect on Human BRAF Melanoma Cells and Potentiates the Cytotoxicity of Current Chemotherapies. Nutrients. 2018;10:1950. doi: 10.3390/nu10121950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahpaz S., Garbacki N., Tits M., Bailleul F. Isolation and pharmacological activity of phenylpropanoid esters from Marrubium vulgare. J. Ethnopharmacol. 2002;79:389–392. doi: 10.1016/S0378-8741(01)00415-9. [DOI] [PubMed] [Google Scholar]
- Samouh Y., Lemrani A., Mimouni H., Mohamad J., Ait Haj Said A. Ethnopharmacological Study of Herbal Medicines used to treat Cancer in Morocco. J. Phytopharmacol. 2019;8:135–141. [Google Scholar]
- Sankar R., Karthik A., Prabu A., Karthik S., Shivashangari K.S., Ravikumar V. Origanum vulgare mediated biosynthesis of silver nanoparticles for its antibacterial and anticancer activity. Colloids Surf B Biointerfaces. 2013;108:80–84. doi: 10.1016/j.colsurfb.2013.02.033. [DOI] [PubMed] [Google Scholar]
- Savini I., Arnone R., Catani M.V., Avigliano L. Origanum Vulgare Induces Apoptosis in Human Colon Cancer Caco2 Cells. Nutr. Cancer. 2009;61:381–389. doi: 10.1080/01635580802582769. [DOI] [PubMed] [Google Scholar]
- Scheffler A., Rauwald H.W., Kampa B., Mann U., Mohr F.W., Dhein S. Olea europaea leaf extract exerts L-type Ca2+ channel antagonistic effects. J. Ethnopharmacol. 2008;120:233–240. doi: 10.1016/j.jep.2008.08.018. [DOI] [PubMed] [Google Scholar]
- Sevgi E., Dag A., Kızılarslan-Hançer Ç., Atasoy S., Kurt B.Z., Aksakal Ö. Evaluation of cytotoxic and antioxidant potential of Dittrichia viscosa (L.) Greuter used in traditional medicine. J. Ethnopharmacol. 2021;276 doi: 10.1016/j.jep.2021.114211. [DOI] [PubMed] [Google Scholar]
- Sharma Y., Fagan J., Schaefer J. Ethnobotany, phytochemistry, cultivation and medicinal properties of Garden sage (Salvia officinalis L.) J Pharmacogn Phytochem. 2019;8:3139–3148. [Google Scholar]
- Shebaby, W.N., El-Sibai, M., Smith, K.B.-, Karam, M.C., Mroueh, M., Daher, C.F., 2013. The antioxidant and anticancer effects of wild carrot oil extract. Phytother Res 27, 737–744. 10.1002/ptr.4776. [DOI] [PubMed]
- Sineh Sepehr, K., Baradaran, B., Mazandarani, M., Khori, V., Shahneh, F.Z., 2012. Studies on the Cytotoxic Activities of Punica granatum L. var. spinosa (Apple Punice) Extract on Prostate Cell Line by Induction of Apoptosis. ISRN Pharm 2012. 10.5402/2012/547942 [DOI] [PMC free article] [PubMed]
- Singla P., Vasudeva N. Origanum majorana L. -Phyto-pharmacological review. Indian Journal of Natural Products and Resources (IJNPR) [Formerly Natural Product Radiance (NPR)] 2015;6:261–267. [Google Scholar]
- Song C., Hong Y.H., Park J.G., Kim H.G., Jeong D., Oh J., Sung G.-H., Hossain M.A., Taamalli A., Kim J.H., Kim J.-H., Cho J.Y. Suppression of Src and Syk in the NF-κB signaling pathway by Olea europaea methanol extract is leading to its anti-inflammatory effects. J. Ethnopharmacol. 2019;235:38–46. doi: 10.1016/j.jep.2019.01.024. [DOI] [PubMed] [Google Scholar]
- Sreekanth D., Arunasree M.K., Roy K.R., Chandramohan R.T., Reddy G.V., Reddanna P. Betanin a betacyanin pigment purified from fruits of Opuntia ficus-indica induces apoptosis in human chronic myeloid leukemia Cell line-K562. Phytomedicine. 2007;14:739–746. doi: 10.1016/j.phymed.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Srinivasan K. Cumin (Cuminum cyminum) and black cumin (Nigella sativa) seeds: traditional uses, chemical constituents, and nutraceutical effects. Food Qual. Saf. 2018;2:1–16. doi: 10.1093/fqsafe/fyx031. [DOI] [Google Scholar]
- Tadesse A., Kagnew B., Kebede F. Ethnobotanical Study of Medicinal Plants used to treat Human Diseases in Enarj Enawga District, East Gojjam Zone, Amhara Region, Ethiopia. J. Pharmacognosy Phytother. 2018;10:64–75. doi: 10.5897/JPP2018.0496. [DOI] [Google Scholar]
- Tai J., Cheung S., Wu M., Hasman D. Antiproliferation effect of Rosemary (Rosmarinus officinalis) on human ovarian cancer cells in vitro. Phytomedicine. 2012;19:436–443. doi: 10.1016/j.phymed.2011.12.012. [DOI] [PubMed] [Google Scholar]
- Talib W.H., Zarga M.H.A., Mahasneh A.M. Antiproliferative, antimicrobial and apoptosis inducing effects of compounds isolated from Inula viscosa. Molecules. 2012;17:3291–3303. doi: 10.3390/molecules17033291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardío J., Pardo de Santayana M. Cultural Importance Indices: A Comparative Analysis Based on the Useful Wild Plants of Southern Cantabria (Northern Spain) Econ. Bot. 2008;62:24–39. doi: 10.1007/s12231-007-9004-5. [DOI] [Google Scholar]
- Teshika J.D., Zakariyyah A.M., Zaynab T., Zengin G., Rengasamy K.R., Pandian S.K., Fawzi M.M. Critical Reviews in Food Science and Nutrition. 2018. Traditional and modern uses of onion bulb (Allium cepa L.): a systematic review. [DOI] [PubMed] [Google Scholar]
- Testai L., Chericoni S., Calderone V., Nencioni G., Nieri P., Morelli I., Martinotti E. Cardiovascular effects of Urtica dioica L. (Urticaceae) roots extracts: in vitro and in vivo pharmacological studies. J. Ethnopharmacol. 2002;81:105–109. doi: 10.1016/S0378-8741(02)00055-7. [DOI] [PubMed] [Google Scholar]
- Thatiparthi J., Dodoala S., Koganti B., Kvsrg P. Barley grass juice (Hordeum vulgare L.) inhibits obesity and improves lipid profile in high fat diet-induced rat model. J. Ethnopharmacol. 2019;238 doi: 10.1016/j.jep.2019.111843. [DOI] [PubMed] [Google Scholar]
- Tilaoui M., Ait Mouse H., Jaafari A., Zyad A. Comparative Phytochemical Analysis of Essential Oils from Different Biological Parts of Artemisia herba alba and Their Cytotoxic Effect on Cancer Cells. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0131799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuncok Y., Kozan O., Cavdar C., Guven H., Fowler J. Urginea Maritima (Squill) Toxicity. J. Toxicol. Clin. Toxicol. 1995;33:83–86. doi: 10.3109/15563659509020221. [DOI] [PubMed] [Google Scholar]
- Vale-Silva L., Silva M.-J., Oliveira D., Gonçalves M.-J., Cavaleiro C., Salgueiro L., Pinto E. Correlation of the chemical composition of essential oils from Origanum vulgare subsp. virens with their in vitro activity against pathogenic yeasts and filamentous fungi. J. Med. Microbiol. 2012;61:252–260. doi: 10.1099/jmm.0.036988-0. [DOI] [PubMed] [Google Scholar]
- Votto, A.P.S., Domingues, B.S., de Souza, M.M., da Silva Júnior, F.M.R., Caldas, S.S., Filgueira, D.M.V.B., Clementin, R.M., Primel, E.G., Vallochi, A.L., Furlong, E.B., Trindade, G.S., 2010. Toxicity mechanisms of onion (Allium cepa) extracts and compounds in multidrug resistant erythroleukemic cell line. Biol. Res. 43, 429–437. /S0716-97602010000400007. [PubMed]
- Wargovich M.J. Diallyl sulfide, a flavor component of garlic (Allium sativum), inhibits dimethyihydrazine-induced colon cancer. Carcinogenesis. 1987;8:487–489. doi: 10.1093/carcin/8.3.487. [DOI] [PubMed] [Google Scholar]
- World Health Organization, 2018. Cancer [WWW Document]. URL https://www.who.int/health-topics/cancer#tab=tab_1 (accessed 2.10.20).
- Wu C.-H., Hong B.-H., Ho C.-T., Yen G.-C. Targeting Cancer Stem Cells in Breast Cancer: Potential Anticancer Properties of 6-Shogaol and Pterostilbene. J. Agric. Food Chem. 2015;63:2432–2441. doi: 10.1021/acs.jafc.5b00002. [DOI] [PubMed] [Google Scholar]
- Wu S.-H., Hang L., Yang J.-S., Chen H.-Y., Lin H.-Y., Chiang J.-H., Lu C.-C., Yang J.-L., Lai T.-Y., Ko Y.-C., Chung J. Curcumin induces apoptosis in human non-small cell lung cancer NCI-H460 cells through ER stress and caspase cascade- and mitochondria-dependent pathways. Anticancer Res. 2010;30:2125–2133. [PubMed] [Google Scholar]
- Yang G.-W., Jiang J.-S., Lu W.-Q. Ferulic Acid Exerts Anti-Angiogenic and Anti-Tumor Activity by Targeting Fibroblast Growth Factor Receptor 1-Mediated Angiogenesis. Int. J. Mol. Sci. 2015;16:24011–24031. doi: 10.3390/ijms161024011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdani E., Jalali Sendi J., Hajizadeh J. Effect of Thymus vulgaris L. and Origanum vulgare L. essential oils on toxicity, food consumption, and biochemical properties of lesser mulberry pyralid Glyphodes pyloalis Walker (Lepidoptera: Pyralidae) J. Plant Prot. Res. 2014;54:53–61. doi: 10.2478/jppr-2014-0008. [DOI] [Google Scholar]
- Yuan H., Zhu M., Guo W., Jin L., Chen W., Brunk U.T., Zhao M. Mustard seeds (Sinapis Alba Linn) attenuate azoxymethane-induced colon carcinogenesis. Redox report : communications in free radical research. 2011;16:38–44. doi: 10.1179/174329211X12968219310918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaini R., Brandt K., Clench M., Le Maitre C. Effects of Bioactive Compounds from Carrots (Daucus carota L.), Polyacetylenes, Beta-Carotene and Lutein on Human Lymphoid Leukaemia Cells. Anti-Cancer Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry - Anti-Cancer Agents) 2012;12:640–652. doi: 10.2174/187152012800617704. [DOI] [PubMed] [Google Scholar]
- Zaoui A., Cherrah Y., Mahassini N., Alaoui K., Amarouch H., Hassar M. Acute and chronic toxicity of Nigella sativa fixed oil. Phytomedicine. 2002;9:69–74. doi: 10.1078/0944-7113-00084. [DOI] [PubMed] [Google Scholar]
- Zarai Z., Kadri A., Ben Chobba I., Ben Mansour R., Bekir A., Mejdoub H., Gharsallah N. The in-vitro evaluation of antibacterial, antifungal and cytotoxic properties of Marrubium vulgare L. essential oil grown in Tunisia. Lipids Health Dis. 2011;10:161. doi: 10.1186/1476-511X-10-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M., Yuan H., Guo W., Li X., Jin L., Brunk U.T., Han J., Zhao M., Lu Y. Dietary mustard seeds (Sinapis alba Linn) suppress 1,2-dimethylhydrazine-induced immuno-imbalance and colonic carcinogenesis in rats. Nutr Cancer. 2012;64:464–472. doi: 10.1080/01635581.2012.658948. [DOI] [PubMed] [Google Scholar]
- Zia T., Hasnain S.N., Hasan S.K. Evaluation of the oral hypoglycaemic effect of Trigonella foenum-graecum L. (methi) in normal mice. J. Ethnopharmacol. 2001;75:191–195. doi: 10.1016/S0378-8741(01)00186-6. [DOI] [PubMed] [Google Scholar]