Abstract
With the widespread use of antibiotics, Gradenigo syndrome is a rare complication of acute otitis media (AOM) and acute mastoiditis. It is an uncommon form of petrous apicitis and can be life-threatening. We report the case of a 14-year-old female with unresolved AOM, who developed otorrhea, ipsilateral headaches, diplopia and raised inflammatory markers. Magnetic Resonance Imaging (MRI) demonstrated features of petrous apicitis and confirmed the suspicion of Gradenigo syndrome. The objective of this clinical case report is to highlight this unusual syndrome together with its radiological appearance to improve its diagnosis and management.
Keywords: Gradenigo syndrome, Petrous apicitis, acute otitis media, VI cranial nerve palsy
Case summary
A 14-year-old girl was admitted to the Ear Nose and Throat (ENT) ward with a history of bilateral ear discharge and ear pain for 3 weeks, which was then associated with left sided headaches and new onset diplopia.
The patient's medical history was significant only for an ear infection complicated by perforation at the age of 9 years. She had no clinical evidence of immunosuppression.
She was initially diagnosed by the general practitioner (GP) with bilateral otitis media and was administered a course of ciprofloxacin ear drops in addition to paracetamol and ibuprofen. However, because of persistent complaints, she presented to the emergency department. Physical examination revealed erythema and bulging of the tympanic membranes B/L and was discharged with aspirin and codeine. Because of a nonrelapsing condition and development of left sided occipital/retro-orbital headaches, her GP then sent her for a computed tomography (CT) scan of the temporal bones, which did not report any bony erosion in these areas.
The next day, the patient began to experience diplopia. Upon evaluation at the ENT clinic, the left eye showed limited abduction consistent with left CN 6 palsy. The remainder of the neurologic examination was normal. She was afebrile, with normal vital signs and otoscopy revealed mucoid discharge from both middle ears associated with bilateral tympanic membrane perforation. An urgent brain and skull base MRI revealed features of left pretrous apicitis.
The patient was admitted to the hospital for concerns of Gradenigo Syndrome and was started on intravenous benzylpenicillin and clindamycin in addition to ciprofloxacin ear drops and oral dexamethasone. Pathology showed raised erythrocyte sedimentation rate (120 mm/h) with mild elevation of C-reactive protein level (23 mg/L) and normal white blood cell count. A previous ear swab returned positive for Group A Streptococcus pyogenes.
The patient responded favorably to conservative medical treatment. After 48 hours of treatment, she obtained full left eye range of motion, her headaches continued intermittently but to a lesser degree, her ear discharge and pain improved significantly and she was discharged after 5 days to continue on IV antibiotics via a peripherally inserted central catheter line until her next MRI scan. She continues to improve and after finishing her 2-week course of IV antibiotics, received amoxicillin for a total of 1 month.
Imaging findings and diagnosis
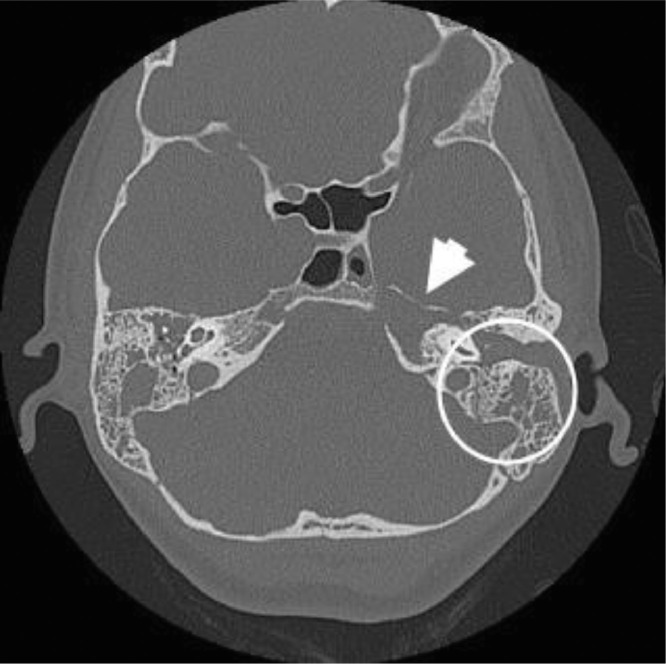
The initial imaging for this patient was a CT temporal bones, which reported extensive bilateral opacification of the external auditory canal, middle ear cavity, aditus ad antrum and mastoid air cells; however, no bony erosion in the petrous appex was reported (Fig. 1).
Fig. 1.
Computed tomography (CT) image before admission. Axial CT temporal bones showing extensive soft tissue opacification in the external auditory canal, middle cavity, aditus ad antrum and mastoid air cells bilaterally (circle). Loss of trabecular pattern near the petrous apex (arrow).
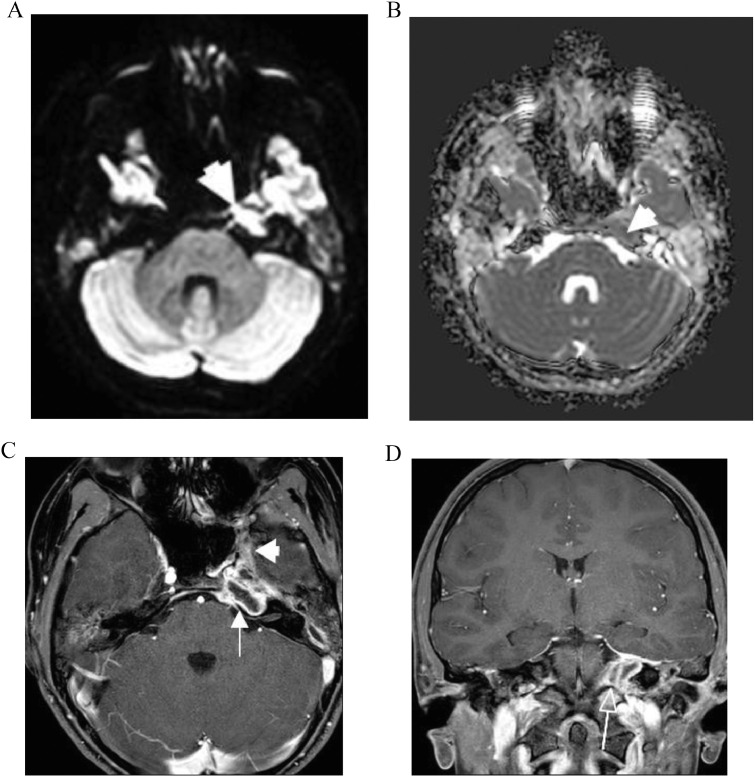
After the patient developed diplopia, an urgent brain and skull base MRI revealed extensive T2 hyper intensity lesion, suggesting fluid was collected throughout the petrous temporal bones bilaterally. High signal on diffusion weighted imaging (DWI) and low signal on the corresponding apparent diffusion coefficient (ADC) map in the petrous apex compatible with restricted diffusion was suggestive of purulent material (Fig. 2A, B). After administration of IV contrast, peripheral enhancement was noted around the hypointense fluid suggesting pus (Fig. 2C). These findings were strongly suggestive of petrous apicitis. Upon review of the initial CT scan, the radiologist also noted loss of the bony trabecular pattern in the left petrous apex (Fig. 1). In addition, abnormal enhancement adjacent to the cavernous sinus was seen, which explains the abducens nerve compromise. Furthermore, there was thickening and enhancement of the dura around the entrance to Meckel's cave (Fig. 2D).
Fig. 2.
(A) and (B) MRI on admission. High signal on diffusion weighted imaging (DWI) sequence and low signal on the corresponding ADC map in the petrous apex compatible with restricted diffusion suggests underlying infection (arrowheads). (C) MRI on admission. Axial T1 fat-saturated contrast-enhanced image shows peripheral enhancement (arrow) around the hypointense fluid suggesting pus. Features are in keeping with an abscess in the petrous apex. In addition, there is abnormal enhancement extending adjacent to the cavernous sinus (arrowhead). (D) MRI on admission. Coronal T1 fat-saturated contrast-enhanced image shows thickening and enhancement of the dura around the entrance to Meckel's Cave (arrow).
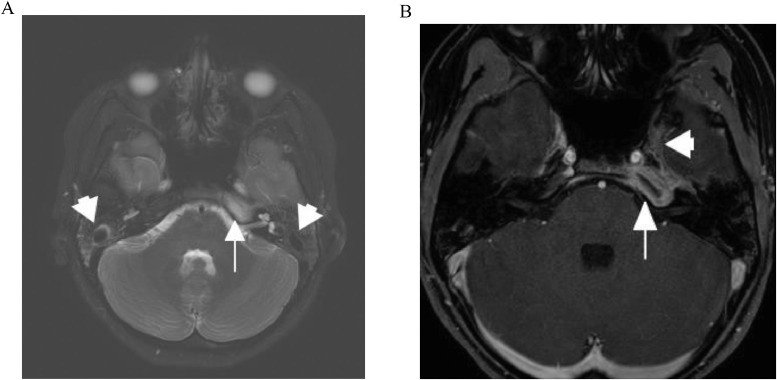
Nine days after discharge, follow-up outpatient MRI scan, indicated interval improvement. There was persistent high T2 signal within the left petrous apex but reduced signal in the mastoid air cells bilaterally, which suggested improved aeration (Fig. 3A). T1 post-gad scans, showed decreased central fluid signal suggesting less pus in the petrous apex and reduced enhancement of this region of the cavernous sinus (Fig. 3B). The ENT clinic confirmed the patient was doing well, IV antibiotics were ceased and she started a 4-week course of amoxicillin.
Fig. 3.
(A) MRI, 2 weeks from admission (9 days post hospital discharge). Axial T2 weighted fat-saturated image showing persistent increased signal in the petrous apex (arrow) but reduced signal in the mastoid air cells (arrowheads) suggesting improved aeration. (B) MRI, 2 weeks from admission (9 days post hospital discharge). Axial T1 fat-saturated contrast-enhanced image showing decreased fluid signal centrally suggesting resolving pus in the petrous apex (arrow) and decreased enhancement in region of the cavernous sinus (arrowhead).
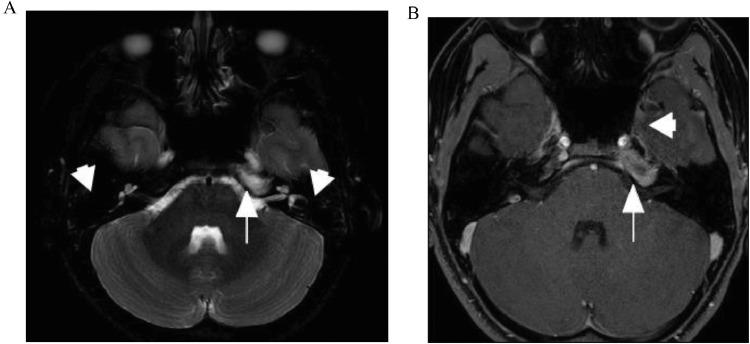
One month later, the final MRI scan revealed improvement of the signal abnormality in the petrous apex (arrow) but resolution of the fluid in the mastoid air cells bilaterally (Fig. 4A). There was also reducing enhancement and near complete resolution of the central fluid component in the petrous apex and no residual abnormal enhancement along the cavernous sinus (Fig. 4B).
Fig. 4.
(A) MRI, 6 weeks from admission. 1 Axial T2 fat-saturated image showing improvement of the signal abnormality in the petrous apex (arrow) but resolution of the fluid in the mastoid air cells (arrowheads). (B) MRI, 6 weeks from admission. Axial T1 fat-saturated contrast-enhanced image shows near complete resolution of the central fluid component in the petrous apex (arrow) and no residual abnormal enhancement along the cavernous sinus (arrowhead).
Discussion
In 1907, Guisseppe Gradenigo described a triad of symptoms consisting of suppurative otitis media, pain in the distribution of the trigeminal nerve, and abducens nerve palsy, all of which formed the Gradenigo syndrome. [1] Gradenigo syndrome is the classical presentation of petrous apicitis, but the full triad of symptoms is rarely observed, especially in the post- antibiotic era. As a result, Gradenigo syndrome is rarely seen nowadays. However, it remains a serious and potentially fatal complication of acute otitis media (AOM) and acute mastoiditis.
A PRISMA flow diagram by Gore [2] identifying 207 manuscripts since 1990, found 37 studies with 38 patients with useful demographic data. Patients were 22.4 years of age on average with an equal gender distribution. The main etiology was described as bacterial otitis media with apex involvement of the petrous part of the temporal bone causing localized osteomyelitis and reactive meningitis [3]. However, atypical causes, with lymphomas, petrous apex pachymeningitis, extradural abscess, cholesteatomas and chronic osteomyelitis have been described.
Gradenigo syndrome has traditionally been treated with mastoidectomy or petrosectomy, but recent advances in imaging, combined with improved antibiotic treatment, allow for conservative management. It is most commonly caused by aerobic microorganisms, but anaerobic microorganisms may also be found, therefore, anaerobic coverage should be considered when determining antibiotic treatment [2].
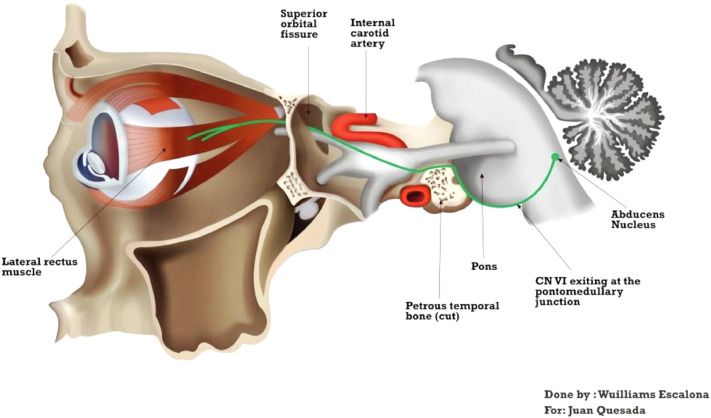
Clinical manifestations are correlated with the particular position of the trigeminal ganglion and the sixth cranial nerve in the bony petrous apex, where they are separated by only a thin layer of dura mater; hence, they are susceptible to compression by inflammatory processes that occur within this region [4] (Figure 5). Although in this case, the AOM explained the ear pain and discharge, involvement of the petrous apex and trigeminal nerve explained the ipsilateral headache, while the compression of the abducens nerve, lead to ipsilateral lateral rectus muscle palsy and consequent diplopia. The delay between otologic symptoms and cranial nerve involvement varies from 1 week to 2 to 3 months [5].
Fig. 5.
Relationship of the abducens nerve and trigeminal nerve with the petrous temporal bone.
The medial part of the temporal bone is the petrous apex, which lies between the inner ear and clivus. In the coronal plane, it is divided by the auditory canal into anterior and posterior compartments. The larger anterior part of the petrous apex is more frequently involved in disease processes. The petrous bone may be pneumatized (filled with mucosa-lined air cells), diploic (filled with bone marrow), or sclerotic [6].
The anterior portion of the petrous apex is filled with marrow in approximately 60% of temporal bones, pneumatized in 33 % and sclerotic in 7 % [7].
Neuroimaging has played a major role for the diagnosis and monitoring of the disease. CT is the first choice of imaging, because it is widely available and has a high sensitivity for detection of changes in bone structures such as erosive lysis with ill-defined irregular edges in the petrous apex in addition to intracranial abscesses. The characteristic feature is permeative destruction of the cortical and cancellous bone in this region, however, detection of bone loss occurs relatively late, with evidence suggesting 30%-50% demineralization before lysis is evident on CT. [8]. Furthermore, the petrous apex is a complex region with an array of variants and pneumatization of the petrous temporal bone is an anatomical variant that might be symmetrical bilaterally or asymmetrical, which can make the interpretation of CT more challenging. Finally, petrous apicitis can be seen in patients without pneumatized petrous apical air cells [7].
MRI is useful for the assessment of inflammatory soft tissue changes; therefore, evaluating the full extent of the lesion in the petrous apex localized on the CT scan is ideal, as is demonstrating features of meningeal involvement such as dural thickening and enhancement. Moreover, MRI is superior for detecting intracranial complications [9,10].
Conclusion
The classical triad of Gradenigo syndrome might not always be present and clinical suspicion for this syndrome and petrous apicitis should be raised in cases with unresolved AOM, especially in the presence of diplopia. The petrous apex is a complex region with multiple anatomical variants, which can be difficult to differentiate from pathological findings on CT. Thus, in cases of clinical suspicion despite nonconclusive CT imaging of the temporal bones, MRI is a reasonable next step.
Footnotes
Competing interests: On behalf of all the authors. I certify that there is no conflict of interest with any financial organization regarding the material in the manuscript.
Patient consent: Appropriate consent has been obtained from the patient's guardian for pubhcation of this case.
References
- 1.Gradenigo G. Ueber die paralyse des Nervus abducens bei Otitis. Gradenigo, G. Arch Ohrenheilunde. 1907;774:149–187. [Google Scholar]
- 2.Gore MR. Gradenigo's Syndrome: A review. Ann Med Health Sci Res. 2018;8:220–224. [Google Scholar]
- 3.Pedroso JL, de Aquino CC, Abrahão A, de Oliveira RA, Pinto LF, Bezerra ML. Gradenigo's syndrome: beyond the classical triad of diplopia, facial pain and otorrhea. Case Rep Neurol. 2011;3(1):45–47. doi: 10.1159/000324179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gillanders DA. Gradenigo's syndrome revisited. J Otolayngol. 1983;12:169–174. [PubMed] [Google Scholar]
- 5.Marianowski R, Rocton S, Ait-Amer JL, Morisseau-Durand MP, Manach Y. Conservative management of Gradenigo syndrome in a child. Int J Pediatr Otorhinolaryngol. 2001;57(1):79–83. doi: 10.1016/s0165-5876(00)00442-0. [DOI] [PubMed] [Google Scholar]
- 6.Chole RA. Petrous apicitis: surgical anatomy. Ann Otol Rhinol Laryngol. 1985;94(3):251–257. [PubMed] [Google Scholar]
- 7.Razek AA, Huang BY. Lesions of the petrous apex: classifications and findings at CT and MRI. Radiographics. 2011;32(1):151–173. doi: 10.1148/rg.321105758. [DOI] [PubMed] [Google Scholar]
- 8.Chapman P, Shah R, Cure JK, Bag AK. Petrous apex lesions: Pictorial review. AJR Am J Roentgenol. 2011;196(3):WS26–WS37. doi: 10.2214/AJR.10.7229. [DOI] [PubMed] [Google Scholar]
- 9.Murakami T, Tsubaki J, Tahara Y, Nagashima T. Gradenigo's syndrome: CT and MRI findings. Pediatric Radiol. 1996;26(9):684–685. doi: 10.1007/BF01356837. [DOI] [PubMed] [Google Scholar]
- 10.Hardjasudarma M, Edwards RL, Ganley JP, Aarstad RF. Magnetic resonance imaging features of Gradenigo's syndrome. Am J Otolaryngol. 1995;16(4):247–250. doi: 10.1016/0196-0709(95)90151-5. [DOI] [PubMed] [Google Scholar]