Abstract
We present the case of a 60-year-old woman who suddenly suffered a witnessed cardiac arrest and did not achieve return of spontaneous circulation despite being given 150-minute ultra-long cardiopulmonary resuscitation (CPR). During CPR, pulmonary embolism was suspected and was eventually diagnosed based on refractory pulseless electrical activity, elevated serum D-dimmer, and a markedly enlarged right ventricle chamber. After rescue thrombolytic alteplase therapy, the patient was successfully resuscitated and had a good neurological recovery.
Keywords: cardiac arrest, pulmonary embolism, thrombolysis
Introduction
Whenever an atraumatic cardiac arrest (CA) happens to a previously healthy adult, it is of paramount importance to analyze the underlying etiology as well as the implementation of standard cardiopulmonary resuscitation (CPR). Unlike coronary artery disease, pulmonary embolism (PE) is an uncommon and easily neglected cause of CA, accounting for only 4–10% of all cases.1 However, the early awareness and timely diagnosis of PE in patients with CA, particularly in those without any previous history of heart disease, may significantly improve outcomes in such patients.2,3 Here, we report on the successful treatment of a 60-year-old woman with sudden CA caused by PE.
Case Report
A 60-year-old woman presented to our department after a witnessed 6-minute CA. She was otherwise healthy and had no history of heart disease, asthma, or deep vein thrombosis. Before her presentation, she experienced chest discomfort and shortness of breath lasted for about an hour, while brushing her teeth in the morning. In the ambulance on the way to hospital, her condition deteriorated and suffered a sudden CA. Standard CPR was carried out by the pre-hospital emergency doctors. Upon arrival at our emergency resuscitation unit, she was immediately intubated and given a single dose of epinephrine (1 mg, i.v.) and continuous manual chest compressions. An arterial blood gas test showed pH 6.87, PaO2 39 mm Hg, PaCO2 49 mm Hg, HCO3− 9.4 mmol/L, BE −24.3 mmol/L, AG 29 mmol/L, K+ 5.2mmol/L, and Lac 14.1mmol/L, and the peripheral oxygen saturation (SpO2) was 0%. The electrocardiogram showed no acute ischemic changes and myocardial enzymes were normal; hence, myocardial infarction appeared less likely. Pulseless electrical activity (PEA) was observed after 11 minutes of CPR, and electrical defibrillation was then implemented, but it was ineffective. Recurrent wide QRS complex PEA, at a rate of 30–120 beats per minute, dominated the rest of the 150-minute ultra-long CPR, at the end of which, an intermittent return of spontaneous circulation was achieved in the patient. During CPR, bedside echocardiography was performed, and the results demonstrated a markedly dilated right ventricle deviating to the left side, which was significantly depressing the left ventricular systolic function. In addition, the patient’s serum D-dimmer showed a significant elevation (3391 μg/L, reference 0–500 ug/L). Based on the above findings, the patient was strongly suspected of having a massive PE and a PE-related CA. We decided to give her rescue thrombolysis treatment using a 5-mg bolus dose of alteplase followed by another 65 mg, which was given continuously. About 20 minutes after the alteplase infusion and the continuous support of a dopamine and intra-aortic balloon pump (IABP), sinus rhythm was restored, and spontaneous circulation was relatively stable, with blood pressure increased to 76/54 mmHg. The patient was then transferred to the emergency intensive care unit for advanced life support.
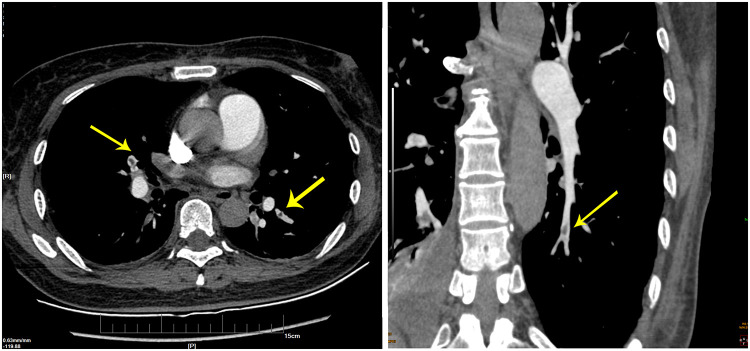
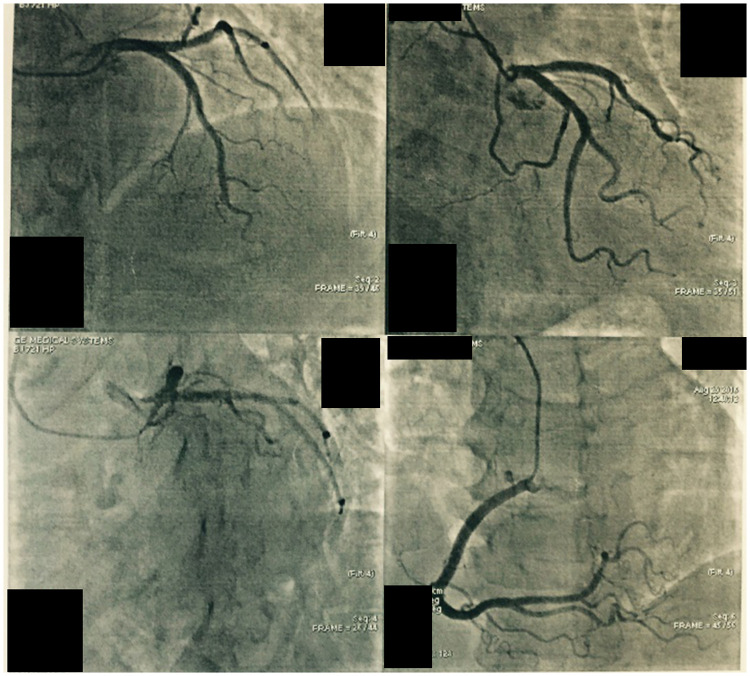
The IABP was withdrawn two days later for the stable spontaneous circulation while heparin infusion was constantly administered. Bedside echocardiography was performed again, and the results showed right ventricular volume overload, an improvement of the mild tricuspid regurgitation, and right ventricular systolic pressure lowered to 40 mmHg. The patient’s consciousness was recovered three days after the resuscitation and was extubated on day 9. After that, she underwent a computed tomography pulmonary angiography, which confirmed the presence of extensive bilateral pulmonary emboli (Figure 1). Coronary angiogram examination on day 24 showed no signs of coronary atherosclerotic heart disease or myocardial infarction (Figure 2). A magnetic resonance imaging scan on day 25 showed ischemic infarction in the globus pallidus caudate nucleus lobe. Fortunately, the patient only manifested a slight decline in memory and complex physical activity but had no major neurological sequelae. The patient finally survived and was discharged from the hospital 45 days after admission.
Figure 1.
Pulmonary artery computerized tomography scan showing filling defects in the bilateral basement segment of the inferior branches of the artery (see arrow).
Figure 2.
A coronary angiogram demonstrating no signs of coronary atherosclerotic heart disease.
Discussion
Pulmonary embolism (PE) is an important cause of cardiac arrest representing at least 4% to 10% of these cases and associated with exceedingly unfavorable prognosis.1 The main reason is that it is usually difficult to make an immediate and confirmed diagnosis of PE in CA patients during resuscitation partly due to fairly non-specific in presentation.4,5 Several lessons have been learned from the successful treatment of this case. First, timely and well-performed CPR is of critical importance, as it could earn valuable time for doctors to perform and comprehensively analyze the work-up results. In our case, laboratory tests and bedside diagnostic instrument played an essential role in making an early diagnosis of PE. Portable bedside echocardiography is a reliable tool as it recognizes the characteristics of PE, even during CPR.6,7 Some studies from case report and case series showed that prolonged CPR, especially in combination with the utility of extracorporeal membrane oxygenation has meaningful survival rates and the survivors are able to regain favorable neurological outcome and return to normal life.8–12 However, successful recovery after extremely long CPR with manual chest compressions, as in our case, has rarely been reported.8,13 Our case suggested that prolonged resuscitation is not futile although it poses a real challenge for doctors with uncertain outcomes. We believe that the duration of CPR should ultimately be determined by the doctors in scene and depend on the specific condition of the patient. Prudent inspection of evidence that is strongly associated with PE helps to establish an early presumptive diagnosis in the absence of clear alternative etiologies. For example, in fulminant PE, up to 90% of CA occurs within one to two hours after the onset of symptoms.1 Scrutinizing any electrocardiogram may also be helpful, as previous studies demonstrated that a right bundle branch block was present in 67% of PE cases and was strongly associated with CA caused by massive PE.1,5 The dilatation of the right ventricle on ultrasound during cardiopulmonary resuscitation is partly associated with pulmonary embolism. Aagaard et al showed that the right ventricular was more dilated when CA was caused by pulmonary embolism compared with other etiologies such as primary arrhythmia.14
Thrombolysis is a potentially beneficial treatment option for patients in cardiac arrest due to presumed PE. Javaudin et al showed that thrombolysis during cardiopulmonary resuscitation may contribute to survival.15 From the experience of our case, rescue thrombolytic therapy should be actively encouraged when a diagnosis, or even a presumed diagnosis, of PE has been made. CPR should be prolonged to make enough time for the thrombolytic therapy to take effect.16 Despite potentially improved outcomes with thrombolytic therapy, this treatment is bearing risks. Patients received thrombolytics may undergo major bleeding events, such as devastating intracranial hemorrhage. To decrease the risk of thrombolytics for therapy of CA due to PE, the clinician must correctly distinguish patients with presumed PE and must also adopt an appropriate thrombolytic agent and dosing regimen. Furthermore, proper high level supportive circulation care, such as IABP in our case, must be considered whenever it is needed.
In summary, the early recognition of etiology is of paramount importance for the successful resuscitation of patients with atraumatic CA and for CA caused by massive PE, and it appeared that a good recovery can be achieved with the use of rescue thrombolysis, even after an ultra-long CPR.
Informed Consent Statement
The written informed consent was obtained for the publication of the case details and relevant images from patients.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kürkciyan I, Meron G, Sterz F, et al. Pulmonary embolism as a cause of cardiac arrest: presentation and outcome. Arch Intern Med. 2000;160(10):1529–1535. PMID: 10826469. doi: 10.1001/archinte.160.10.1529 [DOI] [PubMed] [Google Scholar]
- 2.Logan JK, Pantle H, Huiras P, Bessman E, Bright L. Evidence-based diagnosis and thrombolytic treatment of cardiac arrest or periarrest due to suspected pulmonary embolism. Am J Emerg Med. 2014;32(7):789–796. PMID: 24856738. doi: 10.1016/j.ajem.2014.04.032 [DOI] [PubMed] [Google Scholar]
- 3.Wu JP, Gu DY, Wang S, Zhang ZJ, Zhou JC, Zhang RF. Good neurological recovery after rescue thrombolysis of presumed pulmonary embolism despite prior 100 minutes CPR. J Thorac Dis. 2014;6(12):E289–E293. PMID: 25590010; PMCID: PMC4283306. doi: 10.3978/j.issn.2072-1439.2014.12.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet. 1999;353(9162):1386–1389. PMID: 10227218. doi: 10.1016/s0140-6736(98)07534-5 [DOI] [PubMed] [Google Scholar]
- 5.Tsou PY, Kurbedin J, Chen YS, et al. Accuracy of point-of-care focused echocardiography in predicting outcome of resuscitation in cardiac arrest patients: a systematic review and meta-analysis. Resuscitation. 2017;114:92–99. PMID: 28263791. doi: 10.1016/j.resuscitation.2017.02.021 [DOI] [PubMed] [Google Scholar]
- 6.Blaivas M. Transesophageal echocardiography during cardiopulmonary arrest in the emergency department. Resuscitation. 2008;78(2):135–140. PMID: 18486300. doi: 10.1016/j.resuscitation.2008.02.021 [DOI] [PubMed] [Google Scholar]
- 7.Fair J, Mallin M, Mallemat H, et al. Transesophageal echocardiography: guidelines for point-of-care applications in cardiac arrest resuscitation. Ann Emerg Med. 2018;71(2):201–207. PMID: 29107407. doi: 10.1016/j.annemergmed.2017.09.003 [DOI] [PubMed] [Google Scholar]
- 8.Yu HY, Yeh HL, Wang SS, et al. Ultra long cardiopulmonary resuscitation with intact cerebral performance for an asystolic patient with acute myocarditis. Resuscitation. 2007;73(2):307–308. PMID: 17234321. doi: 10.1016/j.resuscitation.2006.08.012 [DOI] [PubMed] [Google Scholar]
- 9.Rajan S, Folke F, Kragholm K, et al. Prolonged cardiopulmonary resuscitation and outcomes after out-of-hospital cardiac arrest. Resuscitation. 2016;105:45–51. PMID: 27224447. doi: 10.1016/j.resuscitation.2016.05.004 [DOI] [PubMed] [Google Scholar]
- 10.Shida H, Matsuyama T, Kiyohara K, et al. Prehospital cardiopulmonary resuscitation duration and neurological outcome after out-of-hospital cardiac arrest among children by location of arrest: a nationwide cohort study. Scand J Trauma Resusc Emerg Med. 2019;27(1):79. PMID: 31443673; PMCID: PMC6708229. doi: 10.1186/s13049-019-0658-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen YS, Chao A, Yu HY, et al. Analysis and results of prolonged resuscitation in cardiac arrest patients rescued by extracorporeal membrane oxygenation. J Am Coll Cardiol. 2003;41(2):197–203. PMID: 12535808. doi: 10.1016/s0735-1097(02)02716-x [DOI] [PubMed] [Google Scholar]
- 12.Chiu CW, Yen HH, Chiu CC, Chen YC, Siao FY. Prolonged cardiac arrest: successful resuscitation with extracorporeal membrane oxygenation. Am J Emerg Med. 2013;31(11):1627.e5–6. PMID: 24055477. doi: 10.1016/j.ajem.2013.06.040 [DOI] [PubMed] [Google Scholar]
- 13.Nobre C, Thomas B, Santos L, Tavares J. Prolonged chest compressions during cardiopulmonary resuscitation for in-hospital cardiac arrest due to acute pulmonary embolism. Tex Heart Inst J. 2015;42(2):136–138. PMID: 25873823; PMCID: PMC4382878. doi: 10.14503/THIJ-14-4267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aagaard R, Caap P, Hansson NC, Bøtker MT, Granfeldt A, Løfgren B. Detection of pulmonary embolism during cardiac arrest-ultrasonographic findings should be interpreted with caution. Crit Care Med. 2017;45(7):e695–e702. PMID: 28403120. doi: 10.1097/CCM.0000000000002334 [DOI] [PubMed] [Google Scholar]
- 15.Javaudin F, Lascarrou JB, Le Bastard Q, et al; Research Group of the French National Out-of-Hospital Cardiac Arrest Registry (GR-RéAC). Thrombolysis during resuscitation for out-of-hospital cardiac arrest caused by pulmonary embolism increases 30-day survival: findings from the French National Cardiac Arrest registry. Chest. 2019;156(6):1167–1175. PMID: 31381884. doi: 10.1016/j.chest.2019.07.015 [DOI] [PubMed] [Google Scholar]
- 16.Hsin T, Chun FW, Tao HL. Ultra-long cardiopulmonary resuscitation with thrombolytic therapy for a sudden cardiac arrest patient with pulmonary embolism. Am J Emerg Med. 2014;32(11):1443. doi: 10.1016/j.ajem.2014.04.035 [DOI] [PubMed] [Google Scholar]