Abstract
Purpose
The efficacy of neoadjuvant chemotherapy for locally advanced breast cancer (LABC) is limited due to drug resistance and cardiotoxic effects. Preclinical studies have shown that statin induces apoptosis and decreases breast cancer cell growth. This study aims to evaluate the role of statin in combination with fluorouracil, adriamycin, and cyclophosphamide (FAC) therapy in LABC patients.
Materials and Methods
We undertook a randomized, double-blinded, placebo-controlled trial in two centers of Indonesia. Patients were randomly assigned to FAC plus simvastatin (40 mg/day orally) or FAC plus placebo (40 mg/day) for 21 days. The FAC regimen was repeated every 3 weeks. We evaluated the clinical response, pathological response, and toxicities.
Results
The objective response rate (ORR) for FAC plus simvastatin was 90% (95% confidence interval [CI], 0.99 to 1.67) by per-protocol analysis. No complete responses (CR) were recorded, but there were 48 partial responses. No significant difference was observed between the two groups with the ORR (p=0.103). The pathological CR rate was 6.25% (2 in simvastatin group and 1 in placebo group). Adverse events in both arms were generally mild, mainly consisted of myotoxicity. Human epidermal growth factor receptor 2 (HER2) expression was a factor related to the success of therapeutic response (odds ratio, 4.2; 95% CI, 1.121 to 15.731; p=0.033).
Conclusion
This study suggests that simvastatin combined with FAC shows improvements in ORR and pathological response in patients with LABC. Although no statistically significant difference was documented, there was a trend for better activity and tolerability. The addition of 40 mg simvastatin may improve the efficacy of FAC in LABC patients with HER2 overexpression.
Keywords: Neoadjuvant therapy, Breast neoplasms, FAC, Simvastatin
Introduction
Breast cancer is the most commonly occurring cancer with the highest cancer mortality among females worldwide. In developing countries, locally advanced breast cancer (LABC) is a common clinical presentation of breast cancer cases [1]. Among breast cancers, LABC leads to a major clinical challenge. Despite aggressive multimodality treatments, most of the LABC patients undergo relapse and eventually die. Currently, neoadjuvant chemotherapy (NAC) followed by surgery such as mastectomy or breast-conserving surgery is the treatment of choice for patients with LABC [2]. Administration of this systemic therapy prior to surgery has the potential to improve rates of breast-conserving surgery options, increase the chance of early measurement of response, and preferable outcomes for high-risk patients. Fischer et al. reported a NAC response of 80% with a complete clinical response of 36% [3]. Other study reported that doxorubicin combination regiments resulted in 50%–70% clinical response and 3%–16% pathological complete response (pCR) [4]. The pCR obtained after NAC is a surrogate marker for both overall prognosis and disease-free survival in LABC. Doxorubicin and its derivatives are one of the most widely used chemotherapeutics for NAC and the first-line chemotherapy. However, the efficacy of doxorubicin is limited due to the mechanism of drug resistance and cardiotoxic effects that can cause cardiomyopathy and heart failure. Efforts to improve the efficacy of doxorubicin include combinations with chemotherapy and targeted therapy such as anti–human epidermal growth factor receptor 2 (HER2) and bevacizumab. Nevertheless, the regimens are highly toxic, expensive, and not included in Indonesian National Formulary [5]. Statins are widely prescribed, and inexpensive cholesterol-lowering drugs, and is the established therapy for cardiovascular disease prevention and treatment, with a favorable safety profile. Statins’ mechanism of action is the inhibition of 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase, which has a role as the mevalonate pathway rate limiter [6]. Mevalonate is a precursor of farnesyl pyrophosphate and geranyl pyrophosphate, which produces essential substrates for post-translational modification of rat sarcoma viral oncogene homolog (RAS) and RAS homologue (RHO). These regulate the signal transduction of membrane receptors for the transcription of genes essential for cell angiogenesis, proliferation, migration, apoptosis, and differentiation [7]. Based on the role of statin on the post-translational modifications of RAS and RHO, in vitro studies and animal models have demonstrated that statins inhibit tumor growth and induce apoptosis in a number of tumor types such as colorectal cancer, small-cell lung cancer, and breast cancer [8–10]. Research by Garwood et al. confirmed that statin induces apoptosis and decreases tumor proliferation based on clinical preoperative “window-of-opportunity” trials on breast cancer [11]. Moreover, previous research by Yulian et al. [12] reported that statin synergistically interacted with anti-tumor effects (antiproliferative & apoptotic induction) and a non-overlapping toxicity profile. Therefore, we designed a phase II study of conventional fluorouracil, adriamycin, and cyclophosphamide (FAC) NAC plus low-dose simvastatin in LABC patients, to evaluate the role of statin in breast cancer, especially the combination with doxorubicin in LABC patients. This research hopes to improve the FAC’s NAC response and is safe for LABC patients.
Materials and Methods
1. Trial design and eligibility
We conducted a prospective, randomized, double-blinded, placebo-controlled study in two centers in Jakarta, Indonesia. The study protocol was approved by the ethics committee at the Faculty of Medicine, University of Indonesia. This study was registered in ClinicalTrials.gov under identifier NCT04418089. The eligibility criteria were as follows: female patients attending surgical oncology clinic of Dr. Cipto Mangunkusumo General Hospital and Koja District Hospital, with a confirmed diagnosis of LABC (stage IIIA–IIIC) prior to commencing 3 cycles of FAC neoadjuvant chemotherapy; ages ranging from 20–70 years old; normal function of major organs (cardiac, renal, and hepatic functions); and ≤ 2 Eastern Cooperative Oncology Group performance status score. Pregnant patients, lactating patients, and patients with a history of statin or chemotherapy treatments within 30 days were excluded. All patients were enrolled between January 2018 and June 2019. All procedures were double-blinded. Hence neither oncologists nor participants know which type of treatment was administered.
In the beginning, patients’ baseline characteristics were recorded. For the breast cancer molecular subtype, we used St. Gallen criteria for the classification as the following: Luminal A: estrogen receptor (ER) and/or progesterone receptor (PR) positive, HER2 negative, Ki-67 < 14%; Luminal B: (HER2 negative): ER and/or PR positive, HER2 negative, Ki-67 ≥ 14%; (HER2 positive): ER and/or PR positive, any Ki-67, HER2 positive; HER2 positive: ER and PR absent, HER2 positive; Triple-negative: ER and PR absent, HER2 negative.
Moreover, the HER2 positivity was evaluated through immunohistochemistry (IHC) assessment based on 2013 American Society for Clinical Oncology and College of American Pathologists Guideline as the following:
HER2 score 0 (negative): no staining is observed/incomplete membrane staining and barely perceptible within ≤ 10% of tumor cells.
HER2 score 1+ (negative): incomplete membrane staining and barely perceptible within ≥ 10% of tumor cells.
HER2 score 2+ (equivocal): incomplete/weak/moderate circumferential membrane staining within > 10% of tumor cells or complete/intense circumferential membrane staining within ≤ 10% of tumor cells.
The equivocal result in this study is followed by in situ hybridization (ISH) analysis using chromogenic ISH method. HER2/CEP17 ratio ≥ 2.0 with average HER2 copy number ≥ 4.0 signals/cell or < 4.0 signals/cell and HER2/CEP17 ratio < 2.0 with average HER2 copy number ≥ 6.0 signals/cell were considered as ISH positive. HER2/CEP17 ratio < 2.0 with average HER2 copy number ≥ 4.0 and < 6.0 signals/cell were considered ISH equivocal, hence need to be re-test with a new specimen if available. However, HER2/CEP17 ratio < 2.0 with average HER2 copy number < 4.0 signals/cell was considered ISH negative.
HER2 score 3+ (positive): complete/intense/> 10% of tumor cells with circumferential membrane staining.
TOP2A expression was detected from IHC staining as modified from the previous study by An et al. [13]. We considered nuclear staining (active isoform of TOP2A) in five microscopic fields at ×200 magnification and 100 tumor cells per field to assess the positive cells percentage. In our study, > 15% positive cells were regarded as positive expression.
Primary endpoints were objective response rate (ORR), and the secondary endpoint was pCR and tolerability of simvastatin.
2. Treatment protocol
Statin and placebo were provided in the outpatient setting. Patients were assigned to each treatment group (FAC plus simvastatin or placebo) using block randomization. Simvastatin 40 mg and placebo were provided in boxes of identical shape and were sequentially numbered. The chemotherapy regimen was conducted as sandwich NAC therapy method every 3 weeks according to the hospital protocol as follows: cyclophosphamide 500 mg/m2, doxorubicin 50 mg/m2, and 5-fluorouracil (5-FU) 500 mg/m2. The treatment cycles were repeated every 3 weeks. Simvastatin and matching placebos 40 mg/day were orally administered on day 1 until day 21 during the period of chemotherapy continuously. Treatment continued until another termination criteria was met, including unacceptable toxicity, consent withdrawal, or loss to follow-up. All researchers and patients were blinded to the statin and placebo therapy. Pretreatment examinations consisted of history taking, physical examination, complete blood cell (CBC) test and differential counts, blood chemistry, lipid profile, creatinine kinase (CK), chest X-ray, abdominal ultrasonography (USG), pelvic USG, and other indicated procedures. During treatment, history taking, physical examination, toxicity examination, chemistry test, and CBC were performed every 2 weeks prior to each cycle. Imaging examinations (chest X-ray, abdomen, and pelvis USG) were performed after 3 cycles to evaluate treatment response and when indicated.
3. Response and toxicity assessment
The initial evaluation was conducted 1 week before commencing the treatment for each patient. Examinations during treatment were performed every 2 weeks prior to each cycle. Tumor progression assessment by conducting the physical examination at baseline and after every 3 cycles. Abdominal USG, chest X-ray, and bone scan were conducted at the end of the third cycle. Treatment responses were classified based on the solid tumor response evaluation of World Health Organization (WHO)/Union for International Cancer Control (UICC) criteria [14]. Complete response (CR) is a complete disappearance of all known disease for minimally 4 weeks, partial response is a 50% or more of two perpendicular dimensions decrease in tumor size for at least four weeks, stable disease (SD) refers to no significant change for at least 4 weeks, estimated decrease of less than 50% or lesions with an estimated increase of less than 25% in the product of the tumor dimensions, progressive disease (PD) is estimated as an increase of 25% or more in current lesions and the appearance of any new lesions. Then, CR and partial response were grouped as responders and SD and PD as non-responders. ORR was calculated based on both CR+partial response. Pathological tumor response was evaluated according to Miller-Payne (MP) system. The response was evaluated based on the tumor cellularity reduction between biopsy and mastectomy specimens [1]. The system included: grade 1 (no change and no significant cell decrement), grade 2 (tumor cells minor loss [≥ 30%]), grade 3 (30%–90% of tumor cells eduction), and grade 4 (> 90% disappearance of tumor cells), and grade 5 (none of identified cancer cells and ductal carcinoma in situ might be detected). Partial pathological response includes grade 1–4, and pCR includes grade 5 [1]. Adverse events were assessed throughout the treatment and 14 days after the last treatment dose. Adverse events were graded using Common Terminology Criteria for Adverse Events (CTCAE) ver. 4.03.
4. Statistical analysis
The statistical analysis was conducted per protocol by using SPSS ver. 20.0 (IBM Corp., Armonk, NY). Descriptive analysis was used to summarize the patients’ baseline characteristics. The efficacy of simvastatin prior therapy was divided into two groups: responders and non-responders. The primary endpoint was the ORR, which was the ratio of the responders to the total patients assessed for tumor response. The ORR data were analyzed using the Pearson chi-square test. The secondary endpoints were pathological responses based on the MP system, which were then analyzed by using Fisher exact test. Adverse events were graded according to the CTCAE ver. 4.03. Bivariate analysis of clinicopathological variables and molecular biology of both subject’s groups with the therapeutic response was conducted using the continuity correction test and Fisher exact test. Then, a multivariate logistic regression analysis was performed to analyze the patient variables and therapy response associations. All p-values were two-sided. p < 0.05 was considered statistically significant.
Results
1. Patient characteristics
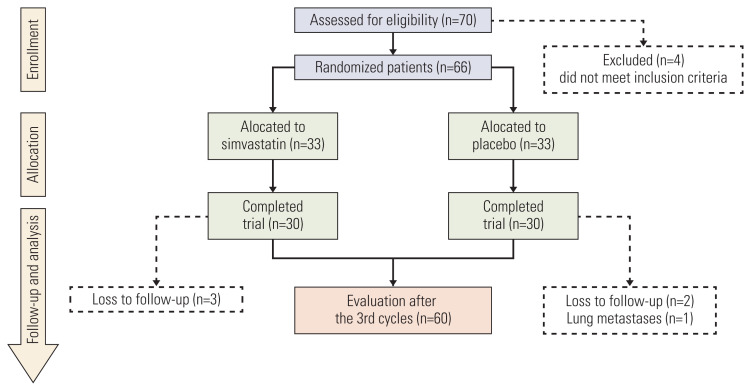
Between January 2018 and June 2019, 70 patients were assessed for eligibility. Four patients were excluded. Sixty-six LABC patients were randomly assigned and received treatment with either FAC-simvastatin (n=33) or FAC-placebo (n=33). Three patients in the FAC-simvastatin group were excluded due to loss to follow up, and three patients in the FAC-placebo group were ineligible because one was diagnosed with lung metastasis, and the other two were lost to follow up. The complete flow chart of patients recruitment in this study is provided in Fig. 1. All patients were assessable for efficacy and safety.
Fig. 1.
Consort flow chart of this study. From 70 eligible patients, 60 patients completed the trial and were evaluated in this study.
In terms of baseline characteristics, the two subjects groups were well balanced, as shown in Table 1. The median age was 46 years (range, 28 to 66 years), 53.3% of the patients were premenopausal, and 56.7% of the patients had a body mass index (BMI) ≤ 25.0. Sixty-three-point-three percent of the patients were in stage IIIB. Moreover, the grading demography of the tumors was different between the FAC-simvastatin group and the FAC-placebo group. The mean tumor size of patients in FAC-simvastatin and FAC-placebo group was 10.4 and 10.1, respectively. The majority of patients (76.7%) had invasive non-specific type carcinoma and had planned to do modified radical mastectomy (70%). Demographic baseline characteristics of immunohistochemistry, lipid profile, and molecular subtype were generally balanced between the two groups. The patient’s lipid profile was assessed before and after the neoadjuvant chemotherapy. Based on the lipid profile results, both groups have mean total cholesterol before the intervention of < 200 (simvastatin, 196.07 and placebo, 195.13), and mean low-density lipoprotein level in both groups was > 100 (simvastatin, 124.23 and placebo, 126.13). Hormonal receptors, ER/PR were positive in > 80% in both groups (simvastatin, 83.3% and placebo, 80%). HER2 expression amplification in placebo group was higher than in simvastatin group (43.3% and 20%, respectively), and the Ki-67 expression was > 14% higher in placebo groups than in simvastatin group (76.6% and 63.3, respectively). The molecular subtype that was commonly found in this study was the Luminal B subtype (simvastatin, 46.7% and placebo, 56.7 %) with the expression of Top2A > 15% (simvastatin, 36.7% and placebo, 53.3%). The surgical procedure mostly conducted in both groups was mastectomy (76.7% in simvastatin group and 43.3% in placebo group).
Table 1.
Baseline characteristics
| Variable | Simvastatin group (n=30) | Placebo group (n=30) | p-value |
|---|---|---|---|
| Age (yr) | |||
| Mean (range) | 49.4 (36–66) | 46.9 (28–65) | 0.293 |
| BMI (kg/m 2 ) | |||
| ≤ 25.0 | 17 (56.7) | 19 (63.3) | 0.598 |
| > 25.0 | 13 (43.3) | 11 (36.7) | |
| Menopausal status | |||
| Premenopausal | 16 (53.3) | 18 (60.0) | 0.602 |
| Postmenopausal | 14 (46.7) | 12 (40.0) | |
| Staging | |||
| IIIA | 11 (36.7) | 10 (33.3) | 0.592 |
| IIIB | 19 (63.3) | 19 (63.3) | |
| IIIC | 0 | 1 (3.3) | |
| Grade | |||
| Low | 13 (43.3) | 17 (56.7) | 0.302 |
| High | 17 (56.7) | 13 (43.3) | |
| Tumor size (cm) | |||
| Mean (range) | 10.4 (4–22) | 10.1 (2.6–24) | 0.737 |
| Histology | |||
| Invasive NST carcinoma | 24 (80.0) | 23 (76.7) | 0.838 |
| Invasive lobular carcinoma | 1 (3.3) | 2 (6.7) | |
| Others | 5 (16.7) | 5 (16.7) | |
| Lipid profile | |||
| LDL | |||
| Mean (range) | 124.23 (71–212) | 126.13 (79–276) | 0.824 |
| Cholesterol | |||
| Mean (range) | 196.07 (134–273) | 195.13 (138–266) | 0.908 |
| ER/PR status | |||
| Positive | 25 (83.3) | 24 (80.0) | 0.739 |
| Negative | 5 (16.7) | 6 (20.0) | |
| HER2 | |||
| Positive | 6 (20.0) | 13 (43.3) | 0.052 |
| Negative | 24 (80.0) | 17 (56.6) | |
| Ki-67 (%) | |||
| ≤ 14 | 19 (63.3) | 23 (76.7) | 0.259 |
| > 14 | 11 (36.7) | 7 (23.3) | |
| Molecular subtype | |||
| Luminal A | 11 (36.6) | 7 (23.4) | 0.418 |
| Luminal B | 14 (46.7) | 17 (56.7) | |
| Triple negative | 4 (13.3) | 2 (9.8) | |
| HER2 enriched | 1 (3.4) | 3 (10.0) | |
| TOP2A | |||
| Positive (> 15%) | 11 (36.7) | 16 (53.3) | 0.194 |
| Negative (≤ 15%) | 19 (63.3) | 14 (46.7) | |
| Type of surgery | |||
| MRM | 23 (76.7) | 13(43.3) | 0.028 |
| CRM | 3 (10.0) | 9 (30.0) | |
| Core biopsy | 4 (13.3) | 8 (26.7) | |
Values are presented as number (%) unless otherwise indicated. BMI, body mass index; CRM, classic radical mastectomy; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; LDL, low-density lipoprotein; MRM, modified radical mastectomy; NST, no special type; PR, progesterone receptor.
2. Treatment outcomes and efficacy
In total, three cycles of NAC were administered to each patient. From 70 patients enrolled in our study, 66 patients (97.6%) received at least 1 cycle of NAC. Sixty of 66 patients (90.9%) were assessed for response evaluation. No patient achieved complete clinical response; however, 48 patients showed partial clinical responses, which were listed in Table 2. In the FAC-Simv group, no patient had a complete response, 27 patients had a partial response, and the ORR was 90% (95% confidence interval [CI], 0.99 to 1.67). Similarly, no patient had a CR in the FAC-placebo group; 21 patients had a partial response, and the ORR was 70% (95% CI, 0.10 to 1.11). There was no significant difference between the two treatment groups concerning the clinical response assessment result (p=0.103). Detailed data is provided in Table 2.
Table 2.
Clinical response (WHO criteria)
| Response | FAC+simvastatin 40 mg (n=30) | FAC+placebo 40 mg (n=30) | p-value |
|---|---|---|---|
| Complete response (CR) | 0 | 0 | |
| Partial response (PR) | 27 (90.0) | 21 (70.0) | |
| Stable disease | 1 (3.3) | 6 (20.0) | |
| Progressive disease | 2 (6.6) | 3 (10.0) | |
| Overall response rate (CR+PR) | 27 (90.0) | 21 (70.0) | |
| 95% Confidential interval | 0.99–1.67 | 0.10–1.11 | 0.103 |
Values are presented as number (%). Odds ratio, 2.571 (range, 0.83 to 7.99); p=0.103. FAC, 5-fluorouracil, adriamycin, and cyclophosphamide; WHO, World Health Organization.
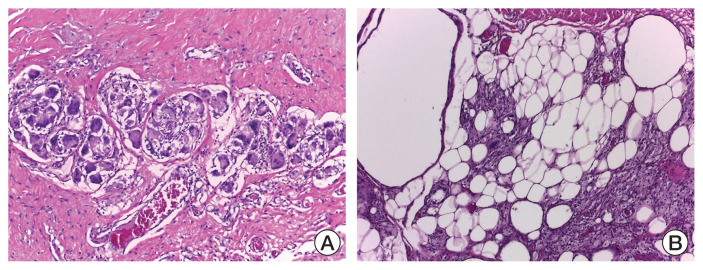
pCR, represented as grade 5 of MP criteria, was found in only three patients (6.25% of total samples), including two patients from simvastatin group and one patient from placebo group. Detailed data listed in Table 3. There was no significant difference in the pathological responses between both groups (p=0.330). The histopathology findings in pCRs in this study showed a picture of atypical cells, including foam and giant cells. Representative histological images of grade 5 MP system are provided in Fig. 2A and B.
Table 3.
Pathological response MP system
| Response | FAC+simvastatin (n=27) | FAC+placebo (n=21) | p-value |
|---|---|---|---|
| Complete response | 2 (4.2) | 1 (2.1) | |
| Partial response | 25 (52.1) | 20 (41.7) | 0.33 |
| Total | 27 | 21 |
Values are presented as number (%). FAC, 5-fluorouracil, adriamycin, and cyclophosphamide; MP, Miller-Payne.
Fig. 2.
Histological image representative of grade 5 Miller-Payne system as a pathological complete response. H&E staining was used in the histopathological evaluation: (A) atypic cells (×100) and (B) foam and giant cells (×100).
3. Safety and tolerability (adverse events)
The safety and tolerability profiles of both treatment arms were similar. Predefined adverse events of special interest in response to the statin listed in Table 4. The same number of patients completed three treatment cycles in both groups (30 patients in simvastatin group and 30 patients in placebo group). Common adverse events were mild to moderate, including nausea (96.7% in both groups), vomiting (86.7% in simvastatin group and 83.3% in placebo group), alopecia (76.7% both group), and fatigue (43.4% in simvastatin group and 46.7% in placebo group). Significant toxicity (grade 3 or 4) was presented as leucopenia (26.6% in simvastatin group and 20% placebo group). There was no significant difference in the incidence of serious adverse events between the two groups. However, a clinically significant increase in the incidence of leukopenia was found in the simvastatin group, compared to the placebo group (26.6% vs. 20%), respectively. Eight leukopenia (grade 3–4) cases were reported for simvastatin, and six cases were reported for placebo. Five patients in the two groups (2 in simvastatin group and 3 in placebo group) experienced treatment delays, from cycle 2 onwards. These were mostly caused by prolonged hematologic toxicities. Two patients experienced grade 1–2 CK elevation, which was considered to be related to the use of simvastatin. There were also patients with grade 1–2 elevated liver enzyme levels (4 patients with alanine aminotransferase [ALT] increment and 3 patients with aspartate aminotransferase [AST] increment). However, abnormal elevations of CK, ALT, and AST were eventually normalized with supportive management. There was no significant difference for simvastatin-specific adverse events between the two treatment groups. In this study, the incidence of myotoxicity that dominantly occurred was myalgia grade 1–2 (36.7%) and muscle cramps grade 1–2 (3.3%). Detailed data on adverse events were listed in Table 4. There were mild increases of CK in two patients (simvastatin groups), but no increase of CK and rhabdomyolysis due to the use of simvastatin was observed. There were no cases of elevated CK level ≥ five times of the upper limit or ALT ≥ three times of the upper limit. Hence, the administration of simvastatin did not cause a significant increase in treatment-related toxicities. FAC and simvastatin combination therapy was well tolerated throughout the study, as shown in Table 4. There were differences in the incidence of cardiac adverse events between the two treatment groups. In heart function toxicity, reductions in heart function were observed in 3.3% cases of simvastatin group and 10% of placebo group. Nevertheless, the reduction of left ventricle ejection fraction (LVEF) in this study did not exceed normal limits (LVEF < 55%).
Table 4.
Adverse event results based on the CTCAE ver. 4.03
| Toxicity | FAC+simvastatin 40 mg (n=30) | FAC+placebo 40 mg (n=30) | ||
|---|---|---|---|---|
|
|
|
|||
| Grade 1–2 | Grade 3–4 | Grade 1–2 | Grade 3–4 | |
| Hematological | ||||
|
| ||||
| Anemia | 10 (33.3) | 0 | 11 (36.6) | 1 (3.3) |
|
| ||||
| Leukopenia | 10 (33.3) | 8 (26.6) | 14 (46.6) | 6 (20.0) |
|
| ||||
| Thrombocytopenia | 3 (10.0) | 0 | 5 (16.0) | 0 |
|
| ||||
| Febrile neutropenia | 0 | 0 | 0 | 0 |
|
| ||||
| Non-hematological | ||||
|
| ||||
| Nausea | 29 (96.7) | 0 | 29 (96.7) | 0 |
|
| ||||
| Vomiting | 26 (86.7) | 0 | 25 (83.3) | 0 |
|
| ||||
| Diarrhea | 1 (3.3) | 0 | 1 (3.3) | 0 |
|
| ||||
| Constipation | 10 (33.3) | 0 | 7 (23.3) | 0 |
|
| ||||
| Mucositis | 3 (10.0) | 0 | 0 | 0 |
|
| ||||
| Fatigue | 13 (43.3) | 0 | 14 (46.7) | 0 |
|
| ||||
| Alopecia | 23 (76.7) | 0 | 23 (76.7) | 0 |
|
| ||||
| Peripheral neuropathy | 2 (6.7) | 0 | 2 (6.7) | 0 |
|
| ||||
| Cardiac function | ||||
|
| ||||
| Decrease EF | 1 (3.3) | 0 | 3 (10.0) | 0 |
|
| ||||
| Muscle toxicities | ||||
|
| ||||
| Myalgia | 11 (36.7) | 0 | 11 (36.7) | 0 |
|
| ||||
| Muscle cramp | 1 (3.3) | 0 | 2 (6.7) | 0 |
|
| ||||
| Rhabdomyolysis | 0 | 0 | 0 | 0 |
|
| ||||
| Statin toxicities | ||||
|
| ||||
| Elevated AST | 2 (6.7) | 0 | 1 (3.3) | 0 |
|
| ||||
| Elevated ALT | 2 (6.7) | 0 | 2 (6.7) | 0 |
|
| ||||
| Elevated CK | 2 (6.7) | 0 | 0 | 0 |
Values are presented as number (%). ALT, alanine aminotransferase; AST, aspartate aminotransferase; CK, creatinine phosphokinase; CTCAE, Common Terminology Criteria for Adverse Events; EF, ejection fraction; FAC, fluorouracil, adriamycin, and cyclophosphamide.
4. Analysis of clinicopathological variables influences
Univariate analysis between clinicopathological parameters, treatment variables including age, menopause status, BMI, staging, lipid profile, tumor grade, ER/PR status, HER2 status, Ki-67, and Top2A and treatment response was observed, and no significant correlation between variables and the treatment response was found (Table 5). A logistic regression model was utilized to analyze the influence of the clinicopathological variables on the treatment response in a multivariate analysis. The multivariate analysis yielded only one independent predictor of treatment response: HER2 (p=0.033) with odds ratio 4.2 (95% CI, 1.121 to 15.731). Patients with HER2 amplification showed a better response to FAC and simvastatin. Univariate and multivariate analyses of clinicopathological factors are summarized in Table 5.
Table 5.
Clinicopathological variables factors influenced response treatment of both groups of subjects
| Variable | Bivariate | Multivariate | ||
|---|---|---|---|---|
|
|
|
|||
| OR (95% CI) | p-value | Adjusted OR (95% CI) | p-value | |
| Age (< 50 yr vs. ≥ 50 yr) | 1.400 (0.394–4.979) | 0.650 | - | - |
|
| ||||
| Menopause status (preM vs. postM) | 0.543 (0.144–2.049) | 0.364 | - | - |
|
| ||||
| BMI (≤ 25 vs. > 25) | 4.231 (0.837–21.397) | 0.100 | 3.559 (0.602–21.032) | 0.161 |
|
| ||||
| Staging (operable vs. inoperable) | 1.645 (0.392–6.904) | 0.743 | - | - |
|
| ||||
| LDL (normal vs. high) | 3.000 (0.442–20.371) | 0.243 | 5.433 (0.621–47.540) | 0.126 |
|
| ||||
| Cholesterol (high vs. normal) | 0.657 (0.183–2.363) | 0.519 | - | - |
|
| ||||
| Grade (low vs. high) | 1.000 (0.282–3.544) | > 0.99 | - | - |
|
| ||||
| ER/PR status (positive vs. negative) | 0.306 (0.035–2.638) | 0.428 | - | - |
|
| ||||
| HER-2 (non-amplified vs. amplified) | 4.200 (1.121–15.731) | 0.026 | 2.753 (0.605–12.524) | 0.190 |
|
| ||||
| TNBC (yes vs. not) | 1.286 (1.115–1.483) | 0.333 | - | - |
|
| ||||
| Ki-67 (< 14% vs. ≥ 14%) | 3.270 (0.379–28.214) | 0.428 | - | - |
|
| ||||
| TOP II alpha (> 15% vs. ≤ 15%) | 0.510 (0.141–1.841) | 0.299 | - | - |
|
| ||||
| NACT (Plb+FAC vs. Simv+FAC) | 3.857 (0.927–16.048) | 0.053 | 3.826 (0.770–19.022) | 0.101 |
BMI, body mass index; CI, confidence interval; ER, estrogen receptor; FAC, fluorouracil, adriamycin, and cyclophosphamide; HER2, human epidermal growth factor receptor 2; LDL, low-density lipoprotein; NACT, neo-adjuvant chemotherapy; OR, odds ratio; Plb, placebo; PR, progesterone receptor; Simv, simvastatin; TNBC, triple-negative breast carcinoma.
Discussion
To the best of our knowledge, this study the first randomized, double-blind, placebo-controlled study to prove the anti-tumor activity efficacy and the safety of an HMG-CoA reductase inhibitor simvastatin (40 mg) combined with the cytotoxic chemotherapy regimen FAC in patients with LABC. We choose FAC because it is a widely used chemotherapy for NAC and the first-line chemotherapy based on National Formulary in Indonesia. HER2 blockage agents was not combined in this study because they have not been included in Indonesian National Formulary and may confound the adverse event analysis in this study. Indonesian national insurance only covers treatments listed in national formulary; hence we only prescribed FAC without any targeted therapy considering the low financial majority condition of our subjects. However, we also realize that this may cause undertreatment and affect the therapeutic response of our subjects. Moreover, we chose to conduct sandwich neo-adjuvant chemotherapy (NACT) using FAC regimen in this study. After finishing the first 3 cycles, the therapeutic responses of subjects were evaluated prior to mastectomy surgeries. This adhered to our hospital protocol and exp-lained why sandwich therapy was chosen instead of total preoperative therapy. Previous study by Pathak et al. [15] proved that the sandwich NACT has no difference from the total preoperative NACT in terms of overall survival, disease-free survival, and time to distant recurrence.
The results of this study demonstrated that statin usage led to a good response in LABC patients given NAC using the FAC regimen. The study yielded superior outcomes for FAC and simvastatin combination over FAC and placebo in terms of ORR. In this study, the clinical ORR in simvastatin group was 90% (95% CI, 0.99 to 1.67), while the ORR in placebo group was only 70% (95% CI, 0.10 to 1.11). Hence, the odds ratio was 2.571, although there was no significant difference between the two treatment groups concerning the clinical response assessment result (p=0.103) (Table 3). The precise molecular mechanism responsible for a better response in statin combination with chemotherapy is not completely clear. Based on the phenomenon observed in the present study, we found that increased response rate depicts the potential cytotoxic effect (apoptosis stimulation and proliferation inhibition) of the simvastatin combination therapy. Based on the currently available literature, we propose potential mechanisms that might explain how simvastatin enhances doxorubicin activity in terms of clinical and pathological response: (1) The chemotherapy induces apoptosis and cell cycle arrest by suppressing cell cycle and regulating the protein RAC1 signaling pathway resulting in decreased of activity of RAC1 in mRNA expression [16]. Then, a study by Sadeghi et al. [17] reported the combination of simvastatin and doxorubicin on HeLa cells (cervix) in longer period could induce apoptosis more than each drug alone through G and G2/M arrests. (2) Cholesterol is an important component for cell growth as well as for lipid rafts that replace the transduction of cellular signaling. Yun et al. [18] found a new pathway that shows doxorubicin inhibiting mevalonate conversion to cholesterol by decreasing Hmgcr downregulation. This leads to cellular cholesterol reduction and lipid raft redistribution that can change cells through the EGFR/Src/HMGCR pathway. (3) Werner et al. [19] reported that simvastatin and doxorubicin-induced apoptosis on human rhabdomyosarcoma cells by enhancing caspase 3 and 9 activity synergistically, translocating from the cytosol into the nucleus, and translocation of Bax from the cytosol into mitochondria. Moreover, lovastatin is able to increase the tumor cells’ vulnerability to chemotherapeutic agents by specifically targeting the tumor cells which express drug-resistant P-glycoprotein. (4) Werner et al. [20] described the intracellular compartmentalization of simvastatin induction on the topoisomerase II nuclear doxorubicin inhibition in human rhabdomyosarcoma cells. It is a new therapeutic concept in overcoming multidrug resistance mediated by ABCB1 through direct inhibition and regulation of ABCB1, enhancement of intracellular doxorubicin concentrations, which might be translated into augmented topoisomerase II inhibition and strengthened further by the replacement of double-stranded DNA. Moreover, statin might also enhance 5-FU chemotherapeutic agent in cancer treatment. Cerivastatin can inhibit nuclear factor-κB DNA-binding activity in chemoresistant colorectal cancer cell lines to increase the cytotoxicity of 5-FU [6]. Moreover, a study by Osman et al. [21] reported the increment of cytotoxic and apoptotic effect of 5-FU combined with simvastatin through the increment of arrested cells in Sub G1. This could be explained by the ability of simvastatin in the modulation of p21 (cip/Waf1) and survivin, including also p53 phosphorylation and acetylation [21].
In our study, there was no patient who achieved a clinical complete response which is not consistent with most of the published reports. There were 48 patients (80%) who showed a partial clinical response, where three (6.25%) of them showed a complete pathological response, and the other 45 patients (93.75%) had residual disease histologically. In the simvastatin group, pCR was achieved in 4.16% of patients. However, different from our study design, they considered pCR based on the histopathology of the operative specimen if there was no residual invasive tumor [6]. Results from other previous study showed pCR was achieved in 20% of all patients after no special type, but pCR rate was largely depended on the breast cancer subtype and stage [22]. The possible reasons are insufficient chemotherapeutic cycles and severe local symptoms, making it difficult to achieve pCR from chemotherapy. In this study, the pathological response of residual invasive tumor in breast tissue according to MP criteria (grade 5 GMP) was observed in only three patients (6.25%) while the clinical response was obtained in 80% of patients; which consisted of two patients in the simvastatin group and one patient in the placebo group (Table 4). Pathological response was lower than in the previous studies (26%) in a study by Rastogi et al. [23]. There were differences in clinical response and pathological response due to maximum tumor diameter being measured on physical examination using calipers, and not using imaging modalities. Evaluations of the clinical response using imaging modalities are recommended, such as computed tomography scan, ultrasound, and magnetic resonance imaging (MRI) before and after treatment. Nevertheless, none of MRI examinations conducted were used for the assessment of NACT. From our perspective, this imaging technique is expensive to conduct. Furthermore, we choose to use WHO criteria to evaluate the tumor response due to this physical examination tumor size measurement. Moreover, WHO criteria have been validated and widely used in prospective randomized clinical trials.
In our study, we found three cases with partial clinical responses but had a complete pathological response. This finding is supported by previous studies suggesting that clinical responses are not correlated to pathological responses. Hence, to assess the therapeutic response, pathological examination on specimens obtained from mastectomy is crucial [24]. There still might be residual tumor which could be palpated in patients who achieve a complete pathological response. The whole tumor cell loss does not always correlate to the size of the tumor. Destroyed tumor cells can still be palpable due to fibrous stromal tissue. Changes in the stroma of specimens after chemotherapy, such as fibromyxoid, stromal fibrosis, microcalcification, and fibroelastotic stroma might cause the bias in clinical response evaluation. Other explanation for the palpable masses in patients with pCR are the general patterns of NAC response. These patterns of a scattergun/honeycomb response or a concentric shrinkage, where the residual carcinoma may present as multiple, scattered foci over an ill-defined tumor bed [25]. The response to NAC is a prognostic factor. The complete pathological response is a marker of increased progression-free survival and increased overall survival [23].
There were no side effects during the study, which required aggressive treatment in the hospital. In this study, a grade 1–2 decrease in hemoglobin (Hb) was obtained > 30% in both groups (simvastatin, 33.3% and placebo, 36.6%), but a grade 3–4 decrease in Hb was only obtained in one case (3.3%) in the placebo group. The grade 1–2 decrease in white blood cell (WBC) (simvastatin 23.3% and placebo 43.3%) was higher in the placebo group; however, greater grade 3–4 decreases in WBC was observed in the simvastatin group than in the placebo group (43.3% and 16.6%). The results were not found to represent the incidence of fever neutropenia in both groups. There were 14 patients who received stimulation factors but five patients were rescheduled for chemotherapy (simvastatin, 2 and placebo, 3). The most common side effects of using simvastatin involved muscle tissue with severity ranging from myalgia to severe rhabdomyolysis. Based on the research of Parkin et al. [26] the incidence of rhabdomyolysis was found in 0.1% in patients with simvastatin consumption. In this study, we found no significant differences in side effects from patients using simvastatin and placebo. The incidence of muscle toxicity that predominantly occurred was grade 1–2 myalgia in 36.7% of both groups, followed by grade 1–2 muscle cramps in 3.3% of the cases in the simvastatin group and 6.7% in the placebo group. The occurrence of side effects on muscles is known to be due to apoptotic events [27]. There was no significant increase in CK levels and the incidence of rhabdomyolysis due to the use of simvastatin, and although there was an increase in CK levels in two patients in the simvastatin group (6.7%), the increase was not more than twice the initial baseline level. Myalgia is the most common side effect due to statin use, with an incidence of around 5%. Doxorubicin is one of the most widely used anticancer drugs with high cardiotoxicity. Preservation of cardiomyocyte function during doxorubicin chemotherapy might help to maintain cardiac function and reduce the development of congestive heart failure. In this study, the two intervention groups (simvastatin and placebo) did not provide a significant change in the fraction-ejection values which might be due to the short follow-up period of the study and the limited measurements of cardiac dysfunction. Ejection fraction reduction in this study was found to be as much as 3.3% in the simvastatin group and 6,1% in the symptomless placebo group. Consistent with the results of the study by Riad et al. [28], fluvastatin pretreatment attenuated doxorubicin-induced cardiomyopathy through antioxidative enzyme mitochondrial superoxide dismutase 2 expression enhancement, oxidative stress reduction, and cardiac inflammation reduction shown by decreased tumor necrosis factor-α expression. In research by Seicean et al. [29], where cardiomyocyte cell cultures are treated with anthracycline, statins reduced oxidative stress by inhibiting the production of RAC1 synthesis and other small G proteins. This is also consistent with a study by Bobrowski et al. [30] which states that statin usage after anthracycline and trastuzumab chemotherapy in early-stage breast cancer is correlated with a lower risk of heart failure that requires hospital care.
There were not any multiple clinicopathological factors and molecular biology that had significant correlations with the response therapy of FAC and simvastatin in this study (Table 5). On the other hand, the cell lines in breast cancer that were the most sensitive in statin therapy were ones with aggressive groups such as ER-negative, HER2-positive, or triple-negative [31]. Moreover, based on a previous study by Yulian et al. [12], it is recommended to use simvastatin as metastases prevention therapy in patients with high cholesterol level, receptor ER/PR negative, and HER2 positive. TOP-2A expression was one of a potential factor that play a crucial role in chromosome instability and tumorigenesis which also as a direct target of anthracycline in causing DNA damage. However, the TOP2A expression also resulted in insignificant correlation to chemotherapy response in this study.
The main strengths of this clinical trial are the randomized blind, double-blind methodology, and a well-tolerated placebo control to be the first clinical study in LABC patients. This study also met the safety and applicability criteria. The present study had limitations, including firstly, a relatively low sample size (n=30) treated with statins—secondly, changes in insurance policies for the sample patients in the hospital. As a result, we do not provide anti-Her2 neu (trastuzumab) therapy in patients with positive HER2, which was positively associated with the increased clinical response when given simvastatin. Lastly, the evaluation of the NAC response in this study was carried out after 3 cycles (sandwich) and not completing up to 6 cycles (total preoperative). In the end, although it was not statistically significant, patients treated with FAC and simvastatin combination showed a trend of improved ORR and pCR compared with patients in placebo group. Although the sample size might be too small, these clinical observations may support the preclinical evidence of the effective apoptotic stimulation and proliferative activity inhibition of statins in LABC. Besides, simvastatin possesses both anticancer potential and can work as a cardioprotective agent.
Moreover, the combination treatment of FAC and simvastatin was generally well tolerated. The adverse events observed were similar to those reported in the FAC and placebo arm. Another consideration for future treatment plans might be reducing the dose of doxorubicin to reduce toxicity without reducing the cytotoxic effectiveness.
The results of the present study suggest that the addition of simvastatin to FAC may improve the ORR and pathological response in patients with LABC, although it has not been proven statistically. HER2 status is a clinicopathological factor related to the clinical response. This combination possesses both anticancer and cardioprotective actions and can be tolerated without any side effects on the patient.
Acknowledgments
We thank all participating patients and their families, the research nurses, study coordinators, trainees of surgical oncology, and all research assistants (dr. Nisa, dr. Syarifah, dr. Hanifah, dr. Rizky, dr. Nadya, dr. Kevin). Other medical staffs, and doctors, particularly Yuli at the Oncology surgery clinic. We also thank dr. Farida Falaivi, a pathologist from Koja Hospital. We also thank Dr. Cipto Mangunkusumo General Hospital for the grant support.
This study was supported by a grant from Dr. Cipto Mangunkusumo General Hospital.
Footnotes
Ethical Statement
The study had been approved by the Medical Faculty of Universitas Indonesia Scientific Research Ethics Committee (Jakarta, Indonesia; Number: 10, Date: November 26, 2018) and performed in accordance with the Declaration of Helsinki ethical standards or any comparable ethical standards. Besides, this study adhered to CONSORT guidelines. All eligible patients had signed the informed consents.
Author Contributions
Conceived and designed the analysis: Yulian ED, Siregar NC, Bajuadji.
Collected the data: Yulian ED, Bajuadji.
Contributed data or analysis tools: Yulian ED, Siregar NC.
Performed the analysis: Yulian ED, Siregar NC.
Wrote the paper: Yulian ED, Siregar NC, Bajuadji.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.
References
- 1.Rahman MS, Akhter PS, Hasanuzzaman M, Rahman J, Bhattacharjee A, Rassell M, et al. Outcome of neoadjuvant chemotherapy in locally advanced breast cancer: a tertiary care center experience. Bangladesh Med J. 2017;45:141–6. [Google Scholar]
- 2.Bonadonna G. Evolving concepts in the systemic adjuvant treatment of breast cancer. Cancer Res. 1992;52:2127–37. [PubMed] [Google Scholar]
- 3.Fisher ER, Wang J, Bryant J, Fisher B, Mamounas E, Wolmark N. Pathobiology of preoperative chemotherapy: findings from the National Surgical Adjuvant Breast and Bowel (NSABP) protocol B-18. Cancer. 2002;95:681–95. doi: 10.1002/cncr.10741. [DOI] [PubMed] [Google Scholar]
- 4.Bolan PJ, Wey A, Eberly LE, Nelson MT, Haddad TC, Yee D, et al. Assessing prognosis and therapy response in primary systemic therapy of breast cancer with magnetic resonance spectroscopy. Cancer Res. 2012;72:P1-14-11. [Google Scholar]
- 5.Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–34. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 6.Altwairgi AK. Statins are potential anticancerous agents (review) Oncol Rep. 2015;33:1019–39. doi: 10.3892/or.2015.3741. [DOI] [PubMed] [Google Scholar]
- 7.Hindler K, Cleeland CS, Rivera E, Collard CD. The role of statins in cancer therapy. Oncologist. 2006;11:306–15. doi: 10.1634/theoncologist.11-3-306. [DOI] [PubMed] [Google Scholar]
- 8.Jang HJ, Hong EM, Park SW, Byun HW, Koh DH, Choi MH, et al. Statin induces apoptosis of human colon cancer cells and downregulation of insulin-like growth factor 1 receptor via proapoptotic ERK activation. Oncol Lett. 2016;12:250–6. doi: 10.3892/ol.2016.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khanzada UK, Pardo OE, Meier C, Downward J, Seckl MJ, Arcaro A. Potent inhibition of small-cell lung cancer cell growth by simvastatin reveals selective functions of Ras isoforms in growth factor signalling. Oncogene. 2006;25:877–87. doi: 10.1038/sj.onc.1209117. [DOI] [PubMed] [Google Scholar]
- 10.Kozar K, Kaminski R, Legat M, Kopec M, Nowis D, Skierski JS, et al. Cerivastatin demonstrates enhanced antitumor activity against human breast cancer cell lines when used in combination with doxorubicin or cisplatin. Int J Oncol. 2004;24:1149–57. [PubMed] [Google Scholar]
- 11.Garwood ER, Kumar AS, Baehner FL, Moore DH, Au A, Hylton N, et al. Fluvastatin reduces proliferation and increases apoptosis in women with high grade breast cancer. Breast Cancer Res Treat. 2010;119:137–44. doi: 10.1007/s10549-009-0507-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yulian ED, Ramli M, Setiabudy R, Siregar NC, Bustami A, Dosan R. The role of simvastatin in inhibiting migration and proliferation of breast cancer cells through Rho/ROCK signaling pathway. J Cancer Ther Res. 2016;5:10. [Google Scholar]
- 13.An X, Xu F, Luo R, Zheng Q, Lu J, Yang Y, et al. The prognostic significance of topoisomerase II alpha protein in early stage luminal breast cancer. BMC Cancer. 2018;18:331. doi: 10.1186/s12885-018-4170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bosco EE, Mulloy JC, Zheng Y. Rac1 GTPase: a “Rac” of all trades. Cell Mol Life Sci. 2009;66:370–4. doi: 10.1007/s00018-008-8552-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pathak M, Deo SV, Dwivedi SN, Sreenivas V, Thakur B. Total preoperative NACT vs sandwich NACT in breast cancer patients: systematic review and meta-analysis. Eur J Cancer. 2017;72:S43. [Google Scholar]
- 16.Yoshida T, Zhang Y, Rivera Rosado LA, Chen J, Khan T, Moon SY, et al. Blockade of Rac1 activity induces G1 cell cycle arrest or apoptosis in breast cancer cells through downregulation of cyclin D1, survivin, and X-linked inhibitor of apoptosis protein. Mol Cancer Ther. 2010;9:1657–68. doi: 10.1158/1535-7163.MCT-09-0906. [DOI] [PubMed] [Google Scholar]
- 17.Sadeghi-Aliabadi H, Minaiyan M, Dabestan A. Cytotoxic evaluation of doxorubicin in combination with simvastatin against human cancer cells. Res Pharm Sci. 2010;5:127–33. [PMC free article] [PubMed] [Google Scholar]
- 18.Yun UJ, Lee JH, Shim J, Yoon K, Goh SH, Yi EH, et al. Anti-cancer effect of doxorubicin is mediated by downregulation of HMG-Co A reductase via inhibition of EGFR/Src pathway. Lab Invest. 2019;99:1157–72. doi: 10.1038/s41374-019-0193-1. [DOI] [PubMed] [Google Scholar]
- 19.Werner M, Sacher J, Hohenegger M. Mutual amplification of apoptosis by statin-induced mitochondrial stress and doxorubicin toxicity in human rhabdomyosarcoma cells. Br J Pharmacol. 2004;143:715–24. doi: 10.1038/sj.bjp.0705928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Werner M, Atil B, Sieczkowski E, Chiba P, Hohenegger M. Simvastatin-induced compartmentalisation of doxorubicin sharpens up nuclear topoisomerase II inhibition in human rhabdomyosarcoma cells. Naunyn Schmiedebergs Arch Pharmacol. 2013;386:605–17. doi: 10.1007/s00210-013-0859-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osman AM, Al-Johani HS, Kamel FO, Ahmed OA, Huwait EA, Sayed-Ahmed M. 5-Fluorouracil and simvastatin loaded solid lipid nanoparticles for effective treatment of colorectal cancer cells. Int J Pharmacol. 2020;16:205–13. [Google Scholar]
- 22.Houssami N, Macaskill P, von Minckwitz G, Marinovich ML, Mamounas E. Meta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. Eur J Cancer. 2012;48:3342–54. doi: 10.1016/j.ejca.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 23.Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26:778–85. doi: 10.1200/JCO.2007.15.0235. [DOI] [PubMed] [Google Scholar]
- 24.Marchio C, Sapino A. The pathologic complete response open question in primary therapy. J Natl Cancer Inst Monogr. 2011;2011:86–90. doi: 10.1093/jncimonographs/lgr025. [DOI] [PubMed] [Google Scholar]
- 25.Sahoo S, Lester SC. Pathology of breast carcinomas after neoadjuvant chemotherapy: an overview with recommendations on specimen processing and reporting. Arch Pathol Lab Med. 2009;133:633–42. doi: 10.5858/133.4.633. [DOI] [PubMed] [Google Scholar]
- 26.Parkin L, Paul C, Herbison GP. Simvastatin dose and risk of rhabdomyolysis: nested case-control study based on national health and drug dispensing data. Int J Cardiol. 2014;174:83–9. doi: 10.1016/j.ijcard.2014.03.150. [DOI] [PubMed] [Google Scholar]
- 27.Corsini A, Bellosta S, Baetta R, Fumagalli R, Paoletti R, Bernini F. New insights into the pharmacodynamic and pharmacokinetic properties of statins. Pharmacol Ther. 1999;84:413–28. doi: 10.1016/s0163-7258(99)00045-5. [DOI] [PubMed] [Google Scholar]
- 28.Riad A, Bien S, Westermann D, Becher PM, Loya K, Landmesser U, et al. Pretreatment with statin attenuates the cardiotoxicity of doxorubicin in mice. Cancer Res. 2009;69:695–9. doi: 10.1158/0008-5472.CAN-08-3076. [DOI] [PubMed] [Google Scholar]
- 29.Seicean S, Seicean A, Plana JC, Budd GT, Marwick TH. Effect of statin therapy on the risk for incident heart failure in patients with breast cancer receiving anthracycline chemotherapy: an observational clinical cohort study. J Am Coll Cardiol. 2012;60:2384–90. doi: 10.1016/j.jacc.2012.07.067. [DOI] [PubMed] [Google Scholar]
- 30.Bobrowski D, Zhou L, Austin P, Arguelles OC, Amir E, Lee D, et al. Statins are associated with lower risk of heart failure after anthracycline and trastuzumab chemotherapy for early stage breast cancer. J Am Coll Cardiol. 2020;75(11 Suppl 2):7. [Google Scholar]
- 31.Budman DR, Tai J, Calabro A. Fluvastatin enhancement of trastuzumab and classical cytotoxic agents in defined breast cancer cell lines in vitro. Breast Cancer Res Treat. 2007;104:93–101. doi: 10.1007/s10549-006-9395-5. [DOI] [PubMed] [Google Scholar]