Abstract
Background
This study provides the first systematic review and meta-analysis to identify the predictors of unfavorable prognosis of COVID-19 in children and adolescents.
Methods
We searched literature databases until July 2021 for studies that investigated risk factors for unfavorable prognosis of children and adolescents with COVID-19. We used random-effects models to estimate the effect size with 95% confidence interval (CI).
Findings
We identified 56 studies comprising 79,104 individuals. Mortality was higher in patients with multisystem inflammatory syndrome (MIS-C) (odds ratio [OR]=58.00, 95% CI 6.39–526.79) and who were admitted to intensive care (OR=12.64, 95% CI 3.42–46.68). Acute respiratry distress syndrme (ARDS) (OR=29.54, 95% CI 12.69–68.78) and acute kidney injury (AKI) (OR=55.02, 95% CI 6.26–483.35) increased the odds to be admitted to intensive care; shortness of breath (OR=16.96, 95% CI 7.66–37.51) increased the need of respiratory support; and neurological diseases (OR=5.16, 95% CI 2.30–11.60), C-reactive protein (CRP) level ≥80 mg/L (OR=11.70, 95% CI 4.37–31.37) and D-dimer level ≥0.5ug/mL (OR=20.40, 95% CI 1.76–236.44) increased the odds of progression to severe or critical disease.
Interpretation
Congenital heart disease, chronic pulmonary disease, neurological diseases, obesity, MIS-C, shortness of breath, ARDS, AKI, gastrointestinal symptoms, elevated CRP and D-dimer are associated with unfavourable prognosis in children and adolescents with COVID-19.
Keywords: Risk factor, Prognosis, Children, Adolescents, COVID-19, Meta-analysis
Research in context.
Evidence before this study
Children and adolescents with COVID-19 experiencing unfavorable prognosis obtained increasing attention worldwide. However, some controversies with respect to some risk factors in the published studies remain. We provided a systematic review and meta-analysis to identify the predictors of unfavorable prognosis of COVID-19 in children and adolescents.
Added value of this study
We report that congenital heart disease, chronic pulmonary disease, neurological diseases, obesity, having multisystem inflammatory syndrome, shortness of breath, acute respiratry distress syndrme, acute kidney injury, gastrointestinal symptoms, elevated C-reactive protein and D-dimer are associated with unfavourable prognosis in children and adolescents with COVID-19. However, the majority of included studies displayed significant risk of bias.
Implications of all the available evidence
Further research on risk factors for poor prognosis of children and adolescents with COVID-19 should be funded with a common definition of outcomes to enhance the homogeneity between the future studies.
Alt-text: Unlabelled box
1. Introduction
Coronavirus Disease 2019 (COVID-19) has caused a truly global pandemic. As of August 2021, there had been more than 197 million confirmed cases of COVID-19 and over 4.2 million deaths worldwide[1]. Findings of a previous study have shown that children with COVID-19 had on average milder clinical symptoms and better prognosis than adults [2]. However, as the number of children with COVID-19 continues to rise globally [3,4], so does the number of children with severe course of disease[5]. Children also sometimes need hospitalization, admission to intensive care unit (ICU), or a ventilator to help them breathe, and may be at increased risk of death [6]. Therefore, despite children being less affected by COVID-19 than adults [7], finding the risk factors for poor prognosis is crucial to identify the children at highest risk as early as possible. Given the growing number of preventive and therapeutic possibilities, a hierarchical prognostic classification can help to identify patient groups suitable for earlier and/or more aggressive intervention. Identification of the children at greatest risk can help to decrease mortality in the affected children, and also reduce the resources needed for intensive care.
Only few guidelines that focus on prognosis in children with COVID-19 exist. The guidelines of the Centers for Disease Control [8] indicate that the risk of developing severe COVID-19 for children was higher if pre-existing conditions, for example, obesity, diabetes, asthma, chronic lung disease or immunosuppression, were present. One consensus statement [9] mentions, amongst other factors, age less than 3 months, poor mental response or lethargy, progressive elevation of lactate levels, and rapid progression of pulmonary lesions in the short term as predictors for severe disease course. However, none of the above recommendations were formulated according to the principles of evidence-based medicine. Although studies on risk factors for poor prognosis in children and adolescents with COVID-19 exist and they sometimes have come to similar conclusions, there remain several controversies or divergences with respect to some factors. For example, Fisler et al. proposed that age above 12 years was associated with a higher risk of ICU admission [10], but Abrams et al. failed to find an association [11].
To our knowledge, only one systematic review on the risk factors for COVID-19 in children has been carried out. Tsabouri et al. [12] summarized the potential risk factors for various indicators (eg. death, ICU admission, progression to critical disease and multisystem inflammatory syndrome [MIS-C]) in children based on 23 studies on children with COVID-19. The study was published on July 30, 2020, and due to heterogeneity between the studies in the definition of outcomes, it did not contain a quantitative analysis. As the prognosis of children affected by COVID-19 obtains increasing attention worldwide, we believe that a meta-analysis on this topic that provides precise and reliable information will be of great clinical value. Therefore, we undertook this study to investigate risk factors for poor prognosis in children and adolescents with COVID-19.
2. Methods
2.1. Protocol and guidance
We report this study in accordance to the Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) 2020 guidelines [13]. The protocol for this study including search strategy is available in Supplementary Materials. Due to the limited time, we did not register the research protocol beforehand. No ethical approval was required as the study include only previously published studies.
2.2. Search strategy and selection criteria
Using the key words “COVID-19″ and (“children” or “adolescent”) and (“risk factor” or “prognosis” or “predictor”), we performed a systematic search of the following databases from their inception to July 23, 2021: MEDLINE (via PubMed), WHO COVID-19 database, Web of Science, the Cochrane library, China Biology Medicine (CBM), China National Knowledge Infrastructure (CNKI), and Wanfang Data [14]. We also searched clinical trial registry platforms (the WHO Clinical Trials Registry Platform and US National Institutes of Health Trials Register); some preprint servers (MedRxiv, BioRxiv and SSRN); and Google. Finally, we reviewed the reference lists of relevant reviews and similar articles of identified studies to find additional records.
Studies on COVID-19 in children and adolescents (aged ≤18 years) that focused on risk factors for poor prognosis were included in our meta-analysis. We defined poor prognosis as experiencing one of the following: (1) death; (2) admission to ICU; (3) receiving respiratory support; or (4) progression to severe or critical disease (regardless of the definition used). The following types of studies were eligiable: randomized controlled trials (RCTs), clinical controlled trials (CCTs), cohort studies, case-control studies and case series. Studies where full text could not be retrieved or data were missing were excluded. Duplicates, articles in languages other than English or Chinese, and conference abstracts were also excluded.
One experienced researcher (QS) searched all the databases. After eliminating duplicates, four researchers in two groups of two (Group1: QS and JL; Group 2: ZW and XW) independently screened first the titles and abstracts, and then the full texts of potentially eligible studies against the pre-defined eligibility criteria. Disagreements were resolved by consensus or appeal to a senior researcher (QZ). The process of study selection was documented using a PRISMA flow diagram.
2.3. Data collection and risk of bias assessment
Two researchers (QS and ZW) independently extracted the following variables: study details, sample size, inclusion/exclusion criteria, age, sex, coexisting medical conditions, clinical symptoms, complications, and laboratory investigations of the participants. Disagreements were resolved by consensus. Before the formal extraction, a pilot test was conducted.
The quality of studies was assessed using the following tools: the Cochrane Risk-of-Bias assessment tool [15] for RCTs (each type of bias graded as “Low”, “Unclear” or “High”); the ROBINS-I tool [16] for CCTs (each type of bias graded as “Low”, “Moderate”, “Serious”, “Critical”, and “No information”); Newcastle-Ottawa Scale (NOS) [17] for cohort and case-control studies (each study rated on a 0–9 scale; with 8 or 9 considered high quality, 7 medium quality, and <7 low quality); and the Institute of Health Economics (IHE) checklist [18] for case series (each study rated on a 0–20 scale, with ≥14 considered acceptable). Two researchers (QS and ZW) independently assessed the quality of all included studies and discussed discrepancies until consensus was reached.
2.4. Statistical analysis
We used random-effects models to conduct the meta-analysis as recommended by the Cochrane Handbook. For dichotomous data, we recorded the number of events and the total number of participants in both groups, and calculated the odds ratio (OR) with 95% confidence intervals (CI); for continuous data, we recorded the mean, standard deviation (SD), and total number of participants in both groups, and calculated mean difference (MD) with 95% CI. For missing SD, standard error (SE) was converted to SD when SE was presented, and if both were missing, we estimated SDs from P values or 95% CI. Missing means were estimated from interquartile ranges and medians [19]. If insufficient information to calculate the primary variables was available, we extracted the reported OR and included it in the meta-analysis.
The I2 statistic was calculated to assess between-study heterogeneity [20]. If I2 was above 75%, we explored possible causes of heterogeneity through sensitivity analyses where we removed one study at a time. If we had enough data, we performed a subgroup analysis removing studies with a considerable risk of bias, containing cohort and case-control studies with high and medium quality only.
As mentioned above, we searched the trial registries to identify completed trials that had not been published elsewhere to minimize publication bias. If heterogeneity was low, we explored the impact of publication bias using the Egger regression asymmetry test (if 5 or more studies were available per outcome) and constructing funnel plots (if 10 or more studies were involved per outcome) [21].
All calculations and graphs were performed using Stata 14 software (Stata Corp LLC). Two-sided P values less than 0.05 were considered statistically significant.
2.5. Assessment of the certainty of evidence
We assessed the certainty of evidence using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach [22]. Two researchers (QS and ZW) with experience in using GRADE rated each domain for each outcome separately and resolved discrepancies by consensus.
2.6. Role of the funding source
The study received no funding. All authors had full access to the full data in the study and accept responsibility to submit for publication.
3. Results
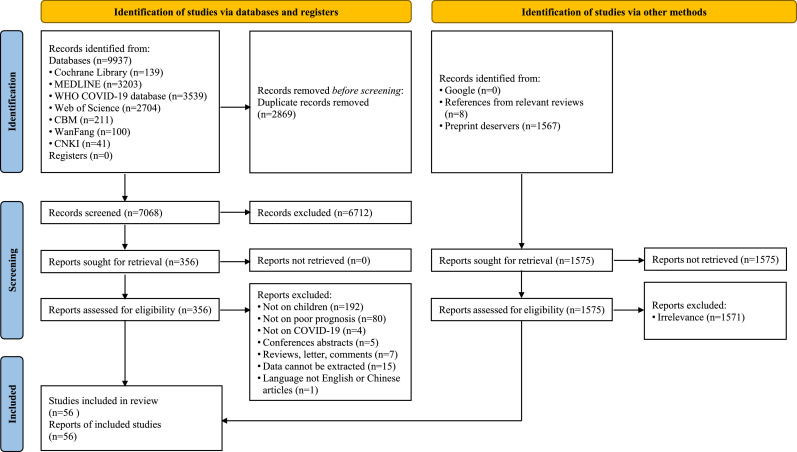
We identified 9937 potentially relevant records from the literature databases and registers, and 1575 records from the additional searches. After screening the titles, abstracts and full texts, 56 studies (22 cohort studies, 9 case-control studies, and 25 case series) with a total of 79,104 patients were included [[10], [11],[23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76]] (Fig. 1).
Fig. 1.
Flow diagram of the literature search. 11,512 records from databases (Cochrane library, MEDLINE, WHO COVID-19, Web of Science, China Biology Medicine, Wanfang Data, China National Knowledge Infrastructure) and additional sources were included in the initial search and 56 studies were finally included after full-text screen.
Table 1 shows the characteristics of the studies and their participants. The number of subjects examined in the individual studies ranged from 19 to 29,886. The highest number of studies were conducted in the USA (n = 21, 37.5%), and more than half of the studies did not report the follow-up time (n = 29, 51.8%). Among those that reported the follow-up time, the time for assessment of risk factors ranged from 2 weeks to 7 months.
Table 1.
Characteristics of the included studies. Characteristics of the included 56 studies (22 cohort studies, 9 case-control studies, and 25 case series) were presented including study design, geographic location, sample size, outcomes, demography (age and gender) and follow up.
| Study ID | Geographic location | Study design | Sample size | Age | Sex (male/female) | Outcomes | Follow-up time |
|---|---|---|---|---|---|---|---|
| Alfraij et al., 2021 [23] | Kuwait and KSA | Cohort study | 25 | 2.8 y (0.2 y–8.5 y)* | 15/10 | I | 5mo |
| Antúnez-Montes et al., 2021 [24] | International | Cohort study | 409 | 3 y (0.6 y–9.0 y)* | 222/187 | I, II | 1mo |
| Bailey et al., 2021 [25] | U.S.A | Cohort study | 5374 | NR | 2672/2699**** | II, IV | NR |
| Bari et al., 2021a [26] | Pakistan | Cohort study | 66 | 7.9 y±4.2 y** | 38/28 | I, II, III, IV | 6mo |
| Basalely et al., 2021 [27] | U.S.A | Cohort study | 97 | 8.2 y (1.5 y–13.8 y)* | 50/47 | I, II, III | NR |
| Besli et al., 2021 [28] | Turkey | Cohort study | 104 | 11.8 y (8.4 y)* | 53/51 | II, III, IV | 3.5mo |
| Farzan et al., 2021 [29] | U.S.A | Cohort study | 38 | NR | 16/22 | I, II, III | NR |
| Fernandes et al., 2021 [30] | U.S.A | Cohort study | 281 | 10 y (1 y–17 y)* | 170/111 | III, IV | NR |
| Götzinger et al., 2020 [31] | International | Cohort study | 582 | 5 y (0.5 y–12.0 y)* | 311/271 | II | 3.5w |
| Graff et al., 2021 [32] | U.S.A | Cohort study | 454 | 11 y (3 y–11 y)* | 262/191**** | III | 4mo |
| Kainth et al., 2020 [33] | U.S.A | Cohort study | 65 | 10.3 y (1.4 mo–16.3 y)* | 33/32 | I, II, III, IV | NR |
| Kari et al., 2021 [34] | Saudi Arabia | Cohort study | 88 | NR | 37/51 | I, II | NR |
| Kelly et al., 2021 [35] | U.S.A | Cohort study | 106 | 4 (NR)* | 59/47 | II | NR |
| Madhusoodhan et al., 2021 [36] | U.S.A | Cohort study | 98 | 2 y–21 y*** | 69/29 | IV | NR |
| Prata-Barbosa et al., 2020 [37] | Brazil | Cohort study | 79 | 4 y (1 y–10.3 y)* | 36/43 | I, III | 3mo |
| Shi et al., 2021 [38] | China | Cohort study | 29,886 | NR | 15,059/14,827 | I | NR |
| Song et al., 2021 [39] | South Korea | Cohort study | 5621 | NR | 2317/3304 | I | NR |
| Surendra et al., 2021 [40] | Indonesia | Cohort study | 217 | NR | NR | I | 5mo |
| Swann et al., 2020 [41] | U.K | Cohort study | 632 | 4.6 y (0.3 y–13.7 y)* | 357/274**** | II | 2w |
| Tripathi et al., 2021 [42] | U.S.A | Cohort study | 394 | 10 y (3.1y–15.0 y)* | 198/186 | III, IV | NR |
| Verma et al., 2021 [43] | U.S.A | Cohort study | 82 | 5 y (2.5 mo–15.2 y)* | 52/30 | II, III | 3mo |
| Yazidi et al., 2021 [44] | Oman | Cohort study | 56 | 1.8 y (0.2 y–6.9 y)* | 36/20 | II | NR |
| Abrams et al., 2021[11] | U.S.A | Case-control study | 1080 | 8 y (4 y–12 y)* | 602/476 | I, II | NR |
| Aykac et al., 2021 [45] | Turkey | Case-control study | 518 | 11 y (5 y–14 y)* | 250/268 | IV | NR |
| Cho et al., 2021[46] | South Korea | Case-control study | 428 | NR | NR | I | NR |
| Chopra et al., 2021 [47] | India | Case-control study | 105 | 6 y (1 y–10 y)* | 57/48 | I, III | 0.5mo |
| Coronado Munoz et al., 2021 [48] | Peru | Case-control study | 47 | 1 mo–16 y*** | 30/17 | I | 5mo |
| Lu et al., 2021 [49] | China | Case-control study | 121 | 6.3 y±4.3 y** | 82/39 | IV | NR |
| Moreira et al., 2021[50] | U.S.A | Case-control study | 20,096 | NR | 9681/10,415 | I | NR |
| Ozsurekci et al., 2020 [51] | Turkey | Case-control study | 30 | 0 y–17 y*** | 14/16 | IV | NR |
| Wang et al., 2020 [52] | China | Case-control study | 43 | NR | 27/16 | IV | NR |
| Bari et al., 2021b [53] | Pakistan | Case series | 83 | 7.0 y±4.3 y** | 51/32 | IV | NR |
| Bhavsar et al., 2021 [54] | U.S.A | Case series | 67 | NR | 36/31 | II | NR |
| Bhumbra et al., 2020 [55] | U.S.A | Case series | 19 | 5 y (0.8 y–16 y)* | 14/5 | IV | NR |
| Bjornstad et al., 2021 [56] | U.S.A | Case series | 106 | 11.0 y (0.1 y–17.8 y)* | 54/52 | III | 1mo |
| Chao et al., 2020 [57] | U.S.A | Case series | 46 | 13.1 y (0.4 y–19.3 y)* | 31/15 | I, II | NR |
| Derespina et al., 2020 [58] | U.S.A | Case series | 70 | 15.0 y (9.0 y–19.0 y)* | 43/27 | I, II, III | 1mo |
| Desai et al., 2020 [59] | U.S.A | Case series | 293 | 5.6 y±6.3 y** | 156/137 | IV | NR |
| Du et al., 2021 [60] | China | Case series | 182 | 3d–15 y*** | 120/62 | I, IV | 2mo |
| Fisler et al., 2020 [10] | U.S.A | Case series | 77 | 9.5 y | 37/40 | II | Unclear |
| Giacomet et al., 2020 [61] | Italy | Case series | 127 | 4.8 y (0.3 y–8.5 y)* | 83/44 | II,IV | NR |
| Haslak et al., 2021 [62] | Turkey | Case series | 76 | 8.2 y±4.4 y** | 52/24 | II | NR |
| Hoseinyazdi et al., 2021 [63] | Iran | Case series | 53 | 9.6 y±5.4 y** | 22/31 | I, II, IV | NR |
| Jimenez et al., 2020 [64] | Spain | Case series | 101 | NR | 58/43 | II | NR |
| Kanburoglu et al., 2020 [65] | Turkey | Case series | 37 | 15.6d±7.7d** | 19/18 | III, IV | 3mo |
| Kompaniyets et al., 2021 [66] | U.S.A. | Case series | 4302 | NR | 1974/2328 | IV | NR |
| Lazzerini et al., 2021 [67] | Italy | Case series | 159 | NR | 77/82**** | IV | NR |
| Ouldali et al., 2021 [68] | France | Case series | 397 | 16 mo (51d–134mo)* | 224/171**** | I, III, IV | NR |
| Parri et al., 2020 [69] | Italy | Case series | 130 | 6 y (0 y–11 y)* | 73/57 | IV | NR |
| Pereira et al., 2020 [70] | Brazil | Case series | 66 | NR | 33/33 | I, II, III | NR |
| Qian et al., 2021 [71] | China | Case series | 127 | 7.3 y (4.9 y)* | 86/41 | IV | NR |
| Rao et al., 2021 [72] | India | Case series | 123 | 3 y (0.7 y–6 y)* | 71/52 | I | NR |
| Ramírez-Soto et al., 2021 [73] | Peru | Case series | 3066 | NR | 1468/1598 | I | NR |
| Rivas-Ruiz et al., 2020 [74] | Mexico | Case series | 1443 | 12 y (5 y–16 y)* | 693/750 | I | NR |
| Sena et al., 2021 [75] | Brazil | Case series | 682 | 9.1 y±7.2 y** | 322/360 | I | 4mo |
| Zachariah et al., 2020 [76] | U.S.A | Case series | 50 | NR | 27/23 | IV | NR |
I: death; II: admission to intensive care unit; III: receiving respiratory support; IV: progression to severe or critical disease; KSA: Kingdom of Saudi Arabia; NR: not reported; U.K: United Kingdom; U.S.A: United States; International means that the study was conducted in more than two countries.
*Median (IQR); **mean ± SD; ***range; “mo” means month, “w” means week, and “d” means day. For the included 56 studies, 28 included patients aged less than 18 years (of which, 1 study included newborns), 9 included patients aged less than 19 years, 10 included patients aged less than 21 years, 2 included patients aged less than 22 years, 1 included patients aged less than 25 years and 6 did not report the age range for their included patients.
**** Sex was missing for some patients.
The median quality score for cohort studies was six (range 5 to 8). Most cohort studies did not control for factors that influence the primary results and had inadequate outcome ascertainment. The median quality score for case-control studies was five (range 4 to 6). Most of the studies had inadequate exposure ascertainment, inadequate control selection, and inconsisteny of non-response rate between groups. The median quality score for case series was nine (range 6 to 12). Most studies did not report or clarify their criteria, interventions, outcome measures, follow-up, or adverse events. Details are available in Supplementary eTables 1–3.
The results of meta-analysis are presented in the following sections and Table 2. The quality of evidence according to GRADE for each factor ranged between very low and moderate. Factors contributing to the downgrading of the quality of evidence included risk of bias, inconsistency or imprecision (due to limitations in study design, wide CI or relatively small sample size, and substantial heterogeneity), whereas for some factors we were able to upgrade the quality due to the large magnitude of effect. Details are available in Supplementary eTable 4 and eFig. 1–18.
Table 2.
Pooled outcomes of the included studies
Meta-analysis showed that male sex, blood group A, underlying conditions (obesity, chronic pulmonary disease, congenital heart disease and neurological diseases), clinical symptoms and complications (ARDS, AKI, MIS-C, shortness of breath, gastrointestinal symptoms, and the need for intensive care), and biomarkers (CRP and D-dimer level at baseline) were associated with poor prognosis in children and adolescents with COVID-19.
| Risk factor | No. of studies reporting the factor | Total no. of patients | Effect size (95% CI) | I2 | Publication bias* | Quality of evidence (GRADE) |
|---|---|---|---|---|---|---|
| Death | ||||||
| AKI | 2 | 201 | OR 3.15 (1.25, 7.90) | 0% | NA | LOW |
| Age less than ten years | 7 | 25,173 | OR 1.76 (1.07, 2.90) | 16% | t = 0.95, p = 0.44 | VERY LOW |
| Underlying conditions | 5 | 20,915 | OR 8.68 (5.27, 14.30) | 0% | t = 134.13, p = 0.005 | VERY LOW |
| Need for intensive care | 5 | 3907 | OR 12.64 (3.42, 46.68) | 69.8% | NA | VERY LOW |
| Age less than four years | 1 | 1443 | OR 4.02 (1.87, 8.65) | 100% | NA | VERY LOW |
| MIS-C | 1 | 66 | OR 58.00 (6.39, 526.79) | 100% | NA | VERY LOW |
| Admitted to intensive care unit | ||||||
| Age less than one month | 3 | 1621 | OR 2.29 (1.48, 3.56) | 0% | NA | MODERATE |
| Underlying conditions | 10 | 2189 | OR 2.41 (1.77, 3.27) | 25.6% | t = 0.29, p = 0.778 | LOW |
| Gastrointestinal symptoms | 6 | 1343 | OR 1.92 (1.30, 2.84) | 9.3% | t = 0.78, p = 0.481 | LOW |
| Suspected or confirmed ARDS | 5 | 842 | OR 29.54 (12.69, 68.78) | 0% | t = 0.00, p = 0.997 | LOW |
| Congenital heart disease | 4 | 1150 | OR 2.90 (1.26, 6.67) | 0% | NA | LOW |
| Chronic pulmonary disease | 3 | 732 | OR 3.45 (1.47, 8.07) | 0% | NA | LOW |
| MIS-C | 3 | 546 | OR 3.83 (1.48, 9.87) | 44.1% | NA | LOW |
| AKI | 2 | 215 | OR 55.02 (6.26, 483.35) | 0% | NA | LOW |
| Male sex | 12 | 3308 | OR 1.20 (1.01, 1.43) | 0% | t = 0.82, p = 0.431 | VERY LOW |
| Obesity | 7 | 2033 | OR 1.66 (1.10, 2.50) | 20.4% | t = 0.40, p = 0.712 | VERY LOW |
| Age (year) | 7 | 1112 | WMD 2.75 (1.63, 3.88) | 0.2% | t = 1.45, p = 0.206 | VERY LOW |
| Shortness of breath | 3 | 1192 | OR 5.28 (1.49, 18.74) | 69.2% | NA | VERY LOW |
| CRP>10 mg/dl (at baseline) | 1 | 54 | OR 8.00 (1.60, 39.97) | 100% | NA | VERY LOW |
| CRP/mg/L (at baseline) | 6 | 365 | WMD 60.04 (23.82, 96.26) | 38.6% | t = 3.26, p = 0.031 | VERY LOW |
| Receiving respiratory support | ||||||
| Neurological diseases | 1 | 435 | OR 2.51 (1.03, 6.15) | 100% | NA | LOW |
| Shortness of breath | 1 | 435 | OR 16.96 (7.66, 37.51) | 100% | NA | LOW |
| Blood group A | 1 | 66 | OR 6.00 (1.78, 20.19) | 100% | NA | VERY LOW |
| CRP/mg/L (at baseline) | 1 | 37 | WMD 18.20 (7.31, 29.09) | 100% | NA | VERY LOW |
| Progression to severe or critical disease | ||||||
| Neurological diseases | 5 | 841 | OR 5.16 (2.30, 11.60) | 27.3% | t = 3.38, p = 0.077 | MODETARE |
| Obesity | 7 | 6228 | OR 2.47 (2.00, 3.04) | 0% | t = 0.58, p = 0.591 | LOW |
| Underlying conditions | 7 | 5375 | OR 3.82 (2.17, 6.71) | 60.6% | NA | LOW |
| Gastrointestinal symptoms | 4 | 363 | OR 2.93 (1.19, 7.22) | 47.2% | NA | LOW |
| Confirmed ARDS | 2 | 225 | OR 48.29 (10.88, 214.33) | 0% | NA | LOW |
| Age less than six months | 2 | 280 | OR 2.54 (1.08, 5.98) | 0% | NA | LOW |
| CRP/mg/L (at baseline) | 5 | 347 | WMD 33.29 (11.25, 55.33) | 94.3% | NA | VERY LOW |
| Shortness of breath | 2 | 342 | OR 8.69 (1.58, 47.70) | 56.1% | NA | VERY LOW |
| MIS-C | 1 | 394 | OR 2.79 (1.84, 4.22) | 100% | NA | VERY LOW |
| Increased level of CRP (at baseline) | 1 | 376 | OR 12.24 (4.51, 33.19) | 100% | NA | VERY LOW |
| CRP≥80 mg/L (at baseline) | 1 | 250 | OR 11.70 (4.37, 31.37) | 100% | NA | VERY LOW |
| Blood group A | 1 | 66 | OR 8.29 (2.40, 28.66) | 100% | NA | VERY LOW |
| D-dimer≥0.5ug/ml (at baseline) | 1 | 43 | OR 20.40 (1.76, 236.44) | 100% | NA | VERY LOW |
OR: odds ratio; WMD: weighted mean difference; CI: confidence interval; AKI: acute kidney injury; ARDS: acute respiratory distress syndrome; CRP: C-reactive protein; GRADE: grading of recommendations assessment, development, and evaluation; MIS-C: multisystem inflammatory syndrome; NA: not applicable.
*The probability of publication bias was tested by using the Egger test.
3.1. Death
A total of 26 studies assessed risk factors for death [11,23,24,26,27,29,33,34,[37], [38], [39], [40],[46], [47], [48],50,57,58,60,63,68,70,[72], [73], [74], [75]]. We found low quality evidence that acute kidney injury (AKI, OR=3.15, 95% CI 1.25 to 7.90, two studies) was associated with an elevated risk of death. Underlying conditions (OR=8.68, 95% CI 5.27 to 14.30, five studies), in need for intensive care (OR=12.64, 95% CI 3.42 to 46.68, five studies) and MIS-C (OR=58.00, 95% CI 6.39 to 526.79, one study) to be associated with increased odds of death (very low-quality evidence).
Eight studies appraised age as a risk factor. Age less than 10 years was associated with a 1.76 times higher odds of death (OR=1.76, 95% CI 1.07 to 2.90, seven studies, very low-quality evidence), while age less than 4 years was associated with a 4.02 times higher odds of death (OR=4.02, 95% CI 1.87 to 8.65, one study, very low-quality evidence). However, no statistically significant difference was found for age less than 1 year (OR=0.89, 95% CI 0.14 to 5.48, three studies, very low-quality evidence) or 2 years (OR=2.02, 95% CI 0.08 to 54.42, one study, very low-quality evidence).
Six studies appraised sex as a risk factor, but no statistically significant association was found (OR=1.12 for males vs females, 95% CI 0.78 to 1.60, very low-quality evidence). Similar findings were also observed for other factors including obesity (OR=1.89, 95% CI 0.60 to 5.91, two studies, very low-quality evidence), chronic pulmonary disease (OR=1.52, 95% CI 0.05 to 43.69, one study, very low-quality evidence), and congenital heart disease (OR=0.43, 95% CI 0.02 to 9.59, one study, very low-quality evidence).
3.2. Admission to ICU
A total of 24 studies assessed factors associated with the risk of admission to ICU [10,11,[24], [25], [26], [27], [28], [29],[31], [32], [33], [34], [35],41,43,44,54,57,58,[61], [62], [63], [64],70]. The pooled results from three studies showed that age less than 1 month was associated with an increased risk of admission to ICU (OR=2.29, 95% CI 1.48 to 3.56, moderate-quality evidence). However, based on results from seven studies, children admitted to ICU were older than those not admitted (WMD=2.75 year, 95% CI 1.63 to 3.88, very low-quality evidence).
Ten studies appraised underlying conditions as a risk factor (OR=2.41, 95% CI 1.77 to 3.27, low-quality evidence), but none of them clarified the specific comorbidities. Having gastrointestinal symptoms (OR=1.92, 95% CI 1.30 to 2.84, six studies), suspected or confirmed ARDS (OR=29.54, 95% CI 12.69 to 68.78, five studies), MIS-C (OR=3.83, 95% CI 1.48 to 9.87, three studies), AKI (OR=55.02, 95% CI 6.26 to 483.35, two studies), congenital heart disease (OR=2.90, 95% CI 1.26 to 6.67, four studies) and chronic pulmonary disease (OR=3.45, 95% CI 1.47 to 8.07, three studies) increased the odds of admission to ICU (low-quality evidence).
Male sex (OR=1.20, 95% CI 1.01 to 1.43, 12 studies), obesity (OR=1.66, 95% CI 1.10 to 2.50, seven studies), shortness of breath (OR=5.28, 95% CI 1.49 to 18.74, three studies) and increased CRP>10 mg/dl (OR=8.00, 95% CI 1.60 to 39.97, one study) at baseline were also associated with elevated risk of admission to ICU (very low-quality evidence). Children admitted to ICU had also higher level of CRP (WMD=60.04 mg/L, 95% CI 23.82 to 96.26, six studies, very low-quality evidence) at baseline when compared to those without.
No significant association with the risk of ICU admission was found for other factors including diabetes (OR=2.42, 95% CI 0.65 to 9.04, four studies, low-quality evidence), neurological diseases (OR=2.03, 95% CI 0.96 to 4.31, five studies, low-quality evidence) and asthma (OR=1.30, 95% CI 0.67 to 2.54, five studies, very low-quality evidence).
3.3. Respiratory support
A total of 16 studies assessed risk factors for receiving respiratory support [[26], [27], [28], [29], [30],32,33,37,42,43,47,56,58,65,68,70] including mechanical ventilation, conventional oxygen therapy. According to the results of meta-analysis, neurological diseases (OR=2.51, 95% CI 1.03 to 6.15, one study) and having shortness of breath (OR=16.96, 95% CI 7.66 to 37.51, one study) were associated with an increased odds of respiratory support (low-quality evidence). Blood group A (OR=6.00, 95% CI 1.78 to 20.19, one study, very low-quality evidence) was also associated with the need of respiratory support. When compared to children not needing respiratory support, those receiving respiratory support had higher level of CRP (WMD=18.20 mg/L, 95% CI 7.31 to 29.09, one study, very low-quality evidence) at baseline.
No significant association with the need of respiratory support was found for other factors including male sex (OR=0.74, 95% CI 0.41 to 1.34, two studies, very low-quality evidence), underlying conditions (OR=1.33, 95% CI 0.45 to 3.91, three studies, very low-quality evidence), or AKI (OR=1.89, 95% CI 0.99 to 3.59, three studies, very low-quality evidence).
3.4. Progression to severe or critical disease
A total of 23 studies assessed risk factors for progression to severe or critical disease [25,26,28,30,33,36,42,49,[51], [52], [53],55,59,60,61,63,[65], [66], [67], [68], [69],71,76]. Neurological diseases (OR=5.16, 95% CI 2.30 to 11.60, five studies, moderate-quality evidence) increased the odds of severe or critical disease. Obesity (OR=2.47, 95% CI 2.00 to 3.04, seven studies), having gastrointestinal symptoms (OR=2.93, 95% CI 1.19 to 7.22, four studies), confirmed ARDS (OR=48.29, 95% CI 10.88 to 214.33, two studies) and age less than 6 months (OR=2.54, 95% CI 1.08 to 5.98, two studies) were also associated with progression to severe or critical disease (low-quality evidence).
Having shortness of breath (OR=8.69, 95% CI 1.58 to 47.70, two studies), MIS-C (OR=2.79, 95% CI 1.84 to 4.22, one study), blood group A (OR=8.29, 95% CI 2.40 to 28.66, one study), CRP level ≥80 mg/L (OR=11.70, 95% CI 4.37 to 31.37, one study) and D-dimer level ≥0.5ug/mL (OR=20.40, 95% CI 1.76 to 236.44, one study) at baseline were associated with progression to severe or critical disease. Fifteen studies appraised sex as a risk factor, but no difference was found (OR=1.12 for males vs females, 95% CI 0.86 to 1.46, very low-quality evidence).
Additionaly, increased level of CRP (OR=12.24, 95% CI 4.51 to 33.19, very low-quality evidence) and underlying conditions (OR=3.82, 95% CI 2.17 to 6.71, low-quality evidence) were appraised as risk factors in one and seven studies, respectively. The studies did not however report the exact CRP level or the specific comorbidities. When compared to children without disease progression, those who progressed into severe or critical disease had higher level of CRP (WMD=33.29 mg/L, 95% CI 11.25 to 55.33, five studies, very low-qulity evidence) on admission to hospital. No significant association was found between disease progression and other factors.
We found considerable heterogeneity (I2=94.3%) between the studies on CRP level and disease progression; and high risk of bias for all five studies. We therefore conducted sensitivity analyses where one study was left out on turn. The result in effect did not differ after exclusion of any study. We also conducted subgroup analyses of cohort and case-control studies with NOS score equal or more than 7 for all outcomes. The results for each risk factor are presented in Table 3.
Table 3.
Pooled outcomes of the included studies in the subgroup analyses. Subgroup analyses suggested that male sex, underlying conditions (obesity, congenital heart disease, and chronic pulmonary disease), clinical symptoms and complications (ARDS, MIS-C, shortness of breath, gastrointestinal symptoms, and the need for intensive care), and biomarkers (CRP level at baseline) were associated with poor prognosis in children and adolescents with COVID-19. While, there was not statistical significance observed for other factors.
| Risk factor | No. of studies reporting the factor | Total no. of patients | Effect size (95% CI) | I2 | Quality of evidence (GRADE) |
|---|---|---|---|---|---|
| Death | |||||
| Need for intensive care | 1 | 409 | OR 352.46 (20.75, 5985.86) | 100% | LOW |
| Age less than ten years | 2 | 489 | OR 4.56 (1.17, 17.71) | 100% | VERY LOW |
| Admitted to intensive care unit | |||||
| Suspected or confirmed ARDS | 2 | 607 | OR 28.44 (7.61, 106.25) | 16.8% | MODERATE |
| Age less than one month | 3 | 1621 | OR 2.29 (1.48, 3.56) | 0% | LOW |
| Congenital heart disease | 2 | 991 | OR 2.76 (1.04, 7.30) | 0% | LOW |
| Obesity | 2 | 668 | OR 2.42 (1.09, 5.40) | 0% | LOW |
| Gastrointestinal symptoms | 2 | 66 | OR 2.01 (1.29, 3.13) | 0% | LOW |
| Shortness of breath | 1 | 991 | OR 6.27 (1.57, 25.05) | 100% | LOW |
| Male sex | 4 | 1688 | OR 1.34 (1.01, 1.80) | 0% | VERY LOW |
| Underlying conditions | 3 | 1622 | OR 2.83 (1.58, 5.06) | 71.7% | VERY LOW |
| Chronic pulmonary disease | 1 | 582 | OR 3.17 (1.23, 8.22) | 100% | VERY LOW |
| MIS-C | 1 | 409 | OR 2.35 (1.27, 4.34) | 100% | VERY LOW |
| CRP/mg/L (at baseline) | 1 | 66 | WMD 125.80 (37.04, 214.56) | 100% | VERY LOW |
| Receiving respiratory support | |||||
| Shortness of breath | 1 | 435 | OR 16.96 (7.66, 37.51) | 100% | LOW |
| Progression to severe or critical disease | |||||
| Confirmed ARDS | 1 | 98 | OR 56.43 (10.27, 310.00) | 100% | LOW |
We did subgroup analyses for cohort and case-control studies with high and medium quality; OR: odds ratio; WMD: weighted mean difference; CI: confidence interval; ARDS: acute respiratory distress syndrome; CRP: C-reactive protein; GRADE: grading of recommendations assessment, development, and evaluation; MIS-C: multisystem inflammatory syndrome.
We found a possibility of publication bias for factor underlying conditions (death) and CRP level at baseline (admission into ICU). However, there was no evidence of publication bias for other factors, either qualitatively based on funnel-plot (eFig. 19 and 20 in Supplementary Materials) or quantitatively (Egger test, Table 3).
4. Discussion
There exist currently only a limited amount of studies investigating risk factors for unfavorable prognosis of COVID-19 in children. This meta-analysis identified 56 studies and revealed that male sex, blood group A, underlying conditions (obesity, chronic pulmonary disease, congenital heart disease and neurological diseases), and biomarkers (CRP and D-dimer level at baseline) were associated with poor prognosis in children and adolescents with COVID-19. Clinical symptoms and complications (ARDS, AKI, MIS-C, shortness of breath, gastrointestinal symptoms, and the need for intensive care) also increased the risk of certain unfavorable outcomes.
Although the SARS-CoV-2 infection is very mild in the overwhelming majority of children, MIS-C, a newly described, life-threatening syndrome has been reported in hundreds of children worldwide [77], [78], [79], [80] and raised much concern. To identify the pathogenesis, Consiglio et al. [81] performed a systems-level analysis of immune cells and suggested multiple autoantibodies being involved in this hyperinflammatory immune state. Our study confirmed the strong association between MIS-C and death, but the sample size was small and the quality of evidence is very low. So far, the incidence of MIS-C is still unknown. In a recent systematic review, Ahmed et al. [82] summarized the clinical presentation and outcomes from 662 children diagnosed with MIS-C and found that many will progress rapidly into shock (n = 398, 60.1%) and cardiorespiratory failure (n = 314 out of 581, 54.0%). Most importantly, the mortality rate of 1.7% (11 of 662) is much higher than 0.09% that observed in children with COVID-19 in general.
Similar to adult patients, age and sex has always been in the focus of analyses in children. On one hand, older age has been confirmed to be significantly associated with an increased risk of severity and mortality of COVID-19 in adults [83]. This is consistent between the published studies [84,85], and may be an adverse outcome of the decline in the immune function (e.g., T-cell and B-cell function) [85]. In children, the majority of studies found that younger children had a worse clinical course. However, our meta-analysis could not quantify a relationship between age and prognosis in children, despite finding some evidence for an association. For example, children admitted to ICU tended to be older than those who were not [41,43,44,57,61,62,64], but children under one month of age were at highest risk [24,31,41]. The reasons for differences observed in disease severity among various age groups is yet to be determined. On the other hand, multiple reports showed higher percentages of hospitalization and mortality among men than women through this pandemic [86,87], indicating that men are more likely to be affected and develop into severe disease. Being male was determined to be a risk factor based on the results of our study, and this is also an established predictor of mortality in adults (RR=1.32, 95% CI 1.13 to 1.54), according to previous reports [85]. However, the association in both children and adults was quite weak. Boys have generally a higher prevalence of underlying childhood diseases than girls, and most importantly, the majority of studies identified in our review had a high risk of bias because of not controlling for some factors that can be expected to influence the outcomes. Altogether, we cannot be sure whether age and gender affects the prognosis of COVID-19, and the use of male sex to identify those who are in the greatest need of protection may be problematic.
Results on other factors were similar to those identified in the studies published before [84,[88], [89], [90]]. These included underlying conditions (obesity, chronic pulmonary disease, congenital heart disease and neurological diseases) and biomarkers (CRP and D-dimer). Elevated CRP has been proposed as predictor of COVID-19 severity. However, the studies of Földi et al. [90] and others [91], [92], [93], [94] did not provide any cut-off value for decision-making from a clinical point of view. For other biomarkers, Zhang et al. [95] found increased leukocyte count, aspartate transaminase, lactate dehydrogenase (LDH) and procalcitonin to be predictors for ICU asmission, while mortality was predicted to be increased by high leukocyte count and LDH. We also observed that blood group A was associated with increased risk of respiratory support and disease progression, in contrast to the study by Wu et al. [96], which found that individuals with blood group AB seemed to have a higher risk to COVID-19 severity and demise.
Furthermore, although gastrointestinal involvement has not been frequently reported in previous studies, Mao and colleagues [97] reported in their findings from 35 studies that such symptoms are not uncommon among children with COVID-19, and children even had a similar prevalence of gastrointestinal symptoms as adults. Our results that newly presenting gastrointestinal symptoms increased the odds to be admitted to ICU are in line with those of others [97], [98]. However, patients with gastrointestinal symptoms had a variety of manifestations, and we were unable to perform subgroup analysis due to not having sufficient data. According to the retrieved studies, possible gastrointestinal symptoms of COVID-19 include abdominal pain, nausea, vomiting and diarrhea [24,31,44,64]. Although our findings support the importance of monitoring for gastrointestinal symptoms in the management of COVID-19, the mechanism of the relationship between gastrointestinal symptoms and disease severity remains unclear.
The results of our meta-analysis can provide precise and reliable evidence for the development of practice guidelines and management of COVID-19 in children and adolescents. However, this study also has some limitations. First, we only included data reported in the studies, and did not contact the authors for unreported data. Second, the retrieval of articles was limited to those published in English and Chinese. Moreover, geographical bias cannot be ruled out as a considerable part of the studies were conducted in the USA, and the Egger test may lack the statistical power to detect bias when the number of studies is small. Third, the criteria to classify whether the patients had poor prognosis varied between studies leading to additional heterogeneity between studies. For example, Kanburoglu et al. [65] defined severe disease as any patient with oxygen saturation <92% or need for nasal continuous positive airway pressure (nCPAP), while Ouldali et al. [68] defined a disease as severe if the patient needed ventilatory or hemodynamic support during hospitalization, or died. This needs to be considered when interpreting the results, as any difference may complicate the analyses and introduce bias. Fourth, there was disagreement in the results for some risk factors between studies, which maybe due to different definitions of these factors or the small sample sizes in some studies. Finally, numerous studies with high risk of bias were included and therefore the level of evidence is on average low.
To address the challenges that COVID-19 poses to our health and economy, the National Institutes of Health (NIH) developed their Strategic Priorities for COVID-19 Research, and emphasized the importance of prevention of poor COVID-19 outcomes in health population [99]. For the already affected children and adolescents, the majority of studies included in this systematic review had higher risk of bias and lower quality of evidence, which limits our abilities to draw robust conclusions. We suggest that in the future: high-quality research should be funded and carried out in an effective manner, adhering to the key methodological principles, such as controlling for the factors that are most likely to influence the study results; studies that investigate topics for clinical practice and decision-making should be conducted more; and the definition of outcomes should be unified to enhance the homogeneity between the future studies.
In conclusion, this systematic review and meta-analysis yields important information regarding the risk factors for unfavorable prognosis in children and adolescents with COVID-19. We are cognizant of the limitations, but believe that this report is useful for clinical decision-making and will contribute to better prevention and screening strategies for poor prognosis in children. In the future, identifying COVID-19 children with predictors of unfavorable outcomes should become a key part of clinical evaluation, and efforts need to be made to improve the methodological quality of studies on children with COVID-19.
Contributors
Qianling Shi: Retrieval, Document selection, Data extraction, Data analysis, Methodology and GRADE assessment, Writing-Original Draft. Zijun Wang: Document selection, Data extraction, Methodology and GRADE assessment. Jiao Liu: Document selection. Xingmei Wang: Document selection. Qi Zhou: Document selection, Writing-Review&Editing. Qinyuan Li: Writing-Review&Editing. Yang Yu: Writing-Review&Editing. Zhengxiu Luo: Writing-Review&Editing. Enmei Liu: Writing-Review&Editing, Supervision. Yaolong Chen: Conceptualization, Supervision.
Funding
There was no funding source for this study.
Data sharing statement
The authors declare that the data collected was gathered from publicly available databases and is available upon reasonable request.
Declaration of Competing Interest
We declare no competing interests.
Acknowledgments
We thank Janne Estill, Institute of Global Health of University of Geneva, Nan Yang, Evidence-based Medicine Center of Lanzhou University, and Jianjian Wang, School of Public Health of Lanzhou University, for providing guidance and comments for our manuscript when revising. And we gratefully acknowledge the assistance of Janne Estill for English language editing.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2021.101155.
Contributor Information
Enmei Liu, Email: emliu186@126.com.
Yaolong Chen, Email: chenyaolong@vip.163.com.
Appendix. Supplementary materials
References
- 1.World Health Organization (WHO) August 2021. Weekly epidemiological update on COVID-19. Available at: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19—3-august-202. Accessed August 3, 2021. [Google Scholar]
- 2.Ludvigsson J.F. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088–1095. doi: 10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Statistica . 2021. Distribution of coronavirus cases in Italy as of May 12, 2021, by age group. Available at: www.statista.com/statistics/1103023/coronavirus-cases-distribution-by-age-group-italy/. Accessed May 20, 2021. [Google Scholar]
- 4.2021. Age distribution of coronavirus (COVID-19) cases in South Korea as of March 24, 2021. Available at: www.statista.com/statistics/1102730/south-korea-coronavirus-cases-by-age/. Accessed March 26, 2021. [Google Scholar]
- 5.Liguoro I., Pilotto C., Bonanni M. SARS-COV-2 infection in children and newborns: a systematic review. Eur J Pediatr. 2020;179(7):1029–1046. doi: 10.1007/s00431-020-03684-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanna G., Serrau G., Bassareo P.P. Children's heart and COVID-19: up-to-date evidence in the form of a systematic review. Eur J Pediatr. 2020;179(7):1079–1087. doi: 10.1007/s00431-020-03699-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang L., Tang K., Levin M. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis. 2020;20(11):e276–e288. doi: 10.1016/S1473-3099(20)30651-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention . 2021. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19)https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html Available at. Accessed April 21, 2021. [Google Scholar]
- 9.Tian M., Feng X.Y., Liu S.Y. Expert consensus on imaging diagnosis and infection control for COVID-19. Chin J Med Imaging. 2020;26(5):401–414. doi: 10.19627/j.cnki.cn31-1700/th.20200309.001. [DOI] [Google Scholar]
- 10.Fisler G., Izard S.M., Shah S. Characteristics and risk factors associated with critical illness in pediatric COVID-19. Ann Intensive Care. 2020;10(1):171. doi: 10.1186/s13613-020-00790-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abrams J.Y., Oster M.E., Godfred-Cato S.E. Factors linked to severe outcomes in multisystem inflammatory syndrome in children (MIS-C) in the USA: a retrospective surveillance study. Lancet Child Adolesc Health. 2021;5(5):323–331. doi: 10.1016/S2352-4642(21)00050-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsabouri S., Makis A., Kosmeri C. Risk Factors for Severity in Children with Coronavirus Disease 2019: a Comprehensive Literature Review. Pediatr Clin N Am. 2021;68(1):321–338. doi: 10.1016/j.pcl.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Page M.J., McKenzie J.E., Bossuyt P.M. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L., Tian J., Tian H. Network meta-analyses could be improved by searching more sources and by involving a librarian. J Clin Epidemiol. 2014;67:1001–1007. doi: 10.1016/j.jclinepi.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Higgins J.P., Altman D.G., Gotzsche P.C. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sterne J.A.C., Hernán M.A., Reeves B.C. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wells G., Shea B.J., O'Connell D. 2021. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses.http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm Available at: Accessed May 1, 2021. [Google Scholar]
- 18.Institute of Health Economics (IHE) 2014. Quality appraisal of case series studies checklist. Edmonton (AB): institute of health economics.http://www.ihe.ca/research-programs/rmd/cssqac/cssqac-about Available at. Accessed May 1, 2021. [Google Scholar]
- 19.Furukawa T.A., Barbui C., Cipriani A. Imputing missing standard deviations in meta-analyses can provide accurate results. J Clin Epidemiol. 2006;59:7–10. doi: 10.1016/j.jclinepi.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Deeks J.J., Higgins J.P.T., Altman D.G. In: Cochrane handbook for systematic reviews of interventions version 6.2 (updated february 2021). cochrane. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. 2021. Chapter 10: analysing data and undertaking meta-analyses. Available at: www.training.cochrane.org/handbook. Accessed May 1, 2021. [Google Scholar]
- 21.Sterne J.A., Sutton A.J., Ioannidis J.P. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 22.Guyatt G., Oxman A.D., Akl E.A. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 23.Alfraij A., Bin Alamir A.A., Al-Otaibi A.M. Characteristics and outcomes of coronavirus disease 2019 (COVID-19) in critically ill pediatric patients admitted to the intensive care unit: a multicenter retrospective cohort study. J Infect Public Health. 2021;14(2):193–200. doi: 10.1016/j.jiph.2020.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antúnez-Montes O.Y., Escamilla M.I., Figueroa-Uribe A.F. COVID-19 and multisystem inflammatory syndrome in Latin American children: a multinational study. Pediatr Infect Dis J. 2021;40(1):e1–e6. doi: 10.1097/INF.0000000000002949. [DOI] [PubMed] [Google Scholar]
- 25.Bailey L.C., Razzaghi H., Burrows E.K. Assessment of 135 794 pediatric patients tested for severe acute respiratory syndrome coronavirus 2 across the United States. JAMA Pediatr. 2021;175(2):176–184. doi: 10.1001/jamapediatrics.2020.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bari A., Ch A., Hareem S. Association of blood groups with the severity and outcome of COVID-19 infection in children. J Coll Phys Surg Pak. 2021;30(1):S57–S59. doi: 10.29271/jcpsp.2021.01.S57. [DOI] [PubMed] [Google Scholar]
- 27.Basalely A., Gurusinghe S., Schneider J. Acute kidney injury in pediatric patients hospitalized with acute COVID-19 and multisystem inflammatory syndrome in children associated with COVID-19. Kidney Int. 2021;100(1):138–145. doi: 10.1016/j.kint.2021.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Besli G.E., Demir S.Ö., Girit S. COVID-19 in children: a single center experience from Istanbul, Turkey. Med J Bakirkoy. 2021;17(1):64–71. doi: 10.5222/BMJ.2021.60490. [DOI] [Google Scholar]
- 29.Farzan S., Rai S., Cerise J. Asthma and COVID-19: an early inpatient and outpatient experience at a US children's hospital. Pediatr Pulmonol. 2021;56(8):2522–2529. doi: 10.1002/ppul.25514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernandes D.M., Oliveira C.R., Guerguis S. Severe acute respiratory syndrome coronavirus 2 clinical syndromes and predictors of disease severity in hospitalized children and youth. J Pediatr. 2021;230 doi: 10.1016/j.jpeds.2020.11.016. 23-31.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Götzinger F., Santiago-García B., Noguera-Julián A. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4(9):653–661. doi: 10.1016/S2352-4642(20)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Graff K., Smith C., Silveira L. Risk factors for severe COVID-19 in children. Pediatr Infect Dis J. 2021;40(4):e137–e145. doi: 10.1097/INF.0000000000003043. [DOI] [PubMed] [Google Scholar]
- 33.Kainth M.K., Goenka P.K., Williamson K.A. Early experience of COVID-19 in a US children's hospital. Pediatrics. 2020;146(4) doi: 10.1542/peds.2020-003186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kari J.A., Shalaby M.A., Albanna A.S. Coronavirus disease in children: a multicentre study from the Kingdom of Saudi Arabia. J Infect Public Health. 2021;14(4):543–549. doi: 10.1016/j.jiph.2021.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelly M.S., Fernandes N.D., Carr A.V. Distinguishing features of patients evaluated for multisystem inflammatory syndrome in children. Pediatr Emerg Care. 2021;37(3):179–184. doi: 10.1097/PEC.0000000000002344. [DOI] [PubMed] [Google Scholar]
- 36.Madhusoodhan P.P., Pierro J., Musante J. Characterization of COVID-19 disease in pediatric oncology patients: the New York-New Jersey regional experience. Pediatr Blood Cancer. 2021;68(3):e28843. doi: 10.1002/pbc.28843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prata-Barbosa A., Lima-Setta F., Santos G.R.D. Pediatric patients with COVID-19 admitted to intensive care units in Brazil: a prospective multicenter study. J Pediatr (Rio J) 2020;96(5):582–592. doi: 10.1016/j.jped.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi F., Wen H., Liu R. The comparison of epidemiological characteristics between confirmed and clinically diagnosed cases with COVID-19 during the early epidemic in Wuhan, China. Glob Health Res Policy. 2021;6(1):18. doi: 10.1186/s41256-021-00200-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song J., Park D.W., Cha J.H. Clinical course and risk factors of fatal adverse outcomes in COVID-19 patients in Korea: a nationwide retrospective cohort study. Sci Rep. 2021;11(1):10066. doi: 10.1038/s41598-021-89548-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Surendra H., Elyazar I.R., Djaafara B.A. Clinical characteristics and mortality associated with COVID-19 in Jakarta, Indonesia: a hospital-based retrospective cohort study. Lancet Reg Health West Pac. 2021;9 doi: 10.1016/j.lanwpc.2021.100108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swann O.V., Holden K.A., Turtle L. Clinical characteristics of children and young people admitted to hospital with covid-19 in United Kingdom: prospective multicentre observational cohort study. BMJ. 2020;370:m3249. doi: 10.1136/bmj.m3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tripathi S., Gist K.M., Bjornstad E.C. Coronavirus disease 2019-associated PICU admissions: a report from the society of critical care medicine discovery network viral infection and respiratory illness universal study registry. Pediatr Crit Care Med. 2021;22(7):603–615. doi: 10.1097/PCC.0000000000002760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verma S., Lumba R., Dapul H.M. Characteristics of hospitalized children with SARS-CoV-2 in the New York city metropolitan area. Hosp Pediatr. 2021;11(1):71–78. doi: 10.1542/hpeds.2020-001917. [DOI] [PubMed] [Google Scholar]
- 44.Al Yazidi L.S., Al Hinai Z., Al Waili B. Epidemiology, characteristics and outcome of children hospitalized with COVID-19 in Oman: a multicenter cohort study. Int J Infect Dis. 2021;104:655–660. doi: 10.1016/j.ijid.2021.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aykac K., Cura Yayla B.C., Ozsurekci Y. The association of viral load and disease severity in children with COVID-19. J Med Virol. 2021;93(5):3077–3083. doi: 10.1002/jmv.26853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cho S.I., Yoon S., Lee H.J. Impact of comorbidity burden on mortality in patients with COVID-19 using the Korean health insurance database. Sci Rep. 2021;11(1):6375. doi: 10.1038/s41598-021-85813-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chopra S., Saha A., Kumar V. Acute kidney injury in hospitalized children with COVID19. J Trop Pediatr. 2021;67(2):fmab037. doi: 10.1093/tropej/fmab037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coronado Munoz A., Tasayco J., Morales W. High incidence of stroke and mortality in pediatric critical care patients with COVID-19 in Peru. Pediatr Res. 2021:.1–.5. doi: 10.1038/s41390-021-01547-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu W., Yang L., Li X. Early immune responses and prognostic factors in children with COVID-19: a single-center retrospective analysis. BMC Pediatr. 2021;21(1):181. doi: 10.1186/s12887-021-02561-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moreira A., Chorath K., Rajasekaran K. Demographic predictors of hospitalization and mortality in US children with COVID-19. Eur J Pediatr. 2021;180(5):1659–1663. doi: 10.1007/s00431-021-03955-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ozsurekci Y., Aykac K., Er A.G. Predictive value of cytokine/chemokine responses for the disease severity and management in children and adult cases with COVID-19. J Med Virol. 2021;93(5):2828–2837. doi: 10.1002/jmv.26683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y., Zhu F., Wang C. Children hospitalized with severe COVID-19 in Wuhan. Pediatr Infect Dis J. 2020;39(7):e91–e94. doi: 10.1097/INF.0000000000002739. [DOI] [PubMed] [Google Scholar]
- 53.Bari A., Ch A., Bano I. Is leukopenia and lymphopenia a characteristic feature of COVID-19 in children? Pak J Med Sci. 2021;37(3):869–873. doi: 10.12669/pjms.37.3.3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhavsar S.M., Clouser K.N., Gadhavi J. COVID-19 in pediatrics: characteristics of hospitalized children in New Jersey. Hosp Pediatr. 2021;11(1):79–87. doi: 10.1542/hpeds.2020-001719. [DOI] [PubMed] [Google Scholar]
- 55.Bhumbra S., Malin S., Kirkpatrick L. Clinical features of critical coronavirus disease 2019 in children. Pediatr Crit Care Med. 2020;21(10):e948–e953. doi: 10.1097/PCC.0000000000002511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bjornstad E.C., Krallman K.A., Askenazi D. Preliminary assessment of acute kidney injury in critically ill children associated with SARS-CoV-2 infection: a multicenter cross-sectional analysis. Clin J Am Soc Nephrol. 2021;16(3):446–448. doi: 10.2215/CJN.11470720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chao J.Y., Derespina K.R., Herold B.C. Clinical characteristics and outcomes of hospitalized and critically ill children and adolescents with coronavirus disease 2019 at a tertiary care medical center in New York City. J Pediatr. 2020;223 doi: 10.1016/j.jpeds.2020.05.006. 14-19.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Derespina K.R., Kaushik S., Plichta A. Clinical manifestations and outcomes of critically ill children and adolescents with coronavirus disease 2019 in New York City. J Pediatr. 2020;226 doi: 10.1016/j.jpeds.2020.07.039. 55–63.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Desai A., Mills A., Delozier S. Pediatric patients with SARS-CoV-2 infection: clinical characteristics in the United States from a large global health research network. Cureus. 2020;12(9):e10413. doi: 10.7759/cureus.10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Du H., Dong X., Zhang J.J. Clinical characteristics of 182 pediatric COVID-19 patients with different severities and allergic status. Allergy. 2021;76(2):510–532. doi: 10.1111/all.14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Giacomet V., Barcellini L., Stracuzzi M. Gastrointestinal symptoms in severe COVID-19 children. Pediatr Infect Dis J. 2020;39(10):e317–e320. doi: 10.1097/INF.0000000000002843. [DOI] [PubMed] [Google Scholar]
- 62.Haslak F., Barut K., Durak C. Clinical features and outcomes of 76 patients with COVID-19-related multi-system inflammatory syndrome in children. Clin Rheumatol. 2021:1–12. doi: 10.1007/s10067-021-05780-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hoseinyazdi M., Esmaeilian S., Jahankhah R. Clinical, laboratory, and chest CT features of severe versus non-severe pediatric patients with COVID-19 infection among different age groups. BMC Infect Dis. 2021;21(1):560. doi: 10.1186/s12879-021-06283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gonzalez Jimenez D., Velasco Rodríguez-Belvís M., Ferrer Gonzalez P. COVID-19 gastrointestinal manifestations are independent predictors of PICU admission in hospitalized pediatric patients. Pediatr Infect Dis J. 2020;39(12):e459–e462. doi: 10.1097/INF.0000000000002935. [DOI] [PubMed] [Google Scholar]
- 65.Kanburoglu M.K., Tayman C., Oncel M.Y. A multicentered study on epidemiologic and clinical characteristics of 37 neonates with community-acquired COVID-19. Pediatr Infect Dis J. 2020;39(10):e297–e302. doi: 10.1097/INF.0000000000002862. [DOI] [PubMed] [Google Scholar]
- 66.Kompaniyets L., Agathis N.T., Nelson J.M. Underlying medical conditions associated with severe COVID-19 illness among children. JAMA Netw Open. 2021;4(6) doi: 10.1001/jamanetworkopen.2021.11182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lazzerini M., Sforzi I., Trapani S. Characteristics and risk factors for SARS-CoV-2 in children tested in the early phase of the pandemic: a cross-sectional study, Italy, 23 February to 24 May 2020. Euro Surveill. 2021;26(14) doi: 10.2807/1560-7917.ES.2021.26.14.2001248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ouldali N., Yang D.D., Madhi F. Factors associated with severe SARS-CoV-2 infection. Pediatrics. 2021;147(3) doi: 10.1542/peds.2020-023432. [DOI] [PubMed] [Google Scholar]
- 69.Parri N., Magistà A.M., Marchetti F. Characteristic of COVID-19 infection in pediatric patients: early findings from two Italian pediatric research networks. Eur J Pediatr. 2020;179(8):1315–1323. doi: 10.1007/s00431-020-03683-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pereira M.F.B., Litvinov N., Farhat S.C.L. Severe clinical spectrum with high mortality in pediatric patients with COVID-19 and multisystem inflammatory syndrome. Clinics (Sao Paulo) 2020;75:e2209. doi: 10.6061/clinics/2020/e2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qian G., Zhang Y., Xu Y. Reduced inflammatory responses to SARS-CoV-2 infection in children presenting to hospital with COVID-19 in China. EClinicalMedicine. 2021;34 doi: 10.1016/j.eclinm.2021.100831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rao S., Gavali V., Prabhu S.S. Outcome of children admitted with SARS-CoV-2 infection: experiences from a pediatric public hospital. Indian Pediatr. 2021;58(4):358–362. doi: 10.1007/s13312-021-2196-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ramírez-Soto M.C., Arroyo-Hernández H., Ortega-Cáceres G. Sex differences in the incidence, mortality, and fatality of COVID-19 in Peru. PLoS One. 2021;16(6) doi: 10.1371/journal.pone.0253193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rivas-Ruiz R., Roy-García I.A., Ureña-Wong K.R. Factors associated with death in children with COVID-19 in Mexico. Gac Med Mex. 2020;156(6):516–522. doi: 10.24875/GMM.M21000478. [DOI] [PubMed] [Google Scholar]
- 75.Sena G.R., Lima T.P.F., Vidal S.A. Clinical characteristics and mortality profile of COVID-19 patients aged less than 20 years old in Pernambuco-Brazil. Am J Trop Med Hyg. 2021;104(4):1507–1512. doi: 10.4269/ajtmh.20-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zachariah P., Johnson C.L., Halabi K.C. Epidemiology, clinical features, and disease severity in patients with coronavirus disease 2019 (COVID-19) in a children's hospital in New York City, New York. JAMA Pediatr. 2020;174(10) doi: 10.1001/jamapediatrics.2020.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Riphagen S., Gomez X., Gonzalez-Martinez C. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395(10237):1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Whittaker E., Bamford A., Kenny J. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324(3):259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Verdoni L., Mazza A., Gervasoni A. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Feldstein L.R., Rose E.B., Horwitz S.M. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383(4):334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Consiglio C.R., Cotugno N., Sardh F. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell. 2020;183(4) doi: 10.1016/j.cell.2020.09.016. 968-981.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ahmed M., Advani S., Moreira A. Multisystem inflammatory syndrome in children: a systematic review. EClinicalMedicine. 2020;26 doi: 10.1016/j.eclinm.2020.100527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wiersinga W.J., Rhodes A., Cheng A.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 84.Gallo Marin B., Aghagoli G., Lavine K. Predictors of COVID-19 severity: a literature review. Rev Med Virol. 2021;31(1):1–10. doi: 10.1002/rmv.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jutzeler C.R., Bourguignon L., Weis C.V. Comorbidities, clinical signs and symptoms, laboratory findings, imaging features, treatment strategies, and outcomes in adult and pediatric patients with COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;37 doi: 10.1016/j.tmaid.2020.101825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Castagnoli R., Votto M., Licari A. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr. 2020;174(9):882–889. doi: 10.1001/jamapediatrics.2020.1467. [DOI] [PubMed] [Google Scholar]
- 87.Sharifi N., Ryan C.J. Androgen hazards with COVID-19. Endocr Relat Cancer. 2020;27(6):E1–E3. doi: 10.1530/ERC-20-0133. [DOI] [PubMed] [Google Scholar]
- 88.Elshazli R.M., Toraih E.A., Elgaml A. Diagnostic and prognostic value of hematological and immunological markers in COVID-19 infection: a meta-analysis of 6320 patients. PLoS One. 2020;15(8) doi: 10.1371/journal.pone.0238160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bellou V., Tzoulaki I., Evangelou E. Risk factors for adverse clinical outcomes in patients with COVID-19: a systematic review and meta-analysis. MedRxiv. 2020 doi: 10.1101/2020.05.13.20100495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Földi M., Farkas N., Kiss S. Obesity is a risk factor for developing critical condition in COVID-19 patients: a systematic review and meta-analysis. Obes Rev. 2020;21(10):e13095. doi: 10.1111/obr.13095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Singh K., Mittal S., Gollapudi S. A meta-analysis of SARS-CoV-2 patients identifies the combinatorial significance of D-dimer, C-reactive protein, lymphocyte, and neutrophil values as a predictor of disease severity. Int J Lab Hematol. 2021;43(2):324–328. doi: 10.1111/ijlh.13354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li Q., Cao Y., Chen L. Hematological features of persons with COVID-19. Leukemia. 2020;34(8) doi: 10.1038/s41375-020-0910-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang G., Wu C., Zhang Q. C-reactive protein level may predict the risk of COVID-19 aggravation. Open Forum Infect Dis. 2020;7(5):ofaa153. doi: 10.1093/ofid/ofaa153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Luo X., Zhou W., Yan X. Prognostic value of C-reactive protein in patients with coronavirus 2019. Clin Infect Dis. 2020;71(16):2174–2179. doi: 10.1093/cid/ciaa641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang J.J.Y., Lee K.S., Ang L.W. Risk factors for severe disease and efficacy of treatment in patients infected with COVID-19: a systematic review, meta-analysis, and meta-regression analysis. Clin Infect Dis. 2020;71(16):2199–2206. doi: 10.1093/cid/ciaa576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu B.B., Gu D.Z., Yu J.N. Association between ABO blood groups and COVID-19 infection, severity and demise: a systematic review and meta-analysis. Infect Genet Evol. 2020;84 doi: 10.1016/j.meegid.2020.104485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mao R., Qiu Y., He J.S. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5(7):667–678. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pan L., Mu M., Yang P. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. 2020;115(5):766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.National Institute of Health (NIH) 2021. Wide strategic plan for COVID-19 research.https://covid19.nih.gov/nih-strategic-response-covid-19 Available at: Accessed May 20, 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.