Abstract
From a morphological point of view, placozoans are among the most simple free-living animals. This enigmatic phylum is critical for our understanding of the evolution of animals and their cell types. Their millimeter-sized, disc-like bodies consist of only three cell layers that are shaped by roughly seven major cell types. Placozoans lack muscle cells and neurons but are able to move using their ciliated lower surface and take up food in a highly coordinated manner. Intriguingly, the genome of Trichoplax adhaerens, the founding member of the enigmatic phylum, has disclosed a surprising level of genetic complexity. Moreover, recent molecular and functional investigations have uncovered a much larger, so-far hidden cell-type diversity. Here, we have extended the microanatomical characterization of a recently described placozoan species—Hoilungia hongkongensis. In H. hongkongensis, we recognized the established canonical three-layered placozoan body plan but also came across several morphologically distinct and potentially novel cell types, among them novel gland cells and “shiny spheres”-bearing cells at the upper epithelium. Thus, the diversity of cell types in placozoans is indeed higher than anticipated.
Keywords: Cell types, Morphology, Physiology, Functional anatomy, Hoilungia hongkongensis, Trichoplax adhaerens, Placozoa, Signaling
Introduction
Placozoans are millimeter-sized disc-shaped marine animals without organs, muscles, or neurons. In the last decades, alongside other aquatic invertebrates, placozoans have become important model organisms to understand the origins and evolution of animal cell types, including the rise of neuronal communication (Varoqueaux and Fasshauer 2017; Moroz 2018; Sebe-Pedros et al. 2018; Arendt 2020).
The first placozoan, Trichoplax adhaerens—an “adhering hairy plate”—was discovered in a seawater aquarium in Graz, Austria, towards the end of the nineteenth century (Schulze 1883) (for a brief history of the discovery of T. adhaerens see Syed and Schierwater (2002)). In a very careful histological study, Franz Eilhard Schulze observed under the light microscope that the flat animal had a highly changeable shape and lacked symmetry (Schulze 1891) and that only top vs. bottom and marginal vs. interior can be distinguished. As he could not place the animal in any known animal phyla, he tentatively suggested that T. adhaerens could be a very early-branching metazoan.
Schulze found that the animal is moving with the help of cilia at its lower surface. He also described that the animal propagates by fission. The flat body of T. adhaerens is organized into just three thin cell layers, an upper (water-facing) squamous epithelium and a lower (surface-contacting) columnar epithelium; in-between the two ciliated epithelia, he observed a loose layer of possibly contractile spindle-or star-like cells. Among other things, he reported that the upper side contains large refractive inclusions, so-called “shiny spheres” (“Glanzkugeln”), while he saw smaller “matte-finished spheres” (“matt-glänzende Kugeln”) embedded in the lower epithelium, where the animal takes up its food. Note that the animal’s upper and lower sides have been described as dorsal and ventral in earlier publications. To prevent a currently unsupported homologization with the bilaterian dorsal/ventral axis, we refrain from using these terms when referring to the top–bottom axis of placozoans.
It was only in the early 1970s that Karl Grell and others started providing the first detailed electron microscopic characterization of T. adhaerens (Grell and Benwitz 1971, 1974; Rassat and Ruthmann 1979; Ruthmann et al. 1986; Thiemann and Ruthmann 1990; Grell and Ruthmann 1991; Buchholz and Ruthmann 1995). They confirmed Schulze’s earlier results that T. adhaerens is composed of three thin cell layers, and identified four different cell types: the flat and T-shaped upper epithelial cells (i), which have a single cilium; embedded in the upper surface, they reported large granules, corresponding to the “shiny spheres.” The cylindrical cells of the lower epithelium (ii) were found to bear one cilium surrounded by microvilli-like structures, later on identified as “fenestrated ledges and folds” (Grell and Benwitz 1981). Between these lower epithelial cells, sporadic cilia-free “gland cells” (iii) were described. Note that “gland cells” since turned as a generic term used to refer to lower epithelial cells that appear to contain a large number of secretory vesicles, independently of the presence/absence of a cilium. In the epithelial interspace, they observed elongated fiber cells (iv), which form a syncytium-like network and can contact other cell types (Grell and Benwitz 1974; Buchholz and Ruthmann 1995).
Grell also reported that the animal grazes on algae. For this, it moves over the algae, releases digestive enzymes, and takes up the predigested food at the lower epithelium. Upon this careful microanatomical analysis, Grell placed T. adhaerens into its own phylum, called Placozoa (Grell 1971).
In 2014, the morphology of T. adhaerens was revisited upon preservation in a living-like state using high-pressure freezing of the live animal combined with electron microscopy (Smith et al. 2014). Two novel cell types were described: lipophil cells (v) and crystal cells (vi). Lipophil cells are sitting in the lower epithelium and extend deep into the interior. They often possess a large vacuolar structure at their distal end. It is likely that these distal vacuole-like structures correspond to the matte-finished spheres described by Schulze (Schulze 1891). These cells are fragile and probably had not been preserved during chemical fixations carried out by Grell and others. Crystal cells contain birefringent crystals that have been reported earlier (Pearse et al. 1994). Those crystals are made of aragonite (Mayorova et al. 2018), which are thought to serve as gravity sensors and contribute to geotaxis. In an earlier EM study, small ovoidal cells (vii) have been observed in the marginal zone, where the two epithelia meet (Guidi et al. 2011).
A recent single-cell transcriptome study has revealed a hidden cell type diversity in Trichoplax (Sebe-Pedros et al. 2018). This study did not identify the distinct expression profile of a putative muscle or neuronal cell, corroborating the morphological notion that placozoans lack such specialized cell types. However, profiles of specialized cells involved in digestion and metabolism and several cells expressing hormones and peptides were readily identified (Sebe-Pedros et al. 2018). The much larger cell diversity was corroborated by studies that investigated the function of the surprisingly diverse repertoire of small peptide hormones in T. adhaerens. Different, non-overlapping populations of peptide-releasing cells exist in fixed locations in all three layers of the animal (Senatore et al. 2017; Varoqueaux et al. 2018). In addition, another study of Mayorova and coworkers revealed the presence of additional cell types in T. adhaerens, including mucus-releasing cells, and distinguishing two types of ciliated gland cells in the lower epithelium (Mayorova et al. 2019).
The type specimens of T. adhaerens originated from the Mediterranean Sea. For their early ultrastructural analyses, Grell and colleagues used a placozoan isolate from the Red Sea (near Elat in Israel), and assigned it to T. adhaerens due to gross morphological similarity to the description by Schulze (1883). Grell’s Red Sea clonal lineage (in the literature referred to the ‘Grell strain’) was used in the majority of studies addressing placozoan ultrastructure, molecular function, and general biology in the last decades. In an initial molecular genetic diversity survey, Voigt et al. (2004) assigned this clone the mitochondrial 16S haplotype H1. Due to the lack of genetic material from the type specimen, the Grell strain is now referred to as Trichoplax adhaerens (H1) sensu Voigt et al. (2004). Broad biodiversity follow-up surveys performed during the last decade identified dozens of 16S haplotypes (Voigt et al. 2004; Signorovitch et al. 2006; Ball and Miller 2010; Eitel and Schierwater 2010; Miyazawa et al. 2012, 2020; Eitel et al. 2013; Kamm et al. 2018). The ecological niche occupied by placozoans covers the sublittoral zone of tropical, subtropical, and some temperate waters from 0.5 to 20 m deep and potentially contains more than one hundred species (Schierwater and DeSalle 2018).
Subsequent comparative genomics analyses identified two additional placozoan genera: Hoilungia (Eitel et al. 2018) and Polyplacotoma (Osigus et al. 2019). The nuclear genomes of both T. adhaerens (Srivastava et al. 2008) and H. hongkongensis have been sequenced (Eitel et al. 2018), confirming clearly distinct molecular features for each genus.
The overall morphology of H. hongkongensis was found to be very similar to that of T. adhaerens (Eitel et al. 2018), including the presence of the six major cell types (Smith et al. 2014). A morphological study comparing different haplotypes of Placozoa (H1, H2, H5, H8, and H16 from different geographical origins (Guidi et al. 2011)) also suggests that the basic bodyplan is conserved in placozoans, while subtle differences might exist between isolates.
In the present study, we used both transmission and scanning electron microscopy to characterize the cellular architecture in H. hongkongensis (Eitel et al. 2018) in more detail. While we did recognize the seven basic cell types described both in T. adhaerens (Smith et al. 2014) and H. hongkongensis (Eitel et al. 2018), we revealed additional populations of cells with distinctive morphological and positional features. These data corroborate the notion that the cellular diversity in placozoans is higher than anticipated.
Material and methods
Culture
We worked with H. hongkongensis (mitochondrial 16S haplotype H13) (Eitel et al. 2018). Animals were cultured at 24 ± 4 °C in 9-cm-diameter Petri dishes containing artificial seawater (ASW; 35 ppm) with rice grains as food source (Heyland et al. 2014). For long-term maintenance, ASW was refreshed every 7 to 10 days (pH 8.0).
Scanning electron microscopy
To achieve fast fixation and preserve morphological features of placozoans in SEM, we used a protocol described elsewhere (Romanova 2019; Starunov 2019). Briefly, 1 day before fixation, animals were transferred in a sterile Petri dish without food. Subsequently, we moved animals into 2-mL Eppendorf tubes with minimum ASW and added 2.5% glutaraldehyde (in 35 ppm ASW) for 60 min at room temperature. Next, the fixative was removed, and individuals were washed three times for 15 min in a 0.3 M NaCl and 0.05 M sodium cacodylate solution. This solution was then gently removed, and we performed a secondary osmium fixation (0.3 M NaCl, 0.05 M cacodylate sodium, 1% OsO4) for 1 h at 4 °C. This solution was gently removed at room temperature and washed in the same previous buffer. The specimens were dehydrated in acetone series (30%, 50%, 70%, 80%, 90%, 95%, 100%) with three 5-min washes. Animals were kept in 100% acetone at 4 °C, for 30 min, and we performed critical point drying (Leica EM CPD300). The specimens were coated with platinum for 10 s and examined under 15 kV using an FEI Quanta 250 scanning electron microscope (FEI, The Netherlands) at the Zoological Institute of Russian Academy of Science (Saint-Petersburg, Russia). In some cases, animals were broken with tungsten needles before platinum coating to reveal the interior structures. About 100 animals were used altogether.
Transmission electron microscopy
Transmission electron microscopy (TEM) was performed exactly as described elsewhere on H. hongkongensis (H13) (Eitel et al. 2018) and T. adhaerens (H1) (Smith et al. 2014). Briefly, animals were rapidly dipped in 20% BSA in ASW and placed in 0.1-mm-deep aluminum planchettes for high-pressure freezing (Wohlwend HPF Compact 02, Switzerland). Samples were processed by freeze-substitution (Leica AFS) for fixation (involving successive treatments in acetone with 0.1% tannic acid and 2% Osmium tetroxide) and Epon embedding. Polymerized samples were cut in 70-nm ultrathin sections collected on coated copper single-slot grids, which were then further contrasted with uranyl acetate and lead citrate and observed in a Philips CM100 transmission electron microscope (see details in (Smith et al. 2014; Eitel et al. 2018).
Light microscopy-differential interference contrast microscopy
Light microscopic observations were made with a Nikon Ti2 fluorescent microscope equipped with a spinning disk and DIC optics.
Results
Gross morphology
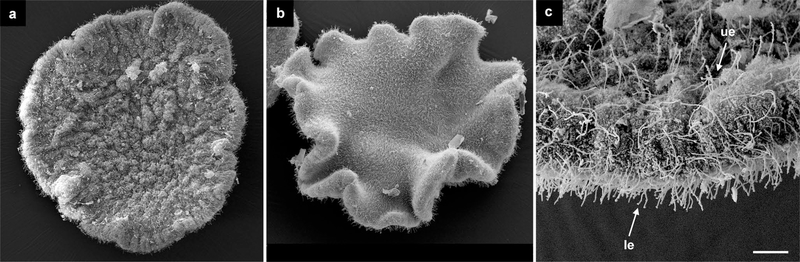
Using scanning electron microscopy (SEM), we first examined the different surfaces of the placozoan H. hongkongensis. The lower epithelium (Fig. 1b) is the locomotory surface, and its high cell density results in a more densely ciliated surface as compared to the upper side of the animal (Fig. 1a), as described for T. adhaerens (Rassat and Ruthmann 1979). The shapes of cells on the upper epithelium are readily visible at high magnification, while on the lower surface, the high density of cilia largely masks the cells’ boundaries (Fig. 1c).
Fig. 1.
Morphology of H. hongkongensis (H13), viewed from the upper (a) and lower (b) sides. Note that the animals were fixed, while they were not adhering to a substrate, thereby showing great variability in body shapes, as they can form invaginations, ripples, curls, and can variably stretch and contract. c Ciliated epithelia in H. hongkongensis (H13). The lower surface (le) is more densely ciliated than the upper surface (ue). Scale bar 50 μm in a, b; 8 μm in c
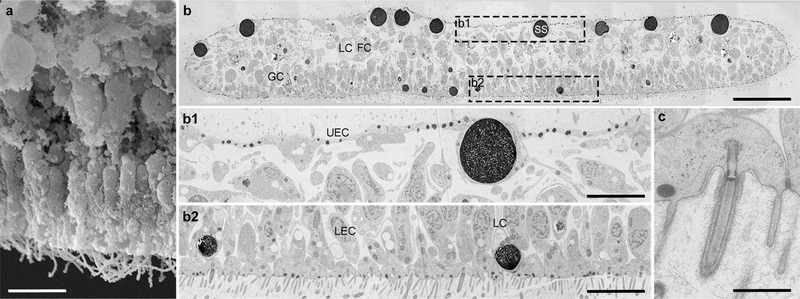
The gross morphology of the placozoan H. hongkongensis had been shown to be similar to that of T. adhaerens using transmission electron microscopy (TEM) (Eitel et al. 2018). When we inspected freeze-fractured animals by SEM, we were able to view the internal organization of the animal (Fig. 2a). While the lower epithelial layer is thick—as it is composed mainly of densely packed columnar cells of different heights—the middle layer is overall loose and comprises very large cells with extensions. Most cells in the upper epithelium form a flat, thin surface, but other cell types can be observed as well, which will be described below.
Fig. 2.
Gross anatomy of H. hongkongensis (H13). (a) A SEM micrograph from the animal’s inside reveals, from top to bottom, the flat cells of the upper epithelium, the bulky cells of the middle layer, and the numerous, mostly elongated cells of the lower epithelium. Note that micro-cavities were often observed between groups of densely packed cells of the middle and lower layers. (b) At the TEM, a full cross-section micrograph of a representative animal (approximately 250 μm wide and 30 μm thick) shows the three-layer bodyplan and upper and lower epithelia merging at the edges. Framed regions of the upper and lower epithelia are shown at higher magnification in (b1, b2) and readily reflect cell diversity. In b1, the shiny sphere-containing cell shows an elongated process toward the middle layer and protrudes between two canonical flat epithelial cells. In b2, the vast majority of columnar cells are ciliated. In contrast, lipophil cells are not ciliated, and their outermost granule (“matte-finished sphere”) abbuting the surface. As illustrated in (c) at the level of the lower epithelium, all cells are joined by adherens junctions, and most bear a cilium and microvilli-like structure (see text for details). Note the ciliary root and the ciliary pit, which can be more or less deep in different cell types, as well as some unidentified glycocalyx-like material outside the membrane. FC fiber cell, GC gland cell, LC lipophil cell, LEC lower epithelial cell, UEC upper epithelial cell, SS shiny sphere. Scale bar 6 μm in a; 18 μm in b; 5 μm in b1, b2; 1 μm in c
We then extended the analysis by imaging at the TEM entire cross-sections of H. hongkongensis (Fig. 2b). These images corroborate a three-layer bodyplan, with two epithelial layers that encompass a more loose middle layer. Also, easily recognized are large osmiophilic vacuole-like structures corresponding to Schulze’s shiny spheres (3–5 μm), on the entire upper side of the animal (Fig. 2b, b1). They belong to a population of cells that has not been characterized yet (see below). In turn, somewhat smaller osmiophilic vacuole-like inclusions (2–3 μm), corresponding to Schulze’s matte-finished spheres, are distributed in the medial part of the lower epithelium, and are close to the lower surface of the animal (Fig. 2b, b2). They have readily been identified as the most distal vacuole of lipophil cells (Smith et al. 2014). At the TEM, both shiny and matte-finished spheres are lipoid in nature and show varying degrees of affinity to osmium (Figs. 2; 3; 6), possibly depending on the preparation procedures or physiological state of the animal. Whether their content—which is not known so far—is secreted by the animal and whether the two cell types producing these structures are related are unclear.
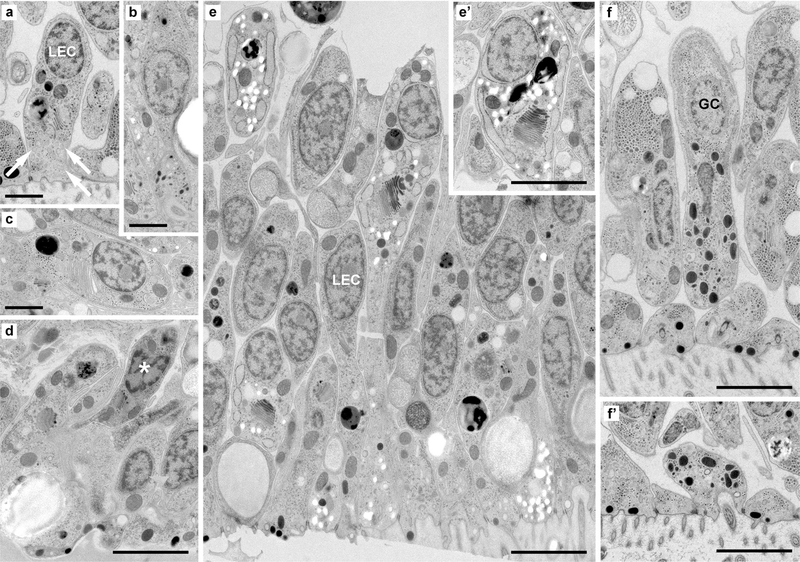
Fig. 3.
Cell diversity in the lower epithelium of H. hongkongensis (H13). (a) Classical lower epithelial cells are columnar and polarized. They often bear clusters of greyish vesicles, possibly reflecting pinocytic events (arrows). (b) Small polarized, ciliated cells present clusters of small dark vesicles near the lower surface of the animal; they likely correspond to an undescribed population of secretory cells. Cells with likely different content (c) or with a dark appearance (d, asterisk) are regularly spotted among the cells of the lower epithelium; they may not reach the lower surface of the animal. (e, e’) In some areas of the epithelium, among lower epithelial cells and lipophil cells, a population of polarized cells contain clusters of very clear vesicles close to the Golgi apparatus or reaching the surface; they may correspond to mucus-secreting cells. (f, f’) Typical gland cells—ciliated, polarized secretory-like cells with large dense vesicles—are present at the inner rim (i.e. at the periphery but not directly at the edge) of the lower epithelium. GC gland cell, LEC lower epithelial cell, Scale bar 3 μm
Fig. 6.
Cell diversity in the upper epithelium of H. hongkongensis (H13). a At the SEM, contours of cells composing the upper epithelium can easily be made out. Most of the cells are ciliated. A globular protrusion-like structure can be seen (arrow), which probably corresponds to a shiny sphere abutting the epithelial surface. b, c Cells from the upper epithelium are essentially bound to other cells at their basal pole (toward the upper surface) by adherens junctions, and the main cell body (including the nucleus) is sunk toward the inside of the animal. They may receive local and maybe transient contact from other cells, such as fiber cells. Canonical upper epithelial cells (“1”) are elongated T-shaped ciliated epithelial cells with pigment granules. They have a large flattened surface area, are not ramified, and their nucleus occupies most of the cytoplasm. Towards the upper surface, they contain electron-dense inclusions. c A variety of other cell types can be identified in the upper epithelium and directly underneath. Some cells (“2”), “sphere cells,” have a large inclusion that might correspond to a shiny sphere; they have no pigment granules, a small nucleus, and a few processes; some cells (“3”) contain small vesicles, reach the surface via a narrow neck, and have no cilium (see Fig. S3 for more details); right underneath the cells forming the surface, populations of small putative secretory cells with numerous small dense vesicles (“4”) or small clear vesicles (“5”) are observed; small rounded cells are seen in pairs (“6”) that may have recently duplicated; other cells, reminiscent of macrophages with an atypical nucleus and numerous ramifications are also observed (“7”). Processes of fiber cells are omnipresent. Scale bar 5 μm in a; 4 μm in b; 3 μm in c
Of note, one can observe at higher magnification that adherens junctions link all cells of the epithelia. The vast majority of these cells possess a single cilium and microvilli-like elements (Fig. 2c); Eitel et al. 2018), which was similar to a system of folds from the columnar cells in T. adhaerens (Grell and Benwitz 1971, 1981), and best described as a “spongy meshwork of fenestrated folds” apparently distinct from microvilli (see details in (Grell and Ruthmann 1991; Smith et al. 2014; Smith and Reese 2016)). In H. hongkongensis, a pronounced glycocalyx-like structure can be seen on the surface of the lower epithelium (Fig. 2c).
Lower epithelium
In H. hongkongensis, as in T. adhaerens, the lower epithelium is mostly composed of columnar lower epithelial cells and lipophil cells (Figs. 2b2, 3e; Eitel et al. 2018). Both cells play roles in external digestion and uptake of nutrients at the basal surface. This process is probably coordinated by different types of gland cells embedded in the lower epithelium (Grell and Ruthmann 1991; Smith et al. 2014; Senatore et al. 2017; Varoqueaux et al. 2018; Mayorova et al. 2019).
Lower epithelial cells are highly polarized in H. hongkongensis and often contain numerous pinocytic-like vesicles at their basal pole (Fig. 3a, e). Lipophil cells—the second most abundant cell type—have somata larger and taller than those of epithelial cells and reach deep into the middle cell layer (Figs. 2a, 3e, 4b, c, 5a). At the SEM, we noted that lipophil cells, together with other cells, delineated numerous microcavities (areas without cells or their processes), which were located in the middle layer between different cell types (Fig. 2a). These microcavities can be seen in SEM micrographs of T. adhaerens as well (Smith et al. 2014), whether they have a role remains elusive, however.
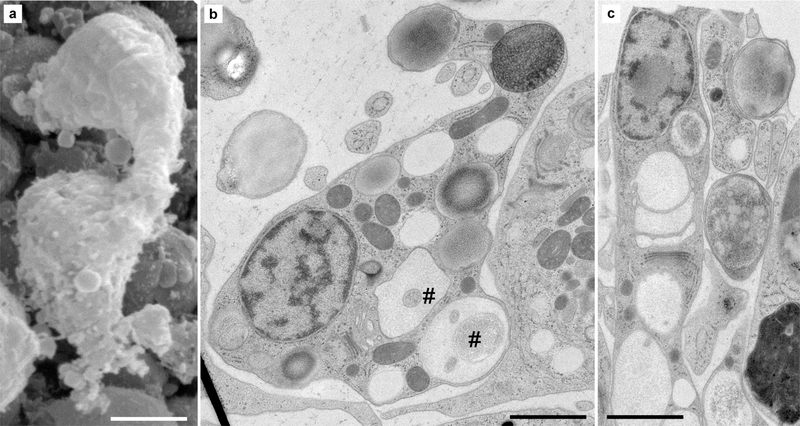
Fig. 4.
Ultrastructure of lipophil cells in H. hongkongensis (H13). a At the SEM, lipophil cells are at times seen bulging from the lower epithelium into the fiber cell layer, often in close vicinity to fiber cells. Their shapes are usually elongated (in the lower epithelium, where they are interspersed between lower epithelial cells (c) or more complex depending on their cellular environment (b) and position in the animal. In all cases, they exhibit large, heterogeneous vacuoles— rather clear for those close to the trans-Golgi network, otherwise with various electron densities (i.e., affinity to osmium) or structural complexity, as some of the vacuoles contain membranous material (#). Their most basal vacuole (matte-finished sphere—asterisk), abutting the lower surface, is often electron-dense but sometimes also clear. Scale bar 2 μm in a; 1.5 μm in b, c
Fig. 5.
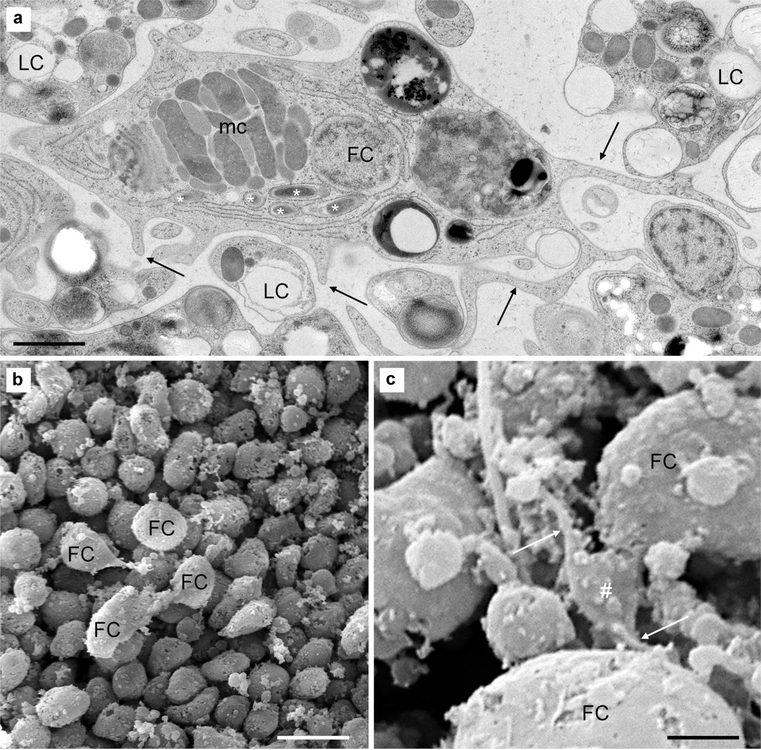
Ultrastructure of cells in the middle layer of H. hongkongensis (H13). a Each fiber cell (FC) has a bulky soma and numerous thin processes (arrows); complex, heterogenous vacuoles in varying numbers; a single large mitochondrial complex (mc); and bacteria inside the endoplasmic reticulum (asterisks). b On this SEM micrograph, a group of four fiber cells (FC) lie on top of cells of the lower epithelium; fiber cells are interconnected, and their processes reach deep between epithelial cells. c Unknown small cell (#) with processes (arrowheads) were observed in the close vicinity of fiber cells (FC). Scale bar 1.5 μm in a; 5 μm in b; 1.5 μm in c
Several other cell types were observed scattered at low density across the lower epithelium in H. hongkongensis (Fig. 3b–f) and reaching the basal surface. Ciliated cells with middle-sized, irregularly shaped, dark granules (Fig. 3f) likely correspond to the gland cells described in T. adhaerens (Smith et al. 2014). In H. hongkongensis, these vesicles are smaller and more irregular, so they might correspond to different cell types.
Apparently, in H. hongkongensis, one type of gland cells is found mostly in the marginal area of the animal, while another type is found throughout the lower epithelium, as in T. adhaerens (Mayorova et al. 2019). Other ciliated cells contained tiny dark granules (Fig. 3b). We also came across long, highly polarized cells with a large Golgi and numerous clear granules accumulating at their basal pole, microvilli, and no cilium (Fig. 3e, e’). These cells might be similar to mucin-releasing cells recently described in T. adhaerens (Mayorova et al. 2019) and/or the gland cells without cilium described by Grell and others (summarized in Grell and Ruthmann 1991). Several additional cells, which can probably be regarded as other cell types, were found embedded in the lower epithelium (and no apparent contact to the outside), such as cells whose cytoplasm is enriched in granular material (Fig. 3c) and cells with a very electron-dense cytoplasm (Fig. 3d).
Of note, we observed on the water-facing side of the lower epithelium, regularly spaced small pores/recesses (approximately 1 μm in diameter; Fig. S1, supplement). These might be sites of the release of different secretory components from lipophil (e.g., be the point where the large vacuoles of lipophil cells lie), mucin-releasing, or other secretory cells.
Lipophil cells received their name from their numerous, large, lipid-rich vacuolar structures (Smith et al. 2014). As illustrated in Fig. 4, lipophil cells are sturdy (a, b), elongated (c), or grossly ramified. In fact, we found that lipophil cells are highly variable in size and shape, and it is conceivable that different stages or even subtypes of lipophil cells exist. Also, their shape may depend on the animal shape/local environment/cellular configuration as lipophil cells are often observed near fiber cells in the middle layer (Figs. 4b, 5a).
Middle layer
In T. adhaerens, the middle layer of the animal is quite loosely packed and consists mostly of fiber cells. Fiber cells are very large cells with uniquely branched cellular extensions and processes (Grell and Benwitz 1974). Their ramification pattern suggests that they contribute to the connectivity of all layers, forming a potential network supporting and capable of coordinated reactions and, perhaps, behavioral integration in general (Grell and Ruthmann 1991; Smith et al. 2014).
In H. hongkongensis, fiber cells were also easily identified in the middle layer, with their distinct shape and subcellular features (Fig. 5a, b). They are large (the soma is 5–15 μm wide), elongated, and asymmetric cells with numerous processes mingling with lipophil cells and reaching laterally and deep in the upper and lower epithelia (Figs. 2b, 5a). They possess a large nucleus; a characteristic mitochondrial complex (a bulky cluster of alternating layers of mitochondria and vacuoles with unknown function); large vacuoles with complex, heterogeneous content (named concrement vacuoles in T. adhaerens (Grell and Ruthmann 1991); and a well-developed endoplasmic reticulum (Fig. 5a) in which bacteria are present. Note that intracellular bacteria in the ER of fiber cells have been described in two Trichoplax species (see Grell and Benwitz 1991; Gruber-Vodicka et al. 2019).
Occasionally, smaller cells were observed in the vicinity of fiber cells. They had a star-like shape with 1–3 thin, long processes (less than 1 μm in diameter and 10 to 50 μm in length; Fig. 5c). Such cells have so far not been reported in placozoans, and this issue should be validated by future studies.
Upper layer
By SEM, the outer upper surface of the animal appeared as a mosaic of large polygonal cells bearing a single cilium, scattered with dome-shaped elements (Fig. 6a). The large contours correspond to upper epithelial cells (Fig. 6b): ciliated, T-shaped cells known to form a thin epithelium, described for T. adhaerens (Grell and Ruthmann 1991; Guidi et al. 2011; Smith et al. 2014), and observed here in H. hongkongensis (Fig. 6b, c). They were connected with each other by adherens junctions and contained numerous dark granules close to the surface as had been reported in T. adhaerens as well (Grell and Ruthmann 1991; Smith et al. 2014).
In T. adhaerens, shiny spheres in the upper epithelium have been studied early on using electron microscopy and often described as extracellular inclusions (Grell and Benwitz 1971; Grell and Ruthmann 1991; Guidi et al. 2011), but were not observed in all studies (Smith et al. 2014). In our specimens, shiny spheres were frequently encountered. In contrast to earlier EM studies that applied conventional fixation methods, using freeze-substitution, we found that shiny spheres correspond to large vacuoles belonging to a specific population of cells. These sphere-bearing cells differ from regular upper epithelial cells as they did not have a large outer surface and sent a few ramifications toward the inner cavity (Fig. S2, supplement). The nucleus of these cells appears almost to be pressed towards the plasma membrane by the large shiny sphere as been described by Tadäus von Garbowski (1903) at the beginning of the twentieth century and, in more detail, in a recent study (Zuccolotto-Arellano and Cuervo-González 2020).
The dome-shaped structures seen by SEM at the animal’s surface (Fig. 6a) may correspond to the shiny spheres abutting the surface (Fig. 2b and b1); Fig. S2, supplement). The position of a shiny sphere within what we term “sphere cells” (close to the outer surface or deeper inside) as well as the sphere’s density may correspond to different maturation states of the cell, to different physiological states of the animal, or to subpopulations of sphere cells.
Within the upper epithelium (Fig. 6c), a few cells bearing clusters of small dense granules reached the surface via a narrow neck. They may represent a specific population of secretory cells. Directly underneath the epithelium, several cell populations with distinct morphological features were observed, which have been identified as fiber cells in a previous study (Guidi et al. 2011). Our data on H. hongkongensis, however, does not show any ultrastructural characteristics of fiber cells, such as a mitochondrial complex, a concrement vacuole, or bacterial cells, in these new cell types. In contrast to the lower epithelium, cells were loosely packed in the upper epithelium, and a clear border to the fiber cell layer could not be recognized. Yet many elongated processes were observed, suggesting that cells in this area, while not tightly opposed, may interact with each other and/or with fiber cells. No specialized subcellular features were observed at these contact points that could reflect a specialized form of local (e.g., chemical or electrical) communication.
These newly identified cell types varied in size, shape, and cellular content. Putative secretory cells were singled-out, which contain clusters of clear vs. electron-dense vesicles of different sizes, suggesting different contents. In each cell type, granules’ population was homogenous. Often, cells with similar subcellular characteristics were close to each other, suggesting that the cells divided and likely differentiated on the spot both across the entire animal and in the marginal zone. It is noteworthy in this respect that in T. adhaerens, lower epithelial cells were shown to split by mitosis (Grell and Benwitz 1981). It is thus likely, in both T. adhaerens and H. hongkongensis, that also other cell types can proliferate by mitosis and give rise to their own cell type outside of the presumed marginal proliferation zone, the location of claimed pluripotent stem cells (Jakob et al. 2004).
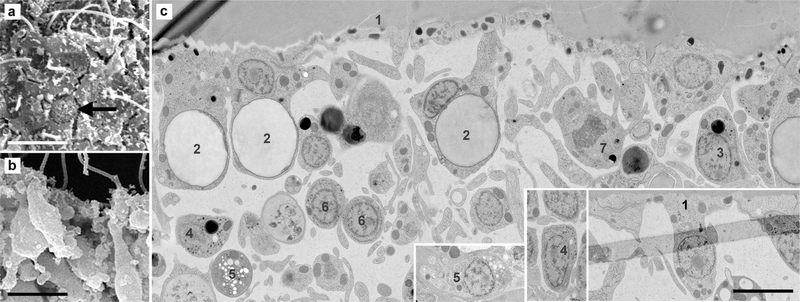
On the upper edge of the central part of the animal disc, just beside the marginal zone, crystal cells were identified in T. adhaerens (Smith et al. 2014). These spherical cells contain a central aragonite crystal and have a flattened, laterally located nucleus (Mayorova et al. 2018). They are thought to function as gravity sensors (Mayorova et al. 2018). As in other placozoans, crystal cells were located under the upper epithelium in H. hongkongensis. They did not have any noticeable direct contact with the external environment (Fig. 7a). Their birefringent crystal can be readily observed using DIC microscopy (Fig. 7b). In H. hongkongensis, crystal cells were quite scarce and usually spread within a concentric band along the marginal zone.
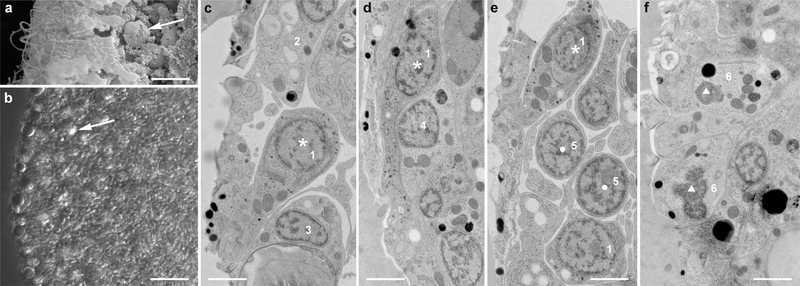
Fig. 7.
Cell diversity in the marginal zone of H. hongkongensis (H13). a Crystal cells (arrow) were rather scarce and located at the marginal edge of animals under the upper epithelium, sometimes close to the rim. b Observation of a live specimen under DIC illumination illustrates the numerous cilia visible at the edge, shiny spheres of the upper epithelium reaching to the periphery, and a bit toward the central part of the animal disc, the typical birefringent crystals of crystal cells (arrow). c–e At the TEM, cells with a dark cytoplasm, numerous irregularly shaped dark granules, a long neck reaching the surface, and a cilium were frequently observed (asterisk). e some round cells with little cytoplasm might correspond to cells that have recently split (dot). f Atypical cells with a poorly defined nucleus, numerous mitochondria, and large dense-core vesicles are also observed (triangle). At least 6 different morphologically distinct cell types (numbered 1–6) can be distinguished in this figure. Scale bar 6 μm in a; 16 μm in b; 1.5 μm in c–f
Marginal zone
As mentioned earlier, the lower epithelium accommodates at its rim concentric rings of gland cells and mucocytes (Mayorova et al. 2019). This zone was also found to contain different populations of peptidergic cells (Senatore et al. 2017; Varoqueaux et al. 2018), suggesting that different gland cells that secrete different signaling molecules exist.
In the marginal zone, which one can define as the outermost rim of the animal and the area where upper and lower epithelia meet (Fig. 2b), small ovoid-like cells were described in T. adhaerens (Guidi et al. 2011). This scantily described area has been presented as a putative proliferative zone (Schwartz 1984) and is thought to contain freshly dividing cells (Schwartz 1984; Jakob et al. 2004).
In H. hongkongensis, we observed in the marginal zone elongated cells with a narrow neck and opening to the surface and a cilium and no microvilli, which contained very small dark granules (Fig. 7c–e). In contrast to marginal mucocytes that were shown to contain clear vesicles (Mayorova et al. 2019), so they likely belong to yet another population of sensory or secretory cells. Small-size cells with a round nucleus and little cytoplasm were frequently observed inwards, near these cells (Fig. 7d). They did not seem to reach the surface. They might correspond to the marginal zone cell population described by Guidi et al. (Guidi et al. 2011) and reflect a previously claimed proliferative zone of pluripotent stem cells (Jakob et al. 2004). Furthermore, atypical cells similar to these observed under the upper epithelial surface were present in this region (Fig. 7f).
Discussion
The placozoan T. adhaerens has a very simple shape, this of a small plate, with an upper (protective) and a lower (nutritive) epithelial layer encompassing a loose middle layer. While not feeding, it presents a constantly changing outline due to its continuous movements and body contractions. As the simple bauplan of T. adhaerens did not correspond to any of the other basic lineages of animals—sponges, cnidarians, ctenophores (commonly known as comb jellies), and bilaterians—the animal was placed into its own phylum Placozoa (Grell 1971). The exact phylogenetic position of the phylum Placozoa is still debated, although a consensus seems to emerge that views Placozoa as the sister group to the clade Cnidaria and Bilateria (Moroz et al. 2014; Whelan et al. 2017; Eitel et al. 2018; Laumer et al. 2018; Nielsen 2019). In recent years, different placozoans species have been discovered, starting to reveal the genetic diversity of the phylum (Eitel and Schierwater 2010; Eitel et al. 2011, 2013; Schierwater and DeSalle 2018; Osigus et al. 2019).
One generation ago, these simple animals were thought to have only four types of cells (Grell and Ruthmann 1991). Yet over the last years, several morphological studies have unraveled several additional major cell types (Guidi et al. 2011; Smith et al. 2014; Mayorova et al. 2019; Smith and Mayorova 2019). A recent single-cell sequencing study has indicated the existence of an even larger variety of cell states, which might support the existence of additional cell (sub)types (Sebe-Pedros et al. 2018). Indeed, it turned out that the lower epithelium, which is specialized for extracellular digestion, contains different types of gland cells and mucin-secreting cells (Smith et al. 2014; Mayorova et al. 2019; Smith and Mayorova 2019). Moreover, the complex and rapid movements, action potentials, and coordinated food uptake of the animal are controlled by integrative systems, including several different types of endocrine cells, suggesting that the animal is much more complex than previously assumed (Smith et al. 2015, 2019; Armon et al. 2018; Varoqueaux et al. 2018; Fortunato and Aktipis 2019; Romanova et al. 2020b).
Here we have taken a closer look at the morphology and cell types of H. hongkongensis (H13 haplotype from the South China Sea), which, based on comparative genomics, belongs to a different genus of placozoans but has a very similar basic bauplan as T. adhaerens (Eitel et al. 2011, 2018). We wanted to describe its morphology in more detail and also search for additional cell types in this species.
Ours and other researcher’s long-term goal is to match the cell types found by single-cell sequencing approaches (Sebe-Pedros et al. 2018) to cell types established on morphological grounds. This will serve as a basis to understand the function of the different cells and how the cells sense their surroundings and communicate with each other to serve food uptake, to trigger movements, and to control other behaviors of placozoans. Addressing this will help shed light on the intriguing question of whether the different cell types and, by extension, the three cell layers in placozoans are homologous or not to cell types and germ layers found in the other major animal lineages (Arendt et al. 2016; Moroz 2018; Sebe-Pedros et al. 2018).
It appears that several specialized cell types, regarded as the”basal building blocks of multicellular organisms,” have already emerged in placozoans. For example, it is being discussed whether the cell types in the nutritive epithelium of placozoans resemble these of the intestine (Varoqueaux and Fasshauer 2017; Kaelberer and Bohorquez 2018). Likewise, it needs to be explored whether small peptide-releasing cells discovered in placozoans are homologous to neuroendocrine or even neuron-like cells (Varoqueaux et al. 2018).
Here, we corroborated the notion that the overall bodyplan and cell types of H. hongkongensis are very similar to that of T. adhaerens (Eitel et al. 2018), suggesting that most findings apply to both species. Nevertheless, subtle morphological differences probably exist and need to be further investigated, including repeated ultrastructural analyses in different placozoan species. Our new observations provide independent morphological support for the previous assignment of the H13 strain to a new species (Hoilungia hongkongensis, Eitel et al. 2018), which was primarily established on the grounds of genomic features (Eitel et al. 2018).
One morphological difference between T. adhaerens and H. hongkongensis is the somewhat different position of crystal cells in the two species. Crystal cells are sparse and located underneath the upper epithelium in both genera. They are thought to function as gravity sensors (Mayorova et al. 2018).
Several other cell types have already been described morphologically, and functions have been ascribed to them, as we will outline below. Moreover, we found ultrastructural hints for additional cell types for which currently no functional description is available.
The lower nutritive epithelium is formed by ciliated columnar epithelial cells that drive the animals’ gliding through ciliary beating. These cells may also be involved in the uptake of nutrients (Grell and Benwitz 1971, 1981; Wenderoth 1986, 1994) after release of digestive enzymes for external digestion by the large lipophil cells (Smith et al. 2014; Mayorova et al. 2019; Smith and Mayorova 2019), which are interspersed in the epithelium. However, to what extent, the digestive system of placozoans is homologous to that of other animals is not clear yet. The cell bodies of lipophil cells reach deep into the middle layer. Eitel et al. (2018) and our study in H. hongkongensis show that the basic morphology of lipophil cells is comparable to that described in T. adhaerens. Yet, we report a remarkable heterogeneity of these cells, which vary in size, position, and shapes. A subset of lipophil cells might not reach the outer epithelial surface and, therefore, differ in their secretion sites. The functional and structural diversity of lipophil cells has not been investigated yet in T. adhaerens, and it would be interesting to identify the molecular markers for different putative subpopulations or different functional states of these cells.
In T. adhaerens as in H. hongkongensis, secretory-like cells corresponding to canonical gland cells are scattered throughout the lower epithelium (Smith et al. 2014; Eitel et al. 2018), which are possibly involved in local regulatory processes. Recently, two different populations of ciliated gland cells were found in T. adhaerens (Mayorova et al. 2019). One population was found near the edge of the animal, whereas the other was found more centrally. In addition, mucocytes without a cilium were identified (Mayorova et al. 2019), concentrated close to the edge of the animal but are also found in the central region of the lower epithelium. Mucus secretion supports adhesion of the animals (Mayorova et al. 2019). In our study, we have established that similar cell types exist in H. hongkongensis as well and have extended their morphological description. Note that these cells morphologically resemble cells of the digestive and respiratory tracts of both vertebrates and invertebrates (Specian and Oliver 1991; Birchenough et al. 2015; Knoop and Newberry 2018; Ma et al. 2018; Caccia et al. 2019; Pereira et al. 2020).
Both in T. adhaerens and H. hongkongensis, the middle layer consists mainly of fiber cells (Grell and Benwitz 1971; Behrendt and Ruthmann 1986; Buchholz and Ruthmann 1995). They are large, have long extensions, and contain a cluster of mitochondria and complex vacuoles. Fiber cells form a syncytium (Grell and Benwitz 1974; Buchholz and Ruthmann 1995) that connect and probably mediate an integration across all other cell types. Hence, fiber cells may support systemic organismal functions and complex behaviors. Occasionally in H. hongkongensis, we came across smaller star-shaped cells with thin, long processes. However, more research is needed to determine whether they are merely smaller fiber cells or constitute a different cell population.
Fiber cells of all placozoan lineages studied so far (including H. hongkongensis) possess a rickettsial endosymbiont inside the endoplasmic reticulum (Grell and Benwitz 1971; Guidi et al. 2011; Driscoll et al. 2013; Gruber-Vodicka et al. 2019; Kamm et al. 2019). Based on bacterial 16S sequencing, the bacteria observed in fiber cells of H. hongkongensis belong to a different Aquarickettsia species than the T. adhaerens endosymbiont (Klinges et al. 2019). No signs for host-specificity were identified that would imply close co-evolution of a specific bacterial species and its host. Of note, in T. adhaerens sp. (16S haplotype H2), an unrelated bacterium (Candidatus Ruthmannia eludens) was also identified in lipophil cells (Gruber-Vodicka et al. 2019). Neither our study on H. hongkongensis nor previous analyses on T. adhaerens (H1) have identified bacteria in lipophil cells. The putative absence in other placozoans, as well as the presence of versatile biosynthesis pathways (Gruber-Vodicka et al. 2019) in Ruthmannia, might, therefore, suggest a parasitic rather than a symbiotic lifestyle of this bacterial species. Future molecular studies on a range of placozoan species will help to elucidate the diversity, distribution, and function of the two placozoan-associated bacterial lineages known to date.
The upper epithelium of T. adhaerens has been described as a homogenous population of flat, ciliated cells forming a protective layer. Their cell bodies with a nucleus hang from the flat upper part, a structure that has been described as T-shape (Grell and Ruthmann 1991; Smith et al. 2014). Very similar epithelial cells form the upper epithelium in H. hongkongensis, but we found several other cell types to be present in and underneath the upper epithelium. One type of cell, described here in detail and termed “sphere cells,” contains shiny spheres, corroborating meticulous early observations (Schulze 1883). In contrast to TEM studies by Grell and colleagues, showing shiny spheres as extracellular lipid globules (Grell and Ruthmann 1991), we could confirm light microscopy observations from more than a hundred years ago in that shiny spheres are actually housed inside cells. This was also suggested for a placozoan specimen isolated recently from Mexico (Zuccolotto-Arellano and Cuervo-González 2020). Although the taxonomic affiliation of their placozoan isolate is unknown, the inclusion of shiny spheres in cells shown independently by multiple authors dealing with different samples indicates that sphere cells are a common placozoan feature. Extracellular spheres were not identified in a single circumstance in H. hongkongensis. The first schematic cross-section of a Placozoon based by Grell (1981) on TEM sections actually showed a membrane surrounding the shiny sphere, that were identified as “remnants of degenerating cells.” Based on our new observations, it is now likely that Grell and others just cut in a plane of the cells that did not include the nucleus of the sphere cell. In their study, Zuccolotto-Arellano and Cuervo-González furthermore identified two populations of sphere cells in live animals using in vivo staining protocols (Zuccolotto-Arellano and Cuervo-González 2020). Our ultrastructural analyses also indicate the presence subtypes of sphere cells.
Note that shiny spheres were not observed in T. adhaerens by Smith and colleagues (Smith et al. 2014), while they are regularly observed by other laboratories using the same clonal lineage (e.g., Jackson and Buss 2009). The presence or absence of shiny spheres within the same strain in conjunction with the presence/absence of concave discs in various placozoan strains (Guidi et al. 2011), therefore, indicates that hidden developmental and/or seasonal stages might exist in placozoans.
The role of shiny spheres remains enigmatic still. It has been suggested that these structures are part of a chemical defense mechanism, which may use paralytic substances toxic for predators of placozoans (Jackson and Buss 2009). As this lipid-rich organelle is not a non-cellular lipid inclusion as suggested earlier (Grell and Ruthmann 1991) but is produced by a distinct cell, it should be possible in the future to identify this cell type from its expression profile. Another intriguing matter is whether the cells that produce shiny spheres in the upper epithelium and lipophil cells, which generate morphologically similar but smaller matte-finished spheres at the lower surface, are related. Do both cell types extrude the content of their large vacuole-like structures into the extracellular medium, or do these prominent structures serve different functions? Some of the cells with larger vacuole-like structures were underneath the upper epithelium but did not reach the upper surface. Whether these cells might correspond to an immature form of shiny-sphere-containing cells, which would further mature and move into the upper epithelium, remains to be clarified. Our data, however, can reject the hypothesis that in H. hongkongensis shiny spheres are produced and released in the middle cell layer and then integrated into the upper epithelium as extracellular inclusions, as suggested for some other placozoans (cf. Guidi et al. 2011).
In our study, we came across putative secretory cells in the lower and the upper epithelium and other cells that are located in the marginal zone. These secretory cells could correspond to one of the several cell populations that contain different secretory signaling peptides ((neuro)peptide-like molecules). About ten different secretory cells are located in distinct regions of both epithelial and inner layers of T. adhaerens (Varoqueaux et al. 2018). Interestingly, many of these lower-frequency cell types are organized in separate concentric circles in the different layers of the animal. They may be rather difficult to tell apart based solely on their morphology, and it is possible that different secretory cells are currently lumped together. For example, different secretory-like cells containing the predicted prohormones for endomorphin (also referred to as YPFF) (Senatore et al. 2017), YYamide, and FFNPamide, and cells containing the insulin-like prohormone (Nikitin 2015) have been found in the lower epithelium, while other peptidergic cells are in the upper epithelium (SITFamide) and marginal region (SIFGamide) (Varoqueaux et al. 2018). In order to distinguish them on an ultrastructural level, specific probes are required. Nevertheless, together with recent microchemical data about the low molecular weight transmitter candidates (Moroz et al. 2020b; Romanova et al. 2020a), the repertoire of secretory cells shows that placozoans are able to release a variety of signaling molecules that probably elicit different behaviors of the animal (Moroz et al. 2021). The target cells for the different peptides and other intercellular messengers, including nitric oxide (Moroz et al. 2020a), need to be identified yet.
Besides clarifying which basic cell types and derivatives exist, where they are located in the body, and if they do belong to the basic makeup of all placozoans, a formidable challenge will be to elucidate what the precise function of each cell type is and how the different cells communicate and coordinate the behavior of the animal. Does most intercellular communication occur mostly by paracrine signaling or do many cells connect to other cells via longer extensions as it has been shown for fiber cells? The loose packing and the many cellular protrusions in the interior of the animal will require three-dimensional reconstructions to shed light on the potential connectivity of the cells and the organization of the bodyplan.
Supplementary Material
Acknowledgements
We thank E. Bedoshvili, A. Miroliubov, and V. Starunov for their help in electron microscopy, sample preparation, and advice for SEM protocols.
Funding
This work was supported by the Human Frontiers Science Program (RGP0060/2017) and National Science Foundation (1146575, 1557923, 1548121, and 1645219) grants to L.L.M., Russian Ministry of Science and High Education (agreement 075–15-2020–801) grant to D.R., and the Swiss National Science Foundation (#31003A_182732) grant to D.F. The research reported in this publication was also supported in part by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Number R01NS114491 (to L.L.M.). M. Eitel received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement no 764840.
Footnotes
Conflict of interest The authors declare no competing interests.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s00441-021-03459-y.
Disclaimer The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data availability
The data that support the findings of this study are available on https://zenodo.org.
References
- Arendt D (2020) The evolutionary assembly of neuronal machinery. Curr Biol 30(10):R603–R616 [DOI] [PubMed] [Google Scholar]
- Arendt D, Musser JM, Baker CVH, Bergman A, Cepko C, Erwin DH, Pavlicev M, Schlosser G, Widder S, Laubichler MD, Wagner GP (2016) The origin and evolution of cell types. Nat Rev Genet 17(12):744–757 [DOI] [PubMed] [Google Scholar]
- Armon S, Bull MS, Aranda-Diaz A, Prakash M (2018) Ultrafast epithelial contractions provide insights into contraction speed limits and tissue integrity. Proc Natl Acad Sci USA 115(44):E10333–E10341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball EE, Miller DJ (2010) Putting placozoans on the (phylogeographic) map. Mol Ecol 19(11):2181–2183 [DOI] [PubMed] [Google Scholar]
- Behrendt G, Ruthmann A (1986) The cytoskeleton of the fiber cells of Trichoplax adhaerens (Placozoa). Zoomorphology 106(2):123130 [Google Scholar]
- Birchenough GM, Johansson ME, Gustafsson JK, Bergstrom JH, Hansson GC (2015) New developments in goblet cell mucus secretion and function. Mucosal Immunol 8(4):712–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz K, Ruthmann A (1995) The mesenchyme-like layer of the fiber cells of Trichoplax adhaerens (Placozoa), a syncytium. Z Naturforsch C Biosci 50c:282–285 [DOI] [PubMed] [Google Scholar]
- Caccia S, Casartelli M, Tettamanti G (2019) The amazing complexity of insect midgut cells: types, peculiarities, and functions. Cell Tissue Res 377(3):505–525 [DOI] [PubMed] [Google Scholar]
- Driscoll T, Gillespie JJ, Nordberg EK, Azad AF, Sobral BW (2013) Bacterial DNA sifted from the Trichoplax adhaerens (Animalia: Placozoa) genome project reveals a putative rickettsial endosymbiont. Genome Biol Evol 5(4):621–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitel M, Francis WR, Varoqueaux F, Daraspe J, Osigus HJ, Krebs S, Vargas S, Blum H, Williams GA, Schierwater B, Worheide G (2018) Comparative genomics and the nature of placozoan species. PLoS Biol 16(7):e2005359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitel M, Guidi L, Hadrys H, Balsamo M, Schierwater B (2011) New insights into placozoan sexual reproduction and development. PLoS ONE 6(5):e19639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitel M, Osigus HJ, DeSalle R, Schierwater B (2013) Global diversity of the Placozoa. PLoS ONE 8(4):e57131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitel M, Schierwater B (2010) The phylogeography of the Placozoa suggests a taxon-rich phylum in tropical and subtropical waters. Mol Ecol 19(11):2315–2327 [DOI] [PubMed] [Google Scholar]
- Fortunato A, Aktipis A (2019) Social feeding behavior of Trichoplax adhaerens. Front Ecol Evol 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tv Garbowski (1903) Morphogenetische Studien: als Betrag zur Meth odologie zoologischer Forschung
- Grell KG (1971) Trichoplax adhaerens F.E. Schulze und die Entste-hung der Metazoen. Naturwiss Rundschau 24:160–161 [Google Scholar]
- Grell K (1981) Trichoplax adhaerens and the origin of Metazoa. In: Lincei Ad C (ed) Origine dei Grandi Phyla dei Metazoi,, eds. 1981. pp.. Accademia Nazionale dei Lincei, Convegno Intern, pp 101–127 [Google Scholar]
- Grell KG, Benwitz G (1971) Die Ultrastruktur von Trichoplax adhaerens F. E Schulze Cytobiologie 4:216–240 [Google Scholar]
- Grell KG, Benwitz G (1974) [Special connecting structures between fiber cells of Trichoplax adhaerens F. E. Schulze (author’s transl)]. Z Naturforsch C Biosci 29(11–12):790. [PubMed] [Google Scholar]
- Grell KG, Benwitz G (1981) Additional investigations on the ultrastructure of Trichoplax adhaerens F.E. Schulze (Placozoa) Zoomorphology 98(1):47–67 [Google Scholar]
- Grell KG, Ruthmann A (1991) Placozoa. In: Harrison FW (ed) Microscopic anatomy of invertebrates. Wiley-Liss, New York, pp 13–27 [Google Scholar]
- Gruber-Vodicka HR, Leisch N, Kleiner M, Hinzke T, Liebeke M, McFall-Ngai M, Hadfield MG, Dubilier N (2019) Two intracellular and cell type-specific bacterial symbionts in the placozoan Trichoplax H2. Nat Microbiol 4(9):1465–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidi L, Eitel M, Cesarini E, Schierwater B, Balsamo M (2011) Ultrastructural analyses support different morphological lineages in the phylum Placozoa Grell, 1971. J Morphol 272(3):371–378 [DOI] [PubMed] [Google Scholar]
- Heyland A, Croll R, Goodall S, Kranyak J, Wyeth R (2014) Trichoplax adhaerens, an enigmatic basal metazoan with potential. Methods Mol Biol 1128:45–61 [DOI] [PubMed] [Google Scholar]
- Jackson AM, Buss LW (2009) Shiny spheres of placozoans (Trichoplax) function in anti-predator defense. Invertebr Biol 128(3):205–212 [Google Scholar]
- Jakob W, Sagasser S, Dellaporta S, Holland P, Kuhn K, Schierwater B (2004) The Trox-2 Hox/ParaHox gene of Trichoplax (Placozoa) marks an epithelial boundary. Dev Genes Evol 214(4):170–175 [DOI] [PubMed] [Google Scholar]
- Kaelberer MM, Bohorquez DV (2018) The now and then of gut-brain signaling. Brain Res 1693(Pt B):192–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamm K, Osigus HJ, Stadler PF, DeSalle R, Schierwater B (2018) Trichoplax genomes reveal profound admixture and suggest stable wild populations without bisexual reproduction. Sci Rep 8(1):11168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamm K, Osigus HJ, Stadler PF, DeSalle R, Schierwater B (2019) Genome analyses of a placozoan rickettsial endosymbiont show a combination of mutualistic and parasitic traits. Sci Rep 9(1):17561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinges JG, Rosales SM, McMinds R, Shaver EC, Shantz AA, Peters EC, Eitel M, Worheide G, Sharp KH, Burkepile DE, Silliman BR, Vega Thurber RL (2019) Phylogenetic, genomic, and biogeographic characterization of a novel and ubiquitous marine invertebrate-associated Rickettsiales parasite, Candidatus Aquarickettsia rohweri, gen. nov., sp. nov. ISME J 13(12):2938–2953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoop KA, Newberry RD (2018) Goblet cells: multifaceted players in immunity at mucosal surfaces. Mucosal Immunol 11(6):1551–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumer CE, Gruber-Vodicka H, Hadfield MG, Pearse VB, Riesgo A, Marioni JC, Giribet G (2018) Support for a clade of Placozoa and Cnidaria in genes with minimal compositional bias. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Rubin BK, Voynow JA (2018) Mucins, mucus, and goblet cells. Chest 154(1):169–176 [DOI] [PubMed] [Google Scholar]
- Mayorova TD, Hammar K, Winters CA, Reese TS, Smith CL (2019) The ventral epithelium of Trichoplax adhaerens deploys in distinct patterns cells that secrete digestive enzymes, mucus or diverse neuropeptides. Biol Open 8(8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayorova TD, Smith CL, Hammar K, Winters CA, Pivovarova NB, Aronova MA, Leapman RD, Reese TS (2018) Cells containing aragonite crystals mediate responses to gravity in Trichoplax adhaerens (Placozoa), an animal lacking neurons and synapses. PLoS ONE 13(1):e0190905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa H, Osigus HJ, Rolfes S, Kamm K, Schierwater B, Nakano H (2020) Mitochondrial genome evolution of placozoans: gene rearrangements and repeat expansions. Genome Biol Evol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa H, Yoshida MA, Tsuneki K, Furuya H (2012) Mitochondrial genome of a Japanese placozoan. Zoolog Sci 29(4):223–228 [DOI] [PubMed] [Google Scholar]
- Moroz LL (2018) Neurosystematics and periodic system of neurons: model vs reference species at single-cell resolution. ACS Chem Neurosci 9(8):1884–1903 [DOI] [PubMed] [Google Scholar]
- Moroz LL, Kocot KM, Citarella MR, Dosung S, Norekian TP, Povolotskaya IS, Grigorenko AP, Dailey C, Berezikov E, Buckley KM, Ptitsyn A, Reshetov D, Mukherjee K, Moroz TP, Bobkova Y, Yu F, Kapitonov VV, Jurka J, Bobkov YV, Swore JJ, Girardo DO, Fodor A, Gusev F, Sanford R, Bruders R, Kittler E, Mills CE, Rast JP, Derelle R, Solovyev VV, Kondrashov FA, Swalla BJ, Sweedler JV, Rogaev EI, Halanych KM, Kohn AB (2014) The ctenophore genome and the evolutionary origins of neural systems. Nature 510(7503):109–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroz LL, Romanova DY, Kohn AB (2021) Neural versus alternative integrative systems: molecular insights into origins of neurotransmitters. Phil Trans R Soc B 376 (1821):20190762; 10.1098/rstb.2019.0762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroz LL, Romanova DY, Nikitin MA, Sohn D, Kohn AB, Neveu E, Varoqueaux F, Fasshauer D (2020a) The diversification and lineage-specific expansion of nitric oxide signaling in Placozoa: insights in the evolution of gaseous transmission. Sci Rep 10(1):13020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroz LL, Sohn D, Romanova DY, Kohn AB (2020b). Microchemical identification of enantiomers in early-branching animals: lineage-specific diversification in the usage of D-glutamate and D-aspartate. Biochem Biophys Res Commun 527(4):947–952 [DOI] [PubMed] [Google Scholar]
- Nielsen C (2019) Early animal evolution: a morphologist’s view. R Soc Open Sci 6(7):190638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikitin M (2015) Bioinformatic prediction of Trichoplax adhaerens regulatory peptides. Gen Comp Endocrinol 212:145–155 [DOI] [PubMed] [Google Scholar]
- Osigus HJ, Rolfes S, Herzog R, Kamm K, Schierwater B (2019) Polyplacotoma mediterranea is a new ramified placozoan species. Curr Biol 29(5):R148–R149 [DOI] [PubMed] [Google Scholar]
- Pearse VB, Uehara T, Miller RL (1994) Birefringent granules in placozoans (Trichoplax adhaerens). Trans Am Microsc Soc 113:385–389 [Google Scholar]
- Pereira RT, Nebo C, de Paula NL, Fortes-Silva R, Cardoso R, de Oliveira I, Paulino RR, Drummond CD, Rosa PV (2020) Distribution of goblet and endocrine cells in the intestine: a comparative study in Amazonian freshwater Tambaqui and hybrid catfish. J Morphol 281(1):55–67 [DOI] [PubMed] [Google Scholar]
- Rassat J, Ruthmann A (1979) Trichoplax adhaerens F.E. Schulze (Placozoa) in the scanning electron microscope. Zoomorpholo-gie 72:59–72 [Google Scholar]
- Romanova DY (2019) Cell types diversity of H4 haplotype Placozoa sp. Marine Biological Journal 4(1):81–90 [Google Scholar]
- Romanova DY, Heyland A, Sohn D, Kohn AB, Fasshauer D, Varoqueaux F, Moroz LL (2020a) Glycine as a signaling molecule and chemoattractant in Trichoplax (Placozoa): insights into the early evolution of neurotransmitters. NeuroReport 31(6):490–497 [DOI] [PubMed] [Google Scholar]
- Romanova DY, Smirnov IV, Nikitin MA, Kohn AB, Borman AI, Malyshev AY, Balaban PM, Moroz LL (2020b) Sodium action potentials in placozoa: insights into behavioral integration and evolution of nerveless animals. Biochem Biophys Res Commun 532(1):120–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthmann A, Behrendt G, Wahl R (1986) The ventral epithelium of Trichoplax adhaerens (Placozoa): Cytoskeletal structures, cell contacts and endocytosis. Zoomorphology 106:115–122 [Google Scholar]
- Schierwater B, DeSalle R (2018) Placozoa. Curr Biol 28(3):R97–R98 [DOI] [PubMed] [Google Scholar]
- Schulze FE (1883) Trichoplax adhaerens, nov. gen., nov. spec. Zool Anz 6:92–97 [Google Scholar]
- Schulze FE (1891) Uber Trichoplax adhaerens Phys Abh Kgl Acad Wiss Berl,:1–23 [Google Scholar]
- Schwartz V (1984) Das radialpolare Differenzierungsmuster bei Trichoplax adhaerens F. E. Schulze (Placozoa) [The Radial Polar Pattern of Differentiation in Trichoplax adhaerens F. E. Schulze (Placozoa)]. Z Naturforsch, B J Chem Sci 39c:818–832 [Google Scholar]
- Sebe-Pedros A, Chomsky E, Pang K, Lara-Astiaso D, Gaiti F, Mukamel Z, Amit I, Hejnol A, Degnan BM, Tanay A (2018) Early metazoan cell type diversity and the evolution of multicellular gene regulation. Nat Ecol Evol 2(7):1176–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senatore A, Reese TS, Smith CL (2017) Neuropeptidergic integration of behavior in Trichoplax adhaerens, an animal without synapses. J Exp Biol 220(Pt 18):3381–3390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signorovitch AY, Dellaporta SL, Buss LW (2006) Caribbean placozoan phylogeography. Biol Bull 211(2):149–156 [DOI] [PubMed] [Google Scholar]
- Smith CL, Mayorova TD (2019) Insights into the evolution of digestive systems from studies of Trichoplax adhaerens. Cell Tissue Res 377(3):353–367 [DOI] [PubMed] [Google Scholar]
- Smith CL, Pivovarova N, Reese TS (2015) Coordinated feeding behavior in Trichoplax, an animal without synapses. PLoS ONE 10(9):e0136098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CL, Reese TS (2016) Adherens junctions modulate diffusion between epithelial cells in Trichoplax adhaerens. Biol Bull 231(3):216–224 [DOI] [PubMed] [Google Scholar]
- Smith CL, Reese TS, Govezensky T, Barrio RA (2019) Coherent directed movement toward food modeled in Trichoplax, a ciliated animal lacking a nervous system. Proc Natl Acad Sci U S A 116(18):8901–8908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CL, Varoqueaux F, Kittelmann M, Azzam RN, Cooper B, Winters CA, Eitel M, Fasshauer D, Reese TS (2014) Novel cell types, neurosecretory cells, and body plan of the early-diverging metazoan Trichoplax adhaerens. Curr Biol 24(14):1565–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specian RD, Oliver MG (1991) Functional biology of intestinal goblet cells. Am J Physiol 260(2 Pt 1):C183–193 [DOI] [PubMed] [Google Scholar]
- Srivastava M, Begovic E, Chapman J, Putnam NH, Hellsten U, Kawashima T, Kuo A, Mitros T, Salamov A, Carpenter ML, Signorovitch AY, Moreno MA, Kamm K, Grimwood J, Schmutz J, Shapiro H, Grigoriev IV, Buss LW, Schierwater B, Dellaporta SL, Rokhsar DS (2008) The Trichoplax genome and the nature of placozoans. Nature 454(7207):955–960 [DOI] [PubMed] [Google Scholar]
- Starunov VV (2019) The organization of musculature and the nervous system in the pygidial region of phyllodocid annelids. Zoomorphology 138(1):55–71 [Google Scholar]
- Syed T, Schierwater B (2002) Trichoplax adhaerens: Discovered as a missing link, forgotten as a hydrozoan, re-discovered as a key to metazoan evolution. Vie Milieu 52:177–187 [Google Scholar]
- Thiemann M, Ruthmann A (1990) Zoomorphology spherical forms of Trichoplax adhaerens (Placozoa). Zoomorphology 110(1):37–45 [Google Scholar]
- Varoqueaux F, Fasshauer D (2017) Getting nervous: An Evolutionary overhaul for communication. Annu Rev Genet 51:455–476 [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Williams EA, Grandemange S, Truscello L, Kamm K, Schierwater B, Jekely G, Fasshauer D (2018) High cell diversity and complex peptidergic signaling Underlie placozoan behavior. Curr Biol 28(21):3495–3501 e3492 [DOI] [PubMed] [Google Scholar]
- Voigt O, Collins AG, Pearse VB, Pearse JS, Ender A, Hadrys H, Schierwater B (2004) Placozoa—no longer a phylum of one. Curr Biol 14(22):R944–945 [DOI] [PubMed] [Google Scholar]
- Wenderoth H (1986) Transepithelial cytophagy by Trichoplax adhaerens F.E. Schulze (Placozoa) feeding on yeast. Z Naturforsch, B J Chem Sci 41c:343–347 [Google Scholar]
- Wenderoth H (1994) Phycoerythrin: Release from cryptophycean algae and bilin storage by the primitive metazoon Trichoplax adhaerens (Placozoa) Zeitschrift für Naturforschung 49c(7–8):458–463 [Google Scholar]
- Whelan NV, Kocot KM, Moroz TP, Mukherjee K, Williams P, Paulay G, Moroz LL, Halanych KM (2017) Ctenophore relationships and their placement as the sister group to all other animals. Nat Ecol Evol 1(11):1737–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccolotto-Arellano J, Cuervo-Gonzalez R (2020) Binary fission in Trichoplax is orthogonal to the subsequent division plane. Mech Dev 162:103608. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on https://zenodo.org.