Abstract
PAS, by replacing part of the plasma in the platelet storage bag, reduces post transfusion allergic reactions and DHTR in the recipient. In this study we compared quality and efficacy of PAS and usual plasma stored platelets. Platelet concentration, content, MPV, pH, swirling, LDH and glucose concentration were tested in SDPs after preparation and on the day of transfusion; and compared between control (plasma-stored SDP) and study (PAS-stored SDP) groups. CCI was compared between the two groups. Transfusion reactions were also noted. In both groups quality parameters were similar except glucose [significantly decreased (p < 0.001) in plasma] and LDH [increased significantly (p: −0.005) in PAS]. CCI was similar in both groups. Transfusion reaction rate were 0.012% and 0.049% in both groups respectively. Quality and post-transfusion efficacy in both groups were similar. PAS stored platelets may be transfused in multi-transfused patients with allergic manifestations and in minor ABO incompatible transfusions.
Keywords: Platelet additive solution, Transfusion reaction, DHTR
Introduction
During platelet storage, platelets produce energy mostly by aerobic respiration (Krebs cycle) [1], for which, oxygen and fuel, are essential. Acetyl Co A is the main fuel and the alternate fuel is free fatty acids available from triglyceride present in plasma, the storage medium. As pyruvate dehydrogenase activity is down regulated during storage, supply of acetyl Co A is sub-optimal [2]. In that condition anaerobic respiration predominates resulting in generation of lactic acid which reduces pH of the medium. The bicarbonate available in plasma buffers the lactic acid preventing lowering of pH and generates volatile CO2 [3]. But gradual consumption of the bicarbonate lowers the pH during storage. pH below 6.2 can result in irreversible platelet damage [4]. The main goal of using platelet additive solutions (PAS) for platelet storage is to maintain pH so as to improve platelet viability. The additive solutions contain acetate as an ingredient which simultaneously acts as an alternate fuel for Krebs cycle as well as buffer (by generating bicarbonate) [5].
During storage, gradual morphological, biochemical as well as functional changes occur. These changes are called platelet storage lesions (PSL) [6]. Platelet count, mean platelet volume (MPV) and platelets distribution width (PDW) are used as markers of quality control of platelet concentrate (PC) and is representative of PSL [7]. Swirling is also considered a marker of shape change [8] and low swirling score is predictive of poor post platelet transfusion increment [9]. Synthetic storage media facilitate extended storage by attenuating PSL. Other than acetate additive solutions of different generations also contain citrate, phosphate, glucose, gluconate, magnesium, potassium etc. Citrate acts as an anticoagulant as well as a fuel for metabolism [10]. and phosphate acts as a buffer [11]. Magnesium and potassium [12] inhibit platelet activation and aggregation. Though glucose used to be a constituent of earlier generations of PAS, it is no longer used as it gets caramelized during heat sterilisation. But glucose is essential for platelet viability. This glucose comes from 20 to 40% plasma carry over [2]. Various studies have been carried out comparing in vitro effect of different additive solutions on platelets during storage [13, 14].
ABO group specific PC is advised to have better platelet increment [15]. But because of poor random donor platelet (RDP) inventory and non-availability of group specific donor for single donor platelet (SDP), out-of-group platelet transfusion is often practised. In minor incompatible PC transfusion, there is chance of haemolysis of the recipient red cells [16] which sometimes turns fatal [17–19]. Chance of haemolytic transfusion reaction (HTR) is more after O group SDP transfusion to a non-O group recipient because of presence of high titre of anti ABO antibody in the donor plasma [20] though titre is not always predictive of hemolysis [21]. Though manipulations of SDPs, like volume reduction, washing or addition of AB plasma instead of donor’s own plasma, have also been tried, the procedures are time consuming and platelet count increment is significantly hampered in some of the practices [22, 23]. Use of PAS is an option which needs active consideration. Similarly, plasma mediated transfusion reactions after platelet transfusion range from mild allergy to rare fatal TRALI (transfusion related acute lung injury) [24]. If PAS is used as the storage medium the chance of transfusion reactions is minimised.
Aims
To compare the quality and efficacy of PAS stored SDPs and plasma stored SDPs.
Materials and Methods
This was an institutional review board approved prospective double arm case–control study. Our hospital is a tertiary care oncology centre in eastern India and we prepare about 9000 RDP PC and 500 SDP PC annually. A total of 520 plateletpheresis procedures were performed between December 2017 and December 2018, of which 73 were included in this study. Of the 73 SDP PCs, 42 were stored in PAS (study group) and 31 in donor plasma (control group). The SDP donor selection and procedure were undertaken as per the departmental standard operating procedure (SOP) in compliance with the regulatory guidelines. The PC recipients included patients of all age groups (range: 2–64 years) with haematological malignancy, undergoing chemotherapy or stem cell transplantation; as post transfusion follow up was easier in this category. The patients already having pruritic rash were excluded from the study.
Platelet Collection and Storage
In the study group 36 apheresis procedures were performed in Haemonetics MCS + 9000® automated cell separator (Haemonetics Corporation, Braintree, Massachusetts, US) and 6 in Trima Accel® (Terumo BCT, Lakewood, Colorado, US). In the control group 7 procedures were performed in Trima® and 24 in Haemonetics®.
In Haemonetics®, Universal Platelet Protocol (UPP®) (UPP is designed to have uniformity across the globe for the Blood Centres to collect all the Components of Blood either concurrently or Individually. It also increases the speed and Efficiency with less troubleshooting.) was used for collection of PAS-stored PC. In this protocol, SSP + PAS (manufactured by Maco Pharma, Rue Lorthiois, 59,420 Mouvaux, France and marketed in India by Maco Pharma India Transfusion Solutions Pvt Ltd) (Table 1) was added by the machine to the product, and final concentration of PAS was around 67%. In Trima®, platelets were collected, concentrated (2000–3000 × 103/µL) than usual (1200–1600 × 103/µL) procedure, after changing the configuration in the machine. After collection, PAS was added to the product manually with the help of sterile connecting device in the same proportion as in Haemonetics. Control group SDPs were stored in 100% donor plasma. All the PCs were stored for a maximum of 5 days in the platelet incubator cum agitator at 20–24 °C and issued on FIFO (first in first out) basis. There is no corelation between high (4.4 × 1011/m2), medium (2.2 × 1011/m2) or low (1.1 × 1011/m2) dose platelet transfusion and incidence of life threatning bleeding episodes [25]. As low dose platelet transfusion can check the flow of inventory, low dose platelet transfusion protocol is practiced at our hospital. So, each SDP unit was divided into two equal volume units and each half unit was transfused on two occasions. Testing of the PC quality parameters was performed on day of collection (DOC) and on day of issue (DOI).
Table 1.
Composition of SSP + PAS
| Serial number | Component | gm/1000 ml |
|---|---|---|
| 1 | Sodium citrate, 2H2O | 3.18 gm |
| 2 | Sodium acetate, 3H2O | 4.42 gm |
| 3 | Sodium dihydrogen phosphate, 2H2O | 1.05 gm |
| 4 | Disodium phosphate anhydrous | 3.05 gm |
| 5 | Potassium chloride | 0.37 gm |
| 6 | Magnesium chloride | 0.3 gm |
| 7 | Sodium chloride | 4.05 gm |
| 8 | Water for injection | 1000 ml |
Sampling
Sample on DOC: 1 h post-procedure, 5–6 ml sample was collected after mixing and tube stripping in an aseptic manner.
Sample on DOI: was taken just before issue from each half unit after mixing and stripping the tube.
Platelet Concentration, Platelet Content, Mean Platelet Volume (MPV), WBC Contamination
Platelet concentration and MPV in the product bag were measured by Beckman Coulter DXH 500® Hematology Analyzer (Danaher Corporation, Brea, California, US) after transferring the sample from sample pouch to a polypropylene/polystyrene Ria vial. Total platelet content was calculated by multiplying the volume of the product and the concentration. WBC contamination was checked by Nageotte Brite Line® (Biotrans GmbH, Dreieich, Germany) chamber under microscope [26].
pH and Swirling
pH of the sample was measured by PH-80® pH meter (HM Digital Inc., 5819 Uplander Way, Culver City, CA 90,230). Swirling was checked by visual inspection holding the bag against light. Swirling score was given as 0, 1, 2 and 3 [27].
Anti ABO Antibody Titration
For performing anti ABO antibody (IgM) titration, master dilution technique [28] was used and it was done at room temperature by column agglutination technique (CAT) [29]by using reverse diluent cassette of Ortho BioView® system (Ortho clinical Diagnostics, Wooburn Green, Buckinghamshire, England). Anti A and anti B antibody titration was done twice only in the group O donors using corresponding antigen-positive pooled in-house red cells. The first titration was done from the donor sample collected during SDP screening tests and the second titration was done from the product just after collection. The titre was reported as the reciprocal of the highest dilution that had a reaction grade of 1+. Standard validated methods were used.
Glucose and LDH
Samples were collected from the sample pouch in a fluoride containing evacuated tube and a plain vial respectively for measuring glucose and LDH. The tests were done by reflectance spectrophotometry in Ortho Vitros® 5600 (Ortho clinical Diagnostics, Wooburn Green, Buckinghamshire, England).
Sterility Testing
Sterility testing was done only while issuing PC. In the biosafety cabinet, 5 ml sample was inoculated in the blood culture bottle by aseptic method. The bottles were put in the BacT Alert® (Bio-merieux, Marcy-l'Étoile, France) machine and incubated at 35 °C for 14 days and checked for any bacteria, yeast or yeast like fungi growth.
Corrected Count Increment (CCI)
1-h post-transfusion sample was obtained for determination of post transfusion platelet count using Beckman Coulter DXH 500 Hematology Analyzer. The pre transfusion platelet count and body surface area was noted from electronic medical records in the Hospital Management System (HMS). CCI was calculated using this formula [30]:
Transfusion Reaction
Any adverse reaction during or within 1 h after completion of transfusion was noted and blood bank was informed.
Statistical Analysis
We used mean, median etc. statistical method in Microsoft excel and used two sample t test for comparison between study and control arms. Statistical software R 4.0.2 was used for p value calculation. If p value was < 0.05 the test result was considered statistically significant.
Results
Between December’17 and December’18, 73 donors were included in the study (Table 2).
Table 2.
Donor parameters
| Parameters | Study arm (n = 42) | Control arm (n = 31) |
|---|---|---|
| Age | Median—34 years | Median—30 years |
| Sex | Male—41, Female—1 | Male—30, Female—1 |
| Hemoglobin | Mean—14.9 gm/dl | Mean—14.9 gm/dl |
| Platelet | Mean—222.5 × 103/µL | Mean—229.2 × 103/µL |
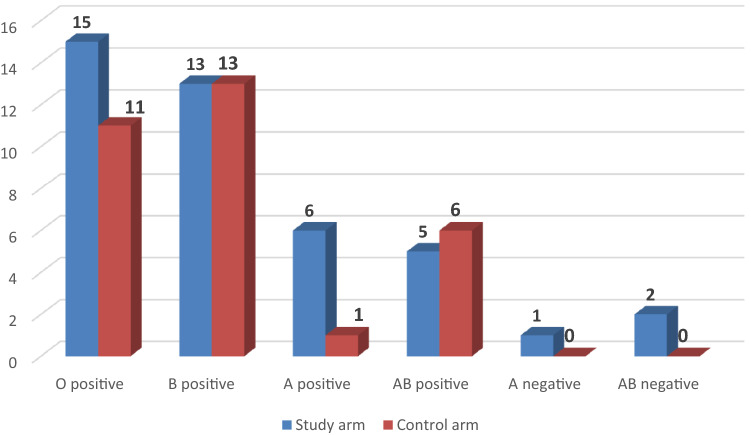
The blood group distribution of the donors is given in Fig. 1.
Fig. 1.
Blood group of the donors
Anti-A and Anti-B Titre Levels
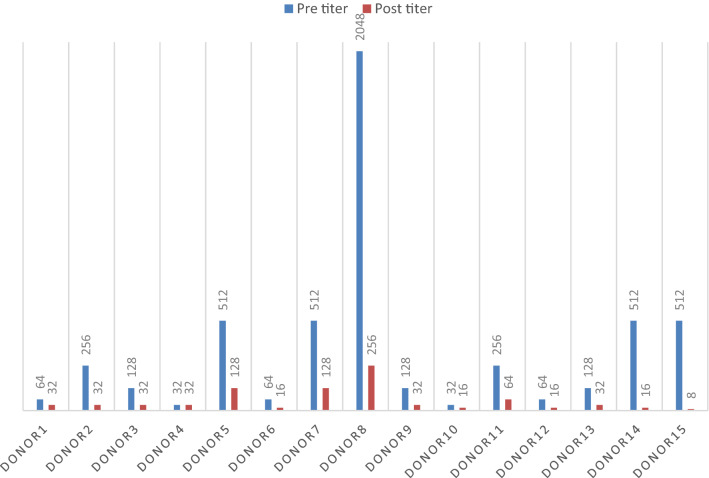
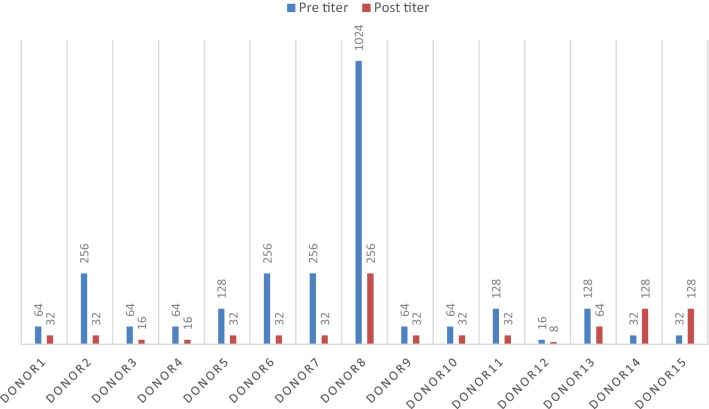
Antibody titration was done in the 26 “O” group donors. The median titre (done from whole blood during SDP screening) of anti-A and anti-B of all donors was 128 (range: 16–2048). In the PC product in the study group (n = 15) after addition of PAS the median titre was 32 (range: 8–256) whereas in the control group it remained 128 (range: 16–2048). Reduction of anti A titre after addition of PAS was statistically significant (p < 0.001), while that of anti B was not (p = 0.18). Out of the 15 donors, in one donor (who donated twice) anti B titer increased after addition of PAS (both the times) by 2 dilutions whereas anti A decreased by 5 and 6 dilutions respectively (Figs. 2, 3). The increase in anti B titer was most probably due to prozone phenomenon. This was the reason of not getting significant reduction in anti B titer. In the control group (n = 11), the titres in the PC, decreased by one dilution in 5 donors and in the rest it remained unchanged. This decrease in titer was due to dilution of plasma with anticoagulant (ACD).
Fig. 2.
Anti A titer in O group donors before and after addition of PAS
Fig. 3.
Anti B titre in O group donors before and after addition of PAS
42 PAS-stored and 31plasma-stored SDPs were transfused (half the volume at a time) on different days of storage i.e., day of collection (D0) through day 5 (D5). Three half SDPs from study group and one-half SDP from control group were excluded from the study as data for them was incomplete. The half SDP PC transfused on D0, were excluded from analysis. Quality and metabolic parameters of the PCs were measured (on D0 and on DOI which could be D1 to D5), analysed and compared using the two-sample t test. If p value was < 0.05 the test result was considered statistically significant.
pH and Swirling
The pH of the platelet storage medium decreased in both the groups during storage. The pH value in the study arm on DOC ranged from 7 to 8.4 and median was 7.4; and that on DOI ranged from 6.4 to 8 and median was 7.4. The pH value in the control arm on DOC ranged from 6.9 to 9 and median was 7.4; and that on DOI ranged from 6.3 to 8.2 and median was 7.5. Most of the SDP units, in both arms, showed swirling score was 3 and in some reduced to 2 on day of issue.
Platelet Concentration, Content, MPV, WBC Contamination
In our study, platelet concentration (per µL) and content (per half SDP) decreased during storage in both the groups. This decrease was more in the study arm and the difference between the groups was not statistically significant (Table 3).
Table 3.
Comparison of different parameters between study and control groups
| Sl no | Parameters for comparison | Study group (confidence interval) | Control group (confidence interval) | p value |
|---|---|---|---|---|
| (i) | Mean plt conc. on DOC | 968.5 × 103/µL (917 × 103/µL, 1026 × 103/µL) | 1255.8 × 103/µL (1193 × 103/µL, 1314 × 103/µL) | < 0.001 (significant) |
| (ii) | Mean decrease in plt conc. on DOI | 89.7 × 103/µL (35 × 103/µL, 129 × 103/µL) | 27.2 × 103/µL (5 × 103/µL, 62 × 103/µL) | 0.09 (NS) |
| (iii) | Mean plt content (per half SDP) on DOC | 1.41 × 1011 (1.33 × 1011, 1.50 × 1011) | 1.83 × 1011 (1.71 × 1011, 1.91 × 1011) | < 0.001 (significant) |
| (iv) | Mean decrease in Plt content (per half SDP) on DOI | 0.18 × 1011 (0.01 × 1011, 0.24 × 1011) | 0.13 × 1011 (0.01 × 1011, 0.20 × 1011) | 0.9 (NS) |
| (v) | Mean MPV on DOC | 10.8 fl (10.47 fl, 11.16 fl) | 9.8 fl (9.42 fl, 10.21 fl) | < 0.001 (significant) |
| (vi) | Mean increase in MPV on DOI | −0.2 fl (−0.31 fl, 0.01 fl) | 0.02 fl (−0.09 fl, 0.03 fl) | 0.07 (NS) |
| (vii) | Mean glucose conc. on DOC | 127.15 mg/dl (123 mg/dl, 132 mg/dl) | 362.87 mg/dl (355 mg/dl, 368 mg/dl) | < 0.001 (significant) |
| (viii) | Mean decrease in glucose conc. on DOI | 14.28 mg/dl (9 mg/dl, 19 mg/dl) | 42.10 mg/dl (29 mg/dl, 54 mg/dl) | < 0.001 (significant) |
| (ix) | Mean LDH conc. on DOC | 352.15U/L (320 U/L, 383 U/L) | 191.93U/L (184 U/L, 204 U/L) | < 0.001 (significant) |
| (x) | Mean increase in LDH conc. on DOI | 152U/L (96 U/L, 207 U/L) | 65U/L (43 U/L, 87 U/L) | 0.005 (significant) |
| (xi) | Mean WBC contamination (in one SDP) | 3.73 × 106 | 3.66 × 106 | 0.49 (NS) |
| (xii) | Median day of storage of SDPs | 2 | 2 | NA |
| (xiii) | Mean CCI | 13,389.38 | 12,337.8 | > 0.1 (NS) |
NS Not significant, NA Not applicable, CCI Corrected count increment
MPV (fl) decreased in majority of the cases in study group and increased in majority of the cases in control group. It was also not statistically significant (Table 3).
Mean WBC contamination (per SDP) in PAS-stored SDPs was less (3.73 × 106) than in plasma-stored ones (3.66 × 106) but the difference was not statistically significant (p > 0.49) (Table 3).
Glucose and LDH
The glucose concentration decreased more in the control group during storage than in the study group and the difference was statistically significant (p value < 0.001) whereas LDH increased more in the study than the control group and the difference was statistically significant (p value = 0.005) (Table 3).
Sterility Testing
No evidence of bacterial or fungal contamination was found in both the groups.
Corrected Count Increment (CCI)
Among 27 patients with haematological malignancies included in the study, most had a diagnosis of acute myeloid leukaemia (AML). They received platelet transfusion on different days of storage. After each transfusion (81 in study, 61 in control arm) 1-h post-transfusion platelet count was checked and CCI was calculated.
Though the mean CCI was more in the study than the control arm, difference was not statistically significant (p > 0.1) (Table 3).
Transfusion Reactions
There was 1 allergic reaction in the study arm from 81 transfusions and 3 allergic reactions in the control arm from 61 transfusions; hence the reaction rate was 0.012% and 0.049%, respectively, though the difference was not statistically significant. The allergic reactions were severe and accompanied with hypotension in 2 of the control arm cases, the other 2 cases were mild.
Discussion
In routine transfusion practice platelets are generally stored for only 5 days. With 5 days of storage, besides the risk of bacterial contamination, platelets develop structural and functional changes called as ‘storage lesions’ which render platelets hemostatically less effective. Synthetic platelet storage media have been developed to improve platelet quality and reduce adverse transfusion reactions in the recipient. Different synthetic storage media, like SSP+, are used to decrease the PSLs and research continues to further improve platelet quality by modifying the constituents of additive solutions so that PSLs can be minimised [31].
For comparison of PAS stored PC and plasma stored PCs, we looked into various platelet quality parameters including platelet concentration and content, pH, swirling, WBC contamination, glucose and LDH levels and antibody titers. In our study, the apheresis platelets were stored at 22–24 °C with continuous agitation for a maximum of 5 days and testing of the PC quality parameters was performed on DOC and on DOI.
Though the mean pH remained above 6.2; all through the storage period, there was decrease in pH in both the groups. Gupta et al. obtained similar results when they compared platelets stored in plasma with that stored in Composol-PS [32]. In another study [33] the authors, found a gradual decline in pH for both plasma and Composol-PS stored platelets. But the pH in PAS was significantly lower as compared to that in plasma (p < 0.001). Tynngard et al.[34] did a comparative study between three PAS media—Composol, T-Sol and SSP+. They found that in all the three solutions change of pH during storage was similar and within acceptable limits (6.4–7.4).
In our study, swirling of platelets was maintained in most of the units except in few and there was no statistically significant difference between the groups. Jain et al. [35] observed maximum swirling on day 0 and day 4 of testing in both groups. Bashir et al. [36] found that swirling was better in platelets stored in PAS than in plasma on day 10 of storage.
In the present study, platelet concentration and content decreased more in the study arm than the control arm; though the difference was not statistically significant. The results of Jain et al. [35] were similar to ours. However, Mokhtar et al. [33] found that platelet content was better in PAS-stored platelet group though the difference was not statistically significant. Hornsey et al. [37] observed a similar decreased platelet count when platelets were stored in SSP + PAS. The variation in results could be due to multiple factors including platelet concentration, capacity of the container and plastic bag quality.
WBC contamination was more in plasma-stored PC than PAS-stored ones in our study but the difference was not statistically significant. Mokhtar et al. [33] and Jain et al. [35] reported similar results. A high residual WBC count in blood products is associated with adverse effects such as febrile non-hemolytic transfusion reaction (FNHTR), human leukocyte antigens (HLA) alloimmunization, transfusion associated graft versus host disease (TA-GVHD) and cytomegalovirus (CMV) transmission to the recipient. We noted a slightly higher transfusion reaction rate in plasma stored platelets in this study. Thus, a lower residual WBC count is desirable to prevent such transfusion-associated reactions.
Though MPV usually increases upon storage of platelets due to disc to sphere transformation [38], in our study MPV in PAS-stored platelets decreased whereas in plasma-stored platelets it increased, though the difference was not statistically significant. Jain et al. [35] found that MPV increased in both arms on day 4 indicating that PAS could not prevent the shape change of platelets from disc to sphere. But Bashir et al.[36] found results in favour of PAS with statistically significant difference (0.001). Sandgren et al. [39] observed that magnesium, potassium containing PAS (PSM1, 2, 3) were equivalent in maintaining MPV to 100% plasma whereas InterSol PAS which did not contain the above ingredients was inferior.
In the present study anti A titre decreased significantly after addition of PAS but anti B titre did not. Jain et al. [35] noted a significant (p < 0.001) reduction in both titres. In transfusion practice, minor ABO incompatible platelet transfusions are often undertaken to minimise platelet outdating, in non-ABO identical apheresis platelet transfusion (where ABO compatible SDP donor is not available) and to provide HLA matched platelets to patients with platelet refractoriness. As there is a reduction of antibody titers, PAS-stored PCs can be used in minor ABO incompatible platelet transfusions. In a recent study by Tynuv et al. [40], all 100 apheresis PC stored in PAS except one, showed titres of 128 or less (p < 0.001). Here the authors claim that minor ABO incompatible platelet transfusion-induced HTR can be minimised by use of PAS. This has also been corroborated by Weisberg et al. [41].
Glucose level decreased significantly in the control arm during storage in our study. Studies by Jain et al. [35]and Gupta et al. [32] had similar results. The study by Sandgren et al. [39] found that in InterSol PAS, where initial glucose level was three times lower than plasma and other PAS (PSM1,2,3)-stored PC, decreased to zero on day 5 of storage. It resulted in decreased lactate production and increased pH which finally increased MPV and surface expression of platelet activation marker CD62P. Glucose is essential for platelet viability and glucose concentration is an important marker for monitoring platelet viability.
In the present study, LDH levels, increased significantly in the study arm in comparison with the control arm. In the study by Gupta et al. [32]. LDH did not increase much in both categories with and without PAS. But Sandgren et al. [39] found that LDH increased in the plasma stored PCs more than PSM1-3 which is contrary to our result. As LDH is a marker of cellular destruction [42, 43], this suggests that there was more platelet destruction in the PAS-stored PCs than the plasma-stored PCs and it reflected as a reduction in the platelet content of PCs in the PAS group. However, it may be noted here that this platelet destruction did not adversely affect post-transfusion CCI, as the 1-h post-transfusion CCI obtained was similar in both the groups.
In the literature though there are reports of decrease in CCI in PAS stored platelets [44, 45], Vasse et al. [46] showed results similar to ours. They found that 24-h CCI of plasma-stored SDP, SSP + PAS-stored SDP and RDP were 10.23, 10.01 and 10.32 respectively. Drawz et al. compared various PAS stored PC and found that PC stored in PAS E (SSP+) showed better CCI (p = 0.01) and percentage of transfusion increment (p < 0.01) as compared with PC stored in PAS C (Intersol) [47].
In our study, adverse transfusion reactions were less frequent with the use of PAS stored platelets. Earlier studies have also shown that adverse reactions are less frequent in PAS-stored PC than in plasma-stored PC [44, 45, 48] and these reactions were predominantly of allergic type, followed by febrile reactions.
In this study we faced certain limitations. Apart from the fewer number of patients, we could not store all PCs for 5 days. Thus, for evaluating the quality and metabolic parameters, we had to depend on the available inventory. Hence, we studied the mean decrease/increase of the specific quality parameter rather than the absolute decrease or increase.
Over the last few decades there have been significant developments in transfusion medicine technology thus improving patient safety. However, it may not always be possible to translate these into routine transfusion practice, keeping in mind the increase in product cost. A study by Kacker et al. [49] discussed the cost-effectiveness of using PAS in preventing allergic transfusion reactions and the authors suggest that PAS is cost saving in patients repeatedly having such problems. However, in India there are no recommendations for the use of PAS stored platelets till date.
Conclusion
Platelet storage solutions are used to enhance platelet quality, minimise allergic transfusion reactions and improve platelet count increment. In our study we found that platelet quality was maintained and there was a reduction in allergic reactions with the use of PAS stored platelets, though our results were statistically insignificant. In addition, both PAS stored platelets and plasma stored platelets showed similar post-transfusion efficacy. The universal use of PAS would certainly add to the cost of the product. Hence in a developing country like India, due to cost constraints; we are not in favour of universal use of PAS stored apheresis platelets. However, we suggest the use of PAS stored platelets in multi-transfused patients with allergic manifestations and in minor ABO incompatible platelet transfusions in platelet transfusion refractory patients.
Acknowledgements
We acknowledge the support rendered by Mr. Kaushik Gupta, Technologist of the blood bank, during data compilation. We also acknowledge the support of Span Health Care for partly funding the study.
Compliance with ethical standards
Conflict of interest
There is no conflict of interest between the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Debapriya Basu, Email: jhumpa.kumar11@gmail.com.
Sabita Basu, Email: drsabitabasu@gmail.com.
Vivek S. Radhakrishnan, Email: Vivek.Radhakrishnan@tmckolkata.com
Sanjay Bhattacharya, Email: sanjay.bhattacharya@tmckolkata.com.
Subhosmito Chakraborty, Email: subhosmito.chakraborty@tmckolkata.com.
Subir Sinha, Email: subir.sinha@tmckolkata.com.
Mammen Chandy, Email: mammen.chandy@tmckolkata.com.
References
- 1.Kilkson H, Holme S, Murphy S. Platelet metabolism during storage of platelet concentrates at 22 °C. Blood. 1984;64:406–414. doi: 10.1182/blood.V64.2.406.406. [DOI] [PubMed] [Google Scholar]
- 2.Vassallo RR. Preparation, preservation, and storage of platelet concentrates. In: Simon TL, McCullough J, Snyder EL, Solheim BG, Strauss RG, editors. Rossi’s principles of transfusion medicine. Chichester: Wiley Blackwell; 2016. pp. 227–234. [Google Scholar]
- 3.Murphy S. Platelet storage for transfusion. BeitrInfusiontherKlinErnahr. 1986;15:93–106. [PubMed] [Google Scholar]
- 4.Solberg C, Holme S, Little C. Morphological changes associated with pH changes during storage of platelet concentrates in first-generation 3-day container. Vox Sang. 1986;50(2):71–77. doi: 10.1111/j.1423-0410.1986.tb04849.x. [DOI] [PubMed] [Google Scholar]
- 5.Gulliksson H. Platelet storage media. Vox Sang. 2014;107(3):205–212. doi: 10.1111/j.1423-0410.1986.tb04849.x. [DOI] [PubMed] [Google Scholar]
- 6.Van der Meer PF. PAS or plasma for storage of platelets? Concise Rev Transfus Med. 2016;26(5):339–342. doi: 10.1111/tme.12325. [DOI] [PubMed] [Google Scholar]
- 7.Singh H, Chaudhary R, Ray V. Evaluation of platelet storage lesions in platelet concentrates stored for seven days. Indian J Med Res. 2003;118:243–246. [PubMed] [Google Scholar]
- 8.Mathai J, Resmi KR, Sulochana PV, et al. Suitability of measurement of swirling as a marker of platelet shape change in concentrates stored for transfusion. Platelets. 2006;17:393–396. doi: 10.1080/09537100600757695. [DOI] [PubMed] [Google Scholar]
- 9.Bertolini F, Agazzi A, Peccatori F, et al. The absence of swirling in platelet concentrates is highly predictive of poor post transfusion platelet count increments and increased risk of a transfusion reaction. Transfusion. 2000;40(1):121–122. doi: 10.1046/j.1537-2995.2000.4001121.x. [DOI] [PubMed] [Google Scholar]
- 10.Alhumaidan H, Sweeney J. Current status of additive solutions for platelets. J Clin Apher. 2012;27:93–98. doi: 10.1002/jca.21207. [DOI] [PubMed] [Google Scholar]
- 11.Gulliksson H, Larsson S, Kumlien G, et al. Storage of platelets in additive solutions: effects of phosphate. Vox Sang. 2000;78(3):176–184. doi: 10.1159/000031177. [DOI] [PubMed] [Google Scholar]
- 12.De Wildt-Eggen J, Schrijver JG, Bins M, et al. Storage of platelets in additive solutions: effects of magnesium and/or potassium. Transfusion. 2002;42:76–80. doi: 10.1046/j.1537-2995.2002.00012.x. [DOI] [PubMed] [Google Scholar]
- 13.Van der Meer PF, Kerkhoffs JL, Curvers J, et al. In vitro comparison of platelet storage in plasma and in four platelet additive solutions, and the effect of pathogen reduction: a proposal for an in vitro rating system. Vox Sang. 2010;98:517–524. doi: 10.1111/j.1423-0410.2009.01283.x. [DOI] [PubMed] [Google Scholar]
- 14.Van der Meer PF, Korte DD. Platelet additive solutions: a review of the latest developments and their clinical implications. Transfus Med Hemother. 2018;45(2):98–102. doi: 10.1159/000487513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heal JM, Rowe JM, McMican A, et al. The role of ABO matching in platelet transfusion. Eur J Haematol. 1993;50(2):110–117. doi: 10.1111/j.1600-0609.1993.tb00150.x. [DOI] [PubMed] [Google Scholar]
- 16.Basu D, Basu S, Roy J, et al. Incompatible crossmatch: first sign of a hemolytic transfusion reaction due to out-of-group platelet transfusion. Asian J Transfus Sci. 2019;13(1):57–59. doi: 10.4103/ajts.AJTS_36_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moinuddin IA, Millward P, Fletcher CH. Acute intravascular hemolysis following an abo non-identical platelet transfusion: a case report and literature review. Am J Case Rep. 2019;20:1075–1079. doi: 10.12659/AJCR.915521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larsson LG, Welsh VJ, Ladd DJ. Acute intravascular hemolysis secondary to out-of-group platelet transfusion. Transfusion. 2000;40(8):902–906. doi: 10.1046/j.1537-2995.2000.40080902.x. [DOI] [PubMed] [Google Scholar]
- 19.Shachner TR, Clark CT. Acute hemolytic transfusion reaction in group b recipient associated with group a apheresis platelet donor: case report and literature review. Hindawi. Case Rep Med. 2018 doi: 10.1155/2018/8259531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Josephson CD, Castillejo MI, Grima K, et al. ABO-mismatched platelet transfusions: strategies to mitigate patient exposure to naturally occurring hemolytic antibodies. Transfus Apher Sci. 2010;42(1):83–88. doi: 10.1016/j.transci.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 21.Karafin MS, Blagg L, Tobian AA, King KE, Ness PM, Savage WJ. ABO antibody titers are not predictive of hemolytic reactions due to plasma-incompatible platelet transfusions. Transfusion. 2012;52(10):2087–2093. doi: 10.1111/j.1537-2995.2012.03574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karafin M, Fuller AK, Savage WJ, et al. The impact of apheresis platelet manipulation on corrected count increment. Transfusion. 2012;52(6):1221–1227. doi: 10.1111/j.1537-2995.2011.03476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romphruk AV, Cheunta S, Pakoate L, et al. Preparation of single donor platelet with low antibody titers for all patients. Transfus Apher Sci. 2012;46(2):125–128. doi: 10.1016/j.transci.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Delaney M, Wendel S, Bercovitz RS, Cid J, Cohn C, Dunbar NM, Apelseth TO, Popovsky M, Stanworth SJ, Tinmouth A, Van De Watering L, Waters JH, Yazer M, Ziman A, Biomedical Excellence for Safer Transfusion (BEST) Collaborative Transfusion reactions: prevention, diagnosis, and treatment. Lancet. 2016;388(10061):2825–2836. doi: 10.1016/S0140-6736(15)01313-6. [DOI] [PubMed] [Google Scholar]
- 25.Slichter SJ, Kaufman RM, Assmann SF, McCullough J, Triulzi DJ, Strauss RG, Gernsheimer TB, Ness PM, Brecher ME, Josephson CD, Konkle BA, Woodson RD, Ortel TL, Hillyer CD, Skerrett DL, McCrae KR, Sloan SR, Uhl L, George JN, Aquino VM, Manno CS, McFarland JG, Hess JR, Leissinger C, Granger S. Dose of prophylactic platelet transfusions and prevention of hemorrhage. N Engl J Med. 2010;362(7):600–613. doi: 10.1056/NEJMoa0904084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.(2014) Counting residual white cells in leukocyte-reduced blood and components—manual method. In: Fung MK, Grossman BJ, Hillyer CD, Westhoff CM (eds) Technical manual. AABB, Bethesda, Maryland, Method 8–11, pp 1–2.
- 27.Singh RP, Marwaha N, Malhotra P, et al. Quality assessment of platelet concentrates prepared by platelet rich plasma-platelet concentrate, buffy coat poor-platelet concentrate (BC-PC) and apheresis-PC methods. Asian J Transfus Sci. 2009;3(2):86–94. doi: 10.4103/0973-6247.53882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.(2014)Antibody titration procedure. In: Fung MK, Grossman BJ, Hillyer CD, Westhoff CM (eds) Technical Manual. AABB, Bethesda, Maryland, Method 3–15, pp 1–3.
- 29.Shah BV, Rajput P, Virani ZA, Warghade S. Baseline anti-blood group antibody titers and their response to desensitization and kidney transplantation. Indian J Nephrol. 2017;27(3):195–198. doi: 10.4103/0971-4065.202402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nester T, Jain S, Poisson J. Hemotherapy decisions and their outcomes. In: Fung MK, Grossman BJ, Hillyer CD, Westhoff CM, editors. Technical manual. Bethesda, Maryland: AABB; 2014. pp. 499–543. [Google Scholar]
- 31.Devine DV, Serrano K. The platelet storage lesion. Clin Lab Med. 2010;30(2):475–487. doi: 10.1016/j.cll.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 32.Gupta A, Chandra T, Kumar A. In vitro function of random donor platelets stored for 7 days in composol platelet additive solution. Asian J Transfus Sci. 2011;5(1):11–14. doi: 10.4103/0973-6247.75969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mokhtar MB, Hashim HB, Joshi SR. Assessment of quality of platelets preserved in plasma and platelet additive solution: a Malaysian experience. Asian J Transfus Sci. 2016;10(1):84–87. doi: 10.4103/0973-6247.172177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tynngård N, Trinks M, Berlin G. In vitro properties of platelets stored in three different additive solutions. Transfusion. 2012;52(5):1003–1009. doi: 10.1111/j.1537-2995.2011.03417.x. [DOI] [PubMed] [Google Scholar]
- 35.Jain P, Tendulkar A, Gupta A. First Indian initiative for preparation of low-titer group “O” single-donor platelets with platelet additive solution. Asian J Transfus Sci. 2018;12:10–16. doi: 10.4103/ajts.AJTS_2_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bashir S, Mohsin S, Amin H, et al. Comparison of changes in platelet count, mean platelet volume and swirling in stored platelet concentrates with and without platelet additive solution. J Appl Hematol. 2014;5(1):10–14. doi: 10.4103/1658-5127.131819. [DOI] [Google Scholar]
- 37.Hornsey VS, McColl K, Drummond O, et al. Extended storage of platelets in SSP platelet additive solution. Vox Sang. 2006;91(1):41–46. doi: 10.1111/j.1423-0410.2006.00771.x. [DOI] [PubMed] [Google Scholar]
- 38.Fijnheer R, Pietersz RN, de Korte D, Roos D. Monitoring of platelet morphology during storage of platelet concentrates. Transfusion. 1989;29(1):36–40. doi: 10.1046/j.1537-2995.1989.29189101162.x. [DOI] [PubMed] [Google Scholar]
- 39.Sandgren P, Mayaudon V, Payrat JM, et al. Storage of buffy-coat-derived platelets in additive solutions: in vitro effects on platelets stored in reformulated PAS supplied by a 20% plasma carry-over. Vox Sang. 2010;98(3 Pt 2):415–422. doi: 10.1111/j.1423-0410.2009.01255.x. [DOI] [PubMed] [Google Scholar]
- 40.Tynuv M, Flegel WA. Quality improvement with platelet additive solution for safer out-of-group platelet transfusions. Immunohematology. 2019;35(3):108–115. doi: 10.21307/immunohematology-2020-024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weisberg SP, Shaz BH, Tumer G, et al. PAS-C platelets contain less plasma protein, lower anti-A and anti-B titers, and decreased HLA antibody specificities compared to plasma platelets. Transfusion. 2018;58(4):891–895. doi: 10.1111/trf.14523. [DOI] [PubMed] [Google Scholar]
- 42.Mittal K, Kaur R. Platelet storage lesion: an update. Asian J Transfus Sci. 2015;9(1):1–3. doi: 10.4103/0973-6247.150933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fisk JM, Pisciotto PT, Snyder EL, Perrotta PL. Platelet and related products. In: Hillyer CD, Silberstein LE, Ness PM, Anderson KC, Roback JD, editors. Blood banking and transfusion medicine. Philadelphia: Churchill Livingstone; 2007. pp. 308–341. [Google Scholar]
- 44.Kerkhoffs JL, van Putten WL, Novotny VM, et al. Clinical effectiveness of leucoreduced, pooled donor platelet concentrates, stored in plasma or additive solution with and without pathogen reduction. Br J Haematol. 2010;150:209–217. doi: 10.1111/j.1365-2141.2010.08227.x. [DOI] [PubMed] [Google Scholar]
- 45.Tobian AA, Fuller AK, Uglik K, et al. The impact of platelet additive solution apheresis platelets on allergic transfusion reactions and corrected count increment. Transfusion. 2014;54:1523–1529. doi: 10.1111/trf.12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vasse J, Gaucheron S, Lebaudy JP, et al. Étude comparative de l’efficacitétransfusionnelleplaquettairebiologique entre les concentrés de plaquettes d’aphérèseconservésen plasma, les concentres de plaquettes d’aphérèse et les mélanges de concentres de plaquettes conservésen solution de conservation SSP+ Transfus Clin Biol. 2012;19:306. doi: 10.1016/j.tracli.2012.08.116. [DOI] [Google Scholar]
- 47.Drawz SM, Marschner S, Yañez M, et al. Observational study of corrected count increments after transfusion of platelets treated with riboflavin pathogen reduction technology in additive solutions. Transfusion. 2015;55:1745–1751. doi: 10.1111/trf.13026. [DOI] [PubMed] [Google Scholar]
- 48.Cohn CS, Stubbs J, Schwartz J, et al. A comparison of adverse reaction rates for PAS C versus plasma platelet units. Transfusion. 2014;54:1927–1934. doi: 10.1111/trf.12597. [DOI] [PubMed] [Google Scholar]
- 49.Kacker S, Ness PM, Savage WJ, et al. The cost-effectiveness of platelet additive solution to prevent allergic transfusion reactions. Transfusion. 2013;53:2609–2618. doi: 10.1111/trf.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]