Abstract
Purpose
Advanced stage clear cell renal cell carcinoma (ccRCC) involves a poor prognosis. Several studies have reported that dysfunctions in iron metabolism–related proteins may cause tumor progression and metastasis of this carcinoma. In this study, we investigated the impact of the expression of iron metabolism–related proteins on patient prognoses in advanced stage ccRCCs.
Materials and Methods
All of 143 advanced stage ccRCC specimens were selected following validation with double blind reviews. Several clinicopathological parameters including nuclear grade, perirenal fat invasion, renal sinus fat invasion, vascular invasion, necrosis, and sarcomatoid/rhabdoid differentiation were compared with the expression of ferroportin (FPN), and F-Box and leucine rich repeat protein 5 (FBXL5), by immunohistochemistry. FPN and FBXL5 mRNA level of ccRCC from The Cancer Genome Atlas database were also analyzed for validation.
Results
FPN and FBXL5 immunohistochemistry showed membrane and cytoplasmic expression, respectively. Based on the H-score, cases were classified as low or high expression with a cutoff value of 20 for FPN and 15 for FBXL5, respectively. Low expression of FPN and FBXL5 were significantly associated with patient death (p=0.022 and p=0.005, respectively). In survival analyses, low expression of FPN and FBXL5 were significantly associated with shorter overall survival (p=0.003 and p=0.004, respectively). On multivariate analysis, low expression of FBXL5 (hazard ratio, 2.001; p=0.034) was significantly associated with shorter overall survival.
Conclusion
FPN and FBXL5 can be used as potential prognostic markers and therapeutic targets for advanced stage ccRCC.
Keywords: Clear cell renal cell carcinoma, Ferroportin, F-Box and leucine rich repeat protein 5, Immunohistochemistry, Prognosis
Introduction
Renal cell carcinoma (RCC) is one of the most common malignancies worldwide, with approximately 400,000 new cases and 175,000 deaths in 2018 [1]. Several studies have changed the concept of RCC from a uniform entity to a spectrum of different histological subtypes with characteristic genetic and molecular features [2,3]. Among the subtypes of RCC, clear cell RCC is the most common subtype and accounts for 75% of all renal neoplasms [4]. Frequent inactivation of the VHL gene causes the overexpression of hypoxia-inducible factor (HIF)-1α and HIF-2α, and activates other oncogenes and downstream cascades, including vascular endothelial growth factor [5,6].
The prognosis of clear cell RCC varies, with more than 90% involving 5-year survival for early stage patients and 12% involving 5-year survival for patients with distant metastases [7]. Because of poor prognoses, several targeted agents and immunomodulatory agents have been used to treat advanced stage clear cell RCC patients. However, definite therapeutic strategies have not yet been established [7–9].
Iron is indispensable for many physiological processes in cells, such as DNA synthesis and mitochondrial oxidative metabolism. Several cancers have alterative pathways to maintain cellular iron balance [10]. Among several iron metabolism–related proteins, ferroportin (FPN) functions as an iron efflux pump, which is diminished in breast cancer, causing tumor growth and metastasis [11]. In addition, a recent study reported that the mechanism of hepatic carcinogenesis involves a deficiency of F-Box and leucine rich repeat protein 5 (FBXL5) [12]. Some studies have reported that FBXL5 and the iron regulatory protein 2 (IRP2) axis controls the expression of FPN in a mouse model [13,14].
Accumulated intracellular iron activates various intracellular signaling pathways [10]. Among these pathways, signal transducer and activator of transcription 3 (STAT3) is activated by Janus kinase 1 (JAK1) and crucial for the development and progression of clear cell RCC [15]. In addition, the excess intracellular iron accounts for the tumorigenesis in colorectal cancer via cyclin dependent kinase 1–JAK1–STAT3 pathway [16]. These findings may indicate the iron metabolism–related proteins as the potential therapeutic target.
Although a recent study validated 25 iron metabolism–related and methylated genes in clear cell RCC [17], studies of the prognostic impacts of FPN and FBXL5 have not yet been reported. In the present study, we therefore characterized the clinicopathological characteristics and prognostic impacts of FPN and FBXL5 expression in advanced stage clear cell RCC patients.
Materials and Methods
1. Patient selection
Tissue specimens from 950 consecutive clear cell RCC patients who underwent radical nephrectomy from 2006 to 2017 at Severance Hospital were used in this study. After the slide review by two independent pathologists (C.K. Park and N.H. Cho), cases with pT3 and pT4 according to pathological tumor, node and metastasis (TNM) staging based on the 8th American Joint Committee on Cancer (AJCC) criteria or those with distant metastases were classified as advanced stage RCC. A total of 143 advanced stage RCC cases were identified and finally selected for the study.
Several pathological factors, including tumor size, nuclear grade according to the World Health Organization (WHO)/International Society of Urologic Pathology (ISUP) grading system [18], sarcomatoid or rhabdoid differentiation, perirenal fat invasion, renal sinus fat invasion, vascular invasion including renal vein thrombi, and pathological TNM staging according to the 8th AJCC criteria were obtained from the slide review by two individual pathologists (C.K. Park and N.H. Cho). Various clinical factors, including age at surgery, sex, regimen of adjuvant treatment, and clinical follow-up data were obtained by medical record review. Relapse–free survival (RFS) was defined as the time from the date of the first curative operation to the date of the first loco-regional recurrence/distant metastasis or to the date of death without any type of relapse. Overall survival (OS) time was calculated from the date of curative resection to the date of the last follow-up or death from any cause.
2. Immunohistochemistry and evaluation
Four μm tissue sections from formalin fixed paraffin embedded blocks were used for immunohistochemistry (IHC). IHC was performed using the Ventana Benchmark XT automated staining system (Ventana Medical Systems, Tucson, AZ) as previously described [19]. Cell Conditioning 1 buffer (EDTA, pH 8.0, Ventana Medical Systems) was used for antigen retrieval. The sections were incubated with primary antibody for FPN (1:200, rabbit polyclonal, Novus Biologicals, Centennial, CO), FBXL5 (1:50, rabbit polyclonal, Abcam, Cambridge, UK), and STAT3 (1:300, mouse monoclonal, 124H6, Cell Signaling Technology, Danvers, MA).
The evaluations of IHC slides were performed by two independent pathologists. Expression of FPN, FBXL5, and STAT3 were evaluated based on the semi-quantitative H-score method with a total score range of 0–300 as previously described [20]. Using X-tile software [21], the cutoff value was optimized for FPN, FBXL5 and STAT3.
3. The Cancer Genome Atlas data
To investigate the relationship between mRNA expression of FPN and FBXL5 and various clinicopathological factors, public data from the Cancer Genome Atlas (TCGA) database were used. The clinical information and RNA sequencing data were obtained from TCGA database as previously described [17]. Detailed clinical and pathological characteristics of the TCGA and Severance Hospital cohort are presented in S1 Table.
4. Statistical analysis
Statistical calculations were performed using SPSS statistical software for Windows ver. 21.0 (IBM Corp., Armonk, NY). The chi-square test or Fisher exact test was used to study the relationship between IHC results or mRNA expression levels and various clinicopathological factors. For the comparison of patient age and tumor size, Student’s t test was used. RFS and OS were estimated by the Kaplan-Meier method using the log-rank test. Multivariate regression was analyzed using the Cox proportional hazards model. Significant comparisons refer to p-values of two-tailed tests < 0.05.
Results
1. FPN and FBXL5 IHC expression in clear cell RCCs
All 143 clear cell RCCs showed membrane expression of FPN with nonspecific nuclear staining. Only membrane expression was considered. The intensities and proportions of FPN expression were diverse. The H-score of FPN varied from 0 to 205 with a cutoff value of 20 optimized by X-tile software. Cases with H-score > 20 were assigned to high FPN expression and others were assigned to low FPN expression.
All clear cell RCCs showed cytoplasmic expression of FBXL5. Similar to FPN, the H-score of FBXL5 varied from 0 to 260 with a cutoff value of 15 optimized by X-tile software. Cases with H-score > 15 were assigned to high FBXL5 expression and others were assigned to low FBXL5 expression.
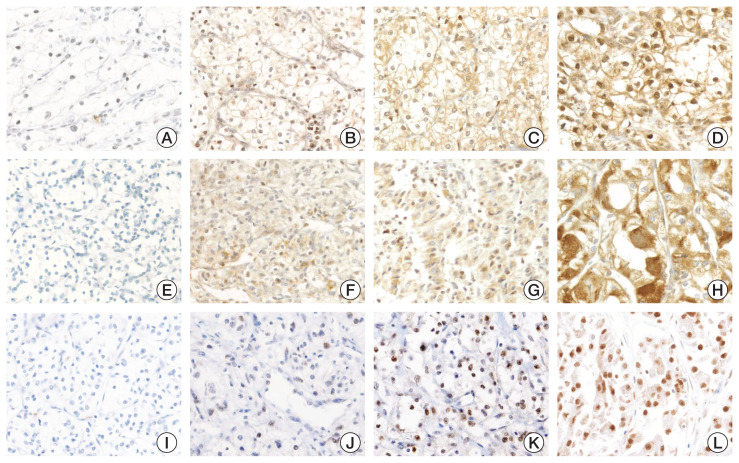
All clear cell RCCs showed nuclear expression of STAT3. The expression of STAT3 varied from 0 to 300 with a cut-off value of 70 optimized by X-tile software. Cases with H-score > 70 were assigned to high STAT3 expression and the others were assigned to low STAT3 expression. The representative cases and the distribution of H-score of each marker are presented as Fig. 1 and S2 Fig., respectively.
Fig. 1.
Representative cases of ferroportin (FPN), F-Box, and leucine rich repeat protein 5 (FBXL5) and signal transducer and activator of transcription 3 (STAT3) expression in clear cell renal cell carcinomas (×200). FPN showed membrane expression with nonspecific nuclear staining: (A) negative, (B) intensity 1+, (C) intensity 2+, and (D) intensity 3+. FBXL5 cytoplasmic expression: (E) negative, (F) intensity 1+, (G) intensity 2+, and (H) intensity 3+. STAT3 showed nuclear expression: (I) negative, (J) intensity 1+, (K) intensity 2+, and (L) intensity 3+.
When analyzing correlations between various clinicopathological factors and FPN expression, only patient survival was significantly associated with high FPN expression (p=0.022). For FBXL5, high expression was significantly associated with higher nuclear grade (p=0.006), high STAT3 expression (p=0.016), and patient survival (p=0.005). The detailed results of chi-square analyses are presented in Table 1.
Table 1.
Clinicopathological characteristics of 143 clear cell renal cell carcinoma cases according to FPN and FBXL5 expression status
| Variable | No. of cases (n=143) | FPN expression | FBXL5 expression | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| High (n=72) | Low (n=71) | p-value | High (n=58) | Low (n=85) | p-value | ||
| Age (yr) | 60.2±12.0 | 59.1±11.7 | 0.581 | 60.4±11.3 | 59.2±12.2 | 0.529 | |
|
| |||||||
| Tumor size (cm) | 9.12±4.00 | 9.06±3.42 | 0.930 | 9.18±3.65 | 9.03±3.76 | 0.804 | |
|
| |||||||
| Sex | |||||||
|
| |||||||
| Female | 40 | 23 (31.9) | 17 (23.9) | 0.287 | 16 (27.6) | 24 (28.2) | 0.932 |
|
| |||||||
| Male | 103 | 49 (68.1) | 54 (76.1) | 42 (72.4) | 61 (71.8) | ||
|
| |||||||
| Nuclear grade | |||||||
|
| |||||||
| 2 | 25 | 14 (19.4) | 11 (15.5) | 0.534 | 4 (6.9) | 21 (24.7) | 0.006 |
|
| |||||||
| 3 and 4 | 118 | 58 (80.6) | 60 (84.5) | 54 (93.1) | 64 (75.3) | ||
|
| |||||||
| Perirenal fat invasion | |||||||
|
| |||||||
| Absent | 67 | 30 (41.7) | 37 (52.1) | 0.211 | 28 (48.3) | 39 (45.9) | 0.778 |
|
| |||||||
| Present | 76 | 42 (58.3) | 34 (47.9) | 30 (51.7) | 46 (54.1) | ||
|
| |||||||
| Sinus fat invasion | |||||||
|
| |||||||
| Absent | 59 | 31 (43.1) | 28 (39.4) | 0.660 | 21 (36.2) | 38 (44.7) | 0.311 |
|
| |||||||
| Present | 84 | 41 (56.9) | 43 (60.6) | 37 (63.8) | 47 (55.3) | ||
|
| |||||||
| Vascular invasion | |||||||
|
| |||||||
| Absent | 80 | 44 (61.1) | 36 (50.7) | 0.210 | 28 (48.3) | 52 (61.2) | 0.127 |
|
| |||||||
| Present | 63 | 28 (38.9) | 35 (49.3) | 30 (51.7) | 33 (38.8) | ||
|
| |||||||
| Sarcomatoid/Rhabdoid differentiation | |||||||
|
| |||||||
| Absent | 128 | 66 (91.7) | 62 (87.3) | 0.397 | 53 (91.4) | 75 (88.2) | 0.547 |
|
| |||||||
| Present | 15 | 6 (8.3) | 9 (12.7) | 5 (8.6) | 10 (11.8) | ||
|
| |||||||
| Necrosis | |||||||
|
| |||||||
| Absent | 122 | 62 (86.1) | 60 (84.5) | 0.786 | 49 (84.5) | 73 (85.9) | 0.816 |
|
| |||||||
| Present | 21 | 10 (13.9) | 11 (15.5) | 9 (15.5) | 12 (14.1) | ||
|
| |||||||
| STAT3 expression | |||||||
|
| |||||||
| Low | 117 | 55 (76.4) | 62 (87.3) | 0.090 | 42 (72.4) | 75 (88.2) | 0.016 |
|
| |||||||
| High | 26 | 17 (23.6) | 9 (12.7) | 16 (27.6) | 10 (11.8) | ||
|
| |||||||
| Recurrence/Metastasis | |||||||
|
| |||||||
| Absent | 54 | 32 (44.4) | 22 (31.0) | 0.097 | 25 (43.1) | 29 (34.1) | 0.276 |
|
| |||||||
| Present | 89 | 40 (55.6) | 49 (69.0) | 33 (56.9) | 56 (65.9) | ||
|
| |||||||
| Survival | |||||||
|
| |||||||
| Alive | 86 | 50 (69.4) | 36 (50.7) | 0.022 | 43 (74.1) | 43 (50.6) | 0.005 |
|
| |||||||
| Expired | 57 | 22 (30.6) | 25 (49.3) | 15 (25.9) | 42 (49.4) | ||
Values are presented as mean±SD or number (%). FBXL5, F-Box and leucine rich repeat protein 5; FPN, ferroportin; STAT3, signal transducer and activator of transcription 3.
2. The mRNA expression levels of FPN and FBXL5 in clear cell RCC
Among 538 patients’ data obtained from TCGA database, 534 patients with available FPN and FBXL5 mRNA expression data were finally selected. All 534 patients were classified into two groups. Cases within the upper 50% of FPN and FBXL5 mRNA expression were assigned to high expression and the others were assigned to low expression.
Using chi-square analysis, low FPN mRNA expression was significantly associated with advanced pathological T stage (p=0.001), recurrence/metastasis (p=0.008), and patient death (p=0.002). In addition, low FPN mRNA expression showed a tendency toward higher WHO/ISUP nuclear grade (p=0.054). For FBXL5, low mRNA expression was significantly associated with male sex (p=0.001), higher WHO/ISUP nuclear grade (p=0.009), advanced pathological T stage (p < 0.001), recurrence/metastasis (p < 0.001), and patient death (p < 0.001). The detailed results of chi-square analysis are presented in S3 Table.
3. Prognostic impact of FPN and FBXL5 expression on clear cell RCC patients’ OS
In survival analysis of the Severance Hospital cohort, low expression of FPN and FBXL5 were significantly associated with shorter OS (p=0.003 and p=0.004, respectively) (Fig. 2A and B). In addition, cases with low expression of both FPN and FBXL5 showed significantly poorer OS than other cases (p < 0.001) (Fig. 2C). High STAT3 expression was significantly associated with shorter OS (p=0.007) (Fig. 2D).
Fig. 2.
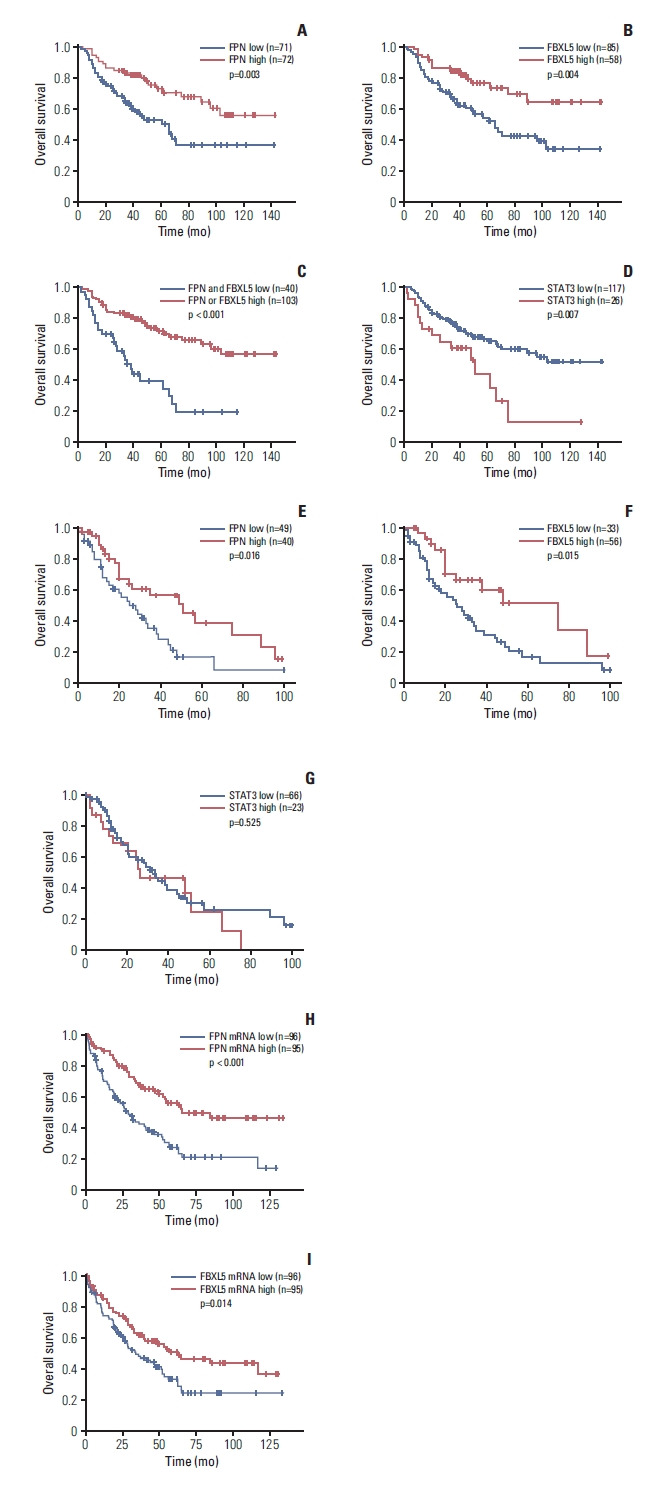
Overall survival (OS) of clear cell renal cell carcinoma patients according to ferroportin (FPN), F-Box, and leucine rich repeat protein 5 (FBXL5) and signal transducer and activator of transcription 3 (STAT3) expression. (A) In 143 clear cell renal cell carcinoma, low expression of FPN was significantly associated with shorter OS (p=0.003). (B) Similar to FPN, low FBXL5 expression was significantly associated with shorter OS (p=0.004). (C) Cases with low expression of both FPN and FBXL5 showed significantly shorter OS than other cases (p < 0.001). (D) High STAT3 expression showed significantly shorter OS (p=0.007). In patients with recurrence or metastasis, those with low FPN (E) and FBXL5 (F) expression showed significantly shorter OS (p=0.016 and p=0.015, respectively). (G) High STAT3 expression did not show significant differences in patients with recurrence or metastasis (p=0.525). (H) When analyzing 191 pT3 and pT4 patients of The Cancer Genome Atlas cohort, low mRNA expression level of FPN was significantly associated with shorter OS (p < 0.001). (I) Similar to FPN, the low mRNA expression level of FBXL5 was significantly associated with shorter OS (p=0.014).
When additionally estimating OS in cases with recurrence or metastasis, those with low expression of FPN and FBLX5 showed significantly shorter OS (p=0.016 and p=0.015, respectively) (Fig. 2E and F). However, high STAT3 expression did not show significant differences in OS (p=0.525) (Fig. 2G).
Using univariate analysis, tumor size > 9 cm (p=0.002), the presence of perirenal fat invasion (p=0.003), the presence of sarcomatoid/rhabdoid differentiation (p < 0.001), low FPN expression (p=0.004), low FBXL5 expression (p=0.005), high STAT3 expression (p=0.009) and the presence of recurrence/metastasis (p < 0.001) were significantly associated with shorter OS. Using multivariate analysis, low FBXL5 expression (p=0.034) and the presence of recurrence/metastasis (p < 0.001) were significantly associated with shorter OS. The presence of perirenal fat invasion (p=0.067), the presence of sarcomatoid/rhabdoid differentiation (p=0.057) and high STAT3 expression (p=0.080) showed a tendency toward shorter OS. The detailed results of univariate and multivariate analysis are presented in Table 2.
Table 2.
Univariate and multivariate analysis of 143 clear cell renal cell carcinoma cases on overall survival
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age (yr) | ||||
|
| ||||
| ≤ 60 | 1 | 0.546 | - | - |
|
| ||||
| > 60 | 0.851 (0.504–1.437) | - | ||
|
| ||||
| Sex | ||||
|
| ||||
| Female | 1 | 0.746 | - | - |
|
| ||||
| Male | 0.909 (0.509–1.622) | - | ||
|
| ||||
| Tumor size (cm) | ||||
|
| ||||
| ≤ 9 | 1 | 0.002 | 1 | 0.899 |
|
| ||||
| > 9 | 2.351 (1.381–4.004) | 1.040 (0.570–1.896) | ||
|
| ||||
| Nuclear grade | ||||
|
| ||||
| 2 | 1 | 0.184 | - | - |
|
| ||||
| 3 and 4 | 1.661 (0.786–3.513) | - | ||
|
| ||||
| Perirenal fat invasion | ||||
|
| ||||
| Absent | 1 | 0.003 | 1 | 0.067 |
|
| ||||
| Present | 2.385 (1.338–4.251) | 1.807 (0.959–3.408) | ||
|
| ||||
| Sinus fat invasion | ||||
|
| ||||
| Absent | 1 | 0.160 | - | - |
|
| ||||
| Present | 0.689 (0.409–1.159) | - | ||
|
| ||||
| Vascular invasion | ||||
|
| ||||
| Absent | 1 | 0.349 | - | - |
|
| ||||
| Present | 1.284 (0.761–2.167) | - | ||
|
| ||||
| Necrosis | ||||
|
| ||||
| Absent | 1 | 0.246 | - | - |
|
| ||||
| Present | 1.504 (0.754–3.000) | - | ||
|
| ||||
| Sarcomatoid/Rhabdoid differentiation | ||||
|
| ||||
| Absent | 1 | < 0.001 | 1 | 0.057 |
|
| ||||
| Present | 3.474 (1.784–6.762) | 1.986 (0.979–4.031) | ||
|
| ||||
| FPN expression | ||||
|
| ||||
| High | 1 | 0.004 | 1 | 0.101 |
|
| ||||
| Low | 2.192 (1.280–3.751) | 1.616 (0.910–2.868) | ||
|
| ||||
| FBXL5 expression | ||||
|
| ||||
| High | 1 | 0.005 | 1 | 0.034 |
|
| ||||
| Low | 2.323 (1.287–4.194) | 2.001 (1.053–3.801) | ||
|
| ||||
| STAT3 expression | ||||
|
| ||||
| Low | 1 | 0.009 | 1 | 0.080 |
|
| ||||
| High | 2.207 (1.217–4.002) | 1.775 (0.934–3.375) | ||
|
| ||||
| Recurrence/Metastasis | ||||
|
| ||||
| Absent | 1 | < 0.001 | 1 | < 0.001 |
|
| ||||
| Present | 9.541 (3.786–24.043) | 7.001 (2.667–18.377) | ||
CI, confidence interval; FBXL5, F-Box and leucine rich repeat protein 5; FPN, ferroportin; HR, hazard ratio; STAT3, signal transducer and activator of transcription 3.
In TCGA cohort, low mRNA expression of FPN and FBXL5 were significantly associated with shorter OS (all, p < 0.001) (S4A and S4B Fig.). When analyzing pT3 and pT4 cases of TCGA cohort, cases with low FPN and FBXL5 mRNA levels showed significantly shorter OS (p < 0.001 and p=0.014, respectively) (Fig. 2H and I). Using univariate analysis, age > 60 years (p < 0.001), WHO/ISUP nuclear grade 3 and 4 (p < 0.001), pathological stage T3 and T4 (p < 0.001), low FPN mRNA level (p < 0.001), low FBXL5 mRNA level (p < 0.001), and the presence of recurrence/metastasis (p < 0.001) were associated with shorter OS. Using multivariate analysis, pathological stage T3 (p=0.024), and low FBXL5 mRNA level (p=0.020) were significantly associated with shorter OS. The low FPN mRNA level showed a tendency toward shorter OS (p=0.085). The detailed results of univariate and multivariate analyses are presented as S5 Table.
4. Prognostic impact of FPN and FBXL5 expression on clear cell RCC patients’ RFS
In survival analysis of the Severance Hospital cohort, only high STAT3 expression showed significant correlation with shorter RFS (p < 0.001). The low FPN expression showed a tendency toward shorter RFS (p=0.055) (S6 Fig.).
Using univariate analysis, tumor size > 9 cm (p=0.001), the presence of perirenal fat invasion (p=0.016), the presence of sarcomatoid/rhabdoid differentiation (p=0.003) and high STAT3 expression (p < 0.001) were significantly associated with shorter RFS. Using multivariate analysis, tumor size > 9 cm (p=0.012), the presence of sarcomatoid/rhabdoid differentiation (p=0.002) and high STAT3 expression (p=0.004) were significantly associated with shorter RFS. The detailed results of univariate and multivariate analysis are presented in S7 Table.
In TCGA cohort, low mRNA expression of FPN and FBXL5 were significantly associated with shorter RFS (p=0.002 and p < 0.001, respectively) (S8A and S8B Fig.). When analyzing pT3 and pT4 cases of TCGA cohort, cases with low FPN mRNA levels showed significantly shorter RFS (p=0.006) (S8C Fig.). However, no significant difference was identified according to FBXL5 mRNA level (p=0.458) (S8D Fig.). Using univariate analysis, age > 60 years (p=0.028), WHO/ISUP nuclear grade 3 and 4 (p < 0.001), pathological stage T3 and T4 (p < 0.001), low FPN mRNA level (p=0.003), and low FBXL5 mRNA level (p < 0.001) were associated with shorter RFS. Using multivariate analysis, WHO/ISUP nuclear grades 3 and 4 (p < 0.001), pathological stage T3 and T4 (p < 0.001) and low FBXL5 mRNA level (p=0.006) were significantly associated with shorter RFS. The low FPN mRNA level showed a tendency toward shorter RFS (p=0.085). The detailed results of univariate and multivariate analyses are presented as S9 Table.
Discussion
Clear cell RCC is the most common tumor among all renal neoplasms; however, its prognoses are poor in patients with advanced stages. Several targeted agents have been used to treat this common but lethal subtype of RCC [7–9]. The maintenance of iron homeostasis is crucial because it is essential for several biological processes. Dysregulation of iron metabolism–related proteins causes tumor growth and metastasis [11,22]. Recent studies have shown the role of the FBXL5-IRP2 axis in the expression of FPN [13,14]. In the present study, we therefore characterized the expression of FPN and FBXL5 in advanced stage clear cell RCCs to elucidate their roles in tumor progression.
We investigated the expression of FPN and FBXL5 in advanced stage clear cell RCC patients and compared the differences with patients in TCGA cohort. Low expression of FPN and FBXL5 showed significantly shorter OS in advanced clear cell RCC patients, which is consistent with TCGA data analysis. In addition, when analyzing pT3 and pT4 cases of TCGA cohort, low FPN and FBXL5 mRNA expression were significantly associated with shorter OS. In our study cohort, FPN and FBXL5 showed variable expression in advanced stage clear cell RCCs with membrane and cytoplasmic expression, respectively.
Low expression of FBXL5 suppresses the degradation of IRP2, causing the overexpression of IRP2. IRP2 causes the degradation of FPN, causing intracytoplasmic accumulation of iron [13,14]. The excess intracytoplasmic iron promotes cancer development via the Fenton reaction by generating reactive oxygen species, hydroxide anion, and hydroxyl radicals [23]. A recent computational study reported that cytosolic and mitochondrial Fenton reactions occurred in 14 cancer types, including clear cell RCC. In the cytosol, to neutralize excess hydroxide anions, glycolysis and nucleotide synthesis increase, which promote cell division and proliferation to eliminate the excess glycolytic products and nucleotides. In addition, ATP is synthesized in mitochondria without oxygen consumption because hydrogen peroxide plays a role as an electron receiver instead of oxygen in the Fenton reaction [24].
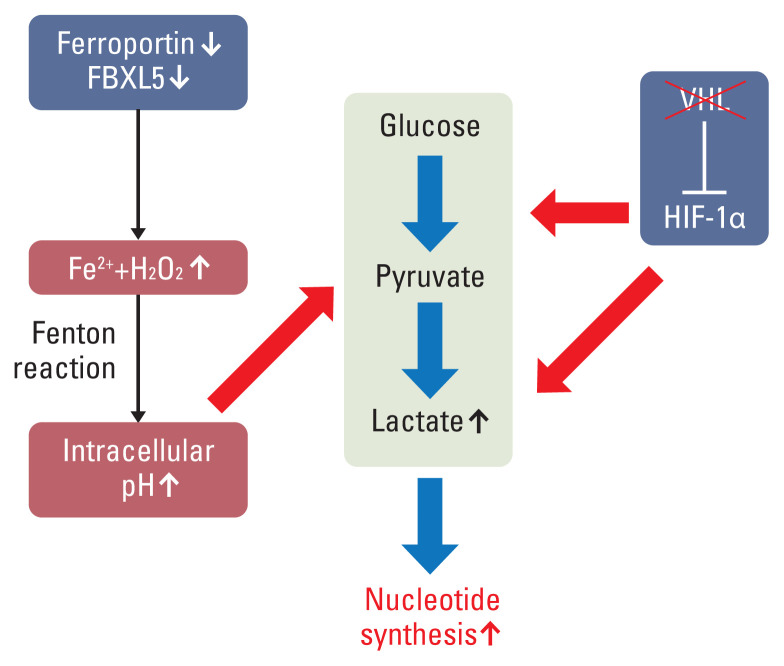
In our speculation, there may be a cross-talk between iron metabolism–related proteins and HIF in clear cell RCC. The inactivation of the VHL gene causes the stabilization and activation of HIF-1α and HIF-2α [5,25]. Especially, HIF-1α stimulates the expression of glycolysis-related genes, such as HK2, PFK, and LDHA [26]. In addition, excess cytosolic iron overload causes glycolysis and nucleotide synthesis via the Fenton reaction [24]. These two pathways, which promote glycolysis and lactate formation, and may induce tumor progression in clear cell RCC (Fig. 3).
Fig. 3.
Schematic diagram of cross-talk between iron-related metabolism and hypoxia-inducible factor 1α (HIF-1α) in clear cell renal cell carcinoma. The cytosolic excess iron and hydrogen peroxide trigger the Fenton reaction with generation of reactive oxygen species, hydroxyl radicals, and hydroxide anions. To maintain the cytosolic and mitochondrial pH, glycolysis, lactate formation, and subsequent nucleotide synthesis is promoted. Inactivation of VHL induces the activation of HIF-1α, which promotes the expression of glycolysis-related genes and LDHA. FBXL5, F-Box and leucine rich repeat protein 5.
Increased intracellular iron also promotes metastasis via downregulation of metastasis suppressor, N-myc, down-stream-regulated gene 1 (NDRG1), and NDRG2 in various types of cancers [27]. NDRG2 inhibits the epithelial-mesenchymal transition and metastasis by inactivating STAT3 and ERK1/2 via reduction of the level of the gp130 receptor [28]. In this regard, it is possible that the low expression of FPN and FBXL5 may be associated with high STAT3 expression. Thus, we performed STAT3 IHC to determine the correlation between iron metabolism–related proteins and STAT3. However, FPN and STAT3 expression showed positive correlations (Pearson’s correlation coefficient, 0.436; p < 0.001). In addition, the correlation between FBXL5 and STAT3 expression was not significant (Pearson’s correlation coefficient, 0.156; p=0.062) (S10 Table). These findings may be due to the upregulation of STAT3 in clear cell RCC [15] or interaction between FPN and FBXL5. Further studies are therefore necessary to investigate the cross-talk between iron metabolism–related proteins and STAT3 in clear cell RCC.
Several studies have reported the expression of iron metabolism–related proteins and their clinical significance in various types of cancers. A recent study reported that the deficiency of FBXL5 causes hepatic carcinogenesis [12]. The expression of FPN is lower in prostate and breast cancers. In addition, the expression level decreases as the tumor grade increases [11,29,30]. Furthermore, a low expression level of FPN in pancreatic cancer is significantly associated with poor patient outcomes [31]. The analysis results of TCGA cohort and our study cohort is consistent with those of previous studies, indicating that the disruption of iron metabolism–related proteins may trigger carcinogenesis and tumor progression. However, some cancers have shown different results. When compared with normal esophagus tissue, FPN is overexpressed in esophageal adenocarcinoma [32]. Thus, the correlation between mRNA or protein expression and patient prognosis may differ according to the subtype of cancer. Further studies are necessary to elucidate the different prognostic impacts of FPN and FBXL5 on patient survival in various cancer types.
Unlike TCGA cohort, only patient survival was significantly associated with high expression of FPN and FBXL5. This may be due to the different composition of the study cohort. Considering the composition of our study cohort, the low expression of FPN and FBXL5 may adversely affect the OS of advanced stage patients. These findings suggested that low expression of these two iron metabolism–related proteins were an independent prognostic predictor of patient survival in advanced clear cell RCC. In addition, our findings suggested the possibility of a FBXL5-IRP2 axis, and FPN as a potential therapeutic target for advanced stage clear cell RCC patients.
Despite being consistent with TCGA cohort, our study had a limitation because it was a retrospective study performed in a single institute with a cohort composed of advanced stage RCC patients. Thus, the correlation between the expression of iron metabolism–related proteins and various clinicopathological factors was not fully elucidated. In addition, the mechanism by which the low expression of FPN and FBXL5 affected patient survival is unclear. Further large series studies with multicenter cohorts are therefore necessary to overcome the limitations of our study.
In conclusion, we showed that low expression of FPN and FBXL5 were significantly associated with shorter OS and that low expression levels predicted the prognoses of RCC patients. Our findings suggested that FPN and FBXL5 can be used as prognostic markers and therapeutic targets for advanced stage clear cell RCC patients, although further studies are necessary to validate our results.
Acknowledgments
This study was supported by the Mid-Career Researcher Program through a grant from the National Research Foundation of Korea (No. 2019R1A2B5B01069934; CNH).
Footnotes
Ethical Statement
This study was approved by the Institutional Review Board of Severance Hospital, Seoul, Republic of Korea (4-2020-0946). Informed consent was obtained from the patients.
Author Contributions
Conceived and designed the analysis: Shin SJ, Cho NH.
Collected the data: Park CK, Heo J, Ham WS, Choi YD.
Contributed data or analysis tools: Park CK, Heo J, Ham WS, Choi YD.
Performed the analysis: Park CK.
Wrote the paper: Park CK, Cho NH.
Financial support: Cho NH.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Linehan WM, Ricketts CJ. The Cancer Genome Atlas of renal cell carcinoma: findings and clinical implications. Nat Rev Urol. 2019;16:539–52. doi: 10.1038/s41585-019-0211-5. [DOI] [PubMed] [Google Scholar]
- 3.Linehan WM, Schmidt LS, Crooks DR, Wei D, Srinivasan R, Lang M, et al. The metabolic basis of kidney cancer. Cancer Discov. 2019;9:1006–21. doi: 10.1158/2159-8290.CD-18-1354. [DOI] [PubMed] [Google Scholar]
- 4.Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO classification of tumours of the urinary system and male genital organs-part A: renal, penile, and testicular tumours. Eur Urol. 2016;70:93–105. doi: 10.1016/j.eururo.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 5.Shen C, Kaelin WG., Jr The VHL/HIF axis in clear cell renal carcinoma. Semin Cancer Biol. 2013;23:18–25. doi: 10.1016/j.semcancer.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qian CN, Huang D, Wondergem B, Teh BT. Complexity of tumor vasculature in clear cell renal cell carcinoma. Cancer. 2009;115:2282–9. doi: 10.1002/cncr.24238. [DOI] [PubMed] [Google Scholar]
- 7.Atkins MB, Tannir NM. Current and emerging therapies for first-line treatment of metastatic clear cell renal cell carcinoma. Cancer Treat Rev. 2018;70:127–37. doi: 10.1016/j.ctrv.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–24. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 9.Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–8. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Yu L, Ding J, Chen Y. Iron metabolism in cancer. Int J Mol Sci. 2018;20:95. doi: 10.3390/ijms20010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo W, Zhang S, Chen Y, Zhang D, Yuan L, Cong H, et al. An important role of the hepcidin-ferroportin signaling in affecting tumor growth and metastasis. Acta Biochim Biophys Sin (Shanghai) 2015;47:703–15. doi: 10.1093/abbs/gmv063. [DOI] [PubMed] [Google Scholar]
- 12.Muto Y, Moroishi T, Ichihara K, Nishiyama M, Shimizu H, Eguchi H, et al. Disruption of FBXL5-mediated cellular iron homeostasis promotes liver carcinogenesis. J Exp Med. 2019;216:950–65. doi: 10.1084/jem.20180900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moroishi T, Nishiyama M, Takeda Y, Iwai K, Nakayama KI. The FBXL5-IRP2 axis is integral to control of iron metabolism in vivo. Cell Metab. 2011;14:339–51. doi: 10.1016/j.cmet.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Liu X, You LH, Ci YZ, Chang S, Yu P, et al. Hepcidin and iron regulatory proteins coordinately regulate ferroportin 1 expression in the brain of mice. J Cell Physiol. 2019;234:7600–7. doi: 10.1002/jcp.27522. [DOI] [PubMed] [Google Scholar]
- 15.Robinson RL, Sharma A, Bai S, Heneidi S, Lee TJ, Kodeboyina SK, et al. Comparative STAT3-regulated gene expression profile in renal cell carcinoma subtypes. Front Oncol. 2019;9:72. doi: 10.3389/fonc.2019.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xue X, Ramakrishnan SK, Weisz K, Triner D, Xie L, Attili D, et al. Iron uptake via DMT1 integrates cell cycle with JAK-STAT3 signaling to promote colorectal tumorigenesis. Cell Metab. 2016;24:447–61. doi: 10.1016/j.cmet.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mou Y, Zhang Y, Wu J, Hu B, Zhang C, Duan C, et al. The landscape of iron metabolism-related and methylated genes in the prognosis prediction of clear cell renal cell carcinoma. Front Oncol. 2020;10:788. doi: 10.3389/fonc.2020.00788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delahunt B, Eble JN, Egevad L, Samaratunga H. Grading of renal cell carcinoma. Histopathology. 2019;74:4–17. doi: 10.1111/his.13735. [DOI] [PubMed] [Google Scholar]
- 19.Park CK, Shin SJ, Cho YA, Joo JW, Cho NH. HoxB13 expression in ductal type adenocarcinoma of prostate: clinicopathologic characteristics and its utility as potential diagnostic marker. Sci Rep. 2019;9:20205. doi: 10.1038/s41598-019-56657-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho YA, Chung JM, Ryu H, Kim EK, Cho BC, Yoon SO. Investigating Trk protein expression between oropharyngeal and non-oropharyngeal squamous cell carcinoma: clinical implications and possible roles of human papillomavirus infection. Cancer Res Treat. 2019;51:1052–63. doi: 10.4143/crt.2018.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio- informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252–9. doi: 10.1158/1078-0432.CCR-04-0713. [DOI] [PubMed] [Google Scholar]
- 22.Wu YY, Jiang JN, Fang XD, Ji FJ. STEAP1 regulates tumorigenesis and chemoresistance during peritoneal metastasis of Gastric Cancer. Front Physiol. 2018;9:1132. doi: 10.3389/fphys.2018.01132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imlay JA, Chin SM, Linn S. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science. 1988;240:640–2. doi: 10.1126/science.2834821. [DOI] [PubMed] [Google Scholar]
- 24.Sun H, Zhang C, Cao S, Sheng T, Dong N, Xu Y. Fenton reactions drive nucleotide and ATP syntheses in cancer. J Mol Cell Biol. 2018;10:448–59. doi: 10.1093/jmcb/mjy039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schodel J, Grampp S, Maher ER, Moch H, Ratcliffe PJ, Russo P, et al. Hypoxia, hypoxia-inducible transcription factors, and renal cancer. Eur Urol. 2016;69:646–57. doi: 10.1016/j.eururo.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC. Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol Cell Biol. 2003;23:9361–74. doi: 10.1128/MCB.23.24.9361-9374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menezes SV, Sahni S, Kovacevic Z, Richardson DR. Interplay of the iron-regulated metastasis suppressor NDRG1 with epidermal growth factor receptor (EGFR) and oncogenic signaling. J Biol Chem. 2017;292:12772–82. doi: 10.1074/jbc.R117.776393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen XL, Lei L, Hong LL, Ling ZQ. Potential role of NDRG2 in reprogramming cancer metabolism and epithelial-to-mesenchymal transition. Histol Histopathol. 2018;33:655–63. doi: 10.14670/HH-11-957. [DOI] [PubMed] [Google Scholar]
- 29.Xue D, Zhou CX, Shi YB, Lu H, He XZ. Decreased expression of ferroportin in prostate cancer. Oncol Lett. 2015;10:913–6. doi: 10.3892/ol.2015.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pinnix ZK, Miller LD, Wang W, D’Agostino R, Jr, Kute T, Willingham MC, et al. Ferroportin and iron regulation in breast cancer progression and prognosis. Sci Transl Med. 2010;2:43ra56. doi: 10.1126/scisignal.3001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toshiyama R, Konno M, Eguchi H, Asai A, Noda T, Koseki J, et al. Association of iron metabolic enzyme hepcidin expression levels with the prognosis of patients with pancreatic cancer. Oncol Lett. 2018;15:8125–33. doi: 10.3892/ol.2018.8357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boult J, Roberts K, Brookes MJ, Hughes S, Bury JP, Cross SS, et al. Overexpression of cellular iron import proteins is associated with malignant progression of esophageal adenocarcinoma. Clin Cancer Res. 2008;14:379–87. doi: 10.1158/1078-0432.CCR-07-1054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.