Abstract
In areas such as eastern Indonesia where both Plasmodium falciparum and Plasmodium vivax occur, rapid antigen detection tests for malaria need to be able to detect both species. We evaluated the new combined P. falciparum-P. vivax immunochromatographic test (ICT Malaria P.f/P.v.) in Radamata Primary Health Centre, Sumba, Indonesia, from February to May 1998 with 560 symptomatic adults and children with a presumptive clinical diagnosis of malaria. Blinded microscopy was used as the “gold standard,” with all discordant and 20% of concordant results cross-checked blindly. Only 50% of those with a presumptive clinical diagnosis of malaria were parasitemic. The ICT Malaria P.f/P.v immunochromatographic test was sensitive (95.5%) and specific (89.8%) for the diagnosis of falciparum malaria, with a positive predictive value (PPV) and a negative predictive value (NPV) of 88.1 and 96.2%, respectively. HRP2 and panmalarial antigen line intensities were associated with parasitemia density for both species. Although the specificity and NPV for the diagnosis of vivax malaria were 94.8 and 98.2%, respectively, the overall sensitivity (75%) and PPV (50%) for the diagnosis of vivax malaria were less than the desirable levels. The sensitivity for the diagnosis of P. vivax malaria was 96% with parasitemias of >500/μl but only 29% with parasitemias of <500/μl. Nevertheless, compared with the test with HRP2 alone, use of the combined antigen detection test would reduce the rate of undertreatment from 14.7 to 3.6% for microscopy-positive patients, and this would be at the expense of only a modest increase in the rate of overtreatment of microscopy-negative patients from 7.1 to 15.4%. Cost remains a major obstacle to widespread use in areas of endemicity.
Microscopy of Giemsa-stained thick and thin films by a skilled microscopist has remained the standard laboratory method for the diagnosis of malaria both in regions where malaria is endemic and in regions where malaria is nonendemic. In eastern Indonesia, as in many other regions where malaria is endemic, there are problems with microscopic diagnosis, particularly at the periphery of the health care system. These include lack of skilled microscopists, limited supply and maintenance of microscopes and reagents, delays in results, and inadequate quality control. Moreover, ready access to these microscopy services is limited for the vast majority of symptomatic, malaria-exposed residents. For these reasons, the Indonesian Malaria Control Operational Policy still recommends early diagnosis and prompt treatment on the basis of a clinical diagnosis of malaria (10). Because of the nonspecific nature of the symptoms and signs of malaria, this results in considerable mistreatment, both overtreatment with antimalarial agents and undertreatment of those with nonmalarial illnesses. The World Health Organization has recently reiterated “the urgent need for simple and cost-effective diagnostic tests for malaria to overcome the deficiencies of [both] light microscopy” and clinical diagnosis (43).
In recent years, multiple studies in areas of both endemicity and nonendemicity have found that rapid dipstick antigen capture tests for the circulating Plasmodium falciparum-specific antigen HRP2 have excellent sensitivity and specificity for the diagnosis of P. falciparum malaria, generally at least as good as microscopy of a thick and thin film by a skilled microscopist (1, 4–6, 9, 11, 13, 15–17, 19, 23, 28–32, 35, 36, 39–42). The two commercially available methods of HRP2 antigen detection, ParaSight-F (1, 4–6, 9, 13, 17, 22, 29–31, 35, 39, 42) and the P. falciparum immunochromatographic test (ICT Malaria Pf) (16, 19, 40, 41) have comparable performances (19, 28, 41), but a major limitation has been their inability to detect malaria caused by other species, particularly Plasmodium vivax. In areas such as eastern Indonesia where both P. falciparum and P. vivax occur (33, 34), rapid antigen detection tests need to be able to detect both species.
We therefore evaluated the new combined P. falciparum-P. vivax immunochromatographic test (ICT Malaria P.f/P.v. [ICT P.f/P.v]) for the detection of malaria. ICT P.f/P.v is based on detection of the P. falciparum-specific HRP2 antigen and a panmalarial antigen, and microscopy is used as the “gold standard.” The test kit is identical to the P. falciparum-specific HRP-2 test described previously (15), but a second monoclonal antibody directed against a panmalarial antigen is added. This antigen is expressed by blood stages of P. falciparum and P. vivax, and probably also by Plasmodium ovale, but no data on antigen expression by Plasmodium malariae are yet available (14).
MATERIALS AND METHODS
Study site.
The study was performed from February to May 1998 in Radamata Primary Health Centre, Laratama subdistrict, West Sumba, East Nusa Tenggara Province, Indonesia, a subdistrict with a Plasmodium infection rate in children 0 to 9 years of age of 5.1% (37). The study was approved by the Ethics Committee of the National Institute of Health Research and Development, Indonesian Ministry of Health, Jakarta, Indonesia, and by the Joint Institutional Ethics Committee of Menzies School of Health Research and Royal Darwin Hospital, Darwin, Northern Territory, Australia.
Patients.
A total of 560 symptomatic adults and children attending the primary health care center were enrolled in the study. Only those who had a presumptive diagnosis of clinical malaria were eligible for the study. A diagnosis of clinical malaria was based on fever or history of fever in the last 48 h and no other evident cause of fever. In routine clinical practice in this setting, all of these people would have been treated empirically with antimalarial drugs. As such, all patients were treated with a standard regimen of chloroquine with or without primaquine according to the malaria treatment protocols of the Indonesian Ministry of Health (10).
Microscopy and immunochromatographic testing.
Thick and thin films were prepared directly from fingerprick blood samples, and immunochromatographic testing was performed directly with the fingerprick blood samples. Thick and thin films were stained with 10% Giemsa solution and examined at ×1,000 by an expert microscopist (S.S.) with 24 years of experience. The microscopist was unaware of the patient’s diagnosis or immunochromatographic test result. The parasite density was counted per 200 leukocytes and was then expressed as the number of trophozoites per microliter by assuming a leukocyte count of 8,000/μl. The initial thick film was considered negative if no parasites were seen in at least 100 high-power fields.
After a period of training, the immunochromatographic test with 15 μl of fingerprick capillary blood was performed by clinic health workers according to the manufacturer’s instructions, and the results were read by the study physician (E.T.), who was blinded to the microscopy results. The MLO2 ICT P.f/P.v test card (AMRAD-ICT, Sydney, Australia) was used. Batch 100088 was used for the first 393 tests, and batch 041388 was used for the remaining 167 tests. The test was considered valid if the control line was visible and positive if the HRP2 and/or panmalarial antigen lines were visible. An immunochromatographic test diagnosis of P. vivax malaria was made if only the panmalarial antigen line was visible. A diagnosis of P. falciparum malaria was made if the HRP2 line was visible, with or without the panmalarial antigen line. Coinfection with both P. falciparum and P. vivax cannot be distinguished from infection with P. falciparum alone: the test interpretation when two lines are visible is P. falciparum malaria. Line intensity was graded into four categories: absent, faint (just visible), intermediate, or greater than or equal to that of the control.
All slides with discordant results and 20% of slides with concordant results were cross-checked by an expert microscopist (M.D.) in Darwin with over 20 years of experience. The microscopist was blinded to the patient’s diagnosis and to previous microscopy and immunochromatographic test results. A thick film was considered negative on cross-checking only if at least 200 high-power fields were negative.
Data analysis.
Epi-Info version 6 (7) was used to calculate test performance and acceptability evaluation indices, with microscopy used as the gold standard. Performance indices were calculated for each of the following microscopic diagnoses: malaria as a whole (diagnosis of either species), P. falciparum malaria (including mixed infection), and P. vivax malaria. The variables measured were the number of true positives (TP), number of true negatives (TN), number of false positives (FP), and number of false negatives (FN). Sensitivity was calculated as TP/(TP + FN), specificity was calculated as TN/(TN + FP), the positive predictive value (PPV) was calculated as TP/(TP + FP), and the negative predictive value (NPV) was calculated as TN/(FN + TN). Sensitivity and specificity were used to calculate the likelihood ratios for a positive test result [sensitivity/(1 − specificity)] and a negative test result [(1 − sensitivity)/specificity] (18). The likelihood ratios were used to determine posttest probabilities by using Fagan’s nomogram (12). Test accuracy, the proportion of all tests that gave a correct result, was defined as (TP + TN)/number of all tests. Reliability was expressed as the J index (TP × TN − FP × FN)/(TP + FN)(TN + FP) (26).
In the analysis for malaria as a whole, results were considered false positive if microscopy detected P. falciparum and the immunochromatographic test detected P. vivax, and vice versa. Because mixed infections are read as P. falciparum alone, when analyzing test performance for the detection of P. vivax, mixed infections detected by microscopy were considered true negative if immunochromatographic testing detected P. falciparum and true positive if immunochromatographic testing detected P. vivax. HRP2 is thought to be present in immature (but not mature) gametocytes (4, 43), which may result in an immunological true-positive antigen-detection test result. However, in clinical evaluations of the ParaSight-F test, mature gametocytemia alone has not been associated with an excess of positive test results (4, 22, 43). Because sexual stages do not cause disease, samples that were HRP2 or panmalarial antigen positive by immunochromatographic tests but that were asexual parasite negative and gametocyte positive on microscopy were considered false positive (43).
The Kruskal-Wallis test was used to examine the overall relationship between parasite density on microscopy and immunochromatographic test line intensity categorized as described above. Stepwise two-sample t tests with log-transformed data were then used to test the significance of differences in mean parasitemia between each category of line intensity. Following Bonferroni adjustment for multiple comparisons, a P value of 0.017 was considered significant.
RESULTS
Of the 560 patients who met the case definition for clinical malaria, 289 (51.6%) were males and 271 (48.4%) were females. The age range was 0 to 80 years, with 50.7% children (age, <13 years) and 49.3% adults (age, ≥13 years). Almost all were Sumbanese (98.2%). Two hundred ninety-four (52.5%) of the 560 people with a presumptive clinical diagnosis of malaria were found to have parasitemia (with or without sexual forms); 279 (49.8%) had asexual-stage parasitemia (with or without sexual forms), and 15 (2.7%) had P. falciparum gametocytes only. Of the 279 people with asexual-stage parasitemia, 230 (82.4%) were infected with P. falciparum as detected by microscopy, 32 (11.5%) were infected with P. vivax, and 17 (6.1%) were infected with both P. falciparum and P. vivax.
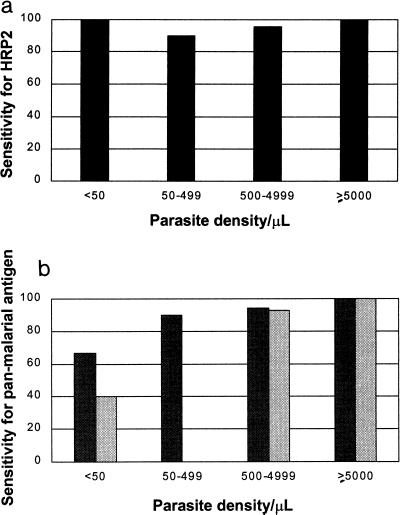
The results of parasite detection by microscopy and immunochromatographic testing are compared in Table 1. The test was sensitive (95.5%) and specific (89.8%) for the diagnosis of falciparum malaria, with a PPV and an NPV of 88.1 and 96.2%, respectively (Table 2). The corresponding sensitivity and specificity for the diagnosis of vivax malaria were 75 and 94.8%, respectively, with an NPV of 98.2% and a PPV of 50%. Other evaluation indices are given in Table 2. Test sensitivity for the detection of P. vivax increased with increasing density of parasitemia (Fig. 1): sensitivity for the detection of P. vivax was 96% with parasitemias of ≥500/μl but only 29% with parasitemias of <500/μl. The mean P. vivax parasitemia (excluding mixed infections) was 7,157/μl, with 22% (7 of 32) of the study subjects having parasitemias below 500/μl. Infections in 6 of the 32 subjects with P. vivax infection were not detected by the panmalarial antigen, 5 subjects had low parasite densities (≤280/μl), and 1 subject had a parasite density of 4,880/μl. Both test antibodies were very sensitive for the detection of P. falciparum antigens: 97% (223 of 230) for HRP2 and 96.1% (221 of 230) for the panmalarial antigen. Of the seven subjects with P. falciparum infection not detected by HRP2 (3%), not all had low parasitemias: four had parasite densities of between 120 and 1,000/μl, but the three others had densities of between 2,200 and 4,080/μl.
TABLE 1.
Comparison of ICT P.f/P.v and microscopic examination for malaria for 560 patients with a presumptive clinical diagnosis of malaria
| Microscopy result | No. of samples with the following result by ICT P.f/P.v:
|
|||
|---|---|---|---|---|
| P. falciparum | P. vivax | Negative | Total | |
| P. falciparum, asexual (±sexuala) | 223 | 4 | 3 | 230 |
| P. vivax, asexual (±sexual) | 2 | 24 | 6 | 32 |
| P. falciparum + P. vivax (±sexual) | 13 | 3 | 1 | 17 |
| P. falciparum sexual only | 11 | 1 | 3 | 15 |
| Negative | 19 | 22 | 225 | 266 |
| Total | 268 | 54 | 238 | 560 |
±sexual, with or without sexual-stage parasites.
TABLE 2.
Performance characteristics of ICT P.f/P.v relative to those of microscopy for 560 patients with a presumptive clinical diagnosis of malaria
| Microscopy result | Sensitivity (% [95% CIa]) | Specificity (% [95% CI]) | PPV (% [95% CI]) | NPV (% [95% CI]) | Accuracy (%) | J index | Likelihood ratio
|
|
|---|---|---|---|---|---|---|---|---|
| Positive test | Negative test | |||||||
| Total | 96.3 (93.2–98.1) | 79.4 (74.2–83.9) | 81.7 (76.9–85.7) | 95.8 (92.2–97.9) | 87.7 | 0.76 | 4.7 | 0.06 |
| P. falciparum, asexual (±sexualb) | 95.5 (92.0–97.6) | 89.8 (85.7–92.8) | 88.1 (83.4–91.6) | 96.2 (93.2–98.0) | 92.3 | 0.85 | 9.4 | 0.05 |
| P. vivax, asexual (±sexual) | 75 (57.5–87.3) | 94.8 (92.5–96.5) | 50.0 (36.3–63.7) | 98.2 (96.5–99.1) | 93.6 | 0.70 | 14.4 | 0.26 |
CI, confidence interval.
±sexual, with or without sexual-stage parasites.
FIG. 1.
Sensitivity of antibodies to the HRP2 and panmalarial antigens at different parasite densities (excluding mixed infections). (a) Sensitivity of tests for HRP2 antigen for detection of P. falciparum. (b) Sensitivity of tests for panmalarial antigen for detection of both species. Dark shading, asexual-stage P. falciparum; pale shading, P. vivax. The numbers in each category for P. falciparum and P. vivax, respectively, are as follows: <50/μl, 3 and 5; 50 to 499/μl, 30 and 2; 500 to 4,999/μl, 86 and 14; >5,000/μl, 107 and 11.
In febrile Sumbanese subjects in Radamata Health Centre the pretest probabilities of falciparum and vivax malaria on the basis of a clinical diagnosis were 44 and 5.7%, respectively. By using the calculated likelihood ratios presented in Table 2 (12), a positive ICT P.f/P.v result for each species gave posttest probabilities for falciparum and vivax malaria of 88 and 44%, respectively. A negative test gave posttest probabilities of 2.7 and 1.3%, respectively.
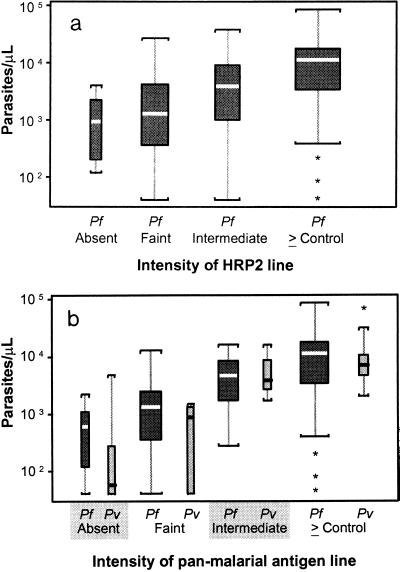
Panmalarial antigen line intensity was associated with parasitemia density for both species (Fig. 2a) (P < 0.0000001 and P < 0.0002 for P. falciparum and P. vivax, respectively, by the Kruskal-Wallis test). The differences in mean parasitemia between absent and faint, faint and intermediate, and intermediate and greater than or equal to that for control panmalarial antigen line intensities were more significant for P. falciparum (P = 0.06, P < 0.001, and P = 0.0024, respectively) than for P. vivax (P = 0.38, P = 0.014, and P = 0.17, respectively). HRP2 line intensity was also associated with P. falciparum parasitemia density (Fig. 2b) (by Kruskal-Wallis testing, P = 0.000005); however, the differences in mean parasitemias between absent and faint, faint and intermediate, and intermediate and greater than or equal to that for control HRP2 line intensities were less than those for the panmalarial antigen and were not significant (P = 0.38, P = 0.07, and P = 0.038, respectively).
FIG. 2.
Box plots of asexual parasite density per microliter by line intensity of the panmalarial antigen (a) and the HRP2 antigen (b), excluding mixed infections. Boxes show the interquartile ranges. The bold horizontal lines indicate medians; vertical lines indicate 1.5 times the interquartile range (or the total range if this is less), with significant outliers indicated by stars. The width of the boxes is proportional to the numbers in each category. For analysis of panmalarial antigen line intensity (a), there were 9 (3.9%), 61 (26.5%), 53 (23.1%), and 107 (46.5%) patients in the line intensity categories of absent, faint, intermediate, and greater than or equal to that for the control, respectively, for P. falciparum (Pf) and 6 (18.8%), 6 (18.8%), 8 (25%), and 12 (37.5%) patients in the four categories, respectively, for P. vivax (Pv). Panmalarial antigen line intensity was associated with increasing parasite density for both P. falciparum (P < 0.0000001) and P. vivax (P = 0.0002). For analysis of HRP2 antigen line intensity (b), there were 7 (3%), 38 (16.5%), 33 (14.4%) and 152 (66.1%) patients in the line intensity categories of absent, faint, intermediate, and greater than or equal to that for the control, respectively, for P. falciparum. HRP2 antigen line intensity was associated with increasing P. falciparum parasite density (P = 0.000005).
One-third (11 of 32) of subjects false positive for falciparum malaria were infected with gametocytes, as detected by microscopy. Of those with no asexual parasites on microscopy but an ICT P.f/P.v result indicating the presence of P. falciparum or P. vivax, approximately half (14 of 30 who were false positive for P. falciparum and 11 of 22 who were false positive for P. vivax) had had chloroquine treatment in the preceding 4 weeks. For microscopy-negative subjects, those with a history of chloroquine treatment in the preceding 4 weeks were twice as likely to be false positive for HRP2 (11.1%) as those without recent treatment (5.4%) (P = 0.17 by χ2 analysis) and twice as likely to be false positive for the panmalarial antigen (20.7 versus 10.8%) (P = 0.03). False-positive test results for panmalarial antigen were equally distributed in both batches of the MLO2 tests used, and the line intensity was faint for almost all (92%) samples that tested false positive. In contrast, the line intensities for only 53% of samples with false-positive HRP2 test results were faint, with 47% being of intermediate intensity or greater. Of the 15 subjects in whom only P. falciparum gametocytes were found by microscopy, 73.3% were positive for HRP2 (with 73% of these having a line intensity of intermediate or above), and 80% were positive for the panmalarial antigen (with 75% of these having a line intensity of intermediate or above).
The addition of the panmalarial antibody to the HRP2 antibody used in current antigen detection tests resulted in a significant reduction in undertreatment at the expense of a modest increase in overtreatment. Plasmodium infections in 41 (14.7%) of the 279 microscopy-positive patients with malaria were not detected by the HRP2 antibody. With the addition of the panmalarial antibody to the test kit, infections in only 10 (3.6%) of these microscopy-positive patients were not detected by the combined ICT P.f/P.v. Of the 266 subjects who were microscopy negative for both asexual and sexual parasites, 19 (7.1%) were false positive by HRP2 testing, and this number increased to 41 (15.4%) with the addition of the panmalarial antibody.
DISCUSSION
Because the monoclonal antibody to HRP2 used in ICT P.f/P.v is identical to that used in the ICT Malaria Pf, it is not surprising that the 96% sensitivity and 90% specificity for the detection of P. falciparum that we found using the combined ICT P.f/P.v were comparable to the high sensitivities (range, 92 to 100%) and specificities (range, 84 to 99%) previously reported for ICT Malaria Pf (11, 15, 16, 19, 23, 28, 32, 36, 40, 41). The sensitivity of ICT P.f/P.v was better than that previously reported for HRP2 antigen detection tests in Indonesia (13, 35). For the detection of P. falciparum, the sensitivity and specificity of ICT P.f/P.v (current study), ICT Malaria Pf (11, 15, 16, 19, 23, 28, 32, 36, 40, 41), and ParaSight-F (1, 4–6, 9, 13, 17, 22, 29–31, 39, 42) for HRP2 are, overall, at least equal to those of microscopy performed in a well-organized malaria diagnostic laboratory and much better than those routinely achieved by microscopy in remote primary health centers (43).
In contrast to the excellent sensitivity for the detection of P. falciparum, the overall sensitivity of ICT P.f/P.v for the detection of P. vivax was less than the desirable level. Although sensitivity was 96% with parasitemias of >500/μl, sensitivity was only 29% when parasitemias were below this level. Because of the relatively few symptomatic patients with vivax malaria and low parasitemias, it was not possible to define the detection threshold with certainty. The utility of the current test for the diagnosis of vivax malaria will depend on the clinical immunity and pyrogenic threshold in the target population. Pyrogenic threshold, which is the density of plasmodia required to invoke a febrile reaction in a given individual (20), is, on average, lower for vivax malaria than for falciparum malaria (20) and is lower in nonimmune subjects than in those with previous exposure (20, 21). For one large series of nonimmune subjects, the mean pyrogenic threshold during initial infection with vivax malaria was <500 parasites/μl (with over 70% of subjects developing fever when densities were <100/μl) (20), suggesting that with its current level of sensitivity, ICT P.f/P.v would miss a significant proportion of symptomatic nonimmune patients with vivax malaria. In populations in whom malaria is endemic, the pyrogenic threshold for vivax malaria is usually higher (20, 21), and in areas of endemicity, the current ICT P.f/P.v may detect vivax malaria in a greater proportion of semiimmune patients who show symptoms of vivax malaria. In our study area, which is hypoendemic for malaria, 78% (25 of 32) of patients with symptoms of vivax parasitemia had parasitemias above 500/μl (mean, 7,157/μl), and parasitemia was detected in 96% of these patients by the immunochromatographic test. As in previous studies with HRP2 (28, 43), occasional false-negative results for HRP2 and panmalarial antigens were found with high falciparum and vivax parasitemias, but the cause for this is not known (43).
The posttest probabilities of detection of vivax and falciparum malaria were such that in febrile Sumbanese, treatment decisions could reliably be made on the basis of the test results. Despite the less than desirable sensitivity of ICT P.f/P.v for the detection of P. vivax, the addition of the antibody to the panmalarial antigen to the monoclonal antibody to the HRP2 antigen significantly improved the probability of diagnosis of malaria in the study area. Compared to diagnosis and treatment decisions based on results of the test with HRP2 alone and despite the relatively low frequency of vivax malaria in Radamata, use of ICT P.f/P.v would significantly reduce the rate of undertreatment (from 14.7 to 3.6%) of microscopy-positive patients at the expense of only a modest increase (7.1 to 15.4%) in the rate of overtreatment of microscopy-negative patients. This increase is modest when one considers that 225 (84.6%) of these 266 microscopy-negative patients would in almost all instances be saved unnecessary antimalarial therapy. Moreover, they would also be more likely to be offered appropriate alternative treatment for their underlying nonmalarial illnesses.
It is possible that the true specificity of ICT P.f/P.v for the detection of P. falciparum is higher than that found by using microscopy as the gold standard. Specificity may have increased had PCR been used as the gold standard for the detection of parasitemia below the detection limit of microscopy. However, the higher rates of both recent treatment and gametocytemia that we found in those subjects with false-positive test results are consistent, with persistent posttreatment antigenemia (43) and gametocytemia (38) being additional likely explanations for false-positive test results. While mature gametocytes do not appear to cause false-positive results by ParaSight-F with HRP2 (4, 22, 43), our longitudinal evaluations of ICT P.f/P.v with symptomatic Sumbanese with microscopy-confirmed malaria have shown that false-positive results by tests with HRP2 and panmalarial antigen 1 week after treatment are significantly associated with the presence of gametocytemia (38). Rheumatoid factor has been found to cross-react with the ParaSight-F immunoglobulin G (IgG) monoclonal antibody to HRP2, causing false-positive results by this test (3, 24). However, rheumatoid factor only rarely cross-reacts or causes false-positive results with the IgM monoclonal antibody to HRP2 used in ICT Malaria Pf and ICT P.f/P.v (2, 25) and is thus very unlikely to be a cause of the false-positive results in tests with HRP2 in this study.
Over half of the positive results by ICT P.f/P.v for the detection of P. vivax were false positive, with the PPV of the test for P. falciparum (88.1%) being much greater than that for P. vivax (50%). Because of the low sensitivity of the test for the panmalarial antigen with P. vivax parasitemias of below 500/μl, vivax parasitemia below the detection limits of microscopy is very unlikely to explain false-positive results of tests with the panmalarial antigen. Although antibodies to both HRP2 and panmalarial antigens have high sensitivities for the detection of P. falciparum, it is possible that parasites in patients with P. falciparum parasitemia below the detection limit for microscopy could in some cases bind to the monoclonal antibody to the panmalarial antigen but not to the antibody to HRP2 and could give a false-positive reading for P. vivax. Alternative explanations are persisting posttreatment panmalarial antigenemia or nonspecific binding to the panmalarial antibody. Although the monoclonal antibody to the panmalarial antigen is IgG, cross-reactivity with rheumatoid factor does not appear to occur (14) and is thus unlikely to explain the false-positive results for vivax malaria.
Line intensities for the HRP2 antigen and particularly the panmalarial antigen were associated with parasite density. Semiquantitative assessment of these antigens in plasma may prove to be useful for the rapid prediction of parasite biomass in and prognosis for patients with severe malaria (8).
An alternative rapid dipstick method (OptiMAL) for the diagnosis of both P. falciparum and P. vivax malaria has also recently been introduced. This test uses a monoclonal antibody to the intracellular antigen parasite lactate dehydrogenase (pLDH). Like ICT P.f/P.v, this test also differentiates species by the use of a P. falciparum-specific antibody and a genus-specific antibody. Initial results for symptomatic Honduran patients have shown sensitivities of 88 and 94% and specificities of 100 and 99% for the diagnosis of falciparum and vivax malaria, respectively (27). Comparative studies will be required to assess the relative utility of the available combined antigen detection tests in areas of endemicity. However, current prices of all antigen detection tests, including ICT P.f/P.v (presently US$1.20 per test for >10,000 tests), are too high to enable widespread use in developing countries. Despite their advantages over microscopy and clinical diagnosis, the cost of all rapid antigen detection tests must be reduced if these tests are to ever become affordable in most areas where malaria is endemic.
ACKNOWLEDGMENTS
We thank Mary Garcia of AMRAD-ICT for providing ICT P.f/P.v; Umar Fahmi, Sumarjati Arjoso, Harijani Marwoto, Thomas Suroso, and Ferdinand Laihad, Ministry of Health, Jakarta, Indonesia, for support; Wang Zhiqiang, James McBroom, Peter Morris, and Jeni Wie for statistical and computing advice; Susan Hutton and Elizabeth Stubbs for logistic help; and Bambang Purnomo, Agus Berek, Frankie Hartanto, Sunarno, Frans Pello, Markus, Wayan, Yulius Weng, and staff, Regional, Provincial, District, and Subdistrict Health Offices, West Sumba, East Nusa Tenggara, Indonesia, for support and technical assistance.
Financial support for the study was received from Northern Territory Government 50th Anniversary of Indonesian Independence Malaria-Tuberculosis Research Fellowships. ICT P.f/P.v kits and some logistical costs were supported by AMRAD-ICT, Sydney, New South Wales, Australia.
REFERENCES
- 1.Banchongaksorn T, Prajakwong S, Rooney W, Vickers P. Operational trial of ParaSight-F (dipstick) in the diagnosis of falciparum malaria at the primary health care level. Southeast Asian J Trop Med Public Health. 1997;28:243–246. [PubMed] [Google Scholar]
- 2.Bartoloni A, Sabatinelli G, Benucci M. Performance of two rapid tests for Plasmodium falciparum malaria in patients with rheumatoid factors. N Engl J Med. 1998;338:1075. doi: 10.1056/NEJM199804093381518. [DOI] [PubMed] [Google Scholar]
- 3.Bartoloni A, Strohmeyer M, Sabatinelli G, Benucci M, Serni U, Paradisi F. False positive ParaSight-F test for malaria in patients with rheumatoid factor. Trans R Soc Trop Med Hyg. 1998;92:33–34. doi: 10.1016/s0035-9203(98)90945-2. [DOI] [PubMed] [Google Scholar]
- 4.Beadle C, Long G W, Weiss W R, McElroy P D, Maret S M, Oloo A J, Hoffman S L. Diagnosis of malaria by detection of Plasmodium falciparum HRP-2 antigen with a rapid dipstick antigen-capture assay. Lancet. 1994;343:564–568. doi: 10.1016/s0140-6736(94)91520-2. [DOI] [PubMed] [Google Scholar]
- 5.Caraballo A, Ache A. The evaluation of a dipstick test for Plasmodium falciparum in mining areas of Venezuela. Am J Trop Med Hyg. 1996;55:482–484. doi: 10.4269/ajtmh.1996.55.482. [DOI] [PubMed] [Google Scholar]
- 6.Craig M H, Sharp B L. Comparative evaluation of four techniques for the diagnosis of Plasmodium falciparum infections. Trans R Soc Trop Med Hyg. 1997;91:279–282. doi: 10.1016/s0035-9203(97)90074-2. [DOI] [PubMed] [Google Scholar]
- 7.Dean A G, Dean J A, Coulombier D, Brendel K A, Smith D C, Burton A H, Dicker R C, Sullivan K, Fagan R F, Arner T G. Epi Info, version 6: a word-prcocessing database, and statistics program for public health on IBM-compatible microcomputers. Atlanta, Ga: Centers for Disease Control and Prevention; 1995. [Google Scholar]
- 8.Desakorn V, Silamut K, Angus B, Sahassananda D, Chotivanich K, Suntharasamai P, Simpson J, White N J. Semi-quantitative measurement of Plasmodium falciparum antigen PfHRP2 in blood and plasma. Trans R Soc Trop Med Hyg. 1997;91:479–483. doi: 10.1016/s0035-9203(97)90292-3. [DOI] [PubMed] [Google Scholar]
- 9.Dietze R, Perkins M, Boulos M, Luz F, Reller B, Corey G R. The diagnosis of Plasmodium falciparum infection using a new antigen detection system. Am J Trop Med Hyg. 1995;52:45–49. doi: 10.4269/ajtmh.1995.52.45. [DOI] [PubMed] [Google Scholar]
- 10.Directorate General of Communicable Disease Control and Environmental Health. Malaria 3: pengobatan. Jakarta, Indonesia: Departemen Kesehatan RI; 1991. [Google Scholar]
- 11.Durrheim D N, la Grange J J, Govere J, Mngomezulu N M. Accuracy of a rapid immunochromatographic card test for Plasmodium falciparum in a malaria control programme in South Africa. Trans R Soc Trop Med Hyg. 1998;92:32–33. doi: 10.1016/s0035-9203(98)90944-0. [DOI] [PubMed] [Google Scholar]
- 12.Fagan T. Nomogram for Bayes’ theorem. N Engl J Med. 1975;293:257. doi: 10.1056/NEJM197507312930513. [DOI] [PubMed] [Google Scholar]
- 13.Fryauff D J, Gomez-Saladin E, Purnomo, Sumawinata I, Sutamihardja M A, Tuti S, Subianto B, Richie T L. Comparative performance of the ParaSight F test for detection of Plasmodium falciparum in malaria-immune and nonimmune populations in Irian Jaya, Indonesia. Bull W H O. 1997;75:547–552. [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia, M. Personal communication.
- 15.Garcia M, Kirimoama S, Marlborough D, Leafasia J, Rieckmann K H. Immunochromatographic test for malaria diagnosis. Lancet. 1996;347:1549. doi: 10.1016/s0140-6736(96)90700-x. [DOI] [PubMed] [Google Scholar]
- 16.Hudson B, Scurr R, Mackertich S, Vinen J. Rapid malaria test guides management. Med J Aust. 1997;167:230. doi: 10.5694/j.1326-5377.1997.tb138861.x. [DOI] [PubMed] [Google Scholar]
- 17.Humar A, Ohrt C, Harrington M A, Pillai D, Kain K C. Parasight F test compared with the polymerase chain reaction and microscopy for the diagnosis of Plasmodium falciparum malaria in travelers. Am J Trop Med Hyg. 1997;56:44–48. doi: 10.4269/ajtmh.1997.56.44. [DOI] [PubMed] [Google Scholar]
- 18.Jaeschke R, Guyatt G, Sackett D L. Users’ guides to the medical literature. III. How to use an article about a diagnostic test. A. Are the results of the study valid? JAMA. 1994;271:389–391. doi: 10.1001/jama.271.5.389. [DOI] [PubMed] [Google Scholar]
- 19.Kilian A H, Mughusu E B, Kabagambe G, von Sonnenburg F. Comparison of two rapid, HRP2-based diagnostic tests for Plasmodium falciparum. Trans R Soc Trop Med Hyg. 1997;91:666–667. doi: 10.1016/s0035-9203(97)90514-9. [DOI] [PubMed] [Google Scholar]
- 20.Kitchen S. Symptomatology: general considerations. In: Boyd M, editor. Malariology. II. Philadelphia, Pa: The W. B. Saunders Co.; 1949. pp. 966–994. [Google Scholar]
- 21.Kitchen S. Vivax malaria. In: Boyd M, editor. Malariology. II. Philadelphia, Pa: The W. B. Saunders Co.; 1949. pp. 1027–1045. [Google Scholar]
- 22.Kodisinghe H M, Perera K L, Premawansa S, de S. Naotunne T, Wickramasinghe A R, Mendis K N. The Parasight-F dipstick test as a routine diagnostic tool for malaria in Sri Lanka. Trans R Soc Trop Med Hyg. 1997;91:398–402. doi: 10.1016/s0035-9203(97)90255-8. [DOI] [PubMed] [Google Scholar]
- 23.Kumar A, Sharma V P, Thavaselvam D, Sumodan P K. Clinical trials of a new immunochromatographic test for diagnosis of Plasmodium falciparum malaria in Goa. Indian J Malariol. 1996;33:166–172. [PubMed] [Google Scholar]
- 24.Laferl H, Kandel K, Pichler H. False positive dipstick test for malaria. N Engl J Med. 1997;337:1635–1636. doi: 10.1056/NEJM199711273372219. [DOI] [PubMed] [Google Scholar]
- 25.Makler M T, Palmer C J, Ager A L. A review of practical techniques for the diagnosis of malaria. Ann Trop Med Parasitol. 1998;92:419–433. doi: 10.1080/00034989859401. [DOI] [PubMed] [Google Scholar]
- 26.Mharakurwa S, Manyame B, Shiff C J. Trial of the ParaSight-F test for malaria diagnosis in the primary health care system, Zimbabwe. Trop Med Int Health. 1997;2:544–550. doi: 10.1046/j.1365-3156.1997.d01-318.x. [DOI] [PubMed] [Google Scholar]
- 27.Palmer C J, Lindo J F, Klaskala W I, Quesada J A, Kaminsky R, Baum M K, Ager A L. Evaluation of the OptiMAL test for rapid diagnosis of Plasmodium vivax and Plasmodium falciparum malaria. J Clin Microbiol. 1998;36:203–206. doi: 10.1128/jcm.36.1.203-206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pieroni P, Mills C D, Ohrt C, Harrington A, Kain K C. Comparison of the ParaSight-F test and the ICT Malaria Pf test with the polymerase chain reaction for the diagnosis of Plasmodium falciparum in travellers. Trans R Soc Trop Med Hyg. 1998;92:166–169. doi: 10.1016/s0035-9203(98)90730-1. [DOI] [PubMed] [Google Scholar]
- 29.Premji Z, Minjas J N, Shiff C J. Laboratory diagnosis of malaria by village health workers using the rapid manual ParaSight-F test. Trans R Soc Trop Med Hyg. 1994;88:418. doi: 10.1016/0035-9203(94)90409-x. [DOI] [PubMed] [Google Scholar]
- 30.Shiff C J, Premji Z, Minjas J N. The rapid manual ParaSight-F test. A new diagnostic tool for Plasmodium falciparum infection. Trans R Soc Trop Med Hyg. 1993;87:646–648. doi: 10.1016/0035-9203(93)90273-s. [DOI] [PubMed] [Google Scholar]
- 31.Singh N, Singh M P, Sharma V P. The use of a dipstick antigen-capture assay for the diagnosis of Plasmodium falciparum infection in a remote forested area of central India. Am J Trop Med Hyg. 1997;56:188–191. doi: 10.4269/ajtmh.1997.56.188. [DOI] [PubMed] [Google Scholar]
- 32.Singh N, Valecha N, Sharma V P. Malaria diagnosis by field workers using an immunochromatographic test. Trans R Soc Trop Med Hyg. 1997;91:396–397. doi: 10.1016/s0035-9203(97)90254-6. [DOI] [PubMed] [Google Scholar]
- 33.Subdirectorate of Malaria Control. Passive case detection (PCD) during 1989 to 1993 in Outer Java-Bali. Jakarta, Indonesia: Directorate General of Communicable Disease Control and Environmental Health, Ministry of Health; 1994. [Google Scholar]
- 34.Subdirectorate of Malaria Control. Recapitulation of malariometric survey during 1989 to 1993 in Outer Java-Bali. Jakarta, Indonesia: Directorate General of Communicable Disease Control and Environmental Health, Ministry of Health; 1994. [Google Scholar]
- 35.Susanto L, Pribadi W, Astuty H. Diagnosis of malaria by rapid manual test. Med J Indonesia. 1995;4:24–29. [Google Scholar]
- 36.Thepsamarn P, Prayoollawongsa N, Puksupa P, Puttoom P, Thaidumrong P, Wongchai S, Doddara J, Tantayarak J, Buchachart K, Wilairatana P, Looareesuwan S. The ICT Malaria PF: a simple, rapid dipstick test for the diagnosis of Plasmodium falciparum malaria at the Thai-Myanmar border. Southeast Asian J Trop Med Public Health. 1997;28:723–726. [PubMed] [Google Scholar]
- 37.Tjitra, E. Unpublished data.
- 38.Tjitra E, Suprianto S, Dyer M, Currie B J, Anstey N M. Abstracts of the Annual Scientific Meeting of the Australasian Society of Infectious Diseases. 1999. Plasmodium falciparum gametocytes are associated with persistent dipstick-positive HRP-2 and panmalarial antigenemia after treatment of P. falciparum malaria, abstr. [Google Scholar]
- 39.Uguen C, Rabodonirina M, De Pina J J, Vigier J P, Martet G, Maret M, Peyron F. ParaSight-F rapid manual diagnostic test of Plasmodium falciparum infection. Bull W H O. 1995;73:643–649. [PMC free article] [PubMed] [Google Scholar]
- 40.Valecha N, Sharma V P, Devi C U. A rapid immunochromatographic test (ICT) for diagnosis of Plasmodium falciparum. Diagn Microbiol Infect Dis. 1998;30:257–260. doi: 10.1016/s0732-8893(98)00003-0. [DOI] [PubMed] [Google Scholar]
- 41.Van den Ende J, Vervoort T, Van Gompel A, Lynen L. Evaluation of two tests based on the detection of histidine rich protein 2 for the diagnosis of imported Plasmodium falciparum malaria. Trans R Soc Trop Med Hyg. 1998;92:285–288. doi: 10.1016/s0035-9203(98)91013-6. [DOI] [PubMed] [Google Scholar]
- 42.Verle P, Binh L N, Lieu T T, Yen P T, Coosemans M. ParaSight-F test to diagnose malaria in hypo-endemic and epidemic prone regions of Vietnam. Trop Med Int Health. 1996;1:794–796. doi: 10.1111/j.1365-3156.1996.tb00112.x. [DOI] [PubMed] [Google Scholar]
- 43.World Health Organization. A rapid dipstick antigen capture assay for the diagnosis of falciparum malaria. WHO Informal Consultation on Recent Advances in Diagnostic Techniques and Vaccines for Malaria. Bull W H O. 1996;74:47–54. [PMC free article] [PubMed] [Google Scholar]