Abstract
Objective
To compare rapid prototyping technology (RP tech) in revision total hip arthroplasty (RTHA) with traditional examination methods and to see how they are different in evaluating acetabular anatomy and designing surgical procedure.
Methods
From February 2014 to March 2018, 43 RTHA patients with complex acetabulum defects were enrolled in this prospective study regardless of age or gender. Incomplete and unclear data were excluded. Three types of radiographic examination were performed on each patient before the revision surgery. Four groups of evaluations were designed: (i) X‐ray; (ii) computed tomography (CT‐scan); (iii) RP tech; and (iv) CT‐aided RP tech. Discrepancies between preoperative radiographic analysis and intra‐operative findings were separately compared by a team of surgeons. Premade surgical plans based on each evaluation method were compared with the final surgical procedure. The compliance of anatomic evaluation and surgical plan‐design based on 3D RP tech and traditional radiographs were ranked manually by a of team surgeons into: (i) complete accordance; (ii) general accordance; and (iii) undetermined structure/procedure. The difference in ranks between RP tech and traditional radiographic methods were analyzed with a nonparametric Kruskal‐Wallis test. P < 0.05 was considered significant. Multiple adjustments were taken for the statistical tests level according to the Bonferroni method.
Results
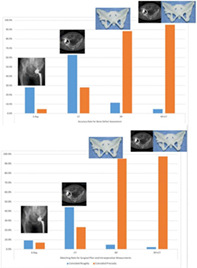
For anatomic analysis, the accordance in four groups of evaluating methods differed from each other (P < 0.05) except for the comparison of RP tech and CT‐aided RP tech. RP tech displayed better anatomic evaluating accuracy than traditional methods (X‐ray and CT) with the “complete accordance” rates of these groups being 88.37%, 4.65% and 27.91%, respectively. But CT‐aided RP tech did not improve accuracy significantly compared with using RP tech individually, although the value seems high in the CT‐aided RP group with the “complete accordance” rate of 95.35%. For surgery design, RP tech significantly showed better applicable surgical design compared with X‐ray and CT (P < 0.05), and the “complete accordance” rates were 88.37%, 6.98% and 23.26%, but no significant difference was observed between RP tech and CT‐aided RP tech, and the “complete accordance” rate of CT‐aided RP tech group was 97.67%. RP tech showed remarkable improvement in bone defect assessment and surgical plan design.
Conclusion
Using RP technology improved both sensibility and accuracy in acetabular defect evaluation with better locating and evaluating efficiency compared with X‐ray and CT‐scans. It also improved surgical schedule designing in complex acetabular defecting revision surgery. In particularly complex cases, CT aided RP tech may increase the accuracy of RP tech.
Keywords: Acetabular defects, Hip arthroplasty, Rapid prototyping, Revision, Surgical procedure design
Rapid prototyping technology improved sensibility and accuracy with far better locating and evaluating efficacy compared with traditional radiographic examinations, but problems still exist. For certain cases, we recommend RP assessment should be used with CT.
Introduction
Procedures of primary and revision hip arthroplasty (RTHA) have been increasing for decades and in some counties are predicted to double in the coming year 1 , 2 , 3 . As the frequency of revision procedure is continually rising, along with a higher occurrence of complication and mortality, how to improve the success rates and clinical results of RTHA has drawn more and more attention in which good surgical procedure design is greatly valued. Previous reports stressed that evaluating the acetabular defects has great significance on designing surgical procedures 4 , 5 .
Total hip arthroplasty (THA) failure may have many kinds of causes, and the aseptic loosening caused by prosthesis instability was the main cause 6 . Prosthesis instability could have many reasons including inadequate mechanical or biological loss of fixator. Osteolysis or unexpected fractures could lead to unstable surrounded structures and patients with unstable prosthesis were likely to experience RTHA, in which periprosthetic acetabular defects were commonly seen. Acetabular defect was considered as the main threat to prosthesis stability as it represented damaged mechanical structure and was difficult to evaluate before surgery.
At present, the evaluation methods on acetabular defect are mainly X‐ray and CT‐scan. Based on X‐rays, the most traditional imaging method used in evaluating skeletal structures, many classic diagnosis and classification methods were built such as the noted Paprosky classification 7 , AAOS classification 8 , etc. With the development of computer science, computed tomography technology (CT‐scan) shows better detailed examination of the hip joint. However, these traditional radiographs still have their drawbacks. X‐rays and CT‐scans have been published for decades, and the limitations of these traditional radiographs are now recognized. For example, in the Paprosky acetabular defect system, it was claimed by Yu et al. that the Paprosky classification system has fewer satisfying correlations between defects at the posterior well and the ischial structure which was photographed in an anteroposterior and oblique position 9 . This situation happens in the AAOS system too 10 , 11 , as the anteroposterior and oblique radiographs have come into use over the years, classic standards based on the old imaging methods seem to be unmatched with the needs of the present day.
Campbell et al. also stated that the traditional classification system exhibited better performances in originators rather than ordinary experts and surgeons 10 . Subjectiveness might be dominant in some diagnosing process, which should be avoided. Compared with X‐rays, CT‐scans were considered a more specific and detailed technology. Ranges of gray values represented density of tissues to mimic the unreachable structures. CT‐scans have been widely applied in surgery and have become the standard examination method. But CT‐scans do not deliver information directly. Rolling and flat images require surgeons to be experienced in inner structures to make appropriate choices on tools and prosthesis. Although, surgeons can build a 3D model on a computer with CT‐scan data, and it has shown that a 3D model eases the diagnosis program in orthopedic surgery. It is still a flat image on the screen, and surgeons still lack intuitionistic experiences on the actual structure. X‐ray and CT‐scan images are not intuitionistic and may require experienced examiners. Limited observational angles in x‐ray images may bring problems in dormant defects in acetabular. CT‐scans require examiners to understand anatomy from transverse images, along with more training requirements for surgeons 9 , 10 , 11 , 12 . Misjudging of acetabular conditions could be seen in these traditional imaging methods 13 .
Thanks to the rise of 3D technology, particularly 3D modeling and 3D print technology, rapid prototyping technology (RP tech) has been proposed to evaluate acetabular bone defects for many cases 14 . Munjal et al., Chana‐Rodríguez et al., and Sánchez‐Pérez et al. reported their attempts to adopt RP tech to evaluate abnormal pelvic structure in complex pelvic fractures 15 , 16 , 17 . It is hoped that this type of technology with an actual model in “the hand” would improve the understanding of patients' hip joint anatomy and hence improve the accuracy and ease in evaluating patients' acetabular defects. Although, evaluation via RP has been accepted in some primary and revision acetabular surgeries as Faur et al, and Zerr' et al. have reported 18 , 19 . it has not been appropriately validated yet. And the feasibility in hip revision surgery is not well compared with traditional radiographic methods. The advantages and disadvantages of traditional radiography are not clearly known yet.
This article aimed to conduct a prospective and consecutive study on the assessments of bone defects in patients via different imaging methods before RTHA to investigate differences among these radiographs. We compared the locations and degrees of bone defects gained by radiographic examinations with intraoperative records and compared results of different radiographic methods to see how results range. The final goal of this research is to; (i) figure out the accuracy and reliability of 3D printed RP tech compared to traditional radiographs in acetabular bone defect assessment; (ii) figure out the accuracy and reliability of 3D printed RP tech compared to traditional radiographs in hip revision surgery procedures design; and (iii) alert the shortcomings which may occur and affect clinical applications.
Patients and Methods
Patients Enrollment
From February 2014 to March 2018, we collected over 50 patients who underwent revision surgery in our hospital. Regardless of age and gender, all enrolled patients met our standard of inclusion criteria: (i) patients presented severe hip pain or mobility dysfunction after primary unilateral/bilateral hip arthroplasty; (ii) cup loosening was confirmed and revision surgery was needed; (iii) acetabular defects were found by X‐ray, CT examinations or analysis on 3D printed hip joint models; (iv) the defects were considered to impair joint stability by the surgeon and were enrolled into surgical consideration; and (v) revision surgery procedure was established based on anatomic analysis of radiographs and 3D printed models. We excluded patients who did not agree to enter this study and those patients who did not have acetabular bone defects but revised for femoral prosthesis loosening. Also, seven patients whose imaging data were incomplete or unclear were excluded. Finally, 43 patients with detailed imaging data and information were gathered in this study.
Radiographic Data Collection and RP Model Building
During our patient admitting process, each of the patients first underwent X‐ray (Siemens, RAX, Erlangen, Germany) examination. By X‐ray, both of implanted prosthesis and surrounding anatomy were examined and for those with acetabulum defects, and CT‐scan (Siemens, SOMATOM Definition Flash, Erlangen, Germany) were performed, followed with more specific examinations on acetabulum conditions. Pelvic CT was performed continuously with patients in a supine position and covered the bilateral anterior iliac spine and the posterior borders of the medial and lateral condyles with 0.5 mm interspacing thickness. All X‐rays and CT scans were performed by the same medical image center with the same scanning parameters. And after the CT‐scan, those with “severe complicated defects” (based on CT‐scan analysis) were selected and provided with modeling analysis by 3D print RP tech (RS4500 Stereolithography, Liantai, Shanghai, China). “Severe complicated defects” are defined as suspicious defects on the essential acetabular ring or load area of the acetabulum, which might make it difficult to place a commercial prosthesis and bring negative effects on initial stability of load area, in which augments might be needed 20 . Thus, based on the existing radiographic data, we selected two traditional evaluation methods (X‐rays and CT‐scans) to make further comparison with RP tech. In addition, another evaluation group of CT‐aided RP tech was added to figure out whether RP tech aided with traditional radiographs improve its accuracy compared with using RP tech individually. In this group, surgeons collected bone defect information both according to RP and CT scans image simultaneously. All radiographs were collected and analyzed respectively by qualified surgeons in our team. The assessment methods were described below which mainly contained defect assessing and surgical procedure design.
Indexes
Surgeon Ranking Criteria
To validate different imaging methods, all results were evaluated to compare with those of intraoperative records with an acetabular scoring table, which covered information about defects' locations, extents, and integrity of load area. In this strategy, after our data collecting process was finished, results were summed up into each group. All radiographic results were compared with intraoperative detection to see the differences with the actual acetabular situation. After that, our team of surgeons manually graded comparison results of defects and surgical design into three grades.
Defect Assessing Criteria
For defect assessment: (i) complete accordance grade indicates that the analyzed results were generally consistent with those found in surgery, including integrity of the acetabular ring and stability of the load area, in which both of the defects' locations and extents were accurately understood; (ii) general accordance grade indicates the methods that were not able to reveal certain parts of acetabular defects that were needed to evaluate acetabular conditions, while the overall evaluation of the acetabulum was still able to be made, which may result in equivocal diagnoses on patients. For them, surgery was still able to be performed, but preparations should be careful in the case of unrevealed instability; and (iii) undetermined structure grade is the worst situation in that no valuable information could be gained from radiographs or models, and surgery without further examination would not be recommended.
Surgical Procedure Design Criteria
For the surgical procedure designing part, choices of cups augments and cages were directly associated with the anatomic structure of the acetabular. Thus, we also designed three grades for evaluating different methods' feasibility to predict surgical plans: (i) complete accordance grade represents an ideal accordance between planned procedure and actual surgery with same the implants and methods; (ii) general accordance grade indicates that a planned procedure has similar choices on implants but not completely the same. Decisions made from this grade are not as firm as (i) and slight changes could be made by surgeons during the surgery; and (iii) undetermined procedure grade radiographs and models are unable to finish surgical plan designing.
In our analysis, surgeons reviewing patients' different radiographic and RP model data were in the same group, which in our expectation it would be better if data were assessed with different groups of surgeons. However, we have a shortage of qualified surgeons with equivalent diagnosing ability. But in our data collecting process, we deliberately make our assessment in the following order, X‐ray, CT‐scan, RP tech, and CT‐aided RP tech, which in our opinion, with the images already analyzed should not affect on the surgeons' decision.
Statistical Analysis
All statistical analyses were performed on a personal computer using SPSS software for Mac (SPSS, version 26.0; Chicago, IL, USA). A Kruskal‐Wallis (KW) test was used to check if there were differences among the four groups of imageological analysis which were groups of related nonparametric samples. P values <0.05 were considered to indicate significant differences. Also, a KW test for two groups were used to compare the consistency of the four groups of imageological analysis with intraoperative findings. Multiple adjustment was taken for the statistical tests level according to the Bonferroni method.
Results
In this study involving 43 RTHA patients, we compared the intraoperative results with previous imaging assessments. The accuracy of different evaluating methods for acetabular defects differed as follows.
Defects Assessment Accordance
In the X‐ray group, only two patients' (4.65%) defect locations and extents coincide precisely with their intraoperative results and obtain: (i) complete accordance. Twelve patients' (27.91%) X‐ray results roughly coincide with intraoperative measurements and get (ii) general accordance. Twenty‐nine patients (67.44%) were unable to provide valuable anatomic information.
In the CT‐scan group, it was better that there were 12 patients' (27.91%) in (i) complete accordance grade where CT‐scan results precisely agreed with intraoperative measurements. Twenty‐seven patients (62.79%) in (ii) the general accordance grade showed CT‐scan results with unsatisfying accordance with intraoperative measurements. Four patients (9.3%) did not receive sufficient evaluation from CT and graded (iii) undetermined structure, which was a remarkable decline compared to X‐ray.
In comparison, using RP technology improved “complete accordance” numbers with far better locating and evaluating efficiency. In the prototype group, 38 patients' (88.37%) prototyping results were precisely in accord with those in intraoperative acetabular detection and their surgical design were all successful, which were graded (i) complete accordance in anatomic assessment. Meanwhile, the other five patients' (11.63%) prototyping results showed several slight differences from operative measurements, three of which were just mildly incompatible with results we measured and graded (ii) general accordance. No patients were graded (iii) undetermined structure under RP technology.
In the additional group of CT‐aided prototyping, 41 patients' (95.35%) results agreed with our intraoperative results and obtained grade (i) complete accordance. But there were still had two (ii) general accordance grade patients (4.65%) whose results that did not completely ag ee intraoperative measurements. No patients were graded (iii) undetermined structure.
Surgical Procedure Accordance
In the surgical procedure design in X‐ray, three patients' (6.98%) surgical procedures remained consistent as we planned and rated (i) complete accordance, while four patients' (9.3%) operations were merely roughly coincident and obtained (ii) general accordance. In addition, there were 36 patients (83.72%) in the X‐ray group unable to be evaluated and graded (iii) undetermined procedure (83.72%), 29 of which (67.44%) could not provide valuable anatomic information and it was difficult to make further precise plans.
For the surgical procedure design, 28 patients (65.11%) received a surgical plan based on CT, and 10 patients (23.26%) among them were perfectly designed compared with intraoperative decisions which were graded (i) complete accordance. Eighteen patients (41.86%) kept (ii) general accordance. Still. there were 15 patients' (34.88%) graded (iii) undetermined procedure which roughly or accordant with actual measured outcomes, which were incapable of designing procedures and prosthesis individually.
In the RP tech group, there were 41 (95.35%) patients in (i) complete accordance in which no modifications were needed in the surgical procedure. The rest two (4.65%) were with more changes in the surgical procedure, and they were graded (ii) general accordance. RP tech significantly improved (i) complete accordance cases both in defect evaluation and surgical procedure design compared with CT‐scan (defect assessment: 27.91% to 88.37%; surgical procedure: 23.26% to 95.35%), which indicated that RP tech offered more detailed information on RTHA and it was easier for surgeons to design procedures with it. However, in one special patient, things were complicated. The patient's model showed different information about the structure of the ramus of ischium compared with intraoperative observation. This situation maked the surgery very difficult even though our surgeons discovered the discrepancy and finally finished the surgery. The detailed information about this patient is introduced in next section. The prototyping group, which should have been the most accurate radiographic method for anatomic detection, still met trouble engaging with unexpected acetabular conditions.
The same thing happened in the CT‐aided RP tech group, 42 patients (97.67%) obtained (i) complete accordance, but in one patient a significant deviation occurred. In that case the surgery was finished, and the case was labeled as (ii) general accordance.
Comparison of X‐ray and CT Scan
In terms of traditional radiographs, based on what we have gained in this study, it reveals that X‐rays are inaccurate and can only roughly locate defects, especially for surgical plan designing, X‐rays offered too limited information that surgeons required on revision surgery. About 83.72% of patients in the X‐ray group could not receive a workable surgical plan, only less than 10% could be designed by X‐ray results. For the CT‐scan, it can generally find normal defects and the evaluation of defects were mostly correct. It did improve evaluating skills as it reduced (iii) undetermined structure grade numbers from 67.44% to 9.3% compared with X‐rays, which might indicate CT‐scans provided more information than X‐rays. But considering the promotion of CT‐scan in (i) complete accordance grade was not as oblivious as (ii) general accordance grade (from 4.65% to 27.91%), CT‐scans might improve less on detailed structures. For surgical planning, CT‐scans significantly reduced (iii) undetermined procedure grade numbers (from 83.72% to 34.88%), which may indicate that CT‐scans provided more anatomic information that RTHA needed. However, CT‐scans are not perfect either, there were still about 1/3 of patients who did not receive a complete surgical plan from CT‐scan radiographs.
Comparison of Traditional Radiographs and RP Tech Model
The results of statistical analysis showed that the consistency of X‐rays, CTs and RP tech were significantly different among each other both for bone defect analysis and operation planning (P < 0.05). Although the difference between the groups of RP tech and CT‐aided RP tech were not significant, we observed higher accuracy in effective host bone analysis for the CT‐aided RP tech group compare with single RP tech group. Also, as we mentioned above, this difference did not affect surgery planning in our research which, was consistent with the K‐W test between the RP tech and CT‐aided RP tech groups for the consistency of operation planning.
From statistical outcomes, although it was seen that groups with RP models involved gained the highest accuracy, although model‐designing errors may still happen. In one imperfect (ii) general accordance grade case in the single RP tech model group, it was the RP models, made of engineering resin, with good rigidity and tenacity, provided inaccurate information of bone mass., as sometimes engineering structures may seem rigid enough while the real bony structure was not. Because of the inevitable osteolysis, reserved bones became relatively thin, and the native skeletal structures failed to sustain the stress, while the RP tech model was made of resin which was more rigid to stress. Even so, participated surgeons thought its adverse effect on surgery planning could be easily avoided in most cases if the surgeons realized this situation, which normally happened when there was severe osteolysis. And our research indeed showed that in most situations, this phenomenon did not affect surgery planning. However, it was extremely complicated for another patient in which both single RP tech and CT‐aided RP tech only got (iii) general accordance grade.
This is the patient we mentioned above, whose RP model provided different information with intraoperative observation. She underwent THA 28 years ago and her prosthesis was loosening, which demanding frevision surgery. In her case, the prototyping built model showed osteolysis on the ischial ramus, but still could provide steady support by screw fixation on ischial branch. Hereby we adopted a customized cage with the ischial fixator, which perfectly matched with the prototyping model. However, in the operative detection, the acetabular ischial ramus was not intact and parts of the branches that were thought steady enough to hold a cage was mostly a mixture of soft tissue and bone cement instead of bone structure, which were not detected in radiographs. The reason why this old type of cement developed in radiation was the radiopacity of bone cement of the early version. The software mistook all high‐density structures as bones. Due to the irreparable mismatch in the surgery, surgeons indicated that although the initial stability was created by the ilium fixator, the prosthesis still could not bear early load. As a result, it was recommended that the patient did not undertake weight‐bearing activity in the short term (Fig. 1).
Fig. 1.
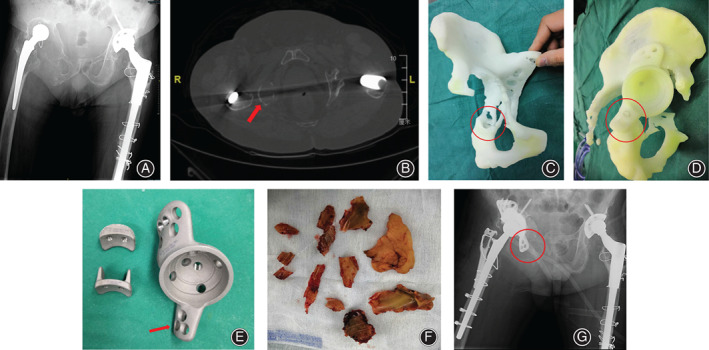
This series showed the details of the patient who was mis‐assessed by RP. (A) The red arrow showed the severely destructed ischial ramus on pre‐operative AP pelvic X‐ray but seemed still had little host bone left. (B) The red arrow showed the remained host bone observed on CT scan which was considered may provide screw fixation for the cage. (C) The red circle area showed the ischial ramus on RP which still seemed had enough cortical bone for screws fixation. (D) The situation of sham operation with RP and the model of custom‐made prosthesis. The red circle area showed the design fixing the flange of the cage on ischial ramus with screws. (F) The red arrow showed the augment flange of the custom‐made cage. The photo showed the old type developed cement removed in surgery. (G) The post‐operative pelvic plan showed the unfixed cage flange and disappeared ischial ramus.
Discussion
Traditional Defect Assessment Method
The number of arthroplasty revision surgeries are rising in many countries 1 , 2 , 3 . With complications in acetabular defects and loosening prosthesis 21 , it is important for surgeons to make the right decisions on types of prosthesis and planning for the surgery, which makes assessing acetabular defects extremely crucial before surgery 22 . Previously, surgeons assessed acetabular defects by classifications via X‐rays and CT‐scans 12 . However, single radiograph evaluation needs a surgeon to be highly trained and very experienced 23 . For RTHA, it is accepted that preoperative acetabular evaluation is an important part of revision surgery and to systematically analyze acetabular condition, classifying different types of damaged acetabulum is the default process 24 .
Normally, based on X‐rays or CT,s two types of acetabular deficiency classification methods, Paprosky and AAOS classification systems are usually used 8 , 9 , 10 , 25 . Both have generally been accepted as preoperative options to predict the acetabular situation 10 . However, since both has been used for decades, along with their validity, the limitation of traditional classification systems has also been focused. For the Paprosky acetabular defect system, Yu claimed it has fewer satisfying correlations between defects at the posterior wall and ischial structure, which might be associated with its x‐ray based radiographic data source 9 . Anteroposterior and oblique radiographs have been used for acetabular surgery for years 26 , and now they are seemingly insufficient. The same is happening to the AAOS system too 10 , 11 . Campbell reported that traditional classification systems were more likely to validate with originators rather than ordinary experts and nonexpert surgeons, which may indicate us sometimes classification systems could be subjective 10 , and a more standardized objective method of examination should be considered.
For now, CT‐scans have been applied to orthopedic surgeries. With better detailed images and accessibilities for surgeons, CT‐scans have better significant reliability on complex acetabular detection, especially for accurate measurements of bone thickness, angles, and density 27 , 28 . Our researchers also agreed with that 3D CT images had provided a more objective method for surgeons to make decisions on surgical procedure designs. However, surgeons are still analyzing on screen with rolling and flat two‐dimension images. It still requires surgeons being well trained and does not provide preoperative matching tests for customized prosthesis.
Advantages and Deficiencies of RP Technique Model
To avoid mistaken diagnosis on complex acetabular conditions, 3D model analysis has been accepted by many institutions in orthopedic surgery. And compared to performing 3D structures on 2D displays, it is preferred to evaluate on a real model with rapid prototype technique. With a real acetabular model, surgeons can observe the 3D anatomy more visually, and work with engineers designing a customized prosthesis, and are able to conveniently design surgical procedure or even do preoperation on the model, which is essentially an important improvement to the surgery's efficiency and safety.
RP technology has been used in several areas, which has shown a high level of ease of use in model building and routine design 14 , 18 , 29 . But problems met in accuracy and deviation factors still exist. Our research proved that RP technology could significantly improve accuracy in evaluation compared to X‐ray and CT‐scans, and surgeons were better able to design surgical procedures and prosthesis. Unfortunately, little research was found for RTHA surgery assisted with RP technology. Investigations on RP technology's usages on spinal and acetabular fracture surgery have been made 15 , 30 , 31 , and RP technology has shown its positive potential on preoperative plan design, with advantages on decreasing diagnosing and preoperative design difficulties 32 . Surgeons are even able to rehearsal on RP model to make surgery more effective 32 . In some cases, it reduces surgical time, and thus, reduces rates of infection and perioperative transfusion requirements 14 , 32 .
But RP technology also has its shortcomings, previous research has indicated that as its resolution is limited, it might be hard for RP models to display structures like cartilage and soft tissue 17 , 31 . The new technology raises the need for expert engineers, and surgeons may also require specialized training 31 . Our institution also agrees that it is important to enroll engineers with experience in cooperation with surgeons in accurate RP modeling. In our opinion, engineers must work with surgeons about at least 10 cases to understand the designs surgeons need in RTHA to create usable RP models. Our institution has had experienced professional engineers that have worked with our team on many cases, which may contribute to the high corrections in the single RP model group. The requirements for experience might be a problem for all types of examination methods. But compared with X‐ray and CT‐scans, when it comes to accurately evaluating complex bone defects, evaluating a RP model is much more intuitionistic, which is of great help for the new surgeons. Also, it might be more costly at the beginning of commercial use 19 .
Other than this, in our research we still found seven patients' prototyping results differed with those of intraoperative detection. The results might be attributed to the fact that the prototyping material has different mechanical properties with normal bone tissue. RP only builds a model based on the sample's CT gray value, while structures with the same gray value are not with equivalent structural rigidity. Sometimes weak structures could be recognized by experienced surgeons on CT‐scans or X‐ray, but the prototyping machine builds whatever it acquires from radiographic data and prints every part that exceeds the gray value baseline. This rigid model's structure can mislead surgeons to adopt inappropriate cage, regarding incompact tissues, such as steady bone structure. In another data group of CT‐aided RP, we re‐compared results with intraoperative results in the same evaluating standard form. We found that with CT‐scans reduced inaccuracy of over‐rigid material, but whether CT‐scan improves the accuracy of RP depends on the surgeon's experience. Although, it is expected that experienced surgeons should be capable of referring types of imaging data and making the right decision, relying on surgeons' professional level is rather of risky. Besides, RP is more time consuming than traditional imaging examination, with commonly 1 to 2 days waiting time for the model to build up an acceptable range for surgeons. Also, there is extra financial burden for patients to bear. Rebuilding a model might not be a preferred choice for patients. Several problems remain to be solved.
Limitations and Prospections
Our research revealed that RP technology has better accuracy and reliability than traditional radiographic examinations with different groups of imaging methods on patients. However, this research is limited. Our research's sample volume was relatively small, and we have not completed a long‐term follow‐up, thus the long‐term results of use of RP tech in revision surgery is not discussed.
The RP technology is of great convenience for surgeons, especially newly qualified ones, to quickly know acetabular conditions and it also shows extraordinary accuracy compared with traditional radiographic methods. It is agreed that it is still challenging to completely reveal postoperative acetabulum conditions by traditional imaging methods, especially when needing valuable information regarding host bone support against cages and the ability of cages obtaining rigid fixation, which were, in our opinion, the main challenges for traditional pre‐operative detection. However, it is with limitation too. At present, materials used for 3D printing are not equivalent to biological bone tissues, prototyping models based on a single gray value does not represent the whole structure's rigidity. To solve this, it is considered that polychrome or even multi‐material prototyping may be applied in model building. Models built with materials of diverse mechanical rigidity can truly display acetabular condition. Not only bone density but bone quality should be considered. To achieve this, a more complex and accurate imaging system is needed, which should be able to aggregate different imaging data and distribute relevant material to the exact structure. Imaging data should be gathered and analyzed by computer to create models with calculable rigidity with techniques like finite element analysis. We will further investigate in forthcoming research.
ETHICAL APPROVAL
This research was approved by the institutional review board of our hospitals, and the written and verbal informed consent from all participants was obtained before the study. All data were collected by researchers from the orthopedics laboratory department of the ninth people's hospital of Shanghai Jiao Tong university.
ACKNOWLEDGMENTS
This study was sponsored by National Nature Science Foundation of China (Grant No. 31900941) and the Interdisciplinary Program of Shanghai Jiao Tong University (project No. ZH2018QNA06).
Disclosure: No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Contributor Information
Meng‐ning Yan, Email: yanmengning@163.com.
Zhe‐nan Zhu, Email: zhuzhenan2006@126.com.
Hui‐wu Li, Email: huiwu1223@163.com.
References
- 1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am, 2007, 89: 780–785. [DOI] [PubMed] [Google Scholar]
- 2. Patel A, Pavlou G, Mújica‐Mota RE, Toms AD. The epidemiology of revision total knee and hip arthroplasty in England and Wales: a comparative analysis with projections for the United States. A study using the National Joint Registry dataset. Bone Joint J, 2015, 97: 1076–1081. [DOI] [PubMed] [Google Scholar]
- 3. Zeng C, Lane NE, Englund M, et al. In‐hospital mortality after hip arthroplasty in China: analysis of a large national database. Bone Joint J, 2019, 101: 1209–1217. [DOI] [PubMed] [Google Scholar]
- 4. Hayashi S, Hashimoto S, Takayama K, Matsumoto T, Nishida K, Kuroda R. Multiple revision surgeries and acetabular bone defect size may predict daily activity after revision total hip arthroplasty. J Arthroplasty, 2017, 32: 1606–1611. [DOI] [PubMed] [Google Scholar]
- 5. Schmolders J, Friedrich MJ, Michel RD, et al. Acetabular defect reconstruction in revision hip arthroplasty with a modular revision system and biological defect augmentation. Int Orthop, 2015, 39: 623–630. [DOI] [PubMed] [Google Scholar]
- 6. Ulrich SD, Seyler TM, Bennett D, et al. Total hip arthroplasties: what are the reasons for revision. Int Orthop, 2008, 32: 597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Paprosky WG, Perona PG, Lawrence JM. Acetabular defect classification and surgical reconstruction in revision arthroplasty. A 6‐year follow‐up evaluation. J Arthroplasty, 1994, 9: 33–44. [DOI] [PubMed] [Google Scholar]
- 8. D'Antonio JA, Antonio JA, Capello WN, et al. Classification and management of acetabular abnormalities in total hip arthroplasty. Clin Orthop Relat Res, 1989, 243: 126–137. [PubMed] [Google Scholar]
- 9. Yu R, Hofstaetter JG, Sullivan T, Costi K, Howie DW, Solomon LB. Validity and reliability of the Paprosky acetabular defect classification. Clin Orthop Relat Res, 2013, 471: 2259–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Campbell DG, Garbuz DS, Masri BA, Duncan CP. Reliability of acetabular bone defect classification systems in revision total hip arthroplasty. J Arthroplasty, 2001, 16: 83–86. [DOI] [PubMed] [Google Scholar]
- 11. Gozzard C, Blom A, Taylor A, Smith E, Learmonth I. A comparison of the reliability and validity of bone stock loss classification systems used for revision hip surgery. J Arthroplasty, 2003, 18: 638–642. [DOI] [PubMed] [Google Scholar]
- 12. Sheth NP, Nelson CL, Springer BD, Fehring TK, Paprosky WG. Acetabular bone loss in revision total hip arthroplasty: evaluation and management. J Am Acad Orthop Surg, 2013, 21: 128–139. [DOI] [PubMed] [Google Scholar]
- 13. Li H, Wang L, Mao Y, Wang Y, Dai K, Zhu Z. Revision of complex acetabular defects using cages with the aid of rapid prototyping. J Arthroplasty, 2013, 28: 1770–1775. [DOI] [PubMed] [Google Scholar]
- 14. Won SH, Lee YK, Ha YC, Suh YS, Koo KH. Improving pre‐operative planning for complex total hip replacement with a rapid prototype model enabling surgical simulation. Bone Joint J, 2013, 95: 1458–1463. [DOI] [PubMed] [Google Scholar]
- 15. Chana‐Rodríguez F, Mañanes RP, Rojo‐Manaute J, Gil P, Martínez‐Gómiz JM, Vaquero‐Martín J. 3D surgical printing and pre contoured plates for acetabular fractures. Injury, 2016, 47: 2507–2511. [DOI] [PubMed] [Google Scholar]
- 16. Munjal S, Leopold SS, Kornreich D, Shott S, Finn HA. CT‐generated 3‐dimensional models for complex acetabular reconstruction. J Arthroplasty, 2000, 15: 644–653. [DOI] [PubMed] [Google Scholar]
- 17. Sánchez‐Pérez C, Rodríguez‐Lozano G, Rojo‐Manaute J, Vaquero‐Martín J, Chana‐Rodríguez F. 3D surgical printing for preoperative planning of trabecular augments in acetabular fracture sequel. Injury, 2018, 49: 36–43. [DOI] [PubMed] [Google Scholar]
- 18. Faur C, Crainic N, Sticlaru C, Oancea C. Rapid prototyping technique in the preoperative planning for total hip arthroplasty with custom femoral components. Wien Klin Wochenschr, 2013, 125: 144–149. [DOI] [PubMed] [Google Scholar]
- 19. Zerr J, Chatzinoff Y, Chopra R, Estrera K, Chhabra A. Three‐dimensional printing for preoperative planning of total hip arthroplasty revision: case report. Skeletal Radiol, 2016, 45: 1431–1435. [DOI] [PubMed] [Google Scholar]
- 20. Van Kleunen JP, Lee GC, Lementowski PW, Nelson CL, Garino JP. Acetabular revisions using trabecular metal cups and augments. J Arthroplasty, 2009, 24: 64–68. [DOI] [PubMed] [Google Scholar]
- 21. Badarudeen S, Shu AC, Ong KL, Baykal D, Lau E, Malkani AL. Complications after revision Total hip arthroplasty in the Medicare population. J Arthroplasty, 2017, 32: 1954–1958. [DOI] [PubMed] [Google Scholar]
- 22. van Haaren EH, Heyligers IC, Alexander FG, Wuisman PI. High rate of failure of impaction grafting in large acetabular defects. J Bone Joint Surg Br, 2007, 89: 296–300. [DOI] [PubMed] [Google Scholar]
- 23. Hüfner T, Pohlemann T, Gänsslen A, Assassi P, Prokop M, Tscherne H. The value of CT in classification and decision making in acetabulum fractures. A systematic analysis. Unfallchirurg, 1999, 102: 124–131. [DOI] [PubMed] [Google Scholar]
- 24. Mallory TH. Preparation of the proximal femur in cementless total hip revision. Clin Orthop Relat Res, 1988, 235: 47–60. [PubMed] [Google Scholar]
- 25. Telleria JJ, Gee AO. Classifications in brief: Paprosky classification of acetabular bone loss. Clin Orthop Relat Res, 2013, 471: 3725–3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jamali AA, Mladenov K, Meyer DC, et al. Anteroposterior pelvic radiographs to assess acetabular retroversion: high validity of the “cross‐over‐sign”. J Orthop Res, 2007, 25: 758–765. [DOI] [PubMed] [Google Scholar]
- 27. Barmeir E, Dubowitz B, Roffman M. Computed tomography in the assessment and planning of complicated total hip replacement. Acta Orthop Scand, 1982, 53: 597–604. [DOI] [PubMed] [Google Scholar]
- 28. Cahir JG, Toms AP, Marshall TJ, Wimhurst J, Nolan J. CT and MRI of hip arthroplasty. Clin Radiol, 2007, 62: 1163–1171 discussion 1172‐1173. [DOI] [PubMed] [Google Scholar]
- 29. Eltorai AE, Nguyen E, Daniels AH. Three‐dimensional printing in orthopedic surgery. Orthopedics, 2015, 38: 684–687. [DOI] [PubMed] [Google Scholar]
- 30. Bagaria V, Deshpande S, Rasalkar DD, Kuthe A, Paunipagar BK. Use of rapid prototyping and three‐dimensional reconstruction modeling in the management of complex fractures. Eur J Radiol, 2011, 80: 814–820. [DOI] [PubMed] [Google Scholar]
- 31. Tong Y, Kaplan DJ, Spivak JM, Bendo JA. Three‐dimensional printing in spine surgery: a review of current applications. Spine J, 2020, 20: 833–846. [DOI] [PubMed] [Google Scholar]
- 32. Liu Q, Leu MC, Schmitt SM. Rapid prototyping in dentistry: technology and application. Int J Adv Manuf Technol, 2005, 29: 317–335. [Google Scholar]


