Abstract
Objective
To study the SARS-CoV-2 infection rate among hospital healthcare workers after the first wave of the COVID-19 pandemic, and provide more knowledge in the understanding of the relationship between infection, symptomatology and source of infection.
Design
A cross-sectional study in healthcare workers.
Setting
Northern Limburg, the Netherlands.
Participants
All employees of VieCuri Medical Center (n=3300) were invited to enrol in current study. In total 2507 healthcare workers participated.
Intervention
Between 22 June 2020 and 3 July 2020, participants provided venous blood samples voluntarily, which were tested for SARS-CoV-2 antibodies with the Wantai SARS-CoV-2 Ig total ELISA test. Work characteristics, exposure risks and prior symptoms consistent with COVID-19 were gathered through a survey.
Main outcome measure
Proportion of healthcare workers with positive SARS-CoV-2 serology.
Results
The overall seroprevalence was 21.1% (n=530/2507). Healthcare workers between 17 and 30 years were more likely to have SARS-CoV-2 antibodies compared with participants >30 years. The probability of having SARS-CoV-2 antibodies was comparable for healthcare workers with and without direct patient (OR 1.42, 95% CI 0.86 to 2.34) and COVID-19 patient contact (OR 1.62, 95% CI 0.80 to 3.33). On the contrary, exposure to COVID-19 positive coworkers (OR 1.83, 95% CI 1.15 to 2.93) and household members (OR 6.09, 95% CI 2.23 to 16.64) was associated with seropositivity. Of those healthcare workers with SARS-CoV-2 antibodies, 16% (n=85/530) had not experienced any prior COVID-19-related symptoms. Only fever and anosmia were associated with seropositivity (OR 1.90, 95% CI 1.42 to 2.55 and OR 10.51, 95% CI 7.86 to 14.07).
Conclusions
Healthcare workers caring for hospitalised COVID-19 patients were not at an increased risk of infection, most likely as a result of taking standard infection control measures into consideration. These data show that compliance with infection control measures is essential to control secondary transmission and constrain the spread of the virus.
Keywords: public health, infectious diseases, COVID-19, infection control
Strengths and limitations of this study.
Large-scaled cross-sectional SARS-CoV-2 antibodies screening of healthcare workers in a highly endemic region in the Netherlands.
Detailed questionnaire of COVID-19 exposures and symptoms consistent with COVID-19.
Screening immediately after the first epidemic wave in the Netherlands and before easing of the national lockdown measures, allowing potential exposure sources to be restricted.
The demographic profile of the spread of the virus among this group of healthcare workers is limited as participants were not asked for their ethnicity, residency or activity in previous festivities.
The probability of having SARS-CoV-2 antibodies on exposure to one specific COVID-19 source might be influenced by other COVID-19 sources, although minimised by including these as confounders in statistical analysis.
Introduction
The SARS-CoV-2 was first reported in Wuhan, China, mid-December 2019. Due to the rapid and worldwide spread of the virus, the WHO officially declared the COVID-19 outbreak a pandemic on 11 March 2020.1 On February 27, the first case of COVID-19 was confirmed in the Netherlands.2 Within a month, more than 10 000 cases had been confirmed.3 The spread of the virus started initially in the southern regions of the Netherlands. VieCuri Medical Centre, located in the province of Limburg in the south of the Netherlands, was located in a highly endemic area.
‘Intelligent lockdown measures’ were announced by the Dutch government on 12 March, targeting transmission in the community,4 whereas infection by patient contact and among hospital employees was primarily addressed by hospital policy. The VieCuri hospital responded quickly to the emerging outbreak and implemented infection prevention and control measures from 4 March 2020. This included low-threshold testing of patients, limitation in numbers of visitors per patient and scaling down (non-urgent) operations, listed in figure 1. The first confirmed COVID-19 case in the hospital concerned a healthcare worker (HCW), on 8 March, only a few days before the first COVID-19 confirmed patient was hospitalised at VieCuri on 11 March. During the first wave of the COVID-19 pandemic in the Netherlands (February to June 2020) a total of 408 patients with COVID-19 have been hospitalised at VieCuri (figure 1). The Netherlands had to contend with insufficient test capacity, therefore only at the end of the first wave increased test capacity allowed HCW to be screened for SARS-CoV-2 again.5 As a result, the overall picture of infections, and therefore risks as well, within hospital settings remained unknown.
Figure 1.
Timeline of hospitalised patients in VieCuri per day and infection prevention measures taken internally over time. The chart shows the number of COVID-19 patients admitted at the hospital each day between March and June 2020. HCWs have been screened on the presence of SARS-CoV-2 antibodies between 22 June 2020 and 3 July 2020. Infection prevention measures taken by the hospital and taken over from the Dutch government policy, are depicted below the chart. *March 7: Testing on SARS-CoV-2 virus of HCW with mild respiratory symptoms; †HCW with mild respiratory symptoms have to work wearing a surgical mask; **27 April: Downscaling IC capacity; ***14 March: FFP1 and surgical type II R face masks for clinical care, FFP2 masks for medical procedures following aerosol release; ****18 May: Start regular care of patients with urgent care; *****12 March: maximum of 1 visitor per patient per day, expect for paediatrics, ICU and terminal patients. FFP, filtering facepiece; HCW, healthcare worker; ICU, intensive care unit.
Caring for patients with COVID-19 (besides exposure to infected family members and colleagues or community transmission) potentially placed front-line HCW at an increased risk of becoming infected with the new virus. Several studies found that the COVID-19 incidence in HCW was higher than in the general population, suggesting nosocomial transmission and emphasises the importance of personal protective equipment (PPE) to prevent HCW infections, subsequently reducing secondary transmission.6–10 A Scottish study showed a higher hospital admission risk in patient facing HCW, as well as in household members of HCW with patient care, compared with non-patient facing HCW.7 Moreover, Shields et al11 identified different risks of seropositivity between different groups of HCW, suggesting different exposure risks exist within the hospital environment.
On the other hand, the WHO-China Joint Mission and preliminary Chinese household transmission studies have shown that transmission within healthcare settings and among HCW was not of major contribution to the spread of SARS-CoV-2 and have suggested HCW to have been infected within the household rather than in healthcare setting.12 Various studies support these data suggesting staff have been most at risk at home and have acquired an infection outside the hospital.13 14 They imply that transmission occurred at specific social events in the community rather than within hospital settings and therefore do not support widespread nosocomial transmission as the source of infection in patients or HCW.15
Due to the fact that front-line HCW in patient-facing roles appeared to be at risk of infection, seroprevalence studies are useful to estimate the risk among HCW and to provide insight into the relationship between infection, symptomatology, and source of infection. Such knowledge is important to reflect on hospital policy regarding infection prevention and control measures, protecting HCW and controlling rates of secondary transmission. Therefore, we conducted a cross-sectional study of hospital staff at VieCuri Medical Centre to determine the seroprevalence of SARS-CoV-2 antibodies and the relationship to prior symptoms, work characteristics and potential exposure, both in the hospital and private situation.
Methods
Study design and population
A cross-sectional study of HCW at VieCuri Medical Centre was performed between 22 June 2020 and 3 July 2020. All employees (n=3300) were invited to participate in current study via internal communication (mail and intranet). Participation was fully voluntary and all participants provided a digital informed consent prior to enrolment in the study. Only individuals employed by the hospital, interns and volunteers, at the moment of blood collection, were included.
All participants (n=2507) voluntarily provided a venous blood sample (10 mL) for SARS-CoV-2 antibody detection, using the Wantai SARS-CoV-2 Total Ab ELISA. Serologic analysis was performed at the Serology division of the department of Medical Microbiology, VieCuri Medical Centre, Venlo. The cut-off value for positive serostatus was set at a ratio of >1.0. Prior validation of this assay in Dutch laboratories showed a sensitivity of 97.5% in patients with severe (PCR-confirmed) infections (n=646) when samples were collected >14 days after onset of illness. The specificity of the ELISA was 99.6% (n=1334).16
Participants were asked to complete an online questionnaire covering exposure risks (COVID-19 confirmed and suspected patient, coworker and household contact), (severity of) prior COVID-19-related symptoms between 1 March and the date of the questionnaire, hospital department and occupation.
Outcomes
The primary outcome was the proportion of HCW with positive SARS-CoV-2 serology. Secondary outcomes consisted of the probability of having SARS-CoV-2 antibodies among HCW according to different age categories, exposure risks (patients, coworkers and household) and work characteristics (department and occupation). Therefore, all participating HCW were clustered. Strict department clustering was challenging due to continuous exchange of staff during the first wave. A general subdivision was used to analyse the differences in seropositivity between HCW with and without direct COVID-19 and non-COVID-19 patient contact. When distinguishing individual hospital departments, we focused only on those wards most and least involved in the care of COVID-19 patients. Since the general internal medicine and pulmonary medicine were both occupied with patients with COVID-19 and personnel continuously exchanged on these wards, we chose to combine these two wards in the analysis. Moreover, symptomatic predictors for the presence of SARS-CoV-2 antibodies were identified.
Under the General Data Protection Regulation, we did not use the hospital HR database for occupational information on all HCW. Occupational roles were broadly categorised according to predesignated occupations stated in the survey. HCW categorised as ‘other’ had been subdivided according to the presence or absence of direct patient contact. Classification bias was minimised by minimal changes to occupational roles reported by all HCW. Nevertheless, all exposures in combination with occupational roles were checked in order to guarantee continuity. We checked whether reported patient contact met the definition of 15 min within 1.5 metres.
Statistical analysis
Collected data were analysed using IBM SPSS Statistics V.24.0. The seroprevalence was calculated and expressed as a percentage. The χ2 test was used to analyse differences in seroprevalence between men and women, independent samples t-test to compare age between seropositive and seronegative HCW. Age was subsequently categorised and analysed with bivariate logistic regression using seropositivity as outcome variable. Multivariate logistic regression was performed to estimate the adjusted OR and 95% CIs to assess differences in probability of a positive test by reported symptoms and their severity, independently, in which all symptoms were included as covariates. Additionally, this analysis was used to examine age differences in absence of (severe) symptoms. Moreover, multivariate logistic regression has also been applied to assess the odds of seropositivity for work characteristics and the potential infection sources (patients, coworkers and household).
When examining the probability of positive serology, distinct reference groups were used. For the analysis of exposure risks we chose to compare HCW exposed to a potential infection source (patient, coworker and household) to HCW reported not to have been exposed to any COVID-19 contact, neither confirmed nor suspected and therefore mutually comparable. When analysing the probability of SARS-CoV-2 seropositivity among different hospital departments, HCW without direct patient contact were used as a reference group, since this subgroup had the least exposure within the hospital.
Covariates
Hospital department, occupation, age and contact with COVID-19 confirmed and suspected individuals were identified as confounders and included in multivariate analyses. The adjustment for COVID-19 suspected contacts was of great importance. HCW had been in contact with patients who were highly suspected of COVID-19 but the diagnosis still had to be confirmed by additional tests. Due to test scarcity in the first few months of the pandemic, only patients were tested by PCR. So HCW were unable to get tested for SARS-CoV-2 when COVID-19 was suspected, and therefore, never confirmed. Moreover, in that period (March–June) hardly any other respiratory viruses were found. Adjustment for work characteristics and age filtered out potential cluster infections within the hospital.
Patient and public involvement
No patients or public were involved in defining research questions or outcome measures, nor were they asked to provide input on the design and conduct of the study. No patients were involved in the interpretation and dissemination of the study results. Results were presented within the hospital and conclusions about specific departments were checked with representatives of the concerning department.
Results
A total of 2507 HCW participated in current study (76%) of which 202 physicians, 745 nurses, 459 paramedical staff and 1101 others including technical and administrative staff, housekeepers, students and volunteers. The median age was 45 years (range 17–80). The seroprevalence among HCW at VieCuri Medical Center between 22 June and 3 July was 21.1% (n=530/2507) (table 1). In total, one HCW (female, 63 years) had to be hospitalised due to COVID-19, but was not admitted to the ICU.
Table 1.
Demographics of study participants
| All HCW | Seropositive | Seronegative | Seroprevalence (%) | P value | |
| n | 2507 | 530 | 1977 | 21.1 | |
| Age (years), median (IQR) | 45 (32–56) | 42 (28–55) | 46 (33–56) | <0.001 | |
| Sex, n (%) | |||||
| Male | 454 (18.1) | 89 (16.8) | 365 (18.5) | 19.6 | 0.376* |
| Female | 2053 (81.9) | 441 (83.2) | 1612 (81.5) | 21.5 |
*χ2=0.786.
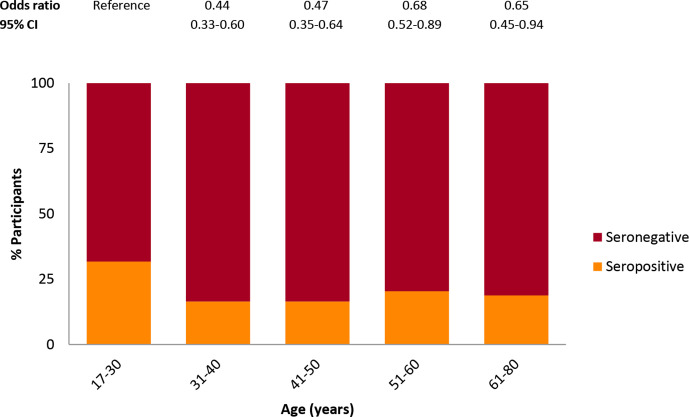
There was no difference in seropositivity between men and women (19.6% vs 21.5%, χ2=0.786, p=0.376). On average seropositive HCW were younger than seronegative HCW (42 vs 46 years; p<0.001). Participants ≤30 years were more likely to have SARS-CoV-2 antibodies compared with participants older than 30 years (figure 2).
Figure 2.
SARS-CoV-2 seropositivity among different age categories. Age categories are defined by years. ORs and 95% CIs are based on multivariate logistic regression analysis, with age group 17–30 years as reference group and adjusted for COVID-19 confirmed and suspected contact, occupation and department as confounders.
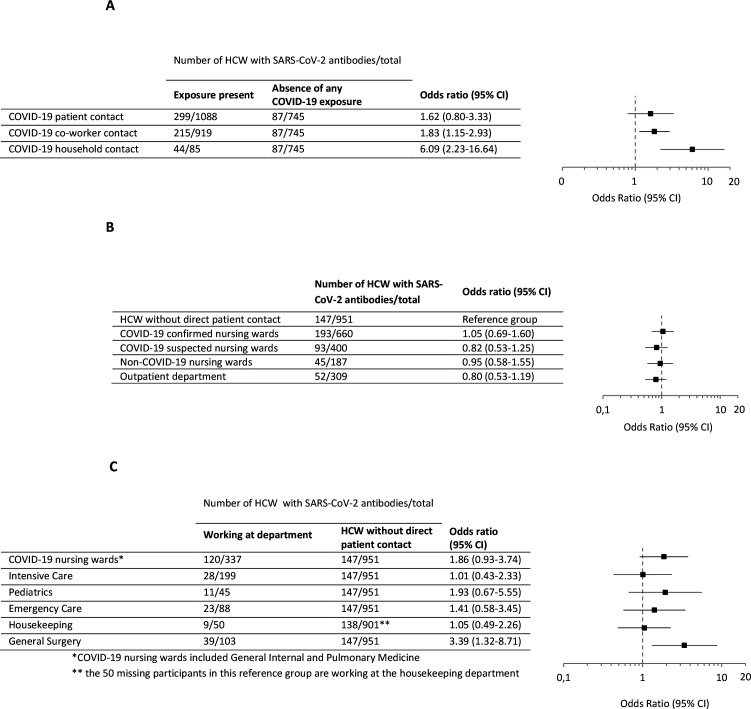
Participants who have worked from home, and who were therefore not present in the hospital in the period March–June, had similar seroprevalence compared with participants who did work in the hospital (17.9% vs 21.2%, χ2=0.370, p=0.543). A total of 745 HCW reported not to have been exposed to any COVID-19 confirmed or suspected contact, of which 11.7% were seropositive (n=87/745). This subpopulation had a significantly lower seroprevalence compared with HCW exposed to at least one potential infection source (11.7% vs 25.1%, χ2=56.936, p<0.001). This subgroup, with no known risk, is used as reference group when examining exposure risks. Of all HCW, 62.1% (n=1556/2,507) was involved in patient care. Seroprevalence among these HCW with direct patient contact was 24.6% (n=383/1556) and the probability of having SARS-CoV-2 antibodies was comparable to HCW without direct patient contact (OR 1.42, 95% CI 0.86 to 2.34). Subsequently, HCW with and without direct contact with COVID-19 patients had similar seropositivity probability (OR 1.62, 95% CI 0.80 to 3.33) (figure 3A). Exposure to COVID-19 positive co-workers (OR 1.83, 95 CI 1.15 to 2.93) or household members (OR 6.09, 95% CI 2.23 to 16.64), on the contrary, was associated with positive serology.
Figure 3.
The probability of SARS-CoV-2 seropositivity among HCW according to different exposure risks and hospital departments. (A) HCW exposed to (COVID-19 positive) HCW exposed to COVID-19 positive patient, coworker and household contact are compared with HCW without either confirmed or suspected COVID-19 exposure. OR and 95% CIs are based on multivariate logistic regression analysis, adjusted for age, COVID-19 confirmed and suspected contacts, occupation and department as confounders. (B, C) HCW working at the department are compared with HCW working without direct patient contact. ORs and 95% CIs are based on multiple logistic regression analysis, adjusted for age, COVID-19 confirmed and suspected contact, and occupation as confounders. HCW, healthcare worker.
Additionally, seroprevalence was mapped across different departments within VieCuri. Multivariate logistic regression, in which the HCW without any patient contact was used as reference group, confirmed that working at COVID-19 departments did not increase the probability of a SARS-CoV-2 infection (figure 3B). In addition, there was no association between occupation and the presence of SARS-CoV-2 antibodies, although paramedical staff seemed to have a lower probability compared with other staff without patient contact (OR 0.66, 95% CI 0.45 to 0.98) (online supplemental figure 1). Examining several individual working departments within VieCuri, focusing on the wards most and least involved in the care of COVID-19 patients, seroprevalence was highest in participants working at COVID-19 nursing wards (35.6%, n=120/337) and general surgery (37.9%, n=39/103) and lowest in the intensive care (14.1%, n=28/199) and housekeeping (18%, n=9/50) (figure 3C). Using HCW without direct patient contact as a reference population in multivariate logistic regression, only an increased OR of seropositivity was observed in HCW working at general surgery (OR 3.39, 95% CI 1.32 to 8.71).
bmjopen-2021-051573supp001.pdf (153.1KB, pdf)
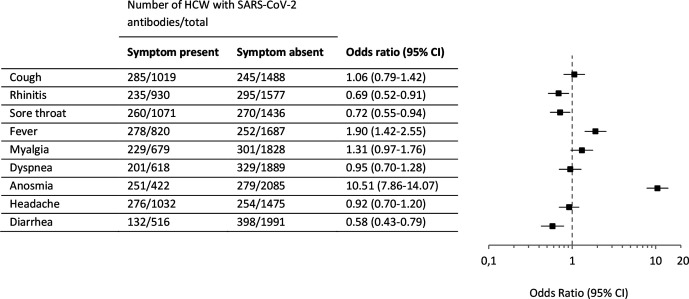
We explored the relationship between seroprevalence and prior symptoms consistent with COVID-19 listed in figure 4. An extensive number of participants had experienced at least one prior symptom (n=1807/2507 (72.1%)). These HCW had a significantly greater seroprevalence compared with the HCW who remained completely asymptomatic since the corona outbreak in the Netherlands (24.6% vs 12.1%, χ2=47.164, p<0.001)(online supplemental figure 2A). Additionally, no age differences are identified among asymptomatic seropositive HCW (OR 1.00, 95% CI 0.849 to 1.181). Besides not experiencing fewer symptoms, seropositive HCW ≤30 years also did not experience less severe symptoms (online supplemental figure 2B, C).
Figure 4.
Symptomatic predictors for the presence of SARS-CoV-2 antibodies. ORs and 95% CIs are based on multivariate logistic regression analysis with all symptoms included in the model. HCW, healthcare worker.
Of those individuals with SARS-CoV-2 antibodies, 16% (n=85/530) had not experienced any prior COVID-19-associated symptoms. Anosmia (n=422) was most strongly associated with seropositivity (59.5% vs 13.4%; OR 10.51, 95% CI 7.86 to 14.07). Furthermore, fever (n=820) was also a symptomatic predictor for positive serology (33.9% vs 14.9%; OR 1.90, 95% CI 1.42 to 2.55) (figure 4).
Participants were also asked to report the severity of their symptoms on a scale of 1–10. Severity scores of 1–3 is categorised as mild, scores 4–7 as moderate and 8–10 as severe. Multiple logistic regression showed an increasing seroprevalence with increasing severity of fever and anosmia (online supplemental figure 3). Severe anosmia (n=210) was highly associated with the presence of SARS-CoV-2 antibodies (78.6% vs 13.4%; OR 23.74, 95% CI 16.67 to 33.78).
Discussion
Overall, 21.1% of HCW in a teaching hospital in a highly endemic region after the first wave was seropositive. The seroprevalence was significantly higher among HCW aged 17–30 years compared with >30 year old HCW, 16% of seropositive participants stayed asymptomatic after a SARS-CoV-2 infection and fever, and especially anosmia, were associated with positive serology. Furthermore, our results show that, when accounting for confounders in a large sample, HCW having contact with COVID-19 positive coworkers and household members were, respectively, twofold and sixfold more likely to be seropositive.
Important here to highlight the implement of adjustments in the analyses. A large amount of HCW reported to have been exposed to a COVID-19 suspected contact additional to a COVID-19 confirmed individual. Creating single exposure subgroups by dismissing these contaminated exposures, did result in too small sample sizes with insufficient statistical power. As a consequence, we were unable to completely isolate exposure sources from each other. Therefore, we chose to redistribute the group of HCW for every exposure, thereby accepting the chance that the outcome might be influenced by other COVID-19 exposures. To account for this, multivariate logistic regressions were adjusted for the remaining COVID-19 contacts. Besides, due to test scarcity only patients were tested for SARS-CoV-2 at the start of the first epidemic wave. Absenteeism among VieCuri staff did not increase in that period, which makes us believe many infections have remained unknown. Moreover, many HCW have been in contact with highly suspected COVID-19no patients pending a PCR. For example at the emergency care department, responsible for the largest COVID-19 patient influx. Therefore, reported suspected COVID-19 contact is an important factor when analysing exposure risks. Nevertheless, the results should be addressed with care.
Differences in study populations and screening period of previous serological studies make results difficult to compare. Similarly, the study region. One month into the pandemic the overall seroprevalence in the Netherlands was 2.7%, based on plasma samples of 7361 regular blood plasma donors, collected between 1 April 2020 and 15 April 2020.17 Regional mapping showed a prevalence of 6.9% in Northern and central Limburg, the region in which VieCuri Medical Centre is located. The difference to our reported seroprevalence could be explained by the time of sample collection. Our study has been performed 2.5 months later into the pandemic, right after the first wave, while the time of testing of Slot et al was relatively early after the outbreak. Also the subjects enrolled in the study were healthy at the time of donation, and had not reported health issues 2 weeks before donation. This could therefore result in an under-representation of donors who suffered or recovered from COVID-19.17 The magnitude of regional differences in serostatus are also reflected by a serological study in HCW of the Amsterdam University Medical Centers between March and June, where the highest seroprevalence observed was 13.2% in HCW who were in direct contact with COVID-19 patients, vs 3.6% in HCW without direct patient contact, while the seroprevalence in our total study population was 21.1%.18 At the beginning of the pandemic, the Southeast region of the Netherlands was considered a COVID-19 hotspot, while community transmission in Amsterdam was still low. Our data suggest most infections arise from the household, while Sikkens et al reported HCW working at COVID-19 wards to be at increased risk of infection, with transmission between HCW as a major contributor.18 Nevertheless, both studies indicate the potential impact of infection risks due to HCW-HCW interaction. Similar results were found by Steensels et al,14 using the same study design, where most infections were most likely acquired in the community. In line with these serological studies, research using PCR to screen mildly symptomatic HCW also reported no nosociomal transmission but many of the infections to be acquired rather in the community and social gatherings.13 15
Previous research has shown that people aged 20–30 have the highest rates of COVID-19 infections.6 19–22 Furthermore, some of the Dutch (healthy and symptom free) blood donors have been tested for the presence of SARS-CoV-2 antibodies. Results show that between 1 April 2020 and 15 April 2020, the seroprevalence in donors aged 18–30 years was significantly higher compared with other age groups (4.2% vs 2.4%).17 However, age differences varied substantially among areas throughout the country. When correcting for region of residence no additional association between age and seropositivity was shown. The celebration of Carnival in the South and Southeaster regions of the country in the last week of February 2020 is thought to have caused the regional variation, even before the start of the epidemic in the Netherlands and the introduction of social distancing and social restrictions.17 23 Carnival is celebrated especially, and more exuberantly, among the younger generation and since they often show no or only mild symptoms, and are unaware they are infected, they could have unknowingly spread the virus. This study supports this hypothesis by observing almost twice as many infections among the participants ≤30 years. Preliminary studies suggest that these young adults will only have few or no symptoms at all, and that the physical effects of the disease are less severe.24–27 However, our data suggest that there is no difference between different age groups regarding the presence and severity of COVID-19-related symptoms. After adjustments for exposures within the hospital and household, the association between serology and age remains, suggesting that the young adults got infected elsewhere and thereby supporting the hypothesis of Carnival as point of origin. Nevertheless, it is important to mention that information on participation in any Carnival festivity of the participated HCW was missing in the survey. Therefore, this assumption has to be addressed with care.
Furthermore, we could not identify significant variations in seroprevalence between different hospital departments apart from HCW working at the general surgery. The higher seropositivity could not be explained based on the survey. Additional inquiries were made to identify the potential origin of the high number of infections. It appeared that in March, before the partial national lockdown and restrictions inside the hospital environment, a social gathering took place among members of this department. Afterwards it appeared that one of the attendees was infected. This could have potentially caused the spread of the virus among general surgery members. Unfortunately, the sample sizes within the department were too small to analyse the risk of exposure to coworkers to confirm this presumption. This cluster at the surgical ward, in combination with no further significant variations among different groups of HCW, emphasises the importance of social distancing in slowing down the spread of COVID-19 and implies proper use of PPE promoted by the hospital when HCW are facing (COVID-19) patients, which included eye protection, long-sleeved disposable apron and gloves in addition to, initially, Filtering Facepice 2 (FFP2) masks, later on surgical mouth-nose masks and good hand hygiene.
Not only proper use of PPE while caring for COVID-19 patients is essential to prevent infection, research shows that break room exposure to another HCW without wearing a mask is associated with increased risk for HCW infection.28 29 The necessity of infection prevention and control measures was emphasised again with multiple clusters of infections among VieCuri employees at the second epidemic wave from October 2020. Inquiries suggest that HCW-HCW interaction might have initiated the spread of the virus.
There are a number of limitations to this study additional to the analysis on exposure risks. Participants could not define the amount of time they were in close contact with a COVID-19 confirmed or suspected patient or colleague. Due to these limitations of the questionnaire, the degree of exposure to COVID-19 contacts could vary between HCW. Serology testing indicates the percentage of participants that developed SARS-CoV-2 antibodies on infection. However, a negative Wantai total Ab ELISA test cannot rule out a possible infection followed by no or no detectable antibody levels. Besides, previous research showed a decline in SARS-CoV-2 antibodies over time following acute infection.30–32 Therefore, the seroprevalence might be underestimated. On the other hand, the cut-off value for positive serostatus was set at a value of 1.0 in this study, while in daily practice values between 1.0 and 3.0 are considered questionable. This may result in an overestimation of true seroprevalence. Finally, participants were not able to indicate their ethnicity or residency in the survey. This data could have been of value in creating an epidemiological profile of the spread of the virus considering some of the surrounding villages were COVID-19 hotspots.
The strengths of the study include the large sample size of this hospital-wide screening study for SARS-CoV-2 antibodies in HCW in a teaching hospital right after the first epidemic wave in the Netherlands and before easing of the national lockdown measures. This restricted the potential exposure sources. The study had high participation rate and in contrast to previous screening studies, participants were not selected based on presence of symptoms or exposures as all HCW at VieCuri were offered serological testing without inclusion criteria. Additionally, COVID-19-related symptoms were queried to detail, allowing for analyses on severity of symptoms.
In conclusion, HCW could face a high risk of SARS-CoV-2 transmission while providing care for suspected or confirmed COVID-19 patients. Yet, they had no increased seroprevalence of previous COVID-19 infection compared with HCW without direct patient contact. Therefore, proper use of PPE and the implementation and compliance to basic infection control precautions are essential to control secondary transmission and constrain the spread of the virus inside the hospital.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the volunteers of the Clinical Chemical Haematology Laboratory (CCHL) of VieCuri Medical Centre for collecting blood of all participating employees, the staff of the Serology department of the Medical Microbiology (MMB) of VieCuri Medical Centre for processing the samples for serological testing and the MMB administrative team for their contribution on the days of blood collection. This research was supported by the VieCuri Corona Foundation and Regio Noord-Limburg. Furthermore we want to thank Deborah Steensels and research group from hospital Oost-Limburg, Genk, Belgium, for sharing their study concept and design.
Footnotes
Contributors: JdV and FC conceived the study and MB, FvO, FC, TT and JdV designed the study. JdV was the guarantor for this study. MB organised blood collection and facilitated the acquisition of samples. MB collated the data. MB and FvO performed data analyses. MB, FvO and JdV interpreted the study results. MB drafted the first version of the manuscript. MB, FvO, FC, TT, JM-S, JPvdB and JdV helped to draft the manuscript. All of the authors contributed to the interpretation of data, and read and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. Data are available upon reasonable request. Proposals should be directed to the corresponding author.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study was approved by the Medical Ethics Committee of the azM and Maastricht University (METC azM/UM, 2020-2304).
References
- 1.World Health Organization (WHO) . Archived: WHO Timeline - COVID-19: WHO, 2020. Available: https://www.who.int/news/item/27-04-2020-who-timeline-covid-19 [Accessed 05 Nov 2020].
- 2.Alderweireld CE, Buiting AG, Murk J, et al. COVID-19: patient zero in the Netherlands. Nederlands tijdschrift voor geneeskunde, 2020: 164. [PubMed] [Google Scholar]
- 3.Rijksinstituut voor Volksgezondheid en Milieu (RIVM) . Actuele cijfers en trends covid 19 in Nederland en wereldwijd: RIVM, 2020. Available: https://app.powerbi.com/view?r=eyJrIjoiMmM0NGQyMTctYWM3Ni00MmI3LTkwY2QtZDYzYTc3ZjM1MDk5IiwidCI6ImVhZTFhNWZlLWZlYTktNGQ3Yy1iMmM2LTkwMjE1NTdlODYwOCIsImMiOjl9&refresh=1 [Accessed 10 Nov 2020].
- 4.Antonides G, van Leeuwen E. Covid-19 crisis in the Netherlands: “Only together we can control Corona”. Mind Soc 2021;20:201–7. 10.1007/s11299-020-00257-x [DOI] [Google Scholar]
- 5.NU.nl. Tijdlijn: Het coronavirus in Nederland: NU.nl, 2020. Available: https://www.nu.nl/coronavirus/6040831/tijdlijn-het-coronavirus-in-nederland.html [Accessed 10 Nov 2020].
- 6.Chou R, Dana T, Buckley DI, et al. Epidemiology of and risk factors for coronavirus infection in health care workers: a living rapid review. Ann Intern Med 2020;173:120–36. 10.7326/M20-1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah ASV, Wood R, Gribben C, et al. Risk of hospital admission with coronavirus disease 2019 in healthcare workers and their households: nationwide linkage cohort study. BMJ 2020;371:m3582. 10.1136/bmj.m3582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061–9. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen LH, Drew DA, Joshi AD, et al. Risk of COVID-19 among frontline healthcare workers and the general community: a prospective cohort study. medRxiv 2020. 10.1101/2020.04.29.20084111. [Epub ahead of print: 25 May 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pouwels KB, House T, Robotham JV, et al. Community prevalence of SARS-CoV-2 in England: results from the ONS coronavirus infection survey pilot. medRxiv 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shields A, Faustini SE, Perez-Toledo M, et al. SARS-CoV-2 seroprevalence and asymptomatic viral carriage in healthcare workers: a cross-sectional study. Thorax 2020;75:1089–94. 10.1136/thoraxjnl-2020-215414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization (WHO) . Report of the WHO-China joint mission on coronavirus disease 2019 (COVID-19), 2020. Available: https://www.who.int/publications/i/item/report-of-the-who-china-joint-mission-on-coronavirus-disease-2019-(covid-19) [Accessed 10 Nov 2020].
- 13.Ran L, Chen X, Wang Y, et al. Risk factors of healthcare workers with coronavirus disease 2019: a retrospective cohort study in a designated hospital of Wuhan in China. Clin Infect Dis 2020;71:2218–21. 10.1093/cid/ciaa287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steensels D, Oris E, Coninx L, et al. Hospital-Wide SARS-CoV-2 antibody screening in 3056 staff in a tertiary center in Belgium. JAMA 2020;324:195–7. 10.1001/jama.2020.11160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sikkema RS, Pas SD, Nieuwenhuijse DF, et al. COVID-19 in health-care workers in three hospitals in the South of the Netherlands: a cross-sectional study. Lancet Infect Dis 2020;20:1273–80. 10.1016/S1473-3099(20)30527-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rijksinstituut voor Volksgezondheid en Milieu (RIVM) . Report status of the validation of ELISA and autoanalyser antibody tests for SARS-CoV-2 diagnostics: considerations for use. In Press 2020.
- 17.Slot E, Hogema BM, Reusken CBEM, et al. Low SARS-CoV-2 seroprevalence in blood donors in the early COVID-19 epidemic in the Netherlands. Nat Commun 2020;11:5744. 10.1038/s41467-020-19481-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sikkens JJ, Buis DTP, Peters EJG, et al. Serologic surveillance and phylogenetic analysis of SARS-CoV-2 infection among hospital health care workers. JAMA Netw Open 2021;4:e2118554. 10.1001/jamanetworkopen.2021.18554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Venkatesan P. The changing demographics of COVID-19. Lancet Respir Med 2020;8:e95. 10.1016/S2213-2600(20)30461-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boehmer TK, DeVies J, Caruso E, et al. Changing Age Distribution of the COVID-19 Pandemic - United States, May-August 2020. MMWR Morb Mortal Wkly Rep 2020;69:1404–9. 10.15585/mmwr.mm6939e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salvatore PP, Sula E, Coyle JP, et al. Recent Increase in COVID-19 Cases Reported Among Adults Aged 18-22 Years - United States, May 31-September 5, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1419–24. 10.15585/mmwr.mm6939e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iversen K, Bundgaard H, Hasselbalch RB, et al. Risk of COVID-19 in health-care workers in Denmark: an observational cohort study. Lancet Infect Dis 2020;20:1401–8. 10.1016/S1473-3099(20)30589-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LUMC-COVID-19 Research Group, Chen Q, Toorop MMA, et al. Why crowding matters in the time of COVID-19 pandemic? - a lesson from the carnival effect on the 2017/2018 influenza epidemic in the Netherlands. BMC Public Health 2020;20:1516. 10.1186/s12889-020-09612-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Huang DQ, Zou B, et al. Epidemiology of COVID-19: a systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J Med Virol 2021;93:1449–58. 10.1002/jmv.26424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu C, Zhou M, Liu Y, et al. Characteristics of asymptomatic COVID-19 infection and progression: a multicenter, retrospective study. Virulence 2020;11:1006–14. 10.1080/21505594.2020.1802194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nikolai LA, Meyer CG, Kremsner PG, et al. Asymptomatic SARS coronavirus 2 infection: invisible yet invincible. Int J Infect Dis 2020;100:112–6. 10.1016/j.ijid.2020.08.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mei X, Zhang Y, Zhu H, et al. Observations about symptomatic and asymptomatic infections of 494 patients with COVID-19 in Shanghai, China. Am J Infect Control 2020;48:1045–50. 10.1016/j.ajic.2020.06.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Çelebi G, Pişkin N, Çelik Bekleviç A, et al. Specific risk factors for SARS-CoV-2 transmission among health care workers in a university hospital. Am J Infect Control 2020;48:1225–30. 10.1016/j.ajic.2020.07.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bielicki JA, Duval X, Gobat N, et al. Monitoring approaches for health-care workers during the COVID-19 pandemic. Lancet Infect Dis 2020;20:e261–7. 10.1016/S1473-3099(20)30458-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Self WH, Tenforde MW, Stubblefield WB, et al. Decline in SARS-CoV-2 Antibodies After Mild Infection Among Frontline Health Care Personnel in a Multistate Hospital Network - 12 States, April-August 2020. MMWR Morb Mortal Wkly Rep 2020;69:1762–6. 10.15585/mmwr.mm6947a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li K, Huang B, Wu M, et al. Dynamic changes in anti-SARS-CoV-2 antibodies during SARS-CoV-2 infection and recovery from COVID-19. Nat Commun 2020;11:6044. 10.1038/s41467-020-19943-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ibarrondo FJ, Fulcher JA, Goodman-Meza D, et al. Rapid decay of anti–SARS-CoV-2 antibodies in persons with mild Covid-19. N Engl J Med Overseas Ed 2020;383:1085–7. 10.1056/NEJMc2025179 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-051573supp001.pdf (153.1KB, pdf)
Data Availability Statement
Data are available on reasonable request. Data are available upon reasonable request. Proposals should be directed to the corresponding author.