Abstract
Introduction
Measurement of blood Favipiravir (FPV) levels and accumulation of data in COVID-19 patients are critical for assessing FPV efficacy and safety. We performed a retrospective study based on measurements of blood levels of FPV and related factors in COVID-19 patients admitted to our hospital. Furthermore, we also investigated the association between blood FPV levels and uric acid level alterations before and after FPV administration.
Methods
We enrolled 27 COVID-19 patients who had received FPV treatment at Hokushin General Hospital from April 1 to December 31, 2020. Age, gender, COVID-19 severity, presence of comorbidities, and laboratory data for each subject were investigated to identify factors that correlate with blood FPV levels. Uric acid levels were measured before and after FPV administration and a difference between the levels (i.e., a change of uric acid level) was evaluated.
Results
When a significant univariate variable was input by the stepwise method and a combination of variables that maintained statistical superiority was searched, serum ferritin was the only factor that independently affected blood FPV level. Furthermore, in the high-FPV group (20 μg/mL or more), a significant increase in uric acid levels was observed after FPV administration. The increment value was significantly larger than that in the low-FPV group (less than 20 μg/mL).
Conclusions
Ferritin level was an important independent factor inversely affecting blood FPV level. Furthermore, a high blood FPV level induced the elevation of uric acid levels in COVID-19 treatment.
Keywords: COVID-19, Favipiravir, Blood level, HPLC, Ferritin, Uric acid
1. Introduction
With the continued spread of the coronavirus disease 2019 (COVID-19) pandemic, the development of a COVID-19 vaccine and therapeutic agents is urgent. Favipiravir (FPV) is a novel influenza therapeutic drug that exhibits growth inhibitory activity against the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), an RNA virus that is the causative agent of COVID-19. Clinical trials testing the use of FPV for the treatment of COVID-19 have been conducted in many countries, including Japan, with significantly improved clinical results such as fever reduction [[1], [2], [3]]. Additionally, an interim report of a non-randomized study of FPV in COVID-19 patients, conducted in Russia, stated that the virus-negative rate up to 5 days was significantly higher in the FPV-treated group (62.5%) than that in the non-treated group (30.0%) [4]. On the other hand, conflicting reports on the lack of clinical benefit from FPV have been published [5,6], with some stating abnormally low blood FPV levels in critically ill COVID-19 patients [7]. Therefore, expanding the indication of FPV for COVID-19 must be carefully considered.
The pharmacokinetics of FPV is complex and exhibits a non-linear time component, along with dose dependence [8]. In clinical trials against the Ebola virus, blood FPV levels were lower than expected [9]. Another trial for FPV against severe influenza reported that the achievement rate of blood FPV level (trough level) of ≥20 μg/mL was <50% [10]. Clearly, low blood FPV levels do not demonstrate sufficient efficacy in patients with Ebola or influenza. Pharmacokinetic/pharmacodynamic data for FPV in the treatment of COVID-19 are currently lacking, and thus the measurement of blood FPV levels and accumulation of FPV-related data in COVID-19 patients are very important for assessing the efficacy and safety of FPV for this indication [11].
To monitor blood FPV levels, we previously developed a rapid, in-hospital (i.e., in our facility) method for quantifying blood FPV levels in COVID-19 patients [12]. This method consists of an initial pretreatment step using solid-phase extraction (SPE) and a subsequent separation and detection step using high-performance liquid chromatography (HPLC). Interestingly, our previous study showed that FPV selectively formed complexes with ferric (Fe3+) and cupric (Cu2+) ions [12]. Based on this finding, we speculated that blood FPV levels may be sensitive to differences in serum ferritin levels considering that ferritin complexes can store Fe3+ ions. Therefore, we performed a retrospective study investigating blood (serum) FPV levels and its clinical factors in COVID-19 patients admitted to our hospital. Furthermore, considering that hyperuricemia is a typical side effect of FPV treatment, we also investigated the possible association between blood FPV levels and uric acid level alterations before and after FPV administration.
2. Materials and methods
2.1. Patients
We enrolled 27 COVID-19 patients who had received FPV treatment at Hokushin General Hospital from April 1 to December 31, 2020. The Ethics Committee of the hospital approved the study protocol (Receipt No. 2021002). Patients’ blood FPV levels were measured from the residual serum collected during examination in the hospital. Informed consent was provided by all study participants or their families.
2.2. Chemicals
FPV was purchased from MedChemExpress Co., Ltd (Monmouth Junction, NJ, USA). Normal human serum was obtained from FUJIFILM Wako Pure Chemical Corp (Osaka, Japan). Acetonitrile (CH3CN; HPLC grade), phosphoric acid (H3PO4; special grade), and 5 mol/L hydrochloric acid (HCl) were purchased from Kanto Chemical Co., Inc. (Tokyo, Japan). Sterile purified water was purchased from Hikari Pharmaceutical Co. (Tokyo, Japan).
2.3. FPV administration
Each patient was administered 1,800 mg of FPV twice on Day 1, followed by 800 mg twice daily from Days 2–10 (up to Day 14 if necessary). FPV was administered orally.
2.4. Measurement of serum FPV levels
The detailed method for quantifying blood (serum) FPV levels was reported previously (The terms “blood” FPV level and “serum” FPV level are interchangeable in this study) [12]. Briefly, a centrifugal spin-cartridge (MonoSpinC18, GL Science, Inc., Tokyo, Japan) was used for the SPE treatment prior to HPLC analysis of FPV. The solution (or patient serum) was passed through the SPE cartridge by centrifugation at 5,000 rpm (2,400×g) using a Himac CT15E centrifuge (Koki Holdings, Tokyo, Japan). Subsequently, FPV in the serum was partially purified and collected in a test tube for HPLC analysis. All HPLC experiments were performed using the Chromaster system (Hitachi High-Tech Science Corporation, Tokyo, Japan). HPLC separation of FPV in the SPE eluates was performed at 30 °C in reverse-phase partition mode using a C18 stationary phase. Analyte containing FPV (injection volume: 20 μL) was eluted with the mobile-phase solvent (0.1% phosphoric acid and CH3CN, 95:5, v/v) at a flow rate of 2 mL/min, and FPV in the eluate was detected at 325 nm using UV absorbance. Patient sera were filtered through a DISMIC 13HP syringe filter (0.45 μm; ADVANTEC, Tokyo, Japan) before SPE pretreatment.
Patient's residual serum from serum collected for performing other tests before FPV oral administration (at trough level) in the morning was used for the abovementioned measurement of blood FPV levels. The patient's residual serum was preserved by freezing and measured within a month (The stability of the FPV was guaranteed in that storage method and period). When multiple measurements of blood FPV levels were performed during the oral administration period after reaching a steady-state of FPV, the blood FPV level is presented as the mean of multiple measurements.
2.5. Search for factors that affect FPV blood levels
Age, sex, COVID-19 severity, presence of comorbidities, and laboratory data for each subject were investigated to search for factors influencing blood FPV levels. In this study, the COVID-19 severity of each subject was classified as mild, moderate, or severe according to the Health, Labor and Welfare Ministry's Guide to the Treatment of COVID-19 (Version 4.1, https://www.mhlw.go.jp/content/000712473.pdf). The presence or absence of comorbidities was classified based on the presence or absence of ≥1 of the following conditions: diabetes, hypertension, cardiovascular disease, chronic lung disease, and immunosuppression. Laboratory data (before FPV administration) included white blood cell (WBC), alanine transaminase (ALT), C-reactive protein (CRP), serum ferritin, D-dimer (μg/mL), serum uric acid, and estimated glomerular filtration rate (eGFR).
2.6. Evaluation of uric acid levels before and after FPV administration
Based on the parameters suggested by a previous report by Wang et al. [10], the subjects were divided into two groups; the first consisted of those with blood FPV levels ≥20 μg/mL, and the second of those with blood FPV levels <20 μg/mL. Uric acid levels were measured before and after FPV administration and the difference between the two (i.e., a change in uric acid levels) was evaluated. The uric acid levels before FPV administration were measured at the time of first hospital admission. Furthermore, when multiple measurements of uric acid levels were performed after FPV administration, the level is presented as the mean of multiple measurements.
2.7. Statistical analyses
Continuous data were reported as the mean ± SD if normally distributed and as the median and interquartile range if not normally distributed. Linear regression analysis was used to analyze the relationship of independent variables to blood FPV levels. The values of blood FPV levels were log-transformed to fit this model. Stepwise multivariate linear regression analysis with significance for entry of variables was performed to study the influence of independent variables on blood FPV level. For the time-series transition analysis of the uric acid levels by FPV blood levels, repeated-measures one-way analysis of variance was performed with the uric acid level as the dependent variable and the “before and after FPV administration,” “blood level [high or low],” and their interaction terms as the fixed effects. A two-sided p value of <0.05 was considered statistically significant. All statistical analyses were performed with SPSS for Windows (version 24.0; IBM Japan, Tokyo, Japan).
3. Results
3.1. Demographics of COVID-19 patients and their blood FPV levels after FPV administration
Table 1 shows the demographics of the 27 patients and the distribution of the major blood test results (including blood FPV levels). Table S1 (Supplementary data) also lists the COVID-19 severities, blood FPV trough levels (after FPV administration), and ferritin levels (before FPV administration) of all patients. Based on the blood FPV level values in Table S1, Table S2 (Supplementary data) shows the patients were divided into two groups: low blood FPV level group (with levels less than 20 μg/mL; n = 6) and high blood FPV level group (with levels of 20 μg/mL or more; n = 21). Furthermore, Table S2 also presents the assorted data (median, % (or IQR), and p-value) of each background item mentioned above.
Table 1.
Background for COVID-19 patents treated with FPV (n = 27).
| Variables | number or median | (% or IQR) |
|---|---|---|
| Gender-no./total no. (%) | ||
| Male | 17/27 | (63.0) |
| Female | 10/27 | (37.0) |
| Median age (IQR) -yr | 63 | (49, 85) |
| Severity of COVID-19-no./total no. (%) | ||
| Mild | 6/27 | (22.2) |
| Moderate | 19/27 | (70.4) |
| Severe | 2/27 | (7.4) |
| Comorbidity-no./total no. (%) | 13/27 | (48.1) |
| Blood levels-median (IQR) | ||
| FPV (μg/mL) | 42.1 | (22.3, 85.5) |
| WBC (/μL) | 5400 | (4300, 6100) |
| ALT (U/L) | 21 | (13, 34) |
| LDH (U/L) | 217 | (181, 265) |
| CRP (mg/dL) | 1.2 | (0.7, 7.7) |
| Ferritin (ng/mL) | 242.1 | (177.5, 835.3) |
| D-dimer (μg/mL) | 0.5 | (0.2, 1.3) |
| Uric acid (mg/dL) | 5.0 | (4.5, 5.7) |
| eGFR (mL/min/1.73m2)-median (IQR) | 67.2 | (59.0, 84.0) |
Data are presented as number (%) or median (interquartile range; IQR).
Blood FPV levels were calculated as the mean trough level after reaching a steady-state of FPV.
3.2. Correlation between FPV and ferritin levels
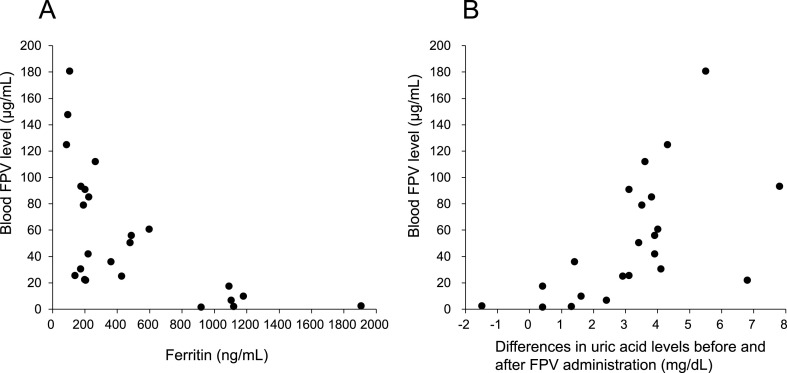
Fig. 1A provides a plot of blood FPV levels and ferritin levels for each patient shown in Table S1 (Supplementary data). Table 2 shows the results of linear regression analyses with the blood FPV levels subjected to logarithmic conversion as the dependent variable. Univariate analysis revealed that ALT, CRP, and ferritin correlated significantly with blood FPV levels. When significant variables were forced into those univariates, all significance disappeared. Therefore, when a significant univariate variable was input by the stepwise method and a combination of variables that maintained statistical superiority was searched, only ferritin was found as a factor that independently affected blood FPV levels.
Fig. 1.
Plots of blood FPV levels of all patients (n = 27) against their serum ferritin levels (A), or of blood FPV levels against changes in blood uric acid levels measured after FPV administration (B).
Table 2.
Linear regression analysis with "ln blood FPV level (trough value)" as the dependent variable.
| Univariate |
Multivariate model 1:Forcibly input a significant variable with a univariate |
Multivariate model 2: Input significant variables in univariates by stepwise method |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | PRC | SE | SPRC | t value | p value | PRC | SE | SPRC | t value | p value | VIF | PRC | SE | SPRC | t value | p value | VIF | |
| Age (per 1 year) | 27 | −0.012 | 0.010 | −0.231 | −1.186 | 0.247 | – | – | ||||||||||
| Gender (Female vs. Male) | 27 | 0.932 | 0.471 | 0.368 | 1.978 | 0.059 | – | – | ||||||||||
| Severity of COVID-19 | 27 | – | – | |||||||||||||||
| Mild | 6 | ref | ||||||||||||||||
| Moderate | 19 | −0.899 | 0.572 | −0.336 | −1.572 | 0.129 | ||||||||||||
| Severe | 2 | −1.382 | 0.998 | −0.296 | −1.386 | 0.179 | ||||||||||||
| Comorbidity | 27 | 0.155 | 0.489 | 0.063 | 0.317 | 0.754 | – | – | ||||||||||
| WBC (per 1000/μL) | 27 | −0.214 | 0.197 | −0.212 | −1.084 | 0.289 | – | – | ||||||||||
| ALT (per 1U/L) | 27 | −0.039 | 0.011 | −0.567 | −3.444 | 0.002 | −0.013 | 0.010 | −0.190 | −1.285 | 0.214 | 1.610 | n.e. | |||||
| CRP (per 1 mg/dl) | 27 | −0.190 | 0.029 | −0.792 | −6.486 | 0.000 | −0.108 | 0.050 | −0.441 | −2.176 | 0.042 | 3.037 | n.e. | |||||
| Ferritin (per 100 ng/mL) | 24 | −0.222 | 0.035 | −0.805 | −6.361 | 0.000 | −0.091 | 0.063 | −0.328 | −1.444 | 0.164 | 3.800 | −0.222 | 0.035 | −0.805 | −6.361 | 0.000 | 1.000 |
| D-dimer (per 1 μg/mL) | 24 | 0.079 | 0.149 | 0.113 | 0.533 | 0.599 | – | – | ||||||||||
| Uric acid (per 1 mg/dL) | 25 | 0.000 | 0.177 | 0.000 | −0.002 | 0.998 | – | – | ||||||||||
| eGFR (per 1 ml/min/1.73m2) | 27 | 0.010 | 0.014 | 0.136 | 0.687 | 0.498 | – | – | ||||||||||
PRC; Partial Regression Coefficient, SE; Standard error, SPRC; Standardized Partial Regression Coefficient, VIF; Variance Inflation Factor, ref: reference standard, n.e.; not entered.
dependent variable:"FPV blood level (trough value)" that has undergone logarithmic conversion.
Multivariate model 1: R2 = 0.729, Adjusted R2 = 0.688.
Multivariate model 2: R2 = 0.648, Adjusted R2 = 0.632.
3.3. Correlation between FPV and uric acid levels
Table S1 (Supplementary data) shows uric acid levels before and after FPV administration in each patient, and the change in uric acid levels after FPV administration. In addition, a plot of blood FPV levels and the change in uric acid level after FPV administration is shown in Fig. 1B. Table S3 (Supplementary data) shows the results of linear regression analyses with the changes in blood uric acid levels measured after FPV administration as the dependent variable. Fig. S1 (Supplementary data) demonstrates the receiver operator characteristic (ROC) curve of blood FPV levels for “2.0 mg/dL or more changes in blood uric acid levels measured after FPV administration”.
Table 3 shows the results of the simultaneous evaluation of the differences in uric acid level (group factor) and blood FPV levels (intergroup factor) before and after FPV administration. In the high blood FPV level group, there was a significant increase in uric acid levels after FPV administration. The magnitude of this increase was significantly larger than that in the low blood FPV level group. There was a single exception, whereby one patient's uric acid level did not increase despite this patient having a baseline blood FPV level exceeding 20 μg/mL (Table S1, No. 13). Notably, this patient had been taking febuxostat (40 mg/day) since admission.
Table 3.
Comparison of uric acid levels of COVID-19 patients between Low blood FPV level group and High blood FPV level group.
| n | Uric acid level (mg/dL) [mean ± SD] |
p value |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Before FPV administration | After FPV administration | Change (after administration) | |||||||||
| Low blood FPV level group (less than 20 μg/mL) | 6 | 5.8 | ± | 1.8 | 6.6 | ± | 2.2 | 0.8 | ± | 1.3 | 0.222 |
| High blood FPV level group (20 μg/mL or more) | 19 | 5.1 | ± | 1.4 | 9.3 | ± | 1.8 | 4.2 | ± | 1.5 | <0.001 |
| p value (High vs. Low) | 0.321 | 0.006 | <0.001 | ||||||||
For the time-series transition analysis of the uric acid levels by FPV blood levels, repeated-measures one-way analysis of variance was performed with the uric acid level as the dependent variable and the “before and after FPV administration,” “blood level [high or low],” and their interaction terms as the fixed effects.
4. Discussion
Two notable findings were obtained from the results described above. First, it has become evident that ferritin is an important independent factor inversely affecting blood FPV levels in COVID-19 patients. In the present study, all patients in the low blood FPV level group (less than 20 μg/mL) had hyperferritinemia (very high ferritin levels). These patients were elderly and had moderate-to-severe COVID-19. Their medical states were very similar to those of the patients described by Taneri et al. [13]. Furthermore, considering our previous in vitro result demonstrating that FPV is selectively complexed with Fe3+ ions in an aqueous buffer (pH 7.4) solution [12], we hypothesized that complexation occurs between stored iron-bound ferritin and FPV in the blood of COVID-19 patients with hyperferritinemia. We also hypothesized that elevated blood FPV levels (after FPV administration) were inversely associated with blood ferritin levels. Interestingly, a previous clinical study reported that FPV is ineffective in patients with moderate-to-severe COVID-19 and elevated ferritin levels (mean 1367 ng/mL) [6]. Furthermore, several reports have described extremely low blood FPV levels after FPV administration in COVID-19 patients [7,14]. In such cases, especially in patients with hyperferritinemia, adjusting the FPV dose based on blood FPV level monitoring or switching from FPV to another antiviral drug (e.g., remdesivir) may be necessary [15,16].
Second, most patients in the high blood FPV level group had hyperuricemia as a typical side effect (with the exception of one patient taking febuxostat 40 mg/day). A previous report showed that the increase in blood uric acid levels caused by FPV is due to the inhibition of the Organic Anion Transporters OAT1 and OAT3, which regulate renal tubular secretion, enhancing uric acid reabsorption of the FPV metabolite M1 via URAT1 and thereby reducing uric acid excretion [17]. In the present study, an increase in uric acid level typically was seen in patients in the high blood FPV level group after FPV administration, with uric acid levels increasing by an average of 4.2 mg/mL (Table 3). Paradoxically, if a COVID-19 patient taking FPV does not display symptoms of hyperuricemia, it is highly likely that such a patient will have an blood FPV level of less than 20 μg/mL.
Given the EC50 (the 50% effective concentration) of FPV for COVID-19 is 61.88 μM (9.72 μg/mL) [10], we hypothesized that blood FPV levels of at least 20 μg/mL must be maintained during COVID-19 treatment with orally administered FPV. Therefore, monitoring blood FPV levels (trough levels) will be very important.
The present study had two limitations. First, this study was a single-center study performed at a single hospital, and the number of COVID-19 patients with low blood FPV levels (n = 6) was far less than that of the patients with high blood FPV levels (n = 21). We believe that more comprehensive research involving a larger study population will be needed to establish clinically conclusive results on the efficacy and safety of FPV against COVID-19. Second, the combination of concomitant medications for COVID-19 treatment at our hospital changed during the present study (performed from April 2020 to December 2020). Given such major background bias, the efficacy and safety of FPV could not be assessed in the present study. However, regarding drug–drug interactions, no patients in this study were concomitantly taking theophylline, which affects aldehyde oxidase activity.
In the present study, the practical FPV quantification method we developed previously [12] was well applied and easily performed by the clinical staff of our hospital, representing little medical or economic burden. In the near future, we expect that this method will be utilized more widely for therapeutic drug monitoring of blood FPV levels and would be clinically useful in assessing COVID-19 treatment with FPV.
Author contributions
GM was main contributor in the conception and preparation. GM and KK contributed to data collection and analysis. GM and KK contributed to the drafting and editing of the manuscript. DK, YT, SM, TK, KO and TC provided advice on the study and revised the manuscript. YM and AY provided advice on blood concentration analysis. AY, KO and TC provided study supervision. All authors read and approved the final manuscript. All authors meet the ICMJE authorship criteria.
Declaration of competing interest
The authors declare that they have no conflicts of interests.
Acknowledgment
We thank the patients and their families, along with all the healthcare personnel who provided care for patients with COVID-19 at Hokushin General Hospital.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jiac.2021.10.011.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Doi Y., Hibino M., Hase R., Yamamoto M., Kasamatsu Y., Hirose M., et al. A prospective, randomized, open-label trial of early versus late favipiravir therapy in hospitalized patients with COVID-19. Antimicrob Agents Chemother. 2020;64 doi: 10.1128/AAC.01897-20. e01897-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Udwadia Z.F., Singh P., Barkate H., Patil S., Rangwala S., Pendse A., et al. Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: a randomized, comparative, open-label, multicenter, phase 3 clinical trial. Int J Infect Dis. 2020;103:62–71. doi: 10.1016/j.ijid.2020.11.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shrestha D.B., Budhathoki P., Khadka S., Shah P.B., Pokharel N., Rashmi P. Favipiravir versus other antiviral or standard of care for COVID-19 treatment: a rapid systematic review and meta-analysis. Virol J. 2020;17:141. doi: 10.1186/s12985-020-01412-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ivashchenko A.A., Dmitriev K.A., Vostokova N.V., Azarova V.N., Blinow A.A., Egorova A.N., et al. AVIFAVIR for treatment of patients with moderate COVID-19: interim results of a phase II/III multicenter randomized clinical trial. Clin Infect Dis. 2021;73:531–534. doi: 10.1093/cid/ciaa1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lou Y., Liu L., Yao H., Hu X., Su J., Xu K., et al. Clinical outcomes and plasma concentrations of baloxavir Marboxil and favipiravir in COVID-19 patients: an exploratory randomized, controlled trial. Eur J Pharmaceut Sci. 2021;157:105631. doi: 10.1016/j.ejps.2020.105631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khamis F., Al Naabi H., Al Lawati A., Ambusaidi Z., Al Sharji M., Al Barwani U., et al. Randomized controlled open label trial on the use of favipiravir combined with inhaled interferon beta-1b in hospitalized patients with moderate to severe COVID-19 pneumonia. Int J Infect Dis. 2021;102:538–543. doi: 10.1016/j.ijid.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irie K., Nakagawa A., Fujita H., Tamura R., Eto M., Ikesue H., et al. Pharmacokinetics of favipiravir in critically ill patients with COVID-19. Clin Transl Sci. 2020;13:880–885. doi: 10.1111/cts.12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madelain V., Guedj J., Mentré F., Nguyen T.H., Jacquot F., Oestereich L., et al. Favipiravir pharmacokinetics in Nonhuman primates and insights for future efficacy studies of hemorrhagic fever viruses. Antimicrob Agents Chemother. 2016;61:e01305–e01316. doi: 10.1128/AAC.01305-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen T.H., Guedj J., Anglaret X., Laouénan C., Madelain V., Taburet A.M., et al. Favipiravir pharmacokinetics in Ebola-Infected patients of the JIKI trial reveals concentrations lower than targeted. PLoS Neglected Trop Dis. 2017;11 doi: 10.1371/journal.pntd.0005389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y., Zhong W., Salam A., Tarning J., Zhan Q., Huang J.A., et al. Phase 2a, open-label, dose-escalating, multi-center pharmacokinetic study of favipiravir (T-705) in combination with oseltamivir in patients with severe influenza. EBioMedicine. 2020;62:103125. doi: 10.1016/j.ebiom.2020.103125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeitlinger M., Koch B.C.P., Bruggemann R., De Cock P., Felton T., Hites M., et al. Pharmacokinetics/pharmacodynamics of antiviral agents used to treat SARS-CoV-2 and their potential interaction with drugs and other supportive measures: a comprehensive review by the PK/PD of anti-infectives study group of the European society of antimicrobial agents. Clin Pharmacokinet. 2020;59:1195–1216. doi: 10.1007/s40262-020-00924-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moriiwa Y., Morikawa G., Okazawa K., Yanagida A. Optimization of analytical procedure for in-hospital rapid quantification of serum level of favipiravir in the pharmacological treatment of COVID-19. Anal Sci. 2021;37:1301–1304. doi: 10.2116/analsci.21N004. [DOI] [PubMed] [Google Scholar]
- 13.Taneri P.E., Gómez-Ochoa S.A., Llanaj E., Raguindin P.F., Rojas L.Z., Roa-Díaz Z.M., et al. Anemia and iron metabolism in COVID-19: a systematic review and meta-analysis. Eur J Epidemiol. 2020;35:763–773. doi: 10.1007/s10654-020-00678-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irie K., Nakagawa A., Fujita H., Tamura R., Eto M., Ikesue H., et al. Population pharmacokinetics of favipiravir in patients with COVID-19. CPT Pharmacometrics Syst Pharmacol. 2021;10:1161–1170. doi: 10.1002/psp4.12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., et al. Remdesivir for the treatment of covid-19 - final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spinner C.D., Gottlieb R.L., Criner G.J., Arribas López J.R., Cattelan A.M., Soriano Viladomiu A., et al. Effect of remdesivir vs standard care on clinical status at 11 Days in patients with moderate COVID-19: a randomized clinical trial. J Am Med Assoc. 2020;324:1048–1057. doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mishima E., Anzai N., Miyazaki M., Abe T. Uric acid elevation by favipiravir, an antiviral drug. Tohoku J Exp Med. 2020;251:87–90. doi: 10.1620/tjem.251.87. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.