Abstract
Signal-induced nuclear expression of the eukaryotic NF-κB transcription factor involves the stimulatory action of select mitogen-activated protein kinase kinase kinases on the IκB kinases (IKKα and IKKβ) which reside in a macromolecular signaling complex termed the signalsome. While genetic studies indicate that IKKβ is the principal kinase involved in proinflammatory cytokine-induced IκB phosphorylation, the function of the equivalently expressed IKKα is less clear. Here we demonstrate that assembly of IKKα with IKKβ in the heterodimeric signalsome serves two important functions: (i) in unstimulated cells, IKKα inhibits the constitutive IκB kinase activity of IKKβ; (ii) in activated cells, IKKα kinase activity is required for the induction of IKKβ. The introduction of kinase-inactive IKKα, activation loop mutants of IKKα, or IKKα antisense RNA into 293 or HeLa cells blocks NIK (NF-κB-inducing kinase)-induced phosphorylation of the IKKβ activation loop occurring in functional signalsomes. In contrast, catalytically inactive mutants of IKKβ do not block NIK-mediated phosphorylation of IKKα in these macromolecular signaling complexes. This requirement for kinase-proficient IKKα to activate IKKβ in heterodimeric IKK signalsomes is also observed with other NF-κB inducers, including tumor necrosis factor alpha, human T-cell leukemia virus type 1 Tax, Cot, and MEKK1. Conversely, the θ isoform of protein kinase C, which also induces NF-κB/Rel, directly targets IKKβ for phosphorylation and activation, possibly acting through homodimeric IKKβ complexes. Together, our findings indicate that activation of the heterodimeric IKK complex by a variety of different inducers proceeds in a directional manner and is dependent on the kinase activity of IKKα to activate IKKβ.
Cell survival largely depends on an innate ability of the cell to rapidly and effectively respond to changes in the external environment. This response can be summarized as perception of the external challenge, elicitation and transmission of an internal signal, and activation of transcription factors leading to alterations in gene expression. The NF-κB/Rel family of inducible transcription factors regulates an array of host genes controlling immune activation, inflammation, and the prevention of apoptosis (1, 17, 37, 57). In unstimulated cells, NF-κB is sequestered in the cytoplasm through its association with proteins of the IκB family of inhibitors (2, 3). Upon exposure to a wide array of stimuli, IκBα becomes phosphorylated on two N-terminal serines (Ser-32 and Ser-36) (7, 13, 50, 55). This modification targets IκBα for rapid degradation by the ubiquitin-proteasome pathway (8, 47), unmasking the nuclear localization signal within the p50-p65 NF-κB heterodimer and allowing its translocation to the nucleus as an active transcription factor.
Tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), lipopolysaccharides (LPS), and ligands recognizing the CD3-CD28 costimulatory T-cell receptor complex represent a subset of the diverse physiological inducers of IκB phosphorylation and subsequent NF-κB activation (53). Several kinases have been implicated as signaling intermediates in the pathway leading to NF-κB activation, most notably select members of the mitogen-activated protein kinase kinase kinase (MAP3K) family, including NF-κB inducing kinase (NIK), MEKK1, and Cot/Tpl-2 (19, 27, 28, 33, 36). NIK has been proposed as a downstream component of the TNF-α signaling pathway (36) which may be activated directly or indirectly by cytoplasmic adaptor proteins like RIP (23, 54) or TRAF2 (20, 45). These proteins are recruited to the cytoplasmic tails of the type 1 TNF-α receptor following ligand binding. Overexpression of wild-type NIK potently activates NF-κB, while a catalytically inactive NIK mutant dominantly interferes with TNF-α and IL-1 induction of NF-κB (36, 49). MEKK1 was originally identified as a key participant in the c-Jun activation pathway but more recently has been shown to also participate in the NF-κB signaling pathway leading to site-specific phosphorylation of IκB and NF-κB activation (19, 27, 28, 40–42). Cot/Tpl-2 is a proto-oncogene kinase that appears to play a role in CD3-CD28 activation of NF-κB (33). Pathological inducers of NF-κB have also been identified, including the human T-cell leukemia virus type 1 (HTLV-1)-encoded Tax protein (10, 16, 56, 61). Gram-negative bacteria contain LPS, which induces NF-κB through interaction with the Toll-like receptor 2, leading to NIK activation (5, 25, 60).
These various MAP3Ks do not directly phosphorylate IκB; rather, they activate a second set of kinases termed IκB kinase α (IKKα) and IKKβ (14, 27, 39, 44, 58, 64). These IKKs interact with each other and reside in a ∼900-kDa multicomponent signaling complex termed the signalsome (14, 27, 39). The predominant IKKα-IKKβ heterodimeric complex also contains NEMO/IKKγ/IKKAP1, a protein that lacks intrinsic kinase activity but is essential for IKK signaling (38, 46, 59), and a scaffolding protein termed IKAP (11). Although this multimeric complex exhibits virtually no basal activity, it readily responds to TNF-α and LPS stimulation (14, 27, 43) as well as to ectopic expression of NIK, Cot, MEKK1, or Tax, but not to functionally defective versions of these inducers (10, 16, 33, 34, 44, 56, 61). Tax induces the sustained nuclear expression of NF-κB/Rel through activation of the IKKs mediated through its assembly with IKKγ/NEMO (9, 18, 22). Recent studies with mice lacking the Ikkβ gene suggest that IKKβ is absolutely required for the kinase activity of the IKK complex and subsequent NF-κB activation in response to proinflammatory cytokines. In contrast, in mice lacking the Ikkα gene, NF-κB is normally induced following TNF-α signaling (21, 30–32, 51, 52). However, interpretation of these results is complicated by earlier studies showing that coexpression of a catalytically inactive form of IKKα (IKKαK44M) or addition of antisense IKKα (IKKα-as) RNA inhibits NF-κB activation in response to TNF-α, IL-1, HTLV-1 Tax or the intermediate kinases NIK, Cot/Tpl2, and MEKK1 (14–16, 28, 41, 44, 56, 58). It seems possible that the formation of IKKβ homodimeric signaling complexes, accentuated in the absence of IKKα, explains these paradoxical results. In this regard, Mercurio and colleagues have identified low-molecular-weight homodimeric IKKβ complexes; however, these particular complexes exhibit diminished IκBα kinase activity in response to TNF-α (38). It seems likely, as in the case of the IKKα−/− mice, that fully functional IKKβ homodimeric signalsomes can also form, although the heterodimeric IKKα-β complex is clearly the most favored and abundant complex formed under normal conditions.
In this study, we explore the biochemical basis for regulation of the heterodimeric IKKα-IKKβ complex resident within the physiologically relevant signalsome. In unstimulated cells, we find that the assembly of IKKα with IKKβ into a heterodimeric complex inhibits the high intrinsic activity of IKKβ. In cells stimulated with such agonists as TNF-α, NIK, Cot, MEKK1, or HTLV-1 Tax, we find that IKKα activation is a prerequisite for stimulation of IKKβ activity. Conversely, IKKβ activation is not required for induction of IKKα by agonists like TNF-α and NIK. In contrast, protein kinase C θ (PKCθ) appears to directly target IKKβ homodimeric complexes. Together these studies demonstrate that signal-coupled activation of the IKKα-IKKβ heterodimeric complex present in signalsomes proceeds in a directional manner through IKKα to IKKβ.
MATERIALS AND METHODS
Expression vectors, biological reagents, and cell cultures.
Wild-type and kinase-deficient constructs of IKKα, IKKβ, NIK, and Cot/Tpl-2 have been described elsewhere (16, 33, 34). Plasmids pCDNA-IKKα(K44M)-HA, pCDNA-IKKα(S176A)-HA, and pCDNA-IKKβ(K44ASTS/AAA) were generated by site-directed mutagenesis using PCR. Mutated residues were confirmed by sequencing. The expression vector encoding MEKK1 was a gift from G. Johnson (National Jewish Medical and Research Center, Denver, Colo.), the IKKα-as construct was kindly provided by Michael Karin (University of California, San Diego), and the PKCθ(A148E) construct was a gift from Amnon Altman (La Jolla Institute for Allergy and Immunology, San Diego, Calif.). Plasmids pCMV4Tax and pCMV4TaxM22 have also been described elsewhere (6, 48). Recombinant human TNF-α was purchased from Endogen (Cambridge, Mass.). The following epitope-specific reagents were used: anti-Flag M2 antibodies conjugated to agarose beads (Sigma, St. Louis, Mo.), polyclonal anti-Flag epitope-specific antibodies, IKKα-, IKKγ-, and c-Myc-specific antibodies (Santa Cruz Biotechnology, Santa Cruz, Calif.), hemagglutinin (HA)-conjugated Sepharose beads, and polyclonal anti-HA antibodies (BabCo, Richmond, Calif.). The 293 human embryonic kidney cell line and HeLa epithelial cell line were maintained in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal bovine serum and antibiotics.
IKKβ kinase assays.
293 cells were transfected with IKKβ-Flag and either IKKα-HA or IKKαK44M-HA expression vectors; 24 h posttransfection, cells were resuspended in lysis buffer (1% Nonidet P-40, 250 mM NaCl, 50 mM HEPES [pH 7.4], 1 mM EDTA) supplemented with a cocktail of protease inhibitors (Roche Biochemicals, Indianapolis, Ind.), 1 mM phenylmethylsulfonyl fluoride, 50 μM dithiothreitol, and 50 μM Na3VO4, freshly prepared before use. Lysates were immunoprecipitated with anti-Flag M2 antibody conjugated to agarose beads. The immunoprecipitates were then incubated with 1 μCi of [γ-32P]ATP and 1 μg of recombinant glutathione S-transferase (GST)–IκBα substrate at 30°C for 30 min. Reactions were stopped by adding 2× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer and boiling for 5 min. Products were separated by SDS-PAGE, electrophoretically transferred to nitrocellulose membranes, and exposed to Hyperfilm MP (Amersham Life Sciences, Piscataway, N.J.). The membranes were subsequently probed with Flag-specific antibodies to determine the amount of IKKβ-Flag present. Cell lysates were similarly examined to confirm the expression of each protein.
IKK complex phosphorylation assays.
To assess the role of IKKα in regulating the activation of IKKβ under both unstimulated and NIK-stimulated conditions, expression vectors encoding IKKβK44A-Flag or IKKβK44A-STS/AAA-Flag were transfected into HeLa cells in the presence or absence of various IKKα constructs as indicated. After 48 h, cells were lysed as described above. Lysates were immunoprecipitated with either anti-Flag M2 antibody-conjugated agarose beads or anti-IKKγ antibodies and protein A-conjugated agarose beads, washed three times in lysis buffer, equilibrated in kinase buffer (10 mM HEPES [pH 7.4], 1 mM MnCl2, 5 mM MgCl2, 12.5 mM β-glycero-2-phosphate, 50 μM Na3VO4, 2 mM NaF, 50 μM dithiothreitol, and resuspended in 20 μl of kinase buffer. The immunoprecipitates were then incubated with 2 μCi of [γ-32P]ATP at 30°C for 30 min. Reactions were stopped and separated as described above. The membranes were subsequently probed with epitope-specific antibodies to determine the amount of IKK present.
IKKβ and IKKα phosphorylation assays.
A kinase-inactive mutant of either IKKβK44A-Flag or IKKαK44M-HA was transfected into HeLa or 293 cells in combination with plasmids encoding either Myc-NIK, Myc-NIK(KK429/430AA), or other agonists including HA-MEKK1, Myc-Cot, PKCθ(A148E), Tax, or Tax M22. IKKα, IKKαK44M, IKKαS176A, IKKα-as, or IKKβK44A constructs were also cotransfected as indicated. At 24 or 48 h posttransfection, IKKγ complexes were immunoprecipitated as described above. Reactions were carried out in ATP-free kinase buffer containing 2 μCi of [γ-32P]ATP. After 30 min, reactions were halted by addition of an equal volume of dissociation buffer (50 mM Tris-Cl [pH 7.4], 20 mM β-mercaptoethanol, 10% SDS) and boiled for 15 min to completely dissociate the immunoprecipitated complex. The dissociated tagged proteins and beads were then washed in 1 ml of lysis buffer and centrifuged for 2 min at maximum speed. The supernatant was collected and incubated for a second immunoprecipitation with antibodies specific for the IKKα or IKKβ epitope tag conjugated to agarose beads. After at least 4 h, the immunoprecipitates were collected, washed with lysis buffer, and resuspended in SDS-PAGE buffer. Products were analyzed as described above.
HeLa cells were transfected with IKKβK44A-Flag and IKKα-HA and with increasing doses of IKKα-as construct. After 48 h, the cells were stimulated with TNF-α (20 ng/ml) for the times indicated. Cells were lysed and prepared as described above.
IKK signalsome purification.
Unstimulated and TNF-α-stimulated HeLa cells (6 × 106 cells) were harvested and resuspended in 400 μl of lysis buffer, spun twice for 10 min each time at 12,000 rpm, and loaded on a phenyl-Superose 6 column (Amersham-Pharmacia, Piscataway, N.J.) equilibrated with lysis buffer containing 10% glycerol. Fractions were collected, boiled in sample buffer, separated by SDS-PAGE, and transferred to nitrocellulose. Membranes were immunoblotted with anti-IKKα antibodies to identify the high-molecular-weight fractions containing the endogenous signalsome. HeLa cells were transfected with IKKβ-K44A and NIK in the presence of either IKKα or IKKα-K44M. After 48 h, lysates were collected and fractionated on a size exclusion column by fast protein liquid chromatography (FPLC). Fractions corresponding to those that contained the endogenous signalsomes, as shown with anti-IKKγ immunoblotting, and the transfected Flag-tagged IKKβ-K44A were collected. These fractions were pooled in pairs and immunoprecipitated with anti-Flag agarose. These immunoprecipitates were then subjected to an in vitro kinase assay followed by heat dissociation and reimmunoprecipitation as described above. Immunoprecipitates were boiled in sample buffer, separated by SDS-PAGE, transferred to nitrocellulose, and exposed to film. The amount of IKKβK44A-Flag in each sample was assessed by immunoblotting.
RESULTS
IKKα negatively regulates the constitutive activity of IKKβ.
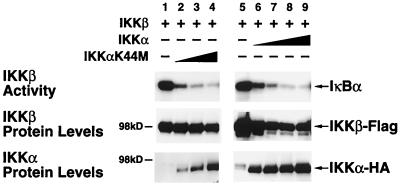
Since IKKβ exhibits high constitutive activity and appears to be a much more potent IκB kinase than IKKα (29), we investigated the possibility that IKKα functions within the heterodimeric complex as a negative regulator of IKKβ activity. To evaluate this possibility, we coexpressed IKKβ with either kinase-proficient or kinase-deficient IKKα in 293 cells. In agreement with prior studies (62), overexpressed IKKβ alone induced significant phosphorylation of IκBα in the absence of other stimuli (Fig. 1, lanes 1 and 5). As shown in Fig. 1, titration of either kinase-active or -inactive IKKα produced a dose-related inhibition of IKKβ basal activity. These studies confirm and extend previous reports (58, 62) demonstrating that IKKα negatively regulates the high constitutive activity of IKKβ observed under basal conditions. The levels of IKKα and IKKβ present in each sample are shown in the lower panels of Fig. 1.
FIG. 1.
IKKα regulates the basal IκB kinase activity of IKKβ. 293 cells were transfected with 0.6 μg of IKKβ-Flag expression vector alone or with increasing doses of either IKKαK44M-HA or IKKα-HA expression plasmids (0.6 μg, 1.2 μg, and 2.4 μg or 0.6 μg, 1.2 μg, 2.4 μg, and 3.6 μg, respectively). After 24 or 48 h, cell lysates were immunoprecipitated with anti-Flag M2-agarose. Immunoprecipitated complexes were assayed for kinase activity by incubation with 0.5 μg of GST-IκBα and [γ-32P]ATP. The resultant products were separated by SDS-PAGE (7.5% gels), transferred to nitrocellulose membranes, and subjected to autoradiography. The levels of IKKβ and IKKα in each lysate were determined by immunoblotting with Flag-specific or HA-specific antibodies (lower panels).
Activation of IKKβ phosphorylation by NIK depends on catalytically active IKKα.
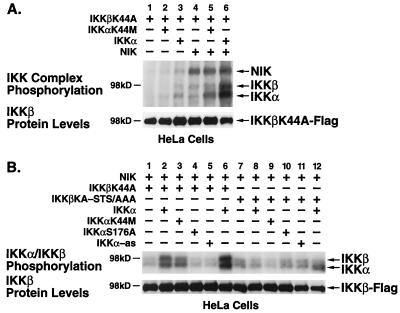
To explore a potential complementary role for IKKα in regulating IKKβ under stimulated conditions, we examined NIK-induced phosphorylation of IKKβ in the presence of functionally active or inactive forms of IKKα. Since wild-type IKKβ exhibits potent autophosphorylation, we used the kinase-deficient mutant IKKβK44A as a substrate in these experiments. As expected, expression of IKKβK44A alone or in combination with IKKαK44M did not result in significant phosphorylation of either IKK (Fig. 2A, lanes 1 and 2). A slight degree of autophosphorylation of kinase-proficient IKKα was detected (Fig. 2A, lane 3). However, in the presence of NIK, phosphorylation of both IKKα and IKKαK44M was significantly enhanced (Fig. 2A, lanes 5 and 6 versus lanes 2 and 3). Conversely, IKKβK44A was not phosphorylated when coexpressed with NIK alone or with combinations of NIK and kinase-deficient IKKαK44M (Fig. 2A, lanes 4 and 5). Notably, a significant level of IKKβ phosphorylation occurred when kinase-proficient IKKα was present with NIK (Fig. 2A, lane 6).
FIG. 2.
NIK-induced phosphorylation of IKKs. (A) HeLa cells were transfected with 1 μg of IKKβK44A-Flag expression vector alone or in combination with 1 μg of IKKα-HA or IKKαK44M-HA with and without 1 μg of Myc-NIK expression plasmids. (B) HeLa cells were transfected with 1 μg of IKKβK44A-Flag or IKKβK44A-STS/AAA-Flag and NIK expression vectors alone or in combination with 1 μg of each IKKα-HA construct as indicated (2 μg of IKKα was used in lanes 6 and 12). Cells were harvested 48 h after transfection, and IKKβK44A was immunoprecipitated with anti-Flag M2-agarose (A) or anti-IKKγ/NEMO (B) antibodies. Immunoprecipitated complexes were subjected to in vitro kinase assay in the presence of [γ-32P]ATP. The products were separated by SDS-PAGE (7.5% gels), transferred to nitrocellulose membranes, and subjected to autoradiography. The level of IKKβ in each lysate was detected by immunoblotting with Flag-specific antibodies (lower panel).
Since this experimental system demonstrating IKKβ phosphorylation involved overexpression of each kinase, it was important to establish whether this NIK-induced phosphorylation of IKKβ was also dependent on IKKα in the context of the physiologically relevant signalsome (14, 38). We used antibodies specific for the NEMO/IKKγ protein component of the complex to immunoprecipitate these signalsomes from HeLa cells transfected with the NIK, IKKα, and IKKβ constructs. These immunoprecipitates were then subjected to an in vitro kinase assay. The kinase-inactive mutant IKKβK44A was not significantly phosphorylated by NIK unless kinase-competent IKKα was coexpressed (Fig. 2B, lanes 2 and 6). In contrast, kinase-deficient IKKα, an IKKα mutant altered at Ser-176 in the activation loop, and IKKα-as constructs all significantly impaired the ability of NIK to phosphorylate IKKβ (Fig. 2B, lanes 3 to 5). The IKKαS176A mutant was evaluated since it represents a key phosphorylation site for NIK (35). This mutant is consistently expressed at a higher level than kinase-inactive IKKαK44M and therefore is a much more effective inhibitor of IKKβ phosphorylation. The IKKβ phosphorylation profile seen with the anti-IKKγ/NEMO immunoprecipitates was identical to that seen with the anti-Flag-agarose immunoprecipitates. Of note, the kinase-inactive mutant of IKKβ did not impede the ability of NIK to phosphorylate IKKα within the signalsome complex.
Previous reports had indicated that serine residues within the activation or T-loop of IKKβ were critical targets for phosphorylation leading to activation of IKKβ (12, 38, 39). In addition, several serine residues in the C terminus of IKKβ have also been implicated as autophosphorylation sites which negatively regulate the activity of IKKβ (12). To map the sites of phosphorylation in IKKβ targeted by IKKα in response to NIK activation, we used a kinase-inactive, T-loop mutant of IKKβ (IKKβK44A-STS/AAA) as a substrate for NIK-induced phosphorylation. As shown in Fig. 2B, mutation of the T-loop residues of IKKβ resulted in a failure of NIK to induce phosphorylation of IKKβ in the presence of kinase-proficient IKKα (Fig. 2B, compare lane 12 with lane 6). Thus, NIK-induced phosphorylation of IKKβ requires intact activation loop residues in both IKKα and IKKβ.
NIK-induced activation of the heterodimeric IKKα-β signalsome is directional.
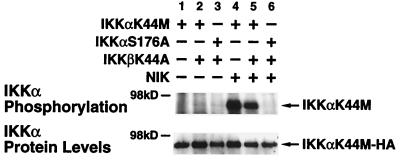
As shown in Fig. 2B, coexpression of kinase-inactive IKKβ did not inhibit the ability of NIK to phosphorylate IKKα, suggesting that the IKK heterodimeric complex was activated in a directional manner from IKKα to IKKβ (Fig. 2B, lanes 2 and 6). To confirm this directionality in a more sensitive manner, we selectively isolated either IKKαK44M or the activation loop mutant IKKαS176A from the other signalsome components. Specifically, anti-IKKγ/NEMO-immunoprecipitated signalsomes were subjected to an in vitro kinase assay. The IKKα substrates were then separated from the other reaction products by heat dissociation followed by reimmunoprecipitation with HA-specific antibodies. As shown in Fig. 3, neither IKKαK44M nor IKKαS176A was phosphorylated when expressed with IKKβK44A (lanes 1 to 3). However, IKKαK44M was robustly phosphorylated by NIK (lane 4), and this phosphorylation was not affected by coexpression of kinase-inactive IKKβ when normalized for the amounts of IKKα present (lane 5). In contrast, IKKαS176A was not phosphorylated by NIK (lane 6), thereby confirming that this activation loop residue serves as the target for NIK in the directional activation of the IKK heterodimeric complex.
FIG. 3.
NIK-induced phosphorylation of IKKα is not blocked by catalytically inactive IKKβ. 293 cells were transfected with 1 μg of IKKαK44M-HA or IKKαS176A alone or in combination with IKKβK44-Flag and Myc-NIK as indicated. Each transfection was supplemented with empty vector to a final total of 4 μg of DNA. Cells were harvested, and signalsomes were immunoprecipitated with anti-IKKγ/NEMO antibodies. Following an in vitro kinase assay and heat dissociation, the tagged IKKα constructs were reimmunoprecipitated with anti-HA-Sepharose. The products were separated by SDS-PAGE (7.5% gels), transferred to nitrocellulose membranes, and subjected to autoradiography. The level of IKKα in each lysate was detected by immunoblotting with HA-specific antibodies (lower panel).
IKKα-dependent NIK-induced phosphorylation of IKKβ occurs in the signalsome.
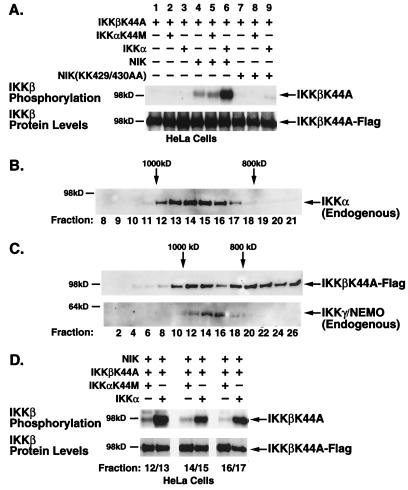
To investigate the directional phosphorylation of IKKβ within the heterodimeric IKK complex in the presence and absence of NIK, we selectively isolated the Flag-tagged IKKβK44A substrate from the other signalsome components as described above. Briefly, the anti-IKKγ/NEMO immunoprecipitates were subjected to an in vitro kinase assay followed by heat dissociation and reimmunoprecipitation with Flag-specific antibodies. As shown in Fig. 4A, the IKKβK44A substrate was not phosphorylated in the presence of kinase-inactive (lane 2) or kinase-proficient (lane 3) IKKα but was slightly phosphorylated in the presence of NIK (lane 4). However, the combination of NIK and IKKα induced robust phosphorylation of the IKKβK44A substrate (lane 6). This phosphorylation of IKKβK44A was dependent on the kinase activity of IKKα, as addition of the IKKαK44M mutant failed to support the NIK-induced response (lane 5). In contrast, a kinase-inactive form of NIK failed to induce IKKβ phosphorylation even in the presence of kinase-proficient IKKα (lanes 7 to 9). Consequently, despite the presence of equivalent levels of IKKβ in the anti-IKKγ immunoprecipitates (Fig. 4A, lower panel), only those signalsomes that contained functional IKKα were able to transmit an activation signal from NIK to IKKβ.
FIG. 4.
NIK-induced phosphorylation of IKKβ requires catalytically active IKKα. (A) HeLa cells were transfected with IKKβK44A alone or with either wild-type or kinase-inactive NIK in combination with wild-type or kinase-inactive IKKα. Cells were lysed 48 h posttransfection. Signalsomes were immunoprecipitated with anti-IKKγ/NEMO antibodies and subjected to an in vitro kinase assay followed by heat dissociation in 10% SDS. IKKβK44A substrates were selectively immunoprecipitated from the disrupted complexes by a second immunoprecipitation with anti-Flag M2-agarose. (B) Unstimulated and TNF-α-stimulated (5 min) HeLa cell lysates were subjected to FPLC size fractionation on a Superose 6 column. Fractions were collected, separated by SDS-PAGE, and immunoblotted with anti-IKKα antibodies to identify fractions containing the endogenous signalsome (fractions 12 to 17, ∼900 kDa). (C) HeLa cells, transfected with Flag-tagged, kinase-inactive IKKβ, NIK, and either kinase-proficient or kinase-defective IKKα, were lysed and size fractionated by FPLC. Fractions were separated by SDS-PAGE followed by immunoblotting with anti-Flag or anti-IKKγ antibodies. (D) Fractions corresponding to those containing the endogenous IKK signalsome, as identified by anti-IKKα and anti-IKKγ antibodies, were collected, pooled, immunoprecipitated, and subjected to an in vitro kinase assay as described for Fig. 2. The level of phosphorylated IKKβ-K44A is shown in the upper panel; the levels of protein as determined by anti-Flag immunoblotting are shown in the lower panel.
We took yet another approach to assessing directionality within the physiological signalsome by isolating the high-molecular-weight complex previously identified to contain TNF-α-responsive IKKα and IKKβ (14, 39). Unstimulated or TNF-α-stimulated HeLa cell lysates were size fractionated by FPLC on a Superose 6 column. Each fraction was subjected to SDS-PAGE, transferred to a membrane, and immunoblotted with an antibody that recognizes endogenous IKKα (H744; Santa Cruz Biotechnology). As seen in Fig. 4B, those fractions that contained the IKK complex (fractions 12 to 17) migrated in the 800- to 1,000-kDa size range in close agreement with prior studies (14, 39). The profiles were not significantly different between unstimulated and stimulated HeLa cells. Lysates from HeLa cells transfected with IKKβK44A and NIK in the presence of either wild-type or kinase-inactive IKKα were similarly fractionated by FPLC. While the transfected Flag-tagged IKKβK44A was distributed across a wider range of fractions (Fig. 4C, upper panel), it was effectively incorporated into the high-molecular-weight signalsome complex confirmed by the presence of endogenous IKKγ/NEMO (Fig. 4C, lower panel). The presence of transfected NIK in these fractions was confirmed by immunoblotting with anti-c-Myc antibodies (data not shown). Fractions corresponding to those containing signalsomes identified by anti-IKKα and IKK-γ antibodies above (fractions 12 to 17) were pooled in pairs and immunoprecipitated with anti-Flag antibodies. The immunoprecipitates were assayed for IKKβ phosphorylation as described above. As with the whole-cell lysates and the immunoprecipitated signalsomes, marked IKKβK44A phosphorylation occurred only in those fractions that contained kinase-proficient IKKα (Fig. 4D). In summary, the ability of NIK to induce IKKβ phosphorylation was severely compromised in heterodimeric IKKα-β signalsomes containing inactive IKKα despite the presence of equivalent levels of IKKβ in each fraction.
IKKα mediates phosphorylation of IKKβ induced by TNF-α and HTLV-1 Tax.
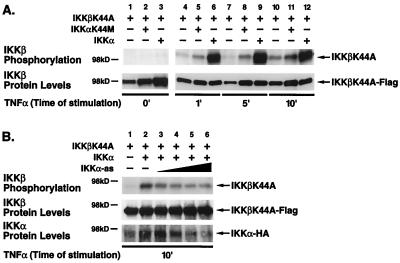
Since overexpression of a MAP3kinase such as NIK represents a somewhat artificial stimulation condition, we tested whether the heterodimeric IKK complex is directionally activated in response to TNF-α, a physiological inducer of NF-κB. HeLa cells were transfected with IKKβK44A alone (Fig. 5A, lanes 1, 4, 7, and 10) or in combination with either kinase-deficient IKKαK44M (lanes 2, 5, 8, and 11) or kinase-proficient IKKα (lanes 3, 6, 9, and 12) and stimulated with TNF-α (20 ng/ml) for 0, 1, 5, or 10 min. Under basal conditions, no phosphorylation on IKKβK44A was observed when this mutant was expressed alone or with either kinase-inactive or kinase-proficient IKKα (Fig. 5A, lanes 1, 2 and 3). In response to addition of TNF-α, coexpression of kinase-proficient IKKα resulted in a marked phosphorylation of IKKβK44A (Fig. 5A, lanes 6, 9, and 12). In contrast, TNF-α induced only minimal phosphorylation of IKKβK44A expressed either alone (lanes 4, 7, and 10) or with kinase-inactive IKKαK44M (Fig. 5A, lanes 5, 8, and 11). The slightly higher levels of IKKβK44A protein (lower panel) probably account for the modestly higher levels of IKKβK44A phosphorylation observed in the presence of IKKαK44M. In addition, disruption of endogenous IKKα protein expression by transfection of an IKKα antisense construct also resulted in a dose-dependent inhibition of IKKβ phosphorylation in response to TNF-α stimulation in the anti-IKKγ/NEMO-immunoprecipitated complexes (Fig. 5B). Thus, IKKβ phosphorylation in response to TNF-α stimulation is dependent on IKKα in the context of the physiological signalsome.
FIG. 5.
IKKβ phosphorylation induced by TNF-α in the presence and absence of IKKα. (A) HeLa cells were transfected with 2 μg of IKKβK44A-Flag and 2 μg of IKKα-HA or 2 μg of IKKαK44M-HA expression vector. Forty-eight hours after transfection, cells were stimulated with TNF-α (20 ng/ml) for 1, 5, and 10 min and lysed. Lysates were immunoprecipitated with anti-Flag M2-agarose and analyzed as for Fig. 4. Levels of IKKβK44A were evaluated by immunoblotting (lower panel). (B) HeLa cells were transfected with 0.5 μg of IKKβK44A-Flag and 1 μg IKKα-HA with increasing amounts of IKKα-as (0.5, 1, 2, and 4 μg). Forty-eight hours after transfection, cells were stimulated with TNF-α (20 ng/ml) for 10 min and lysed. Lysates were immunoprecipitated with anti-IKKγ/NEMO antibodies and analyzed as for Fig. 4. Levels of IKKβK44A-Flag and IKKα-HA were evaluated by immunoblotting (lower panel).
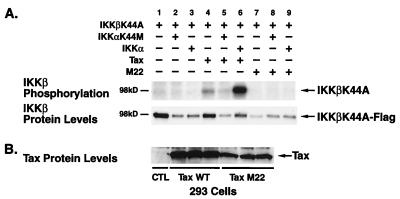
HTLV-1 Tax, a pathological inducer of NF-κB activity, significantly activates both IKKα and IKKβ activity (10, 16, 56) and, alternatively, has been proposed to promote IKKβ, but not IKKα, activation through the induction of MEKK1 (61). Recently, HTLV-1 Tax has been shown to activate the IKKs through its assembly with NEMO/IKKγ (9, 18, 22). To assess the ability of Tax to induce the phosphorylation of kinase-inactive IKKβK44A, wild-type Tax was expressed with either kinase-deficient or kinase-proficient IKKα. In 293 cells, transfected IKKβK44A was only modestly phosphorylated by coexpression of wild-type Tax (Fig. 6A, lane 4), possibly acting through endogenous IKKα since addition of kinase-inactive IKKαK44M markedly suppressed this phosphorylation (Fig. 6A, lane 5). In contrast, in the presence of wild-type IKKα, expression of Tax induced marked phosphorylation of IKKβK44A (Fig. 6A, lane 6). As a control, 293 cells were also transfected with an expression vector encoding the M22 mutant of Tax, which does not induce NF-κB (48). As expected from our previous findings (16), the Tax M22 mutant did not induce phosphorylation of IKKβK44A irrespective of the functional competence of IKKα (Fig. 6A, lanes 7 to 9). An identical pattern of directional phosphorylation of IKKβ by Tax was observed in HeLa cells (data not shown). Levels of Tax protein in the relevant samples are shown in Fig. 6B. These studies indicate that IKKβ phosphorylation induced by both TNF-α and HTLV-1 Tax also proceeds in a directional manner through catalytically competent IKKα to IKKβ in the cell lines studied.
FIG. 6.
IKKβ phosphorylation induced by HTLV-1 Tax. (A) Approximately 3 × 105 293 cells were transfected with 1 μg of kinase-deficient IKKβ (IKKβK44A-Flag) in combination with 1 μg of IKKα-HA or IKKαK44M-HA expression construct in the presence of wild-type Tax (1 μg) or the M22 Tax mutant (2 μg) as indicated. Cell lysates were then immunoprecipitated with anti-Flag M2-agarose and subjected to an in vitro kinase assay with [γ-32P]ATP. The reaction products were separated by SDS-PAGE (7.5% gel), transferred to a nitrocellulose membrane, and analyzed by autoradiography. The amount of IKKβK44A-Flag in each reaction is shown in the lower panel. (B) The levels of wild-type amd mutant Tax proteins in the cell lysates were assessed by immunoblotting with Tax-specific antiserum.
IKKα is required for phosphorylation of IKKβ by Cot/Tpl-2 and MEKK1 but not by PKCθ.
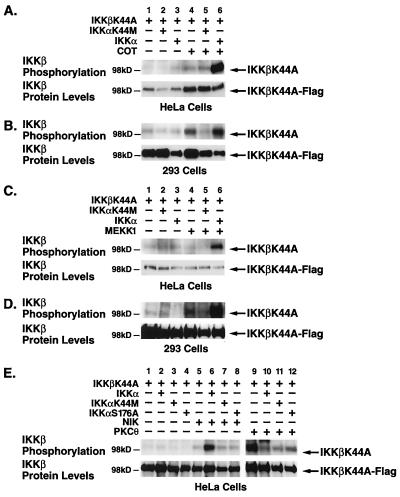
We next investigated whether a similar directional activation of the heterodimeric IKK complex occurs during stimulation by other MAP3Ks like Cot/Tpl-2, MEKK1, and PKCθ (X. Lin, A. O'Mahony, Y. Mu, R. Geleziunas, and W. C. Greene, unpublished data), which represent known inducers of NF-κB. As with NIK, in HeLa cells, IKKβK44A was not phosphorylated when coexpressed with IKKα or Cot alone (Fig. 7A, lanes 3 and 4). However, the combination of Cot and kinase-active IKKα induced potent phosphorylation of IKKβK44A (Fig. 7A, lane 6). This activation failed to occur in the presence of IKKαK44M (Fig. 7A, lane 5). The level of IKKβ phosphorylation did not result from a reduced expression of the IKKβK44A substrate as determined by immunoblotting (Fig. 7A, lower panel). Similarly, MEKK1 coexpressed with wild-type IKKα potently induced phosphorylation of IKKβK44A in HeLa cells (Fig. 7C, lane 6) but failed to do so when expressed either alone or with IKKαK44M (Fig. 7C, lanes 4 and 5). In sharp contrast, a constitutively active PKCθ(A/E) mutant induced phosphorylation of IKKβK44A when expressed alone (Fig. 7E, lane 9). Interestingly, this phosphorylation was inhibited when either wild-type, kinase-inactive, or T-loop mutant IKKα was coexpressed (Fig. 7E, lanes 10 to 12). This pattern of phosphorylation suggests that PKCθ may specifically target signalsomes containing homodimeric IKKβ complexes whereas Cot and MEKK1 operate through the heterodimeric complex in a directional manner.
FIG. 7.
Kinase-deficient IKKα blocks MEKK1- and Cot/Tpl-2-induced, but not PKCθ-induced, phosphorylation of IKKβ. HeLa cells and 293 cells were transfected with 1 μg of IKKβK44A-Flag expression plasmid and 1 μg of HA-tagged wild-type or kinase-deficient IKKα in the presence or absence of the Myc-Cot (A and B), HA-MEKK1 (C and D), and NIK and PKCθ(A/E) (E) expression vectors. After 24 h (293) and 48 h (HeLa), cells were harvested and lysates were immunoprecipitated with anti-Flag M2-agarose (A to D) or with IKKγ-specific antibodies (E). The immunoprecipitated complexes were subjected to an in vitro kinase assay and analyzed as for Fig. 4. The levels of phosphate incorporated into Flag-tagged, kinase-deficient IKKβ are shown in the upper panel, and the levels of Flag-tagged IKKβ are shown in the lower panels.
In 293 cells, both Cot and MEKK1 induced modest phosphorylation of IKKβK44A similar to the result obtained with NIK (Fig. 4B and D). This phosphorylation was blocked by kinase-deficient IKKαK44M but was potently enhanced by wild-type IKKα (Fig. 7B and D, lanes 5 and 6). These findings demonstrate that IKKα kinase activity is required for IKKβ phosphorylation induced by Cot and MEKK1, but not PKCθ, in 293 and HeLa cells.
DISCUSSION
When first identified, IKKα and IKKβ were viewed as functionally interchangeable IκB kinases that coexist within a macromolecular IKK signaling complex termed the signalsome. In the wake of targeted gene disruption studies, it is clear that these kinases play significantly different roles within the heterodimeric signalsome, IKKβ being the principal IκB kinase while the function of IKKα is less clear. We now demonstrate that activation of signalsomes containing heterodimeric IKKα-IKKβ complexes proceeds in a directional manner. Specifically, we show that a wide variety of NF-κB inducing MAP3Ks act through IKKα to induce phosphorylation of the activation loop residues of IKKβ in various cell lines. In contrast, kinase-deficient IKKβ exerts no inhibitory effects on NIK-induced phosphorylation of IKKα, underscoring the directional nature of this activation process. Our studies further indicate that phosphorylation of IKKβ induced by the physiological agonist TNF-α or the pathological stimulant HTLV-1 Tax similarly proceeds through IKKα to IKKβ. Interestingly, not all agonists require IKKα for induction of IKKβ phosphorylation. For example, we found that PKCθ is able to induce phosphorylation of IKKβ in the absence of IKKα. The addition of wild-type IKKα inhibits this PKCθ response, suggesting that expression of IKKα may disrupt IKKβ homodimeric complexes that may be selectively activated by PKCθ. These findings raise the intriguing possibility that different upstream activators couple preferentially to heterodimeric or homodimeric complexes, increasing signalling specificity.
Functional asymmetry within the heterodimeric signalsome was first suggested by the observation that IKKβ is a significantly more potent IκB kinase than IKKα. While both kinases are capable of phosphorylating IκBα in vitro, they do so with dramatically different efficiencies, with IKKβ exhibiting 50- to 60-fold greater activity than IKKα (28, 29, 38, 58). Additional support for disparate roles in NF-κB activation has come from the targeted inactivation of the IKKα and IKKβ genes in mice. Disruption of the Ikkβ locus results in embryonic lethality at ∼14 days of gestation due to massive hepatic cell apoptosis leading to liver degeneration, a phenotype remarkably similar to that seen in mice deficient in the RelA/p65 subunit of NF-κB (4, 31, 32, 52). This enhanced hepatocyte death is likely due to the loss of the antiapoptotic effects of NF-κB since IKKβ-deficient embryonic fibroblasts have severely depressed IκB kinase activity and diminished NF-κB activation in response to either TNF-α or IL-1 (31, 52). Indeed, IKKβ-deficient cells were 30-fold more sensitive to TNF-α-induced apoptosis than their wild-type counterparts (52). The amount of IKKα protein was greater in homozygous IKKβ-deficient embryos than in wild-type embryos, suggesting that there is a selective pressure to enhance IKKα expression in IKKβ-deficient cells, although this up-regulation of IKKα does not fully compensate for the loss of IKKβ activity and therefore is unable to counteract the extensive cell death (52). Of interest is the observation that IKKα continued to assemble into a minimally responsive ∼900-kDa signalsome in these IKKβ-deficient cells (31, 52).
IKKα-defective animals survive to birth but die within 1 to 4 h of birth and exhibit a range of morphogenic abnormalities including a thickened, undifferentiated epidermis that appears to restrict extension of the limbs and a number of skeletal malformations (21, 30, 51). Intriguingly, skin abnormalities, although not identical, have also been reported for mice deficient for IκBα, a negative regulator of NF-κB (26). In this study we, like others, have shown that IKKα can similarly function as a negative regulator of basal IKKβ activity (29, 62). It is interesting to speculate whether these skin abnormalities may emerge as a consequence of disrupting the normal negative regulators of IKKβ activity and NF-κB activation.
Disruption of the Ikkα locus surprisingly does not impair TNF-α induction of NF-κB, a finding confirmed in three independent studies. Of note, there is a quantitative decrease in the total level of NF-κB binding in these IKKα-deficient animals (21, 30, 51). This result seems at odds with the abundance of IKKα expression in the wild-type animals, its tight association with IKKβ expression, and the high degree of sequence similarity shared by these genes. Indeed, the widespread assembly of IKKα with IKKβ in signalsomes in many tissues argues that IKKα plays a broader function than regulating epidermal development (63). Moreover, previous studies with kinase-inactive or activation loop mutants of IKKα (15, 35) as well as transfection of IKKα-as constructs (14) have all reported a negative impact on IKK activity underlying the conditional importance of IKKα expression. In view of our described findings, we propose that the IKKα-deficient animals have likely compensated for the loss of the IKKα regulator by assembling functional homodimeric IKKβ signalsomes (21). These homodimeric IKKβ signalsomes (38) may be positively selected for during embryogenesis in the IKKα-deficient animals to prevent the extensive apoptosis that would result from a loss of IKK activity. In view of the dramatic difference in the IκB-phosphorylating activities of these two kinases, we would argue that IKKα has mainly evolved to negatively regulate the high constitutive activity of IKKβ under basal conditions and to couple its activation in stimulated conditions to many upstream agonists. Likewise, a proportion of complexes consisting of IKKβ homodimers have evolved with an alternative regulatory mechanism, perhaps IKKγ, which also plays a role in coupling of the signalsome to different upstream activators. Therefore, loss of a regulating kinase like IKKα may be compensated for, but loss of the functional kinase, IKKβ, cannot be tolerated. The generation of IKKα and IKKβ conditional knockout and knock-in animals will no doubt clarify the nature of the physiological interplay between these two kinases in the regulation of NF-κB induction.
We have demonstrated directional activation of the heterodimeric IKK complex by a number of MAP3Ks known to play a role in NF-κB activation (19, 27, 28, 33, 36, 40–42, 44). This activation occurs through phosphorylation of the serine residues within the activation loops of the IKKs. One recent report suggests that the activation loop serines of IKKβ are essential for NIK-induced IKK activation (12). We find that these activation loop serines are phosphorylated in the presence of NIK but in an indirect manner dependent on the kinase activity of IKKα. In the same study, Delhase and colleagues report that homologous activation loop mutations in IKKα do not affect IκB phosphorylation (12). This result is at odds with our observations that the activation loop mutant IKKαS176A blocks both IKKβ and IκBα phosphorylation induced by NIK. In support of our data, NIK was previously shown to phosphorylate IKKα on Ser-176 of its activation loop, but it did not phosphorylate IKKβ (35). These data support a dual regulatory role for IKKα leading to the appropriate activation of IKKβ phosphorylation. As such, IKKα could be functionally viewed as a surrogate MAP2-like kinase connecting the upstream MAP3Ks to the downstream MAPK represented by IKKβ.
The precise nature of the interplay of MEKK1 with IKKα or IKKβ remains unclear. Some studies indicate MEKK1 interacts with, and activates, both IKKα and IKKβ (28, 42). However, other reports show that MEKK1 overexpression in 293 or Jurkat cells preferentially stimulates IKKβ kinase activity over IKKα (24, 41). In addition, Tax has been shown to bind and activate MEKK1, which then directly activates IKKβ but not IKKα (61). However, more recent reports indicate that Tax binds to the signalsome by assembling with NEMO/IKKγ rather than by binding to IKKβ directly (9, 18, 22). This interaction may be impaired in the presence of overexpressed upstream kinase-inactive MAP3Ks, which may also interact with IKKγ. We too find that within the heterodimeric signalsome, both MEKK1 and Tax induce IKKβ phosphorylation in a manner dependent on the kinase activity of IKKα. In agreement with our findings, kinase-inactive forms of both IKKα and IKKβ have been shown to block Tax and MEKK1 induction of IKK activity, clearly implicating both kinases in the pathway (10, 16, 24, 56).
Of interest is our finding that not all signals proceed through IKKα. We show that PKCθ appears to selectively target IKKβ for activation. Of note, this reaction may involve IKKβ homodimers since assembly of IKKβ into the heterodimeric complex inhibits its ability to serve as a target for PKCθ-mediated activation. These inconsistencies in activation of IKKα versus IKKβ by various upstream kinases may, in part, be reconciled by the existence of a number of distinct IKK complexes (38). The larger ∼700-kDa TNF-α-responsive complex was found to contain IKKα, IKKβ, and IKKAP1 (NEMO/IKKγ), while a ∼300-kDa complex consisting of only IKKβ and IKKAP1 proved significantly less responsive to TNF-α-coupled induction (38). It is possible, however, that the higher-molecular-weight complex also contains functional IKKβ homodimeric complexes. Moreover, the smaller IKKβ complexes may not respond to TNF-α but may couple to different activators. Different cell lines may contain varying amounts of these IKKα-β heterodimeric versus IKKβ homodimeric complexes, and these complexes may couple differentially to upstream activating signals. Our studies clearly show that, in the 293 and HeLa cell lines studied, transmission of the NF-κB-inducing signal is directional within the heterodimeric IKK signalsome.
In summary, we propose that, when present in the heterodimeric signalsome, IKKα exerts a dominant regulating effect on the phosphorylation and activation of IKKβ kinase activity. This regulatory role of IKKα is further underscored by the finding that mutations in the leucine zipper region of IKKα disrupts dimerization with IKKβ, resulting in a strong diminution of IκB phosphorylation (38, 58, 62). Interestingly, mutations in the helix-loop-helix motifs of either kinase do not abolish their dimerization but do result in the loss of kinase activity (62), likely reflecting a failure of the IKKs to bind NEMO/IKKγ/IKKAP1, an essential component of functional signalsomes (38, 46, 59). IKKα is thus an essential regulatory component of the IKK heterodimeric signalsome that serves to couple the upstream activating signal to the IKKβ catalytic component of the complex.
ACKNOWLEDGMENTS
We thank Wolfgang Fischle for assistance with the FPLC, Bobby Benitez for technical help, John Carroll, Neile Shea, Stephen Gonzales, and Chris Goodfellow for preparation of the figures, and Robin Givens for assistance in preparation of the manuscript. We also thank G. Johnson for providing the MEKK1 expression vector, Michael Karin for the IKKα antisense construct, and Amnon Altman for the PKCθ construct.
This work was partially supported by the Gladstone Institutes, a grant from Pfizer, and core support from the UCSF Center for AIDS Research (P30A127763).
REFERENCES
- 1.Baeuerle P A, Baltimore D. NF-κB: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin A. The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 3.Beg A A, Baldwin A J. The IκB proteins: multifunctional regulators of Rel/NF-κB transcription factors. Genes Dev. 1993;7:2064–2070. doi: 10.1101/gad.7.11.2064. [DOI] [PubMed] [Google Scholar]
- 4.Beg A A, Sha W C, Bronson R T, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature. 1995;376:167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- 5.Belvin M P, Anderson K V. A conserved signaling pathway: the Drosophila toll-dorsal pathway. Annu Rev Cell Dev Biol. 1996;12:393–416. doi: 10.1146/annurev.cellbio.12.1.393. [DOI] [PubMed] [Google Scholar]
- 6.Beraud C, Sun S C, Ganchi P, Ballard D W, Greene W C. Human T-cell leukemia virus type I Tax associates with and is negatively regulated by the NF-κB2 p100 gene product: implications for viral latency. Mol Cell Biol. 1994;14:1374–1382. doi: 10.1128/mcb.14.2.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Control of IκB-alpha proteolysis by site-specific, signal-induced phosphorylation. Science. 1995;267:1485–1488. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- 8.Chen Z, Hagler J, Palombella V J, Melandri F, Scherer D, Ballard D, Maniatis T. Signal-induced site-specific phosphorylation targets IκB alpha to the ubiquitin-proteasome pathway. Genes Dev. 1995;9:1586–1597. doi: 10.1101/gad.9.13.1586. [DOI] [PubMed] [Google Scholar]
- 9.Chu Z-L, Shin Y-A, Yang J-M, DiDonato J A, Ballard D A. IKKγ mediates the interaction of cellular IκB kinases with the Tax transforming protein of human T cell leukemia virus type 1. J Biol Chem. 1999;274:15297–15300. doi: 10.1074/jbc.274.22.15297. [DOI] [PubMed] [Google Scholar]
- 10.Chu Z L, DiDonato J A, Hawiger J, Ballard D W. The tax oncoprotein of human T-cell leukemia virus type 1 associates with and persistently activates IκB kinases containing IKKα and IKKβ. J Biol Chem. 1998;273:15891–15894. doi: 10.1074/jbc.273.26.15891. [DOI] [PubMed] [Google Scholar]
- 11.Cohen L, Henzel W J, Baeuerle P A. IKAP is a scaffold protein of the IκB kinase complex. Nature. 1998;395:292–296. doi: 10.1038/26254. [DOI] [PubMed] [Google Scholar]
- 12.Delhase M, Hayakawa M, Chen Y, Karin M. Positive and negative regulation of the IκB kinase activity through IKKβ subunit phosphorylation. Science. 1999;284:309–313. doi: 10.1126/science.284.5412.309. [DOI] [PubMed] [Google Scholar]
- 13.DiDonato J, Mercurio F, Rosette C, Wu L J, Suyang H, Ghosh S, Karin M. Mapping of the inducible IκB phosphorylation sites that signal its ubiquitination and degradation. Mol Cell Biol. 1996;16:1295–304. doi: 10.1128/mcb.16.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiDonato J A, Hayakawa M, Rothwarf D M, Zandi E, Karin M. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 15.Fischer C, Page S, Weber M, Eisele T, Neumeier D, Brand K. Differential effects of lipopolysaccharide and tumor necrosis factor on monocytic IκB kinase signalsome activation and IκB proteolysis. J Biol Chem. 1999;274:24625–24632. doi: 10.1074/jbc.274.35.24625. [DOI] [PubMed] [Google Scholar]
- 16.Geleziunas R, Ferrell S, Lin X, Mu Y, Cunningham E J, Grant M, Connelly M A, Hambor J E, Marcu K B, Greene W C. Human T-cell leukemia virus type 1 Tax induction of NF-κB involves activation of the IκB kinase alpha (IKKα) and IKKβ cellular kinases. Mol Cell Biol. 1998;18:5157–5165. doi: 10.1128/mcb.18.9.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghosh S, May M J, Kopp E B. NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 18.Harhaj E W, Sun S-C. IKKγ serves as a docking subunit of the IκB kinases (IKK) and mediates interaction of IKK with the human T-cell leukemia virus Tax protein. J Biol Chem. 1999;274:22911–22914. doi: 10.1074/jbc.274.33.22911. [DOI] [PubMed] [Google Scholar]
- 19.Hirano M, Osada S, Aoki T, Hirai S, Hosaka M, Inoue J, Ohno S. MEK kinase is involved in tumor necrosis factor alpha-induced NF-κB activation and degradation of IκB-α. J Biol Chem. 1996;271:13234–13238. doi: 10.1074/jbc.271.22.13234. [DOI] [PubMed] [Google Scholar]
- 20.Hsu H, Shu H B, Pan M G, Goeddel D V. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 21.Hu Y, Baud V, Delhase M, Zhang P, Deerinck T, Ellisman M, Johnson R, Karin M. Abnormal morphogenesis but intact IKK activation in mice lacking the IKKα subunit of the IκB kinase. Science. 1999;284:316–320. doi: 10.1126/science.284.5412.316. [DOI] [PubMed] [Google Scholar]
- 22.Jin D-Y, Giordano V, Kibler K V, Nakano H, Jeang K-T. Role of adapter function in oncoprotein-mediated activation of NF-κB. J Biol Chem. 1999;274:17402–17405. doi: 10.1074/jbc.274.25.17402. [DOI] [PubMed] [Google Scholar]
- 23.Kelliher M A, Grimm S, Ishida Y, Kuo F, Stanger B Z, Leder P. The death domain kinase RIP mediates the TNF-induced NF-κB signal. Immunity. 1998;8:297–303. doi: 10.1016/s1074-7613(00)80535-x. [DOI] [PubMed] [Google Scholar]
- 24.Kempiak S J, Hiura T S, Nel A E. The Jun kinase cascade is responsible for activating the CD28 response element of the IL-2 promoter: proof of cross-talk with the IκB kinase cascade. J Immunol. 1999;162:3176–3187. [PubMed] [Google Scholar]
- 25.Kirschning C J, Wesche H, Merrill Ayres T, Rothe M. Human toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J Exp Med. 1998;188:2091–2097. doi: 10.1084/jem.188.11.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klement J F, Rice N R, Car B D, Abbondanzo S J, Powers G D, Bhatt H, Chen C-H, Rosen C A, Stewart C L. IκB alpha deficiency results in a sustained NF-κB response and severe widespread dermatitis in mice. Mol Cell Biol. 1996;16:2341–2349. doi: 10.1128/mcb.16.5.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee F S, Hagler J, Chen Z J, Maniatis T. Activation of the IκB alpha kinase complex by MEKK1, a kinase of the JNK pathway. Cell. 1997;88:213–222. doi: 10.1016/s0092-8674(00)81842-5. [DOI] [PubMed] [Google Scholar]
- 28.Lee F S, Peters R T, Dang L C, Maniatis T. MEKK1 activates both IκB kinase α and IκB kinase β. Proc Natl Acad Sci USA. 1998;95:9319–9324. doi: 10.1073/pnas.95.16.9319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, Peet G W, Pullen, Schembri-King S S, J, Warren T C, Marcu K B, Kehry M R, Barton R, Jakes S. Recombinant IκB kinases α and β are direct kinases of IκBα. J Biol Chem. 1998;273:30736–30741. doi: 10.1074/jbc.273.46.30736. [DOI] [PubMed] [Google Scholar]
- 30.Li Q, Lu Q, Hwang J Y, Buscher D, Lee K-F, Izpisua-Belmonte J C, Verma I M. The IKK1-deficient mice exhibit abnormal development of skin and skeleton. Genes Dev. 1999;13:1322–1328. doi: 10.1101/gad.13.10.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Q, Van Antwerp D, Mercurio F, Lee K-F, Verma I M. Severe liver degeneration in mice lacking the IκB kinase 2 gene. Science. 1999;284:321–325. doi: 10.1126/science.284.5412.321. [DOI] [PubMed] [Google Scholar]
- 32.Li Z-W, Chu W, Hu Y, Delhase M, Deerinck T, Ellisman M, Johnson R, Karin M. The IKKβ subunit of IκB kinase (IKK) is essential for nuclear factor-κB activation and prevention of apoptosis. J Exp Med. 1999;189:1839–1845. doi: 10.1084/jem.189.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin X, Cunningham E T, Mu Y, Geleziunas R, Greene W C. The proto-oncogene Cot kinase participates in CD3/CD28 induction of NF-κB acting through the NF-κB-inducing kinase and IκB kinases. Immunity. 1999;10:271–280. doi: 10.1016/s1074-7613(00)80027-8. [DOI] [PubMed] [Google Scholar]
- 34.Lin X, Mu Y, Cunningham E T, Marcu K B, Geleziunas R, Greene W C. Molecular determinants of NF-κB-inducing kinase action. Mol Cell Biol. 1998;18:5899–5907. doi: 10.1128/mcb.18.10.5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ling L, Cao Z, Goeddel D V. NF-κB-inducing kinase activates IKK-α by phosphorylation of Ser-176. Proc Natl Acad Sci USA. 1998;95:3792–3797. doi: 10.1073/pnas.95.7.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malinin N L, Boldin M P, Kovalenko A V, Wallach D. MAP3K-related kinase involved in NF-κB induction by TNF, CD95 and IL-1. Nature. 1997;385:540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- 37.May M J, Ghosh S. Rel/NF-κB and IκB proteins: an overview. Semin Cancer Biol. 1997;8:63–73. doi: 10.1006/scbi.1997.0057. [DOI] [PubMed] [Google Scholar]
- 38.Mercurio F, Murray B W, Shevchenko A, Bennett B L, Young D B, Li J W, Pascual G, Motiwala A, Zhu H, Mann M, Manning A M. IκB kinase (IKK)-associated protein 1, a common component of the heterogeneous IKK complex. Mol Cell Biol. 1999;19:1526–1538. doi: 10.1128/mcb.19.2.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mercurio F, Zhu H, Murray B W, Shevchenko A, Bennett B L, Li J, Young D B, Barbosa M, Mann M, Manning A, Rao A. IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-κB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 40.Meyer C F, Wang X, Chang C, Templeton D, Tan T H. Interaction between c-Rel and the mitogen-activated protein kinase kinase kinase 1 signaling cascade in mediating κB enhancer activation. J Biol Chem. 1996;271:8971–8976. doi: 10.1074/jbc.271.15.8971. [DOI] [PubMed] [Google Scholar]
- 41.Nakano H, Shindo M, Sakon S, Nishinaka S, Mihara M, Yagita H, Okumura K. Differential regulation of IκB kinase alpha and beta by two upstream kinases, NF-κB-inducing kinase and mitogen-activated protein kinase/ERK kinase kinase-1. Proc Natl Acad Sci USA. 1998;95:3537–3542. doi: 10.1073/pnas.95.7.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nemoto S, DiDonato J A, Lin A. Coordinate regulation of IκB kinases by mitogen-activated protein kinase kinase kinase 1 and NF-κB-inducing kinase. Mol Cell Biol. 1998;18:7336–7343. doi: 10.1128/mcb.18.12.7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Connell M A, Bennett B L, Mercurio F, Manning A M, Mackman N. Role of IKK1 and IKK2 in lipopolysaccharide signaling in human monocytic cells. J Biol Chem. 1998;273:30410–30414. doi: 10.1074/jbc.273.46.30410. [DOI] [PubMed] [Google Scholar]
- 44.Regnier C H, Song H Y, Gao X, Goeddel D V, Cao Z, Rothe M. Identification and characterization of an IκB kinase. Cell. 1997;90:373–383. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- 45.Rothe M, Sarma V, Dixit V M, Goeddel D V. TRAF2-mediated activation of NF-κB by TNF receptor 2 and CD40. Science. 1995;269:1424–1427. doi: 10.1126/science.7544915. [DOI] [PubMed] [Google Scholar]
- 46.Rothwarf D M, Zandi E, Natoli G, Karin M. IKK-γ is an essential regulatory subunit of the IκB kinase complex. Nature. 1998;395:297–300. doi: 10.1038/26261. [DOI] [PubMed] [Google Scholar]
- 47.Scherer D C, Brockman J A, Chen Z, Maniatis T, Ballard D W. Signal-induced degradation of I κBα requires site-specific ubiquitination. Proc Natl Acad Sci USA. 1995;92:11259–11263. doi: 10.1073/pnas.92.24.11259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith M R, Greene W C. Identification of HTLV-I tax trans-activator mutants exhibiting novel transcriptional phenotypes. Genes Dev. 1990;4:1875–85. doi: 10.1101/gad.4.11.1875. [DOI] [PubMed] [Google Scholar]
- 49.Song H Y, Regnier C H, Kirschning C J, Goeddel D V, Rothe M. Tumor necrosis factor (TNF)-mediated kinase cascades: bifurcation of nuclear factor-κB and c-jun N-terminal kinase (JNK/SAPK) pathways at TNF receptor-associated factor 2. Proc Natl Acad Sci USA. 1997;94:9792–9796. doi: 10.1073/pnas.94.18.9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun S, Elwood J, Greene W C. Both amino- and carboxyl-terminal sequences within IκBα regulate its inducible degradation. Mol Cell Biol. 1996;16:1058–1065. doi: 10.1128/mcb.16.3.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takeda K, Takeuchi O, Tsujimura T, Itami S, Adachi O, Kawai T, Sanjo H, Yoshikawa K, Terada N, Akira S. Limb and skin abnormalities in mice lacking IKKα. Science. 1999;284:313–316. doi: 10.1126/science.284.5412.313. [DOI] [PubMed] [Google Scholar]
- 52.Tanaka M, Fuentes M E, Yamaguchi K, Durnin M H, Dalrymple S A, Hardy K L, Goeddel D V. Embryonic lethality, liver degeneration and impaired NF-κB activation in IKK-beta-deficient mice. Immunity. 1999;10:421–429. doi: 10.1016/s1074-7613(00)80042-4. [DOI] [PubMed] [Google Scholar]
- 53.Thanos D, Maniatis T. NF-κB: a lesson in family values. Cell. 1995;80:529–532. doi: 10.1016/0092-8674(95)90506-5. [DOI] [PubMed] [Google Scholar]
- 54.Ting A T, Pimentel M F, Seed B. RIP mediates tumor necrosis factor receptor 1 activation of NF-κB but not Fas/APO-1-initiated apoptosis. EMBO J. 1996;15:6189–6196. [PMC free article] [PubMed] [Google Scholar]
- 55.Traenckner E B, Pahl H L, Henkel T, Schmidt K N, Wilk S, Baeuerle P A. Phosphorylation of human IκB-α on serines 32 and 36 controls IκB-α proteolysis and NF-κB activation in response to diverse stimuli. EMBO J. 1995;14:2876–2883. doi: 10.1002/j.1460-2075.1995.tb07287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Uhlik M, Good L, Xiao G, Harhaj E W, Zandi E, Karin M, Sun S C. NF-κB-inducing kinase and IκB kinase participate in human T-cell leukemia virus I Tax-mediated NF-κB activation. J Biol Chem. 1998;273:21132–21136. doi: 10.1074/jbc.273.33.21132. [DOI] [PubMed] [Google Scholar]
- 57.Verma I M, Stevenson J K, Schwarz E M, Van A D, Miyamoto S. Rel/NF-κB/IκB family: intimate tales of association and dissociation. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 58.Woronicz J D, Gao X, Cao Z, Rothe M, Goeddel D V. IκB kinase-β: NF-κB activation and complex formation with IκB kinase-α and NIK. Science. 1997;278:866–869. doi: 10.1126/science.278.5339.866. [DOI] [PubMed] [Google Scholar]
- 59.Yamaoka S, Courtois G, Bessia C, Whiteside S T, Weil R, Agou F, Kirk H E, Kay R J, Israel A. Complementation cloning of NEMO, a component of the IκB kinase complex essential for NF-κB activation. Cell. 1998;93:1231–1240. doi: 10.1016/s0092-8674(00)81466-x. [DOI] [PubMed] [Google Scholar]
- 60.Yang R B, Mark M R, Gray A, Huang A, Xie M H, Zhang M, Goddard A, Wood W I, Gurney A L, Godowski P J. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature. 1998;395:284–288. doi: 10.1038/26239. [DOI] [PubMed] [Google Scholar]
- 61.Yin M J, Christerson L B, Yamamoto Y, Kwak Y T, Xu S, Mercurio F, Barbosa M, Cobb M H, Gaynor R B. HTLV-1 Tax protein binds to MEKK1 to stimulate IκB kinase activity and NF-κB activation. Cell. 1998;93:875–884. doi: 10.1016/s0092-8674(00)81447-6. [DOI] [PubMed] [Google Scholar]
- 62.Zandi E, Chen Y, Karin M. Direct phosphorylation of IκB by IKKα and IKKβ: discrimination between free and NF-κB-bound substrate. Science. 1998;281:1360–1363. doi: 10.1126/science.281.5381.1360. [DOI] [PubMed] [Google Scholar]
- 63.Zandi E, Karin M. Bridging the gap: composition, regulation and physiological function of the IκB kinase complex. Mol Cell Biol. 1999;19:4547–4551. doi: 10.1128/mcb.19.7.4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zandi E, Rothwarf D M, Delhase M, Hayakawa M, Karin M. The IκB kinase complex (IKK) contains two kinase subunits, IKKα and IKKβ, necessary for IκB phosphorylation and NF-κB activation. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]