Graphical abstract
Abbreviations: NSCLC, non-small cell lung cancer; GPA, graded prognostic assessment; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; SREs, skeletal-related events; OS, overall survival; BOM, bone only metastasis; TNM, tumor-node-metastasis; SM, sensitive mutations; KRAS, kristen rat sarcoma; TKIs, tyrosine kinase inhibitors; MST, median survival time; ECOG, Eastern Cooperative Oncology Group
Keywords: Non-small cell lung cancer, Bone-only metastasis, Prognostic factors, Graded prognostic assessment model
Highlights
-
•
Studies about prognostic factors of NSCLC patients with BOM are lacking.
-
•
Five significant independent prognostic factors for them were found.
-
•
We developed a GPA model to estimate life expectancy and to guide interventions.
-
•
The more distal metastases to the spine, the worse the prognosis.
Abstract
Objectives
This retrospective study investigated prognostic factors in advanced non-small cell lung cancer (NSCLC) with bone-only metastasis, and developed a graded prognostic assessment (GPA) model to estimate patient survival.
Methods
The primary endpoint was overall survival. We investigated the patients with advanced NSCLC with bone-only metastasis at the initial diagnosis and diagnosed between 2013 and 2019 in our hospital. A log-rank test and Cox proportional hazards model were used to examine factors. A GPA model was developed in the training set based on the factors that were determined significant according to their hazard ratios and verified by the validation set.
Results
We finally included 220 patients for analysis. These patients were divided into two groups, 147 cases for the training cohort and 73 for the validation cohort. The following were significant independent prognostic factors, and were included in the GPA model: smoking; EGFR (epidermal growth factor receptor) sensitive/ALK (anaplastic lymphoma kinase) mutations; loss of weight; hypoalbuminemia; and primary site treated by surgery or radiotherapy. GPA score of nil was assigned to smoking, without sensitive mutations, loss of weight, hypoalbuminemia, and without local treatment of primary site; the corresponding superior alternatives were scored 1.5, 2.0, 1.5, 1.5, and 1.5, respectively. The median survival times of patients with GPA scores of nil to 3.0, 3.5 to 6.0, and 6.5 to 8.0 were 14.2, 29.5, and 56.6 months in the training set (P < 0.001) and 15.2, 31.2, and 54.0 months in the validation set (P < 0.001).
Conclusion
The survival time of patients with NSCLC with bone-only metastasis was dramatically influenced by the presence of the determined prognostic factors. The GPA model developed in this study may be a useful clinical tool to estimate the life expectancy of these patients, and guide treatment.
1. Introduction
Non-small cell lung cancer (NSCLC) is a common malignant tumor, and accounts for 70% to 80% of all lung cancer cases worldwide. The rates of morbidity and mortality are high [1]. At the time of diagnosis, 70% to 80% of NSCLC cases are advanced [2], with a common site of metastasis being the skeletal system (30%–40% of cases) [3].
In NSCLC with skeletal metastasis, many factors may impinge the patient's quality of life and performance status, and can affect overall survival (OS). These include epidemiological history, molecular alteration, distribution of metastasis, major skeletal-related events (SREs) at initial diagnosis, and disease progression [4], [5]. Thus, the duration of survival of patients with bone metastasis from NSCLC varies greatly. The optimal clinical treatment of these patients should be based on life expectancy [6], [7], [8], [9]. Predicting survival time is of great significance, but this estimation is complicated by many variables.
Notably, SREs and prognosis are affected by metastatic spread of NSCLC to sites other than bone, such as brain or liver [10]. Thus, focusing on patients with bone-only metastasis (BOM) is best for studies of survival time in NSCLC with skeletal metastasis, although this has rarely been considered. The present study investigated the demographic and clinical prognostic factors of patients with advanced NSCLC with BOM, recorded at the initial diagnosis. To guide physicians in estimating the survival time of these patients, a graded prognostic assessment (GPA) model was developed.
2. Materials & methods
2.1. Selection of study population
Data were retrospectively collected from the records of consecutive patients who received a diagnosis of advanced NSCLC in our hospital from 2013 to 2019. Clinical staging of the disease was conducted renewedly with reference to the eighth edition for tumor-node-metastasis (TNM) classification [11], at the time of data collection. The inclusion criteria in this study were: (1) a diagnosis of NSCLC confirmed from pathological or cytological specimens, or both; (2) evidences at bone metastasis confirmed by imaging examinations, such as plain radiograph, CT, PET-CT, MRI and bone scan, or a bone biopsy performed during surgery; (3) a data of gene mutations status identified via next-generation sequencing; (4) did not receive immunotherapy in the first-line. Patients were excluded if they had second primary tumor; a site of metastasis other than bone; without gene sequence result; or incomplete medical records.
2.2. Definition of special concept
In this study, positively sensitive mutations (SM+) included: EGFR (epidermal growth factor receptor) exon 19 deletion, EGFR exon 21 Leu858Arg mutation, and ALK (anaplastic lymphoma kinase) mutation. EGFR uncommon mutations, such as exon 18 mutations, exon 20 insertion mutations and so on, KRAS (kristen rat sarcoma) mutation or without any mutation, were defined as sensitive mutation negative (SM–).
The concept of GPA model was firstly proposed in brain metastasis by Sperduto et al in 2008 [12]. In present study, we developed a GPA model for NSCLC patients with bone-only metastasis by referring to its strategy. The GPA was the sum of scores for five factors according to their hazard ratios in multivariate analysis (using “integer unchanged” and “the decimal place if < 0.5, counted as 0; if greater than 0.5, counted as 0.5 ”method). Components of the GPA were smoking (no or yes); EGFR sensitive/ALK mutations (present or none); loss of weight (no or yes); hypoalbuminemia (no or yes) and primary site treated by surgery or radiotherapy (yes or no).
2.3. First-line systemic treatment strategy
All patients with EGFR non-sensitive mutations, KRAS mutation or without mutation, underwent first-line chemotherapy after confirmation of the initial NSCLC diagnosis. The treatment included platinum-based doublet chemotherapy such as pemetrexed, paclitaxel, docetaxel or gemcitabine combined with cisplatin, carboplatin, or nedaplatin. Each chemotherapy session was separated by an interval of 3–4 weeks.
Patients with EGFR-sensitive mutations (exon 19 deletion, exon 21 Leu858Arg mutations) were administered first-line treatment with EGFR tyrosine kinase inhibitors (TKIs), such as osimertinib, gefitinib, erlotinib, and icotinib; or with chemotherapy mentioned above and then TKIs after disease progression. All patients with ALK mutation were administered first-line treatment with crizotinib or ceritinib, or with chemotherapy as aforesaid and then TKIs after disease progression.
2.4. Data analysis and statistical considerations
OS was the primary endpoint, defined as the time from the date of diagnosis until death or the last follow-up. The follow-up schedule began from the time of treatment to the final follow-up on 28 September 2021. The data on the date of death or at the final follow-up visit were acquired from hospital records or through direct correspondence with the family of the patient. The chi-squared test (or Fisher’s exact test as applicable) and independent-samples T test were used to compare the clinical characteristics and outcomes. The estimation of OS was performed using the Kaplan-Meier method. The log-rank test was used to compare the survival curves and to assess the significance. A multivariate analysis was conducted using the Cox proportional hazards model to examine factors associated with increased hazard of death. All P-values were two-sided, with P < 0.05 considered statistically significant. SPSS 24.0 software (IBM, IL, USA) was used to perform the statistical analyses.
3. Results
3.1. Patient characteristics
Altogether, data were collected for 983 patients with advanced NSCLC, who had been treated in our hospital from January 2013 to December 2019. Among them were excluded 656, 72, 18, and 17 patients due to other-site metastasis, without gene sequence results, with second primary tumors, and incomplete medical records, respectively. Ultimately, 220 patients with NSCLC with BOM fulfilled the inclusion criteria for this study. The demographic and clinicopathological features of the patients were displayed in Table 1.
Table 1.
Patient characteristics.
| Characteristics | All (N = 220) | Training set (N = 147) | Validation set (N = 73) | P value |
|---|---|---|---|---|
| No. of patients (%) | No. of patients (%) | No. of patients (%) | ||
| Gender (male/female) | 128/92 (58.2/41.8) | 85/62 (57.8/42.2) | 43/30 (58.9/41.1) | 0.878 |
| Age | 59.4 ± 9.30 | 58.9 ± 9.62 | 60.4 ± 8.60 | 0.270 |
| KPS score (<80/≥80) | 19/201 (8.6/91.4) | 15/132 (10.2/89.8) | 4/69 (5.5/94.5) | 0.240 |
| Smoking history | 99 (45.0) | 64 (43.5) | 35 (49.7) | 0.536 |
| Loss of weight | 47 (21.4) | 35 (23.8) | 12 (16.4) | 0.209 |
| Histopathological type | 0.199 | |||
| Adenocarcinoma | 182 (82.7) | 125 (85.0) | 57 (78.1) | |
| Non-adenocarcinoma | 38 (17.3) | 22 (15.0) | 16 (21.9) | |
| Gene alternation status | 0.070 | |||
| EGFR-sensitive mutations | 97 (44.1) | 70 (47.6) | 27 (37.0) | |
| ALK mutation | 9 (4.1) | 4 (2.7) | 5 (6.8) | |
| EGFR unsensitive mutations | 15 (6.8) | 6 (4.1) | 9 (12.3) | |
| KRAS mutation | 2 (0.9) | 1 (0.7) | 1 (1.4) | |
| No | 97 (44.1) | 66 (44.9) | 31 (42.5) | |
| T stage | 0.597 | |||
| T1 | 64 (29.1) | 39 (26.5) | 25 (34.2) | |
| T2 | 88 (40.0) | 59 (40.1) | 29 (39.7) | |
| T3 | 38 (17.3) | 27 (18.4) | 11 (15.1) | |
| T4 | 30 (13.6) | 22 (15.0) | 8 (11.0) | |
| N stage | 0.012 | |||
| N0-N1 | 69 (31.4) | 38 (25.9) | 31 (42.5) | |
| N2-N3 | 151 (68.6) | 109 (74.1) | 42 (57.5) | |
| Number of bone metastasis | 0.733 | |||
| Single | 69 (31.4) | 45 (30.6) | 24 (32.9) | |
| Multiple | 151 (68.6) | 102 (69.4) | 49 (67.1) | |
| Axial skeleton metastasis | 184 (83.6) | 124 (84.4) | 60 (82.2) | 0.683 |
| Appendicular skeleton metastasis | 125 (56.8) | 85 (57.8) | 40 (54.8) | 0.669 |
| Spine only metastasis | 43 (19.5) | 25 (17.0) | 18 (24.7) | 0.178 |
| AXS-OM | 95 (43.2) | 62 (42.2) | 33 (45.2) | 0.669 |
| APS-OM | 34 (15.5) | 22 (15.0) | 12 (16.4) | 0.776 |
| Primary site treatment | 0.217 | |||
| Surgery | 12 (5.5) | 8 (5.4) | 4 (5.5) | |
| Radiotherapy | 62 (28.2) | 36 (24.9) | 26 (35.6) | |
| No | 146 (66.4) | 103 (70.1) | 43 (58.9) | |
| First-line treatment for SM+ patients | 0.098 | |||
| Icotinib | 37 (16.8) | 28 (19.0) | 9 (12.3) | |
| Gefitinib | 11 (5.0) | 10 (6.8) | 1 (3.1) | |
| Erlotinib | 8 (3.6) | 7 (4.8) | 1 (3.1) | |
| Osimertinib | 1 (0.5) | 1 (0.7) | 0 (0) | |
| Crizotinib | 1 (0.5) | 1 (0.7) | 0 (0) | |
| Ceritinib | 1 (0.5) | 0 (0) | 1 (3.1) | |
| Chemotherapy | 47 (21.4) | 27 (18.4) | 20 (27.4) | |
| Immunotherapy history | 19 (8.6) | 10 (6.8) | 9 (12.3) | 0.169 |
| Antiresorptive drugs | 182 (82.7) | 126 (85.7) | 56 (76.7) | 0.096 |
| ALP (U/L) | 134.76 ± 95.32 | 135.75 ± 98.77 | 132.78 ± 88.57 | 0.828 |
| White blood cell count (×109/L) | 7.56 ± 2.44 | 7.57 ± 2.08 | 7.55 ± 3.06 | 0.955 |
| Neutrophils (×109/L) | 5.11 ± 2.16 | 5.17 ± 1.89 | 4.98 ± 2.62 | 0.554 |
| Albuminemia (g/L) | 41.56 ± 5.71 | 41.43 ± 4.17 | 41.80 ± 8.00 | 0.637 |
| Calcium (mmol/L) | 2.35 ± 0.13 | 2.35 ± 0.13 | 2.35 ± 0.12 | 0.699 |
KPS: karnofsky performance status; AXS-OM: axial skeleton only metastasis; APS-OM: appendicular skeleton only metastasis.
SM+: EGFR-sensitive mutations/ ALK mutation; ALP: alkaline phosphatase.
The entire cohort was randomly divided into two groups, 147 cases in the training cohort and 73 cases in the validation cohort, respectively. The patient characteristics in the two cohorts were shown in Table 1. In the training set, the mean ± SD age of the training set was 58.9 ± 9.62, and it ranged from 25 to 84 years. Among them, 66 had no mutation; and 70, 6, 4, and 1 had EGFR-sensitive mutations, EGFR non-sensitive mutation, ALK mutation and KRAS mutation, respectively. After diagnosis, most patients received a systemic antiresorptive treatment (about once a month); either pamidronic acid (n = 100, 68.0%) or zoledronic acid (n = 25, 17.0%). In addition, ten patients (6.8%) had a history of immunotherapy.
3.2. Distribution of skeletal metastases
In this study population, there were 151 (68.6%), 69 (31.4%), and 43 (19.5%) patients with, respectively, multiple bone, a single bone, and vertebral-only metastases (Table 1). By standard anatomical definition, the axial skeleton consists of cervical vertebrae, thoracic vertebrae, lumbar vertebrae, sacrum, coccyx, ribs, sternum, and skull. The appendicular skeleton comprises the collar, hip bones, and limb bones. In this study, 95 patients (43.2%) had axial skeleton only metastasis, and 34 (15.5%) had appendicular skeleton only metastasis (Table 1). The most common site of bone metastasis was the spine (65.5%, Table A1). The MST of patients with spine-only metastasis, axial skeleton only metastases other than those of the spine, and appendicular skeleton only metastasis was 35.6, 29.7 and 21.7 months, respectively (P = 0.075, Fig. A1). Although not statistically significant, this seemed to mean that the more distal metastases to the spine, the worse the prognosis.
3.3. Survival and prognostic factors of survival in the training set
In the training set, the median follow-up time was 31.9 months (range, 24.4 to 125.9 months). The median OS was 29.5 months, and the 1-, 3- and 5-year OS rates were 78.3, 41.3, and 18.2%, respectively. For patients with single bone metastasis, the median survival time (MST) was 31.2 months, and the 1-, 3-, 5-year survival rates were 73.9, 44.4, and 21.9%.
The univariate analysis included the following: gender, age, smoking status, Karnofsky performance status scores, loss of weight, histopathological type, gene mutation status, T stage, N stage, number of bone metastases, alkaline phosphatase level, calcium level, albuminemia level, white blood cell count level, neutrophil level, primary site treatment and antiresorptive drugs treatment. The result showed that the following were significant prognostic factors for OS: smoking status, loss of weight, gene mutation status, T stage, serum albumin level and white blood cell count level (Table 2). Then, the covariates with P < 0.1 from the univariate analysis were further analyzed using a multivariate Cox proportional hazards model. The multivariate analysis showed that the following were significant prognostic factors for OS: smoking; gene mutation status; loss of weight; serum albumin level; and primary site treated by surgery or radiotherapy (Table 3).
Table 2.
Survival-related factors on OS in univariate analysis (Training set).
| Factors | MST (months) | P value |
|---|---|---|
| Gender | 0.271 | |
| Male | 22.6 | |
| Female | 34.5 | |
| Age | 0.920 | |
| <65 | 31.2 | |
| ≥65 | 22.6 | |
| Smoking status | <0.001* | |
| Yes | 17.0 | |
| No | 35.6 | |
| KPS score | 0.356 | |
| <80 | 35.6 | |
| ≥80 | 29.3 | |
| Loss of weight | <0.001* | |
| Yes | 18.5 | |
| No | 33.5 | |
| Histopathological type | 0.141 | |
| Adenocarcinoma | 31.2 | |
| Non-adenocarcinoma | 14.7 | |
| Gene alternation | <0.001* | |
| SM+ | 42.6 | |
| SM- | 16.7 | |
| T stage | 0.041* | |
| T1-2 | 35.5 | |
| T3-4 | 17.8 | |
| N stage | 0.391 | |
| N0-N1 | 31.3 | |
| N2-N3 | 29.3 | |
| Number of bone metastasis | 0.377 | |
| <5 | 31.5 | |
| ≥5 | 21.4 | |
| ALP level | 0.919 | |
| Normal | 29.3 | |
| Elevated | 32.4 | |
| Calcium level | 0.621 | |
| Normal | 29.3 | |
| Elevated | 29.5 | |
| Albuminemia level | 0.007* | |
| Normal | 42.6 | |
| Reduced | 21.9 | |
| WBC level | 0.042* | |
| Normal | 31.3 | |
| Elevated | 14.2 | |
| Neutrophil level | 0.318 | |
| Normal | 29.9 | |
| Elevated | 22.9 | |
| Primary site treatment status | 0.073 | |
| Yes | 36.4 | |
| No | 28.2 | |
| Antiresorptive drugs treatment | 0.818 | |
| Yes | 31.2 | |
| No | 28.3 | |
Cutoff value for biological serum data: ALP: 125 U/L; Calcium: 2.35 mmol/L; Albuminemia: 42.5 g/L; WBC: 10.0 × 10^9/L; Neutrophil: 6.3 × 109/L.
KPS: karnofsky performance status; ALP: alkaline phosphatase.
SM-: EGFR unsensitive mutations/KRAS mutation/no mutation.
SM+: EGFR-sensitive mutations/ALK mutation.
WBC: white blood cell.
*: p value < 0.05 considered statistically significant.
Table 3.
Survival-related factors on OS in multivariate analysis (Training set).
| Factors | B value | P value | Hazard Ratio | 95%CI |
|---|---|---|---|---|
| Smoking (yes vs. no) | 0.568 | 0.009* | 1.765 | 1.154 ∼ 1.699 |
| SM (- vs. + ) | 0.763 | 0.001* | 2.145 | 1.393 ∼ 3.302 |
| Loss of weight (yes vs. no) | 0.649 | 0.005* | 1.914 | 1.214 ∼ 3.018 |
| Albumin (reduced vs. normal) | 0.506 | 0.019* | 1.658 | 1.087 ∼ 2.531 |
| Primary site treatment status (no vs. yes) | 0.589 | 0.015* | 1.803 | 1.119 ∼ 2.903 |
SM-: EGFR unsensitive mutations/KRAS mutation/no mutation; SM+: EGFR-sensitive mutations/ALK mutation.
*: p value < 0.05 considered statistically significant.
3.4. Development and validation of graded prognostic assessment
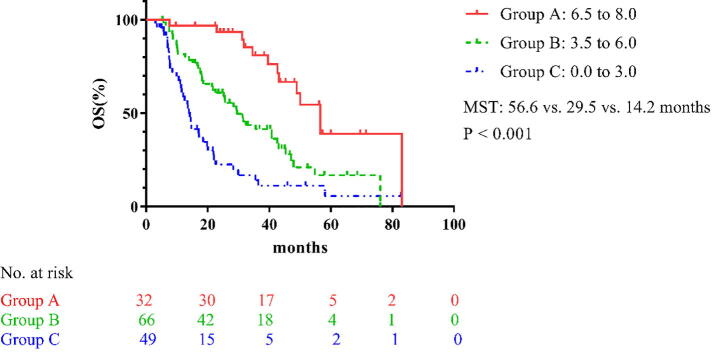
A GPA model was developed using the training cohort based on the 5 significant factors identified from the multivariate analysis, according to their hazard ratios (Table 4). The GPA scores were assigned as follows. Regarding smoking, patients who smoking received 0 point, and others were given a score of 1.5 points. Patients with EGFR-sensitive mutations or ALK mutation received 2.0 points, and others without these mutations scored 0 points. Patients with loss of weight received 0 point, and others were scored 1.5 points. Patients with hypoalbuminemia received 0 point, and others were scored 1.5 points. Patients who experienced local treatment to primary site received 1.5 point, and patients who had not been treated were scored 0 points (Table 4). Ultimately, patients in the training set were divided into 3 groups according to the sum of their GPA score: nil to 3.0, 3.5–6.0, and 6.5–8.0 (Table 5). The MST of the 3 groups were 14.2, 29.5, and 56.6 months, respectively (P < 0.001; Fig. 1).
Table 4.
Score of significant survival factors (Training set).
| Factors | Subgroup | Score | Hazard Ratio |
|---|---|---|---|
| Smoking | No | 1.5 | 1.765 |
| Yes | 0 | ||
| SM | + | 2.0 | 2.145 |
| – | 0 | ||
| Loss of weight | No | 1.5 | 1.914 |
| Yes | 0 | ||
| Albumin | Normal | 1.5 | 1.658 |
| Reduced | 0 | ||
| Primary site treatment status | Yes | 1.5 | 1.803 |
| No | 0 | ||
SM-: EGFR unsensitive mutations/KRAS mutation/no mutation.
SM+: EGFR-sensitive mutations/ALK mutation.
Table 5.
Score and survival of different groups.
| Subgroup | Score | Training set |
Validation set |
||||
|---|---|---|---|---|---|---|---|
| No. patients | MST (months) | P value | No. patients | MST (months) | P value | ||
| Group A | 6.5 to 8.0 | 32 | 56.6 | <0.001* | 16 | 54.0 | <0.001* |
| Group B | 3.5 to 6.0 | 66 | 29.5 | 25 | 31.2 | ||
| Group C | nil to 3.0 | 49 | 14.2 | 32 | 15.2 | ||
MST: median survival time.
*: p value < 0.05 considered statistically significant.
Fig. 1.
Survival curves of the different groups in the training set. MST: median survival time; OS: overall survival.
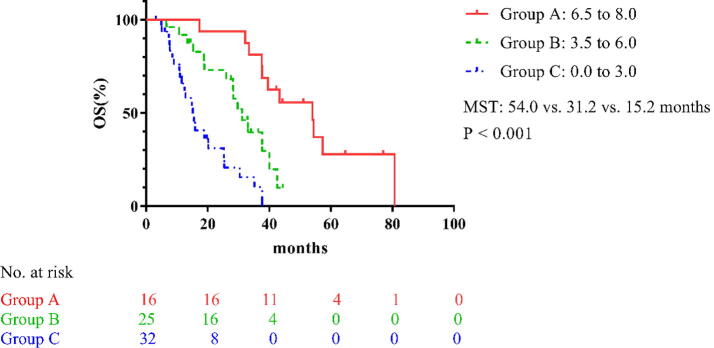
According to the above score criteria, the validation set patients were also successfully divided into three groups and the MST of the 3 groups were 15.2, 31.2, and 54.0 months, respectively (P < 0.001; Table 5 and Fig. 2).
Fig. 2.
Survival curves of the different groups in the validation set. MST: median survival time; OS: overall survival.
3.5. Characteristics of SREs
At initial diagnosis or during the course of treatment, 104 patients (47.3%) experienced SREs. Seventy-three patients (33.2%) underwent bone metastasis radiotherapy. Spinal cord compression occurred in 22 patients (10.0%). There were 28 patients (12.7%) with hypercalcemia, 8 patients (3.6%) with pathologic fracture, and 8 (3.6%) underwent surgical treatment, respectively. Thus, 139 SREs were observed in the entire group. As it has been reported that genetic mutations may had the potential to affect the occurrence of SREs of patients with NSCLC bone metastasis [10], [13], therefore, a further analysis was performed to investigate the difference between Group SM+ and Group SM–, but there was no statistically significant difference regarding the incidence of SREs (Table A2).
4. Discussion
In the current study, we established a GPA model to predict the prognosis and estimate the life expectancy of advanced NSCLC patients with BOM. A total of 220 cases were included, and 17 important prognosis factors that represent demographic, pathological, and treatment data were identified by conducting univariate and subsequent multivariable analysis in the training set. Eventually, five significant independent prognostic factors included in the GPA model were: smoking; EGFR-sensitive/ALK mutations; loss of weight; hypoalbuminemia; and primary site treated by surgery or radiotherapy. The validation showed that the GPA model is of excellent discrimination ability with a P-value of < 0.001 in the training set and the validation set.
Numerous factors which were reported that suggest a good prognosis for lung cancer after bone metastasis were: an ECOG (Eastern Cooperative Oncology Group) score of 1 or 2; no smoking; EGFR sensitive mutations; single bone metastasis; good nutritional status; and female gender [13], [14], [15], [16]. The current result was in keeping with previous studies—that no smoking, sensitive mutations, normal albumin level, and without loss of weight are good independent prognostic factors. Furthermore, local treatment of the primary site was a significantly good prognostic factor. That may be expected, as several prospective and retrospective clinical studies have shown that local consolidation therapy can improve the outcome of patients, especially those at an oligometastatic status [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27].
As previously reported in bone-metastasis lung cancer, systemic inflammation, as evidenced by leukocytosis or neutrophilia, were associated with poor OS [16]. Nevertheless, these did not remain significant in multivariate analysis, contrary to nutritional parameters (serum albumin levels and weight change), which may be related to the use of antiresorptive agents. In the setting of bone metastatic lung cancer, systemic inflammation was mainly due to increased tumor-induced bone resorption through the activation of a vicious circle between bone and metastases [28], [29]; tumor cells produce inflammatory cytokines, such as interleukin-1, interleurkin-6, transforming growth factor-β, and tumor necrosis factor-α that promote formation and activation of osteoclasts leading to bone destruction [30]. This vicious cycle can be inhibited by antiresorptive agents and these parameters were subsequently improved [31], [32], [33]. Hence, nutrition may reflect the initial health status for patients with bone-only metastases who represent a specific group.
Chambard and colleagues once reported that patients with a weight bearing bone involvement had a higher risk of death than others [16]. In this study, we found that the more distal metastases to the spine, the worse the prognosis (P = 0.075). It may suggest that appendicular skeleton metastasis, compared with other metastasis distribution status, significantly affect the patient's daily activities, quality of life, and bone metabolism, such as blood calcium and dickkopf-related protein 1, and thus further affecting survival.
Ideally the optimal clinical treatment (such as dose or frequency of radiotherapy, or surgery), should be based on the patient's expected survival time [7], [8], [9]. Several scoring systems for evaluating life expectancy have been proposed for this purpose [14], [34], [35]. For example, Tokuhashi et al. [34] developed a system to guide decisions regarding surgical intervention for spinal metastasis. The 6 parameters that were considered in their assessment system were: general condition; number of extraspinal bone metastases; number of metastases in the vertebral body; metastasis to major internal organs (lungs, liver, kidneys, and brain); primary site of the cancer; and the severity of spinal cord palsy [34]. Katagiri et al. [35] proposed a prognostic scoring system for general skeletal metastasis that relied on the type of primary cancer, whether the metastasis is visceral or cerebral, normality of laboratory data, performance status, previous chemotherapy, and presence of multiple skeletal metastases. In addition, a scoring system for bone metastasis from lung cancer, based on significant prognostic factors (gender, ECOG score), was developed by Pruksakorn et al [14].
On the one hand, the above scoring systems included many types of primary cancer, or metastasis of other sites, or both. On the other hand, the evaluation scale was not anymore valid since the pathology does not refer to mutated status. So, we recognized a need for a scoring system that is specific for patients with NSCLC with BOM based on molecular markers. In present study, patients in the training set were divided into 3 groups by GPA score (nil to 3.0, 3.5 to 6.0, and 6.5 to 8.0) and the MST were 14.2, 29.5, and 56.6 months, respectively (P < 0.001). Similarly, in the validation set, patients could be significantly stratified (P < 0.001). Since 48.2% patients with sensitive mutations (SM+; which included EGFR-sensitive mutations and ALK mutation) who could benefit from targeted therapy and many patients with oligometastases in our study, it prolonged survival.
With regard to the influence of bone metastasis on survival, most attention has been given to SREs, which impair quality of life and are understood to affect survival directly or indirectly [4], [5]. In the present study, 47.3% patients experienced SREs, which is similar to other reports [5], [10], [13], [15], [36]. The retrospective study by Lagana et al. [10] revealed that, compared to the historical record, patients with NSCLC with bone metastasis and EGFR mutated disease, and treated with TKIs, were at high risk to develop SREs. While, according to a retrospective study by Sun et al. [13], the absence of TKI therapy for bone metastatic NSCLC patients who had EGFR-sensitive mutations was an independent risk factor of developing SREs throughout the disease course. In the present study, we investigated differences in SRE incidence in our population according to EGFR/ALK mutation status. Curiously, the results showed that there was no statistically significant difference between the SM+ and SM– groups. One possible reason is that only patients with BOM were included.
5. Limitations
There are several limitations to this analysis. Most importantly, due to its retrospective nature, the bone metastatic status was assessed by non-homogeneous imaging techniques which had the different diagnosis capacity. Secondly, we were lacking of some data, such as C-reactive protein, cachexia, sarcopenia, and KRAS mutation status which were essential to survival. Thirdly, treatments were also inconsistent, which may influence survival. Finally, this study was based on the experience of a single institution, and the number of patients was limited. Further studies involving larger samples are needed to confirm these findings.
6. Conclusions
The prognosis of patients with advanced NSCLC with BOM at initial diagnosis is significantly influenced by smoking, EGFR-sensitive/ALK mutations, loss of weight, hypoalbuminemia and primary site treated by surgery or radiotherapy. A GPA model was developed in this study to guide physicians when estimating survival time for these patients.
Funding
This study was found by Chinese National Key Research and Development Project (Grant No. 2018YFC1315601). The funding sources is Ministry of Science and Technology of the People's Republic of China.
CRediT authorship contribution statement
Chunliu Meng: Conceptualization, Methodology, Formal analysis, Investigation, Writing – original draft. Jia Wei: Investigation. Jia Tian: Investigation. Jintao Ma: Investigation. Ningbo Liu: Methodology. Zhiyong Yuan: Methodology. Lujun Zhao: Writing - review & editing. Ping Wang: Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
Thanks for the funding support of the Ministry of Science and Technology of the People's Republic of China.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jbo.2021.100394.
Contributor Information
Chunliu Meng, Email: MCLTJMU@163.com.
Jia Wei, Email: weijia199409@163.com.
Jia Tian, Email: 690651842@qq.com.
Jintao Ma, Email: 932768329@qq.com.
Ningbo Liu, Email: liuningbo@tjmuch.com.
Zhiyong Yuan, Email: yuanzhiyong@tjmuch.com.
Lujun Zhao, Email: zhaolujun@tjmuch.com.
Ping Wang, Email: wangping@tjmuch.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Sung H., Ferlay J., Siegel R.L. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021 doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J., Steliarova-Foucher E., Lortet-Tieulent J. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur. J. Cancer. 2013;49(6):1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 3.Confavreux C.B., Girard N., Pialat J.B. Mutational profiling of bone metastases from lung adenocarcinoma: results of a prospective study (POUMOS-TEC) Bonekey Rep. 2014;3:580. doi: 10.1038/bonekey.2014.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sugiura H., Yamada K., Sugiura T. Predictors of survival in patients with bone metastasis of lung cancer. Clin. Orthop. Relat. Res. 2008;466(3):729–736. doi: 10.1007/s11999-007-0051-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cetin K., Christiansen C.F., Jacobsen J.B. Bone metastasis, skeletal-related events, and mortality in lung cancer patients: a Danish population-based cohort study. Lung Cancer. 2014;86(2):247–254. doi: 10.1016/j.lungcan.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 6.Suva L.J., Griffin R.J., Makhoul I. Mechanisms of bone metastases of breast cancer. Endocr. Relat. Cancer. 2009;16(3):703–713. doi: 10.1677/ERC-09-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lutz S., Berk L., Chang E. Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int. J. Radiat. Oncol. Biol. Phys. 2011;79(4):965–976. doi: 10.1016/j.ijrobp.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 8.Pin Y., Paix A., Le Fèvre C. A systematic review of palliative bone radiotherapy based on pain relief and retreatment rates. Crit. Rev. Oncol./Hematol. 2018;123:132–137. doi: 10.1016/j.critrevonc.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Patchell R.A., Tibbs P.A., Regine W.F. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366(9486):643–648. doi: 10.1016/S0140-6736(05)66954-1. [DOI] [PubMed] [Google Scholar]
- 10.Lagana M., Gurizzan C., Roca E. High prevalence and early occurrence of skeletal complications in EGFR mutated NSCLC patients with bone metastases. Front. Oncol. 2020;10 doi: 10.3389/fonc.2020.588862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.K. Chansky, F.C. Detterbeck, A.G. Nicholson, et al., The IASLC Lung Cancer Staging Project: External Validation of the Revision of the TNM Stage Groupings in the Eighth Edition of the TNM Classification of Lung Cancer, J Thorac Oncol 12(7) (2017) 1109-1121. [DOI] [PubMed]
- 12.Sperduto P.W., Berkey B., Gaspar L.E. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int. J. Radiat. Oncol. Biol. Phys. 2008;70(2):510–514. doi: 10.1016/j.ijrobp.2007.06.074. [DOI] [PubMed] [Google Scholar]
- 13.Sun J.M., Ahn J.S., Lee S. Predictors of skeletal-related events in non-small cell lung cancer patients with bone metastases. Lung Cancer. 2011;71(1):89–93. doi: 10.1016/j.lungcan.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Pruksakorn D., Phanphaisarn A., Settakorn J. Prognostic score for life expectancy evaluation of lung cancer patients after bone metastasis. J. Bone Oncol. 2018;10:1–5. doi: 10.1016/j.jbo.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bae H.M., Lee S.H., Kim T.M. Prognostic factors for non-small cell lung cancer with bone metastasis at the time of diagnosis. Lung Cancer. 2012;77(3):572–577. doi: 10.1016/j.lungcan.2012.05.094. [DOI] [PubMed] [Google Scholar]
- 16.Chambard L., Girard N., Ollier E. Bone, muscle, and metabolic parameters predict survival in patients with synchronous bone metastases from lung cancers. Bone. 2018;108:202–209. doi: 10.1016/j.bone.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Iyengar P., Wardak Z., Gerber D.E. Consolidative radiotherapy for limited metastatic non-small-cell lung cancer: a phase 2 randomized clinical trial. JAMA Oncol. 2018;4(1) doi: 10.1001/jamaoncol.2017.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bauml J.M., Mick R., Ciunci C. Pembrolizumab after completion of locally ablative therapy for oligometastatic non-small cell lung cancer: a phase 2 trial. JAMA Oncol. 2019 doi: 10.1001/jamaoncol.2019.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomez D., Tang C., Zhang J. Local consolidative therapy Vs maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: long-term results of a multi-institutional, Phase II. Randomiz. Study. 2019;37(18):1558–1565. doi: 10.1200/JCO.19.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez Guerra J.L., Gomez D., Zhuang Y. Prognostic impact of radiation therapy to the primary tumor in patients with non-small cell lung cancer and oligometastasis at diagnosis. Int. J. Radiat. Oncol. Biol. Phys. 2012;84(1):e61–e67. doi: 10.1016/j.ijrobp.2012.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu Q., Zhou F., Liu H. Consolidative local ablative therapy improves the survival of patients with synchronous oligometastatic NSCLC harboring EGFR activating mutation treated with first-line EGFR-TKIs. J. Thorac. Oncol. 2018;13(9):1383–1392. doi: 10.1016/j.jtho.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 22.Liu L.F., Li Q.S., Hu Y.X. Prognostic model to predict overall survival for metastatic non-small cell lung cancer patients treated with chemotherapy combined with concurrent radiation therapy to the primary tumor: analysis from two prospective studies. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.625688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weickhardt A.J., Scheier B., Burke J.M. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J. Thorac. Oncol. 2012;7(12):1807–1814. doi: 10.1097/JTO.0b013e3182745948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedes C., Mai N., Fu W. Isolated progression of metastatic lung cancer: Clinical outcomes associated with definitive radiotherapy. Cancer. 2020;126(20):4572–4583. doi: 10.1002/cncr.33109. [DOI] [PubMed] [Google Scholar]
- 25.Guo T., Ni J., Yang X. Pattern of recurrence analysis in metastatic EGFR-mutant NSCLC treated with osimertinib: implications for consolidative stereotactic body radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2020;107(1):62–71. doi: 10.1016/j.ijrobp.2019.12.042. [DOI] [PubMed] [Google Scholar]
- 26.Chan O.S.H., Lam K.C., Li J.Y.C. ATOM: a phase II study to assess efficacy of preemptive local ablative therapy to residual oligometastases of NSCLC after EGFR TKI. Lung Cancer. 2020;142:41–46. doi: 10.1016/j.lungcan.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Xu Q., Liu H., Meng S. First-line continual EGFR-TKI plus local ablative therapy demonstrated survival benefit in EGFR-mutant NSCLC patients with oligoprogressive disease. J. Cancer. 2019;10(2):522–529. doi: 10.7150/jca.26494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mundy G.R. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat. Rev. Cancer. 2002;2(8):584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 29.Fornetti J., Welm A.L., Stewart S.A. Understanding the Bone in Cancer Metastasis. J. Bone Miner. Res. 2018;33(12):2099–2113. doi: 10.1002/jbmr.3618. [DOI] [PubMed] [Google Scholar]
- 30.A.J.N.E.J.o.M. Stewart, Clinical practice. Hypercalcemia associated with cancer, 352(4) (2005) 373-9. [DOI] [PubMed]
- 31.Rosen L.S., Gordon D., Tchekmedyian S. Zoledronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: a phase III, double-blind, randomized trial–the Zoledronic Acid Lung Cancer and Other Solid Tumors Study Group. J. Clin. Oncol. 2003;21(16):3150–3157. doi: 10.1200/JCO.2003.04.105. [DOI] [PubMed] [Google Scholar]
- 32.Matsumoto S., Kimura S., Segawa H. Efficacy of the third-generation bisphosphonate, zoledronic acid alone and combined with anti-cancer agents against small cell lung cancer cell lines. Lung Cancer. 2005;47(1):31–39. doi: 10.1016/j.lungcan.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 33.Hirsh V., Major P.P., Lipton A. Zoledronic acid and survival in patients with metastatic bone disease from lung cancer and elevated markers of osteoclast activity. J. Thorac. Oncol. 2008;3(3):228–236. doi: 10.1097/JTO.0b013e3181651c0e. [DOI] [PubMed] [Google Scholar]
- 34.Y. Tokuhashi, H. Matsuzaki, S. Toriyama, et al., Scoring System for the Preoperative Evaluation of Metastatic Spine Tumor Prognosis, 15(11) (1990) 1110-1113. [DOI] [PubMed]
- 35.Katagiri H., Okada R., Takagi T. New prognostic factors and scoring system for patients with skeletal metastasis. Cancer Med. 2014;3(5):1359–1367. doi: 10.1002/cam4.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oliveira M.B.D.R., Mello F.C.D.Q., Paschoal, M.E.M. The relationship between lung cancer histology and the clinicopathological characteristics of bone metastases. Lung Cancer. 2016;96:19–24. doi: 10.1016/j.lungcan.2016.03.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.