Abstract
Conventional agriculture solely depends upon highly chemical compounds that have negatively ill-affected the health of every living being and the entire ecosystem. Thus, the smart delivery of desired components in a sustainable manner to crop plants is the primary need to maintain soil health in the upcoming years. The premature loss of growth-promoting ingredients and their extended degradation in the soil increases the demand for reliable novel techniques. In this regard, nanotechnology has offered to revolutionize the agrotechnological area that has the imminent potential over conventional agriculture and helps to reform resilient cropping systems withholding prominent food security for the ever-growing world population. Further, in-depth investigation on plant-nanoparticles interactions creates new avenues toward crop improvement via enhanced crop yield, disease resistance, and efficient nutrient utilization. The incorporation of nanomaterial with smart agrochemical activities and establishing a new framework relevant to enhance efficacy ultimately help to address the social acceptance, potential hazards, and management issues in the future. Here, we highlight the role of nanomaterial or nanocomposite as a sustainable as well stable alternative in crop protection and production. Additionally, the information on the controlled released system, role in interaction with soil and microbiome, the promising role of nanocomposite as nanopesticide, nanoherbicide, nanofertilizer, and their limitations in agrochemical activities are discussed in the present review.
Keywords: Conventional agriculture, Agrochemicals, Nanomaterial, Crop improvement, Sustainable
Introduction
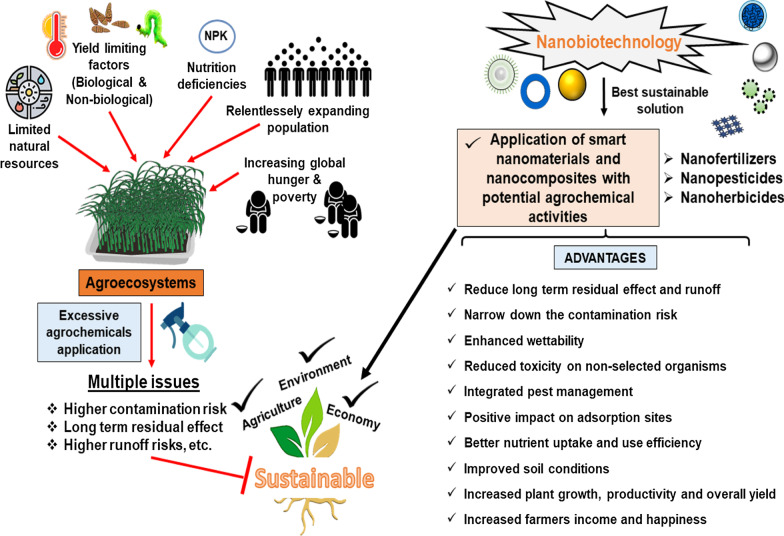
Globally, people are employed in agriculture for the cultivation of fundamental food crops and various essential forms of products such as fibers, fuels, fodders, and raw materials. Limited resources and an exponentially growing population, which is estimated to mark 9.6 billion by 2050, enforce the areas derived demanding the elaboration of very sustainable agriculture while permitting declination of global hunger and poverty [1, 2]. To fulfill this demand of relentlessly expanding population, there is an urgent prerequisite to enhance food production by more than 50% [2, 3]. Due to the limited number of natural resources (water, land, soil, forest, etc.) and ceiling in crop productivity, there is a huge demand for effective agricultural approaches that are viable and liable economically and eco-friendly. To overcome these dilemmas, synthetic agrochemicals (herbicides, insecticides, fungicides, and fertilizers) have been developed and used to increase agricultural yields [4, 5]. However, the application of such agrochemicals had been instrumental for elevating food quality and quantity in past decades to evaluate the long-term ill effect of such agrochemicals on soil health and the ecosystem [6]. However, research on nanoparticle application as chemical alternatives for utility in the agriculture sector has become enhancing popularity over the past decade, later referred to as nanoagrochemicals [7]. The intentional and directional delivery in the environment, nanoagrochemicals may be considered specific in terms of expectable environmental issues, as they would represent the single diffuse cause of engineered nanoparticles (NPs) [8, 9]. Given this, one such initiative taken is the forefront of smart nanomaterials for revolutionizing current agriculture practices that contain good reactivity due to their substantial surface area to volume ratio and exceptional physicochemical characteristics that offer the novel advantage of modification according to increasing demand [2].
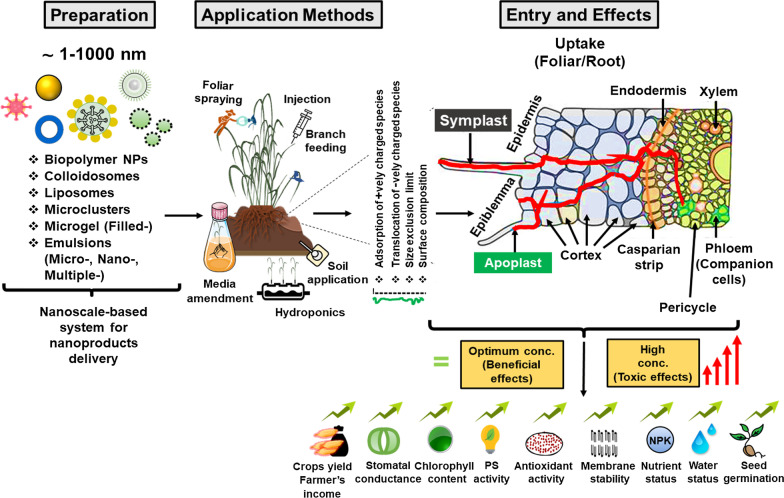
Modern agriculture is renovating into sustainable agriculture with the use of these modern age materials that are empowering to attain maximum output from limited resources [10]. Generally, agrochemical is essential to increase crop productivity but contrary, their application decline soil fertility by hindering soil mineral balance [11]. Moreover, the direct foliar or sprayed application can be cost-effective and very high, which run off and need to be controlled [12]. The nanomaterials-based chemicals developed in agriculture regulate nutrient depletion rate, yield reduction, input cost for crop raising, protection, production, and minimizing post-harvest loss [3]. Nanocomposites have become a key component of nanomaterials for scrutinizing and stimulating the plant life cycle because of their intrinsic unique thermal, electrical, chemical, and mechanical properties. The translocation in size-dependent lies in the range of 0.1–1000 nm within plant parts and altered according to surface compositions, a charge of NPs (highly negatively charged shows more translocation), and plant size exclusion limit [10, 13]. These routes of penetration are confirmed via different in vitro (Filter paper, hydroponics, agar media, Hoagland solution, Mursashige and Skoog media, nutrient solution) and in vivo (foliar uptake, branch feeding, trunk injection, and root uptake) experiments using nanopesticide, nanoherbicide, nanoherbicides, and nanogrowth-promoting compounds [2, 9]. However, in certain cases the size exclusion is high so, it’s difficult to limits the specific passage and concentration that affect the growth phase of plants both positively and negatively (Fig. 1).
Fig. 1.
Diagrammatic illustration of nanoparticles transport, and their interactions in crop plant
Many successful examples of utilizing smart nanomaterial in agriculture have been reported in recent years including multi-walled carbon nanotubes [5, 14], metal-based nanocomposites [15], silver inhibits fungus germination [16], and many more. This new-age nanoformulation has the potential to fine-tune the physiology just entering the soil–plant complex that can be solely exploited to spotify the lateral effect [17].
The nanoparticle-based products (NMs) including smart agrochemical delivery systems having nanocomposites as chief ingredients are being constantly developed. Much intensive research is still required to achieve the practical advantages of nanoagrochemicals with improved working design, regulation of commercialization, and risk assessment of nanofertilizer, nanopesticide, and nanoherbicide [18, 19]. New crop cultivars, that can sustain heat, drought, salinity, and other unresolved challenges in farming systems disturb the whole spectrum of major cultivation practices worldwide. Moreover, it is expected that the implementation of NMs in the natural environment decline the chemicals-based hazardous level [12]. We surely believe, their application in agriculture will narrow down the gap between sustainable and chemical-based agriculture systems. Besides this, it boosts food production and quality globally in an eco-friendly manner by resolving water and soil contamination [20]. Thus, practically they could provide novel avenues regarding developing new NMs-based products [14]. Conventional agrochemical has offered numerous drawbacks regarding the non-selectively and adsorption rate of active ingredients (AIs).
It has been reported that more than 99.9% pesticides are failed to be delivered at target sites and cause a hazardous impact on the health of the soil, water, air with enhances pathogenic resistance and biodiversity loss [12, 21, 22]. Overall, we aimed to highlight the current information on facts that nanomaterial or nanocomposite deliver an efficient solution to upgrade and advanced the agriculture innovations, food systems, sustainable crop protection, and production. Moreover, information on the controlled released system, role in interaction with soil and microbiome, the promising role of nanocomposite as nanopesticide, nanoherbicide, nanofertilizer, and limitation in agrochemical activities are also discussed in the present review.
Nanostructure compounds with the controlled released system (CRS)
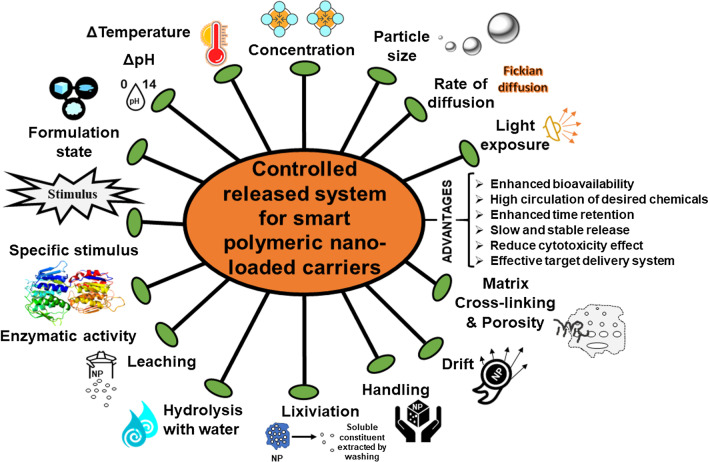
Due to several advantages over conventional chemical application approaches, many researchers have put forward the model of the controlled release system [15, 23–29] to offer substitutes to reduce environmental pollution. The controlled release (CR) allows efficient delivery of an AI more actively in soil and plant for the desired interval of time, resulting in the decreases of the amounts of agrochemicals used, energy, manpower, or other resources crucial to operate the application instruments as well as in enhancement in safety to humans who deal with their application [26, 29–32]. Additionally, CR shows many advantages over conventional methods including decrease phytotoxicity, reduce agrochemical loss due to volatilization, lixiviation, drift, improper handling, and degradation in soil and controlled delivery coincides with a suitable concentration in the plant to prevent unpredictable losses in form of evaporation, leaching and weather (Fig. 2) [16, 33].
Fig. 2.
Types of nanoparticle delivery system
Comprehensive characterization is a significant prerequisite to predict or explain the efficiency and behaviour of smart nano-loaded agrochemicals. In particular, retention of AIs, behaviour, composition and phase, zeta potential, and internal structure of polymeric nanocarriers, and their release in particle environment conditions are summarized as important properties [30, 34–36]. The rate of loading and release for AIs from nanocarriers plays a central role in predicting or assessing their efficacy. These can be evaluated by ingredients concentration remaining within polymeric matrix and amount of released ingredients [37, 38]. The mechanism of release can be achieved via different modes such as:
Diffusion via relaxation/swelling of NPs
In the concentration gradient phenomena (or fickian diffusion), the release would occur at a high rate when nanocarriers are diluted using either concentrated or solid formulations even under irrigation or rainfall events. The diffusion can be slowdown by enhancing the nanoparticle size or enhancing the distance within media in which diffusion of AI occurs observed in poly lactic acid (PLA) loaded metazachlor [32, 39, 40]. Similarly, enhanced cross-linking has been suggested as an efficient method to delay diffusion by increasing the tortuosity or decreasing the porosity via the polymer matrix, as indicates by methomyl-loaded chitosan (azidobenzaldehyde-carboxymethyl) pesticide before and after polymer crosslinking [40–43].
Burst release
The most commonly rapid release method in which AI release undesirably, if an initial high amount of AI is not favorable for the application of target. The phenomena would show enhance the concentration of AIs present near or on the surface of the NPs indicates high significant burst release. For example, PLA-loaded metazachlor (herbicides) nanocapsule or surface coating has been recommended to inhibits the initial rapid burst that is frequently noted for nanospheres [35].
Degradation
Nanoparticle release can be triggered or accelerated by physical, chemical, and biological degradation that can be achieved by hydrolysis with water, light exposure, temperature, pH, specific stimulus, and enzymatic activities. For example, PLGA (Poly lactic co-glycolic acid) NPs show increased hydrolytic degradation with enhancing surface area- volume ratio for water, and their diffusion rate might be fine-tuned with appropriate nanocarriers [44]. Moreover, the mPEG (methoxy polyethylene glycol) incorporated in PLGA-NPs increases the degradation rate of NPs via enhanced hydrophilicity and ultimately accessibility for hydrolysis in hydrolytic degradation type. In enzymatic degradation, the events lead by the activities of phosphatases, glycosidases, and protease viz: PCL (poly(ε-caprolactone) degradation enhance with the activity of lipase activity [44]. Similarly, γ-PGA (poly (γ-glutamic acid) degradation mediated by γ-GTP (γ-glutamyl transpeptidase) is considered as a most common enzyme that causes rapid degradation [38]. In another study, zein nanoparticle shows rapid and extensive degradation and release of encapsulated ciprofloxacin antibiotic, in presence of trypsin enzyme than collagenase [37].
In some cases, stimuli-response release can be observed using photosensitive polymers such as micellar or UV (Ultraviolet) labile core–shell NPs were produced to PEG and nitrobenzyl to carboxymethyl chitosan. Thus, stimuli-based nanocomposite can intelligently react to the stimulus produced by the target or the adjoining environment that eventually triggers the AIs release to regulate the pest effectively [45, 46]. However, physical stability in some NPs altered by pH, when the polymer is weak basic or acidic such that electrostatic and charge will reliable on pH [40, 41, 47]. For instance, carboxymethyl cellulose and feather keratin were loaded with avermectin. The diffusion rate was observed to be faster at low pH (Fickian transport) and higher pH (non-Fickian) [46].
Nanoformulations as a promising tool in an agricultural system
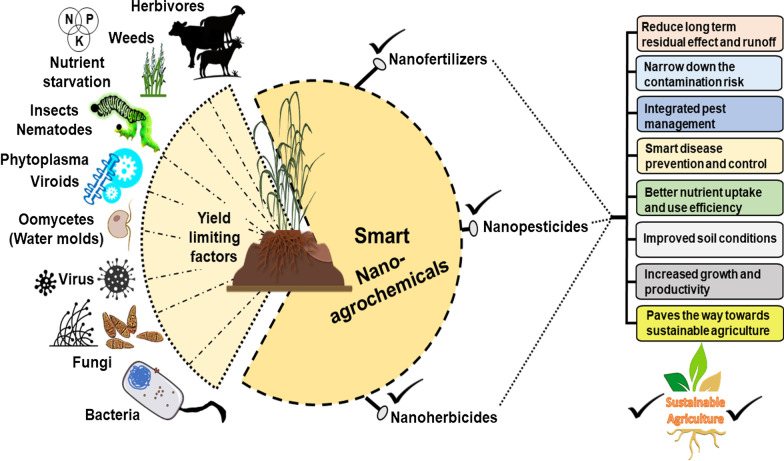
Agrochemicals includes pesticides, herbicides, fungicides, bactericides, nematicides, rodenticides that are used to target pest, weed, pathogenic fungus, bacteria, nematodes and rodents (Fig. 3) [48–50]. Globally, the herbicide market is expanding and is estimated to lies between $27.21 and $39.15 billion at a compound annual growth rate (CAGR) of 6.25% in the expected period 2016–2022. Besides this, the global pesticides market was accounted to reach $70.57 billion by 2021 at a CAGR of 5.15% estimated between 2016 and 2021. Besides this, the global market of encapsulated pesticides grows exponentially at reach benchmark of US $800 million by 2025 expectedly and gains 11.8% CAGR in the tenure of 2019–2025 [18, 19, 48, 49].
Fig. 3.
Applications of different nanoparticles for regulation of plant growth, pathogen management, and nutrient uptake in sustainable agriculture
The families represented by inorganic chemicals are triazines, phenoxy, and benzoic acid chloroacetanilides representing herbicides, phenylpyrrole, benzimidazoles, dithiocarbamates, and nitriales for fungicide, carbamate, organophosphates, organochlorines relating to insecticide. Smart nanoagrochemicals with nanoformulations must offer a broad variety of benefits including enhanced durability, effectiveness, wettability, good dispersion, less toxicity, good biodegradable ability in soil and environment, and photogenerative nature with the least residues compared to conventional chemicals [51–53]. Over the past, extensive studies were carried out on nanoagrochemicals to access their significant role and contamination range in affecting soil–plant nutrient cycles [19].
Nanopesticide
The potential utility of nanochemicals in integrated pest management (IPM) depends upon targeted delivery of AIs with increased activity at least drug concentration and proficient monitoring of pesticides interactions with the surroundings. Under harsh conditions, the chemical stability can be achieved by efficient nanocarriers having enhanced dispersal range, wettability, and more protectivity to pesticides without risk of runoff [54–57]. Other noteworthy characteristics of pesticidal nanocompositions can be observed in thermal stability, large surface area, increased target affinity, and biodegradable nature after successful delivery. These delivery systems can be regulated for single goals or multiple combinations viz; spatially target release, time-controlled release, remotely or self-regulated release to overcome the biological barriers in the successful target [21, 58–60]. However, the efficacy of nanoencapsulation or nanocarriers is (1) to prevent pre-degradation of AI in the carrier before their release in the target (2) to improve penetration and ease solubility of AIs within the target site (3) to monitor or regulate the degradation of AIs in the desired site [61, 62].
According to Kremer et al. [63] the adsorptive interaction between pesticides and NPs showing discrete molecular dynamics. Such interactions should have a positive impact on adsorption sites via physiological morphology, binding ability, antioxidant systems, and transportability of pesticides in plants [64]. In Arabidopsis thaliana, the antagonistic effect between silver NPs and Diclofop-methyl (post-emergence herbicide) in which herbicides presence decline or affected the Ag+ from silver NPs. Moreover, a decrease in pesticide concentration is imperative to avoid their toxicity on non-selected organisms and narrow down contamination risk [65–67]. Several nanocompositions of pesticides have been developed such as nanoemulsions, nanosuspensions, and nanocapsulations. Such nanomaterials are prepared specifically to maintain the regulated release of AIs in several ways including magnetic release, ultrasound release, pH release, heat release, moisture release, DNA-based release, specific release, quick and slow-release [19].
In some cases, nanoparticle delivery in hollow silica NPs are used to prevent avermectin from UV radiation and provide photostability to nanopesticides causes long-term effects on the target organism. Several NPs used various forms of encapsulations including (1) Lipid nanomaterial-based encapsulation. (2) Metal–organic framework-based encapsulation. (3) Polymer-based 6encapsulation. (4) Clay nanomaterial-based encapsulation. (4) Greener encapsulation [9, 42, 43, 45, 47, 68–70].
Nanofertilizer
Besides plant protection, these smart NPs are extensively used to regulate the physiological process. For example, SiO2 NPs (silicon dioxide NPs) elevates seed germination rate in Lycopersicon esculentum [71, 72], chitosan-polymethacrylic-NPK increase biomass, nutrient uptake and antioxidant enzymes in Phaseolus vulgaris [73, 74], Au-NPs (gold NPs) promotes seed germination, seedling growth, enzymatic activity and nutrient uptake in Zea mays [75, 76], SiO2-NPs improve uptake of NPK, increase enzymatic activity and seed germination rate in Hyssopus officinalis and Z. mays [77–79], chitosan-CuNPs (copper NPs) enhance seed germination, activation of α-amylase, protease and activity of various antioxidant enzymes in Z. mays [2, 80, 81], chitosan-ZnNPs (zinc NPs) increase accumulation of zinc content and defense enzymes in Triticum durum [82, 83], chitosan-γ-polyglutamic acid-gibberellic acid NPs promotes seed germination, root development, leaf area, hormonal efficiency, extracellular enzymes and nutrient efficiency [83, 84], Chitosan-polymethacrylic acid-NPK NPs promotes protein content and nutrient uptake [74, 85], ZnO-NPs (zinc oxide NPs) increase activity of catalase (60.7%), superoxide dismutase (22.8%) and nutrient acquisition [86, 87], CeO2-NPs (cerium oxide NPs) enhance seed germination and vigour, enzymatic activity and nutrient uptake in Spinacia oleracea and Z. mays [88–91], AuNPs increase chlorophyll content and antioxidant enzyme activities in Brassica juncea [92] and TiO2 NPs (titanium oxide NPs) enhance chlorophyll content, nutrient uptake, activity of Rubisco and antioxidant enzymes in S. oleracea and Cicer arietinum [89, 93] (Table 1).
Table 1.
Successful use of nanoformulation used in crop plant as plant growth promoters
| Nanoformulation | Mode of applications | Targeted crop | Properties (size/shape/Molecular weight/ pH) | Effect on Plant physiological processes | Key references | ||
|---|---|---|---|---|---|---|---|
| Growth phases | Enzyme activities | Nutrient uptake and release | |||||
| SiO2 NPs | Seed treatment | L. esculentum Mill | 12 nm | Enhance seed germination | No visible effect on enzyme activity | – | [71, 72] |
| Nano-chitosan | Seed treatment | C. arietinum L | pH 4.8 | Promote total biomass, germination, and vigor index up to (57%) | Increase activities of catalase and superoxide dismutase | – | [83, 170] |
| Chitosan-polymethacrylic acid-NPK NP | Foliar spray | P. vulgaris L | 20 nm | Enhance plant growth and total biomass | Enhance the antioxidant enzyme activities | Promote nutrient uptake and accumulation | [73, 74] |
| Au NPs | Seed imbibition | Z. mays L | 10–30 nm, spherical | Promote germination and seedling growth | Promote activity of Superoxide dismutase, peroxidase, and catalase | Increased nutrient uptake of maize excluding iron | [75, 76] |
| SiO2NPs | Seed imbibition | H. officinalis L | 10–20 nm, spherical | Improve plant growth and seed germination | Enhance total soluble proteins | – | [77, 78] |
| SiO2 NPs | Seed Imbibition | Z. mays L | 10–20 nm, spherical | Promotes seed germination | Enhanced the activities of antioxidant enzymes | Increase uptake of nitrogen, phosphorus, and potassium | [77, 79] |
| Chitosan-Cu NPs | Seed treatment | Z. mays L | Low molecular weight, 80% | Promote seedling growth | Enhance activity of α-amylase and protease | – | [2, 80] |
| Chitosan-Cu NPs | Foliar spray | Z. mays L | 50–190 kDa, 80% | Promote seedling growth, overall plant height, and biomass | Enhance activities of defense enzymes | – | [2, 81] |
| Chitosan-Zn NPs | Foliar spray | T. durum L | 60 kDa, 85% | Stomatal localization of nanoparticles | Promote defense enzyme activities | Enhance zinc content accumulation by 42% | [82, 83] |
| Nano-chitosan | Seed treatment | Z. mays L | pH 7.0–9.0 | Enhance plant height, leaf area, and seed germination | Promote activities of glucose-6-phosphate dehydrogenase, succinate dehydrogenase, and superoxide dismutase | Enhance accumulate of potassium inside the plant | [102, 171] |
|
Chitosan-γ-polyglutamic acid-gibberellic Acid-NPs |
Seed treatment | P. vulgaris L | 290 kDa, 75%–85%, pH 4.5 | Promote seed germination, root development, and total leaf area | Enhance the hormonal efficiency, enhance extracellular enzymes, such as cutinase, lipase, and esterase | Increase efficiency of nutrients | [83, 84] |
| Chitosan-gibberellic acid NPs | Seed treatment | P. vulgaris L | 27 kDa, 75%–85%, pH 4.5 | Promote leaf area, carotenoid and chlorophyll content | Enhance the hormonal efficiency by 90% | Not significant effect on nutrient uptake | [172, 173] |
| Nano-chitosan | Seed treatment | P. vulgaris L | 100–399 kDa | Increase seed germination and radical length | Enhance the activity of peroxidase and catalase | Increase Zinc uptake in plant | [174, 175] |
| Nano-chitosan | Seed treatment | Capsicum annuum L | 110 kDa, 85–90%, pH 4.0 | Enhance the root biomass (77%) and fresh leaf biomass (28%) | Increase the activity of catalase and peroxidase 33% and 23% respectively | Not much significant effect on nutrient uptake | [176, 177] |
| Chitosan-polymethacrylic acid-NPK NP | Seed treatment | Pisum sativum var. Master B | 20 nm | Enhance mitotic cell division about 1.5 fold | Enhance total soluble proteins like legumin, convicilin and β, vicilin 1, 2 and 3 | Enhances the efficiency of plants for the uptake of nutrients | [74, 85] |
| ZnO NPs | Seed soaked | Avena sativa L | 20–50 nm, spherical shape | Promote percent germination | – | Modulate uptake of nitrogen and phosphorus in plants | [178, 179] |
| ZnO NPs | Seed imbibition | T. aestivum L | 30–40 nm, sphere-crystal | Improve shoot length and total plant biomass | Enhance the activity of superoxide dismutase (22.8%) and catalase (60.7%) | Enhance nutrient acquisition in wheat | [86, 87] |
| Chitosan-thiamine NP | Seed treatment | C. arietinum L | 27 kDa, 85% | Promote seed germination and plant growth | Enhance activities of peroxidase, polyphenol oxidase, chitinase, and protease enzyme | Enhance nutrient uptake | [83, 180] |
| CeO2 NPs | 50 Mg-Ce per L hydroponic | Z. mays L | 2–4 nm, crystal | Promote photosynthesis and gas exchange | Accumulation of hydrogen peroxidase enzyme | Increase nutrient uptake of maize | [91, 181] |
| CeO2 NPs | Foliar spray | S. oleracea L | 4–7 nm | Enhance percent germination (4%) and vigor index | Catalase activity significantly increased | Enhance nutrient uptake | [89, 182] |
| Nano-chitosan | Foliar spray | Coffea canephora Piere var Robusta | 600 kDa, 85%, pH 6.0 | Increase (30–50%) chlorophyll content and photosynthetic rate (30%) | Enhance the enzymatic activities | Enhance nutrient uptake (Nitrogen 10–27%, Phosphorus 17–30% and Potassium 30–45%) | [83] |
| Chitosan-polymethacrylic acid-NPK NP | Foliar spray | T. aestivum L | 20 nm | Increase crop yield (50%) and harvest index (24%) | Increase polysaccharides and total saccharides (11%), nitrate reductase enzyme | Accumulation of nitrogen, phosphorus, and potassium in plant | [83] |
| AuNPs | Spray on leaves | B. juncea L. Czern | 10–20 nm, spherical | Enhance chlorophyll content and plant growth | Enhance antioxidative enzymes, proline and hydrogen peroxide | – | [92] |
| Carbon (CNTs) | Seeds in culture media | L. esculentum Mill | Nanotubes | Enhance seed germination and vigor index | Increase activities of peroxidase, catalase, and superoxide dismutase | Percentage concentration of nutrient elements present in germinated tomato increased | [182] |
| TiO2 NPs | Seeds soaked | S. oleracea L | 30–60 nm, crystal shape | Enhance seedling growth, biomass, and chlorophyll content | Promote activities of antioxidant enzymes (0.25%) | Enhance nutrient uptake | [88] |
| TiO2 NPs | Spray on leaves | C.arietinum L | 5–20 nm | Reduce membrane damage during cold stress | Increase activity of Rubisco enzyme | Increase the mineral uptake in plant | [183] |
Nanoinsecticides
As the trends and demand of encapsulated NPs exponentially increased the regulatory pressure for their management also enhanced simultaneously. Encapsulated insecticides share more than 42% of total pesticide revenue up to 2017 [60, 94, 95]. Recently, in 2019 pesticide manual online classified encapsulated insecticides contain hazardous toxic AIs like pendimethalin, acetochlor, dichlobenil, tefluthrin, etofenprox, chlorpyrifos, carbosulfan, and furathiocarb at the commercial level [19]. The toxicity level of AIs not only depends upon encapsulation material but it helps in adjusting the dynamics of the target species exposure to AIs in vivo conditions [21, 25, 96]. The use of styrene and methylmethacrylate as encapsulation wall material increased the nematicidal activity to suppress the growth of the wheat rust-causing pathogen, Puccinia reconditea. Similarly, the effect of urea–formaldehyde and polyuria resin wall on stomatal toxicity, contact toxicity, phoxim loaded microcapsule efficacy, and photolysis properties was reported by Zhang et al. [97]. In another study, improved pest efficiency and poor cytotoxicity of sodium alginate imidachloroprid encapsulation were observed that favored direct application of imidachloroprid [68].
Another study shows a decrease in picloram toxicity to soil microbiota with silica gel encapsulation in comparison to free-form picloform. The silica NPs bioavailability to the non-selected organism can be enhanced by tunning the wall properties of the silica shell [98]. In a study, Jacques et al. [99] reported the atrazine toxicity in encapsulated polymeric and lipid nanocompositions against nematodes, Caenorhabditis elegans, but comparably no toxicity was observed in tripolyphosphate/chitosan-based encapsulation that itself can be attributed to low toxicity. Moreover, the oil encapsulated PCL neem-derived nanoencapsulation did not exhibit any adverse effect of stomatal conductance, the photosynthetic ability of maize after exposure up to 300 days. These findings suggest the careful selection of wall material/encapsulation and physicochemical properties of AIs and their composition and application sites [19, 100].
The Si-NPs (silicon NPs) have been efficiently reported to protect infestation from stored beetle Callosobruchus maculatus in pulses like Vigna unguiculata, V. mungo, V. radiate, Macrotyloma uniflorum, C. arietinum, and Cajanus cajan [101]. Despite their excellent performance, nanopesticides show poor commercialization and stability. The pH, temperature, humidity, UV radiation influence AIs availability and influence physiochemical characteristics. Besides these quantity, quality, strict legislation, expensiveness and degradation period of AIs are emerging issues while using nanopesticides [19, 54, 79].
Nanofungicides
Beyond the nanocarriers application, nanomaterial as AIs for crop protection is a major aspect of research. The broad spectrum of antifungal properties of nanofungicides can improve their efficiency as a pesticide. For instance, copper, silver, and zinc NPs resolve the disadvantages of chemical AIs for pathogenic resistance with sharp antimicrobial activity and non-toxicity [19]. Moreover, chitosan-based NPs (Ch-NPs) showed effective antifungal activity and restrict growth reported by many research workers in the last decade. For example, Ch-NPs against Alternaria alternata, Macrophomina phaseolina, Rhizoctonia solani [102], Pyricularia grisea, Alternaria solani, Fusarium oxysporum [102, 103], Pyricularia grisea, Copper–chitosan NPs against Fusarium solani [104], Cu-chitosan NPs- against R. solani and Sclerotium rolfsii [105], chitosan-saponin NPs [102], oleoyl-chitosan NPs against Verticillium dahaliae [106], salicylic acid-loaded chitosan NPs against Fusarium verticillioides [107], Ag-chitosan NPs against R. solani, Aspergillus flavus and A. alterneta [108], silica-chitosan NPs against Phomopsis asparagi [109] chitosan-pepper tree (Schinus molle) essential oil (CS-EO) NPs against Aspergillus parasiticus [110], chitosan boehmite alumina nanocomposites films and thyme oil against Monilinia laxa [111] fungicide zineb (Zb) and chitosan-Ag NPs against Neoscytalidium dimidiatum [112], chitosan-Thyme-oregano, thyme-tea tree and thyme-peppermint EO mixtures against Aspergillus niger, A. flavus, A. parasiticus, and Penicillium chrysogenum, [113], chitosan-thymol NPs against Botrytis cinerea [39], chitosan-Cymbopogon martinii essential oil against Fusarium graminearum [114].
In comparison to conventional agrochemicals, the nanoparticle was confirmed to be highly effective in crop protection even at minute concentration viz: 0.43 and 0.75 mg/plate concentration of Ag-doped hollow titanium-oxide (TiO2) nanoformulation against Potato pathogens such as Venturia inaequalis and F. solani [115] (Table 2). Moreover, several successful examples of NPs were studied extensively for abiotic stress tolerance in recent years [116–118]. To cope with drought tolerance, several reports published in past decades on the application of NPs such as TiO2 application in Linum usitatissimum via elevating pigmentation and reducing the activity of Malondialdehyde (MDA) and Hydrogen peroxide (H2O2) [119], ZnO promotes effective seed germination in Glycine max [120], CuNPs improve pigmentation, biomass and grain yield in Z. mays [121]. In case of salinity stress, seed soaking, nutrient solutions, and seed priming methods are used for evaluation in G. max, S. lycopersicum, and Gossypium hirsutum respectively [122–124].
Table 2.
Successful application of nanocomposites for biotic stress tolerance
| Pathogen type | Nanoparticles used | Plant disease | Mechanism of action | Key references |
|---|---|---|---|---|
| Fungus | ||||
| Bipolaris sorokiniana | AgNPs biosynthesized with Serratia sp. | Spot blotch pathogen of wheat | Enhance lignification of vascular bundles | [92] |
| Gloeophyllum abietinum | Green-synthesize AgNPs extracted with turnip leaf | Wood-rotting | Inhibit the conidia development | [184] |
| Phytophthora capsici | Ag core-DHPAC shell nanocluster | Blight diseases in Solanaceae | Reduce mycelial growth and sporangial production | [185] |
|
Escherichia coli, Bacillus Subtilis and F. oxysporum |
Cu(OH)2NPs | Corn leaf blight | Decrease number of conidia | [125] |
| F. oxysporum | Cu3(PO4)2·3H2O nanosheets | Root fungal disease in watermelon | Inhibit the fungus growth | [125] |
| F. graminearum | Multiwalled carbon nanotubes, graphene oxide, reduced graphene oxide, and fullerene | Fusarium head blight in wheat | Inhibit spore germination of Fusarium graminearum | [96] |
| F. oxysporum | CeO2NPs | Panama disease | Enhance antioxidant enzyme activity | [186] |
| Aspergillus spp. | SiNPs | Black mold | Inhibit fungus proliferation | [187] |
| R. solani | Calcium carbonate | Brown rot of stems | Reduce rot growth and recover sucrose level | [188] |
| Phytophthora | Green-synthesize AgNPs extracted with Artemisia absinthium | Seed rots | Effect zoospore development | [189] |
| Bacteria | ||||
| X. perforans | Ag nanoparticles along with graphene oxide | Bacterial spot of tomato | Significantly decrease the activity of X. perforans | [190] |
| B. sorokiniana | AgNPs biosynthesized with Serratia sp. | Spot blotch pathogen of wheat | Inhibit conidial germination | [191] |
| Clavibacter michiganensis | CuNPs and K2SiO3NPs | Bacterial ring rot in potato | Decrease bacterial cell viability | [192] |
| X. perforans |
Photochemically active TiO2NPs |
Spot disease in tomato | Due to high photocatalytic activity, reduction in bacterial spot | [193] |
| Ralstonia solanacearum | MgONPs | Vascular wilt disease | Inhibit bacterial activity | [185] |
| Xanthomonas campestris pv. campestris | Silver (Ag) NPs | Bacterial blight | Enhance antioxidant enzyme activity | [194] |
| Colletotrichum gloeosporioides | Chitosan NP | Disease in Chile | Inhibition growth of mycelia | [195] |
| A. alternata | Chitosan NP | Leaf spot | Inhibit spore germination | [83] |
| Xanthomonas alfalfae | Synthesized Mg(OH)2NPs | Bacterial leaf spot | Significantly decrease the activity of X. alfalfae | [11] |
| Target species | Nanoparticles used | Mechanism of action | References |
|---|---|---|---|
| Insects | |||
| Aedes aegypti and Anopheles stephensi | Microbial synthesized Ag, Au, and ZnO-NPs | Epithelial cell, midgut, cortex damage, and thorax shape change | [196] |
| Aedes albopictus and Culex pipiens pallens | Ag synthesized using Cassia fistula extract | Total protein level, acetylcholinesterase, and α- and decreased activity of ß-carboxylesterase | [197] |
| Chironomus riparius | AgNPs | Modulates GST genes expression, upregulated mRNA expression in delta3, Sigma4 and Epsilon1 GST class | [198] |
| C. riparius | AgNPs | Downregulated activity of ribosomal gene protein, activation of gonadotrophin through upregulation of Balbiani ring protein gene (CrBR2.2) and gonadotrophin-releasing hormone gene (CrGnRH1) | [198] |
| C. riparius | AgNPs | Enhance expression of epsilon-1, sigma-4, and delta-3 and transcript levels of catalase, thioredoxin reductase 1, Mn superoxide dismutase | [198] |
| Drosophila melanogaster | AgNPs | Reduce Cu-dependent enzyme activity, couple with membrane-bound Cu transport protein results in Cu sequestration | [199] |
| D. melanogaster | AgNPs | Cause pigmentation defects and flies locomotive ability | [200] |
| D. melanogaster | AgNPs | DNA-damage, autophagy, ROS-mediated apoptosis | [201] |
| D. melanogaster | Ag and TiO2 NPs | Effect developmental processes of flies | [202] |
| Sitophilus oryzae | Nanostructured Al2O3 | Absorbing wax layer that results in insect dehydration | [93] |
| Aedes albopictus | Ag NPs prepared using 3,5-dinitrosalicylic acid and salicylic acid | Total protein, esterase, phosphatase, and acetylcholine esterase enzyme activity decreased | [203] |
The application improves stress tolerance by enhancing chlorophyll content, biomass number, soluble sugar content, seed germination [125–127]. According to Shoemaker [128] application of AgNPs (silver NPs) in Triticum aestivum increases seedling growth and leaf area whereas foliar application of SeNPs (selenium NPs) improves antioxidant enzyme activity and thylakoid membrane stability in Sorghum bicolor under heat stress [129] (Table 3).
Table 3.
Successful application of nanocomposites for abiotic stress tolerance
| Stress type | Nanoparticles | Plant species | Mode of application | Results | Key references |
|---|---|---|---|---|---|
| Drought | TiO2 | L. usitatissimum L. | Foliar spray | Enhance chlorophyll and carotenoid content as well as lowers the activity of MDA and H2O2 | [204] |
| TiO2 |
T. aestivum L. c.v Pishtaz |
Foliar spray | Enhance starch content, growth, and yield | [76] | |
| TiO2 | T. aestivum L. | Amended soil | Increased seedling growth, antioxidant enzymes, total chlorophyll, and carotenoid content | [205] | |
| ZnO | G. max L. | Seed soaking | Enhance germination rate and percent germination | [119] | |
| Fe2O3 | Mentha piperita L. | Hoagland solution | Increase activity of antioxidant enzymes | [206] | |
| Cu | Z. mays L. | Plant priming | Improve plant biomass, chlorophyll, anthocyanins, and grain yield | [207] | |
| CNTs, graphene | G. hirsutum L. | Seed priming | Increase seedling growth and biomass | [123] | |
| Chitosan NPs | Hordeum vulgare L. | Foliar spray | Increase proline content, CAT and SOD | [208] | |
| Salinity | Ag | Trigonella foenum-graecum | Seed soaking | Improve percent germination, fresh and dry weight of seedlings | [209] |
| ZnO | Abelmoschus esculentus L. | Foliar spray | Increase activity of superoxide dismutase, catalase, and photosynthetic pigments | [210] | |
| ZnO and Si | Mangifera indica L. | Foliar spray | Enhance nutrient uptake, carbon assimilation in plants | [211] | |
| SiO2 | Solanum lycopersicum L. | Seed soaking | Upregulation of stress tolerance genes | [212] | |
| SiO2 | Fragaria ananassa L. | Soil application | Enhance growth, proline, chlorophyll, epicuticular wax layer and leaf relative water content | [213] | |
| SiO2 | Musa acuminate L. | Seed priming | Enhance chlorophyll content, shoot growth | [214] | |
| Ag | T. aestivum L. | Seed priming | Enhance total soluble sugars, proline content, and peroxidase activity | [185] | |
| Fe2O3 | Helianthus annuus L. | Foliar spray | Enhance dry weight, leaf area, chlorophyll content | [215] | |
| Fe2O3 | Dracocephalum moldavica L. | Foliar spray | Increase the enzymatic activity of guaiacol peroxidase, catalase, ascorbate peroxidase, and glutathione reductase | [215] | |
| Mn | C. annuum L. | Nanopriming | Improve plant growth | [216] | |
| CeO | G. hirsutum L. | Seed priming | Improve root growth and decrease ROS level | [121] | |
| CNTs, graphene | Catharanthus roseus L. | Murashige and Skoog medium | Enhance the number of leaves and flowers | [124] | |
| Chitosan-PVA and CuNPs | S. lycopersicum L. | Nutrient solution | Enhance chlorophyll, carotenoids, and lycopene content | [122] | |
| Heat | Ag | T. aestivum L. | Soil application | Promote the root number, seedling length, and leaf area | [127] |
| Se | S. bicolor L. Moench | Foliar spray | Improve thylakoid membrane stability and activity of antioxidant enzymes | [217] | |
| Heavy metal | Fe | T. aestivum L. | Soil application | Increase rate of photosynthesis, chlorophyll content, and plant growth | [87] |
| UV-B | Si | T. aestivum L. | Nutrient solution | Improve antioxidant defense system | [218] |
| Cold | TiO2 | C. arietinum L. | Amended soil | Decrease MDA levels and electrolyte leakage index | [219] |
| Flooding | Al2O3 | G. max L. | Seed soaking | Increased hypocotyl length, mitochondrial membrane proteins | [220] |
Nanoherbicide
These NPs inhibit the physiological processes and growth phases in several weed species. For example, Ch-NPs retard germination and growth phases in Bidens pilosa [130, 131] NPs atrazine disrupts PSII activity in Amaranthus viridus [132], Fe3O4 NPs (Iron oxide NPs) + purified diatomite + glyphosate decrease pH level in Cynodon dactylon [133], Zero valent Fe NPs (Iron NPs) retard germination in Lolium perenne [32]. The efficacy of metribuzan, (a commercial herbicide) was enhanced via using NPs to maintain the growth of the weed population including Melilotus album, T. aestivum, Agrostis stolonifera, and Setaria macrocheata [19].
The atrazine-loaded nanocarriers are used to penetrate the stomatal region, hydathodes and ensure their direct entry into vascular tissues. It ensures the targeting, cellular uptakes, and overcomes intracellular trafficking due to certain properties of NPs: (1) Interaction affinity. (2) Mechanical effect of form and size. (3) catalytic effect. (4) Surface charges/hydrophobicity. Fraceto et al. [19] describing decreased toxicity level of paraquat in non-targeted plants preferring Triphosphate/chitosan nanocarriers application over conventional spray system in Brassica sp. Similarly, in B. pilosa and C. dactylon mortality rate of seedlings was enhanced using encapsulated glyphosate magnetic nanocarriers [19, 131]. The nanoencapsulation uses low doses of herbicide and could effectively reduce the long-term residual effect of herbicides in target species as well as in agricultural land. Conclusively, nanoherbicide can enhance the delivery of AIs in plant tissues and comparatively declined the chance of environmental toxicity [60, 94, 95].
Impact on plants-soil microbiome
NPs face numerous experience transformation, dissolution aggregation in soil microbiota, adsorption with key regulators that mediate the fate of degradation for organic content, pH, divalent cations, and clay (most important for retention of NPs). According to Asadishad et al. [134], the toxicity of AgNPs depends upon microbial substrate-dependent respiration toward ammonia-oxidizing bacteria decreased with elevation pH content and clay content. Low pH causes the dissolution of AgNPs whereas high soil pH value enhances the negative charge site numbers and leads to increase Ag sorption [19]. In a study, similar results were reported about CuONPs (Copper oxide NPs) on low clay content and organic matter with coarse soil texture. Such acidic soil favors the dissolution of Ag and CuNPs with free ionic liberation, which can elevate the short-duration impact of NPs [9]. Zhai et al. [135] also concluded that nanoformulations of ionic pesticides can show the variable impact, more commonly associated with the fractional ion release. Other authors noted the difference and similarities of ionic and nanoforms of AgNPs with variation in antibacterial activity or the effect on a soil-borne microbial community and their response in in-vitro conditions [19, 136, 137].
In long-term studies, Guilger et al. [66], ensuring routes predictably depend on biogenic NPs, that show the least effect on human cells and denitrification process but are likely to show more impact on plant fungus relationship. At the microscale level, denitrification is a prime microbial activity that gets affected by AgNPs by modulating hydric conditions, pH and creating a devoid zone for fundamental accessories (carbon, nitrate, and oxygen). However, by high soil redox potential value and sandy texture soil favored denitrification, whereas textured clay soils provided offers low redox potential and lies in range for biological transformation [19]. Such impact is correlated by the affinity of AgNPs to denitrification and physicochemical properties ex: surface charge, coating, size, sedimentation rate, dispersibility, and solubility [138]. The biogenic AgNPs are derived from the green process and have no effect on N-cycle reported by Kumar et al. [67]. While the effect of nanocapsules, nanogels, nanometal, and nonmetal particles on soil microbiota as non-selected microbes has been documented. Li et al. [139] evidenced the negative impact of nanopesticide CM-β-CD-MNPs-Diuron complex (carboxymethyl-hdroxypropyl-β-cyclodextrin magnetic NPs) on the activity of the urease enzyme.
The Diuron NPs complex causes declined in the population status of soil bacteria except for actinobacteria with an increase in reactive oxygen species. All these indicate toxicity of CM-β-CD-MNPs-Diuron exert stress on soil microbes and did not reduce even by using Diuron nanoencapsulation [12, 19]. The bionanopesticides treatment was confirmed to improve soil microbiome including weight gain and survival percentages in beneficial earthworm Eudrilus eugeniae. It also shows excellent larvicidal, antifeedant, and pupicidal activities against Helicoverpa armigera and Spodoptera sp. at 100 ppm nanoformulation dose [19, 50, 55].
Drawbacks using nanoagrochemicals on plants
The nanopesticides are also showing some adverse effects on crop plants directly or indirectly. The most favorable and used AgNPs and their complex nanoparticle have been attributed to their diverse range in each class of pesticides due to low toxicity but still many reported published that explained the drawback of these smart nanoagrochemicals [61, 140, 141] (Table 4). For example, In Vicia faba, the AgNPs internalization in leaves can abrupt the stomatal conductance CO2 assimilation rate and photosystem II [142]. Furthermore, the binding of AgNPs attaches with Chlorophyll forming a hybrid, that excites electrons 10 times due to fast electron–hole separation and plasmon resonance effect. In another study, AgNPs and AgNPs-graphene oxide GO (Ag@dsDNA GO) effect also observed in L. esculentum exhibit antibacterial activity toward Xanthomonas perforans [143]. Various reports were submitted in recent years such as ZnO NPs reduced root growth in Allium cepa [89], Ch-NPs + paraquat biomass reduction, lipid peroxidation, genotoxicity and leaf necrosis in Brassica sp. [144], SiO2NPs affect biomass, germination, protein content, photosynthetic pigment in Taraxacum officinale and Amaranthus retroflexus [76], AgNPs cause lipid peroxidation, leaf damages and alters catalase activity in G. max [145], NPP ATZ + AMZ Raphanus raphanistrum suppresses plant growth [146].
Table 4.
Adverse effect of nanoparticles on targeted crop and soil health
| NPs | Size (nm) | Targeted crop | Adverse effect on plant | Degradation time in soil (days) | Effect on soil | Key references |
|---|---|---|---|---|---|---|
| Al2O3 | 50 | Nicotiana tabacum L. | Reduce the germination percentage, biomass per seedling, and average root length | 3 | Reduce the activity of bacteria Bacillus cereus and Pseudomonas stutzeri | [147, 148] |
| C60-fullerence | 50 | G. max (L.) Merr | Reduced biomass | 60 | Reduction of 20–30% in fast-growing protozoa and bacteria | [58, 149] |
| CuO, Ni, ZnO and Cr2O3 | 100 | Oryza sativa L. | Effect the activities of antioxidant enzymes in plant | 24 | Activity of enzyme dehydrogenase and urease reduced to 75% and 44% respectively | [221, 222] |
| ZnO and TiO2 | 10- 20 | T. aestivum L. | Reduced the root growth by 75% | 60 | Adversely affect the growth of earthworms, traces of ZnO and TiO2 were found inside the body | [61, 223] |
| Zn2+, Zn, and ZnO | 50 | Z. mays L. | 50% reduction in photosynthesis, leaf stomatal conductance, transpiration rate, and intercellular CO2 concentration | 56 | Reduce enzymes like β-glucosidase, phosphatase, and dehydrogenase present in the soil | [51, 150] |
| nZVI (zero valent iron) | 20–100 | Salix alba L. | Effect seedling growth | 7 | At 750 mg/kg, mortality rate of Lumbricus rubellus and Eisenia fetida was 100% | [53, 224] |
| Au | 25 | O. sativa L. | Damage to the root cell wall due to accumulation of Au across xylem | 30 | Effect the soil microbes and edaphic factors of soil | [52, 225] |
| TiO2, Ag, and CeO2 | 7–45 | A. cepa L. | Increase in DNA damage as well as lipid peroxidation in roots | 14 | Reduced the survival, growth and fertility of nematodes | [226] |
| SnO2, CeO2 and Fe3O4 | 61 (SnO2), 50–100 (CeO2), 20–30 (Fe3O4) | Z. mays L. | Fe3O4 results in accumulation of Al in plant roots and negatively affects plant growth | 63 | Inhibits microbial growth | [141] |
| Ag | 10–20 | P. vulgaris L. | Disrupt chlorophyll synthesis, nutrient uptake, and hormone regulation | 30 | 50% reduction in the activity of nitrifying bacteria | [158] |
Besides these, NPs show an adverse impact on plant physiology, soil microbiota, and declined enzymatic population. For instance; Al2O3 (Aluminium oxide) reduces bacterial growth and reduces seedling growth [147, 148], C60 fullerene restricts bacterial growth up to 20–30% [149], ZnNPs decrease enzymatic activities in soil and reduces transpiration rate and photosynthetic rate in Z. mays [150]. Conclusively, NPs are very reactive and variable in nature, so always a concerning risk for workers who may come across during their application.
Limitation and challenges at commercial scale implementation
As with documentation, the lack of finding on behavior and fate in the environment of nanoagrochemicals and their impact on faunal diversity may put challenges on their incorporation in agriculture. Instead of the benefits of using nanoencapsulation systems, their implementation requires caution, since it is mandatory to calculate their behavior in the environment and non-targeted communities to develop safer product development policies [54]. Although, it needs to develop smart nanoagrochemicals that are focused on biological nanoformulation and that offer a simple handling process, low cost, more AIs persistence with a sharp release system, and high degradation rate without leaving any residue [148]. Besides these, poor demonstrations at field conditions, cost-effectiveness, consumer acceptance, and feasibility of technology are major constraints on commercial implementation [152].
The limited management guidelines, inconsistence legislative framework, and regulatory models, and lack of public awareness campaign creates inconsistent marketing of such incipient nanoagricultural products. The national and international arrangement that fits at ground level is the only way that supports Nanotechnological development [49]. However, the community seeking approval for nanoagrochemicals must demonstrate the precautionary uses of these new products by proposing unjustifiable safety risks to the user and environment. Thus regulatory guidelines and frameworks are becoming primarily important to resolve the emerging issues of nanoagrochemicals [153]. Moreover, the need for collaboration, discussion, and information exchange forums among countries to ensure threat mitigating strategies should be considered as a milestone in nanoagrochemicals. So consolidates efforts of governmental organizations, scientists, and social communities are needed to preventing the adverse effect of nanoagrochemicals on humans and the environment [59].
In this scenario, the toxicity measuring instrumental setup is used in the characterization of toxicity type and their level to access the potential intrinsic hazards [59]. Currently, the main focus of experimental investigation on nanomaterial translocation in biotic/abiotic systems, monitoring and revealing interaction Among nanotoxicity and nanomaterial in the physical and chemical environment [48, 54, 151–153].
Transformation
Due to high reactivity, the interaction of nanocomponents with organic and inorganic components in the soil as well as for plants is undetermined and unregulated. The changing in physiochemical properties and transformation behavior after implementation creates chances of heavy metal toxicity. Biotransformation was demonstrated in Cucumis sativa, using CeO2 bioavailability cause 20% to Ce(III) in the shoots and 15% of Ce(IV) being reduced to Ce(III) in the roots [154]. In another study, AgNPs were oxidized and forming the Ag-glutathione complex in the lettuce plant [154].
Accumulation of NPs
Because of variability in binding, the accumulation of NPs causes toxicity in plants, humans, and animals. In soybean, CeO2 application shut down the Nitrogen fixation cycles and causes toxicity. However, ROS production, growth inhibition, cellular toxicity, and other phytotoxic effect were reported in Amaranthus tricolor. The application of C60 fullerene enhanced DDT accumulation in soybean, tomato, and zucchini plants [155].
Time to switch toward more sustainability
Most agrochemicals are not fully utilized by plants or seep off into the soil, air and water unintendedly causes toxic ill effects and accumulated through biomagnification. Moreover, global pesticide rise threatened biodiversity and led to the adverse effect on human intelligence quotient and fecundity in recent years. Still, it’s also enhancement the resistance in weeds and plant pathogen against agrochemical turn them to super pathogen/weed. New doses after the changing in strategies of pathogens or new strain resurgence enhance cost-effectiveness and put the question on existing regulatory recommendations. [14, 106, 156–158].
The chemicals persist in soil particles, agricultural residues, irrigation water and migrates into the different layers of soils turns into a serious threat to the ecosystem. Leaching of synthetic pesticides, abrupting soil-pest, soil-microbe activities, algal blooms formation, eutrophication, altering soil physiochemical properties [159], and salt toxicity via creating salt buildup in soil [160].
Low-cost oxides of Mg, Al, Fe, Ti, Ce, and Zn (Magnesium, Aluminium, Iron, Titanium, Cerium, Zinc) are ideal candidates and provides greater affinity, a large number of active sites, minimum intraparticle diffusion distance, and maximum specific surface area [160]. NP implementation help to successfully chase down the inorganic residues of various chemicals such as permethrin, 2–4 Dichlorophenoxy acetic acid (2–4-D), Dichlorodiphenyltrichloroethane (DCPT), Diuron (Adsorption), Chlorpyrifos, Chloridazon, Methomyl (Photocatalysis) from the soil. Some nanocomposites are used for complete degradation of lethal agrochemicals for example silver- doped TiO2 and gold doped TiO2, Zerovalent Fe (nZVI), endosulfan, TiO2, nZVI for atrazine, Ag for chlorpyrifos, Pd–Mg, Ni–Fe bimetallic system, nZVI for DDT, nZVI, nitrogen-doped TiO2, Fe–Pd (iron–palladium), Fe–S (Iron-sulfur) for Lindane [161] (Table 5).
Table 5.
Agrochemicals (insecticides, herbicides, and other fungicides) used to regulate the activity of crop pests under a sustainable agriculture approach
| Chemical | Trade manufacture company | Nanocomposites used | Crop | Target | Key references |
|---|---|---|---|---|---|
| Insecticides | |||||
| Inorganic | |||||
| Chlorpyrifos | Dow Chemical Company | PVC | G. hirustum L. | Aphis gossypii, Spodoptera frugiperda, and Lygus lineolaris | [227] |
| Dow AgroSciences | Chitosan/PLA | Solanum melongena L. | Pseudococcidae | [228] | |
| Chlorfenapyr | Super Bio Tech Marketing Company | Silica | Brassica rapa | Helicoverpa armigera | [94] |
| Avermectin | Super Bio Tech Marketing Company | Polystyrene nanoparticles (PHSN) | G. hirustum L. | Tetranychus urticae | [229] |
| Super Bio Tech Marketing Company | Polydopamine | Brassica oleraceae | Thysanoptera | [230] | |
| Azadirachtin | Ecobiocides & Botanicals Pvt Ltd | Chitosan | Ricinus communis L. | Spodoptera litura | [231] |
| Deltamethrin | Crop Chemicals India Limited | Chitosan-coated beeswax SLN (Solid–liquid nanoparticles) | G. hirustum L., S. lycopersicum L. | Helicoverpa zea, Leucinodes orbonalis | [185] |
| Imidacloprid | Chemet Wets & Flows Pvt. Ltd | Sodium alginate | Nicotiana tobacum L. | Cicadellidae | [232] |
| Geraniol | Otto Chemie Pvt Ltd | Chitosan/Gum Arabic | G. hirustum L. | Bemisia tabaci | [233] |
| Nicotine | Alchem International Pvt. Ltd | Chitosan/TPP | S. lycopersicum L. | Musca domestica | [234] |
| Organic | |||||
| Garlic essential oil | Arishtha Organics Pvt | PEG | O. sativa L. | Tribolium castaneum | [235] |
| A. arborescens L. essential oil | Priority Biocidal, LLC | SLN | S. lycopersicum L. | Sitophilus zeamais | [236] |
| Nanopermethrin | Jerobin J (Hamad medical corporation) | PEG | – | Culex quinquefasciatus | [237] |
| Geranium essential oils | India aroma oils and company | PEG | T. aestivum L. | Rhyzopertha dominica | [238] |
| Citrus peel essential oil | India aroma oils and company | PEG | S. lycopersicum L. | Tuta absoluta | [239] |
| Rosmarinus officinalis essential oil | Rosemary essential oil manufacturers & oem manufacturers India | PEG | O. sativa L. | Tribolium castaneum | [240] |
| Herbicides | |||||
| Paraquat | Syngenta | Montmorillonite | G. max L. | Plantago lanceolata L | [241] |
| Syngenta | Chitosan/tripolyphosphate | Brassica rapa L. | Soil sorption microalga | [242] | |
| Atrazine | Syngenta | Poly(ε-caprolactone) | S. bicolor L. | Stellaria media L., Trifolium repens L., Lamium amplexicaule L | [63] |
| SLN | Brassica napus | Raphanus raphanistrum L | |||
| Poly (lactic-co-glycolic acid) | Solanum tuberosum L. | Croton setigerus L., Oxalis corniculata L | |||
| Imazapic, Imazapyr | Avansagro chemicals shanghai limited | Alginate/chitosanChitosan/tripolyphosphate | A. cepa L. | B. pilosa L | [243] |
| Diuron | Adama Agan Ltd | Chitosan | Z. mays L. | Echinochloa crus-galli L. Beauv | [241] |
| 2,4-D | FirmLimited Company | Nanosized rice husk | T. repens L., Stellaria media L | ||
| Fungicides | |||||
| Tebuconazole | Super bio tech | PVP and PVP copolymer | Pinus taeda L. | Gloeophyllum trabeum | [244] |
| Bacterial ghosts | T. aestivum L., H. vulgare L. and Cucumis sativus L. | Erysiphe graminis and Sphaerotheca fuliginea | |||
| Super bio tech | Polymeric and SLN | P. vulgaris L. | A. niger | [11] | |
| Chlorothalonil | Rallis india limited | PVP and PVP copolymer | Pinus taeda L. | Gloeophyllum trabeum | [244] |
| Kathon 930 | Rohm and haas company | PVC | Betula alleghaniensis Britt | Trametes versicolor | [245] |
| Validamycin | Indochem agri science private limited | PHSN | S. tuberosum L. | R. solani | [246] |
| Pyraclostrobin | Shijiazhuangdailunchemical co. Ltd | Chitosan–PLA graft copolymer | Abelmoschus esculentus L. | Colletotrichum gossypii | [247] |
| Chitosan/MSN | S. lycopersicum L. | Puccinia asparagi | [248] | ||
| Ferbam | Loveland Products Canada Inc | Gold | Camellia sinensis | Pythium aphanidermatum | [249] |
| Carbendazim | Chemet Wets & Flows Pvt. Ltd | Chitosan/Pectin | Z. mays L. | F. oxysporum | [250] |
| Prochloraz | Lanfeng biochemical co., Ltd | PHSN | C. sativus L. | B. cinerea | [251] |
Smart agrochemical: a step ahead toward more sustainability
Al-Barly et al. reported the slow release of nanocomposite fertilizers to depend upon phosphate and nitrogen content availability in soil [162]. TiO2 NPs derived from Moringa oleifera leaf extract are used to control the red palm weevil (Rhynchophorus ferrugineus) and exhibits antioxidant and larvicidal activities. In the case of Zanthoxylum rhoifolium, nano-encapsulated essential oil was reported to maintain the population of Bemisia tabaci [19, 163]. Nanopesticides derived from pyrethrum insecticides cause an impact on the population status of honey bees. Except for these studies, agrochemical degradation can also be accomplished using adsorption, membrane filtration, catalytic degradation, oxidation, and biological treatment. Since, adsorption using smart Nanosorbents also relies on environmental factors including pH, temperature, and competitive adsorbing molecules [19]. At low pH, the protonated charged active site of NPs disturbs the binding ability of positively charge agrochemical whereas, high temperature creates hinders the electrochemical interactions between active sites and agrochemicals due to elevated vibrate energy of active site of adsorbent and kinetic energy of agrochemicals [79]. Moreover, chitosan-coated and cross-linked chitosan-Ag NPs used as composite microbeads that incorporated into reverse osmosis filters help in the effective removal of atrazine content from the water. According to Aseri et al. [164] integration of membrane filters and magnetic NPs-based beads enhances microbial elimination and resonance activation of water, respectively.
Secondly, targeting a not selected species with possible adverse effect is a key issue emerging that put a loophole of criticism for these smart nanoagrochemicals. For example; 1–10 mg L−1 of Polyhydroxybutyrate-co-hydroxyvalerate (PBHA) encapsulation for atrazine in lactuca sativa for 24 h reduced genotoxicity in plants [165], PCL atrazine nanocapsules ill effect on Daphnia similis and Pseudokirchneriella subcapitata, after exposure up to 24 h [166], Solid lipid NPs encapsulating simazine 0.025–0.25 mg mL−1 exhibits Caenorhabditis elegans Induction of mortality and decrease in the body length after exposure of 48 h [167]. The uncontrolled non-targeted release of AIs in plant cells causes lysosomal damage with increasing pH. After the cellular compartment, nanoagrochemicals may bind or channelization into cell organelles and causes damage to protein, pigments, and DNA [98].
The binding ability of nanocompositions with selected and non-selected binding helps to recognize its distribution, bioavailability, toxicity level, and exclusion from the plant cell. Several proteins acquire a wide range of functional and structural properties including ligand boding, metabolite production, catalysis, cellular and molecular reorganization [19]. The protein- nanopesticide complex can cause minor structural configuration and denaturation of proteins. Similarly, conformational changes and movement of the genomic DNA mediated through NPs also induced cytogenetic abnormalities. These nanopesticide toxicity are solely dependent upon the balance between key factors like biodegradability, concentration, and size of incorporated AIs. In Prochilodus lineatus 20 μg L−1 concentration using PCL nanocapsules containing atrazine up to 24–48 h declined toxicity, as they did not induce carbonic anhydrase activity, alterations in glycemia and antioxidant response [168], in Enchytraeus crypticus causes a decrease in hatching due to the delayed number of adults and juveniles [19, 158, 169].
No doubt, intervention of nanoagrochemicals, resolve many threats mitigation put forward by the implementation of agrochemical but still more validation is required to lowering the agroecological risks. The persistent use of novel monitoring applications always knocks down the door of improvement of sustainable crop production and protection without creating the threats of NPs as a new contaminant.
Conclusion and future perspectives
During the entire course of million years of evolution, the green plants had evolved without any interference from other eukaryotes. However, for the last fifty years, continuous human activities have introduced many contaminants in the environment that altered the ecological balance and raised the eye-brows of researchers towards combating the new pathovars and pathotypes. These thrusting biological stresses have severely damaged global crop production. Concerning, the environmental penalty of conventional agrochemicals at present, nanoformulations seem to be a potential applicant for plant protection. The use of controlled biodegradable polymers especially polyhydroxyalkanoates shows significant and attractive properties of biocompatibility, biosorption rate, low-cost synthesis, thermoplastic nature, and ease in biodegradation rate that have popular advantages conventional chemical delivery systems. However, sustainable and efficient utilization with promising target delivery and low toxic effects are prerequisites of commercial implementation. Although, the studies on the soil–plant microbiome and nanoscale characterization highlight the impact of chemical agrochemical on the environment.
The use of nanocoated AIs biopesticides is expected to surpass the challenges of chemical residual management gap and premature degradation of AIs. Instead, these, applying new nanocomponents along with existing chemicals should follow regular checks on resistance strategies of targeted organisms, new resistance pathways, and revolutionized pest strains. Although, smart agrochemicals or nanoagrochemicals resolve so many issues and gives an instant solution.
To ensure these, it is essential to develop more international and national risk assessment, management, and mitigating strategies. Beyond these challenges, social acceptance with reduced environmental cost chiefly soil deterioration, microbiome disruption, depleted water resources need keen monitoring. Ecologically, the continuum uses of agrochemical put the question on survival challenges result in more resistance races creating a vicious loop in which pesticides concentration help to revolutionizing the organism more toward superiority.
For this, alternative strategies with strong monitoring are required, together recommendations of IPM practices help to eliminate shortcomings in individual practices. Despite the advancement in studies on nanoformulation and plant response more extensions in genomic, proteomics, physiological, and metabolic studies help to understand the interaction in the mechanism.
Acknowledgements
Not applicable.
Abbreviations
- NPs
Nanoparticles
- NMs
Nanomaterils-based products
- AIs
Active ingreadents
- CRS
Controlled release system
- CR
Controlled release
- PLA
Poly lactic acid
- PLGA
Poly(lactic-co-glycolic acid)
- mPEG
Methoxy polyethylene glycol
- PCL
Poly(ε-caprolactone
- γ-PGA
(Poly (γ-glutamic acid)
- γ-GTP
(γ-Glutamyl transpeptidase)
- UV
Ultraviolet
- PEG
Polyethylene glycol
- CAGR
Compound annual growth rate
- IPM
Integrated pest management
- Ag+
Silver
- SiO2NPs
Silicon dioxide nanoparticles
- Ch-polymethacrylic NPK
Chitosan polymethacrylic nitrogen phosphorus potassium
- Au-NPs
Gold nanoparticles
- ZnO NPs
Zinc oxide nanoparticles
- CeO2-NPs
Cerium dioxide nanoparticles
- TiO2NPs
Titanium oxide nanoparticles
- S. oleracea
Spinacia oleracea
- Si NPs
Silicon nanoparticles
- V. mungo
Vigna mungo
- V. radiate
Vigna radiate
- C. arietinum
Cicer arietinum
- Ch-NPs
Chitosan nanoparticles
- CS-EO
Chitosan essential oil
- MDA
Malondialdehyde
- H2O2
Hydrogen peroxide
- PS II
Photosystem II
- Fe3O4 NPs
Iron oxide nanoparticles
- Fe NPs
Iron nanoparticles
- T. aesitivum
Triticum aestivum
- B. pilosa
Bidens pilosa
- C. dactylon
Cynodon dactylon
- AgNPs
Silver nanoparticles
- CM-β-CD-MNPs-Diuron complex
Carboxymethyl-hdroxypropyl-β-cyclodextrin magnetic nanoparticles diuron complex
- Ag@dsDNA GO
Ag@dsDNA-graphene oxide
- L. esculemtum
Lycopersicon esculentum
- Z. mays
Zea mays
- CeO2
Cerium dioxide
- ROS
Reactive oxygen species
- Mg
Magnesium
- Al
Aluminium
- Fe
Iron
- Ti
Titanium
- Ce
Cerium
- Zn
Zinc
- 2-4-D
2-4 Dichlorophenoxy acetic acid
- DCPT
DDT- Dichlorodiphenyltrichloroethane
- nZVI
Zerovalent iron
- Fe-Pd
Iron-palladium
- Fe-S
Iron-Sulphur
- PBHA
Polyhydroxybutyrate-co-hydroxyvalerate
- P. vulgaris
Phaseolus vulgaris
- C. annum
Capsicum annum
- S. oleracea
Spinacia oleracea
- B. juncea
Brassica juncea
- CNTs
Carbon nanotubes
- Cu3(PO4)2
Copper(II) phosphate
- X. perforans
Xanthomonas perforans
- B. sorokiniana
Bipolaris sorokiniana
- X. alfalfa
Xanthomonas alfalfa
- C. riparius
Chironomus riparius
- CrBR2.2
Balbiani ring protein gene
- CrGnRH1
Gonadotrophin-releasing hormone gene
- D. melanogaster
Drosophila melanogaster
- L. usitatissimum
Linum usitatissimum
- G. max
Glycine max
- SLN
Solid lipid nanoparticles
- G. hirusutum
Gossypium hirusutum
- PVA
Poly vinyl alcohol
- S. lycopersicum
Solanum lycopersicum
- S. bicolor
Sorghum bicolor
- PVC
Polyvinyl chloride
- PHSN
Polystyrene nanoparticles
- O. sativa
Oryza sativa
- SnO2
Stannic oxide
- H. vulgare
Hordeum vulgare
- A. cepa
Allium cepa
- T. repens
Trifolium repens
- H. vulgare
Hordeum vulgare
- S. tuberosum
Solanum tuberosum
- MSN
Mesoporous silica nanoparticles
- C. sativus
Cucumis sativus
- B. cinerea
Botrytis cinerea
Authors' contributions
The primary draft of the manuscript was prepared by AH and established by AC, AK, HK, and SM. SM reviewed the literature, AK and AC examined the manuscript and designed the table and figure section. AK, AC, and SM were involved in manuscript writing, and AH offered crucial advice and examined the entire manuscript on every step of writing. All authors read and approved the final version of the manuscript.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Declarations
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Antul Kumar, Email: antulkumar007@gmail.com.
Anuj Choudhary, Email: ajchoudhary784@gmail.com.
Harmanjot Kaur, Email: harmanjot.gill94@gmail.com.
Sahil Mehta, Email: sahilmehtasm21@gmail.com.
Azamal Husen, Email: adroot92@yahoo.co.in.
References
- 1.Zulfiqara F, Navarro M, Ashrafd M, Akrame NA, Munné-Boschb S. Nanofertilizer use for sustainable agriculture: advantages and limitations. Plant Sci. 2019;289:110270. doi: 10.1016/j.plantsci.2019.110270. [DOI] [PubMed] [Google Scholar]
- 2.Yu J, Wang D, Geetha N, Khawar KM, Jogaiah S, Mujtaba M. Current trends and challenges in the synthesis and applications of chitosan-based nanocomposites for plants: a review. Carbohydr Polym. 2021;261:117904. doi: 10.1016/j.carbpol.2021.117904. [DOI] [PubMed] [Google Scholar]
- 3.Mittal D, Kaur G, Singh P, Yadav K, Ali SA. Nanoparticle-based sustainable agriculture and food science: recent advances and future outlook. Front Nanotechnol. 2020;2:579954. doi: 10.3389/fnano.2020.579954. [DOI] [Google Scholar]
- 4.Husen H, Iqbal M. Nanomaterials and plant potential. Cham: Springer; 2019. [Google Scholar]
- 5.Husen H, Jawaid M. Nanomaterials for agriculture and forestry applications. Cambridge: Elsevier; 2020. [Google Scholar]
- 6.Husen H. Harsh environment and plant resilience (Molecular and Functional Aspects) Cham: Springer; 2021. [Google Scholar]
- 7.Husen H. Plant performance under environmental stress (Hormones, Biostimulants and Sustainable Plant Growth Management) Cham: Springer; 2021. [Google Scholar]
- 8.Bachheti RK, Fikadu A, Bachheti A, Husen A. Biogenic fabrication of nanomaterials from flower-based chemical compounds, characterization and their various applications: a review. Saudi J Biol Sci. 2020;27:2551–2562. doi: 10.1016/j.sjbs.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siddiqui MH, Al-Whaibi MH. Role of nano-SiO2 in germination of tomato (Lycopersicum esculentum seeds Mill) Saudi J Biol Sci. 2014;21:13–17. doi: 10.1016/j.sjbs.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sonika D, Saurav K, Aakash G, Uttam L, Ranjita T, Shankar J, Ganesh L, Deval PB, Niranjan P. Current research on silver nanoparticles: synthesis, characterization, and applications. J Nanomat. 2021;2021:6687290. doi: 10.1155/2021/6687290. [DOI] [Google Scholar]
- 11.Salem SS, Fouda A. Green synthesis of metallic nanoparticles and their prospective biotechnological applications: an overview. Biol Trace Elem Res. 2021;199:344–370. doi: 10.1007/s12011-020-02138-3. [DOI] [PubMed] [Google Scholar]
- 12.He X, Deng H, Hwang H. The current application of nanotechnology in food and agriculture. J Food Drug Anal. 2019;27:1–21. doi: 10.1016/j.jfda.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salem SS, Fouda MMG, Fouda A. Antibacterial, cytotoxicity and larvicidal activity of green synthesized selenium nanoparticles using Penicillium corylophilum. J Clust Sci. 2021;32:351–361. doi: 10.3390/jof7050372. [DOI] [Google Scholar]
- 14.Husen A, Siddiqi KS. Plants and microbes assisted selenium nanoparticles: characterization and application. J Nanobiotechnol. 2014;12:28. doi: 10.1186/s12951-014-0028-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khot LR, Sankaran S, Maja JM, Ehsani R, Schuster EW. Applications of nanomaterials in agricultural production and crop protection: a review. Crop Prot. 2012;35:64–70. doi: 10.1007/978-3-030-31938-0_7. [DOI] [Google Scholar]
- 16.Nair R, Varghese SH, Nair BG, Maekawa T, Yoshida Y, Kumar DS. Nanoparticulate material delivery to plants. Plant Sci. 2010;179:154–163. doi: 10.1016/j.plantsci.2010.04.012. [DOI] [Google Scholar]
- 17.Sharma A, Bachheti A, Sharma P, Bachheti RK, Husen A. Phytochemistry, pharmacological activities, nanoparticle fabrication, commercial products and waste utilization of Carica papaya L.: a comprehensive review. Curr Res Biotechnol. 2020;2:145–160. doi: 10.1016/j.crbiot.2020.11.001. [DOI] [Google Scholar]
- 18.Pandey A, Srivastava S, Aggarwal N. Assessment of the pesticidal behaviour of diacyl hydrazine-based ready-to-use nanoformulations. Chem Biol Technol Agric. 2020;7:10. doi: 10.1186/s40538-020-0177-9. [DOI] [Google Scholar]
- 19.Fraceto LF, Pascoli M, de Albuquerque FP, Calzavara AK, Tinoco-Nunes B, Oliveira WHC, Gonçalves KC. The potential of nanobiopesticide based on zein nanoparticles and neem oil for enhanced control of agricultural pests. J Pest Sci. 2020;93:793–806. doi: 10.1007/978-3-030-61985-5_17. [DOI] [Google Scholar]
- 20.Kamle M, Mahato DK, Devi S, Soni R, Tripathi V, Mishra AK, Kumar P. Nanotechnological interventions for plant health improvement and sustainable agriculture. Biotech. 2020;10:1–1. doi: 10.1007/s13205-020-2152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Özkara A, Akyıl D, Konuk M (2016) Pesticides, environmental pollution, and health. In: Environmental health risk-hazardous factors to living species 2016, p 16. 10.5772/63094
- 22.Titir G, Geetha G, Rita K, Amitava M. Nanocomposites for delivering agrochemicals: a comprehensive review. J Agric Food Chem. 2020;68:3691–3702. doi: 10.1021/acs.jafc.9b06982. [DOI] [PubMed] [Google Scholar]
- 23.Aouada FA, de Moura MR, Orts WJ, Mattoso LHC. Polyacrylamide and methylcellulose hydrogel as delivery vehicle for the controlled release of paraquat pesticide. J Mater Sci. 2010;45:4977–4985. doi: 10.1002/app.30339. [DOI] [Google Scholar]
- 24.Bortolin A, Aouada FA, de Moura MR, Ribeiro C, Longo E, Mattoso LHC. Application of polysaccharide hydrogels in adsorption and controlled-extended release of fertilizers processes. J Appl Polym Sci. 2012;123:2291–2298. doi: 10.1002/app.34742. [DOI] [Google Scholar]
- 25.Ghazali SAISM, Hussein MZ, Sarijo SH. 3,4-Dichlorophenoxyacetate interleaved into anionic clay for controlled release formulation of a new environmentally friendly agrochemical. Nanosc Res Lett. 2013;8:362. doi: 10.1186/1556-276X-8-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chuxiang S, Ke S, Wei W, Zhao Y, Tian L, Yuxiang G, Hua Z, Yihua Y. Encapsulation and controlled release of hydrophilic pesticide in shell cross-linked nanocapsules containing aqueous core. Int J Pharm. 2014;463:108–114. doi: 10.1016/j.ijpharm.2013.12.050. [DOI] [PubMed] [Google Scholar]
- 27.Wanyika H. Controlled release of agrochemicals intercalated into montmorillonite interlayer space. Sci World J. 2014;2014(1–15):656287. doi: 10.1155/2014/656287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cartmill AD, Cartmill DL, Alarcon A. Controlled release fertilizer increased phytoremediation of petroleum-contaminated sandy soil. Int J Phytorem. 2014;16:285–301. doi: 10.1080/15226514.2013.773280. [DOI] [PubMed] [Google Scholar]
- 29.Carson LC, Ozores-Hampton M, Morgan KT, Sargent SA. Effect of controlled-release and soluble fertilizer on tomato production and postharvest quality in seepage irrigation. Hort Sci. 2014;49:89–95. doi: 10.21273/HORTSCI.49.1.89. [DOI] [Google Scholar]
- 30.Sopena F, Maqueda C, Morillo E. Controlled release formulations of herbicides based on micro-encapsulation. Cienc Investig Agrar. 2009;35:27–42. doi: 10.4067/S0718-16202009000100002. [DOI] [Google Scholar]
- 31.Chevillard A, Angellier-Coussy H, Guillard V, Gontard N, Gastaldi E. Controlling pesticide release via structuring agropolymer and nanoclays based materials. J Hazard Mater. 2012;205:32–39. doi: 10.1016/j.jhazmat.2011.11.093. [DOI] [PubMed] [Google Scholar]
- 32.El-Temsah YS, Joner EJ. Effects of nano-sized zero-valent iron (nZVI) on DDT degradation in soil and its toxicity to collembola and ostracods. Chemosphere. 2013;92:131–137. doi: 10.1016/j.chemosphere.2013.02.039. [DOI] [PubMed] [Google Scholar]
- 33.Aouada FA, de Moura MR (2015) Nanotechnology applied in agriculture: controlled release of agrochemicals. In: Rai M, et al (eds) Nanotechnologies in food and agriculture. Springer. 10.1007/978-3-319-14024-7
- 34.Fauzia S, Furqani F, Zein R, Munaf E. Adsorption and reaction kinetics of tatrazine by using Annona muricata L. seeds. J Chem Pharm Res. 2015;7:573–582. [Google Scholar]
- 35.Stloukal P, Kucharczyk P, Sedlarik V, Bazant P, Koutny M. Low molecular weight polyijlactic acid) microparticles for controlled release of the herbicide metazachlor: preparation, morphology, and release kinetics. J Agric Food Chem. 2012;60:4111–4119. doi: 10.1021/jf300521j. [DOI] [PubMed] [Google Scholar]
- 36.Zielińska A, Carreiró F, Oliveira AM, Neves A, Pires B, Venkatesh DN, Durazzo A, Lucarini M, Eder P, Silva AM, Santini A, Souto EB. Polymeric nanoparticles: production, characterization, toxicology and ecotoxicology. Molecules. 2020;25:3731–3951. doi: 10.3390/molecules25163731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fu JX, Wang HJ, Zhou YQ, Wang JY. Antibacterial activity of ciprofloxacin-loaded zein microsphere films. Mater Sci Eng. 2009;29:1161–1166. doi: 10.1016/j.msec.2008.09.031. [DOI] [Google Scholar]
- 38.Hou Y, Hu J, Park H, Lee M. Chitosan based nanoparticles as a sustained protein release carrier for tissue engineering applications. J Biomed Mater Res Part A. 2012;100:939–947. doi: 10.1002/jbm.a.34031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalagatur NK, Nirmal Ghosh OS, Sundararaj N, Mudili V. Antifungal activity of chitosan nanoparticles encapsulated with Cymbopogon martinii essential oil on plant pathogenic fungi Fusarium graminearum. Front Pharmacol. 2018;9:610. doi: 10.3389/fphar.2018.00610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.El-Hamshary H, Fouda MMG, Moydeen M, El-Newehy MH, Al-Deyab SS, Megeed M. Synthesis and antibacterial of carboxymethyl starch-grafted poly (vinyl imidazole) against some plant pathogens. Int J Biol Macromol. 2016;72:1466–1472. doi: 10.1016/j.ijbiomac.2014.10.051. [DOI] [PubMed] [Google Scholar]
- 41.Ianchis R, Ninciuleanu CM, Gifu IC, Alexandrescu E, Somoghi R, Gabor AR, Preda S, Nistor CL, Nitu S, Petcu C, Icriverzi M, Florian PE, Roseanu AM. Novel hydrogel-advanced modified clay nanocomposites as possible vehicles for drug delivery and controlled release. NANO. 2017;7:443. doi: 10.3390/nano7120443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng M, Falkeborg M, Zheng Y, Yang T, Xu X. Formulation and characterization of nanostructured lipid carriers containing a mixed lipids core. Colloids Surf Physicochem Eng Asp. 2013;430:76–84. doi: 10.1016/j.jconrel.2013.01.018. [DOI] [Google Scholar]
- 43.Pan Y, Tikekar RV, Nitin N. Distribution of a model bioactive within solid lipid nanoparticles and nanostructured lipid carriers influences its loading efficiency and oxidative stability. Int J Pharm. 2016;511:322–330. doi: 10.1016/j.ijpharm.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 44.Chawla JS, Amiji MM. Biodegradable poly (ε- caprolactone) nanoparticles for tumor-targeted delivery of tamoxifen. Int J Pharm. 2002;249:127–138. doi: 10.1016/s0378-5173(02)00483-0. [DOI] [PubMed] [Google Scholar]
- 45.Orellana-Tavra C, Baxter EF, Tian T, Bennett TD, Slater NKH, Cheetham AK, Fairen-Jimenez D. Amorphous metal-organic frameworks for drug delivery. Chem Commun. 2015;51:13878–13881. doi: 10.1039/c5cc05237h. [DOI] [PubMed] [Google Scholar]
- 46.Lin G, Chen X, Zhou H, Zhou X, Xu H, Chen H. Elaboration of a feather keratin/carboxymethyl cellulose complex exhibiting ph sensitivity for sustained pesticide release. J Appl Polym Sci. 2019;136:47160. doi: 10.1002/app.47160. [DOI] [Google Scholar]
- 47.Ramasamy T, Ruttala HB, Gupta B, Poudel BK, Choi HG, Yong CS, Kim JO. Smart chemistry-based nanosized drug delivery systems for systemic applications: a comprehensive review. J Control Release. 2017;258:226–253. doi: 10.1016/j.jconrel.2017.04.043. [DOI] [PubMed] [Google Scholar]
- 48.Henchion M, McCarthy M, Dillon EJ. Big issues for a small technology: consumer trade-offs in acceptance of nanotechnology in food. Innov Food Sci Emerg Technol. 2019;58:102210. doi: 10.1016/j.ifset.2019.102210. [DOI] [Google Scholar]
- 49.Lai RWS, Yeung KWY, Yung MMN. Regulation of engineered nanomaterials: current challenges, insights and future directions. Environ Sci Pollut Res Int. 2018;25:3060–3077. doi: 10.1007/s11356-017-9489-0. [DOI] [PubMed] [Google Scholar]
- 50.Kamaraj C, Gandhi PR, Elango G, Karthi S, Chung IM, Rajakumar G. Novel and environmental friendly approach; Impact of Neem (Azadirachta indica) gum nanoformulation (NGNF) on Helicoverpa armigera (Hub.) and Spodoptera litura (Fab.) Int J Biol Macromol. 2018;107:59–69. doi: 10.1016/j.ijbiomac.2017.08.145. [DOI] [PubMed] [Google Scholar]
- 51.Vishnu D, Tatiana M, Arvind B, Svetlana NS, Saglara M, Ritu S, Andrey G, Viktoriia ST, William OP, Karen AG, Hasmik SM. Effects of zinc-oxide nanoparticles on soil, plants, animals and soil organisms: a review. Environ Nanotech Monitor Manag. 2018;9:76–84. doi: 10.1016/j.enmm.2017.12.006. [DOI] [Google Scholar]
- 52.Zoya J, Kavya D, Mansi M, Vinayak DF, Ayushi S. Effect of accumulation of nanoparticles in soil health- a concern on future. Front Nanosci Nanotech. 2019 doi: 10.15761/FNN.1000182. [DOI] [Google Scholar]
- 53.Zand AD, Tabrizi AM, Heir AV. Incorporation of biochar and nanomaterials to assist remediation of heavy metals in soil using plant species. Environ Technol Innov. 2020;20:101134. doi: 10.1016/j.eti.2020.101134. [DOI] [Google Scholar]
- 54.Pandey S, Giri K, Kumar R. Nanopesticides: opportunities in crop protection and associated environmental risks. Proc Natl Acad Sci India Sect B Biol Sci. 2018;88:1287–1308. doi: 10.1007/s40011-016-0791-2. [DOI] [Google Scholar]
- 55.Pavela R, Benelli G. Essential oils as eco-friendly biopesticides? Challenges and constraints. Trends Plant Sci. 2016;21:1000–1007. doi: 10.1016/j.tplants.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 56.Kremer RJ (2020) Bioherbicides and nanotechnology: current status and future trends. In: Nano-biopesticides today and future perspectives, Academic Press, pp 353–366. 10.1016/B978-0-12-815829-6.00015-2
- 57.Grillo R, Fraceto LF, Amorim MJ, Scott-Fordsmand JJ, Schoonjans R, Chaudhry Q. Ecotoxicological and regulatory aspects of environmental sustainability of nanopesticides. J Hazard Mater. 2020;404:124148. doi: 10.1016/j.jhazmat.2020.124148. [DOI] [PubMed] [Google Scholar]
- 58.Torre-Roche RDL, Hawthorne J, Deng Y, Xing B, Cai W, Newman LA, Wang Q, Ma X, Hamdi H, White JC. Multiwalled carbon nanotubes and C60 fullerenes differentially impact the accumulation of weathered pesticides in four agricultural plants. Environ Sci Technol. 2013;12:12539–12547. doi: 10.1021/es4034809. [DOI] [PubMed] [Google Scholar]
- 59.Kah M, Tufenkji N, White JC. Nano-enabled strategies to enhance crop nutrition and protection. Nat Nanotechnol. 2019;14:532–540. doi: 10.1038/s41565-019-0439-5. [DOI] [PubMed] [Google Scholar]
- 60.de Oliveira RL, de Mello PR, Felisberto G, Checchio MV, Gratão PL. Silicon mitigates manganese deficiency stress by regulating the physiology and activity of antioxidant enzymes in sorghum plants. J Soil Sci Plant Nutr. 2019;19:524–534. doi: 10.1007/s11738-013-1367-x. [DOI] [Google Scholar]
- 61.Ahmed B, Ameen F, Rizvi A, Ali K, Sonbol H, Zaidi A, Musarrat J. Destruction of cell topography, morphology, membrane, inhibition of respiration, biofilm formation, and bioactive molecule production by nanoparticles of Ag, ZnO, CuO, TiO2, and Al2O3 toward beneficial soil bacteria. ACS Omega. 2020;5:7861–7876. doi: 10.1021/acsomega.9b04084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chung I, Rekha K, Venkidasamy B, Thiruvengadam M. Effect of copper oxide nanoparticles on the physiology, bioactive molecules, and transcriptional changes in Brassica rapa ssp. rapa seedlings. Water Air Soil Pollut. 2019;230:48. doi: 10.1007/s11270-019-4084-2. [DOI] [Google Scholar]
- 63.Kremer RJ (2020) Bioherbicides and nanotechnology: current status and future trends. In: Nano-biopesticides today and future perspectives. Academic Press, pp 353–366. 10.1016/B978-0-12-815829-6.00015-2.
- 64.Fadoju OM, Osinowo OA, Ogunsuyi OI. Interaction of titanium dioxide and zinc oxide nanoparticles induced cytogenotoxicity in Allium cepa. Nucleus. 2020;63:159–166. doi: 10.1007/s13237-020-00308-1. [DOI] [Google Scholar]
- 65.Youssef MS, Elamawi RM. Evaluation of phytotoxicity, cytotoxicity, and genotoxicity of ZnO nanoparticles in V. faba. Environ Sci Pollut Res Int. 2018;5:1–13. doi: 10.1007/s11356-018-3250-1. [DOI] [PubMed] [Google Scholar]
- 66.Guilger M, Pasquoto-Stigliani T, Bilesky-Jose N, Grillo R, Abhilash PC, Fraceto LF, Lima R. Biogenic silver nanoparticles based on trichoderma harzianum: synthesis, characterization, toxicity evaluation and biological activity. Sci Rep. 2017;7:44421. doi: 10.1038/srep44421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kumar S, Bhanjana G, Sharma A, Dilbaghi N, Sidhu MC, Kim KH. Development of nanoformulation approaches for the control of weeds. Sci Total Environ. 2017;586:1272–1278. doi: 10.1016/j.scitotenv.2017.02.138. [DOI] [PubMed] [Google Scholar]
- 68.Chevillard H, Angellier-Coussy V, Guillard N, Gontard EG. Controlling pesticide release via structuring agropolymer and nanoclays based materials. J Hazard Mater. 2012;205:32–39. doi: 10.1016/j.jhazmat.2011.11.093. [DOI] [PubMed] [Google Scholar]
- 69.Wilpiszewska K, Spychaj T, Paździoch W. Carboxymethyl starch/montmorillonite composite microparticles: properties and controlled release of isoproturon. Carbohydr Polym. 2016;136:101–106. doi: 10.1016/j.carbpol.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 70.Francis S, Joseph S, Koshy EP, Mathew B. Green synthesis and characterization of gold and silver nanoparticles using Mussaenda glabrata leaf extract and their environmental applications to dye degradation. Environ Sci Pollut Res Int. 2017;24:17347–17357. doi: 10.1038/srep44421. [DOI] [PubMed] [Google Scholar]
- 71.Nafees M, Ali S, Rizwan M, Aziz A, Adrees M, Hussain S, Junaid M (2020) Effect of nanoparticles on plant growth and physiology and on soil microbes. In: Nanomaterials and environmental biotechnology. Springer, Cham, pp. 65–85. 10.1007/978-3-030-34544-0_5
- 72.Hasaneen M, Abdel-aziz HMM, Omer AM. Effect of foliar application of engineered nanomaterials: carbon nanotubes NPK and chitosan nanoparticles NPK fertilizer on the growth of French bean plant. Biochem Biotechnol Res. 2016;4:68–76. [Google Scholar]
- 73.Mujtaba M, Khawar KM, Camara MC, Carvalho LB, Fraceto LF, Morsi RE, Wang D. Chitosan-based delivery systems for plants: a brief overview of recent advances and future directions. Int J Biol Macromol. 2020;154:683–697. doi: 10.1016/j.ijbiomac.2020.03.128. [DOI] [PubMed] [Google Scholar]
- 74.Mahakham W, Theerakulpisut P, Maensiri S, Phumying S, Sarmah AK. Environmentally benign synthesis of phytochemicals-capped gold nanoparticles as nanopriming agent for promoting maize seed germination. Sci Total Environ. 2016;573:1089–1102. doi: 10.1016/j.scitotenv.2016.08.120. [DOI] [PubMed] [Google Scholar]
- 75.Ullah H, Li X, Peng L, Cai Y, Mielke HW. In vivo phytotoxicity, uptake, and translocation of PbS nanoparticles in maize (Z. mays L.) plants. Sci Total Environ. 2020;737:139558. doi: 10.1016/j.scitotenv.2020.139558. [DOI] [PubMed] [Google Scholar]
- 76.Sharif-Rad J, Sharif-Rad M, Teixeira da Silva JA. Morphological, physiological and biochemical responses of crops (Z. mays L., Phaseolus vulgaris L.), medicinal plants (Hyssopus officinalis L., Nigella sativa L.), and weeds (Amaranthus retroflexus L., Taraxacum officinale F. H. Wigg) exposed to SiO2 nanoparticles. J Agric Sci Technol. 2016;18:1027–1040. [Google Scholar]
- 77.Rezaei S, Ariaii P, Charmchian LM. The effect of encapsulated plant extract of hyssop (Hyssopus officinalis L.) in biopolymer nanoemulsions of Lepidium perfoliatum and Orchis mascula on controlling oxidative stability of soybean oil. Food Sci Nutr. 2020;8:1264–1271. doi: 10.1002/fsn3.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sabir S, Zahoor MA, Waseem M, Siddique MH, Shafique M, Imran M, Muzammil S. Biosynthesis of ZnO nanoparticles using Bacillus subtilis: characterization and nutritive significance for promoting plant growth in Z. mays L. Dose-Response. 2020;18:1559325820958911. doi: 10.1007/s00253-012-3934-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Saharan V, Mehrotra A, Khatik R, Rawal P, Sharma SS, Pal A. Synthesis of chitosan based nanoparticles and them in vitro evaluation against phytopathogenic fungi. Int J Biol Macromol. 2013;62:677–683. doi: 10.1016/j.ijbiomac.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 80.Choudhary RC, Kumaraswamy R, Kumari S, Sharma S, Pal A, Raliya R, Biswas P, Saharan V. Cu-chitosan nanoparticle boost defense responses and plant growth in maize (Z. mays L.) Sci Rep. 2017;7:9754. doi: 10.1038/s41598-017-08571-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Deshpande P, Dapkekar A, Oak MD, Paknikar KM, Rajwade JM. Zinc complexed chitosan/TPP nanoparticles: a promising micronutrient nanocarrier suited for foliar application. Carbohydr Polym. 2017;165:394–401. doi: 10.1016/j.carbpol.2017.02.061. [DOI] [PubMed] [Google Scholar]
- 82.Maluin FN, Hussein MZ. Chitosan-based agronanochemicals as a sustainable alternative in crop protection. Molecules. 2020;25:1611. doi: 10.3390/molecules25071611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pereira A, Sandoval-Herrera I, Zavala-Betancourt S, Oliveira H, Ledezma-Pérez A, Romero J, Fraceto L. γ-Polyglutamic acid/chitosan nanoparticles for the plant growth regulator gibberellic acid: characterization and evaluation of biological activity. Carbohydr Polym. 2017;157:1862–1873. doi: 10.1016/j.carbpol.2016.11.073. [DOI] [PubMed] [Google Scholar]
- 84.Khalifa NS, Hasaneen MN. The effect of chitosan–PMAA–NPK nanofertilizer on Pisum sativum plants. Biotech. 2018;8:193. doi: 10.1007/s13205-018-1221-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mansoor N, Younus A, Jamil Y, Shahid M. Impact of nanosized and bulk ZnO on germination and early growth response of T. aestivum. Pak J Agric Sci. 2019;56:879–884. [Google Scholar]
- 86.Adrees M, Khan ZS, Hafeez M, Rizwan M, Hussain K, Asrar M, Ali S. Foliar exposure of zinc oxide nanoparticles improved the growth of wheat (T. aestivum L.) and decreased cadmium concentration in grains under simultaneous Cd and water deficient stress. Ecotoxic Environ Saf. 2020;208:111627. doi: 10.1016/j.ecoenv.2020.111627. [DOI] [PubMed] [Google Scholar]
- 87.Wu F, Fang Q, Yan S, Pan L, Tang X, Ye W. Effects of zinc oxide nanoparticles on arsenic stress in rice (Oryza sativa L.): germination, early growth, and arsenic uptake. Environ Sci Pollut Res Int. 2020;27:26974–26981. doi: 10.1007/s11356-020-08965-0. [DOI] [PubMed] [Google Scholar]
- 88.Singh D, Kumar A. Quantification of metal uptake in Spinacia oleracea irrigated with water containing a mixture of CuO and ZnO nanoparticles. Chemosphere. 2020;243:125239. doi: 10.1016/j.chemosphere.2019.125239. [DOI] [PubMed] [Google Scholar]
- 89.Spielman-sun E, Avellan A, Bland GD, Tappero RV, Acerbo AS, Unrine JM, Giraldo JP, Lowry GV. Nanoparticle surface charge influences translocation and leaf distribution in vascular plants with contrasting anatomy. Environ Sci Nano. 2019;6:2508–2519. doi: 10.1039/C9EN00626E. [DOI] [Google Scholar]
- 90.Fox JP, Capen JD, Zhang W, Ma X, Rossi L. Effects of cerium oxide nanoparticles and cadmium on corn (Z. mays L.) seedlings physiology and root anatomy. NanoImpact. 2020;20:100264. doi: 10.1016/j.impact.2020.100264. [DOI] [Google Scholar]
- 91.Prakash S, Deswal R. Analysis of temporally evolved nanoparticle-protein corona highlighted the potential ability of gold nanoparticles to stably interact with proteins and influence the major biochemical pathways in Brassica juncea. Plant Physiol Biochem. 2020;146:143–156. doi: 10.1016/j.plaphy.2019.10.036. [DOI] [PubMed] [Google Scholar]
- 92.Ghorbani R, Movafeghi A, Gangeali A, Nabati J. Effects of TiO2 nanoparticles on morphological characteristics of chickpea (Cicer arietinum L.) under drought stress. Environ Stresses Crop Sci. 2021;14:85–98. doi: 10.22077/ESCS.2020.2485.1654. [DOI] [Google Scholar]
- 93.Buteler M, Gitto JG, Stadler T. Enhancing the potential use of microparticulate insecticides through removal of particles from raw grain. J Stored Prod Res. 2020;89:101707. doi: 10.1016/j.jspr.2020.101707. [DOI] [Google Scholar]
- 94.Kandil MAH, Sammour EA, Abdel-Aziz NF. Comparative toxicity of new insecticides generations against tomato leafminer Tuta absoluta and their biochemical effects on tomato plants. Bull Natl Res Cent. 2020;44:126. doi: 10.1186/s42269-020-00382-0. [DOI] [Google Scholar]
- 95.Li Z, Sellaoui L, Franco D, Netto MS, Georgin J, Dotto GL, Li Q. Adsorption of hazardous dyes on functionalized multiwalled carbon nanotubes in single and binary systems: experimental study and physicochemical interpretation of the adsorption mechanism. Chem Eng J. 2020;389:124467. doi: 10.1016/j.molliq.2020.114348. [DOI] [Google Scholar]
- 96.Zhang DX, Li BX, Zhang XP, Zhang ZQ, Wang WC, Liu F. Phoxim microcapsules prepared with polyurea and urea–formaldehyde resins differ in photostability and insecticidal activity. J Agric Food Chem. 2016;64:2841–2846. doi: 10.1021/acs.jafc.6b00231. [DOI] [PubMed] [Google Scholar]
- 97.Wibowo D, Zhao CX, Peters BC, Middelberg AP. Sustained release of fipronil insecticide in vitro and in vivo from biocompatible silica nanocapsules. J Agric Food Chem. 2014;12:504–511. doi: 10.1021/jf504455x. [DOI] [PubMed] [Google Scholar]
- 98.Jacques MT, Oliveira JL, Campos EVR, Fraceto LF, Ávila DS. Safety assessment of nanopesticides using the roundworm Caenorhabditis elegans. Ecotoxicol Environ Saf. 2017;139:245–253. doi: 10.1016/j.ecoenv.2017.01.045. [DOI] [PubMed] [Google Scholar]
- 99.Pasquoto-Stigliani T, Campos EVR, Oliveira JL, Silva CMG, Bilesky-José N, Guilger M, Troost J, Oliveira HC, Stolf-Moreira R, Fraceto LF, de Lima R. Nanocapsules containing neem (Azadirachta indica) oil: development, characterization, and toxicity evaluation. Sci Rep. 2017;7:5929. doi: 10.1038/s41598-017-06092-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mittal AK, Chisti Y, Banerjee UC. Synthesis of metallic nanoparticles using plant extracts. Biotechnol Adv. 2013;31:346–356. doi: 10.1016/j.biotechadv.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 101.Sebastian A, Nangia A, Prasad MNV. Cadmium and sodium adsorption properties of magnetite nanoparticles synthesized from Hevea brasiliensis Muell. Arg. Bark: relevance in amelioration of metal stress in rice. J Hazard Mater. 2019;371:261–272. doi: 10.1016/j.jhazmat.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 102.Sathiyabama M, Parthasarathy R. Biological preparation of chitosan nanoparticles and its in vitro antifungal efficacy against some phytopathogenic fungi. Carbohydr Polym. 2016;151:321–325. doi: 10.1016/j.carbpol.2016.05.033. [DOI] [PubMed] [Google Scholar]
- 103.Vokhidova NR, Sattarov ME, Kareva ND, Rashidova SS. Fungicide features of the nanosystems of silkworm (Bombyx mori) chitosan with copper ions. Microbiol. 2014;83:751–753. doi: 10.1134/S0026261714060204. [DOI] [PubMed] [Google Scholar]
- 104.Rubina MS, Vasil’kov AY, Naumkin AV, Shtykova EV, Abramchuk SS, Alghuthaymi MA, Abd-Elsalam KA. Synthesis and characterization of chitosan–copper nanocomposites and their fungicidal activity against two sclerotia-forming plant pathogenic fungi. J Nanostruct Chem. 2017;7:249–258. doi: 10.1007/s40097-017-0235-4. [DOI] [Google Scholar]
- 105.Xing K, Liu Y, Shen X, Zhu X, Li X, Miao X, Qin S. Effect of O-chitosan nanoparticles on the development and membrane permeability of Verticillium dahliae. Carbohydr Polym. 2017;165:334–343. doi: 10.1016/j.carbpol.2017.02.063. [DOI] [PubMed] [Google Scholar]
- 106.Kumaraswamy RV, Kumari S, Choudhary RC, Sharma SS, Pal A, Raliya R, Saharan V. Salicylic acid functionalized chitosan nanoparticle: a sustainable biostimulant for plant. Int J Biol Macromol. 2019;123:59–69. doi: 10.1016/j.ijbiomac.2018.10.202. [DOI] [PubMed] [Google Scholar]
- 107.Kaur P, Thakur R, Choudhary A. An in vitro study of the antifungal activity of silver/chitosan nanoformulations against important seed borne pathogens. Int J Sci Technol Res. 2012;1:83–86. [Google Scholar]
- 108.Cao L, Zhang H, Cao C, Zhang J, Li F, Huang Q. Quaternized chitosan-capped mesoporous silica nanoparticles as nanocarriers for controlled pesticide release. Nanomater. 2016;6:126. doi: 10.3390/nano6070126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Luque-Alcaraz AG, Cortez-Rocha MO, Velázquez-Contreras CA, Acosta-Silva AL, Santacruz-Ortega HDC, Burgos-Hernández A, Argüelles-Monal WM, Plascencia-Jatomea M. Enhanced antifungal effect of chitosan/pepper tree (Schinus molle) essential oil bionanocomposites on the viability of Asppergillus parasiticus Spores. J Nanomat. 2016;1:1–10. doi: 10.1007/s11356-020-10716-0. [DOI] [Google Scholar]
- 110.Cindi MD, Shittu T, Sivakumar D, Bautista-Banos S. Chitosan boehmite-alumina nanocomposite films and thyme oil vapour control brown rot in peaches (Prunus persica L.) during postharvest storage. Crop Prot. 2015;72:127–131. doi: 10.1016/jcropro.2015.03.011. [DOI] [Google Scholar]
- 111.Ngoc UTP, Nguyen DH. Synergistic antifungal effect of fungicide and chitosan-silver nanoparticles on Neoscytalidium dimidiatum. Green Proc Synth. 2018;7:132–138. doi: 10.1515/gps-2016-0206. [DOI] [Google Scholar]
- 112.Hossaina F, Follettb P, Salmieria S, Vua KD, Fraschinic C, Lacroixa M. Antifungal activities of combined treatments of irradiation and essential oils (EOs) encapsulated chitosan nanocomposite films in in vitro and in situ conditions. Int J Food Microbiol. 2019;295:33–40. doi: 10.3389/fmicb.2021.613155. [DOI] [PubMed] [Google Scholar]
- 113.Medina E, Caro N, Abugoch L, Gamboa A, Diaz-Dosque M, Tapia C. Chitosan thymol nanoparticles improve the antimicrobial effect and the water vapour barrier of chitosan-quinoa protein films. J Food Eng. 2019;240:191–198. doi: 10.1016/j.jfoodeng.2018.07.023. [DOI] [Google Scholar]
- 114.Arumugam G, Velayutham V, Shanmugavel S, Sundaram J. Efficacy of nanostructured silica as a stored pulse protector against the infestation of bruchid beetle, Callosobruchus maculatus (Coleoptera: Bruchidae) Appl Nanosci. 2016;6:445–450. doi: 10.1007/s13204-015-0446-2. [DOI] [Google Scholar]
- 115.Ahmad J, Qamar S, Kausar N, Qureshi MI (2020). Nanoparticles: the magic bullets in mitigating drought stress in plants. In: Nanobiotechnology in agriculture. Springer, Cham, pp 145–161. 10.1007/978-3-030-39978-8_8
- 116.Singh S, Husen A. Behavior of agricultural crops in relation to nanomaterials under adverse environment al conditions. In: Husen A, Jawaid M, editors. Nanomaterials for agriculture and forestry applications. Cambridge: Elsevier; 2020. pp. 219–256. [Google Scholar]
- 117.Singh S, Husen A. Role of nanomaterials in the mitigation of abiotic stress in plants. In: Husen A, Iqbal M, editors. Nanomaterials and plant potential. Cham: Springer; 2019. pp. 441–471. [Google Scholar]
- 118.Husen A (2021) The Harsh environment and resilient plants: an overview. In: Husen A (Eds.) Harsh environment and plant resilience, pp 1–23. 10.1007/978-3-030-65912-7_1
- 119.Yusefi-Tanha E, Fallah S, Rostamnejadi A, Pokhrel LR. Zinc oxide nanoparticles (ZnONPs) as a novel nanofertilizer: Influence on seed yield and antioxidant defense system in soil grown soybean (Glycine max cv. Kowsar) Sci Total Environ. 2020;738:140240. doi: 10.1016/j.scitotenv.2020.140240. [DOI] [PubMed] [Google Scholar]
- 120.Van HC, Van ND, Nguyen HM, Le NT, Nguyen KH, Le HM. Copper nanoparticle application enhances plant growth and grain yield in maize under drought stress conditions. bioRxiv. 2020 doi: 10.1101/2020.02.24.963132. [DOI] [Google Scholar]
- 121.An J, Hu P, Li F, Wu H, Shen Y, White JC, Giraldo JP. Emerging investigator series: molecular mechanisms of plant salinity stress tolerance improvement by seed priming with cerium oxide nanoparticles. Environ Sci Nano. 2020;7:2214–2228. doi: 10.1039/D0EN00387E. [DOI] [Google Scholar]
- 122.Attia MS, Osman MS, Mohamed AS, Mahgoub HA, Garada MO, Abdelmouty ES. Impact of foliar application of chitosan dissolved in different organic acids on isozymes, protein patterns and physio-biochemical characteristics of tomato grown under salinity stress. Plants. 2021;10:388. doi: 10.3390/plants10020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sikder RK, Wang X, Zhang H, Gui H, Dong Q, Jin D, Song M. Nitrogen enhances salt tolerance by modulating the antioxidant defense system and osmoregulation substance content in Gossypium hirsutum. Plants. 2020;9:450. doi: 10.3390/plants9040450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ioannou A, Gohari G, Papaphilippou P, Panahirad S, Akbari A, Dadpour MR, Fotopoulos V. Advanced nanomaterials in agriculture under a changing climate: the way to the future? Environ Exp Bot. 2020;176:104048. doi: 10.1016/j.envexpbot.2020.104048. [DOI] [Google Scholar]
- 125.Khan N, Bano AMD, Babar A. Impacts of plant growth promoters and plant growth regulators on rainfed agriculture. PLoS ONE. 2020;15:e0231426. doi: 10.1371/journal.pone.0231426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Soliman M, Qari SH, Abu-Elsaoud A, El-Esawi M, Alhaithloul H, Elkelish A. Rapid green synthesis of silver nanoparticles from blue gum augment growth and performance of maize, fenugreek, and onion by modulating plants cellular antioxidant machinery and genes expression. Acta Physiol Plant. 2020;42:1–16. doi: 10.3390/plants10040790. [DOI] [Google Scholar]
- 127.Iftikhar A, Rizwan M, Adrees M, Ali S, Rehman MZ, Qayyum MF, Hussain A. Effect of gibberellic acid on growth, biomass, and antioxidant defense system of wheat (T. aestivum L.) under cerium oxide nanoparticle stress. Environ Sci Pollution Res. 2020;27:33809–33820. doi: 10.1007/s11356-020-09661-9. [DOI] [PubMed] [Google Scholar]
- 128.Shoemaker AG. The effects of titanium dioxide nanoparticles on the growth and development of Sorghum Bicolor (L.) Moenech. Adv Agric Hortic Entomol. 2020;132:1–15. [Google Scholar]
- 129.Maruyama CR, Guilger M, Pascoli M, Bileshy-José M. Nanoparticles based on chitosan as carriers for the combined herbicides imazapic and imazapyr. Sci Rep. 2016;6:19768. doi: 10.1038/srep19768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Preisler AC, Pereira AES, Campos EVR, Dalazen G, Fraceto LF, Oliveira HC. Atrazine nanoencapsulation improves pre-emergence herbicidal activity against Bidens pilosa without enhancing long-term residual effect on Glycine max. Pest Manag Sci. 2020;76:141–149. doi: 10.1002/ps.5482. [DOI] [PubMed] [Google Scholar]
- 131.Sousa GFM, Gomes DG, Campos EVR, Oliveira JL, Fraceto LF, Stolf-Moreira R, Oliveira HC. Post-emergence herbicidal activity of nanoatrazine against susceptible weeds. Front Environ Sci. 2018;6:1–6. doi: 10.1007/s10311-019-00912-x. [DOI] [Google Scholar]
- 132.Xiang Y, Zhang G, Chi Y, Cai D, Wu Z. Fabrication of a controllable nanopesticide system with magnetic collectability. Chem Eng J. 2017;328:320–330. doi: 10.1021/acssuschemeng.7b00348. [DOI] [Google Scholar]
- 133.Schlich K, Hund-Rinke K. Influence of soil properties on the effect of silver nanomaterials on microbial activity in five soils. Environ Pollut. 2015;196:321–330. doi: 10.1016/j.envpol.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 134.Asadishad B, Chahal S, Akbari A, Cianciarelli V, Azodi M, Ghoshal S, Tufenkji N. Amendment of agricultural soil with metal nanoparticles: effects on soil enzyme activity and microbial community composition. Environ Sci Technol. 2018;52:1908–1918. doi: 10.1021/acs.est.7b05389. [DOI] [PubMed] [Google Scholar]
- 135.Zhai Y, Hunting ER, Wouters M, Peijnenburg WJGM, Vijver MG. Silver nanoparticles, ions, and shape governing soil microbial functional diversity: nano shapes micro. Front Microbiol. 2016;7:1123. doi: 10.3389/fmicb.2016.01123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kędziora A, Speruda M, Krzyżewska E, Rybka J, Łukowiak A, Bugla-Płoskońska G. Similarities and differences between silver ions and silver in nanoforms as antibacterial agents. Int J Mol Sci. 2018;19:444. doi: 10.3390/ijms19020444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.VandeVoort AR, Skipper H, Arai Y. Macroscopic assessment of nanosilver toxicity to soil denitrification kinetics. J Environ Qual. 2014;43:1424–1430. doi: 10.2134/jeq2013.12.0524. [DOI] [PubMed] [Google Scholar]
- 138.El-Temsah YS, Joner EJ. Impact of Fe and Ag nanoparticles on seed germination and differences in bioavailability during exposure in aqueous suspension and soil. Environ Toxicol. 2012;27:42–49. doi: 10.1002/tox.20610. [DOI] [PubMed] [Google Scholar]
- 139.Li F, Chen Y, Tang DM, Jian Z, Liu C, Golberg D, Yamada A, Zhou H. Performance-improved Li–O 2 battery with Ru nanoparticles supported on binder-free multi-walled carbon nanotube paper as cathode. Energy Environ Sci. 2014;7:1648–1652. doi: 10.1039/C3EE44043E. [DOI] [Google Scholar]
- 140.Dogaroglu ZG, Koleli N (2014) Effect of different zinc oxide nanoparticles on germination, plant growth and chlorophyll content of wheat. In: International congress on green infrastructure and sustainable socities/cities greinsus, p 78. 10.13254/j.jare.2020.0099
- 141.Lei Y, Peiye L, Xiaopeng Z, Rong J, Lijuan Z. Physiological and metabolic responses of maize (Z. mays) plants to Fe3O4 nanoparticles. Sci Total Environ. 2020;718:1–36. doi: 10.1016/j.scitotenv.2020.137400. [DOI] [PubMed] [Google Scholar]
- 142.Falco WF, Scherer MD, Oliveira SL, Wender H, Colbeck I, Lawson T, Caires ARL. Phytotoxicity of silver nanoparticles on V. faba: evaluation of particle size effects on photosynthetic performance and leaf gas exchange. Sci Total Environ. 2019;701:134816. doi: 10.1016/j.scitotenv.2019.134816. [DOI] [PubMed] [Google Scholar]
- 143.Oliveira E, Núñez C, Santos HM, Fernández-Lodeiro J, Fernández-Lodeiro A, Capelo JL, Lodeiro C. Revisiting the use of gold and silver functionalised nanoparticles as colorimetric and fluorometric chemosensors for metal ions. Sens Actuators B Chem. 2015;212:297–328. doi: 10.1016/j.snb.2015.02.026. [DOI] [Google Scholar]
- 144.Scherer MD, Sposito JC, Falco WF, Grisolia AB, Andrade LH, Lima SM, Machado G, Nascimento VA, Gonçalves DA, Wender H, Oliveira SL. Cytotoxic and genotoxic effects of silver nanoparticles on meristematic cells of Allium cepa roots: a close analysis of particle size dependence. Sci Total Environ. 2019;660:459–467. doi: 10.1016/j.scitotenv.2018.12.444. [DOI] [PubMed] [Google Scholar]
- 145.Galazzi RM, Arruda MAZ. Evaluation of changes in the macro and micronutrients homeostasis of transgenic and non-transgenic soybean plants after cultivation with silver nanoparticles through ionomic approaches. J Trace Elem Med Biol. 2018;48:181–187. doi: 10.1016/j.jtemb.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 146.Pereira AE, Grillo R, Mello NF, Rosa AH, Fraceto LF. Application of poly(epsilon-caprolactone) nanoparticles containing atrazine herbicide as an alternative technique to control weeds and reduce damage to the environment. J Hazard Mater. 2014;268:207–215. doi: 10.1016/j.jhazmat.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 147.Latef AAHA, Zaid A, Alhmad MFA, Abdelfattah KE. The impact of priming with Al2O3 nanoparticles on growth, pigments, osmolytes, and antioxidant enzymes of Egyptian Roselle (Hibiscus sabdariffa L.) cultivar. Agronomy. 2020;10:681. doi: 10.3390/agronomy10050681. [DOI] [Google Scholar]
- 148.Burklew CE, Ashlock J, Winfrey WB, Zhang B. Effects of aluminum oxide nanoparticles on the growth, development, and microRNA expression of tobacco (Nicotiana tabacum) PLoS ONE. 2012;7:e34783. doi: 10.1371/journal.pone.0034783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ahmadi SZ, Ghorbanpour M, Aghaee A, Hadian J. Deciphering morpho-physiological and phytochemical attributes of Tanacetum parthenium L. plants exposed to C60 fullerene and salicylic acid. Chemosphere. 2020;259:127406. doi: 10.1016/j.chemosphere.2020.127406. [DOI] [PubMed] [Google Scholar]
- 150.Caldelas MC, Poitrasson F, Viers J, Araus OJL. Stable Zn isotopes reveal the uptake and toxicity of zinc oxide engineered nanomaterials in Phragmites australis. Environ Sci Nano. 2020;7:1927–1941. doi: 10.1039/D0EN00110D. [DOI] [Google Scholar]
- 151.Lombi E, Donner E, Dusinska M. One health approach to managing the applications and implications of nanotechnologies in agriculture. Nat Nanotechnol. 2019;14:523–531. doi: 10.1038/s41565-019-0460-8. [DOI] [PubMed] [Google Scholar]
- 152.Mitter N, Hussey K. Moving policy and regulation forward for nanotechnology applications in agriculture. Nat Nanotech. 2019;14:508–510. doi: 10.1038/s41565-019-0464-4. [DOI] [PubMed] [Google Scholar]
- 153.Kumar JN, Bora A, Kumar RN, Amb MK, Khan S. Toxicity analysis of pesticides on cyanobacterial species by 16S rDNA molecular characterization. Proc Int Acad Ecol Environ Sci. 2013;3:101. [Google Scholar]
- 154.Hong JR. Evidence of translocation and physiological impacts of foliar applied CeO2 nanoparticles on cucumber (Cucumis sativus) plants. Environ Sci Technol. 2014;48:4376–4385. doi: 10.1021/es404931g. [DOI] [PubMed] [Google Scholar]
- 155.Torre-Roche RDL. Fullerene-enhanced accumulation of p, p’-DDE in agricultural crop species. Environ Sci Technol. 2012;46:9315–9323. doi: 10.1021/es301982w. [DOI] [PubMed] [Google Scholar]
- 156.Kumar SS, Venkateswarlu P, Rao VR, Rao GN. Synthesis, characterization and optical properties of zinc oxide nanoparticles. Int Nano Lett. 2013;3:1–6. doi: 10.1186/2228-5326-3-30. [DOI] [Google Scholar]
- 157.Pejam F, Ardebili ZO, Ladan-Moghadam A, Danaee E. Zinc oxide nanoparticles mediated substantial physiological and molecular changes in tomato. PLoS ONE. 2021;16(3):e0248778. doi: 10.1371/journal.pone.0248778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Prażak R, Święciło A, Krzepiłko A, Michałek S, Arczewska M. Impact of Ag nanoparticles on seed germination and seedling growth of green beans in normal and chill temperatures. Agriculture. 2020;10:312. doi: 10.3390/agriculture10080312. [DOI] [Google Scholar]
- 159.Sebastian A, Prasad MNV. Trace element management in rice. Agron. 2015;5:374–404. doi: 10.3390/agronomy5030374. [DOI] [Google Scholar]
- 160.Khan NM, Mobin M, Zahid A, Alamri S. Fertilizers and their contaminants in soils, surface, and groundwater. Encycl Anthropocene. 2018;5:225–240. doi: 10.1016/B978-0-12-409548-9.09888-2. [DOI] [Google Scholar]
- 161.Sebastian A, Nangia A, Majeti N, Vara P. Advances in agrochemical remediation using nanoparticles Agrochemicals Detection. Treat Remediat. 2020 doi: 10.1016/B978-0-08-103017-2.00018-0465-484. [DOI] [Google Scholar]
- 162.Al-Barly AMF, Hamza RZ. Larvicidal, ani-oxidant activities and perturbation of transaminases activities of titanium dioxide nanoparticles synthesized using Moringa oleifera leaves extract against the red palm weevil (Rhynchophorus ferrugineus) Eur J Pharm Med Res. 2015;2:49–54. doi: 10.1080/21691401.2017.1408121. [DOI] [Google Scholar]
- 163.Christofoli M, Costa ECC, Bicalho KU, de Cassia DV, Peixoto MF, Alves CCF, de Melo CC. Insecticidal effect of nanoencapsulated essential oils from Zanthoxylum rhoifolium (Rutaceae) in Bemisia tabaci populations. Ind Crops Prod. 2015;70:301–308. doi: 10.1016/j.indcrop.2015.03.025. [DOI] [Google Scholar]
- 164.Aseri A, Garg SK, Nayak A, Trivedi SK, Mohamed A. Magnetic nanoparticles: magnetic nano-technology using biomedical applications and prospects. Int J Pharm Sci Rev Res. 2015;31:119–131. doi: 10.1002/adhm.201700845. [DOI] [Google Scholar]
- 165.You G, Hou J, Xu Y, Miao L, Ao Y, Xing B. Surface properties and environmental transformations controlling the bioaccumulation and toxicity of cerium oxide nanoparticles: a critical review. Rev Environ Contam Toxicol. 2021;253:155–206. doi: 10.1007/398_2020_42. [DOI] [PubMed] [Google Scholar]
- 166.Grillo R, de Melo NF, de Araújo DR, de Paula E, Rosa AH, Fraceto LF. Polymeric alginate nanoparticles containing the local anesthetic bupivacaine. J Drug Target. 2010;18:688–699. doi: 10.3109/10611861003649738. [DOI] [PubMed] [Google Scholar]
- 167.Clemente Z, Castro VL, Moura MA, Jonsson CM, Fraceto LF. Toxicity assessment of TiO2 nanoparticles in zebrafish embryos under different exposure conditions. Aquat Toxicol. 2014;147:129–139. doi: 10.1016/j.aquatox.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 168.Ahsan SM, Rao CM, Ahmad MF. Nanoparticle-protein interaction: the significance and role of protein corona. Adv Exp Med Biol. 2018;1048:175–198. doi: 10.1007/978-3-319-72041-8_11. [DOI] [PubMed] [Google Scholar]
- 169.Severino P, da Silva CF, Andrade LN, de Lima OD, Campos J, Souto EB. Alginate nanoparticles for drug delivery and targeting. Curr Pharm Des. 2019;25:1312–1334. doi: 10.2174/1381612825666190425163424. [DOI] [PubMed] [Google Scholar]
- 170.Silva AM, Alvarado HL, Abrego G, Martins-Gomes C, Garduño-Ramirez ML, García ML, Calpena AC, Souto EB. In vitro cytotoxicity of oleanolic/ursolic acids-loaded in PLGA nanoparticles in different cell lines. Pharmaceutics. 2019;11:362. doi: 10.3390/pharmaceutics11080362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Khati P, Chaudhary P, Gangola S, Bhatt P, Sharma A (2017) Nanochitosan supports growth of Z. mays and also maintains soil health following growth. Biotech 7:81. doi: 10.1007/s13205-017-0668-y. [DOI] [PMC free article] [PubMed]
- 172.Kubavat D, Trivedi K, Vaghela P, Prasad K, Vijay Anand GK, Trivedi H, Ghosh A. Characterization of a chitosan-based sustained release nanofertilizer formulation used as a soil conditioner while simultaneously improving biomass production of Z. mays L. Land Degrad Dev. 2020;31:2734–2746. doi: 10.1002/ldr.3629. [DOI] [Google Scholar]
- 173.Santo Pereira AE, Silva PM, Oliveira JL, Oliveira HC, Fraceto LF. Chitosan nanoparticles as carrier systems for the plant growth hormone gibberellic acid. Colloids Surf B Biointerfaces. 2017;150:141–152. doi: 10.1016/j.colsurfb.2016.11.027. [DOI] [PubMed] [Google Scholar]
- 174.El-Gazzar N, Almaary K, Ismail A, Polizzi G. Influence of Funneliformis mosseae enhanced with titanium dioxide nanoparticles (TiO2NPs) on Phaseolus vulgaris L under salinity stress. PLoS ONE. 2020;15:e0235355. doi: 10.1371/journal.pone.0235355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zayed MF, Eisa WH, Hezma AM. Spectroscopic and antibacterial studies of anisotropic gold nanoparticles synthesized using Malva parviflora. J Appl Spect. 2017;83:1046–1050. doi: 10.1007/s10812-017-0406-6. [DOI] [Google Scholar]
- 176.Mirbolook A, Rasouli-Sadaghiani M, Sepehr E, Lakzian A, Hakimi M. Synthesized Zn (II)-amino acid and-chitosan chelates to increase Zn uptake by bean (Phaseolus vulgaris) plants. J Plant Growth Regul. 2020 doi: 10.1007/s00344-020-10151-y. [DOI] [Google Scholar]
- 177.Asgari-Targhi G, Iranbakhsh A, Ardebili ZO. Potential benefits and phytotoxicity of bulk and nano-chitosan on the growth, morphogenesis, physiology, and micropropagation of Capsicum annuum. Plant Physiol Biochem. 2018;127:393–402. doi: 10.1016/j.plaphy.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 178.Esyanti RR, Farah N, Bajra BD, Nofitasari D, Martien R, Sunardi S, Safitri R (2020) Comparative study of nano-chitosan and synthetic bactericide application on chili pepper (Capsicum annuum L.) infected by Xanthomonas campestris. Agrivita 42:13. 10.17503/agrivita.v42i1.1283
- 179.Maity A, Natarajan N, Vijay D, Srinivasan R, Pastor M, Malaviya DR. Influence of metal nanoparticles (NPs) on germination and yield of Oat (Avena sativa) and Berseem (Trifolium alexandrinum) Proc Natl Acad Sci India Sect B Biol Sci. 2018;88:595–607. doi: 10.1007/s40011-016-0796-x. [DOI] [Google Scholar]
- 180.Shahhoseini R, Azizi M, Asili J, Moshtaghi N, Samiei L. Effects of zinc oxide nanoelicitors on yield, secondary metabolites, zinc and iron absorption of Feverfew (Tanacetum parthenium (L.) Schultz Bip.) Acta Physiol Plant. 2020;42:1–18. doi: 10.1007/s11738-020-03043-x. [DOI] [Google Scholar]
- 181.Muthukrishnan S, Murugan I, Selvaraj M. Chitosan nanoparticles loaded with thiamine stimulate growth and enhances protection against wilt disease in Chickpea. Carbohydr Polym. 2019;212:169–177. doi: 10.1016/j.carbpol.2019.02.037. [DOI] [PubMed] [Google Scholar]
- 182.Zhang H, Lu L, Zhao X, Zhao S, Gu X, Du W, Wei H, Ji R, Zhao L. Metabolomics reveals the “invisible” responses of spinach plants exposed to CeO2 nanoparticles. Environ Sci Technol. 2019;53:6007–6017. doi: 10.1021/acs.est.9b00593. [DOI] [PubMed] [Google Scholar]
- 183.Samadi S, Saharkhiz MJ, Azizi M, Samiei L, Ghorbanpour M. Multi-walled carbon nanotubes stimulate growth, redox reactions and biosynthesis of antioxidant metabolites in Thymus daenensis celak in vitro. Chemosphere. 2020;249:126069. doi: 10.1016/j.chemosphere.2020.126069. [DOI] [PubMed] [Google Scholar]
- 184.Mondal AH, Yadav D, Ali A, Khan N, Jin JO, Haq QMR. Anti-bacterial and anti-candidal activity of silver nanoparticles biosynthesized using Citrobacter spp MS5 culture supernatant. Biomolecules. 2020;10:944. doi: 10.3390/biom10060944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tailor G, Yadav BL, Chaudhary J, Joshi M, Suvalka C. Green synthesis of silver nanoparticles using Ocimum canum and their anti-bacterial activity. Biochem Biophys Rep. 2020;24:100848. doi: 10.1016/j.bbrep.2020.100848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Francisco J, Frank LWT. Biocontrol by Fusarium oxysporum using endophyte-mediated resistance. Front Plant Sci. 2020 doi: 10.3389/fpls.2020.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Madany MM, Saleh AM, Habeeb TH, Hozzein WN, AbdElgawad H. Silicon dioxide nanoparticles alleviate the threats of broomrape infection in tomato by inducing cell wall fortification and modulating ROS homeostasis. Environ Sci Nano. 2020;7:1415–1430. doi: 10.1039/C9EN01255A. [DOI] [Google Scholar]
- 188.Enyedi NT, Makk J, Kótai L. Cave bacteria-induced amorphous calcium carbonate formation. Sci Rep. 2020;10:8696. doi: 10.1038/s41598-020-65667-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Baran M, Keskin C, Atalar M, Baran A. Environmentally friendly rapid synthesis of gold nanoparticles from Artemisia absinthium plant extract and application of antimicrobial activities. J Inst Sci Technol. 2020;11:365–375. doi: 10.21597/jist.779169. [DOI] [Google Scholar]
- 190.Tan S, Wu X, Xing Y, Lilak S, Wu M, Zhao JX. Enhanced synergetic antibacterial activity by a reduce graphene oxide/Ag nanocomposite through the photothermal effect. Colloids Surf B: Biointerfaces. 2020;185:110616. doi: 10.1016/j.colsurfb.2019.110616. [DOI] [PubMed] [Google Scholar]
- 191.Ali M, Ahmed T, Wu W, Hossain A, Hafeez R, Islam M, Li B. Advancements in plant and microbe-based synthesis of metallic nanoparticles and their antimicrobial activity against plant pathogens. Nanomaterials. 2020;10:1146. doi: 10.3390/nano10061146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Orzali L, Valente MT, Scala V, Loreti S, Pucci N. Antibacterial activity of essential oils and Trametes versicolor extract against Clavibacter michiganensis subsp. michiganensis and Ralstonia solanacearum for seed treatment and development of a rapid in vivo assay. Antibiotics. 2020;9:628. doi: 10.3390/antibiotics9090628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhao L, Lu L, Wang A, Zhang H, Huang M, Wu H, Ji R. Nano-biotechnology in agriculture: use of nanomaterials to promote plant growth and stress tolerance. J Agric Food Chem. 2020;68:1935–1947. doi: 10.1021/acs.jafc.9b06615. [DOI] [PubMed] [Google Scholar]
- 194.Asma N, Mudassar I, Crispin H, Hassan W. Biogenic AgNPs—a nano weapon against bacterial canker of tomato (bct) Adv Agric. 2020;2020(1–11):9630785. doi: 10.1155/2020/9630785. [DOI] [Google Scholar]
- 195.Sonika D, Saurav K, Aakash G, Uttam L, Ranjita T, Shankar J, Ganesh L, Deval PB, Niranjan P. Current research on silver nanoparticles: synthesis, characterization, and applications. J Nanomat. 2021 doi: 10.1155/2021/6687290. [DOI] [Google Scholar]
- 196.Verma R, Chauhan A, Shandilya M, Li X, Kumar R, Kulshrestha S. Antimicrobial potential of Ag-doped ZnO nanostructure synthesized by the green method using Moringa oleifera extract. J Environ Chem Eng. 2020;8:103730. doi: 10.1016/j.jece.2020.103730. [DOI] [Google Scholar]
- 197.Jyoti K, Arora D, Fekete G, Lendvai L, Dogossy G, Singh T. Antibacterial and anti-inflammatory activities of Cassia fistula fungal broth-capped silver nanoparticles. Mater Technol. 2020;1:11. doi: 10.1080/10667857.2020.1802841. [DOI] [Google Scholar]
- 198.Khosrovyan A, Gabrielyan B, Kahru A. Ingestion and effects of virgin polyamide microplastics on Chironomus riparius adult larvae and adult zebrafish Danio rerio. Chemosphere. 2020;259:127456. doi: 10.1016/j.chemosphere.2020.127456. [DOI] [PubMed] [Google Scholar]
- 199.Alaraby M, Demir E, Domenech J, Velázquez A, Hernández A, Marcos R. In vivo evaluation of the toxic and genotoxic effects of exposure to cobalt nanoparticles using Drosophila melanogaster. Environ Sci Nano. 2020;7:610–622. doi: 10.1039/C9EN00690G. [DOI] [Google Scholar]
- 200.Raj A, Shah P, Agrawal N (2020) Impact of nanoparticles on behavior and physiology of Drosophila melanogaster. In: Toxicology of nanoparticles: insights from Drosophila. Springer, Singapore, pp. 59–67
- 201.Sahu S, Mishra M. Hydroxyapatite nanoparticle causes sensory organ defects by targeting the retromer complex in Drosophila melanogaster. NanoImpact. 2020;19:100237. doi: 10.1016/j.impact.2020.100237. [DOI] [Google Scholar]
- 202.Demir E. An in vivo study of nanorod, nanosphere, and nanowire forms of titanium dioxide using Drosophila melanogaster: toxicity, cellular uptake, oxidative stress, and DNA damage. J Toxicol Environ Health, Part A. 2020;83:456–469. doi: 10.1080/15287394.2020.1777236. [DOI] [PubMed] [Google Scholar]
- 203.Kumar D, Kumar P, Singh H. Biocontrol of mosquito vectors through herbal-derived silver nanoparticles: prospects and challenges. Environ Sci Pollut Res. 2020;27:25987–26024. doi: 10.1007/s11356-020-08444-6. [DOI] [PubMed] [Google Scholar]
- 204.Ahmad J, Qamar S, Kausar N, Qureshi MI (2020) Nanoparticles: the magic bullets in mitigating drought stress in plants. In: Nanobiotechnology in agriculture. Springer, Cham, pp 145–161
- 205.Faraji J, Sepehri A. Exogenous nitric oxide improves the protective effects of tio 2 nanoparticles on growth, antioxidant system, and photosynthetic performance of wheat seedlings under drought stress. J Soil Sci Plant Nutr. 2020;20:703–714. doi: 10.1007/s42729-019-00158-0. [DOI] [Google Scholar]
- 206.Tombuloglu H, Anıl I, Akhtar S, Turumtay H, Sabit H, Slimani Y, Baykal A. Iron oxide nanoparticles translocate in pumpkin and alter the phloem sap metabolites related to oil metabolism. Sci Hortic. 2020;265:109223. doi: 10.3390/nano10091654. [DOI] [Google Scholar]
- 207.Taran N, Storozhenko V, Svietlova N, Batsmanova L, Shvartau V, Kovalenko M. Effect of zinc and copper nanoparticles on drought resistance of wheat seedlings. Nanosc Res Lett. 2017;12:60. doi: 10.1186/s11671-017-1839-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kolbert Z, Szőllősi R, Feigl G, Kónya Z, Rónavári A. Nitric oxide signalling in plant nanobiology: current status and perspectives. J Exp Bot. 2021;72:928–940. doi: 10.1093/jxb/eraa470. [DOI] [PubMed] [Google Scholar]
- 209.Soliman M, Qari SH, Abu-Elsaoud A, El-Esawi M, Alhaithloul H, Elkelish A. Rapid green synthesis of silver nanoparticles from blue gum augment growth and performance of maize, fenugreek, and onion by modulating plants cellular antioxidant machinery and genes expression. Acta Physiol Plant. 2021;42:1–16. doi: 10.3390/plants9040431. [DOI] [Google Scholar]
- 210.Alabdallah NM, Alzahrani HS. The potential mitigation effect of ZnO nanoparticles on [Abelmoschus esculentus L. Moench] metabolism under salt stress conditions. Saudi J Biol Sci. 2020;27:3132–3137. doi: 10.1016/j.sjbs.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Elsheery NI, Helaly MN, El-Hoseiny HM, Alam-Eldein SM. Zinc oxide and silicone nanoparticles to improve the resistance mechanism and annual productivity of salt-stressed mango trees. Agronomy. 2020;10:558. doi: 10.3390/agronomy10040558. [DOI] [Google Scholar]
- 212.Hoffmann J, Berni R, Hausman JF, Guerriero G. A review on the beneficial role of silicon against salinity in non-accumulator crops: tomato as a model. Biomolecules. 2020;10:1284. doi: 10.3390/biom10091284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zahedi SM, Karimi M, da Teixeira SJA. The use of nanotechnology to increase quality and yield of fruit crops. J Sci Food Agric. 2020;100:25–31. doi: 10.1002/jsfa.10004. [DOI] [PubMed] [Google Scholar]
- 214.Mahmoud LM, Dutt M, Shalan AM, El-Kady ME, El-Boray MS, Shabana YM. Silicon nanoparticles mitigate oxidative stress of in vitroderived banana (Musa acuminata ‘Grand Nain’) under simulated water deficit or salinity stress. South Afr J Bot. 2020;132:155–163. doi: 10.1016/j.sajb.2020.04.027. [DOI] [Google Scholar]
- 215.Moradbeygi H, Jamei R, Heidari R, Darvishzadeh R. Investigating the enzymatic and non-enzymatic antioxidant defense by applying iron oxide nanoparticles in Dracocephalum moldavica L. plant under salinity stress. Sci Hortic. 2020;272:109537. doi: 10.1016/j.scienta.2020.109537. [DOI] [Google Scholar]
- 216.Ye Y, Cota-Ruiz K, Hernández-Viezcas JA, Valdés C, Medina-Velo IA, Turley RS. Manganese nanoparticles control salinity modulated molecular responses in Capsicum annuum L. through priming: a sustainable approach for agriculture. ACS Sustain Chem Eng. 2020;8:1427–1436. doi: 10.1021/acssuschemeng.9b05615. [DOI] [Google Scholar]
- 217.Shoemaker AG (2020) The effects of titanium dioxide nanoparticles on the growth and development of Sorghum Bicolor (L.) Moenech. Adv Agric Hortic Entomol. 10.37722/AAHAE.202052
- 218.Thomas TD, Dinakar C, Puthur JT. Effect of UV-B priming on the abiotic stress tolerance of stress-sensitive rice seedlings: priming imprints and cross-tolerance. Plant Physiol Biochem. 2020;147:21–30. doi: 10.1016/j.plaphy.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 219.Kardavan GV, Karamian R. Effects of TiO2 nanoparticles and spermine on antioxidant responses of Glycyrrhiza glabra L. to cold stress. Acta Bot Croat. 2020 doi: 10.37427/botcro-2020-025. [DOI] [Google Scholar]
- 220.Iqbal MS, Singh AK, Singh SP, Ansari MI. Nanoparticles and plant interaction with respect to stress response. Nano Environ Biotechnol. 2020 doi: 10.1007/978-3-030-34544-0_1. [DOI] [Google Scholar]
- 221.Jośko I, Oleszczuk P, Futa B. The effect of inorganic nanoparticles (ZnO, Cr2O3, CuO and Ni) and their bulk counterparts on enzyme activities in different soils. Geoderma. 2014;232:528–537. doi: 10.1016/j.geoderma.2014.06.012. [DOI] [Google Scholar]
- 222.Jośko I, Dobrzyńska J, Dobrowolski R, Kusiak M, Terpiłowski K. The effect of pH and ageing on the fate of CuO and ZnO nanoparticles in soils. Sci Total Environ. 2020;721:137771. doi: 10.1016/j.scitotenv.2020.137771. [DOI] [PubMed] [Google Scholar]
- 223.Dogaroglu ZG, Koleli N (2014) Effect of different zinc oxide nanoparticles on germination, plant growth and chlorophyll content of wheat. In: International congress on green infrastructure and sustainable socities/cities greinsus, vol 14, pp 78–84. 10.1080/02772248.2013.803796
- 224.Mokarram-Kashtiban S, Hosseini SM, Tabari Kouchaksaraei M, Younesi H. The impact of nanoparticles zero-valent iron (nZVI) and rhizosphere microorganisms on the phytoremediation ability of white willow and its response. Environ Sci Pollut Res Int. 2019;26:10776–10789. doi: 10.1016/j.chemosphere.2020.126909. [DOI] [PubMed] [Google Scholar]
- 225.Szymanski M, Dobrucka R. Evaluation of phytotoxicity of bimetallic Ag/Au nanoparticles synthesized using Geum urbanum L. J Inorg Organomet Polym Mater. 2020;1:12. doi: 10.1016/j.chemosphere.2009.01.078. [DOI] [Google Scholar]
- 226.Heikal YM, Şuţan NA, Rizwan M, Elsayed A. Green synthesized silver nanoparticles induced cytogenotoxic and genotoxic changes in Allium cepa L. varies with nanoparticles doses and duration of exposure. Chemosphere. 2020;243:125430. doi: 10.1016/j.chemosphere.2019.125430. [DOI] [PubMed] [Google Scholar]
- 227.Taheri SM, Aramideh S, Akbarian J, Pirsa S. Effects of ZnO nanoparticles and kaolin in combination with Neem Azal-T/S against Bemisia tabaci and its parasitoid Eretmocerus mundus on cotton. Chem Rev Lett. 2020;3:131–139. doi: 10.22034/CRL.2020.235381.1066. [DOI] [Google Scholar]
- 228.Hafiz UR, Waqas A, Wahab N, Mansur AS, Anwaar A, Nauman K. A comprehensive review on chlorpyrifos toxicity with special reference to endocrine disruption: evidence of mechanisms, exposures and mitigation strategies. Sci Total Environ. 2021;755:142649. doi: 10.1016/j.scitotenv.2020.142649. [DOI] [PubMed] [Google Scholar]
- 229.Lozano-Pérez AA, Pagán A, Zhurov V. The silk of gorse spider mite Tetranychus lintearius represents a novel natural source of nanoparticles and biomaterials. Sci Rep. 2020;10:18471. doi: 10.1038/s41598-020-74766-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Sun C, Yu M, Zeng Z, Francis F, Cui H, Verheggen F. Biocidal activity of polylactic acid-based nano-formulated abamectin on Acyrthosiphon pisum (Hemiptera: Aphididae) and the aphid predator Adalia bipunctata (Coleoptera: Coccinellidae) PLoS ONE. 2020;15:e0228817. doi: 10.1371/journal.pone.0228817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Soudagar ME, Mujtaba MA, Safaei MR, Afzal A, Ahmed W, Banapurmath NR, Hossain N, Bashir S, Badruddin IA, Goodarzi M, Shahapurkar K. Effect of Sr@ ZnO nanoparticles and Ricinus communis biodiesel-diesel fuel blends on modified CRDI diesel engine characteristics. Energy. 2021;215:119094. doi: 10.1016/j.energy.2020.119094. [DOI] [Google Scholar]
- 232.Singh BK, Pandey R, Singh AK, Mishra MK. Effectiveness of flonicamid 50 wg and flupyradifurone 200 SL against leafhopper and whitefly in okra. J Entomol Zool Stud. 2020;8:181–185. [Google Scholar]
- 233.Da Silva CL, Henriques RO, Rios JV, Lerin LA, de Oliveira D, Furigo A. Lipase-catalyzed esterification of geraniol and citronellol for the synthesis of terpenic esters. App Biochem Biotechnol. 2020;190:574–583. doi: 10.1007/s12010-019-03102-1. [DOI] [PubMed] [Google Scholar]
- 234.Attaullah MKZ, Muhammad AZ, Muhammad SM, Hina R, Humara NM, Muhammad Z, Kanwal R, Kishwar S, Muhammad I, Samina Q. Insecticidal, biological and biochemical response of Musca domestica (Diptera: Muscidae) to some indigenous weed plant extracts. Saudi J Biol Sci. 2020;27:106–116. doi: 10.1016/j.sjbs.2019.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Sabbour MMA. Efficacy of nano-formulated certain essential oils on the red flour beetle Tribolium castaneum and confused flour beetle, Tribolium confusum (Coleoptera: Tenebrionidae) under laboratory and storage conditions. Bull Natl Res Cent. 2020;44:111. doi: 10.1186/s42269-020-00336-6. [DOI] [Google Scholar]
- 236.Raveau R, Fontaine J, Lounès H, Sahraoui A. Essential oils as potential alternative biocontrol products against plant pathogens and weeds: a review. Foods. 2020;9:365. doi: 10.1186/s42269-020-00336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Nazima S, Prasanta KR, Diganta G, Dipankar D, Saidul I, Varun T, Bodhaditya D, Hemanta KG, Pronobesh C, Pakalapati SR. Bio-nanoparticle assembly: a potent on-site biolarvicidal agent against mosquito vectors. RSC Adv. 2020;10:9356–9368. doi: 10.1039/C9RA09972G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Abouelatta AM, Keratum AY, Ahmed SI, El-Zun HM. Repellent, contact and fumigant activities of geranium (Pelargonium graveolens L’.Hér) essential oils against Tribolium castaneum (Herbst) and Rhyzopertha dominica (F.) Int J Tropical Insect Sci. 2020;40:1021–1030. doi: 10.1007/s42690-020-00161-4. [DOI] [Google Scholar]
- 239.Campolo O, Puglisi I, Barbagallo RN, Cherif A, Ricupero M, Biondi A, Zappala L. Side effects of two citrus essential oil formulations on a generalist insect predator, plant and soil enzymatic activities. Chemosphere. 2020;257:127252. doi: 10.1016/j.chemosphere.2020.127252. [DOI] [PubMed] [Google Scholar]
- 240.Ikawati S, Himawan T, Abadi AL, Tarno H. Toxicity nanoinsecticide based on clove essential oil against Tribolium castaneum (Herbst) J Pest Sci. 2020;46:222–228. doi: 10.1584/jpestics.D20-059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Leslie B, Mark M, Leonard L, Matteo S. Efficacy of various herbicides for the control of perennial Plantago spp. and effects on alfalfa damage and yield. Agronomy. 2020;10:1710. doi: 10.3390/agronomy10111710. [DOI] [Google Scholar]
- 242.Thongpitak J, Pumas P, Pumas C. Paraquat degradation by biological manganese oxide (BioMnOx) catalyst generated from living microalga pediastrum duplex AARL G060. Front Microbiol. 2020;11:575361. doi: 10.3389/fmicb.2020.575361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Francisco CF, María EAG, Claudia LA, Balam RR, Roberto LVG, Patricia RC, Rocío ACS, Alexey P, Yanis TM, Juan CGR, Nina B. ArgovitTM silver nanoparticles effects on Allium cepa: plant growth promotion without cyto genotoxic damage. Nanomaterials. 2020;10:1386. doi: 10.3390/nano10071386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Broda M. Natural compounds for wood protection against fungi—a review. Molecules. 2020;25:3538. doi: 10.3390/molecules25153538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kıvrak I, Kivrak S, Karababa E. Assessment of bioactive compounds and antioxidant activity of turkey tail medicinal mushroom Trametes versicolor (Agaricomycetes) Int J Med Mushrooms. 2020;22:559–571. doi: 10.1615/IntJMedMushrooms.2020035027. [DOI] [PubMed] [Google Scholar]
- 246.Cui J, Sun C, Wang A, Wang Y, Zhu H, Shen Y, Li N, Zhao X, Cui B, Wang C, Gao F, Zeng Z, Cui H. Dual-functionalized pesticide nanocapsule delivery system with improved spreading behavior and enhanced bioactivity. Nanomaterials. 2020;10:220. doi: 10.3390/nano10020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Luis AP, Ana AFP, Ramón G, Sandra M, Karen E. Nanoparticles in agroindustry: applications, toxicity, challenges, and trends. Nanomaterials. 2020;10:1654. doi: 10.3390/nano10091654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Marcela VH, Israel MB, Ramon GG, Enrique RG, Rosalia VOV, Luciano AJ, Irineo TP. Nanoparticles as potential antivirals in agriculture. Agriculture. 2020;10:444. doi: 10.3390/agriculture10100444. [DOI] [Google Scholar]
- 249.Arshad A, Temoor A, Wenge W, Afsana H, Rahila H, Md. Mahidul IM, Yanli W, Qianli A, Guochang S, Bin L. Advancements in plant and microbe-based synthesis of metallic nanoparticles and their antimicrobial activity against plant pathogens. Nanomaterials. 2020;10:1146. doi: 10.3390/nano10061146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Pieła A, Żymańczyk DE, Brzezińska RM, Duda M, Grzesiak J, Saeid A, Klimek OM (2020) Biogenic synthesis of silica nanoparticles from corn cobs husks. Dependence of the productivity on the method of raw material processing. Bioorg Chem 99:103773. 10.1016/j.bioorg.2020.103773. [DOI] [PubMed]
- 251.Hafez YM, Attia KA, Kamel S, Alamery SF, El-Gendy S, Al-Doss AA, Abdelaal KA. Bacillus subtilis as a bio-agent combined with nano molecules can control powdery mildew disease through histochemical and physiobiochemical changes in cucumber plants. Physiol Mol Plant Pathol. 2020;111:101489. doi: 10.1016/j.pmpp.2020.101489. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.