Abstract
Elevation in hyperglycemia-associated methylglyoxal level can trigger vascular endothelial cells oxidative stress and apoptosis. The present work assesses the cell proliferative, anti-oxidative and anti-apoptotic potential of Suaeda monoica derived four new terpenes: a norsesquaterpenol (normonisesquaterpenol), a monocyclic triterpenoid (suaedanortriterpene dione), an aromatic monoterpenic ester and a labdane-type norditerpenic xyloside as well as two new phenols: an alkylated β-naphthol and a β-methoxy naphthalene in cultured human umbilical vein endothelial cells (HUVEC). Of these, suaedanortriterpenedione (53.7%), normonisesquaterpenol (51.4%) and norditerpenic xyloside (48%) showed the most promising cell proliferative activities compared to others. Moreover, normonisesquaterpenol, norditerpenic xyloside and suaedanortriterpenedione efficiently reversed the oxidative and apoptotic cell damage via downregulation of capase-3/7 by 44.3%, 42.2% and 39.4%, respectively against dichlorofluorescin, whereas by 46.2%, 43.5% and 42.5%, respectively against methylglyoxal. Aminoguanidine, the reference drug inhibited caspase-3/7 activity by 56.2% and 54.7% through attenuation of dichlorofluorescin and methylglyoxal, respectively. Further in silico molecular docking analysis revealed formation of stable complexes between the tested compounds and caspase-3/7. Conclusively, we for the first time demonstrate the growth stimulatory, anti-oxidative and anti-apoptotic salutations of S. monoica derived novel compounds in human endothelial cells. This warrants their further assessment as vascular cell protective and rejuvenating therapeutics, especially in hyperglycemic conditions.
Keywords: Suaeda monoica, Terpenes, Methylglyoxal, Dichlorofluorescin, Endothelial cells, Apoptosis
1. Introduction
In recent times, several plant extracts and their bioactive constituents have shown promising growth stimulatory or cytoprotective potential (Kong et al., 2004, Kim et al., 2013, Arbab et al., 2016, Parvez et al., 2018) warranting their further exploitation as anti-oxidative, anti-inflammatory or tissue-rejuvenating agents. The mangrove herb Suaeda monoica J. F. Gmel (Chenopodiaceae) is traditionally used to treat sore throat, rheumatism, asthma, snake-bites, skin disease, ulcer, hepatotoxicity, and microbial infections (Kathiresan and Ramanathan, 1997, Muthazhagan et al., 2014, Lakshmi and Narsimha Rao, 2013). In addition, its flavonoids, saponins, alkaloids, polyphenols, resins, tannins, coumarins, cardiac glycosides, and fatty acids are characterized as therapeutic phytoconstituents (Kokpal et al., 1990, Lakshmanan et al., 2013, Muthazhagan et al., 2014). Recently, we have reported isolation and preliminary bioactivity of S. monoica derived four new terpenes: a norsesquaterpenol (normonisesquaterpenol), a monocyclic triterpenoid (suaedanortriterpene dione), an aromatic monoterpenic ester, an unknown labdane-type norditerpenic xyloside as well as two new phenols: an alkylated β-naphthol and a β-methoxy naphthalene derivative (AlSaid et al., 2017, Siddiqui et al., 2020). Notably, a novel pentacyclic triterpenedione from Picea jezoensis with unknown bioactivity has been previously reported (Tanaka et al., 1997).
The vascular endothelial cells, the inner layer of blood vessel is crucial in modulating vascular function and homeostasis (Choy et al., 2001). In conditions with hyperglycemia, retardation of endothelial cells proliferation or apoptosis often leads to diabetic microvascular lesions and cardiovascular complications. Methylglyoxal (MGO) is a highly reactive aldehyde that is produced as a byproduct of several metabolic pathways, including lipid peroxidation (Thornalley and Rabbani, 2014). Also, it is a major precursor of advanced glycation end products implicated in the development of type-2 diabetic complications (Vander Jagt and Hunsaker, 2003) as well oxidative stress and apoptosis in endothelial cells (Bourajjaj et al., 2003, Thornalley and Rabbani, 2014). In addition, high level of MGO is demonstrated to cause in vitro hyperglycemia and oxidative damages in human umbilical vein endothelial cells (Bourajjaj et al., 2003). In endothelial cells, MGO-induced oxidative stress and apoptosis is suggested mainly through the generation of reactive-oxygen species (Phalitakul et al., 2013, Figarola et al., 2014, Kim et al., 2004).
Dichlorofluorescin (DCF), is generally used to measure cellular oxidative stress as a result of H2O2-dependent reactions, including cytochrome C and Fe2+ (Royall and Ischiropoulos, 1993, Carter et al., 1994, LeBel et al., 1992). In line with this, we have recently reported DCFH and MGO induced oxidative stress and apoptosis in a variety of cells, including HUVEC (Arbab et al., 2016, Shahat et al., 2016; Parvez et al., 2018, Parvez et al., 2019, Alqahtani et al., 2019, Parvez and Al-Dosari., M.S., Ahmed. S., Rehman, M.T., Al-Rehaily, A.J., , 2020). In this report, we have investigated the cytoprotective potential of S. monoica derived six novel compounds against MGO and DCFH induced oxidative and apoptotic damages in cultured HUVEC cells.
2. Materials and methods
2.1. Extraction, isolation and structure elucidation of the compounds
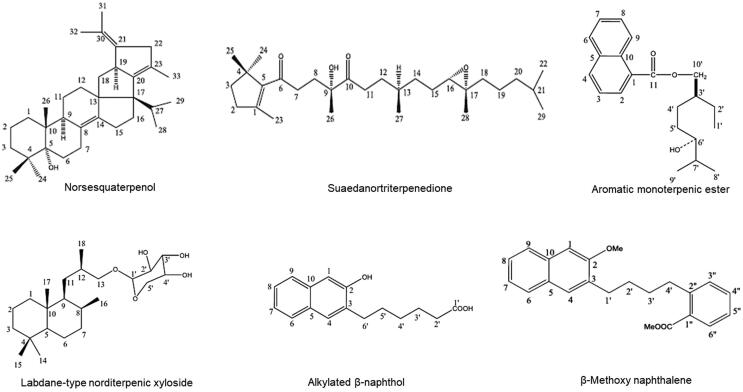
The extraction and isolation of six novel compounds: a norsesquaterpenol (normonisesquaterpenol), a monocyclic triterpenoid (suaedanortriterpenedione), an aromatic monoterpenic ester, an unknown labdane-type norditerpenic xyloside, an alkylated β-naphthol and a β-methoxy naphthalene derivative from the aerial parts of S. monoica (voucher specimen no. 15135), including their structure elucidations (Fig. 1) have been previously reported by us (AlSaid et al., 2017, Siddiqui et al., 2020).
Fig. 1.
Chemical structure of Suaeda monoica derived new terpenic and phenolic compounds: normonisesquaterpenol, suaedanortriterpenedione, aromatic monoterpenic ester, norditerpenic xyloside, alkylated β-naphthol and β-methoxy naphthalene.
2.2. Cell culture
Human umbilical vein epithelial cells (HUVEC 16549; ATCC, USA) were maintained in DMEM-Glutmax medium (Gibco, USA), supplemented with 10% fetal calf serum (Gibco, USA) and 1x penicillin–streptomycin mix (Invitrogen, USA) at 37 °C with 5% CO2 supply. HUVEC cells (0.5x105/100 μl/well) were seeded in a 96-well flat-bottom plate (Becton-Dickinson Labware, USA) and grown overnight for all experiments.
2.3. Natural compounds and drugs preparations
The S. monoica derived norsesquaterpenol (NSQ), suaedanortriterpenedione (SND), aromatic monoterpenic ester (AES), norditerpenic xyloside (NDX), alkylated β-naphthol (ABN) and β-methoxy naphthalene (BMN) were first dissolved in 50 μl dimethyl sulfoxide (DMSO, Sigma-Alderich, Germany) and reconstituted in culture media (1 mg/ml, each). Based on our previously assessed non-toxic concentrations on liver cancer cells (AlSaid et al., 2017, Siddiqui et al., 2020), only three working doses (50, 25 and 12.5 μg/ml; DMSO < 0.5% final) were further prepared in culture media. Likewise, DCF and MGO (Sigma-Alderich, Germany) were prepared to be used as inducers of oxidative and apoptotic cell damage, whereas aminoguanidine (AG; Sigma-Alderich, Germany) served as anti-apoptotic agent (positive control).
2.4. Microscopy
Visual monitoring of the treated cells for any morphological changes, cytotoxicity or proliferation cells was made under an inverted microscope (Optica, 40x and 100x).
2.5. Cell proliferation assay of S. Monoica derived compounds
The S. monoica derived compounds: NSQ, SND, AES, NDX, ABN, BMN were tested for their cell proliferative or growth stimulatory activities in cultured HUVEC cells. Cells were treated with the different doses of the compounds, including untreated control (0.5% DMSO) for 3 days. MTT assay (TACS MTT Cell Proliferation Assay Kit, Tervigen, USA) was performed as per the kit’s manual. Briefly, the MTT reagent (10 μl/well) was added and incubated in dark for about 4 h at room temperature (RT) until purple color appeared. Further the detergent solution (100 μl/well) was added and the cells were incubated for another 1.5 h at 37 °C. The optical density (OD; λ = 570) was measured (Microplate reader ELx800; BioTek, USA) and data was analyzed by non-linear regression (Excel software 2010; Microsoft, USA) to determine the cell proliferation in relation to the untreated control [%Cell proliferation = (ODsample − ODblank/ODcontrol − ODblank) x100]. All samples were tested in triplicate and repeated.
2.6. Assessment of anti-oxidative and cytoprotective activity of S. Monoica derived compounds
HUVEC cell grown in a 96-well plate were treated with DCF (IC50: 32.5 μg/ml) as described elsewhere (Parvez et al., 2020) plus a dose of NSQ, SND, AES, NDX, ABN or BMN. DCFH and DMSO served as negative and untreated control, respectively. The culture was incubated for 3 days at 37 °C and MTT assay was performed to determine the cell survival (%) as above. All samples were tested in triplicate and repeated.
2.7. Anti-apoptotic activity assay of S. Monoica derived compounds
HUVEC cells grown in a 96-well plate were treated with MGO (0.5 mM) as described elsewhere (Alqahtani et al., 2019) plus a dose of NSQ, SND, AES, NDX, ABN or BMN. MGO and AG (0.05 mM; Alqahtani et al., 2019) served as negative and positive control, respectively. The treated cells were incubated for 3 days and MTT assay was performed to determine the cell survival (%) as above. All samples were tested in triplicate and repeated.
2.8. Assessment of caspase-3/7 modulating activity of S. Monoica derived compounds
Based on the promising anti-oxidative and anti-apoptotic activities, an optimal dose (25 μg/ml) of the compounds was tested for cellular caspase-3/7 activation in HUVEC cells in 96-well plates (Set-I: DCF treated and Set-II: MGO treated). Day 3 post-incubation, cellular caspase expressions were measured (Apo-ONE-cas3/7 assay kit; Promega, USA) as per the kit’s manual., Briefly, 100 μl of caspase-3/7 reagent was added to each well, mixed by gentle rocking and incubated in dark for ~ 6 h at RT. Caspase reagent plus culture medium served as blank while reagent plus DMSO treated cells acted as negative control. The OD was measured (Microplate reader ELx800; BioTek, USA) and data was analyzed. All samples were tested in triplicate and repeated.
2.9. Virtual preparation of proteins and ligands
The interactions S. monoica derived compounds with caspase-3 and caspase-7 were elucidated by performing molecular docking using AutoDock 4.2 as described elsewhere (Al-Shabib et al., 2020). Briefly, the three-dimensional coordinates of caspase-3 (PDB Id: 2XYG) and caspase-7 (PDB Id: 3IBC) were retrieved from PDB RCSB database (www.rcsb.org). The proteins were pre-processed by removing water molecules or bound hetero atoms, if any, including addition of hydrogens and assigning Kollman charges. The structure of protein molecules was finally energy-minimized using Merck Molecular Force Field (MMFF). The 2D structures of all compounds were drawn in ChemDraw. All the compounds, including control ligands such as TQ8 (bound to Caspase-3 active site in the crystal structure) and Acetyl-YVAD-CHO (bound to Caspase-7 in the crystal structure) were prepared for docking by assigning bond orders and angles. For all structures, Gasteiger partial charges were defined and the energies of were minimized using UFF (Universal Force Field).
2.10. Molecular docking
Grids around the active site of the targets were defined by selecting the amino acid residues that interacted with the bound ligand. For caspase-3 and Caspase-7, grid boxes 33.3 × 28.8 × 28.3 Å and 25.1 × 34.5 × 29.8 Å, centered at 36.4 × 37.4 × 31.5 Å and 49.8 × -26.4 × -2.3 Å with 0.375 Å, respectively were used for molecular docking in AutoDcok 4.2 (Morris et al., 2009). The van der Waals’ and electrostatic parameters were calculated with the help of distance-dependent dielectric function. Docking was performed using Lamarck Genetic Algorithm (LGA) and Solis-Wets local search methods. A total of 10 docking runs were performed with 2.5 × 106 energy calculations for each. The population size (1 5 0), translational step (0.2), quaternions (5.0) and torsions (5.0) were set. The docking affinity (Kb) of ligands for proteins was estimated from docking energy (ΔG) using the equation: (Boltzmann gas constant, R = 1.987 cal/mol/K and temperature, T = 298 K). The molecular docking procedure generated several low energy binding poses for each ligand, of which the complex with the lowest energy was selected for the analysis.
2.11. Statistical analysis
All data in triplicate were presented as mean ± SD and analyzed using one-way analysis of variance. Differences between two groups were compared using Student's t-test (SPSS software; Version 25; IBM, USA), and p < 0.05 was considered significant.
3. Results
3.1. Endothelial cell proliferative activities of S. Monoica derived compounds
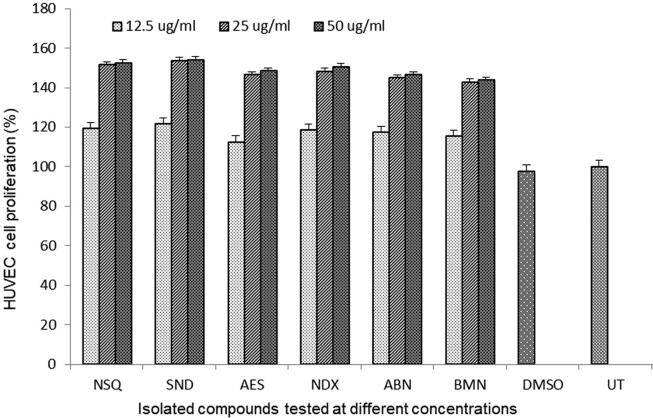
All tested compounds (NSQ, SND, AES, NDX, ABN and BMN) were non-toxic to HUVEC cells even at the highest dose (50 μg/ml) in line with microscopic observations (data not shown). Our MTT assay showed dose-dependent cell proliferative activities of all compounds. Of these, SND (53.7%), NSQ (51.4%) and NDX (48%) exhibited relatively higher effects than AES (46.2%), ABN (44.8%) and BMN (42.8%) at 25 μg/ml in relation to untreated control (Fig. 2). There were no significant changes in growth enhancement at 50 μg/ml dose.
Fig. 2.
Cell proliferative (MTT) assay showing dose-dependent growth stimulatory activity of Suaeda monoica derived new compounds (12.5, 25 and 50 μg/ml): norsesquaterpenol (NSQ), suaedanortriterpenedione (SND), aromatic monoterpenic ester (AES), norditerpenic xyloside (NDX), alkylated β-naphthol (ABN) and β-methoxy naphthalene (BMN) in cultured human endothelial cells (HUVEC). UT: un-treated. Values on Y-axis: means of three determinations. All samples in triplicate were test-repeated twice.
3.2. Attenuation of oxidative cell damage by S. Monoica derived compounds
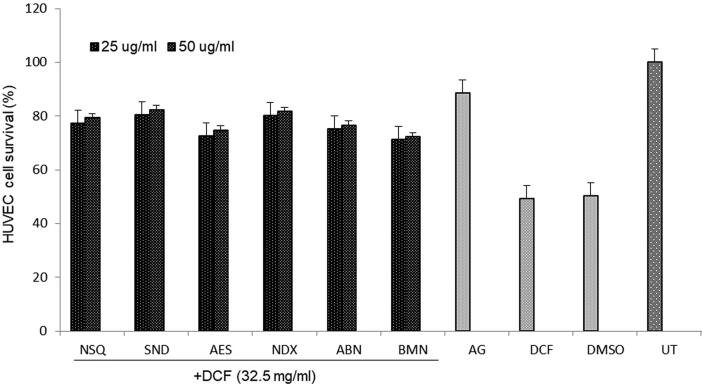
Based on the promising cell proliferative activities, all six compounds (25 and 50 μg/ml, each) were evaluated for their cytoprotective potential against DCF-induced oxidative damage in HUVEC cells. As shown by MTT assay, cell viability was restored (in the order) by SND (80.5%), NSQ (80%), NDX (77%), ABN (75.2%), AES (72.5%) and BMN (71.35%) at 25 μg/ml dose. Notably, SND and NSQ showed the best activities as compared to the reference drug AG (88.5%) through attenuation of DCF (Fig. 3). Treatment with 50 mg/ml dose however, did not show significant enhancement in their activities.
Fig. 3.
Cell proliferative (MTT) assay showing cytoprotective activity of Suaeda monoica derived new compounds (25 and 50 μg/ml): norsesquaterpenol (NSQ), suaedanortriterpenedione (SND), aromatic monoterpenic ester (AES), norditerpenic xyloside (NDX), alkylated β-naphthol (ABN) and β-methoxy naphthalene (BMN) against dichlorofluorescin (DCF; 32.5 ug/ml) induced oxidative stress in cultured human endothelial cells (HUVEC). DMSO: vehicle control; UT: un-treated control. Values on Y-axis: means of three determinations. All samples in triplicate were test-repeated twice.
3.3. Reversal of apoptotic cell death by S. Monoica derived compounds
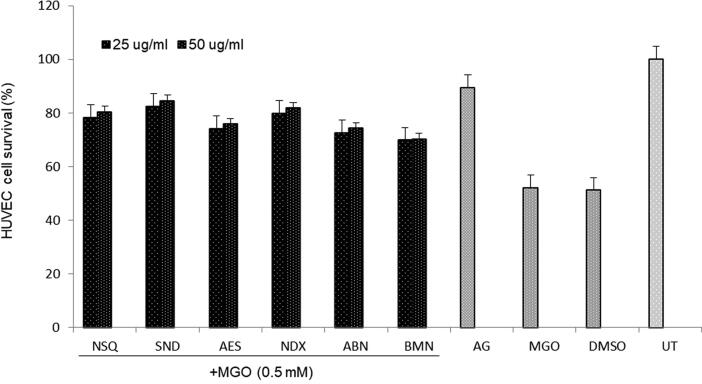
Further, when tested against MGO-induced apoptosis, HUVEC cell death were reversed and rejuvenated (in the order) by SND (82.3%), NSQ (78.3%), NDX (79.8%), AES (74%), ABN (72.6%) and BMN (69.8%) at 25 μg/ml dose. Notably, SND, NSQ and NDX showed the best activities as compared to the reference drug AG activity (89.4%) (Fig. 4). The 50 mg/ml dose did not show significant additive effect.
Fig. 4.
Cell proliferative (MTT) assay showing cytoprotective activity of Suaeda monoica derived new compounds (25 and 50 μg/ml): norsesquaterpenol (NSQ), suaedanortriterpenedione (SND), aromatic monoterpenic ester (AES), norditerpenic xyloside (NDX), alkylated β-naphthol (ABN) and β-methoxy naphthalene (BMN) against Methylglyoxal (MGO; 0.05 mM) triggered apoptosis in cultured human endothelial cells (HUVEC). DMSO: vehicle control; UT: un-treated control. Values on Y-axis: means of three determinations. All samples in triplicate were test-repeated twice.
3.4. S. monoica derived compounds effectively down regulated cellular caspase-3/7
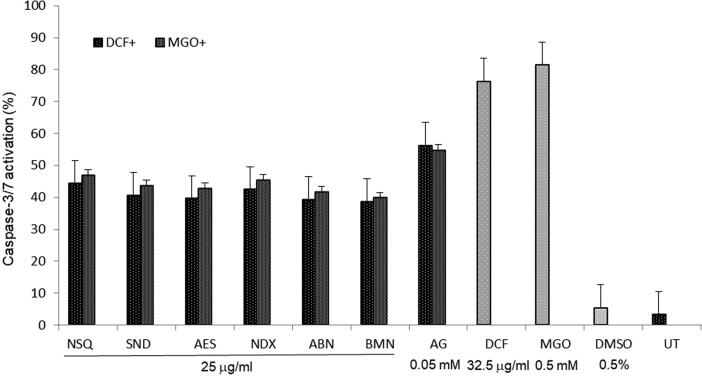
Further insight into the anti-apoptotic mechanism of the three most active terpenic compounds (25 μg/ml) showed modulation of caspase-3/7 activities in both DCF and MGO treated HUVEC cells. DCF and MGO induced cellular caspases by 76.3% and 81.3%, respectively (Fig. 5). NSQ, NDX and SND efficiently down regulated caspase-3/7 expressions by 44.3%, 42.2% and 39.4%, respectively against DCF, whereas by 46.2%, 43.5% and 42.5%, respectively against MGO (Fig. 5). The reference drug AG downregulated caspase-3/7 by 56.2% and 54.7% through attenuation of DCF and MGO, respectively.
Fig. 5.
The anti-apoptotic assay showing inhibition of dichlorofluorescin (DCF; 32.5 μg/ml) and methylglyoxal (MGO; 0.05 mM) induced cellular caspase-3/7 expressions by Suaeda monoica derived new compounds (25 μg/ml): suaedanortriterpenedione (SND), norsesquaterpenol (NSQ) and norditerpenic xyloside (NDX) in cultured human endothelial cells (HUVEC). DMSO: vehicle control; UT: un-treated control. Values on Y-axis: means of three determinations. All samples in triplicate were test-repeated twice.
3.5. Interaction between caspase-3 and S. Monoica derived compounds
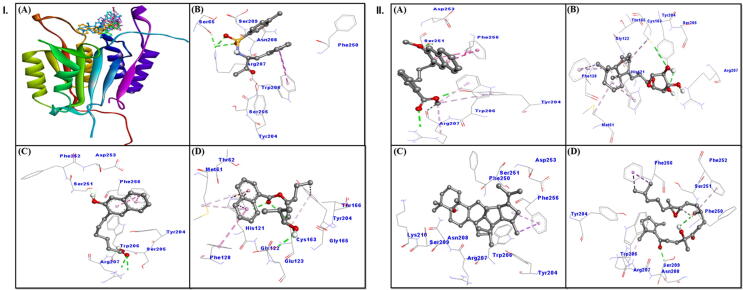
Our molecular docking analysis revealed that all the compounds were able to bind to the active site of caspase-3 (Fig. 6, IA), and their binding energy and corresponding binding affinity towards caspase-3 were estimated (Table 1). The interaction between caspase-3 and TQ8 (ligand control) suggested involvement of three hydrogen bonds with Arg207, and two hydrophobic interactions with Trp206. Some other residues such as Ser65, Tyr204, Ser205, Asn208, Ser209, and Phe250 formed van der Waals’ interactions (Fig. 6, IB; Table 2). The binding energy and affinity of TQ8 and caspase-3 complex were estimated to be −5.8 kcal mol−1 and 1.79 × 104 M−1, respectively (Table 1).
Fig. 6.
The in silico molecular docking analysis showing interaction of caspase-3 with Suaeda monoica derived compounds. Panel I: (A) all compounds, (B) ligand control TQ8, (C) Alkylated β-naphthol, (D) Aromatic monoterpenic; Panel II: (A) β-methoxy naphthalene, (B) Norditerpenic xyloside, (C) Norsesquaterpenol, (D) Suaedanortriterpenedione.
Table 1.
Molecular docking analysis of complexes formed by S. monoica derived compounds with caspase-3 and caspase-7.
| Ligands |
Caspase-3 |
Caspase-7 |
||
|---|---|---|---|---|
|
Binding energy (kcal mol−1) |
Binding affinity (M−1) |
Binding energy (kcal mol−1) |
Binding affinity (M−1) |
|
| Ligand control* | −5.8 | 1.79 × 104 | −9.6 | 1.10 × 107 |
| Norsesquaterpenol | −7.4 | 2.68 × 105 | −8.0 | 7.37 × 105 |
| Suaedanortriterpenedione | −5.5 | 1.08 × 104 | −6.2 | 3.53 × 104 |
| Aromatic monoterpenic ester | −5.5 | 1.08 × 104 | −5.7 | 1.52 × 104 |
| Norditerpenic xyloside | −6.6 | 6.93 × 104 | −7.6 | 3.75 × 105 |
| Alkylated β-naphthol | −6.1 | 2.98 × 104 | −6.6 | 6.93 × 104 |
| β-methoxy naphthalene | −5.8 | 1.79 × 104 | −6.3 | 4.18 × 104 |
*Ligand controls: TQ8 (N-[(2S)-4-chloro-3-oxo-1-phenyl-butan-2-yl]-4-methyl-benzenesulfonamide) for caspase- 3 and Acetyl-YVAD-CHO for caspase-7.
Table 2.
Molecular docking parameters for the interaction between caspase-3 and S. monoica derived compounds.
| Ligands | Donor-Acceptor pair | Distance (Å) | Type of interaction | Van der Waals’ interaction |
|---|---|---|---|---|
| Control* | ARG207:HN - LIG:O ARG207:HH11 - LIG:O ARG207:HH21 - LIG:O TRP206:CZ3 - LIG TRP206 - LIG |
1.8753 2.9212 2.2992 3.6914 5.0512 |
Conventional Hydrogen Bond Conventional Hydrogen Bond Conventional Hydrogen Bond Hydrophobic (Pi-Sigma) Hydrophobic (Pi-Pi T-shaped) |
SER65, TYR204, SER205, ASN208, SER209, PHE250 |
| ABN | ARG207:HE - LIG:O ARG207:HH22 - LIG:O ARG207:HN - LIG:O SER251:HG - LIG:O LIG:H - SER251:OG PHE256 - LIG PHE256 - LIG |
2.4690 2.4750 2.4534 2.4498 1.8630 3.8742 4.0116 |
Conventional Hydrogen Bond Conventional Hydrogen Bond Conventional Hydrogen Bond Conventional Hydrogen Bond Conventional Hydrogen Bond Hydrophobic (Pi-Pi Stacked) Hydrophobic (Pi-Pi Stacked) |
TYR204, SER205, TRP206, PHE252, ASP253 |
| AES | HIS121:HD1 - LIG:O CYS163:SG - LIG:O LIG:H - GLU123:OE1 HIS121:NE2 - LIG HIS121 - LIG HIS121 - LIG PHE128 - LIG TYR204 - LIG:C LIG - MET61 LIG - MET61 |
2.3773 3.6886 2.3259 4.3581 4.0199 4.6979 5.0433 4.9384 5.3853 4.9636 |
Conventional Hydrogen Bond Conventional Hydrogen Bond Conventional Hydrogen Bond Electrostatic (Pi-Cation) Hydrophobic (Pi-Pi Stacked) Hydrophobic (Pi-Pi Stacked) Hydrophobic (Pi-Pi T-shaped) Hydrophobic (Pi-Alkyl) Hydrophobic (Pi-Alkyl) Hydrophobic (Pi-Alkyl) |
THR62, GLY122, GLY165, THR166 |
| BMN | TYR204:HH - LIG:O ARG207:HH22 - LIG:O SER251:HG - LIG:O LIG:C - ARG207:O PHE256 - LIG PHE256 - LIG LIG:C - ARG207 TYR204 - LIG:C TRP206 - LIG:C |
2.0032 2.4339 2.3132 3.5213 3.7981 3.8563 4.5903 5.3747 5.0178 |
Conventional Hydrogen Bond Conventional Hydrogen Bond Conventional Hydrogen Bond Carbon Hydrogen Bond Hydrophobic (Pi-Pi Stacked) Hydrophobic (Pi-Pi Stacked) Hydrophobic (Alkyl) Hydrophobic (Pi-Alkyl) Hydrophobic (Pi-Alkyl) |
ASP253 |
| NDX | ARG207:HH22 - LIG:O CYS163:SG - LIG:O TYR204:HH - LIG:O MET61 - LIG CYS163 - LIG HIS121 - LIG HIS121 - LIG:C PHE128 - LIG:C PHE128 - LIG:C |
2.3814 3.4607 2.7000 4.7801 5.1548 4.4606 4.9783 5.0270 4.1657 |
Conventional Hydrogen Bond Conventional Hydrogen Bond Conventional Hydrogen Bond Hydrophobic (Alkyl) Hydrophobic (Alkyl) Hydrophobic (Pi-Alkyl) Hydrophobic (Pi-Alkyl) Hydrophobic (Pi-Alkyl) Hydrophobic (Pi-Alkyl) |
GLY122, THR166, SER205 |
| NSQ | LIG:C - PHE256 PHE256 - LIG |
3.5475 4.4100 |
Hydrophobic (Pi-Sigma) Hydrophobic (Pi-Alkyl) |
TYR204, TRP206, ARG207, ASN208, SER209, LYS210, PHE250, SER251, ASP253 |
| SND | SER209:HN - LIG:O LIG:H - PHE250:O LIG:C - PHE250:O LIG:C - PHE256 LIG:C - PHE256 LIG:C - ARG207 TRP206 - LIG:C PHE252 - LIG:C |
2.2032 2.3776 3.2851 3.9312 3.7106 4.5144 4.4890 4.8691 |
Conventional Hydrogen Bond Conventional Hydrogen Bond Carbon Hydrogen Bond Hydrophobic (Pi-Sigma) Hydrophobic (Pi-Sigma) Hydrophobic (Alkyl) Hydrophobic (Pi-Alkyl) Hydrophobic (Pi-Alkyl) |
TYR204, ASN208, SER251 |
*Chemically the control TQ8 is N-[(2S)-4-chloro-3-oxo-1-phenyl-butan-2-yl]-4-methyl-benzenesulfonamide.
Alkylated β-naphthol formed a stable complex with caspase-3 mainly through hydrogen bonding with Arg207 and Ser251, including other hydrophobic interactions (Fig. 6, IC; Table 2). The complex was further stabilized by van der Waals’ interactions with Tyr204, Ser205, Trp206, Phe252, and Asp253. The binding energy and affinity of the complex were estimated to be −6.1 kcal mol−1 and 2.98 × 104 M−1, respectively (Table 1).
Aromatic monoterpenic ester and caspase-3 complex was stabilized by an electrostatic interaction (Pi-Cation) with His121 and three hydrogen bonds with His121, Cys163 and Glu123. Some other residues such as Thr62, Gly122, Gly165, and Thr166 formed van der Waals’ interactions (Fig. 6 ID; Table 2). The docking energy and affinity of the complex were estimated to be −5.5 kcal mol−1 and 1.08 × 104 M−1, respectively (Table 1).
β-methoxy naphthalene formed a stable complex with caspase-3 mainly through hydrogen bonding with Tyr204 and Arg207 as well as hydrophobic interactions (Fig. 6, IIA; Table 2). The complex was further stabilized by van der Waals’ interactions with Asp253. The estimated binding energy and affinity of the complex were −5.8 kcal mol−1 and 1.79 × 104 M−1, respectively (Table 1).
Norditerpenic xyloside and caspase-3 complex was formed through three hydrogen bonds involving Arg207, Cys163 and Tyr204 as well as six hydrophobic interactions with Met61, His121, Phe128 and Cys163. Some other residues also formed van der Waals’ interactions (Fig. 6, IIB; Table 2). The docking energy and affinity of the complex were estimated to be −6.6 kcal mol−1 and 6.93 × 104 M−1, respectively (Table 1).
Norsesquaterpenol formed a stable complex with caspase-3 mainly through hydrophobic interactions with Phe256 (Fig. 6, IIC; Table 2). The complex was further stabilized by van der Waals’ interactions involving Tyr204, Trp206, Arg207, Asn208, Ser209, Lys210, Phe250, Ser251 and Asp253. The calculated binding energy and affinity of the complex were −7.4 kcal mol−1 and 2.68 × 105 M−1, respectively (Table 1).
Suaedanortriterpenedione and caspase-3 formed complex via two hydrogen bonds involving Ser209 and Phe250 as well as through hydrophobic interactions with Trp206, Arg207, Phe252 and Phe256. Some residues like Tyr204, Asn208 and Ser251 also showed van der Waals’ interactions (Fig. 6, IID; Table 2). The docking energy and affinity of the complex were estimated to be −5.5 kcal mol−1 and 1.08 × 104 M−1, respectively (Table 1).
3.6. Interaction between caspase-7 and S. Monoica derived compounds
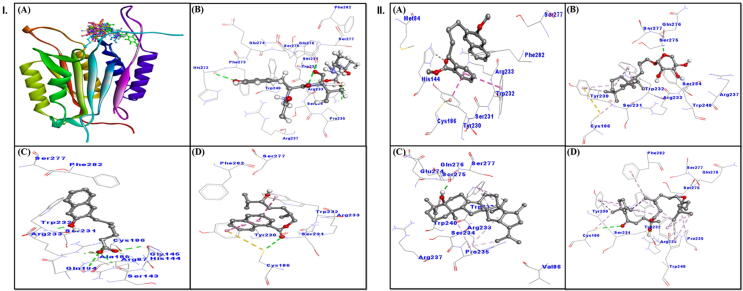
All tested compounds showed good interaction with caspase-7 active site (Fig. 7, IA), and their binding energy and corresponding binding affinity towards caspase-7 were calculated (Table 1). The interaction between Acetyl-YVAD-CHO (ligand control) and caspase-7 suggested the involvement of hydrogen bonds with Arg233, Gln276 and His272. Some other residues such as Ser231, Trp232, Ser234, Pro235, Arg237, Trp240, Phe273, Glu274, Ser275, Ser277 and Phe282 formed van der Waals’ interactions (Fig. 7, IB; Table 3). The docking energy and docking affinity of Acetyl-YVAD-CHO for caspase-7 were estimated to be −9.6 kcal mol−1 and 1.10 × 107 M−1, respectively (Table 1).
Fig. 7.
The in silico molecular docking analysis showing interaction of caspase-7 with Suaeda monoica derived compounds. Panel I: (A) all compounds, (B) ligand control Acetyl-YVAD-CHO, (C) Alkylated β-naphthol, (D) Aromatic monoterpenic; Panel II: (A) β-methoxy naphthalene, (B) Norditerpenic xyloside, (C) Norsesquaterpenol, (D) Suaedanortriterpenedione.
Table 3.
Molecular docking parameters for the interaction between caspase-7 and S. monoica derived compounds.
| Ligand | Donor-Acceptor pair | Distance (Å) | Type of interaction | Van der Waals’s interaction |
|---|---|---|---|---|
| Control* | ARG233:HN - LIG:O ARG233:HH11 - LIG:O ARG233:HH21 - LIG:O LIG:O – GLN276 LIG:H - HIS272:O LIG:HO - ARG233:O |
2.0867 2.7924 2.5474 3.0801 2.5836 2.7121 |
Conventional Hydrogen Bond Conventional Hydrogen Bond Conventional Hydrogen Bond Conventional Hydrogen Bond Conventional Hydrogen Bond Conventional Hydrogen Bond |
SER231, TRP232, SER234, PRO235, ARG237, TRP240, PHE273, GLU274, SER275, SER277, PHE282 |
| ABN | ARG87:HE - LIG:O ARG87:HH22 - LIG:O HIS144:HD1 - LIG:O ARG233:HN - LIG:O ARG233:HE - LIG:O ARG233:HH22 - LIG:O HIS144:CA - LIG:O |
2.3632 2.8463 2.6366 1.9248 2.1267 2.1997 3.3939 |
Conventional Hydrogen Bond Conventional Hydrogen Bond Conventional Hydrogen Bond Conventional Hydrogen Bond Conventional Hydrogen Bond Conventional Hydrogen Bond Carbon Hydrogen Bond |
SER143, GLY145, GLN184, ALA185, CYS186, SER231, TRP232, SER277, PHE282 |
| AES | CYS186:SG - LIG:O CYS186:SG - TYR230 TYR230 - LIG TYR230 - LIG TRP232 - LIG |
3.5904 4.6432 4.2061 4.5641 4.8124 |
Conventional Hydrogen Bond Hydrophobic (Pi-Sulfur) Hydrophobic (Pi-Pi Stacked) Hydrophobic (Pi-Pi Stacked) Hydrophobic (Pi-Pi T-shaped) |
SER231, ARG233, SER277, PHE282 |
| BMN | HIS144:CE1 - LIG:O TYR230 - LIG TRP232 - LIG |
3.6982 3.7578 5.0180 |
Carbon Hydrogen Bond Hydrophobic (Pi-Pi Stacked) Hydrophobic (Pi-Pi T-shaped) |
MET84, SER231, ARG233, SER277, PHE282 |
| NDX | TRP240:HE1 - LIG:O GLN276:HN - LIG:O LIG:C - CYS186 TYR230 - LIG TYR230 - LIG:C TRP232 - LIG:C TRP232 - LIG TRP232 - LIG:C |
2.1034 2.0006 4.6176 4.6001 4.7870 4.9544 4.6991 4.4321 |
Conventional Hydrogen Bond Conventional Hydrogen Bond Hydrophobic (Alkyl) Hydrophobic (Pi-Alkyl) Hydrophobic (Pi-Alkyl) Hydrophobic (Pi-Alkyl) Hydrophobic (Pi-Alkyl) Hydrophobic (Pi-Alkyl) |
SER231, ARG233, SER234, ARG237, SER275, SER277 |
| NSQ | LIG:H - GLN276:O PRO235 - LIG LIG:C - PRO235 LIG:C - PRO235 TRP232 - LIG TRP232 - LIG:C TRP232 - LIG:C TRP240 - LIG:C TRP240 - LIG:C |
1.7762 5.4848 4.7898 3.5114 5.3563 4.8321 4.9250 4.5652 5.3680 |
Conventional Hydrogen Bond Hydrophobic (Alkyl) Hydrophobic (Alkyl) Hydrophobic (Alkyl) Hydrophobic (Pi-Alkyl) Hydrophobic (Pi-Alkyl) Hydrophobic (Pi-Alkyl) Hydrophobic (Pi-Alkyl) Hydrophobic (Pi-Alkyl) |
VAL86, ARG233, SER234, ARG237, GLU274, SER275, SER277 |
| SND | CYS186:SG - LIG:O ARG233:HN - LIG:O LIG:C - TRP232 LIG:C - CYS186 LIG:C - PRO235 TYR230 - LIG TYR230 - LIG:C TYR230 - LIG:C TRP232 - LIG:C TRP232 - LIG:C TRP232 - LIG:C TRP240 - LIG:C TRP240 - LIG:C TRP240 - LIG:C TRP240 - LIG:C PHE282 - LIG:C |
3.2926 2.1417 3.5878 4.8621 4.4650 3.8562 4.6311 4.5072 4.3383 5.1841 5.0696 5.0866 4.5228 5.1945 4.7176 5.3566 |
Conventional Hydrogen Bond Conventional Hydrogen Bond Hydrophobic (Pi-Sigma) Hydrophobic (Alkyl) Hydrophobic (Alkyl) Hydrophobic (Pi-Alkyl) Hydrophobic (Pi-Alkyl) Hydrophobic (Pi-Alkyl) Hydrophobic (Pi-Alkyl) Hydrophobic (Pi-Alkyl) Hydrophobic (Pi-Alkyl) Hydrophobic (Pi-Alkyl) Hydrophobic (Pi-Alkyl) Hydrophobic (Pi-Alkyl) Hydrophobic (Pi-Alkyl) Hydrophobic (Pi-Alkyl) |
SER231, SER275, GLN276, SER277 |
* The chemical nature of Caspase 7 control ligand (peptide based inhibitor) is Acetyl-YVAD-CHO.
Alkylated β-naphthol formed a stable complex with caspase-7 mainly through hydrogen bonding which involved Arg87, His144 and Arg233, wherein His114 also formed a carbon-hydrogen bond (Fig. 7, IC; Table 3). The ABN-Caspase 7 complex was further stabilized by van der Waals’ interactions with SER143, GLY145, GLN184, ALA185, CYS186, SER231, TRP232, SER277, and PHE282. The binding energy of ABN-Caspase 7 complex formation was estimated to be −6.6 kcal mol−1 while the binding affinity was determined to be 6.93 × 104 M−1 (Table 1).
Aromatic monoterpenic ester and caspase-7 complex was stabilized by a hydrogen bond involving Cys186, and hydrophobic interactions with Tyr230 and Trp232. Residues such as Ser231, Arg233, Ser277 and Phe282 formed van der Waals’ interactions (Fig. 7, ID; Table 3), Table 3). The estimated binding energy and docking affinity of the complex were −5.7 kcal mol−1 and 1.52 × 104 M−1, respectively (Table 1).
β-methoxy naphthalene formed a stable complex with caspase-7 mainly through a carbon- hydrogen bond involving His144 and hydrophobic interactions with Cys186, Tyr230 and Trp232 (Fig. 7, IIA; Table 3). The complex was further stabilized by van der Waals’ interactions involving Met84, Ser231, Arg233, Ser277 and Phe282. The binding energy and affinity of the complex was estimated to be −6.3 kcal mol−1 and 4.18 × 104 M−1, respectively (Table 1).
Norditerpenic xyloside and caspase-7 formed complex via two hydrogen bonds with Trp240 and Gln276 as well as. Through hydrophobic interactions involving Cys186, Tyr230 and Trp232 (Fig. 7, IIB; Table 3). Also, it showed van der Waals’ interactions with Ser231, Arg233, Ser234, Arg237, Ser275 and Ser277. The calculated docking energy and affinity of the complex were −7.6 kcal mol−1 and 3.75 × 105 M−1, respectively (Table 1).
Norsesquaterpenol formed a stable complex with caspase-7 mainly through hydrophobic interactions, involving Trp232, Pro235, Trp240 and others as well as one hydrogen bond with Gln276, (Fig. 7, IIC; Table 3). The complex was further stabilized by van der Waals’ interactions with Val86, Arg233, Ser234, Arg237, Glu274, Ser275 and Ser277. The binding energy and affinity of the complex were −8.0 kcal mol−1 and 7.37 × 105 M−1, respectively (Table 1).
Suaedanortriterpenedione and caspase-7 complex was formed with two hydrogen bonds involving Cys186 and Arg233 as well as hydrophobic interactions with Cys186, Tyr230, Trp232, Pro235, Trp240 and Phe282. Some residues such as Ser231, Ser275, Gln276 and Ser277 showed van der Waals’ interactions (Fig. 7, IID; Table 3). The estimated docking energy and affinity of the complex were −6.2 kcal mol−1 and 3.53 × 104 M−1, respectively (Table 1).
4. Discussions
Several natural or plants products are known to have cell proliferative and cytoprotective potential via anti-oxidative, anti-inflammatory and tissue-rejuvenating/regenerative activities (Kong et al., 2004, Kim et al., 2013, Parvez et al., 2018, Parvez et al., 2019, Alqahtani et al., 2019, Parvez and Al-Dosari., M.S., Ahmed. S., Rehman, M.T., Al-Rehaily, A.J., , 2020). Plant secondary metabolites have high chemical diversity and biochemical specificity, which often act more effectively than synthetic drugs (Ganesan, 2008). In the present study, S. monoica derived new four terpenes (a norsesquaterpenol, a monocyclic triterpenoid, an aromatic monoterpenic ester and a norditerpenic xyloside) and two phenols (an alkylated β-naphthol and a β-methoxy naphthalene) were studied for their cell proliferative and cytoprotective efficacies in cultured endothelial cells. Notably, we have used the non-cytotoxic optimal dose of 50 μg/ml for all tested compounds as compared to previously reported maximal non-cytotoxic concentrations of monoterpenes up to 60 μg/ml (Astani and Schnitzler, 2014).
Plant essential oils comprising of a diverse group of terpenes (monoterpenes and sesquiterpenes) and phenylpropanoids including carbohydrate, alcohol, ether, aldehyde and ketones are attributed to fragrance and flavor as well as a wide range of medicinal applications. Cellular accumulation of highly toxic reactive oxygen species (ROS) can damage lipids, proteins or nucleic acids, and normal cell growth and function leading to tissue damages (Opara and Rockway, 2006). In in vitro settings, DCF is generally used for estimating free–radical triggered oxidative stress (LeBel et al., 1992, Oyama et al., 1994, Rota et al., 1999). In cultured endothelial cells, its oxidation is suggested as a result of H2O2 dependent reactions involving cytochrome c and Fe2+ (Royall and Ischiropoulos, 1993, Carter et al., 1994). Here we demonstrate the maximal endothelial cell proliferation and cytoprotection by suaedanortriterpenedione, norsesquaterpenol and norditerpenic xyloside, whereas moderately by aromatic monoterpenic ester, alkylated β-naphthol and β-methoxy naphthalene via attenuation of DCF in line with our recent reports on other phytoproducts (Alqahtani et al., 2019, Parvez and Al-Dosari., M.S., Ahmed. S., Rehman, M.T., Al-Rehaily, A.J., , 2020).
In hyperglycemia, the role of endogenous aldehydes and their end-products, including the highly reactive MGO is suggested as a prime inducer of vascular endothelial cell damage via oxidative stress and apoptosis (Bourajjaj et al., 2003, Kim et al., 2004, Phalitakul et al., 2013, Figarola et al., 2014). Recently, significant reversal of MGO induced HUVEC cell apoptosis by pyrrophenone has been demonstrated (Ravikumar et al., 2010, Yuan et al., 2017). In addition, we have also reported promising cytoprotection of HUVEC cells against MGO by rhuspartin (Alqahtani et al., 2019) and oncoglabrinol C (Parvez et al., 2020). In line with this, we demonstrate the maximal HUVEC cell proliferation and cytoprotection by suaedanortriterpenedione, norsesquaterpenol and norditerpenic xyloside, whereas moderately by aromatic monoterpenic ester, alkylated β-naphthol and β-methoxy naphthalene through amelioration of MGO.
Caspases belong to cysteine-aspartate proteases, which play crucial roles in maintaining cellular homeostasis by inducing apoptotic cell death and tissue inflammation (Kumar, 2006). All caspases are synthesized as inactive enzymes where activation of effector caspase-3 or 7 is performed by the initiator caspase-9 that itself is autoactivated under oxidative or apoptotic conditions (Boatright and Salvesen, 2003, Shi, 2000). Therefore, the therapeutic intervention that could inhibit caspase expressions in acute and chronic diseases are very much desirable. To have an insight into the plausible underlying mechanisms involved in anti-oxidative and anti-apoptotic salutations, suaedanortriterpenedione, norsesquaterpenol and norditerpenic xyloside, the most active terpenes were further assessed for caspase-3/7 modulating potential. Our data showed that the three terpenes effectively downregulated DCF and MGO activated caspase-3/7 expressions in HUVEC cells, endorsing our previous observation (Alqahtani et al., 2019, Parvez and Al-Dosari., M.S., Ahmed. S., Rehman, M.T., Al-Rehaily, A.J., , 2020). Furthermore, in silico docking results also confirmed that suaedanortriterpenedione, norsesquaterpenol and norditerpenic xyloside as well the control ligands (TQ8 and Acetyl-YVAD-CHO) interacted with key substrate-binding and catalytic residues of caspase-3 and 7. The complexex between caspase-37 and the phytocompounds were stabilized by hydrogen bondings, hydrophobic interactions and van der Waals’ interactions. Interestingly, some amino acid residues of caspase-3 were commonly involved in the interaction with TQ8 as well as suaedanortriterpenedione (Tyr204, Trp206, Arg207, Asn208, Ser209, and Phe250), norsesquaterpenol (Tyr204, Trp206, Arg207, Asn208, Ser209, Phe250) and norditerpenic xyloside (Tyr204, Ser205, Arg207, and Phe250). Similarly, the amino acid residues Ser231, Trp232, Arg233, Pro235, Trp240, Ser275, Gln276, Ser277 and Phe282 of caspase-7 were involved in the interaction with Acetyl-YVAD-CHO and suaedanortriterpenedione. For norsesquaterpenol, the interacting residues were Trp232, Arg233, Ser234, Pro235, Arg237, Trp240, Glu274, Ser275, and Ser277, whereas for norditerpenic xyloside, those were Ser231, Trp232, Arg233, Ser234, Arg237, Trp240, Ser275, Gln276 and Ser277. This is in line with our previous study where Oncoglabrinol C, a flavan isolated from Oncocalyx glabratus strongly interacted with the substrate binding sites of caspase 3/7, and suggested inhibition of their catalytic acivities (Parvez et al., 2020).
5. Conclusion
Our data for the first time demonstrate in vitro cell proliferative, anti-oxidative and anti-apoptotic efficacies of Suaeda monoica derived novel terpenes viz., suaedanortriterpenedione, normonisesquaterpenol, and norditerpenic xyloside in human primary endothelial cells. This warrants their further molecular and pharmacological assessment as vascular cell protective as well as tissue-rejuvenating therapeutics, especially in hyperglycemic conditions.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors thankfully acknowledge the Researchers Supporting Project (RSP-2021/379), King Saud University, Riyadh for funding this work.
Footnotes
Peer review under responsibility of King Saud University.
References
- AlSaid M.S., Siddiqui N.A., Mukhair M.A., Parvez M.K., Alam P., Ali M., Haque A. A novel monocyclic triterpenoid and a norsesquaterpenol from the aerial parts of Suaeda monoica Forssk. ex J. F. Gmel with cell proliferative potential. Saudi Pharm. J. 2017;25:1005–1010. doi: 10.1016/j.jsps.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alqahtani A.S., Abdel-mageed W.M., Shahat A.A., Parvez M.K., Al-Dosari M.S., Malik A., Abdel-Kader M.S., Alsaid M.S. Proanthocyanidins from the stem bark of Rhus tripartita and their amelioration of methylglyoxal-induced apoptosis of endothelial cells. J. Food Drug Anal. 2019;27:358–365. doi: 10.1016/j.jfda.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shabib, N.A,, Khan, J.M., Malik, A., Rehman, M.T., AlAjmi. M.F., Husain, F.M., Ahmad, A. Sen P., 2020. Investigating the effect of food additive azo dye “tartrazine” on BLG fibrillation under in-vitro condition. A biophysical and molecular docking study. J. King Saud Univ.–Sci. 32, 2034-2040.
- Arbab A.H., Parvez M.K., Al-Dosari M.S., Al-Rehaily A.J., Ibrahim K.E., Alam P., AlSaid M.S., Rafatullah S. Therapeutic efficacy of ethanolic extract of Aerva Javanica aerial parts in the amelioration of CCl4-induced hepatotoxicty and oxidative damage in rats. Food Nutr. Res. 2016;60:30864. doi: 10.3402/fnr.v60.30864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astani A., Schnitzler P. Antiviral activity of monoterpenes beta-pinene and limonene against herpes simplex virus in vitro. Iranian J. Microbiol. 2014;6:149–155. [PMC free article] [PubMed] [Google Scholar]
- Boatright, K.M., Salvesen, G,S., 2003. Mechanisms of caspase activation. Curr. Opin. Cell Biol. 15, 725-731. [DOI] [PubMed]
- Bourajjaj M., Stehouwer C.D., van Hinsbergh V.W., Schalkwijk C.G. Role of methylglyoxal adducts in the development of vascular complications in diabetes mellitus. Biochem. Soc. Trans. 2003;31:1400–1402. doi: 10.1042/bst0311400. [DOI] [PubMed] [Google Scholar]
- Carter W.O., Narayanan P.K., Robinson J.P. Intracellular hydrogen peroxide and superoxide anion detection in endothelial cells. J. Leuk. Biol. 1994;55:253–258. doi: 10.1002/jlb.55.2.253. [DOI] [PubMed] [Google Scholar]
- Choy J.C., Granvilleab D.J., Hunt D.W.C., McManus B.M. Endothelial cell apoptosis: biochemical characteristics and potential implications for atherosclerosis. J. Mol. Cell. Cardiol. 2001;33:1673–1690. doi: 10.1006/jmcc.2001.1419. [DOI] [PubMed] [Google Scholar]
- Figarola, J.L., Singhal, J., Rahbar, S., Awasthi, S., Singha,l S.S., 2014. LR-90 prevents methylglyoxal-induced oxidative stress and apoptosis in human endothelial cells. Apoptosis. 19, 776-788. [DOI] [PMC free article] [PubMed]
- Ganesan A. The impact of natural products upon modern drug discovery. Curr. Opin Chem. Biol. 2008;12:306–317. doi: 10.1016/j.cbpa.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Kim J., Son J.W., Lee J.A., Oh Y.S., Shinn S.H. Methylglyoxal induces apoptosis mediated by reactive oxygen species in bovine retinal pericytes. J. Korean Med. Sci. 2004;19:95–100. doi: 10.3346/jkms.2004.19.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.R., Kim H.Y., Park J.K., Park S.K., Chang M.S., Jeon J.Y. Aconiti lateralis preparata radix activates the proliferation of mouse bone marrow mesenchymal stem cells and induces osteogenic lineage differentiation through the bone morphogenetic protein-2/smad-dependent runx2 pathway. Evid. Based Compl. Alter. Med. 2013;2013:86741. doi: 10.1155/2013/586741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X., Hu Y., Rui R., Wang D., Li X. Effects of Chinese herbal medicinal ingredients on peripheral lymphocyte proliferation and serum antibody titer after vaccination in chicken. Intl. Immunopharmacol. 2004;4:975–982. doi: 10.1016/j.intimp.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Kathiresan K., Ramanathan T. India, Annamalai University; Tamil Nadu: 1997. Medicinal Plants of Parangipettai Coast; pp. 72–76. [Google Scholar]
- Kokpal V., Miles D.H., Payne A.M., Chittarwong V. Chemical constituents and bioactive compounds from mangrove plants. Stud. Nat. Prod. Chem. 1990;7:175–199. [Google Scholar]
- Kumar S. Caspase function in programmed cell death. Cell Death Differen. 2006;14:32–43. doi: 10.1038/sj.cdd.4402060. [DOI] [PubMed] [Google Scholar]
- Lakshmanan G., Rajeshkannan C., Kavitha A., Mekala B., Kamaladevi N. Preliminary screening of biologically active constituents of Suaeda monoica and Sesuvium portulacastrum from palayakayal mangrove forest of Tamilnadu. J. Pharmacog. Phytochem. 2013;2:149–152. [Google Scholar]
- Lakshmi K.P., Narsimha Rao G.M. Antimicrobial activity of Suaeda monoica (Forsst ex Geml) against Human and plant pathogens. Res. J. Pharm. Biol. Chem. Sci. 2013;4:680–685. [Google Scholar]
- LeBel C.P., Ischiropoulos H., Bondy S.C. Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem. Res. Toxicol. 1992;5:227–231. doi: 10.1021/tx00026a012. [DOI] [PubMed] [Google Scholar]
- Morris G.M., Huey R., Lindstrom W., Sanner M.F., Belew R.K., Goodsell D.S., Olson A.J. Autodock4 and AutoDockTools4: automated docking with selective receptor flexiblity. J. Comput. Chem. 2009;16:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthazhagan K., Thirunavukkarasu P., Ramanathan T., Kannan D. Studies on phytochemical screening, antimicrobial and antiradical scavenging effect of a coastal salt mash plant Suaeda monoica. Res. J. Phytochem. 2014;8:102–111. [Google Scholar]
- Opara, E.C., Rockway, S.W., 2006. Antioxidants and micronutrients. Dis, Mon. 52, 151-63. [DOI] [PubMed]
- Oyama Y., Hayashi A., Ueha T., Maekawa K. Characterization of 2′,7′-dichlorofluorescin fluorescence in dissociated mammalian brain neurons: estimation on intracellular content of hydrogen peroxide. Brain Res. 1994;635:113–117. doi: 10.1016/0006-8993(94)91429-x. [DOI] [PubMed] [Google Scholar]
- Parvez M.K., Arbab A.H., Al-Dosari M.S., Al-Rehaily A.J., Alam P., Ibrahim K.E., AlSaid M.S., Rafatullah S. Protective effect of Atriplex suberecta extract against oxidative and apoptotic hepatotoxicty. Exp. Therap. Med. 2018;15:3883–3891. doi: 10.3892/etm.2018.5919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvez M.K., Al-Dosari M.S., Arbab A.H., Alam P., Alsaid M.S., Khan A.A. Hepatoprotective efficacy of Solanum surattense extract against chemical–induced oxidative and apoptotic injury in rats. BMC Compl. Alter. Med. 2019;19:155–162. doi: 10.1186/s12906-019-2553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvez, M.K., Al-Dosari., M.S., Ahmed. S., Rehman, M.T., Al-Rehaily, A.J., 2020. Oncoglabrinol C, a new flavan from Oncocalyx glabratus protects human endothelial cells against oxidative and apoptotic damages and modulated hepatic CYP3A4 activity. Saudi Pharm. J. 28, 646-656. [DOI] [PMC free article] [PubMed]
- Phalitakul S., Okada M., Hara Y., Yamawaki H. Vaspin prevents methylglyoxal-induced apoptosis in human vascular endothelial cells by inhibiting reactive oxygen species generation. Acta Physiol. (Oxford) 2013;209:212–219. doi: 10.1111/apha.12139. [DOI] [PubMed] [Google Scholar]
- Ravikumar S., Gnanadesigan M., Serebiah J., Inbaneson S.J. Hepatoprotective effect of an Indian salt marsh herb Suaeda monoica Forrsk ex. Gmel against concanavalin-A induced toxicity in rats. Life Sci. Med. Res. 2010;2:1–9. [Google Scholar]
- Rota C., Chignell C.F., Mason R.P. Evidence for free radical formation during the oxidation of 2'-7'-dichlorofluorescin to the fluorescent dye 2'-7'-dichlorofluorescein by horseradish peroxidase: possible implications for oxidative stress measurements. Free Rad. Biol. Med. 1999;27:873–881. doi: 10.1016/s0891-5849(99)00137-9. [DOI] [PubMed] [Google Scholar]
- Royall J.A., Ischiropoulos H. Evaluation of 2′,7′-dichlorofluorescin and dihydro-rhodamine 123 as fluorescent probes for intracellular H2O2 in cultured endothelial cells. Arch. Biochem. Biophys. 1993;302:348–355. doi: 10.1006/abbi.1993.1222. [DOI] [PubMed] [Google Scholar]
- Shahat A.A., Alsaid M.S., Rafatullah S., Al-Sohaibani M.O., Parvez M.K., Al-Dosari M.S., Exarchou V., Pieters L. Treatment with Rhus tripartita extract curtails isoproterenol-elicited cardiotoxicity and oxidative stress in rats. BMC Compl. Alter. Med. 2016;2016:351. doi: 10.1186/s12906-016-1318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y. Caspase activation, inhibition, and reactivation: A mechanistic view. Protein Sci. 2000;13:1979–1987. doi: 10.1110/ps.04789804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui N.A., Mothana R.A., Al-Said M.S., Parvez M.K., Alam P., Rehman M.T., Ali M., Alajmi M.F., Al-Dosari M.S., Al-Rehaily A.J., Khalid J.M. Cell proliferation activity delineated by molecular docking of four new compounds isolated from Suaeda monoica Forssk. ex. J.F. Gmel (aerial parts) Saudi Pharm. J. 2020;28:172–186. doi: 10.1016/j.jsps.2019.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka R., Tsujimoto K., In Y., Matsunaga S. New Methoxytriterpene Dione from the cuticle of Picea jezoensis var. jezoensis. J. Nat. Prod. 1997;60:319–322. [Google Scholar]
- Thornalley P.J., Rabbani N. Assay of methylglyoxal and glyoxal and control of peroxidase interference. Biochem. Soc. Trans. 2014;42:504–510. doi: 10.1042/BST20140009. [DOI] [PubMed] [Google Scholar]
- Yuan J., Zhu C., Hong Y., Sun Z., Fang X., Wu B., Li S. The role of cPLA2 in Methylglyoxal-induced cell apoptosis of HUVECs. Toxicol. Appl. Pharmacol. 2017;23:44–52. doi: 10.1016/j.taap.2017.03.020. [DOI] [PubMed] [Google Scholar]
- Vander Jagt D.L., Hunsaker L.A. Methylglyoxal metabolism and diabetic complications: roles of aldose reductase, glyoxalase-I, betaine aldehyde dehydrogenase and 2-oxoaldehyde dehydrogenase. Chem. Biol. Interact. 2003;143:341–351. doi: 10.1016/s0009-2797(02)00212-0. [DOI] [PubMed] [Google Scholar]