Summary
An analytical approach combining the statistical distributions of the sleep-wake bouts and the Markov transition matrix is used to explain the under-examined association between the microarchitecture of the sleep-wake cycle and susceptibility to chronic social stress in C57BL/6J mice. We separated the sleep-wake transitions into distinct sleep-wake sequences, NREM↔Wake and NREM→REM→Wake, which are controlled by independent neural circuits. Our findings imply greater pull toward the wake leading to early termination and fragmentation of the sleep bouts in the light in both sleep-wake sequences pre- and post-stress. Moreover, the stability of NREM in the NREM↔Wake transition was lower, and the probability of transitioning to wake was higher in susceptible relative to resilient or stress-naïve mice pre- and post-stress. Our findings help elucidate the mechanistic interplay between sleep and mood by suggesting the potential neural underpinnings of sleep disturbances responsible the aberrant transitions of sleep-wake bouts exhibited by the stress-susceptible phenotype.
Subject areas: behavioral neuroscience, systems neuroscience
Graphical abstract
Highlights
-
•
Transition between sleep and wake are segregated into a short and long sleep states
-
•
NREM stability during short sleep is lower in susceptible mice pre- and post-stress
-
•
More NREM and wake bouts during short sleep in susceptible mice pre- and post-stress
-
•
Increased fragmented sleep in susceptible mice pre- and post-stress
Behavioral neuroscience; Systems neuroscience
Introduction
Sleep disturbances are a diagnostic criterion for stress-related disorders (Association and Association, 2000). Insomniacs are nine times more likely to concurrently have major depression than those who do not suffer from insomnia (Kaneita et al., 2006; Peterson and Benca, 2006; Taylor et al., 2005). Moreover, sleep disturbances such as insomnia and hypersomnia are a risk factor for major depressive disorder (Perlis et al., 1997; Jackson et al., 2003). Specifically, insomnia is associated with recurrent depressive episodes and precedes recurrent mood episode in patients diagnosed with bipolar disorder (Bauer et al., 2006).
Mice can be either resilient or susceptible to the chronic social defeat (CSD) stress paradigm (Krishnan et al., 2007; Chaudhury et al., 2013). Moreover, these mice exhibit differential physiological and molecular change, and it is hypothesized that resilient mice express larger homeostatic adaptive changes that buffer against stress induced changes observed in susceptible mice (Walsh et al., 2014; Friedman et al., 2014). We recently identified a potential vulnerability electroencephalogram (EEG) marker using the CSD stress paradigm where mice susceptible to future stress exhibited greater fragmented NREM sleep as shown by similar increases in the number of NREM and wake bouts due to increasing switching between both states together with the prevalence of shorter duration NREM bouts (Radwan et al., 2021). However, the microarchitecture of the sleep and wake states was not investigated in our previous study, which is crucial for developing insights into the neural mechanisms of transition between sleep and wake states. Therefore, in an attempt to infer the potential neural circuitry regulating these states, we investigated the dynamics of sleep-wake states in stress-susceptible and -resilient mice. Our analysis sorted the sleep-wake transitions into two separate sleep-wake sequences that are differentially and independently associated with the susceptible phenotype. Specifically, we noticed that sleep occurs either in series of NREM bouts interrupted by wake bouts or in a series of NREM bouts followed by REM bouts which are then followed by wake bouts. Therefore, we labeled the transition of vigilance states in NREM↔Wake, as short sleep state, and NREM→REM→Wake, as long sleep state. Indeed, the transition of NREM to either wake or REM is controlled by two separate neural circuits. The switch between NREM and Wake states was shown to be controlled by thalamic reticular nucleus (TRN) cells (Herrera et al., 2016), while the switch between the NREM and REM is controlled by dorsomedial hypothalamus (DMH) galaninergic neurons (Chen et al., 2018).
In order to better investigate the dynamical properties of sleep-wake states and their transitions, we assessed the distribution of pooled bouts duration in stress-susceptible, stress-resilient, and stress-naïve mice pre- and post-exposure to chronic social stress. In agreement with previous work, sleep bouts exhibited an exponential distribution, while the wake bouts exhibited power law distribution (Blumberg et al., 2005; Lo et al., 2004). We also used statistical tools such as discrete time Markov Chains, which is widely used to model sleep-wake states stability and transition (Kim et al., 2009; Perez-Atencio et al., 2018). Previous studies analyzed sleep-wake dynamics to elucidate differences in the control mechanisms of sleep and wake among the mammalian species during development (Lo et al., 2004; Blumberg et al., 2005, Mcshane et al., 2010). Here, we assessed how exposure, and adaptation, to chronic stress influence sleep and wake dynamics. Additionally, by having sleep-wake data pre-exposure to chronic social stress, we investigated the association between changes in sleep-wake dynamics and vulnerability to future stress. Our current study expands on our previous, and novel, observations that abnormal sleep is a marker of vulnerability to future stress. Specifically, we are the first to quantitatively analyze EEG data to investigate the association between abnormalities in sleep wake dynamics and susceptibility to stress, prior and post-exposure to CSD stress, a widely validated preclinical model of major depressive disorders (Krishnan et al., 2007; Golden et al., 2011; Chaudhury et al., 2013). With further investigation of the sleep-wake transitions in this current study, we are laying the groundwork for linking the changes in sleep-wake microarchitecture pre- and post-CSD to potential underlying neural circuitry. A potential application of these findings could be to screen people likely to be exposed to high stress situations.
Results
We analyzed the sequence of vigilance states across the light and dark cycles, pre- and post-CSD (Figure S1), and found that the vast majority of transitions occurs between NREM and Wake or from NREM to REM to Wake (Figure S2). We then separately analyzed the distributions of the sleep and wake bouts from pooled data across all the mice in each phenotypic group during NREM↔Wake, and NREM→REM→Wake transitions. Generally, sleep bouts displayed an exponential distribution while wake bouts displayed a power law distribution (Blumberg et al., 2005; Stephenson et al., 2013).
Shorter NREM bouts in NREM↔Wake transitions in stress-susceptible mice pre- and post-exposure to stress
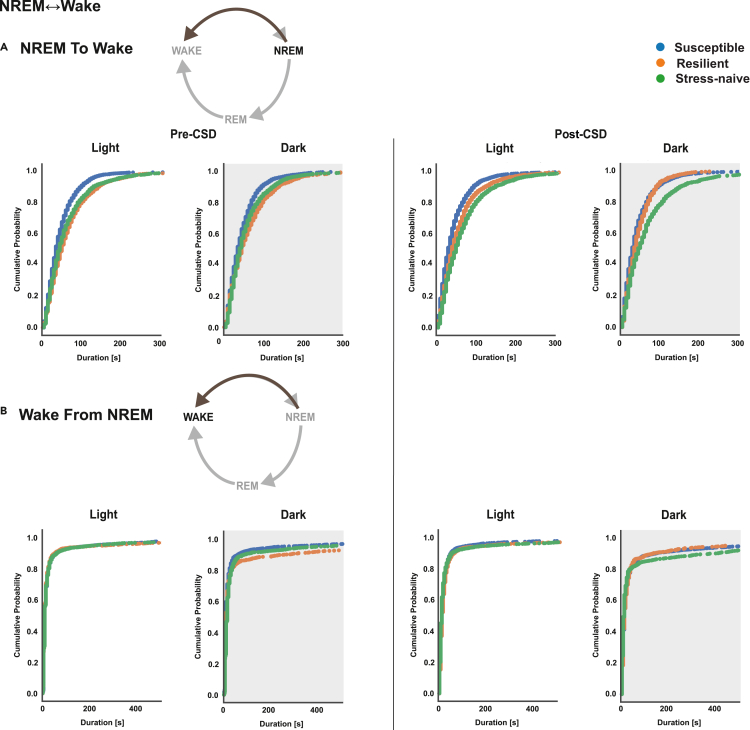
Pre-CSD, susceptible mice exhibited significantly shorter NREM bouts in NREM↔Wake transitions compared with resilient and stress-naïve mice (p < 0.05 for both comparisons; Figures 1A and S3A) in the light and the dark. Post-CSD, in the light, susceptible mice still exhibited shorter NREM bouts compared with resilient and stress-naïve mice (p < 0.05 for both comparisons; Figures 1A and S3A). Additionally, resilient mice exhibited shorter NREM bouts in NREM↔Wake transitions compared with stress-naïve mice (p < 0.05). In the dark, susceptible and resilient mice exhibited shorter NREM bouts in NREM↔Wake transitions compared with stress-naïve mice (p < 0.05 for both, bootstrap; Figures 1A and S3A). Analysis of wake bouts that transitioned from NREM bouts showed differences in wake distribution between the phenotypes only in the dark either pre- or post-CSD. Pre-CSD, in the dark, the wake bouts in NREM↔Wake transitions were shorter in susceptible mice compared with resilient and stress-naïve mice (p < 0.05 for both; Figures 1B and S3B). Post-CSD, in the dark, the wake bouts in NREM↔Wake transitions were longer in stress-naive mice compared with susceptible and resilient mice (p < 0.05 for both; Figures 1B and S3B). p values were computed via bootstrap method and Bonferroni-corrected for multiple comparisons.
Figure 1.
NREM bouts in NREM↔Wake are shorter in duration in susceptible mice compared to resilient and stress-naïve mice in the light and dark, while wake bouts of susceptible mice are shorter in the dark pre- and post-CSD
(A) NREM: Pre-CSD: Susceptible mice exhibited significantly shorter NREM bouts duration in NREM↔Wake compared with resilient and stress-naïve mice in the light and the dark (p < 0.05 for both). Post-CSD: Light: Susceptible mice exhibit significantly shorter NREM bouts duration in NREM↔Wake compared with resilient and stress-naïve mice (p < 0.05 for both). Resilient mice have shorter NREM bouts, in NREM↔Wake, compared with stress-naïve mice (p < 0.05). Dark: Susceptible mice exhibit shorter NREM bout durations in NREM↔Wake compared with resilient and stress-naïve mice (p < 0.05 for both).
(B) Wake: Susceptible mice exhibit shorter wake bouts in NREM↔Wake compared to resilient and stress-naive mice in the dark pre-CSD. Stress-naïve mice exhibit longer wake bouts in NREM↔Wake compared to susceptible and resilient mice in the dark post-CSD. Pre-CSD: Light: the duration of wake bouts in NREM↔Wake was comparable between phenotypes. Dark: Wake bouts in NREM↔Wake in susceptible mice are shorter than in resilient and stress-naïve mice (p < 0.05 for both). Post-CSD: Light: the duration of wake bouts in NREM↔Wake was comparable between phenotypes. Dark: Wake bouts in NREM↔Wake in stress-naive mice are longer than in susceptible and resilient mice (p < 0.05 for both). Values are pooled individual bouts duration within each phenotype in the light and dark separately pre- and post-CSD. p values are computed via bootstrap method and Bonferroni-corrected for multiple comparisons. N = 7-8 for each group.
Shorter NREM and REM bouts in NREM→REM→Wake transitions in stress-susceptible mice pre- and post-exposure to stress
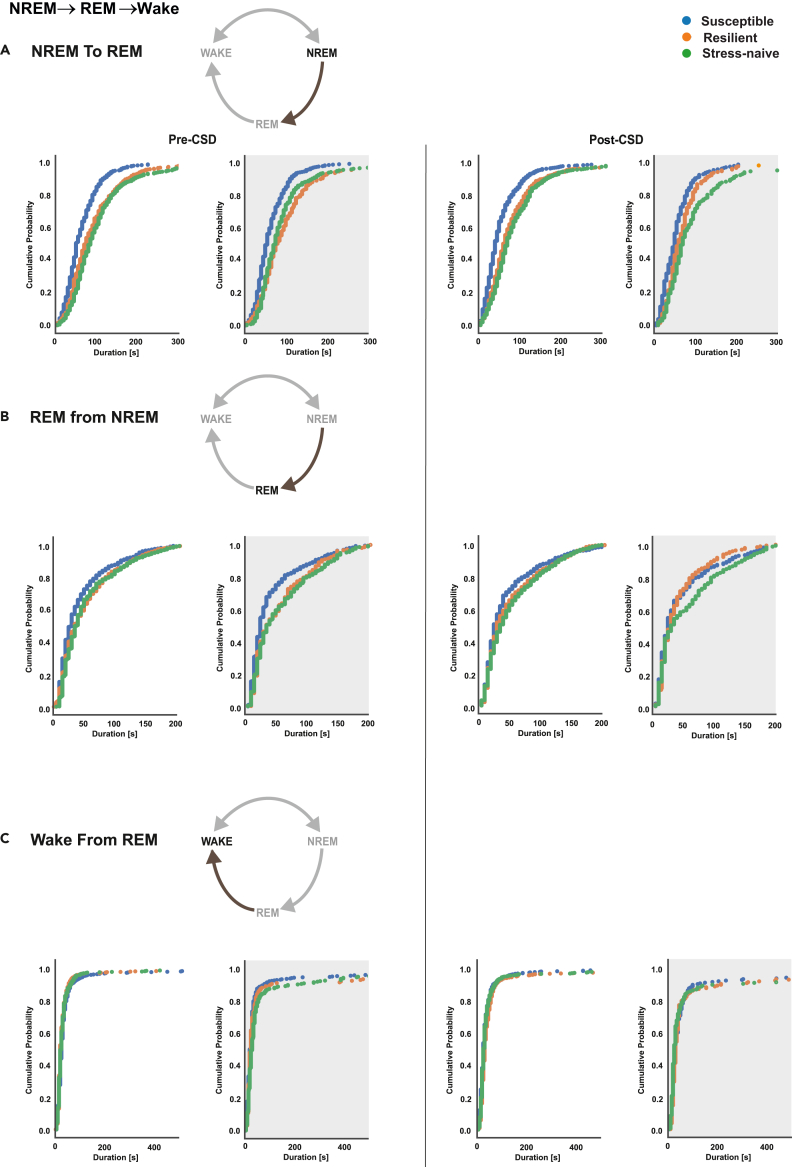
Pre-CSD, susceptible mice exhibited significantly shorter NREM bouts in NREM→REM→Wake transitions compared to resilient and stress-naïve mice (p < 0.05 for both comparisons; Figure 2A) in the light and the dark. Moreover, the distribution of NREM bouts duration of resilient mice was different than that of stress-naïve mice in the light (p < 0.05; Figures 2A and S4A). Post-CSD, this pattern persisted as the NREM bouts in NREM→REM→Wake transitions of susceptible mice were shorter relative to resilient and stress-naïve mice (p < 0.05 for both; Figure 2A) in the light and the dark. Pre-CSD, susceptible mice exhibited significantly shorter REM bouts compared with resilient and stress-naïve mice (p < 0.05 for both; Figures 2B, S4B and S5) in the light and the dark. Post-CSD, susceptible mice exhibited significantly shorter REM bouts in NREM→REM→Wake transitions compared with stress-naïve mice in the light (p < 0.05; Figures 2B, S4B and S5). In the dark, resilient mice exhibited shorter REM bouts compared with stress-naïve mice (p < 0.05). No difference was detected in the wake bouts between the phenotypes either pre- or post-CSD. However, there was a significant difference between the distribution of wake bouts of both susceptible and stress-naïve in the light pre-CSD (p < 0.05; Figures 2C and S4C). p values were computed via bootstrap method and Bonferroni-corrected for multiple comparisons.
Figure 2.
Susceptible mice exhibit shorter NREM and REM bouts in NREM→REM→Wake compared to resilient and stress-naïve mice pre- and post-CSD
The duration of wake bouts was comparable among the phenotypes.
(A) NREM: Susceptible mice exhibit shorter duration of NREM bouts in NREM→REM→Wake compared to resilient and stress-naïve mice in the light and the dark pre- and post-CSD (p < 0.05 for all comparisons).
(B) REM: Pre-CSD: Susceptible exhibit shorter REM bouts compared to resilient and stress-naïve mice in the light and dark (p < 0.05 for both). Post-CSD: Light: Susceptible mice exhibit shorter REM bouts compared to stress-naïve (p < 0.05). Dark: Resilient mice exhibit shorter REM bouts compared to stress-naïve (p < 0.05).
(C) Wake: No difference in the duration of wake bouts between the phenotypes pre- and post-CSD either in the light or the dark in general, except for a difference between susceptible and stress-naïve mice pre-CSD in the light (p < 0.05). Values are pooled individual bouts duration for each phenotype separated for the light and dark separately pre- and post-CSD. p values are computed via bootstrap method and Bonferroni-corrected for multiple comparisons. N = 7-8 for each group.
Lower stability of NREM bouts in NREM↔Wake transitions only in susceptible mice due to greater probability of transition to wake pre- and post-exposure to stress
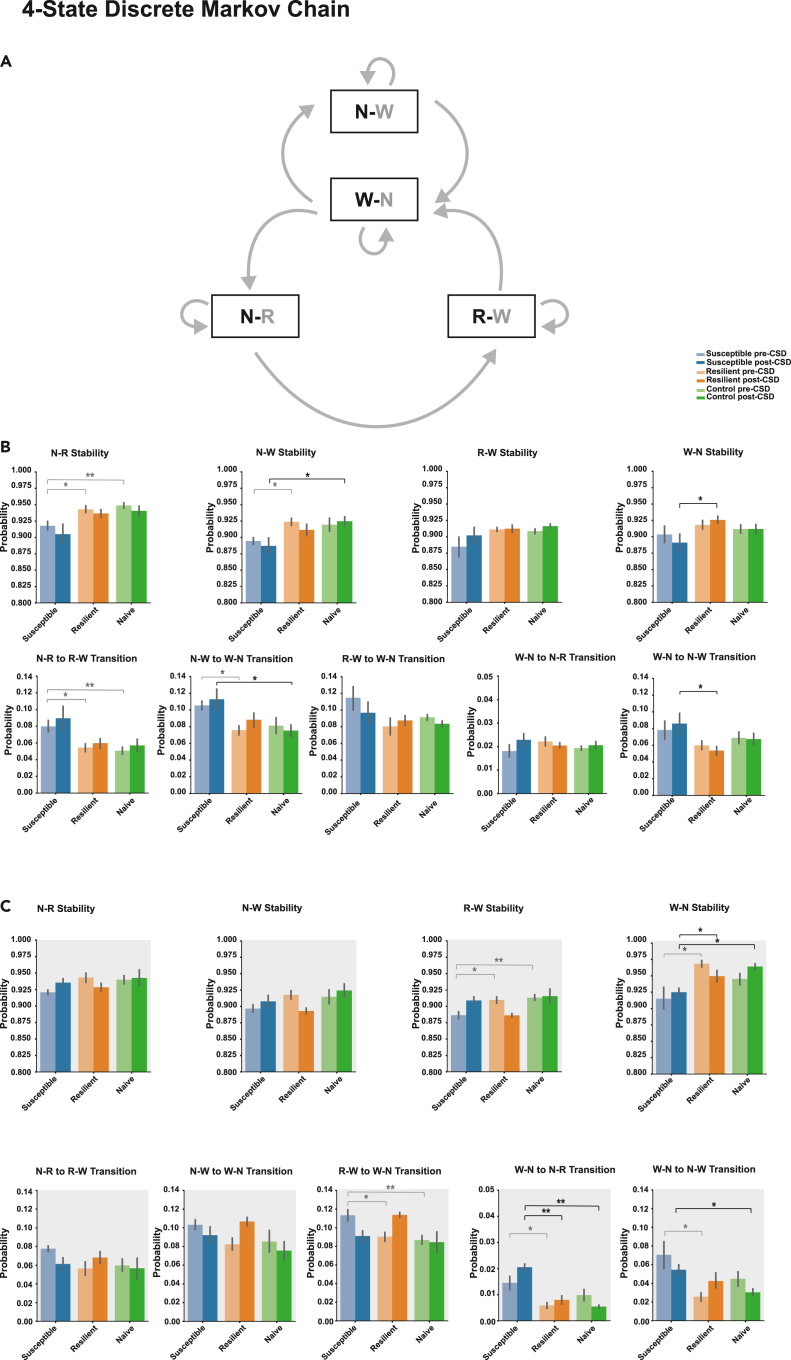
Based on the previous results that susceptible mice generally exhibited shorter sleep bouts duration in NREM↔Wake and NREM→REM→Wake transitions compared with resilient and stress-naïve mice we assessed the stability of the sleep states and the probability of transitioning to the wake state. A three-state discrete Markov chain was used to model the transition between NREM, REM, and wake states, using discrete 5 s time steps (Figures S6A and S7A). Markov modeling of the discrete states allowed us to compute the probability of remaining in one state, which is related to state duration, and the probability of transition to and from that state (Perez-Atencio et al., 2018). Markov Transition matrices were computed across all phenotypes pre- and post-CSD in the light and dark (Figures S6B and S7B). Pre-CSD, in the light, the NREM state stability, as depicted by the probability of NREM transitioning to itself, was lower in susceptible mice compared with resilient and control mice (p < 0.05 for both; Figure S6C). Moreover, the probability of transitioning from NREM to Wake was significantly higher in susceptible mice relative to resilient mice and a trend of being higher relative to stress-naïve mice (p < 0.01 and p = 0.05; Figure S6C). In the dark, the stability of the sleep and wake states was generally lower, while the probability of transitioning from sleep to wake was higher in the susceptible mice. Specifically, there was a trend showing lower stability of NREM states in susceptible, compared with resilient, mice (p = 0.055; Figure S6D). REM stability of susceptible mice was lower relative to resilient and stress-naïve mice (p < 0.05 for both; Figure S6D). Wake stability was lower in susceptible mice compared with resilient mice (p < 0.05; Figure S6D). The probability of transition from wake to NREM was higher in susceptible mice compared with resilient mice, and the probability of transition from REM to wake was higher in susceptible mice compared with resilient and stress-naïve mice (p < 0.05 for all comparisons; Figure S6D). Post-CSD, there was no difference between the stability and the transition of the sleep and wake states between the phenotypes (Figures S7C and S7D). In order to separately assess the sleep-wake states in NREM↔Wake and NREM→REM→Wake transitions, we used a more granular four-state discrete Markov model to model the transitions between NREM→Wake (N-W) and Wake→NREM (W-N) in NREM↔Wake and NREM→REM (N-R), REM→Wake (R-W) and Wake→NREM (W-N) in NREM→REM→Wake transitions. The sleep-wake bouts of interest are marked in bold. Both transition paths converge on wake bouts (W-N) that transition to either NREM→Wake (N-W) in NREM↔Wake or NREM→REM (N-R) in NREM→REM→Wake. The direct transitions of REM→NREM or Wake→REM were rarely observed and were omitted from the granular 4-state Markov model (Figures 3A and S8A). Pre-CSD, in the light, the NREM bouts in both NREM↔Wake and NREM→REM→Wake transitions were less stable in susceptible compared with resilient mice. Specifically, NREM→REM (N-R) states were less stable in susceptible mice compared with resilient and stress-naïve mice (p < 0.05 and p < 0.01; Figures 3B and S8B). This is because susceptible mice exhibited higher probability of transition of NREM→REM (N-R) to REM→wake (R-W) compared with resilient and stress-naïve mice (p < 0.05 and p < 0.01; Figures 3B and S8B). Additionally, NREM→wake (N-W) is less stable in susceptible mice compared with resilient mice (p < 0.05; Figure 3B) as susceptible mice exhibited greater probability of transitions of NREM→wake (N-W) to Wake→NREM (W-N) compared with resilient mice (p < 0.05; Figures 3B and S8B). Post-CSD, in the light, the stability of NREM→wake (N-W) was lower and the probability of transition of NREM→wake (N-W) to Wake→NREM (W-N) was greater in susceptible mice compared with stress-naïve mice (p < 0.05 for both; Figures 3B and S8B). Additionally, the stability of Wake→NREM (W-N) was lower (p < 0.05; Figures 3B and S8B) because the probability of transition of Wake→NREM (W-N) to NREM→wake (N-W) was greater in susceptible compared with resilient mice (p < 0.05; Figures 3B and S8B). In contrast, pre-CSD, in the dark, the stability of REM→wake (R-W) was lower in susceptible compared with resilient and stress-naïve mice (p < 0.05 for both; Figures 3C and S8C). This is because the transition of REM→wake (R-W) to Wake→NREM (W-N) was greater in susceptible mice compared with resilient and stress-naïve mice (p < 0.05 for both; Figure 3C). Moreover, the stability of Wake→NREM (W-N) in susceptible mice was lower compared with resilient mice (p < 0.05; Figures 3C and S8C). This is because the probability of transitioning of Wake→NREM (W-N) to NREM→REM (N-R) or to NREM→wake (N-W) was greater in susceptible mice compared with resilient mice (p < 0.05 for both; Figures 3C and S8C). Post-CSD, in the dark, the stability of Wake→NREM was lower and the probability of transition of Wake→NREM (W-N) to NREM→wake (N-W) was greater in susceptible versus resilient mice (p < 0.05 for both). Moreover, susceptible mice exhibited greater probability of transition of Wake→NREM (W-N) to NREM→REM (N-R) compared with resilient and stress-naïve mice (p < 0.01 for both; Figures 3C and S8C). Tukey's multiple comparisons tests were used for all the comparisons.
Figure 3.
Susceptible mice exhibit lower stability of NREM bouts relative to resilient or stress-naïve mice in the light pre- and post-CSD
(A) We fit a four-state discrete Markov chain to model the state transitions in the sequence of sleep and wake bouts in 5-s discrete steps. The sleep-wake bouts of interest are marked in bold.
(B) Pre-CSD: Light: Susceptible mice exhibit lower stability of NREM bouts (N-R) in NREM→REM→Wake compared to resilient and stress-naïve mice (p < 0.05 and p < 0.01 respectively). Susceptible mice exhibit lower stability of NREM bouts (N-W) in NREM↔Wake compared to resilient mice (p < 0.05). Susceptible mice exhibit greater probability of transition of NREM (N-R) to REM (R-W) in NREM→REM→Wake relative to resilient and stress-naïve mice (p < 0.05 and p < 0.01 respectively). Susceptible mice exhibit greater probability of transition of NREM (N-W) in NREM↔Wake to wake (W-N) relative to resilient mice (p < 0.05). Post-CSD: Light: Susceptible mice exhibit lower stability of NREM bouts (N-W) in NREM↔Wake relative to that of stress-naïve mice (p < 0.05), while the stability of wake (W-N) was lower in susceptible compared to resilient mice (p < 0.05). Susceptible mice exhibit greater probability of transition of NREM bouts in NREM↔Wake to wake (W-N) relative to stress-naïve (p < 0.05). Susceptible mice exhibit greater probability of transition wake (W-N) to NREM (N-W) in NREM↔Wake relative to resilient mice (p < 0.05).
(C) Pre-CSD: Dark: Susceptible mice exhibit lower stability of REM bouts in NREM→REM→Wake relative to resilient and stress-naïve mice (p < 0.05 and p < 0.01 respectively) due to greater probability of transition of REM bouts to wake (W-N) in susceptible mice relative to resilient and stress-naïve mice (p < 0.05 and p < 0.01, respectively). The stability of wake bouts (W-N) is lower in susceptible mice relative to resilient mice (p < 0.05) due to greater probability of transition of wake bouts (W-N) to either NREM bouts (N-R) in NREM→REM→Wake or to NREM bouts (N-W) in NREM↔Wake relative to resilient mice (p < 0.05 for both). Post-CSD: Dark: Susceptible mice exhibit lower stability of wake bouts (W-N) compared with resilient and stress-naïve mice (p < 0.05 for both). Susceptible mice exhibit greater probability of transition of wake (W-N) to NREM (N-R) in NREM→REM→Wake relative to resilient and stress-naïve mice (p < 0.01 for both). Susceptible mice exhibit greater probability of transition of wake (W-N) to NREM (N-W) in NREM↔Wake relative to stress-naïve mice (p < 0.05). Values are expressed as mean (±sem) of probability of state transitions. Light gray asterisks represent the statistical significance of comparison between pre-CSD probability values, while black asterisks represent the statistical significance of comparison between post-CSD probability values. ∗ and ∗∗ denote, p < 0.05, and p < 0.01, respectively. One-way ANOVAs followed by post hoc Tukey tests were performed for multiple comparisons. N = 7-8 for each group.
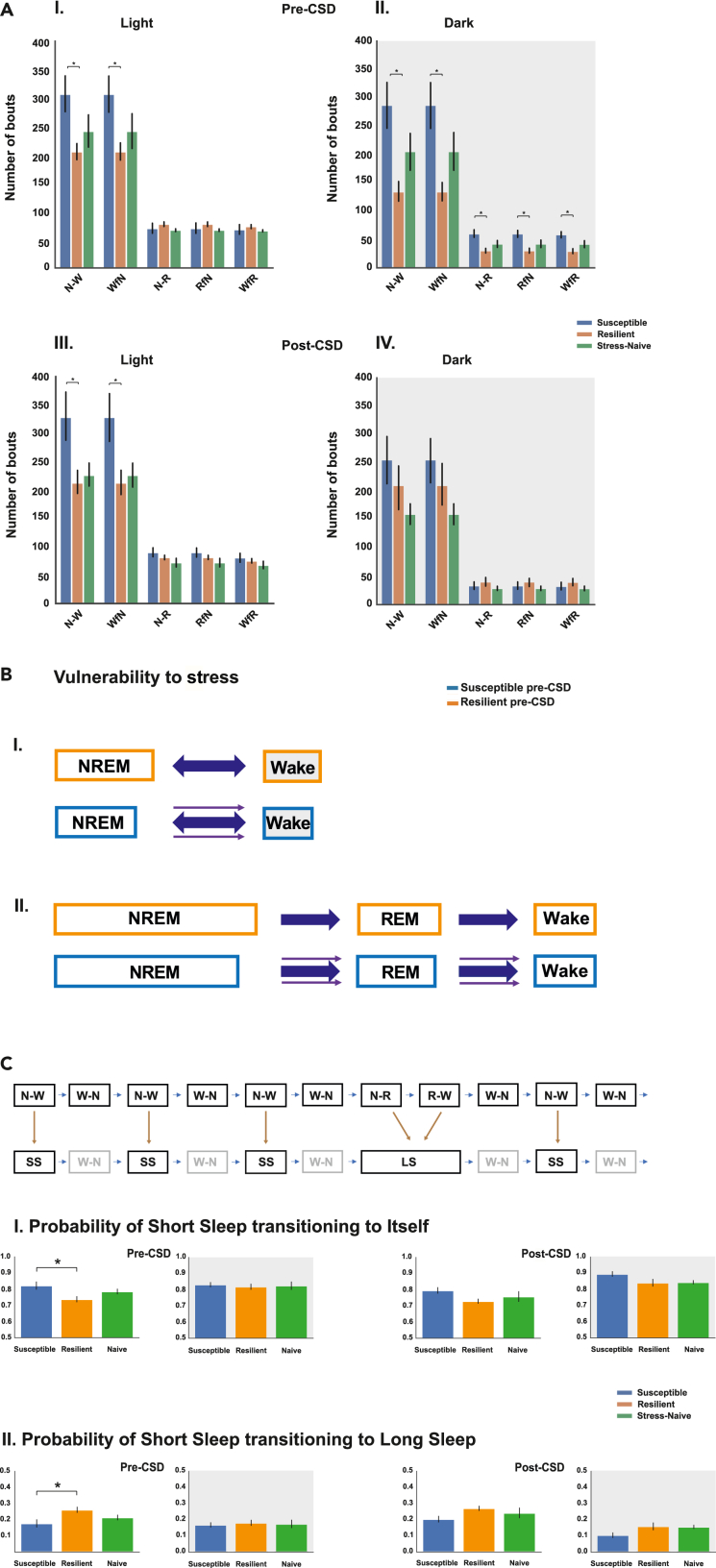
Susceptible mice exhibited greater number of NREM and wake bouts in NREM↔Wake only in the light pre- and post-CSD
Based on the previous findings of shorter sleep bouts, specifically the lower stability of NREM bouts, in the susceptible mice, we investigated whether the lower stability and higher probability of transitions of the sleep bouts were accompanied by greater switching between states. Pre-CSD, the number of bouts of both NREM (p < 0.05) and wake during NREM↔Wake transitions (p < 0.05) was greater in susceptible compared with resilient mice in the light and the dark (F2,21 = 3.71, p < 0.05; Figure 4AI). However, in the dark, susceptible mice exhibited greater number of bouts during NREM→REM→Wake transitions relative to resilient mice (p < 0.05 for all bouts; Figure 4AII). Post CSD, in the light, the number of bouts of both NREM and wake during NREM↔Wake transitions was greater in susceptible mice compared with resilient mice (p < 0.05 for both; Figure 4AIII). In the dark, there was no difference in the number of bouts either during NREM↔Wake or NREM→REM→Wake transitions between the phenotypes. Tukey's multiple comparisons test were used across all the comparisons post-ANOVA (Figure 4AIV). Using our findings, we constructed a simple model highlighting the predominant differences in the dynamics of sleep-wake bouts in susceptible versus either resilient or stress-naïve mice pre-CSD (Figure 4B) and post-CSD (Figure S9). Pre-CSD, mice susceptible to future stress exhibited shorter NREM bout duration during both NREM↔Wake and NREM→REM→Wake transitions compared with resilient mice. In contrast, the duration of the wake bouts was only shorter in susceptible compared with resilient mice during NREM↔Wake in the dark. Additionally, the number of bouts of NREM and Wake in NREM↔Wake was greater in susceptible mice relative to resilient mice. In summary, we hypothesize that in susceptible mice there might be a greater pull toward the wake state leading to early termination, or shorter duration, of the sleep bouts in both NREM↔Wake and NREM→REM→Wake transitions (Luthi, 2016). The greater pull was accompanied by a greater number of transitions only during NREM↔Wake (Figures 4B and S10). Post-CSD, the mice resilient to stress exhibited shorter NREM bouts in NREM↔Wake in the light relative to stress-naïve mice, while all the sleep and wake bouts were shorter in the dark relative to stress-naïve. This suggests that the sleep bouts in the light in NREM↔Wake are more sensitive to the effects of chronic stress (Figure S9).
Figure 4.
Susceptible mice exhibit increased switching between NREM and wake bouts in NREM↔Wake
(A I-IV). Susceptible mice exhibit greater number of bouts of NREM and Wake in NREM↔Wake compared with resilient mice pre- and post-CSD in the light. Pre-CSD, in the dark, the number of bouts of all vigilance states was greater in susceptible compared to resilient mice (p < 0.05 for all comparisons).
(B) Pre-CSD: Model summarizing the findings of sleep wake dynamics associated with vulnerability to stress. A greater pull towards the wake state in the sleep wake flip flop switch might lead to early termination of the sleep bouts or shorter duration with no apparent change in the wake bouts duration in the light. The greater pull leads to increased switching between the sleep and wake states only in NREM↔Wake.
(C) Modeling of the sleep state(s) in NREM↔Wake as “short sleep” and in NREM→REM→Wake as “long sleep” and the use of 2-state discrete Markov model to assess the stability of “short sleep” state and the probability of transition from “short sleep” to “long sleep.” (CI) Susceptible mice exhibited greater stability of “short sleep” state and (CII) lower probability of transition to “long sleep” state relative to resilient mice pre-CSD only in the light. ∗ denotes, p < 0.05. One-way ANOVAs followed by post hoc Tukey tests were performed for multiple comparisons. N = 7-8 for each group.
Next, we modeled the sleep states during NREM↔Wake and NREM→REM→Wake as “short sleep” and “long sleep” global states respectively transitioning to wake states. The global sleep bouts in the transitions are described in bold. As previously mentioned, both these global states converge on wake bouts (W-N) that transition to NREM either in NREM↔Wake or in NREM→REM→Wake (Figure S11). We assessed how many “short sleep” events/states occur on average before the occurrence of a “long sleep” event/state across the different phenotypes (Table S1). Pre-CSD, in the light, there was a trend showing greater number of “short sleep” occurrences in susceptible relative to naïve mice (p = 0.06, Tukey's multiple comparison test, one-way-ANOVA: F2,21 = 3.017, p = 0.07; Table S1). In the dark, there was no difference between the phenotypes in the number of “short sleep” occurrences before “long sleep” event (F2,21 = 0.953, p = 0.41; Table S1). Post-CSD, in the light, susceptible mice have greater number of “short sleep” occurrences before “long sleep” occurrences relative to resilient mice (p = 0.049, Tukey's multiple comparison test, one-way-ANOVA: F1,21 = 3.93, p = 0.04; Table S1). In the dark, there was no difference among the phenotypes in the number of “short sleep” occurrences before “long sleep” event (F1,21 = 1.28, p = 0.31; Table S1). An additional way to assess the differences in the occurrence of the global sleep states is to model the sequence of “short sleep” and “long sleep” episodes using the 2-state discrete Markov model, by removing the intervening wake bouts, to assess the probability of maintaining the “short sleep” state and the probability of transitioning from “short sleep” to “long sleep.” Pre-CSD, in the light, susceptible mice exhibited greater probability of maintaining “short sleep” state relative to resilient mice (p < 0.05, Tukey's multiple comparison test, one-way-ANOVA: F2,21 = 4.53, p < 0.05; Figure 4CI). Moreover, susceptible mice displayed a lower probability of transitioning to “long sleep” from “short sleep” relative to resilient mice (p < 0.05, Tukey's multiple comparison test, one-way-ANOVA: F2,21 = 4.528, p < 0.05; Figure 4CII). No difference was detected between the phenotypes in the dark. Post-CSD, no difference was detected between the phenotypes in the light and the dark (Figure 4C). Pre-CSD, in the light, susceptible mice spent less time in “long sleep” by spending less time in NREM and REM states during NREM→REM→Wake transitions relative to resilient mice (p < 0.05 for both comparisons, Tukey's multiple comparison test; Figure S12I), while in the dark, susceptible mice spent more time in “short sleep” by spending more time in NREM bouts in NREM→Wake (“short sleep”) relative to resilient mice (p < 0.05; Figure S12II). Post-CSD, in the light, the time spent in wake, following “short sleep” in NREM↔Wake, by susceptible mice is greater than the time spent by stress-naïve mice (p < 0.05, Figure S12III).
Discussion
Previous studies have focused on identifying macro-changes in the sleep-wake features of mice pre- and post-exposure to CSD stress (Henderson et al., 2017; Olini et al., 2017; Radwan et al., 2021). One of the first approaches for elucidating sleep-wake dynamics is by investigating the statistical properties of the sleep and wake bouts. Prior work showed that the sleep bouts follow an exponential distribution, where the mean changes are based on the size across species and on the age within the same species (Blumberg et al., 2005; Lo et al., 2004). Since we previously found that NREM bouts duration were fragmented in mice vulnerable to chronic stress (Radwan et al., 2021), we then investigated the “fine temporal structure” of sleep and wake bouts pre-and post-exposure to stress.
Based on the sleep and wake state transitions in our data, we segregated them into two separate sleep-wake sequences, either a “short sleep” state in NREM ↔ Wake, or a “long sleep” state in NREM→REM→Wake. The “short sleep” state consists of one sleep state, NREM that transitions to wake, while the “long sleep” state consists of 2 sleep states followed by wake. We initially compared the exponential distribution of sleep bout durations during NREM↔Wake and NREM→REM→Wake transitions and found that susceptible mice exhibit shorter sleep bouts duration (NREM and REM) pre- and post-CSD relative to resilient and stress-naïve mice, especially in the light cycle. The power law distribution of the wake bouts did not change between the phenotypes pre- and post-CSD, except in the dark. We hypothesize there is a stronger pull towards the wake state in the sleep-wake flip flop switch leading to the earlier termination of the NREM or REM sleep bouts. Our hypothesis further suggests testing whether the stronger pull toward the wake state is accompanied with both a greater number and probability of transitions between the sleep and wake states. Interestingly, in susceptible mice, pre- and post-CSD in the light, the number of transitions between NREM and wake was greater relative to resilient mice only during NREM↔Wake but not in NREM→REM→Wake transitions. In summary, our data suggests a strong association between susceptibility to stress and changes in duration and number of NREM sleep bouts during NREM↔Wake transitions. Moreover, our findings imply that NREM bouts during NREM↔Wake transitions are more sensitive to the effect of chronic exposure to stress as the mice resilient to stress exhibited shorter NREM during NREM↔Wake in the light relative to stress-naïve post-, but not, pre-CSD.
The Markov model is a powerful tool for evaluating sleep-wake dynamics, and we are among the first to use it to assess the change in the sleep-wake dynamics associated with vulnerability to stress or post-exposure to chronic social stress. We fitted a 4-state discrete Markov model to fully account for the two separate sleep-wake bouts transitions during NREM↔Wake and NREM→REM→Wake since analysis using the 3-state discrete Markov model is incapable of capturing such granularity. NREM bouts during NREM↔ Wake exhibited lower stability and greater probability of transitioning to wake in susceptible mice relative to resilient mice pre-CSD in the light and relative to stress-naïve post-CSD in the light. Additionally, NREM bouts during NREM→REM→Wake are less stable in susceptible mice pre-CSD in the light with greater probability of transitioning to REM relative to resilient mice. No change in the stability of the wake bouts between the phenotypes in the light was observed which is due to intact wake bout duration.
Activity of galaninergic neurons in the DMH controls the switch between NREM and REM. Within the DMH two sub-populations of galaninergic neurons exist that project to the preoptic area (POA) or raphe pallidus (RPA) (Chen et al., 2018). These are REM-Off and REM-ON, respectively as optogenetic stimulation of POA-projecting neurons increases the probability of maintaining NREM state during NREM→REM→Wake transitions, while optogenetic stimulation of RPA-projecting neurons increase the switch from NREM to REM. Conversely, a separate neural circuit controls the NREM↔Wake transitions. Activity of TRN neurons were shown to control the transition of NREM to wake state. Specifically, optogenetic stimulation of GABAergic neurons of the lateral hypothalamus (LH) projecting to the TRN induces rapid transition from NREM to wake (Herrera et al., 2016). The increased transitions from NREM to Wake and Wake to NREM in susceptible mice may be due to rapid switching between active and silence states of LHGABA cells projecting to TRN cells. Our data suggest that these areas might be a more appropriate target for investigating the link between sleep disturbances and susceptibility to future stress such as CSD.
Medium spiny neurons (MSNs) of the nucleus accumbens (NAc) contain two subtypes of neurons that express either D1 or D2 dopamine receptors (Muir et al., 2018). Previous work showed that NAc D1 and D2 MSNs display different neural activity in the mice that develop depressive-like symptoms post-CSD. For example, D1 but not, D2 MSNs, of mice susceptible to social stress exhibit increased intrinsic excitability (Francis et al., 2015). Moreover, the neurotrophic factor BDNF acts predominantly on D1 MSNs in stress susceptible mice, which is likely responsible for increased levels of phosphorylated (active) extracellular signal-regulated kinase (p-ERK) in these cells (Wook Koo et al., 2016). In a separate study, D1-MSNs were shown to be important in promoting wakefulness while D2-MSNs activity promotes NREM sleep (Luo et al., 2018). Thus, pathophysiological functioning of these cells may be responsible, in part, for differences in sleep architecture observed in susceptible and resilient mice. D1 receptor activation is predominantly excitatory while D2 receptor activation is predominantly inhibitory (Martel and Gatti Mcarthur, 2020). Moreover, ventral tegmental area (VTA) dopaminergic cells projecting to the NAc of susceptible mice exhibit increased burst firing (Chaudhury et al., 2013). Thus, it is possible that burst firing of VTA dopamine input to the NAc in susceptible mice activates D1 MSNs leading to a net increased drive for wakefulness. This may be partly responsible for our observation of stronger pull toward the wake leading to shorter sleep bouts duration and increase number of transitions. Further investigation is required to elucidate the differential effect of D1 and D2 MSNs activation in susceptible mice and its association with the aberrant sleep-wake behavior.
We next labeled the NREM bouts in NREM↔Wake as “short sleep” state and the NREM and REM bouts in NREM→REM→Wake as “long sleep” state. We hypothesized that increased switching of NREM and wake during NREM ↔Wake, would lead to a greater number of “short sleep” states before the occurrence of “long sleep” state in susceptible mice pre- and post-CSD. Indeed, there were greater occurrences of “short sleep” in susceptible mice post-CSD. Moreover, there was a trend showing greater occurrences of short sleep before the occurrence of “long sleep” pre-CSD, in the light, relative to resilient mice. The “short sleep” state was more stable and exhibited lower probability of transition to “long sleep” state in susceptible mice pre-CSD. It will be interesting to investigate the neural mechanisms that control the transition between these two distinct “global” sleep states in the mice and how chronic stress exposure differentially modulates the transition in both the susceptible and resilient phenotypes.
In summary, we show lower NREM stability together with higher probability of transition from NREM to wake in NREM↔Wake in susceptible mice relative to resilient or stress-naïve mice pre- and post-CSD. Additionally, the number of bouts of NREM and wake in NREM↔Wake only was greater in susceptible relative to resilient mice. We modeled the sleep states in NREM↔Wake and NREM→REM→Wake as “short sleep” and “long sleep” global states, respectively, transitioning to wake. Susceptible mice exhibited greater numbers of occurrences of “short sleep” before “long sleep” pre-CSD. Our current analysis emphasizing the use of statistical tools and separating the states based on their transition provides a window into the potential neural mechanisms underlying the sleep-wake dynamics exhibited by stress-susceptible and stress-resilient mice. The distinction between the sleep and wake states in NREM↔Wake and NREM→REM→Wake is supported by findings that the transition of NREM to either wake or REM is controlled by two separate neural circuits. Understanding the distinct neural mechanisms controlling transitions during NREM↔Wake and NREM→REM→Wake and the transition between the distinct global sleep states could provide a glimpse into a better understanding of the mechanistic interplay between stress and sleep-wake transitions.
Limitations of the study
Our study is correlational, so any conclusion made in our paper requires further validation to determine the causal link between changes in specific neural circuits leading to susceptibility to stress and changes in sleep-wake cycle. Additionally, to simplify our analysis, we eliminated the rare, but existing, transitions from REM→Wake or REM→NREM. Detailed analysis of these transitions would be needed in the future.
STAR★Methods
Key resource table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Tools | ||
| Head connector MS363 Pedestal | PlasticsOne | www.plasticsone.com |
| Stainless screws head diameter 2.5 mm, shaft diameter 1.57 mm and shaft length 1.6 mm | Bilaney | www.bilaney.com |
| Stereotactic frames | Kopf Instruments | www.kopfinstruments.com |
| wires, 0.001” bare, 0.0055” coated | A-M Systems | www.a-msystems.com |
| Sleep Chamber | Viewpoint | www.viewpoint.ffr |
| Metabond | Parkell Inc. | www.parkell.com |
| Dental Cement | Stoelting | https://stoeltingco.com |
| Software and algorithms | ||
| TopScan video Tracking | CleverSyst. Inc. | www.cleversysinc.com |
| SeepScore | Viewpoint | www.viewpoint.ffr |
| Python version 3.0 | Python Software Foundation | https://python.org |
| Package 'markovchain' | R version 4.0.3 | https://cran.r-project.org |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Dipesh Chaudhury (dc151@nyu.edu).
Material availability
This study did not generate unique reagents.
Experimental model and subject details
Ethics statement
All experiments performed were approved by the NYUAD Animal Care and Use Committee, and all experimental protocols were conducted according to the National Institute of Health Guide for Care and Use of Laboratory Animals (IACUC Protocol: 150005A2).
Animals
CD1 retired male breeders (Charles River, UK), and C57BL/6J male mice (10-16 weeks; Jackson Laboratories, ME, USA) were used in this study. The following number of animals were used in the study: CD1 = 22; C57BL/6JL, Susceptible = 7, Resilient = 8, stress-naïve (Controls) = 7. All mice were maintained in the home cages, with ad libitum access to food and water in temperature (21 ± 2 °C)- and humidity (50 ± 10%)-controlled facilities with 12-h light-dark (L/D) cycles (lights on at 7:00 AM and lights off at 7:00 PM, zeitgeber time (ZT 0 = lights on, ZT 12 = light off). Zeitgeber time is a unit of time based on 12:12 light: dark cycle. All behavioral experiments were conducted during the light cycle (ZT 5 to ZT 10).
Method details
CSD stress paradigm
CSD stress paradigm was performed according to previously published protocols (Golden et al., 2011; Krishnan and Nestler, 2011; Chaudhury et al., 2013). The CD1 mice were screened upon arrival and the aggressive ones were selected. CD1 aggressor mice were single housed to habituate at least 48-72 h on one side of a clear perforated plexiglass divider. Experimental C57BL/6J mice were introduced into the cage of a novel and aggressive CD1 mouse for 10-min during which time they were physically attacked by the CD1 mouse. After 10-min of physical contact, the C57BL/6J mice were separated by a clear perforated plexiglass divider for the following 24-h allowing sensory, but not physical contact. The social defeat stress was repeated for 15-d for each C57BL/6J mouse, using a novel aggressor daily. We chose the 15-d protocol of CSD to ensure the chronicity of stress and its potential impact on sleep and wake. The stress-naïve/control mice were housed in pairs within a cage continuously separated by a clear perforated plexiglass divider. Additionally, the stress-naïve mice were moved daily to a different room for the duration of the defeat (10 min) to eliminate the passive stress effect. On the last day of defeat (Day 15), the mice were singly housed in new cages. The current study was conducted across a total of 8 blocks, each containing 2-4 mice in a staggered approach following the same timeline. CD1 mice were screened for frequency and latency for attacks before each CSD such that those mice that exhibited similar attack latencies of equal to or less than 30-s with comparable number of attacks were used. This ensured that the C57BL/6J mice were exposed to similar level of aggression. Additionally, all C57BL/6J used in this study started around the same age (10 ± 2 weeks).
Social interaction test
On recovery day 16 (following the CSD paradigm), social-avoidance behavior towards a novel non-aggressive but active CD1 mouse was measured in a two-trial social-interaction test. In the first 2.5 min trial, the experimental mouse was allowed to freely explore a square-shaped arena (44 × 44 cm) containing a perforated plexiglass cage (10 × 6 cm) along on one side of the arena (“No target” condition). In the second 2.5 min trial, the experimental mouse was reintroduced back into the arena with an unfamiliar CD1 non-aggressive mouse placed in the plexiglass cage (“Target” condition). Between trials, the behavioral apparatus was cleaned with MB-10 solution (Quip Laboratories, Inc. USA) to avoid persistence of olfactory cues. TopScan video tracking system (CleverSys. Inc.) was used to automatically monitor and record the amount of time the experimental mouse spent in the ‘interaction zone’ (14 × 26 cm) interacting with the unfamiliar CD1 mouse, ‘corner zone’ (10 × 10 cm) and ‘total travel’ within the arena for the duration in both trials. Interaction zone time, corner zone time, total distance traveled were collected and analyzed. The classification of susceptible and resilient mice was based on the social interaction (SI) ratio, which was calculated as [100 × (time spent in the interaction zone during social target session)/(time spent in the interaction zone during no social target session)] as described previously (Golden et al., 2011; Krishnan et al., 2007; Chaudhury et al., 2013). All mice with scores ˂ (100 – threshold) were classified as ‘susceptible’ and those with scores ≥ (100 + threshold) were classified as ‘resilient’. Threshold was used to avoid using mice with a score close to 100. We set a threshold of 1.0%, which led us to exclude one mouse with a SI score 100.95.
Surgery and electrode implantation
The animals were anesthetized with an intraperitoneal (IP) injection consisting of a mixture of ketamine (100 mg kg−1) and xylazine (10 mg kg−1). The mice were fixated in a stereotactic frame (Kopf instruments) at a sufficient level of anesthesia. The head was shaved, and the scalp was opened medially and the periosteum was removed. We used a dental precision driller (Stoelting) to drill 4 holes into the skull. The EEG electrodes were placed in the left and right part of the parietal lobe (from Bregma/caudal: −2 mm, medio-lateral: ± 1.5 mm) and the right frontal lobe (from Bregma/rostral: +1 mm, medio-lateral: ± 1 mm) and the grounding/reference electrode was placed in the cerebellum. Two EMG electrodes, gold plated, were lowered bilaterally into the neck muscle, directly caudal to the occipital bone. All EEG recording electrodes consisted of stainless-steel screws (Bilaney) with the following dimensions: head diameter 2.5 mm, shaft diameter: 1.57 mm, shaft length: 1.6 mm. All wires (0.001” bare, 0.0055” coated, A-M Systems) were connected to a head connector (MS 363 Pedestal,PlasticsOne), which was secured over the skull using acrylic C and B Metabond (Parkell Inc.). Next, dental cement (Stoelting) was applied around the head connected to protect all the wires and the connector.
Timeline of EEG sleep recording
Following a minimum duration of 7 days of postoperative recovery, mice were allowed to habituate (Hab) to the sleep chambers (Viewpoint, Lyon France) for 48-h after which, 24-h recording of EEG and EMG was performed. After 15-d of CSD and social interaction (SI) test, 24-h recording of EEG and EMG was performed after 48-h of Hab in the sleep chambers.
Electroencephalogram (EEG)/electromyogram (EMG) recording
After the postoperative recovery period, mice were transferred to a quasi-soundproof isolation sleep chamber (Viewpoint, Lyon France) under the standard laboratory conditions (12/12 h light-dark cycle, lights on at 7 am, 21 ± 2°C). Mice were connected to a cable plugged to a rotating commutator (SL-89-Opt-6, Dragonfly, Ridgeley, WV USA) to allow free movement in all three dimensions during the chronic recording sessions (video monitored). Unipolar EEG and bipolar EMG signals were amplified 800× (TBSI, part of HBIO, Cambridge, MA USA). The digitization was performed using a DAQ card (TBSI, part of HBIO, Cambridge, MA USA). The data were sampled at 30 kHz. The videos were synchronized with the EEG recording via an output TTL signal (5V pulse) to trigger the start and the end of the video.
Preprocessing, visualization
The electrophysiological signals were filtered with a low-pass filter (cutoff frequency 7kHz) and subsampled at 250 Hz. Next, the electrophysiological signals, the actimetry and the video were imported into a custom software program (SleepScore, Viewpoint, Lyon, France).
Scoring of the vigilance states
The vigilance states Wake, NREM sleep and REM sleep were visually scored off-line using the EEG and EMG signals according to standard criteria and methods(Franken et al., 1991) with a 5-s scoring window using custom software (SleepScore, Viewpoint, Lyon France). Sleep and wake states were visually analyzed and scored by the first author. The analysis was performed blind to eliminate experimenter's bias. The occurrence of artifacts was very low (∼1-3%) in all of the mice used in the study and concentrated primarily in the wake states (motion-related artifacts). Sleep epochs containing artifacts were excluded from the spectral analysis.
Quantification and statistical analysis
Statistical analysis
Sleep and wake bouts were pooled from all the mice in each group (susceptible (n = 7), resilient (n = 8) and stress-naïve (n = 7)) to plot the cumulative distribution of the bouts duration in both light and dark separately pre- and post-CSD. States were separated based on the two separate sequences of sleep and wake bouts in NREM↔Wake by isolating the NREM transitioning to wake and isolating the wake bouts transitioning from NREM, and in NREM→REM→Wake by isolating the NREM transitioning to REM, the REM bouts transitioning from NREM and isolating the wake bouts transitioning from REM. To test the statistical significance of the difference between the distribution, we had to choose between using either Kolmogorov-Smirnoff Test (KS) or a two-sample bootstrap hypothesis test. KS would've been used if the goal is to compare whether the 2 samples belong to the same distribution. Since we are interested in comparing the centrality/the difference in means between the distributions, comparison between the distribution of the groups was achieved using a two-sample bootstrap hypothesis test. For two-sample bootstrap test, the null hypothesis is that both distributions have the same mean. Therefore, the null hypothesis distribution was simulated by concatenating and scrambling together both distributions, bootstrap samples are drawn out of the null hypothesis distribution and the difference in means was computed. This bootstrapped difference in means along with the difference of the mean between the two distributions (empirical difference in means) were used to compute the p value. Specifically, 10,000 bootstrap samples were drawn from the null hypothesis and p value was computed as the percentage where the bootstrapped difference in means is greater or equal to the empirical difference in means. For the wake bouts, bouts duration above the 99 percentile were excluded from the two-sample bootstrap test. Bonferroni correction was used for the multiple comparison testing.
To model the stochastic nature of the transition between sleep-wake states, a discrete time Markov chain was used. We used 2 different discrete Markov models to model the transition between the sleep and wake states: A) a discrete-3-state-Markov model, the transition probabilities of Markov chains between the 3-state were arranged in a matrix with the form:
State stability describes the probability of one state transitioning to itself in a discrete time step of 5 seconds: Pii = Pr(Xn = i/Xo = i). State transition probabilities describe the probability of going from one state i to state j in a discrete time step of 5 seconds: Pij = Pr(Xn = j/Xo = i).
We next separated the sleep-wake sequences into 2 separate transition states: NREM↔Wake, and NREM→REM→Wake. The states were defined as following: NREM transitioning to Wake defined as N-W, NREM transitioning to REM defined as N-R, wake transitioning to NREM defined as W-N, REM transitioning to wake as R-W. The transitions wake→REM and REM→NREM were rarely observed and were hence omitted from the sequences. It is worth mentioning that wake transitioning to NREM defined as W-N comprises wake transitioning from NREM and wake transitioning from REM. Based on these distinct sleep-wake transitions, we defined another discrete 4-state-Markov model, the transition probabilities of Markov chains between the 4-state were arranged in a matrix with the form:
Markov analysis was performed using the markovchain package (R environment), where the probabilities of state maintenance and transitions can be reasonably approximated by fitting a stochastic Markov process(Perez-Atencio et al., 2018; Stephenson et al., 2013). Statistical analysis was performed for each state for a given condition (pre or post) for a given phase (light or dark) separately. One-way ANOVA was used to assess the phenotypic effect. Tukey's multiple comparison test was used to compare the stability of states, the probability of state transitions, the number of state transitions and the % duration of between the phenotypes. ∗ and ∗∗ denote, p < 0.05, and p < 0.01, respectively.
To model the ‘short sleep’ and ‘long sleep’ state transition, N-W in NREM↔Wake was replaced with ‘short sleep’, and N-R and R-W in NREM→REM→Wake were replaced with ‘long sleep’ and W-N were intervening bouts between ‘short sleep’ and ‘long sleep.’ For each mouse within a phenotype, the average number of ‘short sleep’ occurrences before ‘long sleep’ event was calculated. Tukey's multiple comparison test was used to compare between the average number of ‘short sleep’ occurrence before long sleep between the phenotypes. ∗ and ∗∗ denote, p < 0.05, and p < 0.01, respectively. Additionally, a discrete two-state Markov model was fit on the sequences of ‘short sleep’ and ‘long sleep’, by removing the intervening wake bouts, to compute the probability of stability of ‘short sleep’ and the probability of transition of ‘short sleep’ to ‘long sleep.’
Acknowledgments
The authors would like to thank all the members of the Chaudhury lab for their continuous support. This study was supported in part by grants from: (NYUAD Annual Research Budget, Brain and Behavior Research Foundation (NARSAD: 22,715), NYUAD Research Enhancement Fund, NYU Research Enhancement Fund, Al Jalila Research Foundation (AJF20163): DC) and L’OREAL-UNESCO (4500385541) “For Women in Science (FWIS)” Middle East Fellowship (BR).
Author contributions
B.R. and D.C. designed the experiments and wrote the manuscript; B.R. and G.J. conducted the experiment and collected the data; B.R. analyzed the data.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
One or more of the authors of this paper received support from a program designed to increase minority representation in science.
Published: October 22, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.103204.
Supplemental information
Data and code availability
All data reported in this paper and any additional information and code will be shared by the lead contact upon request.
References
- Association A., Association A. 4th Text Revision ed. American Psychiatric Association; 2000. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Bauer M., Grof P., Rasgon N., Bschor T., Glenn T., Whybrow P.C. Temporal relation between sleep and mood in patients with bipolar disorder. Bipolar Disord. 2006;8:160–167. doi: 10.1111/j.1399-5618.2006.00294.x. [DOI] [PubMed] [Google Scholar]
- Blumberg M.S., Seelke A.M., Lowen S.B., Karlsson K.A. Dynamics of sleep-wake cyclicity in developing rats. Proc. Natl. Acad. Sci. U S A. 2005;102:14860–14864. doi: 10.1073/pnas.0506340102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury D., Walsh J.J., Friedman A.K., Juarez B., Ku S.M., Koo J.W., Ferguson D., Tsai H.C., Pomeranz L., Christoffel D.J. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2013;493:532–536. doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K.S., Xu M., Zhang Z., Chang W.C., Gaj T., Schaffer D.V., Dan Y. A hypothalamic switch for REM and non-REM sleep. Neuron. 2018;97:1168–1176 e4. doi: 10.1016/j.neuron.2018.02.005. [DOI] [PubMed] [Google Scholar]
- Francis T.C., Chandra R., Friend D.M., Finkel E., Dayrit G., Miranda J., Brooks J.M., Iniguez S.D., O'donnell P., Kravitz A., Lobo M.K. Nucleus accumbens medium spiny neuron subtypes mediate depression-related outcomes to social defeat stress. Biol. Psychiatry. 2015;77:212–222. doi: 10.1016/j.biopsych.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken P., Dijk D.J., Tobler I., Borbely A.A. Sleep deprivation in rats: effects on EEG power spectra, vigilance states, and cortical temperature. Am. J. Physiol. 1991;261:R198–R208. doi: 10.1152/ajpregu.1991.261.1.R198. [DOI] [PubMed] [Google Scholar]
- Friedman A.K., Walsh J.J., Juarez B., Ku S.M., Chaudhury D., Wang J., Li X., Dietz D.M., Pan N., Vialou V.F. Enhancing depression mechanisms in midbrain dopamine neurons achieves homeostatic resilience. Science. 2014;344:313–319. doi: 10.1126/science.1249240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S.A., Covington H.E., 3rd, Berton O., Russo S.J. A standardized protocol for repeated social defeat stress in mice. Nat. Protoc. 2011;6:1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson F., Vialou V., El Mestikawy S., Fabre V. Effects of social defeat stress on sleep in mice. Front. Behav. Neurosci. 2017;11:227. doi: 10.3389/fnbeh.2017.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera C.G., Cadavieco M.C., Jego S., Ponomarenko A., Korotkova T., Adamantidis A. Hypothalamic feedforward inhibition of thalamocortical network controls arousal and consciousness. Nat. Neurosci. 2016;19:290–298. doi: 10.1038/nn.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A., Cavanagh J., Scott J. A systematic review of manic and depressive prodromes. J. Affect Disord. 2003;74:209–217. doi: 10.1016/s0165-0327(02)00266-5. [DOI] [PubMed] [Google Scholar]
- Kaneita Y., Ohida T., Uchiyama M., Takemura S., Kawahara K., Yokoyama E., Miyake T., Harano S., Suzuki K., Fujita T. The relationship between depression and sleep disturbances: a Japanese nationwide general population survey. J. Clin. Psychiatry. 2006;67:196–203. doi: 10.4088/jcp.v67n0204. [DOI] [PubMed] [Google Scholar]
- Kim J.W., Lee J.S., Robinson P.A., Jeong D.U. Markov analysis of sleep dynamics. Phys. Rev. Lett. 2009;102:178104. doi: 10.1103/PhysRevLett.102.178104. [DOI] [PubMed] [Google Scholar]
- Krishnan V., Han M.H., Graham D.L., Berton O., Renthal W., Russo S.J., Laplant Q., Graham A., Lutter M., Lagace D.C. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Krishnan V., Nestler E.J. Animal models of depression: molecular perspectives. Curr. Top. Behav. Neurosci. 2011;7:121–147. doi: 10.1007/7854_2010_108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo C.C., Chou T., Penzel T., Scammell T.E., Strecker R.E., Stanley H.E., Ivanov P. Common scale-invariant patterns of sleep-wake transitions across mammalian species. Proc. Natl. Acad. Sci. U S A. 2004;101:17545–17548. doi: 10.1073/pnas.0408242101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y.J., Li Y.D., Wang L., Yang S.R., Yuan X.S., Wang J., Cherasse Y., Lazarus M., Chen J.F., Qu W.M., Huang Z.L. Nucleus accumbens controls wakefulness by a subpopulation of neurons expressing dopamine D1 receptors. Nat. Commun. 2018;9:1576. doi: 10.1038/s41467-018-03889-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthi A. Sleep: switching off the off-switch. Curr. Biol. 2016;26:R765–R767. doi: 10.1016/j.cub.2016.06.059. [DOI] [PubMed] [Google Scholar]
- Martel J.C., Gatti Mcarthur S. Dopamine receptor subtypes, physiology and pharmacology: new ligands and concepts in schizophrenia. Front. Pharmacol. 2020;11:1003. doi: 10.3389/fphar.2020.01003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcshane B.B., Galante R.J., Jensen S.T., Naidoo N., Pack A.I., Wyner A. Characterization of the bout durations of sleep and wakefulness. J. Neurosci. Methods. 2010;193:321–333. doi: 10.1016/j.jneumeth.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir J., Lorsch Z.S., Ramakrishnan C., Deisseroth K., Nestler E.J., Calipari E.S., Bagot R.C. In vivo fiber photometry reveals signature of future stress susceptibility in nucleus accumbens. Neuropsychopharmacology. 2018;43:255–263. doi: 10.1038/npp.2017.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olini N., Rothfuchs I., Azzinnari D., Pryce C.R., Kurth S., Huber R. Chronic social stress leads to altered sleep homeostasis in mice. Behav. Brain Res. 2017;327:167–173. doi: 10.1016/j.bbr.2017.03.022. [DOI] [PubMed] [Google Scholar]
- Perez-Atencio L., Garcia-Aracil N., Fernandez E., Barrio L.C., Barios J.A. A four-state Markov model of sleep-wakefulness dynamics along light/dark cycle in mice. PLoS One. 2018;13:e0189931. doi: 10.1371/journal.pone.0189931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis M.L., Giles D.E., Buysse D.J., Tu X., Kupfer D.J. Self-reported sleep disturbance as a prodromal symptom in recurrent depression. J. Affect Disord. 1997;42:209–212. doi: 10.1016/s0165-0327(96)01411-5. [DOI] [PubMed] [Google Scholar]
- Peterson M.J., Benca R.M. Sleep in mood disorders. Psychiatr. Clin. North Am. 2006;29:1009–1032. doi: 10.1016/j.psc.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Radwan B., Jansen G., Chaudhury D. Abnormal sleep signals vulnerability to chronic social defeat stress. Front. Neurosci. 2021;14:1–16. doi: 10.3389/fnins.2020.610655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson R., Famina S., Caron A.M., Lim J. Statistical properties of sleep-wake behavior in the rat and their relation to circadian and ultradian phases. Sleep. 2013;36:1377–1390. doi: 10.5665/sleep.2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D.J., Lichstein K.L., Durrence H.H., Reidel B.W., Bush A.J. Epidemiology of insomnia, depression, and anxiety. Sleep. 2005;28:1457–1464. doi: 10.1093/sleep/28.11.1457. [DOI] [PubMed] [Google Scholar]
- Walsh J.J., Friedman A.K., Sun H., Heller E.A., Ku S.M., Juarez B., Burnham V.L., Mazei-Robison M.S., Ferguson D., Golden S.A. Stress and CRF gate neural activation of BDNF in the mesolimbic reward pathway. Nat. Neurosci. 2014;17:27–29. doi: 10.1038/nn.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wook Koo J., Labonte B., Engmann O., Calipari E.S., Juarez B., Lorsch Z., Walsh J.J., Friedman A.K., Yorgason J.T., Han M.H., Nestler E.J. Essential role of mesolimbic brain-derived neurotrophic factor in chronic social stress-induced depressive behaviors. Biol. Psychiatry. 2016;80:469–478. doi: 10.1016/j.biopsych.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data reported in this paper and any additional information and code will be shared by the lead contact upon request.