Abstract
Lysosomes are organelles involved in cell metabolism, waste degradation, and cellular material circulation. They play a key role in the maintenance of cellular physiological homeostasis. Compared with the lysosomal content of other organs, that of the kidney is abundant, and lysosomal abnormalities are associated with the occurrence and development of certain renal diseases. Lysosomal structure and function in intrinsic renal cells are impaired in diabetic kidney disease (DKD). Promoting lysosomal biosynthesis and/or restoring lysosomal function can repair damaged podocytes and proximal tubular epithelial cells, and delay the progression of DKD. Lysosomal homeostasis maintenance may be advantageous in alleviating DKD. Here, we systematically reviewed the latest advances in the relationship between lysosomal dyshomeostasis and progression of DKD based on recent literature to further elucidate the mechanism of renal injury in diabetes mellitus and to highlight the application potential of lysosomal homeostasis maintenance as a new prevention and treatment strategy for DKD. However, research on screening effective interventions for lysosomal dyshomeostasis is still in its infancy, and thus should be the focus of future research studies. The screening out of cell-specific lysosomal function regulation targets according to the different stages of DKD, so as to realize the controllable targeted regulation of cell lysosomal function during DKD, is the key to the successful clinical development of this therapeutic strategy.
Subject terms: Mechanisms of disease, Chronic kidney disease
Facts
AGE–RAGE interaction is a potential mechanism underlying lysosomal dysfunction.
The screening out of cell-specific lysosomal function regulation targets according to different DKD stages is key to the successful clinical development of lysosomal homeostasis maintenance as a therapeutic strategy for DKD.
Screening effective interventions for lysosomal dyshomeostasis should be the focus of future research efforts.
Open Questions
What’s the mechanisms of CTSD protecting PTECs from apoptosis and LMP?
What’s the cell-specific lysosomal function regulation targets in different DKD stages?
How to combine lysosome function regulation with cell-specific drug delivery?
Introduction
Lysosomes are important regulatory platforms in numerous vesicle transport pathways, including endocytosis, phagocytosis, and autophagy. Their ability to fuse with endosomes, phagosomes, and autophagosomes enables them to break down a variety of endogenous and exogenous substances, including macromolecules, certain pathogens, and damaged organelles1. Owing to their central position in complex intracellular trafficking networks, lysosomes have become central signaling nodes for sensing and coordinating cellular metabolism, intra- and inter-cellular signaling, and membrane repair1.
Diabetic kidney disease (DKD) is a common consequence of type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM)2. It has attracted much attention in recent years owing to its high incidence, poor prognosis, and heavy economic burden. DM-mediated changes in extracellular and intracellular metabolism and hemodynamics in intrinsic renal cells have a considerable and lasting impact on DKD progression3.
Autophagy protects intrinsic renal cells from injury and cell death in several renal diseases, including DKD4–6. Our previous study showed that lysosomal depletion induced by lysosomal membrane permeabilization (LMP) weakens intrinsic renal cell autophagy protection in DKD7. We found that long-term proteinuria (a secondary nephrotoxin factor that aggravates DKD progression) inhibited autophagy in intrinsic renal cells by impairing lysosomal structure and function8. Alternatively, preventing lysosomal depletion by eliminating damaged lysosomes or replenishing intact lysosomes improves autophagy flux, thereby protecting intrinsic renal cells from damage during DKD9,10.
The clinical application of lysosome dysfunction for the prevention and treatment target of DKD has not yet been realized. To promote the development of therapeutic lysosome targeting, in this review we summarize the latest advances in lysosomal dyshomeostasis in intrinsic renal cells, including podocytes, proximal tubular epithelial cells (PTECs), and macrophages during DKD, to highlight the key mechanism of interaction between lysosomal dyshomeostasis and progression of DKD.
New understanding of lysosomal function and regulation
Recent findings on lysosomal function
Lysosomes are important sub-organelles of single-layer capsulated vesicles containing >60 acidic hydrolases, including proteases, phosphatases, and lipases11. Lysosomes are involved in the degradation of cellular waste products, and play a crucial role in maintaining cellular homeostasis12. However, the role of lysosomal dysfunction in the pathogenesis of different diseases has not yet been elucidated, as lysosomes have only been considered end-degradation compartments involved in the elimination of cellular waste. Yet, lysosomes have several other important biological functions, including membrane repair, secretion, energy metabolism, and signal transduction (Fig. 1B)1,13.
Fig. 1. Schematic diagram of TFEB regulation and lysosomal function.
A Under physiological (nutrient) conditions, phosphorylated TFEB is inactive and degradation occurs via the proteasomal pathway. Under starvation or stress, TFEB is activated by kinase inactivation or phosphatase activation-mediated dephosphorylation, and is subsequently transferred to the nucleus to upregulate the expression of its target genes. TFEB acetylation at K274 and K279 blocks TFEB nucleation28–31,33. B Lysosomes have several biological functions, including membrane repair, secretion, energy metabolism, and signal transduction1,13–23. AMPK, adenosine 5′-monophosphate (AMP)-activated protein kinase; ROS, reactive oxygen species; STUB1, STIP1-homologous U-Box containing protein 1; TFEB, transcription factor EB.
An important marker of plasma membrane repair is the initiation of lysosome recruitment and exocytosis at the damaged site14. As lysosomes are able to respond to Ca2+ influx, rapid lysosomal exocytosis promotes plasma membrane repair, possibly by providing membrane support and tension release14–16. Lysosomes are essential for the maintenance of plasma membrane integrity, thus avoiding cytoplasm leakage and cell death.
Previously, lysosomal exocytosis was considered a unique cell secretory function, and myeloid cells were thought to contain a unique type of lysosome with exocytosis ability17. However, several subsequent studies showed that following the fusion of lysosomes with the plasma membrane, all cell types secrete lysosomal contents under different stimuli18,19.
Lysosomes are terminal degradation components that participate in the degradation of damaged cell structures, senescent organelles, and biological macromolecules, and produce small molecules (e.g., amino acids and fatty acids), which are subsequently transported to the cytoplasm for cell reuse and ATP generation to provide energy, thereby ensuring energy metabolism of cells1,20.
Lysosomal damage activates AMPK, a regulator of autophagy21. Upon lysosomal injury, galectin 9 increases the association with lysosomal glycoproteins, whereas it decreases interactions with the deubiquitinase USP9X; K63 ubiquitination of TAK1 activates AMPK in damaged lysosomes22,23. Furthermore, lysosomes also participate in immune processes24 and form exosomes25.
Transcription factor EB (TFEB)-mediated lysosomal biogenesis regulation
Lysosomal genes share a 10-base E-box-like palindrome sequence (5′-GTCACGTGAC-3′), which is usually found within 200 bp of the transcription initiation site. This motif, termed coordinated lysosomal expression and regulation (CLEAR) element, consists of an E-box (CANNTG) identified by MIT/TFE family transcription factors (e.g., TFEB and TFE3)26. TFEB promotes transcription and expression of its target genes by specifically binding to the distinct CLEAR motif in the target promoter26, which increases the biogenesis of lysosomes and improves their degradation ability27.
TFEB activity primarily depends on its phosphorylation state and cytoplasmic-nuclear shuttling (Fig. 1A)28. Under physiological conditions, phosphorylated TFEB is inactive and predominantly located in the cytoplasm. Under stress, TFEB is activated by kinase inactivation or phosphatase activation-mediated dephosphorylation, and is subsequently transferred to the nucleus and combines with the CLEAR motif to upregulate its target gene expression29,30. TFEB degradation occurs via the proteasomal pathway31. Proteasome inhibition induces TFEB accumulation, dephosphorylation, and subsequent nuclear translocation, which remarkably increases the expression of TFEB downstream genes32. Wang et al. further reported that TFEB acetylation at K274 and K279 disrupted the dimerization of TFEB and its DNA‐binding activity, leading to inhibition of lysosome biogenesis33.
DKD and lysosome function in intrinsic renal cells
Epidemiological characteristics and primary pathogenesis of DKD
DKD is the leading cause of end-stage renal disease (ESRD). Up to 50% of patients with diabetes eventually develop DKD, leading to a considerable increase in the risk of mortality in these patients34,35. According to the International Diabetes Federation, the number of people with diabetes worldwide will increase from 382 million in 2013 to 592 million in 203536, which will impose a huge economic burden on the family of the patients and on society.
DKD pathogenesis is extremely complex. Preliminary signs and symptoms of DKD generally appear 10–20 years after the onset of diabetes37. DKD occurs through various mechanisms, including hemodynamic changes, altered metabolic factors, and pro-inflammatory molecules38. Moreover, activation of the renal renin–angiotensin system (RAS)39, mitochondrial dysfunction40,41, and endoplasmic reticulum stress40,42 are also involved in DKD injury.
DKD pathogenesis has traditionally been characterized by glomerular pathology (e.g., mesangial hypertrophy, glomerular ultrafiltration, and proteinuria)43. However, recent research has suggested that glomerular alterations are not the main inducing factors of DKD, and renal tubular injury, especially proximal tubule injury, is likely to be a key inducer of major pathological events of progression to diabetic kidney failure44. The cellular morphology alteration of renal proximal tubules is considered an early symptom of DKD, and subsequent tubulo-interstitial fibrosis may play an important role in progression to end-stage renal failure45,46.
The prevention and treatment of DKD are conducted in a multi-target manner, including promoting a healthy lifestyle and targeting molecular factors associated with the pathogenesis of the disease. Intensive interventions (e.g., blood pressure control, glycemic control, and RAS suppression) reduce the risk of proteinuria progression; however, these therapies have failed to prevent DKD progression in some patients with refractory proteinuria47. Consequently, there is a necessity to find more effective treatment options to improve the prognosis of DKD.
Emphasis of lysosomal dysfunction in DKD
The autophagy-lysosome pathway has been one of the main focuses of studies on DKD pathogenesis48–52. When renal cells are exposed to various stressors, such as oxidative stress, hypoxia, and toxic damage, autophagy is induced and plays an important role in cell survival53–55. However, reports on changes in autophagy activity in intrinsic renal cells during DKD are inconsistent. Zhan et al. showed that autophagy was inhibited in tubular epithelial cells (TECs) of diabetic animals56, whereas the opposite was observed by Zhao et al.57. Fang et al. suggested that hyperglycemia disrupted autophagy in podocytes58, whereas Ma et al. reported that high glucose (HG) promoted autophagy59. These differences may be caused because these studies primarily evaluated the upstream, rather than downstream, autophagy-lysosome pathway.
The autophagy-lysosome pathway involves autophagic vacuole induction, a fusion of autophagic vacuoles with lysosomes, and lysosomal degradation of autophagic vacuoles60. Lysosomes, located at the end of the autophagy-lysosome pathway, play an important role in the degradation of impaired organelles and macromolecules61. A decrease in lysosomal enzyme activity62,63 and lysosome-mediated degradation of albumin in the kidney64 under diabetic conditions have been reported. Furthermore, lysosomal damage leads to reduced renal protein degradation, resulting in diabetic renal hypertrophy63. Examination of renal parenchyma from 12 patients with DM showed a 50% reduction in TFEB mRNA levels versus 12 individuals without DM. A reduction in TFEB protein levels in the kidney tubulointerstitium was also observed in DM patients65. These findings suggest that considerable attention should be paid to the role of lysosomal dysfunction in DKD pathogenesis.
Lysosomal dysfunction and enzyme abnormalities of podocytes in patients with DKD
Podocytes are highly differentiated cells on the lateral surface of the glomerular basement membrane and play an important role in maintaining the structure and function of the glomerular filtration barrier66. Podocyte dysfunction and loss because of apoptosis can contribute to massive proteinuria in DKD patients67,68. Various metabolites [e.g., advanced glycation end products (AGEs), uremic toxin, and methylglyoxal] can damage podocytes and lead to DKD69–71. Lysosomes participate in the processing of albumin in podocytes. Inhibition of lysosomal degradation can increase albumin accumulation, aggravating podocyte injury and glomerulosclerosis72. Consequently, lysosomal dysfunction in podocytes caused by etiological factors under diabetic conditions can aggravate podocyte lesions.
Lysosomal degradation dysfunction
Restoration of lysosomal function can activate podocyte autophagy and alleviate podocyte apoptosis in DKD73. Substantial time-dependent decreases in lysosomal enzyme activity [e.g., cathepsin B, D, and L (CTSB, CTSD, and CTSL)], LMP, and autophagy inhibition in podocytes were detected following AGE treatment. L-leucyl-L-leucine-O-methyl ester, known as an LMP inducer, inhibits autophagy in podocytes and aggravates podocyte apoptosis, revealing that LMP may be a critical factor triggering podocyte injury after AGE exposure under diabetic conditions. Notably, resveratrol plus vitamin E treatment in AGE-treated podocytes may increase CTSB/CTSL enzymatic activity and DQ-ovalbumin degradation, rescue actin cytoskeleton changes, and alleviate podocyte apoptosis. However, these cytoprotective effects were blocked by the addition of the lysosomal inhibitor leupeptin, suggesting that LMP-related lysosomal degradation dysfunction is a crucial contributor to podocyte damage during DKD occurrence and progression (Fig. 2)73.
Fig. 2. Mechanism of lysosomal dyshomeostasis and its effect on podocyte during DKD.
LMP-related lysosomal degradation dysfunction is a crucial contributor to podocyte damage; mTOR inhibition and the PI3K/Akt-GSK3β axis prevent TFEB nuclear translocation and lysosomal biogenesis73–76. CTSL can degrade synaptopodin-inducing albuminuria by presenting a migration of podocyte foot processes86–90. AGEs, advanced glycation end products; CTSL, cathepsin L; DKD, diabetic kidney disease; LMP, lysosomal membrane permeabilization; RAGE, AGE receptor; TFEB, transcription factor EB.
TFEB inactivation mediated lysosomal biogenesis obstruction
TFEB is the master regulator of the autophagy-lysosome pathway. Chen et al. proposed that catalpol stabilized the cytoskeleton, ameliorated podocyte injury, and recovered kidney damage in DKD by inhibiting mTOR activity and promoting TFEB nuclear translocation74. Phosphorylated p70s6k levels (p-p70s6k, a downstream target of mTOR) considerably increased in podocytes from DKD mice and cultured podocytes treated with HG content, with TFEB nuclear translocation also being affected. However, catalpol treatment reversed these changes. Zhao et al. demonstrated that AGEs triggered podocyte injury and pathological injury of the kidney by activating mTOR and subsequently inactivating TFEB75. Co-immunoprecipitation results indicated that TFEB interacted with mTOR in the glomeruli of db/db mice and AGE-stimulated podocytes. Torin1 (a strong inhibitor of mTOR activity) recovered TFEB nuclear expression in db/db mice and AGE-stimulated cultured podocytes. Hou et al. found that hepatocyte growth factor improved lysosome function by promoting TFEB nuclear translocation via the PI3K/Akt-GSK3β-TFEB axis in podocytes, which decreased urinary albumin excretion, alleviated matrix expansion, and rescued podocyte loss in DKD mice (Fig. 2)76. Collectively, TFEB activation, lysosomal biogenesis, and lysosomal function enhancement play key roles in preventing podocyte injury in DKD.
Inhibition of mTOR activity reduces podocyte injury by activating TFEB under diabetic conditions. However, inhibition of mTORC1 under non-diabetic conditions exerts serious adverse effects on podocytes77,78. The absence of mTORC1 activity in podocyte-specific Raptor-deficient mice resulted in severe podocyte injury, proteinuria, and glomerulosclerosis79. This highlights the importance of regulation of the mTOR signaling pathway to ensure normal renal function. The strong protective effect of activated TFEB on DKD should be emphasized, and other signaling pathways that activate TFEB should be actively explored.
Abnormalities of lysosomal enzyme CTSL during DKD and its role in DKD pathogenesis
CTSL is a key lysosomal enzyme, and a cysteine protease of the cathepsin family80. Increased CTSL expression in podocytes was observed in patients with DKD, highlighting the clinical relevance of these findings81. Urine CTSL concentrations were lower in children with DKD than in children without DKD, which raises the possibility that CTSL may be an early predictor of DKD82. High CTSL levels in urine were associated with albuminuria improvement after four years of DKD diagnosis in patients83. The increase in serum CTSL activity positively correlated with the hospitalization rate of DKD patients, and serum CTSL levels positively correlated with proteinuria severity84. Regarding the role of CTSL in renal filtration function, studies have shown that CTSL inhibitors can reduce experimental proteinuria85.
A study using a streptozotocin (STZ)-induced CTSL-deficient diabetic mouse model and an STZ-induced wild-type (WT) diabetic mouse model showed that CTSL was associated with podocyte injury by aggravating proteinuria, mesangial matrix expansion, and tubular fibrosis86. Following diabetes induction, cortical CTSL activity and mRNA expression notably increased in WT mice86. CTSL-deficient diabetic mice did not develop albuminuria, displayed better renal function with normal plasma creatinine and blood urea nitrogen concentrations, and did not suffer from DKD86. Podocyte-specific calcineurin-CTSL interference was sufficient to induce albuminuria, also indicating that CTSL plays a key role in albuminuria pathogenesis87.
Proteinuria occurrence represented a migration of podocyte foot processes, caused by CTSL (Fig. 2)88. Notably, CTSL can degrade CD2-related proteins, synaptopodin, and dynamin, which are crucial for the normal architecture of the podocyte cytoskeleton81,87,89. Synaptopodin, an antagonist of RhoA and Cdc42 signaling, stabilizes renal filtration by preventing the podocyte actin cytoskeleton from reorganizing to form a migratory phenotype90. Consistently, synaptopodin protein expression was remarkably reduced in WT diabetic mice compared to CTSL-deficient diabetic mice86. Lysosomal CTSL plays an important role in DKD development by inducing albuminuria, which degrades important structural proteins in podocytes.
Lysosomal dysfunction and enzyme abnormalities of PTECs in DKD
Phenotypic changes in renal PTECs are the first signs of DKD91, and the consequent tubulointerstitial injury plays a key role in the progression of DKD to ESRD92,93. AGEs are generated because of chronically high sugar levels, degraded by lysosomes via endocytosis in PTECs, and relevant to the progression of DKD94. In addition to elevated AGE production in the hyperglycemic state, the decreased lysosome clearance rate contributes to AGE accumulation in PTECs with DKD95.
LMP occurrence
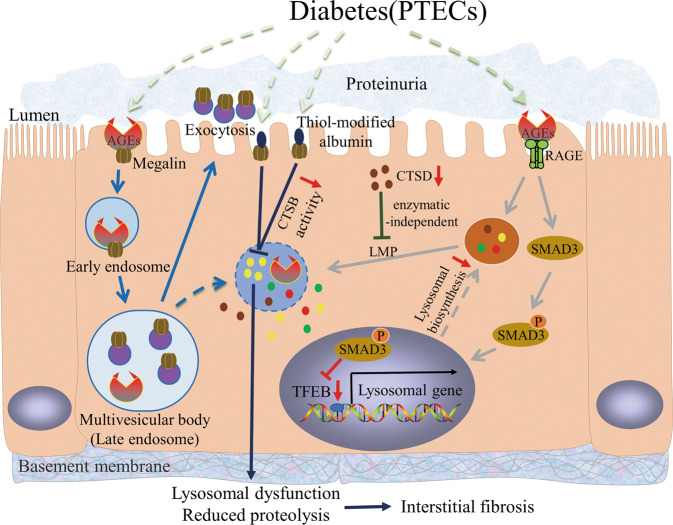
Our previous study demonstrated the close association between lysosome impairment and abnormal AGE accumulation in PTECs and its subsequent contribution to tubular injury in DKD7. In human PTECs (HK-2), AGE exposure caused a significant reduction in CTSB and CTSL activity. The degradation ability of lysosomes was partially improved by anti-AGE receptor (RAGE) antibody pretreatment, demonstrating that the AGE–RAGE interaction is a potential mechanism underlying lysosomal dysfunction. AGE–RAGE axis played a crucial role in LMP occurrence by promoting oxidative stress generation7. Furthermore, quantitative cytoplasmic active cathepsins released from destructive lysosomes might play an important role in triggering TEC apoptosis and subsequent tubulointerstitial injury96. These results suggest that AGEs can induce LMP through the AGE–RAGE interaction, subsequently triggering further accumulation of AGEs and exacerbating DKD progression (Fig. 3).
Fig. 3. Mechanism of lysosomal dyshomeostasis and its effect on PTECs during DKD.
AGEs can induce LMP through the AGE-RAGE interaction, triggering lysosomal dysfunction and tubular injury7. Smad3 binds with TFEB and inhibits its expression, resulting in inhibition of lysosome biosynthesis obstacle95. Megalin-mediated uptake of AGEs causes lysosomal dysfunction, leading to increased multivesicular body formation and exocytosis-mediated urinary megalin excretion. Renal PTECs lack protein endocytosis megalin receptors, resulting in proteinuria persistence104,105. LMP caused by AGEs can marginally improve by CTSD upregulation, independent of its enzymatic activity112. CTSB activity decreases by megalin-mediated uptake of thiol-modified albumin. Subsequently, the rate of lysosomal CTSB proteolysis is compromised, contributing to renal tubulointerstitial fibrosis119. AGEs, advanced glycation end products; CTSB, cathepsin B; CTSD, cathepsin D; DKD; diabetic kidney disease; LMP, lysosomal membrane permeabilization; PTECs, proximal tubular epithelial cells; RAGE, AGE receptor; TFEB, transcription factor EB.
Singh et al.97 investigated the urinary proteome of 220 adolescents and children; half had T1DM for an average of seven years and a half were healthy siblings. Eight of the fifteen proteins most substantially elevated in the T1DM cohort were lysosomal-related, including lysosomal enzymes or present in lysosomes, supporting the concept that damage of lysosomal membrane in PTECs occurs in T1DM patients. Urinary lysosomal enzyme loss and lysosomal enzymuria are early biomarkers of DKD98. Therefore, LMP or lysosomal dysfunction in PTECs must be considered during DKD development.
Lysosomal dysfunction results in increased urinary protein excretion
Proteinuria is the result of increased glomerular capillary wall permeability and/or decreased proximal tubule endocytosis99,100. Under normal physiological conditions, the proximal tubule plays an important role in reabsorbing filtered low-molecular-weight (LMW) proteins (<65 kDa) and albumin via an accurate mechanism of receptor-mediated endocytosis (e.g., megalin and cubicin, two types of multiligand receptors)101. The dysfunction of renal proximal tubular endocytosis in DKD animals is associated with the excretion of high levels of urinary total protein, albumin, and transferrin102.
Megalin is involved in AGE uptake filtered by glomeruli in PTECs103. In a high-fat diet (HFD) diabetic mouse model, megalin-mediated lysosomal dysfunction in PTECs by ingestion of pathogenic ligands led to dysfunctional renal tubules compared with the megalin-KO mice group104. In AGE-treated cultured immortalized rat proximal tubule cells (IRPTCs), megalin-mediated uptake of AGEs caused lysosomal dysfunction, leading to increased multivesicular body (MVB) formation and exocytosis-mediated urinary megalin excretion (Fig. 3)105. Exosomes are formed in the endosomal network, with late endosomes fusing with lysosomes for further degradation or with plasma membranes for exosome excretion. Lysosomal dysfunction leads to increased MVB production, megalin recruitment in MVB, and release of exosomes containing megalin105. When IRPTCs were treated with two potent lysosome inhibitors, bafilomycin A1 (BAFA) and chloroquine (CQ) phosphate, lysosomal dysfunction increased MVB formation and exocytosis-mediated urinary megalin excretion105. Figueira et al. showed reduced mRNA and protein levels of the endocytic apparatus (e.g., megalin and cubicin) in the renal cortex and proximal tubules in the early stages of T1DM102. Urinary full-length megalin excretion via extracellular vesicles increased in HFD-fed mice105. The urinary level of megalin positively correlated with the DKD progression in T2DM patients105.
A urinary megalin ELISA has potential value for early diagnosis and severity assessment of DKD in patients with T2DM106. Notably, lysosomal dysfunction can be caused by megalin-mediated uptake of toxic ligands in DM, and increased megalin excretion in urine is because of lysosomal dysfunction in proximal tubules. Renal PTECs lack protein endocytosis receptors, resulting in proteinuria persistence, which accelerates DKD progression107. Therefore, these studies highlight the importance of lysosome function in proximal tubules to maintain endocytic protein receptor integrity, and the key role of lysosomes in DKD pathophysiology and diagnosis.
TFEB-induced lysosomal biosynthesis alleviates autophagy stress in PTECs
Takahashi et al. demonstrated that more AGEs accumulated in the tubular cells of diabetic STZ-treated Atg5-deficient mice compared to control mice6. The protein levels of Lamp1 and TFEB, and nuclear translocation of TFEB, were decreased in AGE-exposed Atg5-deficient PTECs and tubular cells of diabetic Atg5-deficient mice. This indicated that autophagy contributed to the upregulation of TFEB expression, while promoting nuclear translocation of TFEB and lysosomal biogenesis to clearing the accumulated AGEs, which also highlights the importance of TFEB expression and lysosomal biogenesis in DKD.
We found that lysosomes in PTECs occur via LMP in the diabetic state7,8. Severely damaged lysosomes can be removed by lysophagy, and normal lysophagy propulsion requires intact and functional lysosomes108,109. Unfortunately, our in vivo and in vitro studies found that the number and proportion of primary lysosomes decreased with lysosome accumulation in diabetic PTECs. We found that Smad3 inhibited TFEB expression in DKD (Fig. 3), whereas inhibition of SMAD3 activity increased TFEB expression and rescued PTEC lysosomal renewal disorder in DKD95. Zhao et al. demonstrated that high-dose vitamin E therapy could alleviate autophagic stress, ameliorate proteinuria, and improve renal function in diabetic rats110. Autophagic stress is a continuous imbalance in which the formation rate of autophagosomes exceeds its lysosomal pathway degradation rate111. Interestingly, vitamin E treatment reduced the accumulation of autophagic vacuoles and autophagic substrates. Notably, the decline in CTSB and CTSL activity caused by AGE exposure was reversed following vitamin E treatment110. Thus, the improvement of lysosomal biosynthesis and lysosomal function provides a promising new option for DKD treatment to alleviate autophagic stress.
CTSD improves PTEC injury independent of enzymatic activity
CTSD is a major aspartate protease within lysosomes80. Du et al. demonstrated that CTSD protected renal PTECs from injury induced by HG and AGE exposure, independent of its enzymatic catalytic activity under diabetic conditions112. Immunohistochemical staining of renal tissues from DM patients and non-DM subjects revealed relatively low expression of CTSD with a uniformly distributed pattern in non-DM tubules, whereas this was considerably reduced in disordered DM tubules. CTSD overexpression showed elevated cell viability and decreased apoptosis following AGE exposure in CTSD-transduced HK-2 cells. Furthermore, when HK-2 cells were treated with pepstatin A, a CTSD inhibitor, or transduced with a lentiviral vector encoding an inactive mutant of CTSD, the protective effect of CTSD persisted, indicating that CTSD protection is independent of its enzymatic activity112.
LMP contributes to CTSD release from lysosomes into the cytoplasm, and consequently triggers the mitochondrial apoptotic cascade113. Here, LMP caused by AGEs in HK-2 cells improved marginally by CTSD upregulation, indicating a potential benefit of CTSD in tubular damage in DKD (Fig. 3)112. Thus, the mechanisms by which CTSD protects PTECs from apoptosis and LMP require further investigation.
Impaired CTSB activity inhibits protein degradation
CTSB is the primary lysosomal cysteine protease involved in lysosomal protein degradation and is primarily expressed in the cytoplasmic vesicles of PTECs in renal tissues114. CTSB promotes the degradation of reabsorbed urine protein, which is internalized by proximal tubular endocytosis. The reabsorbed urine protein is degraded in lysosomes to LMW fragments and returned to the tubular lumen115. A decrease in CTSB activity in PTECs accompanied by excretion of high-molecular-weight urinary proteins was noted in early DKD rats116. The degradation rate of long-lived proteins in PTECs was drastically reduced, accompanied by decreased CTSB activity, which led to the inhibition of protein degradation in PTECs, ultimately leading to diabetic renal hypertrophy63. CTSB activity considerably decreased when exposed to AGEs, resulting in decreased cell viability of PTECs114.
AGEs can inhibit protein degradation by inhibiting CTSB expression and activity117. In the STZ-induced DKD rat model, Sebekova et al.118 found that the cell volume of PTECs incubated with AGEs increased remarkably, intracellular and extracellular protein synthesis increased, protein degradation rate decreased, and CTSB activity decreased. A correlation between AGEs and CTSB was established by LMP occurrence when exposed to AGEs, resulting in lysosomal alkalization and CTSB inactivation7. Furthermore, Medina-Navarro et al. demonstrated that reduced CTSB activity in PTECs with DKD was closely related to the tertiary structure of reabsorbed albumin (Fig. 3)119. Albumin collected from stage 4 DKD patients presented >50% higher thiol-dependent changes in the albumin tertiary structure than that collected from stages 0 and 1. CTSB activity in isolated lysosomes revealed a significant inhibitory effect in HK-2 cells treated with albumin from stage 4 DKD patients and with albumin that was intentionally modified119. Subsequently, the rate of lysosomal CTSB proteolysis was compromised; thus, an imminent overload of proteins was induced, which facilitated transdifferentiation of epithelial tubular cells to myofibroblasts and contributed to renal tubulointerstitial fibrosis and DKD progression119. These findings suggest that CTSB may function as a therapeutic target in DKD.
Macrophage iysosomal dysfunction and enzyme abnormalities in DKD
DKD is a chronic inflammatory disease characterized by numerous inflammatory cell infiltrates and overexpression of pro-inflammatory factors120,121. Numerous clinical and animal studies have found that most patients or animal renal tissues with DKD are associated with different levels of macrophage infiltration122,123. The degree of macrophage infiltration is associated with renal damage severity123,124. Therefore, the prevention of macrophage infiltration is crucial for DKD treatment.
Normal iysosomal function alleviates macrophage infiltration and renal injuries
Normal lysosomal function plays a crucial role in maintaining and controlling adhesion and migration of macrophages, thus alleviating renal injury. In a diabetic rat model, macrophage infiltration increased in renal tissue, characterized by the macrophage marker CD68+. In an in vitro experiment, adhesion and migration of macrophages increased under HG stimulation. The number of adhesion and migration macrophages further increased when BAFA or CQ were added simultaneously, compared with the HG group125. Furthermore, Yuan et al. reported that mesenchymal stem cells could elicit M1/M2 macrophages into the M2 phenotype and alleviate renal injury in DKD mice by activating TFEB and subsequently restoring the lysosomal substrate degradation function in macrophages (Fig. 4)126.
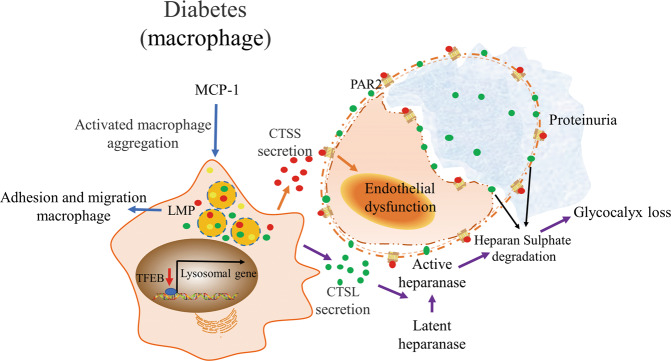
Fig. 4. Mechanism of lysosomal dyshomeostasis in macrophages and its effect on renal endothelial cells during DKD.
Lysosomal dysfunction promotes adhesion and migration of macrophages125. Decreased TFEB activity in macrophages is involved in kidney injury126. MCP-1 promotes activated macrophage glomerular aggregation133,134. Macrophage-derived CTSS could induce diabetic renal endothelial cell damage by activating PAR2 on the surface of endothelial cells132. CTSL can be secreted into the extracellular space of the kidney by glomerular macrophage infiltration, resulting in renal heparanase activation and subsequent glycocalyx loss and proteinuria137,138. CTSL, cathepsin L; CTSS; cathepsin S; DKD, diabetic kidney disease; MCP-1, monocyte chemotactic protein-1; PAR2, protease-activated receptor-2; TFEB, transcription factor EB.
Macrophage-derived CTSS induces endothelial cell damage
CTSS is a member of the cysteine cathepsin protease family. CTSS degrades proteins via the endosomal/lysosomal pathway and can be secreted into the extracellular environment for biological activity127. Unlike that of the other members of the cathepsin family, the enzyme activity of CTSS operates within a large pH range such that it can function outside the cell128. A prominent feature of CTSS is that its expression is primarily limited to leukocyte subsets, especially macrophages129. Elevated serum CTSS levels increase the risk of T2DM, suggesting a clinical correlation between CTSS suppression and reducing or delaying type 2 diabetes and kidney disease development in obese individuals130. In patients with ESRD, followed by an increase in the serum level of CTSS, the glomerular filtration rate and cysteine protease inhibitor C level decreased, indicating that CTSS activity increased with the progression of chronic kidney disease131.
Kumar et al. demonstrated that macrophage-derived CTSS could induce diabetic renal endothelial cell damage by activating protease-activated receptor-2 on the surface of endothelial cells (Fig. 4)132. In db/db mice, proteinuria, glomerulosclerosis, and renal inflammation were alleviated by treatment with RO5461111, a selective CTSS activity inhibitor, and PAR2 inhibition was effective in attenuating glomerulosclerosis. In an in vitro experiment, CTSS specifically triggered epithelial cell dysfunction through PAR2; however, it had no effect on TECs and podocytes132. Collectively, the inhibition of CTSS-mediated PAR2 activation in endothelial cells plays an important role in the prevention and improvement of DKD.
MCP-1/Macrophage-derived CTSL injures glomerular barrier
Monocyte chemotactic protein-1 (MCP-1) is associated with monocyte recruitment from the circulatory system and monocyte and macrophage migration133,134. In patients with DKD, increased MCP-1 levels in renal tissue and urine suggested that macrophages play an important role in DKD development135. Glomerular macrophages have been observed in biopsies of mild DKD, suggesting that macrophages play a role in early diabetes-induced impairment136.
Boels et al.137 demonstrated that CTSL could be secreted into the extracellular space of the kidney by glomerular macrophage infiltration, making them candidates for renal heparanase activation and subsequent glycocalyx loss (Fig. 4). MCP-1 inhibition can reduce albuminuria and restore glomerular endothelial glycocalyx in DKD. Glomerular heparanase and CTSL protein expression was increased in diabetic mice, whereas it was decreased in diabetic mice treated with NOX-E36 (emapticap pegol, an MCP-1 inhibitor)137. Previous immunohistochemical studies showed that the macrophage marker F4/80 co-localizes with CTSL138. The above studies suggest that MCP-1 inhibition can restore glomerular barrier function by influencing macrophage CTSL secretion and reducing heparanase activation, highlighting the potential pathogenicity of CTSL released from infiltrated renal macrophages in DKD.
Conclusions and future prospects
Despite the use of multiple intensive therapies, the incidence of diabetes continues to increase globally. DKD, a serious diabetic complication, is still the primary cause of ESRD, with high morbidity and mortality. Therefore, there is an urgent need to identify new effective therapeutic targets for the prevention and treatment of DKD. In this review, we provide an in-depth understanding of the role of lysosomes in DKD pathogenesis, identify potential mechanisms that promote the regulation of lysosome biogenesis and function in diabetic kidneys, and highlight a new research direction for the effective control of diabetic nephropathy.
However, valuable results of research on pathogenesis should eventually be applied to improve the treatment and prognosis of diseases. Currently, research on screening effective interventions for lysosomal dyshomeostasis is still in its infancy, and thus should be the focus of future research studies. The screening out of cell-specific lysosomal function regulation targets according to the different stages of DKD, so as to realize the controllable targeted regulation of cell lysosomal function during DKD, is the key to the successful clinical development of this therapeutic strategy.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing. This research was funded by the National Natural Science Foundation of China (81974095 and 82000647).
Author contributions
H.L. and X.L.: design and conception; X.L.: review and revision of the paper; M.W. and M.Z.: writing and revision of the paper; Y.Z., Z.L., X.L. and Z.L.: technical and material support; All authors have read and approved the final version of the paper.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Competing interests
The authors declare no competing interests.
Footnotes
Edited by Professor Alessandro Finazzi-Agrò
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Man Wu, Minjie Zhang.
Contributor Information
Huafeng Liu, Email: liuhf@gdmu.edu.cn.
Xiaoyu Li, Email: lixiaoyu1226@gmail.com.
References
- 1.Meyer-Schwesinger C. Lysosome function in glomerular health and disease. Cell Tissue Res. 2021. https://link.springer.com/article/10.1007/s00441-020-03375-7. [DOI] [PMC free article] [PubMed]
- 2.Giandalia A, Giuffrida AE, Gembillo G, Cucinotta D, Squadrito G, Santoro D, et al. Gender differences in diabetic kidney disease: focus on hormonal, genetic and clinical factors. Int J Mol Sci. 2021;22:5808. [DOI] [PMC free article] [PubMed]
- 3.Kumar Pasupulati A, Chitra PS, Reddy GB. Advanced glycation end products mediated cellular and molecular events in the pathology of diabetic nephropathy. Biomol Concepts. 2016;7:293–309. doi: 10.1515/bmc-2016-0021. [DOI] [PubMed] [Google Scholar]
- 4.Ding Y, Choi ME. Autophagy in diabetic nephropathy. J Endocrinol. 2015;224:R15–30. doi: 10.1530/JOE-14-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu WJ, Luo MN, Tan J, Chen W, Huang LZ, Yang C, et al. Autophagy activation reduces renal tubular injury induced by urinary proteins. Autophagy. 2014;10:243–56.. doi: 10.4161/auto.27004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahashi A, Takabatake Y, Kimura T, Maejima I, Namba T, Yamamoto T, et al. Autophagy inhibits the accumulation of advanced glycation end products by promoting lysosomal biogenesis and function in the kidney proximal tubules. Diabetes. 2017;66:1359–72.. doi: 10.2337/db16-0397. [DOI] [PubMed] [Google Scholar]
- 7.Liu WJ, Shen TT, Chen RH, Wu HL, Wang YJ, Deng JK, et al. Autophagy-lysosome pathway in renal tubular epithelial cells is disrupted by advanced glycation end products in diabetic nephropathy. J Biol Chem. 2015;290:20499–510. doi: 10.1074/jbc.M115.666354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu WJ, Xu BH, Ye L, Liang D, Wu HL, Zheng YY, et al. Urinary proteins induce lysosomal membrane permeabilization and lysosomal dysfunction in renal tubular epithelial cells. Am J Physiol Ren Physiol. 2015;308:F639–49. doi: 10.1152/ajprenal.00383.2014. [DOI] [PubMed] [Google Scholar]
- 9.Yang D, Livingston MJ, Liu Z, Dong G, Zhang M, Chen JK, et al. Autophagy in diabetic kidney disease: regulation, pathological role and therapeutic potential. Cell Mol Life Sci. 2018;75:669–88.. doi: 10.1007/s00018-017-2639-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang W, Li X, Wang S, Chen Y, Liu H. Regulation of TFEB activity and its potential as a therapeutic target against kidney diseases. Cell Death Discov. 2020;6:32. [DOI] [PMC free article] [PubMed]
- 11.Xu H, Ren D. Lysosomal physiology. Annu Rev Physiol. 2015;77:57–80. doi: 10.1146/annurev-physiol-021014-071649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davidson SM, Vander, Heiden MG. Critical functions of the lysosome in cancer biology. Annu Rev Pharm Toxicol. 2017;57:481–507. doi: 10.1146/annurev-pharmtox-010715-103101. [DOI] [PubMed] [Google Scholar]
- 13.Kreher C, Favret J, Maulik M, Shin D. Lysosomal functions in glia associated with neurodegeneration. Biomolecules. 2021;11:400. [DOI] [PMC free article] [PubMed]
- 14.Tan JMJ, Mellouk N, Brumell JH. An autophagy-independent role for ATG16L1: promoting lysosome-mediated plasma membrane repair. Autophagy. 2019;15:932–3. doi: 10.1080/15548627.2019.1586261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corrotte M, Castro-Gomes T. Lysosomes and plasma membrane repair. Curr Top Membr. 2019;84:1–16. doi: 10.1016/bs.ctm.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Encarnação M, Espada L, Escrevente C, Mateus D, Ramalho J, Michelet X, et al. A Rab3a-dependent complex essential for lysosome positioning and plasma membrane repair. J Cell Biol. 2016;213:631–40.. doi: 10.1083/jcb.201511093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffiths GM. Secretion from Myeloid Cells: Secretory Lysosomes. Microbiol Spectr. 2016;4. [DOI] [PubMed]
- 18.Goodridge JP, Jacobs B, Saetersmoen ML, Clement D, Hammer Q, Clancy T, et al. Remodeling of secretory lysosomes during education tunes functional potential in NK cells. Nat Commun. 2019;10:514. doi: 10.1038/s41467-019-08384-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Villeneuve J, Bassaganyas L, Lepreux S, Chiritoiu M, Costet P, Ripoche J, et al. Unconventional secretion of FABP4 by endosomes and secretory lysosomes. J Cell Biol. 2018;217:649–65.. doi: 10.1083/jcb.201705047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Settembre C, Zoncu R, Medina DL, Vetrini F, Erdin S, Erdin S, et al. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. Embo j. 2012;31:1095–108.. doi: 10.1038/emboj.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jia J, Abudu YP, Claude-Taupin A, Gu Y, Kumar S, Choi SW, et al. Galectins control MTOR and AMPK in response to lysosomal damage to induce autophagy. Autophagy. 2019;15:169–71.. doi: 10.1080/15548627.2018.1505155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jia J, Bissa B, Brecht L, Allers L, Choi SW, Gu Y, et al. AMPK, a regulator of metabolism and autophagy, is activated by lysosomal damage via a novel galectin-directed ubiquitin signal transduction system. Mol Cell. 2020;77:951–69.e9. doi: 10.1016/j.molcel.2019.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jia J, Bissa B, Brecht L, Allers L, Choi SW, Gu Y, et al. AMPK is activated during lysosomal damage via a galectin-ubiquitin signal transduction system. Autophagy. 2020;16:1550–2. doi: 10.1080/15548627.2020.1788890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Z, Yue P, Lu T, Wang Y, Wei Y, Wei X. Role of lysosomes in physiological activities, diseases, and therapy. J Hematol Oncol. 2021;14:79. doi: 10.1186/s13045-021-01087-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buratta S, Tancini B, Sagini K, Delo F, Chiaradia E, Urbanelli L, et al. Lysosomal exocytosis, exosome release and secretory autophagy: the autophagic- and endo-lysosomal systems go extracellular. Int J Mol Sci. 2020;21:2576. [DOI] [PMC free article] [PubMed]
- 26.Sardiello M, Palmieri M, di Ronza A, Medina DL, Valenza M, Gennarino VA, et al. A gene network regulating lysosomal biogenesis and function. Science. 2009;325:473–7. doi: 10.1126/science.1174447. [DOI] [PubMed] [Google Scholar]
- 27.Chen M, Dai Y, Liu S, Fan Y, Ding Z, Li D. TFEB Biology and Agonists at a Glance. Cells. 2021;10:333. [DOI] [PMC free article] [PubMed]
- 28.Puertollano R, Ferguson SM, Brugarolas J, Ballabio A. The complex relationship between TFEB transcription factor phosphorylation and subcellular localization. Embo j. 2018;37:e98804. [DOI] [PMC free article] [PubMed]
- 29.Zheng Q, Li J, Wang X. Interplay between the ubiquitin-proteasome system and autophagy in proteinopathies. Int J Physiol Pathophysiol Pharmacol. 2009;1:127–42. [PMC free article] [PubMed] [Google Scholar]
- 30.Behrends C, Sowa ME, Gygi SP, Harper JW. Network organization of the human autophagy system. Nature. 2010;466:68–76. doi: 10.1038/nature09204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sha Y, Rao L, Settembre C, Ballabio A, Eissa NT. STUB1 regulates TFEB-induced autophagy-lysosome pathway. Embo j. 2017;36:2544–52.. doi: 10.15252/embj.201796699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li C, Wang X, Li X, Qiu K, Jiao F, Liu Y, et al. Proteasome inhibition activates autophagy-lysosome pathway associated with TFEB dephosphorylation and nuclear translocation. Front Cell Dev Biol. 2019;7:170. doi: 10.3389/fcell.2019.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Huang Y, Liu J, Zhang J, Xu M, You Z, et al. Acetyltransferase GCN5 regulates autophagy and lysosome biogenesis by targeting TFEB. EMBO Rep. 2020;21:e48335. doi: 10.15252/embr.201948335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pippias M, Kramer A, Noordzij M, Afentakis N, Alonso de la Torre R, Ambühl PM, et al. The european renal association - european dialysis and transplant association registry annual report 2014: a summary. Clin Kidney J. 2017;10:154–69.. doi: 10.1093/ckj/sfw135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koye DN, Shaw JE, Reid CM, Atkins RC, Reutens AT, Magliano DJ. Incidence of chronic kidney disease among people with diabetes: a systematic review of observational studies. Diabet Med. 2017;34:887–901. doi: 10.1111/dme.13324. [DOI] [PubMed] [Google Scholar]
- 36.Shi Y, Hu FB. The global implications of diabetes and cancer. Lancet. 2014;383:1947–8. doi: 10.1016/S0140-6736(14)60886-2. [DOI] [PubMed] [Google Scholar]
- 37.Rossing P, Hougaard P, Parving HH. Progression of microalbuminuria in type 1 diabetes: ten-year prospective observational study. Kidney Int. 2005;68:1446–50. doi: 10.1111/j.1523-1755.2005.00556.x. [DOI] [PubMed] [Google Scholar]
- 38.Lim A. Diabetic nephropathy - complications and treatment. Int J Nephrol Renovasc Dis. 2014;7:361–81. doi: 10.2147/IJNRD.S40172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu CC, Hu ZB, Wang R, Hong ZH, Lu J, Chen PP, et al. Gut microbiota dysbiosis-induced activation of the intrarenal renin-angiotensin system is involved in kidney injuries in rat diabetic nephropathy. Acta Pharm Sin. 2020;41:1111–8. doi: 10.1038/s41401-019-0326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang XS, Xiang XY, Chen XM, He JL, Liu T, Gan H, et al. Inhibition of soluble epoxide hydrolase attenuates renal tubular mitochondrial dysfunction and ER stress by restoring autophagic flux in diabetic nephropathy. Cell Death Dis. 2020;11:385. doi: 10.1038/s41419-020-2594-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu X, Lu J, Liu S, Huang D, Chen M, Xiong G, et al. Huangqi-Danshen decoction alleviates diabetic nephropathy in db/db mice by inhibiting PINK1/Parkin-mediated mitophagy. Am J Transl Res. 2020;12:989–98. [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Y, Xu D, Wang L, Du W, Zhang L, Xiang X. MBTPS2 exacerbates albuminuria in streptozotocin-induced type I diabetic nephropathy by promoting endoplasmic reticulum stress-mediated renal damage. Arch Physiol Biochem. 2020:1–8. [DOI] [PubMed]
- 43.Kimmelstiel P, Wilson C. Intercapillary lesions in the glomeruli of the kidney. Am J Pathol. 1936;12:83–98.7. [PMC free article] [PubMed] [Google Scholar]
- 44.Liberti ME, Sagliocca A, Palmisano R, Pirro L, Provenzano M, Minutolo R, et al. Prevention of diabetic nephropathy: from bench to bedside. G Ital Nefrol. 2013;30:gin/30.4.2. [PubMed]
- 45.Gilbert RE. Proximal tubulopathy: prime mover and key therapeutic target in diabetic kidney disease. Diabetes. 2017;66:791–800. doi: 10.2337/db16-0796. [DOI] [PubMed] [Google Scholar]
- 46.Haraguchi R, Kohara Y, Matsubayashi K, Kitazawa R, Kitazawa S. New Insights into the pathogenesis of diabetic nephropathy: proximal renal tubules are primary target of oxidative stress in diabetic kidney. Acta Histochem Cytochem. 2020;53:21–31. doi: 10.1267/ahc.20008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dagar N, Das P, Bisht P, Taraphdar AK, Velayutham R, Arumugam S. Diabetic nephropathy: a twisted thread to unravel. Life Sci. 2021;278:119635. doi: 10.1016/j.lfs.2021.119635. [DOI] [PubMed] [Google Scholar]
- 48.Xu Y, Zhou Q, Xin W, Li Z, Chen L, Wan Q. Autophagy downregulation contributes to insulin resistance mediated injury in insulin receptor knockout podocytes in vitro. PeerJ. 2016;4:e1888. doi: 10.7717/peerj.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xin W, Li Z, Xu Y, Yu Y, Zhou Q, Chen L, et al. Autophagy protects human podocytes from high glucose-induced injury by preventing insulin resistance. Metabolism. 2016;65:1307–15.. doi: 10.1016/j.metabol.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 50.Ji J, Zhao Y, Na C, Yang M, Zhu X, Shi H, et al. Connexin 43‑autophagy loop in the podocyte injury of diabetic nephropathy. Int J Mol Med. 2019;44:1781–8. doi: 10.3892/ijmm.2019.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lenoir O, Jasiek M, Hénique C, Guyonnet L, Hartleben B, Bork T, et al. Endothelial cell and podocyte autophagy synergistically protect from diabetes-induced glomerulosclerosis. Autophagy. 2015;11:1130–45.. doi: 10.1080/15548627.2015.1049799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu Y, Liu L, Xin W, Zhao X, Chen L, Zhen J, et al. The renoprotective role of autophagy activation in proximal tubular epithelial cells in diabetic nephropathy. J diabetes its complications. 2015;29:976–83.. doi: 10.1016/j.jdiacomp.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 53.Duan X, Kong Z, Mai X, Lan Y, Liu Y, Yang Z, et al. Autophagy inhibition attenuates hyperoxaluria-induced renal tubular oxidative injury and calcium oxalate crystal depositions in the rat kidney. Redox Biol. 2018;16:414–25.. doi: 10.1016/j.redox.2018.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shirazi MK, Azarnezhad A, Abazari MF, Poorebrahim M, Ghoraeian P, Sanadgol N, et al. The role of nitric oxide signaling in renoprotective effects of hydrogen sulfide against chronic kidney disease in rats: Involvement of oxidative stress, autophagy and apoptosis. J Cell Physiol. 2019;234:11411–23.. doi: 10.1002/jcp.27797. [DOI] [PubMed] [Google Scholar]
- 55.Hadj Abdallah N, Baulies A, Bouhlel A, Bejaoui M, Zaouali MA, Ben Mimouna S, et al. Zinc mitigates renal ischemia-reperfusion injury in rats by modulating oxidative stress, endoplasmic reticulum stress, and autophagy. J Cell Physiol. 2018;233:8677–90.. doi: 10.1002/jcp.26747. [DOI] [PubMed] [Google Scholar]
- 56.Zhan M, Usman IM, Sun L, Kanwar YS. Disruption of renal tubular mitochondrial quality control by Myo-inositol oxygenase in diabetic kidney disease. J Am Soc Nephrol. 2015;26:1304–21.. doi: 10.1681/ASN.2014050457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao X, Liu G, Shen H, Gao B, Li X, Fu J, et al. Liraglutide inhibits autophagy and apoptosis induced by high glucose through GLP-1R in renal tubular epithelial cells. Int J Mol Med. 2015;35:684–92.. doi: 10.3892/ijmm.2014.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hou Y, Lin S, Qiu J, Sun W, Dong M, Xiang Y, et al. NLRP3 inflammasome negatively regulates podocyte autophagy in diabetic nephropathy. Biochem Biophys Res Commun. 2020;521:791–8. doi: 10.1016/j.bbrc.2019.10.194. [DOI] [PubMed] [Google Scholar]
- 59.Ma T, Zhu J, Chen X, Zha D, Singhal PC, Ding G. High glucose induces autophagy in podocytes. Exp Cell Res. 2013;319:779–89.. doi: 10.1016/j.yexcr.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoshii SR, Mizushima N. Monitoring and measuring autophagy. Int J Mol Sci. 2017;18:1865. [DOI] [PMC free article] [PubMed]
- 61.Huang Y, Li Y, Qu Y, Zheng Y, Ouyang M, Zhang Y, et al. UVA-induced photoaging inhibits autophagic degradation by impairing lysosomal function in dermal fibroblasts. Biochem Biophys Res Commun. 2019;518:611–8. doi: 10.1016/j.bbrc.2019.08.103. [DOI] [PubMed] [Google Scholar]
- 62.Osicka TM, Kiriazis Z, Pratt LM, Jerums G, Comper WD. Ramipril and aminoguanidine restore renal lysosomal processing in streptozotocin diabetic rats. Diabetologia. 2001;44:230–6. doi: 10.1007/s001250051604. [DOI] [PubMed] [Google Scholar]
- 63.Shechter P, Boner G, Rabkin R. Tubular cell protein degradation in early diabetic renal hypertrophy. J Am Soc Nephrol. 1994;4:1582–7. doi: 10.1681/ASN.V481582. [DOI] [PubMed] [Google Scholar]
- 64.Osicka TM, Houlihan CA, Chan JG, Jerums G, Comper WD. Albuminuria in patients with type 1 diabetes is directly linked to changes in the lysosome-mediated degradation of albumin during renal passage. Diabetes. 2000;49:1579–84.. doi: 10.2337/diabetes.49.9.1579. [DOI] [PubMed] [Google Scholar]
- 65.Brijmohan AS, Batchu SN, Majumder S, Alghamdi TA, Thieme K, McGaugh S, et al. HDAC6 inhibition promotes transcription factor EB activation and is protective in experimental kidney disease. Front Pharmacol. 2018;9:34. doi: 10.3389/fphar.2018.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blaine J, Dylewski J. Regulation of the actin cytoskeleton in podocytes. Cells. 2020;9:1700. [DOI] [PMC free article] [PubMed]
- 67.Qu H, Gong X, Liu X, Zhang R, Wang Y, Huang B, et al. Deficiency of mitochondrial glycerol 3-phosphate dehydrogenase exacerbates podocyte injury and the progression of diabetic kidney disease. Diabetes. 2021;70:1372–87.. doi: 10.2337/db20-1157. [DOI] [PubMed] [Google Scholar]
- 68.Imeri F, Stepanovska Tanturovska B, Schwalm S, Saha S, Zeng-Brouwers J, Pavenstädt H, et al. Loss of sphingosine kinase 2 enhances Wilm’s tumor suppressor gene 1 and nephrin expression in podocytes and protects from streptozotocin-induced podocytopathy and albuminuria in mice. Matrix Biol. 2021;98:32–48. doi: 10.1016/j.matbio.2021.05.003. [DOI] [PubMed] [Google Scholar]
- 69.Kikuchi K, Saigusa D, Kanemitsu Y, Matsumoto Y, Thanai P, Suzuki N, et al. Gut microbiome-derived phenyl sulfate contributes to albuminuria in diabetic kidney disease. Nat Commun. 2019;10:1835. doi: 10.1038/s41467-019-09735-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim J, Sohn E, Kim CS, Kim JS. Renal podocyte apoptosis in Zucker diabetic fatty rats: involvement of methylglyoxal-induced oxidative DNA damage. J Comp Pathol. 2011;144:41–7. doi: 10.1016/j.jcpa.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 71.Zhou LL, Cao W, Xie C, Tian J, Zhou Z, Zhou Q, et al. The receptor of advanced glycation end products plays a central role in advanced oxidation protein products-induced podocyte apoptosis. Kidney Int. 2012;82:759–70. doi: 10.1038/ki.2012.184. [DOI] [PubMed] [Google Scholar]
- 72.Carson JM, Okamura K, Wakashin H, McFann K, Dobrinskikh E, Kopp JB, et al. Podocytes degrade endocytosed albumin primarily in lysosomes. PLoS One. 2014;9:e99771. doi: 10.1371/journal.pone.0099771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu WJ, Gan Y, Huang WF, Wu HL, Zhang XQ, Zheng HJ, et al. Lysosome restoration to activate podocyte autophagy: a new therapeutic strategy for diabetic kidney disease. Cell Death Dis. 2019;10:806. doi: 10.1038/s41419-019-2002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen Y, Liu Q, Shan Z, Mi W, Zhao Y, Li M, et al. Catalpol ameliorates podocyte injury by stabilizing cytoskeleton and enhancing autophagy in diabetic nephropathy. Front Pharmacol. 2019;10:1477. doi: 10.3389/fphar.2019.01477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao X, Chen Y, Tan X, Zhang L, Zhang H, Li Z, et al. Advanced glycation end-products suppress autophagic flux in podocytes by activating mammalian target of rapamycin and inhibiting nuclear translocation of transcription factor EB. J Pathol. 2018;245:235–48. doi: 10.1002/path.5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hou B, Li Y, Li X, Zhang C, Zhao Z, Chen Q, et al. HGF protected against diabetic nephropathy via autophagy-lysosome pathway in podocyte by modulating PI3K/Akt-GSK3β-TFEB axis. Cell Signal. 2020;75:109744. doi: 10.1016/j.cellsig.2020.109744. [DOI] [PubMed] [Google Scholar]
- 77.Huber TB, Walz G, Kuehn EW. mTOR and rapamycin in the kidney: signaling and therapeutic implications beyond immunosuppression. Kidney Int. 2011;79:502–11. doi: 10.1038/ki.2010.457. [DOI] [PubMed] [Google Scholar]
- 78.Lieberthal W, Levine JS. The role of the mammalian target of rapamycin (mTOR) in renal disease. J Am Soc Nephrol. 2009;20:2493–502. doi: 10.1681/ASN.2008111186. [DOI] [PubMed] [Google Scholar]
- 79.Gödel M, Hartleben B, Herbach N, Liu S, Zschiedrich S, Lu S, et al. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J Clin Invest. 2011;121:2197–209. doi: 10.1172/JCI44774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dana D, Pathak SK. A review of small molecule inhibitors and functional probes of human cathepsin L. Molecules. 2020;25:698. [DOI] [PMC free article] [PubMed]
- 81.Sever S, Altintas MM, Nankoe SR, Möller CC, Ko D, Wei C, et al. Proteolytic processing of dynamin by cytoplasmic cathepsin L is a mechanism for proteinuric kidney disease. J Clin Invest. 2007;117:2095–104. doi: 10.1172/JCI32022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sołtysiak J, Skowrońska B, Fichna P, Stankiewicz W, Lewandowska-Stachowiak M, Ostalska-Nowicka D, et al. Neutrophil gelatinase-associated lipocalin and Cathepsin L as early predictors of kidney dysfunction in children with type 1 diabetes. Endokrynol Pol. 2014;65:479–84. doi: 10.5603/EP.2014.0067. [DOI] [PubMed] [Google Scholar]
- 83.Brings S, Fleming T, Herzig S, Nawroth PP, Kopf S. Urinary cathepsin L is predictive of changes in albuminuria and correlates with glucosepane in patients with type 2 diabetes in a closed-cohort study. J Diabetes Complications. 2020;34:107648. doi: 10.1016/j.jdiacomp.2020.107648. [DOI] [PubMed] [Google Scholar]
- 84.Cao Y, Liu X, Li Y, Lu Y, Zhong H, Jiang W, et al. Cathepsin L activity correlates with proteinuria in chronic kidney disease in humans. Int Urol Nephrol. 2017;49:1409–17. doi: 10.1007/s11255-017-1626-7. [DOI] [PubMed] [Google Scholar]
- 85.Baricos WH, Cortez SL, Le QC, Wu LT, Shaw E, Hanada K, et al. Evidence suggesting a role for cathepsin L in an experimental model of glomerulonephritis. Arch Biochem Biophys. 1991;288:468–72. doi: 10.1016/0003-9861(91)90222-5. [DOI] [PubMed] [Google Scholar]
- 86.Garsen M, Rops AL, Dijkman H, Willemsen B, van Kuppevelt TH, Russel FG, et al. Cathepsin L is crucial for the development of early experimental diabetic nephropathy. Kidney Int. 2016;90:1012–22. doi: 10.1016/j.kint.2016.06.035. [DOI] [PubMed] [Google Scholar]
- 87.Faul C, Donnelly M, Merscher-Gomez S, Chang YH, Franz S, Delfgaauw J, et al. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med. 2008;14:931–8. doi: 10.1038/nm.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bao H, Ge Y, Peng A, Gong R. Fine-tuning of NFκB by glycogen synthase kinase 3β directs the fate of glomerular podocytes upon injury. Kidney Int. 2015;87:1176–90. doi: 10.1038/ki.2014.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yaddanapudi S, Altintas MM, Kistler AD, Fernandez I, Möller CC, Wei C, et al. CD2AP in mouse and human podocytes controls a proteolytic program that regulates cytoskeletal structure and cellular survival. J Clin Invest. 2011;121:3965–80. doi: 10.1172/JCI58552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ning L, Suleiman HY, Miner JH. Synaptopodin Is Dispensable for Normal Podocyte Homeostasis but Is Protective in the Context of Acute Podocyte Injury. J Am Soc Nephrol. 2020;31:2815–32. doi: 10.1681/ASN.2020050572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Coughlan MT, Nguyen TV, Penfold SA, Higgins GC, Thallas-Bonke V, Tan SM, et al. Mapping time-course mitochondrial adaptations in the kidney in experimental diabetes. Clin Sci. 2016;130:711–20. doi: 10.1042/CS20150838. [DOI] [PubMed] [Google Scholar]
- 92.Xiao L, Xu X, Zhang F, Wang M, Xu Y, Tang D, et al. The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/PINK1. Redox Biol. 2017;11:297–311. doi: 10.1016/j.redox.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhou L, Xu DY, Sha WG, Shen L, Lu GY. Long non-coding RNA MALAT1 interacts with transcription factor Foxo1 to regulate SIRT1 transcription in high glucose-induced HK-2 cells injury. Biochem Biophys Res Commun. 2018;503:849–55. doi: 10.1016/j.bbrc.2018.06.086. [DOI] [PubMed] [Google Scholar]
- 94.Rabbani N, Thornalley PJ. Advanced glycation end products in the pathogenesis of chronic kidney disease. Kidney Int. 2018;93:803–13. doi: 10.1016/j.kint.2017.11.034. [DOI] [PubMed] [Google Scholar]
- 95.Yang C, Chen XC, Li ZH, Wu HL, Jing KP, Huang XR, et al. SMAD3 promotes autophagy dysregulation by triggering lysosome depletion in tubular epithelial cells in diabetic nephropathy. Autophagy. 2020:1–20. [DOI] [PMC free article] [PubMed]
- 96.Repnik U, Hafner Česen M, Turk B. Lysosomal membrane permeabilization in cell death: concepts and challenges. Mitochondrion. 2014;19 Pt A:49–57. doi: 10.1016/j.mito.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 97.Singh H, Yu Y, Suh MJ, Torralba MG, Stenzel RD, Tovchigrechko A, et al. Type 1 diabetes: urinary proteomics and protein network analysis support perturbation of lysosomal function. Theranostics. 2017;7:2704–17. doi: 10.7150/thno.19679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Omozee EB, Okaka EI, Edo AE, Obika LF. Urinary N-acetyl-beta-d-glucosaminidase levels in diabetic adults. J Lab Physicians. 2019;11:1–4. doi: 10.4103/JLP.JLP_164_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zeng B, Chen GL, Garcia-Vaz E, Bhandari S, Daskoulidou N, Berglund LM, et al. ORAI channels are critical for receptor-mediated endocytosis of albumin. Nat Commun. 2017;8:1920. doi: 10.1038/s41467-017-02094-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Terryn S, Tanaka K, Lengelé JP, Olinger E, Dubois-Laforgue D, Garbay S, et al. Tubular proteinuria in patients with HNF1α mutations: HNF1α drives endocytosis in the proximal tubule. Kidney Int. 2016;89:1075–89. doi: 10.1016/j.kint.2016.01.027. [DOI] [PubMed] [Google Scholar]
- 101.Weyer K, Andersen PK, Schmidt K, Mollet G, Antignac C, Birn H, et al. Abolishment of proximal tubule albumin endocytosis does not affect plasma albumin during nephrotic syndrome in mice. Kidney Int. 2018;93:335–42. doi: 10.1016/j.kint.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 102.Figueira MF, Castiglione RC, de Lemos Barbosa CM, Ornellas FM, da Silva Feltran G, Morales MM, et al. Diabetic rats present higher urinary loss of proteins and lower renal expression of megalin, cubilin, ClC-5, and CFTR. Physiol Rep. 2017;5:e13335. [DOI] [PMC free article] [PubMed]
- 103.Obeid R, Shannan B, Herrmann W. Advanced glycation end products overload might explain intracellular cobalamin deficiency in renal dysfunction, diabetes and aging. Med Hypotheses. 2011;77:884–8. doi: 10.1016/j.mehy.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 104.Kuwahara S, Hosojima M, Kaneko R, Aoki H, Nakano D, Sasagawa T, et al. Megalin-mediated tubuloglomerular alterations in high-fat diet-induced kidney disease. J Am Soc Nephrol. 2016;27:1996–2008. doi: 10.1681/ASN.2015020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.De S, Kuwahara S, Hosojima M, Ishikawa T, Kaseda R, Sarkar P, et al. Exocytosis-mediated urinary full-length megalin excretion is linked with the pathogenesis of diabetic nephropathy. Diabetes. 2017;66:1391–404. doi: 10.2337/db16-1031. [DOI] [PubMed] [Google Scholar]
- 106.Ogasawara S, Hosojima M, Kaseda R, Kabasawa H, Yamamoto-Kabasawa K, Kurosawa H, et al. Significance of urinary full-length and ectodomain forms of megalin in patients with type 2 diabetes. Diabetes Care. 2012;35:1112–8. doi: 10.2337/dc11-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Saito A, Sato H, Iino N, Takeda T. Molecular mechanisms of receptor-mediated endocytosis in the renal proximal tubular epithelium. J Biomed Biotechnol. 2010;2010:403272. doi: 10.1155/2010/403272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hasegawa J, Maejima I, Iwamoto R, Yoshimori T. Selective autophagy: lysophagy. Methods. 2015;75:128–32. doi: 10.1016/j.ymeth.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 109.Maejima I, Takahashi A, Omori H, Kimura T, Takabatake Y, Saitoh T, et al. Autophagy sequesters damaged lysosomes to control lysosomal biogenesis and kidney injury. EMBO J. 2013;32:2336–47. doi: 10.1038/emboj.2013.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhao Y, Zhang W, Jia Q, Feng Z, Guo J, Han X, et al. High dose vitamin E attenuates diabetic nephropathy via alleviation of autophagic stress. Front Physiol. 2018;9:1939. doi: 10.3389/fphys.2018.01939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pilapong C, Phatruengdet T, Krungchanuchat S. Autophagic stress; a new cellular response to nanoparticles. Could it be a new strategy for inhibition of liver cancer cell invasion and metastasis? Nanoscale. 2020;12:6556–61. doi: 10.1039/C9NR10131D. [DOI] [PubMed] [Google Scholar]
- 112.Du F, Wang T, Li S, Meng X, Zhang HY, Li DT, et al. Cathepsin D protects renal tubular cells from damage induced by high glucose independent of its enzymatic activity. Am J Transl Res. 2017;9:5528–37. [PMC free article] [PubMed] [Google Scholar]
- 113.Guicciardi ME, Leist M, Gores GJ. Lysosomes in cell death. Oncogene. 2004;23:2881–90. doi: 10.1038/sj.onc.1207512. [DOI] [PubMed] [Google Scholar]
- 114.Peres GB, Schor N, Michelacci YM. Impact of high glucose and AGEs on cultured kidney-derived cells. Effects on cell viability, lysosomal enzymes and effectors of cell signaling pathways. Biochimie. 2017;135:137–48. doi: 10.1016/j.biochi.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 115.Desmond MJ, Lee D, Fraser SA, Katerelos M, Gleich K, Martinello P, et al. Tubular proteinuria in mice and humans lacking the intrinsic lysosomal protein SCARB2/Limp-2. Am J Physiol Ren Physiol. 2011;300:F1437–47. doi: 10.1152/ajprenal.00015.2011. [DOI] [PubMed] [Google Scholar]
- 116.Peres GB, Juliano MA, Simões MJ, Michelacci YM. Lysosomal enzymes are decreased in the kidney of diabetic rats. Biochim Biophys Acta. 2013;1832:85–95. doi: 10.1016/j.bbadis.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 117.Yuan Y, Sun H, Sun Z. Advanced glycation end products (AGEs) increase renal lipid accumulation: a pathogenic factor of diabetic nephropathy (DN) Lipids Health Dis. 2017;16:126. doi: 10.1186/s12944-017-0522-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sebeková K, Schinzel R, Ling H, Simm A, Xiang G, Gekle M, et al. Advanced glycated albumin impairs protein degradation in the kidney proximal tubules cell line LLC-PK1. Cell Mol Biol. 1998;44:1051–60. [PubMed] [Google Scholar]
- 119.Medina-Navarro R, Torres-Ramos YD, Guzmán-Grenfell AM, Díaz-Flores M, León-Reyes G, Hicks GJ. Lysosomal dysfunction induced by changes in albumin’s tertiary structure: Potential key factor in protein toxicity during diabetic nephropathy. Life Sci. 2019;230:197–207. doi: 10.1016/j.lfs.2019.05.069. [DOI] [PubMed] [Google Scholar]
- 120.Jha JC, Banal C, Okabe J, Gray SP, Hettige T, Chow BSM, et al. NADPH oxidase nox5 accelerates renal injury in diabetic nephropathy. Diabetes. 2017;66:2691–703. doi: 10.2337/db16-1585. [DOI] [PubMed] [Google Scholar]
- 121.de Torre-Minguela C, Barberà-Cremades M, Gómez AI, Martín-Sánchez F, Pelegrín P. Macrophage activation and polarization modify P2X7 receptor secretome influencing the inflammatory process. Sci Rep. 2016;6:22586. doi: 10.1038/srep22586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang X, Yao B, Wang Y, Fan X, Wang S, Niu A, et al. Macrophage cyclooxygenase-2 protects against development of diabetic nephropathy. Diabetes. 2017;66:494–504. doi: 10.2337/db16-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chow F, Ozols E, Nikolic-Paterson DJ, Atkins RC, Tesch GH. Macrophages in mouse type 2 diabetic nephropathy: correlation with diabetic state and progressive renal injury. Kidney Int. 2004;65:116–28. doi: 10.1111/j.1523-1755.2004.00367.x. [DOI] [PubMed] [Google Scholar]
- 124.Szeto HH, Liu S, Soong Y, Seshan SV, Cohen-Gould L, Manichev V, et al. Mitochondria protection after acute ischemia prevents prolonged upregulation of IL-1β and IL-18 and arrests CKD. J Am Soc Nephrol. 2017;28:1437–49. doi: 10.1681/ASN.2016070761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jiang Y, Zhao Y, Zhu X, Liu Y, Wu B, Guo Y, et al. Effects of autophagy on macrophage adhesion and migration in diabetic nephropathy. Ren Fail. 2019;41:682–90. doi: 10.1080/0886022X.2019.1632209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yuan Y, Li L, Zhu L, Liu F, Tang X, Liao G, et al. Mesenchymal stem cells elicit macrophages into M2 phenotype via improving transcription factor EB-mediated autophagy to alleviate diabetic nephropathy. Stem Cells. 2020;38:639–52. doi: 10.1002/stem.3144. [DOI] [PubMed] [Google Scholar]
- 127.Wang X, Xiong L, Yu G, Li D, Peng T, Luo D, et al. Cathepsin S silencing induces apoptosis of human hepatocellular carcinoma cells. Am J Transl Res. 2015;7:100–10. [PMC free article] [PubMed] [Google Scholar]
- 128.Andrault PM, Panwar P, Brömme D. Characterization of cathepsin S exosites that govern its elastolytic activity. Biochem J. 2020;477:227–42. doi: 10.1042/BCJ20190847. [DOI] [PubMed] [Google Scholar]
- 129.Thanei S, Theron M, Silva AP, Reis B, Branco L, Schirmbeck L, et al. Cathepsin S inhibition suppresses autoimmune-triggered inflammatory responses in macrophages. Biochem Pharmacol. 2017;146:151–64. doi: 10.1016/j.bcp.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 130.Jobs E, Risérus U, Ingelsson E, Sundström J, Jobs M, Nerpin E, et al. Serum cathepsin S is associated with decreased insulin sensitivity and the development of type 2 diabetes in a community-based cohort of elderly men. Diabetes Care. 2013;36:163–5. doi: 10.2337/dc12-0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Steubl D, Kumar SV, Tato M, Mulay SR, Larsson A, Lind L, et al. Circulating cathepsin-S levels correlate with GFR decline and sTNFR1 and sTNFR2 levels in mice and humans. Sci Rep. 2017;7:43538. doi: 10.1038/srep43538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kumar VrS, Darisipudi MN, Steiger S, Devarapu SK, Tato M, Kukarni OP, et al. Cathepsin S cleavage of protease-activated receptor-2 on endothelial cells promotes microvascular diabetes complications. J Am Soc Nephrol. 2016;27:1635–49. doi: 10.1681/ASN.2015020208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Biswas SK, Sodhi A, Paul S. Regulation of nitric oxide production by murine peritoneal macrophages treated in vitro with chemokine monocyte chemoattractant protein 1. Nitric Oxide. 2001;5:566–79. doi: 10.1006/niox.2001.0370. [DOI] [PubMed] [Google Scholar]
- 134.Biswas SK, Sodhi A. In vitro activation of murine peritoneal macrophages by monocyte chemoattractant protein-1: upregulation of CD11b, production of proinflammatory cytokines, and the signal transduction pathway. J Interferon Cytokine Res. 2002;22:527–38. doi: 10.1089/10799900252982007. [DOI] [PubMed] [Google Scholar]
- 135.Tashiro K, Koyanagi I, Saitoh A, Shimizu A, Shike T, Ishiguro C, et al. Urinary levels of monocyte chemoattractant protein-1 (MCP-1) and interleukin-8 (IL-8), and renal injuries in patients with type 2 diabetic nephropathy. J Clin Lab Anal. 2002;16:1–4. doi: 10.1002/jcla.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Klessens CQF, Zandbergen M, Wolterbeek R, Bruijn JA, Rabelink TJ, Bajema IM, et al. Macrophages in diabetic nephropathy in patients with type 2 diabetes. Nephrol Dial Transplant. 2017;32:1322–9. doi: 10.1093/ndt/gfw260. [DOI] [PubMed] [Google Scholar]
- 137.Boels MGS, Koudijs A, Avramut MC, Sol W, Wang G, van Oeveren-Rietdijk AM, et al. Systemic monocyte chemotactic protein-1 inhibition modifies renal macrophages and restores glomerular endothelial glycocalyx and barrier function in diabetic nephropathy. Am J Pathol. 2017;187:2430–40. doi: 10.1016/j.ajpath.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 138.Boels MG, Avramut MC, Koudijs A, Dane MJ, Lee DH, van der Vlag J, et al. Atrasentan reduces albuminuria by restoring the glomerular endothelial glycocalyx barrier in diabetic nephropathy. Diabetes. 2016;65:2429–39. doi: 10.2337/db15-1413. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.