Abstract
Purpose
Reduced quality of life after cystectomy has made bladder preservation a popular research topic for muscle-invasive bladder cancer (MIBC). Previous research has indicated significant tumor downstaging after neoadjuvant chemotherapy (NAC). However, maximal transurethral resection of bladder tumor (TURBT) was performed before NAC to define the pathology, impacting the real evaluation of NAC. This research aimed to assess real NAC efficacy without interference from TURBT and apply combined modality therapies guided by NAC efficacy.
Materials and Methods
Patients with cT2-4aN0M0 MIBC were confirmed by cystoscopic biopsy and imaging. NAC efficacy was assessed by imaging, urine cytology, and cystoscopy with multidisciplinary team discussion. Definite responders (≤ T1) underwent TURBT plus concurrent chemoradiotherapy. Incomplete responders underwent radical cystectomy or partial cystectomy if feasible. The primary endpoint was the bladder preservation rate.
Results
Fifty-nine patients were enrolled, and the median age was 63 years. Patients with cT3-4 accounted for 75%. The median number of NAC cycles was three. Definite responders were 52.5%. The complete response (CR) was 10.2%, and 59.3% of patients received bladder-sparing treatments. With a median follow-up of 44.6 months, the 3-year overall survival (OS) was 72.8%. Three-year OS and relapse-free survival were 88.4% and 60.0% in the bladder-sparing group but only 74.3% and 37.5% in the cystectomy group. The evaluations of preserved bladder function were satisfactory.
Conclusion
After stratifying MIBC patients by NAC efficacy, definite responders achieved a satisfactory bladder-sparing rate, prognosis, and bladder function. The CR rate reflected the real NAC efficacy for MIBC. This therapy is worth verifying through multicenter research.
Keywords: Muscle-invasive bladder cancer, Neoadjuvant therapy, Chemoradiotherapy, Combined modality therapy, Organ sparing treatments
Introduction
Bladder cancer is one of the most common types of urinary cancer, and muscle-invasive bladder cancer (MIBC) is an aggressive type with a high mortality rate [1]. The current standard treatment for MIBC is neoadjuvant chemotherapy (NAC), followed by radical cystectomy (RC) [2]. Compared with RC, NAC plus RC can increase the 10-year survival rate by 6% [3]. However, the incidence of adverse events from RC and urinary diversion is high, at approximately 49.1% to 69.0% [4,5], and urinary diversion significantly affects quality of life and social function [6]. Therefore, combined modality therapy to preserve the bladder without affecting survival has been the focus of MIBC research [7].
A series of studies from Massachusetts General Hospital (MGH) investigated a bladder-sparing therapy known as tri-modality therapy (TMT), evaluating the efficacy of monopolar maximal transurethral resection of bladder tumor (mTURBT) with or without NAC, along with concurrent cisplatin-based chemoradiotherapy (CRT). Bladder-sparing therapy was performed if concurrent CRT achieved a complete response (CR), and the bladder preservation rate reached approximately 70% [8,9]. However, all patients in the MGH study received CRT without being screened for CRT sensitivity. Therefore, MIBC patients who were not sensitive to CRT initially went undetected. Consequently, these insensitive patients had an increased risk of tumor recurrence or metastasis, rendering them a less-than-ideal population for bladder preservation protocols. NAC alone was reported to have promising efficacy in terms of tumor downstaging, with 64% to 73% of MIBC patients achieving downstaging from T2–T4a to pTa or pT1 and 15% to 31% achieving pathologic complete response (pCR) [4,10–14]. Furthermore, MIBC prognosis was closely related to the downstaging effects of NAC [10,12,13]. However, with current therapy, mTURBT is always performed before NAC [4,10,13,14], and 15% of MIBC patients already achieved pCR through mTURBT confirmed by the pathology of subsequent cystectomy [10]. Therefore, the real efficacy of NAC is affected by mTURBT. Although most urologists consider mTURBT conducive to T staging, the stages of other cancers, such as gastric cancer and breast cancer, mostly depend on imaging.
With the rapid development of multiparameter MRI (mpMRI), the accuracy of the radiological staging of the bladder has risen sufficiently (73% to 96%), positioning clinical staging in close correspondence with pathological staging [15–17]. Moreover, mpMRI was able to distinguish residual tumors from treatment effects in MIBC patients with NAC (sensitivity, 75%; specificity, 100%) [18]. The mpMRI has been a powerful tool in clinical diagnosis. Furthermore, identification of MIBC patients by clinical staging can reveal the actual efficacy of NAC and screen out patients with significant downstaging. On this basis, we applied an innovative combined modality protocol that was implemented in a multidisciplinary team (MDT) discussion mode, with imaging and cystoscopic biopsy confirming the clinical staging and pathology, followed by NAC. Bladder-sparing therapy or RC was performed guided by NAC efficacy. Herein, our research report is as follows.
Materials and Methods
1. Patients
This prospective, single-center, phase II trial aimed to assess the real efficacy of NAC for MIBC before TURBT, followed by the bladder-sparing rate and prognosis with combined modality therapies. This institutional review board-approved study planned to enroll MIBC (cT2-4aN0M0) patients between September 2015 and September 2018. All patients underwent cystoscopic biopsy (not TURBT) in the outpatient department. The key inclusion criteria were as follows: (1) urothelial carcinoma with the absence of carcinoma in situ confirmed by tumor plus random biopsies; (2) no ureteral orifice invasion; and (3) no other history of malignant tumors.
2. Treatment protocol
The combined modality treatments in this study were based on the efficacy of NAC. Two to four courses of NAC were performed with a gemcitabine/cisplatin (GC) regimen. The GC regimen consisted of 1,000 mg/m2 gemcitabine on days 1 and 8 and 75 mg/m2 cisplatin on day 2 administered every 21 days. The evaluation was performed after 2 cycles and consisted mainly of imaging examinations, along with cystoscopy if necessary. Dose reduction of chemotherapy was carried out only in patients who suffered grade 3 adverse events. Imaging examinations, urine cytology, and cystoscopy were performed to assess the efficacy of NAC.
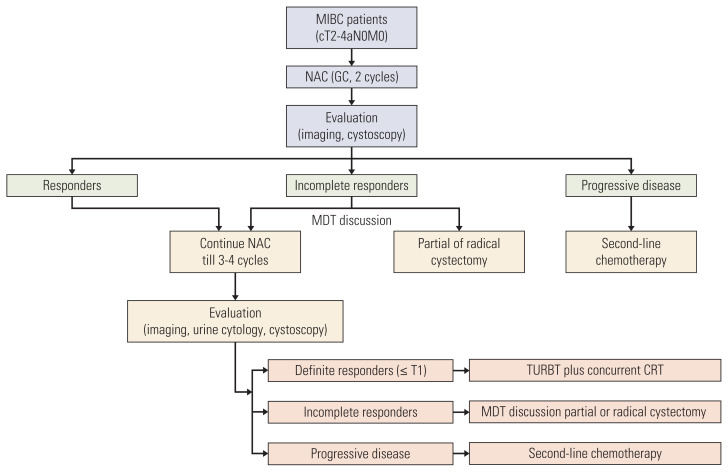
The efficacy of NAC was determined by imagological assessments including (1) mpMRI of the bladder; and (2) enhanced computed tomography; computed tomography urography of the thoracic and abdominopelvic cavities. Then, clinical T downstaging was confirmed by mpMRI and discussion within the MDT. Patients with obvious tumor shrinkage or downstaging after 2 cycles of NAC were defined as responders. Patients whose primary tumors were not significantly downstaged were defined as incomplete responders (IR). If imaging examinations suggested that the maximum diameter of the primary tumor increased by at least 20% or a new lesion appeared, patients were classified as having progressive disease (PD). Patients with definite tumor downstaging (≤ cT1) after total cycles were categorized as definite responders (DR). Responders, IR, and patients with PD were stratified at the first-time evaluation after 2 cycles.
After the first-time evaluation, responders continued NAC. IR continued NAC or received surgery according to MDT discussion. Patients with PD accepted second-line chemotherapy. After the second-time evaluation, DR received TURBT plus concurrent CRT. Clinical complete response (cCR) was defined as no visible tumor on imaging examinations and cystoscopy, in addition to negative pathology of TURBT. IR could receive partial cystectomy plus pelvic lymphadenectomy (PLND) if the patient’s desire for organ preservation was strong and partial cystectomy plus PLND was feasible. Other IR and those who refused bladder-sparing treatment received RC. Patients with PD received second-line chemotherapy.
Concurrent CRT consisted of image-guided intensity-modulated conformal radiotherapy to the true pelvis with 45 Gy in 25 fractions plus the local lesion with 20 Gy in 10 fractions and concurrent cisplatin (40 mg/m2, once per week). The regimen schema is shown in Fig. 1.
Fig. 1.
Regimen schema of 59 MIBC patients. Different therapies were performed according to the evaluation of NAC efficacy. CRT, chemoradiotherapy; GC, gemcitabine/cisplatin; MDT, multidisciplinary team; MIBC, muscle-invasive bladder cancer; NAC, neoadjuvant chemotherapy; TURBT, transurethral resection of bladder tumor.
3. Trial assessments
Oncology outcomes included the bladder preservation rate, overall survival (OS), and relapse-free survival (RFS). Physical examination, blood sampling, and imaging examinations were performed every 3 months for 2 years after combined modality treatments, every 6 months in the third year, and annually afterward. A questionnaire assessment with the 36-item Short Form Health Survey (SF-36) was used for all of the patients. The Overactive Bladder Symptom Score (OABSS) was used for patients who underwent bladder-sparing treatment at the 6th month after enrollment. The actual scores of the SF-36 were first obtained and then transformed to a percentage scale of score from 0 to 100, with a higher number representing better quality of life. The OABSS was scored from 0 to 15, with a lower number representing better bladder function. Adverse events were graded using Common Terminology Criteria for Adverse Events (CTCAE, ver. 4.0). The primary endpoint was the bladder preservation rate. All of the follow-ups and data were concluded on January 15, 2021.
4. Statistical analysis
To be considered feasible, we assumed a 70% bladder preservation rate, referring to the data from MGH research [8]. Fifty-six patients were required (90% power, one-sided α=0.05). Considering the 5% loss to follow-up rate, the final sample size was set at 59 patients.
OS was defined as the period from the beginning of NAC to the date of death or the last follow-up, while RFS was defined as the date of tumor relapse or the last follow-up. Kaplan-Meier analysis was used to analyze OS and RFS. The log-rank test was used to estimate differences between curves and subgroups. Continuous variables are reported as the mean±standard deviation. The differences in the mean values between groups were analyzed with the Mann-Whitney U test. Statistical analysis was performed using SPSS software ver. 21.0 (IBM Corp., Armonk, NY). All applicable tests were two-tailed. p < 0.05 were considered to indicate statistical significance.
Results
1. Patient characteristics and NAC efficacy
The trial was closed to recruitment on August 31, 2018, with 59 patients enrolled. The clinical characteristics and allocated treatments of patients are summarized in Table 1. The median age was 63 years old (range, 39 to 70 years), and 53 patients were male (89.8%). All of the cases were diagnosed as urothelial carcinoma. The maximum diameter of the tumors was 6.0 cm (median, 3.8 cm; range, 2.0 to 6.0 cm). High T staging of cT3 and cT4 accounted for 75% of cases. High-grade tumors confirmed by biopsy accounted for 81.4%.
Table 1.
Baseline characteristics and allocated treatments
| Variable | No. (%) (n=59) |
|---|---|
| Age, median (range, yr) | 63.0 (39.0–70.0) |
| Sex | |
| Male | 53 (89.8) |
| Female | 6 (10.2) |
| Body mass index, median (range, kg/m 2 ) | 24.3 (18.0–33.0) |
| ECOG performance score | |
| 0 | 8 (13.6) |
| 1 | 51 (86.4) |
| Clinical stage | |
| cT2 | 15 (25.4) |
| cT3 | 39 (66.1) |
| cT4a | 5 (8.5) |
| Biopsy differentiation | |
| High grade | 48 (81.4) |
| Low grade | 11 (18.6) |
| Tumor number | |
| Single | 41 (69.5) |
| Multiple | 18 (30.5) |
| NAC courses | |
| 2 | 18 (30.5) |
| 3 | 23 (39.0) |
| 4 | 18 (30.5) |
| NAC efficacy | |
| Definite responder (≤ T1) | 31 (52.5) |
| Incomplete responder | 26 (44.1) |
| Progressive disease | 2 (3.4) |
| Type of treatments | |
| NAC+TURBT+concurrent CRT | 25 (42.4) |
| NAC+TURBT | 2 (3.4) |
| NAC+TURBT+BCG instillation | 4 (6.8) |
| NAC+partial cystectomy+PLND | 4 (6.8) |
| NAC+RC+PLND | 20 (33.9) |
| NAC+second-line chemotherapy | 2 (3.4) |
| NAC+Chinese medicine | 2 (3.4) |
| Pathological stages of 20 RC patients | |
| pT1 | 1 (5.0) |
| pT2 | 5 (25.0) |
| pT3 | 12 (60.0) |
| pT4 | 2 (10.0) |
| pN0 | 14 (70.0) |
| pN1 | 6 (30.0) |
BCG, bacillus Calmette-Guerin; CRT, chemoradiotherapy; ECOG, Eastern Cooperative Oncology Group; NAC, neoadjuvant chemotherapy; PLND, pelvic lymphadenectomy; RC, radical cystectomy; TURBT, transurethral resection of bladder tumor.
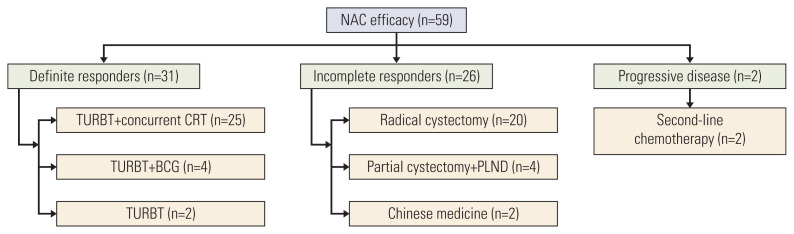
All of the enrolled patients met cisplatin eligibility. After 2 cycles of NAC, there were 31 responders, 27 IR, and one patient with PD. All responders and 10 IR continued NAC, resulting in 31 DR, nine IR, and one patient with PD. The median number of NAC cycles was three. DR, IR, and patients with PD accounted for 52.5% (31/59), 44.1% (26/59), and 3.4% (2/59) of patients, respectively. The median time interval between the last clinical T staging assessment with MR and pathologic staging assessment with surgery was 12 days (range, 8 to 37 days). All DR received bladder-sparing treatments, and most received TURBT plus concurrent CRT (25/31, 80.6%). However, others who refused CRT of their own volition received TURBT plus bacillus Calmette-Guerin (BCG) instillation (4/31, 12.9%) or TURBT alone (2/31, 6.5%). The reasons for refusal of concurrent CRT included the long period of radiotherapy, resistance to radiotherapy-related adverse reactions, and a high level of patient satisfaction with the current efficacy. The postoperative pathological stages of all DR were ≤ 1T. Six patients achieved cCR (6/59, 10.2%), and five of them accepted concurrent CRT.
In the IR (26 patients), it was difficult to define muscular invasion after MDT discussion for six patients with obvious downstaging, and we fully discussed the NAC results with them. Four patients chose partial cystectomy plus PLND, resulting in two of pT1N0 and two of pT2aN0. The other two patients chose RC plus an ileal conduit, resulting in pT1N0 and pT2aN0. Eighteen patients without obvious downstaging underwent RC plus ileal conduit, and two refused surgeries. The median interval from NAC finish to RC was 36 days (range from 18 to 64 days). Among the IR without obvious downstaging, 88.9% (16/18) of them had consistency between clinical T staging and pathological T staging, while the other two cases were both cT3 but pT2. The clinical diagnostic accuracy of muscle invasion reached 100% (18/18). Pelvic lymph node metastasis was diagnosed in six patients after RC with no imaging signs.
One patient exhibited bladder tumor enlargement followed by aggravating hematuria after 2 cycles and was considered to have PD. Internal iliac artery embolization and second-line chemotherapy were performed because cystectomy was refused. Another patient was considered to have PD after 4 cycles because pelvic and retroperitoneal lymph nodes were newly found; the patient subsequently received second-line chemotherapy. Fig. 2 shows specific treatments after NAC.
Fig. 2.
Specific treatments after NAC. Presented by allocated therapy for different groups stratified by NAC efficacy. Definite responders mainly received TURBT plus concurrent TURBT. Incomplete responders received radical cystectomy or partial cystectomy plus pelvic lymphadenectomy. BCG, Bacillus Calmette-Guerin; CRT, chemoradiotherapy; NAC, neoadjuvant chemotherapy; PLND, pelvic lymphadenectomy; TURBT, transurethral resection of bladder tumor.
2. Cancer control and oncologic outcomes
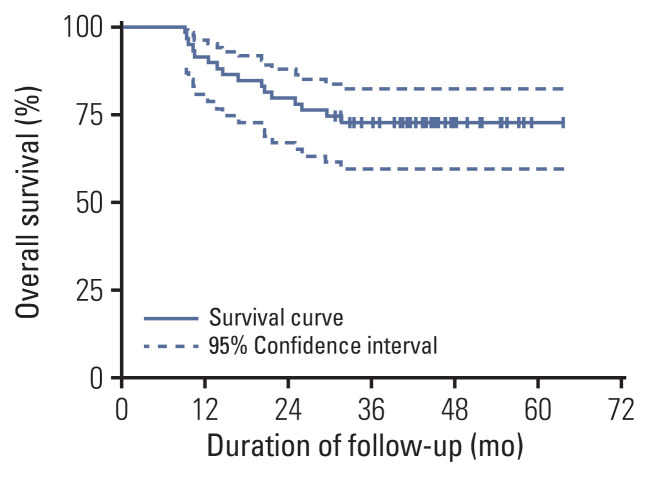
All patients were followed up regularly. The median follow-up was 44.6 months (range from 9.2 to 63.7 months). By the last follow-up, 16 patients died because of bladder cancer, and the 3-year OS rate was 72.8% (Fig. 3). The maximum tumor diameter shrinkage is shown in S1 Fig.
Fig. 3.
Kaplan-Meier plot of the 59 muscle-invasive bladder cancer patients. Error bars are defined as 95% confidence intervals and denoted with a dashed line. The 3-year overall survival rate was 72.8%.
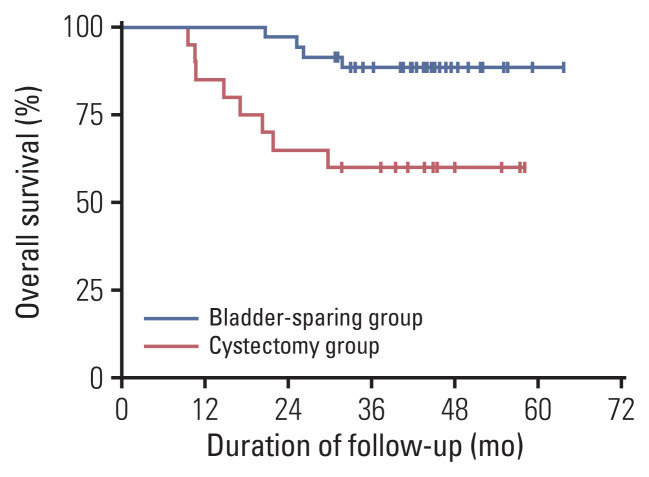
Ultimately, 59.3% (35/59) of patients successfully underwent bladder-sparing treatments, and the 3-year OS was 88.4% (Fig. 4). Furthermore, 74.3% (26/35) of them maintained tumor-free survival with a 3-year RFS of 74.3% (Fig. 5). Conversely, 14.3% (5/35) of these patients experienced recurrence in the bladder and then received TURBT plus BCG instillation (4/5) or salvage cystectomy (1/5) depending on whether the bladder muscle was invaded. In addition, 11.4% (4/35) of patients had distant metastasis with no sign of local recurrence and received second-line chemotherapy, targeted therapy plus immunotherapy, or best supportive treatment (Table 2). By the last follow-up, 97.1% (34/35) of patients who underwent bladder-sparing treatment still maintained their bladders, and 85.7% (30/35) of them were tumor-free.
Fig. 4.
Kaplan-Meier curves of overall survival with log-rank statistics for the two groups of bladder-sparing treatment and cystectomy. Patients in the bladder-sparing group had significantly better prognosis than those of the cystectomy group (p=0.007).
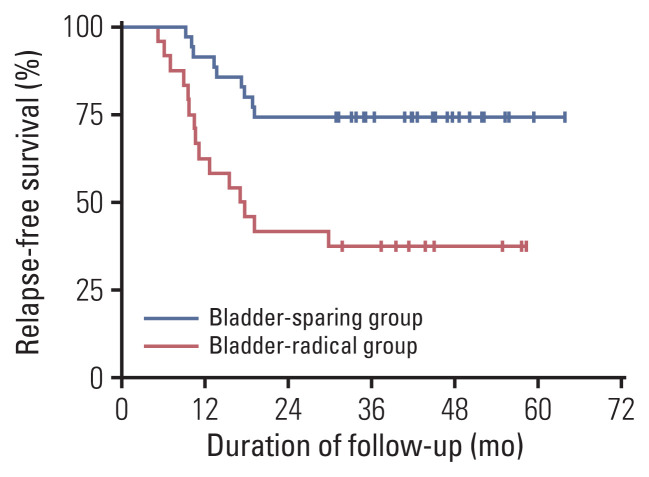
Fig. 5.
Kaplan-Meier curves of relapse-free survival with log-rank statistics for the two groups of bladder-sparing treatment and cystectomy. Patients in the bladder-sparing group had significantly better relapse-free survival than those of the cystectomy group (p=0.002).
Table 2.
Subsequent treatments after the failure of bladder preservation in the bladder-sparing group and the failure of RC in the cystectomy group
| Treatment | No. (%) | |
|---|---|---|
| Bladder-sparing group | 35 | |
| Local recurrence | TURBT+BCG instillation | 4 (11.4) |
| Salvage cystectomy | 1 (2.9) | |
| Distant metastasis | Second-line chemotherapy | 2 (5.7) |
| Targeted therapy plus immunotherapy (levatinib+pembrolizumab) | 1 (2.9) | |
| Best supportive treatment | 1 (2.9) | |
| Cystectomy group | 20 | |
| Distant metastasis | Second-line chemotherapy | 10 (50.0) |
| Best supportive treatment | 1 (5.0) |
BCG, bacillus Calmette-Guerin; RC, radical cystectomy; TURBT, transurethral resection of bladder tumor.
The 3-year OS rate of the RC group was 60.0% (Fig. 4), which was statistically significantly poorer than that of the bladder-sparing treatment group (p=0.007). The distant metastasis rate after RC was 55.0% (11/20), and the second-line chemotherapy rate was 50.0% (10/20), while 5.0% (1/20) of patients accepted best supportive treatment (Table 2). The 3-year RFS rate was only 37.5% which was also poorer than bladder-sparing group (p=0.002) (Fig. 5).
Two IR patients and two patients with PD did not accept combined modality therapies after NAC. The median survival time was 9.7 months (95% confidence interval, 6.37 to 13.03). The OS times of the two IR patients were 12.6 months and 13.9 months, and those of the two patients with PD were 9.2 months and 9.7 months.
3. Functional outcomes
On the SF-36 questionnaire, all of the patients had favorable total physical health (actual score, 59.4±6.1; percentage scale, 70.4±11.3) and total mental health (actual score, 54.8±5.4; percentage scale, 69.6±10.9) when evaluated at 6 months. Patients in the bladder-sparing group had better total physical health (p < 0.001), total mental health (p < 0.001), and overall health (p < 0.001) than those in the cystectomy group. The comparisons of data from the SF-36 were shown in Table 3 by item. Of the patients who received bladder-sparing treatment, compared with the pretreatment mean score of 3.29±1.0, the mean score of OABSS was 2.14±0.7 at 6 months (p < 0.001) and 1.91±1.4 at 1 year (p < 0.001). However, there was no significant difference between the scores at 6 months and 1 year (p=0.078).
Table 3.
Comparison of data from the Short Form Health Survey (SF-36) between the bladder-sparing and cystectomy groups at 6 months
| Item | Bladder-sparing group (n=35) | Cystectomy group (n=20) | p-value | ||
|---|---|---|---|---|---|
|
|
|
||||
| Actual score | Percentage scale | Actual score | Percentage scale | ||
| Physical functioning | 21.9±1.7 | 59.4±8.7 | 19.7±3.8 | 48.5±19.0 | 0.01 |
|
| |||||
| Role-physical | 7.1±0.8 | 77.1±21.0 | 5.9±0.8 | 47.5±19.2 | < 0.001 |
|
| |||||
| Bodily pain | 11.0±1.0 | 90.0±10.3 | 6.2±0.7 | 41.5±7.4 | < 0.001 |
|
| |||||
| General health | 22.8±1.2 | 88.9±6.2 | 20.9±1.8 | 79.5±9.1 | < 0.001 |
|
| |||||
| Vitality | 20.4±1.1 | 82.0±5.4 | 16.1±0.4 | 60.5±2.2 | < 0.001 |
|
| |||||
| Social functioning | 9.1±0.7 | 52.9±37.7 | 8.7±0.5 | 35.0±22.9 | 0.076 |
|
| |||||
| Role-emotional | 5.3±0.5 | 78.1±15.8 | 4.6±0.5 | 53.3±16.3 | < 0.001 |
|
| |||||
| Mental health | 23.6±2.3 | 74.4±9.3 | 21.5±0.7 | 65.8±2.7 | 0.005 |
|
| |||||
| Overall health | 121.1±4.7 | 77.1±4.5 | 103.5±5.7 | 60.1±5.5 | < 0.001 |
|
| |||||
| Total physical health | 62.7±2.8 | 77.3±5.3 | 52.7±5.4 | 58.6±9.9 | < 0.001 |
|
| |||||
| Total mental health | 58.4±3.2 | 76.8±6.4 | 50.9±0.8 | 61.7±1.6 | < 0.001 |
Values are presented as mean±standard deviation.
4. Toxicity
Most adverse events of NAC were grade 1 (13/59, 22.0%) or 2 (34/59, 57.6%) as estimated by CTCAE ver. 4.0 criteria, and included bone marrow hypocellularity, gastrointestinal disorders (diarrhea, nausea, vomiting, or oral mucositis), acute kidney injury, and hepatobiliary disorders (elevated alanine aminotransferase). Five patients (8.5%) experienced grade 3 toxicity of bone marrow hypocellularity or gastrointestinal disorders (vomiting or diarrhea) and accepted a dose reduction of gemcitabine or cisplatin. One 54-year-old man suffered acute coronary syndrome of grade 4, manifesting as unstable angina and hemodynamic instability in the second course, but he fully recovered and received RC. Otherwise, the side effects of concurrent CRT were mild, and all were grades 1–2 (28.0%, 7/25), including diarrhea, nausea or vomiting, rash, and rectitis (Table 4). For the 20 patients undergoing RC, one patient experienced partial intestinal obstruction, one patient experienced incision infection with delayed healing, and one patient experienced a fungal infection of the digestive tract. All three patients were safely discharged and recovered well.
Table 4.
Adverse events of NAC and CRT
| No. (%) | |
|---|---|
| Grade of NAC adverse events | 59 |
| Grade 1 | 13 (22.0) |
| Bone marrow hypocellular | 6 (10.2) |
| Gastrointestinal disorders | 6 (10.2) |
| Acute kidney injury | 1 (1.7) |
| ALT elevation | 1 (1.7) |
| Grade 2 | 34 (57.6) |
| Bone marrow hypocellular | 23 (39.0) |
| Gastrointestinal disorders | 13 (22.0) |
| Grade 3 | 5 (8.5) |
| Bone marrow hypocellular | 3 (6.8) |
| Gastrointestinal disorders | 2 (1.7) |
| Grade 4 | 1 (1.7) |
| Acute coronary syndrome | 1 (1.7) |
| Total grade 1–2 | 47 (79.6) |
| Total grade 3–4 | 6 (10.2) |
| Grade of CRT adverse events | 25 |
| Grade 1 | 5 (20.0) |
| Gastrointestinal disorders | 5 (20.0) |
| Skin rash | 2 (8.0) |
| Rectitis | 2 (8.0) |
| Grade 2 | 2 (8.0) |
| Gastrointestinal disorders | 2 (8.0) |
| Total grade 1–2 | 7 (28.0) |
ALT, alanine aminotransferase; CRT, chemoradiotherapy; NAC, neoadjuvant chemotherapy.
Discussion
On the premise of not affecting survival, organ-sparing combined modality therapies have been successfully applied in several cancers, such as breast cancer and rectal cancer, to improve quality of life. In the research field of bladder cancer, the well-studied regimens of bladder preservation include mTURBT, partial cystectomy, or concurrent CRT, accompanied by either adjuvant or neoadjuvant chemotherapy. In this prospective phase II study, we explored a combined modality therapy: in the MDT discussion mode, imaging and biopsy pathology confirmed the clinical staging, followed by NAC; then, according to the real NAC efficacy, appropriate bladder-sparing treatments were performed. The results were promising, with a 10.2% cCR rate, which reflected the real CR rate of NAC for MIBC. The 3-year OS rate of the intention-to-treat (ITT) population was 72.8%, and 59.3% (35/59) of them had successfully preserved bladders, with a 3-year OS rate of 88.4%. Assessments of bladder function and quality of life were satisfactory. In medical centers with prolific imaging experiences or other large centers, this type of combined modality therapy is promising.
Of bladder-sparing therapies for MIBC patients, the most studied regimen is TMT. In TMT, mTURBT is performed to determine the pathological T stage followed by concurrent CRT [19–21]. Related research from MGH enrolled 475 MIBC patients, and cT2 accounted for 66% [9]. The overall 5-year OS rate was 57%, and the bladder preservation rate reached approximately 70%. For the bladder-sparing group, the 3-year OS rate was approximately 85%, and the 5-year OS rate was 75%. Compared with TMT, our study adopted a protocol of combined modality therapy guided by actual NAC efficacy. A total of 59.3% of patients successfully underwent bladder preservation therapies with a similar 3-year OS rate of 88.4%. By the last follow-up, 97.1% (34/35) of patients who underwent bladder-sparing treatment still maintained their bladders, and 85.7% (30/35) of them were tumor-free and alive. Although the bladder preservation rate for the ITT population did not reach the primary endpoint, patients who received bladder-sparing therapies had an optimistic bladder preservation rate and OS. Our outcomes were similar to those of the TMT regimen, but we mainly focused on patients with higher T staging (T3–T4, 75%) since mTURBT was difficult and a high risk in these patients. Considering the above, a similar outcome may indicate that our protocol had better survival benefits with the screening of MIBC patients by NAC efficacy. Prospective studies explored the efficacy of NAC combined with concurrent CRT in MIBC patients and indicated that patients with effective NAC were often sensitive to concurrent CRT with a lower recurrence [22]. Furthermore, TMT required TURBT twice, while DR patients received TURBT only once because we adopted the cystoscopic biopsy instead of mTURBT in our protocol. Moreover, patients who underwent bladder-sparing treatment had better quality of life than patients in the cystectomy group according to the SF-36 questionnaire and maintained favorable bladder function. The OABSS was significantly better than pretreatment (2.14 vs. 3.29, p < 0.001) at 6 months and 1 year (1.91 vs. 3.29, p < 0.001).
Among IR patients screened by NAC, 20 patients received RC, and the 3-year OS rate was worse than that of the bladder-sparing group (60.0% vs. 88.4%, p=0.007) and worse than that of previous RC studies (5-year OS rate, 62% to 68%) [21,23]. The 3-year RFS rate was only 37.5%, indicating a higher metastasis rate after treatments. Therefore, patients who were not suitable for bladder preservation could be screened out by NAC efficacy, and these patients had poor survival after RC. Therefore, more effective treatment options must be explored to improve their prognoses. Several clinical trials have begun to explore the efficacy of neoadjuvant immunotherapy or chemotherapy combined with immunotherapy in patients with MIBC [24]. It is hoped that these studies yield satisfactory outcomes to provide medical evidence that avails more clinical strategies for IR.
Although mTURBT became the traditional method to define T staging in bladder cancer, biopsy plus imaging were widely used in multiple cancers, such as rectal, laryngeal, esophageal, and breast cancer, before organ-sparing surgeries. Meanwhile, bladder mpMRI was becoming common and useful to evaluate the response to bladder-sparing treatments [25,26]. In terms of the coincidence rate between clinical staging and pathological staging, previous studies have demonstrated that the accuracy of mpMRI for clinical staging of bladder cancer could be as high as 96% [15]. In our center, Zhang et al. [27] concluded the diagnostic efficacy of mpMRI and confirmed mpMRI was reliable to predict T staging. In this study, the pathologies of all DR were ≤ T1, consistent with the preoperative assessments of cT staging (100%). Moreover, 88.9% of IR patients had the same clinical T staging and pathological T staging after RC, and the accuracy of muscle invasion identification was 100% (18/18). The consistency indicated that a promising accuracy of clinical T staging could be achieved through mpMRI. Although it was likely that overstaging existed before NAC, CRT also offered a curative bladder-sparing treatment option for T1 bladder cancer, with an optimistic CR rate of 90.9% [28]. Pathologies of six patients after cystectomy showed pelvic lymph node metastasis, so it was necessary to perform PLND for IR, indicating that the N staging assessment was still flawed by imaging examination alone.
The side effects in this study were related to NAC, concurrent CRT, and surgical complications. Meleis and colleagues retrospectively analyzed the safety of the GC regimen as NAC in MIBC patients, and grade 3–4 events accounted for 41% (7/17) [29]. Huddart et al. evaluated CRT-related toxicity, and grade 3–4 events accounted for 36% (8/22), including common fatigue and nocturia [30]. In this research, the NAC of the GC regimen had acceptable adverse events, and the grade 3/4 rate was relatively lower (10.2%). The most common type was bone marrow hypocellularity (54.2%, 32/59). CRT-related adverse events were all grade 1–2 (28%). Since only five patients received dose reduction during NAC, it was difficult to assess the effect of dose reduction on clinical prognosis due to the low rate of dose reduction. All patients completed 2–4 cycles of NAC, so we believed our NAC and CRT regimens were practicable in medical centers with prolific oncology treatment experiences. Among 20 patients with RC, only three patients experienced short-term complications, and all recovered well before discharge, so the safety of RC after NAC was optimistic.
Some limitations warrant discussion. As biopsies were bitten through the cystoscope, tumor tissues obtained were superficial resulting in 18.6% of low-grade differentiation, and the real degree of the tumor may be underestimated. In fact, a tumor biopsy plus a random biopsy were mainly used to confirm urothelial cancer without carcinoma in situ. Radiological staging was adopted to evaluate the baseline and NAC efficacy of MIBC patients. Nevertheless, this process might lead to inaccurate T staging. Several factors weakened this bias: (1) all patients were evaluated by mpMRI; (2) all patients underwent MDT discussion with clinical T staging further defined; and (3) the ITT population mainly covered T3 or T4a patients (75%), which weakened the difficulty of distinguishing muscle invasion. Another limitation was the inadequate follow-up period, but optimistic RFS and preservation rates of the bladder-sparing group portends the good outcomes of further follow-ups. This result was preliminary, and enrollment will enlarge the group, while multicenter research is also actively being conducted.
In conclusion, this study confirmed the innovative protocol of combined modality treatment guided by NAC efficacy in MIBC. The outcomes demonstrated that patients with definite downstaging screened by real NAC efficacy could benefit from a satisfactory bladder-sparing rate, fair survival, and functional outcomes. Since IR had disappointing survival after RC, these patients demand better clinical strategies to improve prognosis. This protocol of combined modality treatment deserves to be verified through multicenter studies.
Acknowledgments
The authors thank the patients and their families and all investigators and site personnel.
The work was supported by the Beijing Hope Run Special Fund of the Cancer Foundation of China (LC2015L12) to support patients to accomplish therapies and to improve follow-up.
Footnotes
Ethical Statement
This study has been retrospectively registered at ClinicalTrials.gov under the unique identifier NCT02861196. This institutional review board approved this study (approval number: NCC2015 YL-05). All of the patients provided written informed consent for enrollment in this protocol.
Author Contributions
Conceived and designed the analysis: Shi H, Zhang W, Liu Y, Zhou A, Shou J.
Collected the data: Shi H, Zhang W, Bai H, Hu L, Cao C, Jiang W, Hu Z, Zhang J, Chen Y, Zheng S, Feng X.
Contributed data or analysis tools: Shi H, Zhang W, Bi X, Wang D, Xiao Z, Guan Y, Guan K, Tian J.
Performed the analysis: Shi H, Zhang W, Li C, Li Y, Ma J, Liu Y, Zhou A, Shou J.
Wrote the paper: Shi H, Zhang W.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
References
- 1.Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol. 2017;71:96–108. doi: 10.1016/j.eururo.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Flaig TW. NCCN guidelines updates: management of muscle-invasive bladder cancer. J Natl Compr Canc Netw. 2019;17:591–3. doi: 10.6004/jnccn.2019.5017. [DOI] [PubMed] [Google Scholar]
- 3.International Collaboration of Trialists; Medical Research Council Advanced Bladder Cancer Working Party; European Organisation for Research and Treatment of Cancer Genito-Urinary Tract Cancer Group; Australian Bladder Cancer Study Group; National Cancer Institute of Canada Clinical Trials Group; Norwegian Bladder Cancer Study Group; Club Urologico Espanol de Tratamiento Oncologico Group, et al. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol. 2011;29:2171–7. doi: 10.1200/JCO.2010.32.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parekh DJ, Reis IM, Castle EP, Gonzalgo ML, Woods ME, Svatek RS, et al. Robot-assisted radical cystectomy versus open radical cystectomy in patients with bladder cancer (RAZOR): an open-label, randomised, phase 3, non-inferiority trial. Lancet. 2018;391:2525–36. doi: 10.1016/S0140-6736(18)30996-6. [DOI] [PubMed] [Google Scholar]
- 5.Yu HY, Hevelone ND, Lipsitz SR, Kowalczyk KJ, Nguyen PL, Choueiri TK, et al. Comparative analysis of outcomes and costs following open radical cystectomy versus robot-assisted laparoscopic radical cystectomy: results from the US Nationwide Inpatient Sample. Eur Urol. 2012;61:1239–44. doi: 10.1016/j.eururo.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 6.Winters BR, Wright JL, Holt SK, Dash A, Gore JL, Schade GR. Health related quality of life following radical cystectomy: comparative analysis from the medicare health outcomes survey. J Urol. 2018;199:669–75. doi: 10.1016/j.juro.2017.08.111. [DOI] [PubMed] [Google Scholar]
- 7.Kulkarni GS, Hermanns T, Wei Y, Bhindi B, Satkunasivam R, Athanasopoulos P, et al. Propensity score analysis of radical cystectomy versus bladder-sparing trimodal therapy in the setting of a multidisciplinary bladder cancer clinic. J Clin Oncol. 2017;35:2299–305. doi: 10.1200/JCO.2016.69.2327. [DOI] [PubMed] [Google Scholar]
- 8.Efstathiou JA, Spiegel DY, Shipley WU, Heney NM, Kaufman DS, Niemierko A, et al. Long-term outcomes of selective bladder preservation by combined-modality therapy for invasive bladder cancer: the MGH experience. Eur Urol. 2012;61:705–11. doi: 10.1016/j.eururo.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Giacalone NJ, Shipley WU, Clayman RH, Niemierko A, Drumm M, Heney NM, et al. Long-term outcomes after bladder-preserving tri-modality therapy for patients with muscle-invasive bladder cancer: an updated analysis of the Massachusetts General Hospital experience. Eur Urol. 2017;71:952–60. doi: 10.1016/j.eururo.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 10.Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–66. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 11.Yeshchina O, Badalato GM, Wosnitzer MS, Hruby G, RoyChoudhury A, Benson MC, et al. Relative efficacy of perioperative gemcitabine and cisplatin versus methotrexate, vinblastine, adriamycin, and cisplatin in the management of locally advanced urothelial carcinoma of the bladder. Urology. 2012;79:384–90. doi: 10.1016/j.urology.2011.10.050. [DOI] [PubMed] [Google Scholar]
- 12.Galsky MD, Pal SK, Chowdhury S, Harshman LC, Crabb SJ, Wong YN, et al. Comparative effectiveness of gemcitabine plus cisplatin versus methotrexate, vinblastine, doxorubicin, plus cisplatin as neoadjuvant therapy for muscle-invasive bladder cancer. Cancer. 2015;121:2586–93. doi: 10.1002/cncr.29387. [DOI] [PubMed] [Google Scholar]
- 13.Lee FC, Harris W, Cheng HH, Shenoi J, Zhao S, Wang J, et al. Pathologic response rates of gemcitabine/cisplatin versus methotrexate/vinblastine/adriamycin/cisplatin neoadjuvant chemotherapy for muscle invasive urothelial bladder cancer. Adv Urol. 2013;2013:317190. doi: 10.1155/2013/317190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peyton CC, Tang D, Reich RR, Azizi M, Chipollini J, Pow-Sang JM, et al. Downstaging and survival outcomes associated with neoadjuvant chemotherapy regimens among patients treated with cystectomy for muscle-invasive bladder cancer. JAMA Oncol. 2018;4:1535–42. doi: 10.1001/jamaoncol.2018.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stenzl A, Cowan NC, De Santis M, Jakse G, Kuczyk MA, Merseburger AS, et al. The updated EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol. 2009;55:815–25. doi: 10.1016/j.eururo.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 16.van der Pol CB, Chung A, Lim C, Gandhi N, Tu W, McInnes MD, et al. Update on multiparametric MRI of urinary bladder cancer. J Magn Reson Imaging. 2018;48:882–96. doi: 10.1002/jmri.26294. [DOI] [PubMed] [Google Scholar]
- 17.Takeuchi M, Sasaki S, Ito M, Okada S, Takahashi S, Kawai T, et al. Urinary bladder cancer: diffusion-weighted MR imaging: accuracy for diagnosing T stage and estimating histologic grade. Radiology. 2009;251:112–21. doi: 10.1148/radiol.2511080873. [DOI] [PubMed] [Google Scholar]
- 18.Donaldson SB, Bonington SC, Kershaw LE, Cowan R, Lyons J, Elliott T, et al. Dynamic contrast-enhanced MRI in patients with muscle-invasive transitional cell carcinoma of the bladder can distinguish between residual tumour and post-chemotherapy effect. Eur J Radiol. 2013;82:2161–8. doi: 10.1016/j.ejrad.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Shipley WU, Winter KA, Kaufman DS, Lee WR, Heney NM, Tester WR, et al. Phase III trial of neoadjuvant chemotherapy in patients with invasive bladder cancer treated with selective bladder preservation by combined radiation therapy and chemotherapy: initial results of Radiation Therapy Oncology Group 89–03. J Clin Oncol. 1998;16:3576–83. doi: 10.1200/JCO.1998.16.11.3576. [DOI] [PubMed] [Google Scholar]
- 20.Mathieu R, Lucca I, Klatte T, Babjuk M, Shariat SF. Trimodal therapy for invasive bladder cancer: is it really equal to radical cystectomy? Curr Opin Urol. 2015;25:476–82. doi: 10.1097/MOU.0000000000000203. [DOI] [PubMed] [Google Scholar]
- 21.Ploussard G, Daneshmand S, Efstathiou JA, Herr HW, James ND, Rodel CM, et al. Critical analysis of bladder sparing with trimodal therapy in muscle-invasive bladder cancer: a systematic review. Eur Urol. 2014;66:120–37. doi: 10.1016/j.eururo.2014.02.038. [DOI] [PubMed] [Google Scholar]
- 22.Zapatero A, Martin De Vidales C, Arellano R, Ibanez Y, Bocardo G, Perez M, et al. Long-term results of two prospective bladder-sparing trimodality approaches for invasive bladder cancer: neoadjuvant chemotherapy and concurrent radio-chemotherapy. Urology. 2012;80:1056–62. doi: 10.1016/j.urology.2012.07.045. [DOI] [PubMed] [Google Scholar]
- 23.Hautmann RE, de Petriconi RC, Pfeiffer C, Volkmer BG. Radical cystectomy for urothelial carcinoma of the bladder without neoadjuvant or adjuvant therapy: long-term results in 1100 patients. Eur Urol. 2012;61:1039–47. doi: 10.1016/j.eururo.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 24.Massari F, Santoni M, di Nunno V, Cheng L, Lopez-Beltran A, Cimadamore A, et al. Adjuvant and neoadjuvant approaches for urothelial cancer: updated indications and controversies. Cancer Treat Rev. 2018;68:80–5. doi: 10.1016/j.ctrv.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Panebianco V, Barchetti F, de Haas RJ, Pearson RA, Kennish SJ, Giannarini G, et al. Improving staging in bladder cancer: the increasing role of multiparametric magnetic resonance imaging. Eur Urol Focus. 2016;2:113–21. doi: 10.1016/j.euf.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 26.Panebianco V, Narumi Y, Altun E, Bochner BH, Efstathiou JA, Hafeez S, et al. Multiparametric magnetic resonance imaging for bladder cancer: development of VI-RADS (Vesical Imaging-Reporting And Data System) Eur Urol. 2018;74:294–306. doi: 10.1016/j.eururo.2018.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang M, Chen Y, Cong X, Zhao X. Utility of intravoxel incoherent motion MRI derived parameters for prediction of aggressiveness in urothelial bladder carcinoma. J Magn Reson Imaging. 2018;48:1648–56. doi: 10.1002/jmri.26165. [DOI] [PubMed] [Google Scholar]
- 28.Inoue M, Ishioka J, Fukuda H, Kageyama Y, Saito Y, Higashi Y. Clinical outcome of chemoradiotherapy for T1G3 bladder cancer. Int J Urol. 2008;15:747–50. doi: 10.1111/j.1442-2042.2008.02077.x. [DOI] [PubMed] [Google Scholar]
- 29.Meleis L, Moore R, Inman BA, Harrison MR. Retrospective analysis of the efficacy and safety of neoadjuvant gemcitabine and cisplatin in muscle-invasive bladder cancer. J Oncol Pharm Pract. 2020;26:330–7. doi: 10.1177/1078155219845434. [DOI] [PubMed] [Google Scholar]
- 30.Huddart RA, Birtle A, Maynard L, Beresford M, Blazeby J, Donovan J, et al. Clinical and patient-reported outcomes of SPARE: a randomised feasibility study of selective bladder preservation versus radical cystectomy. BJU Int. 2017;120:639–50. doi: 10.1111/bju.13900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.