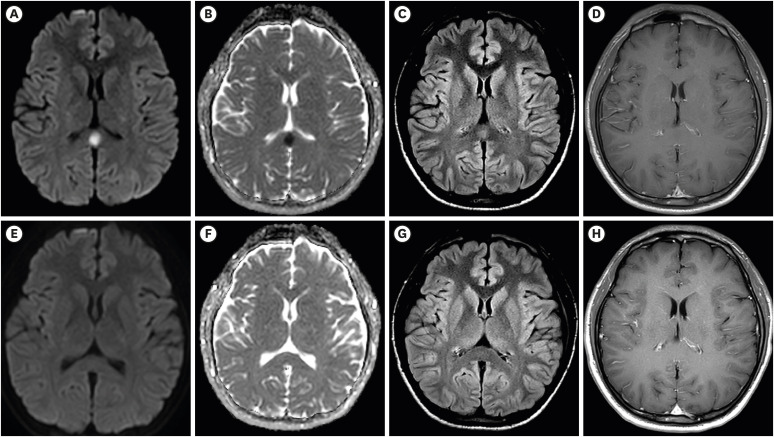
With interest we read the article by Youn et al.1 about a 22 years old male who developed fever and headache three days after having been vaccinated with the first jab of an mRNA-based severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) vaccine (Pfizer). Clinical neurologic exam only revealed mild nuchal rigidity but multimodal magnetic resonance imaging (MRI) showed a cytotoxic lesion in the splenium of the corpus callosum.1 The patient was discharged after two days without therapy and the clinical presentation had not changed at a follow-up one week after discharge.1 The study is appealing but raises the following comments and concerns.
A limitation is that no follow up MRIs were provided. Assuming that the corpus callosum lesion was ischemic in nature, the hypointensity on apparent diffusion coefficient (ADC) maps, as shown in Fig. 1, will turn into a hyperintensity after about 8 hours from the event. The hyperintensity on diffusion weighted imaging (DWI) will turn into an isointensity after a few days. T2-weighted or fluid attenuation inversion recovery (FLAIR) images will show the lesion as hyperintensity after a few hours.
Missing is the exclusion of a venous sinus thrombosis (VST), which is increasingly recognised as a complication of SARS-CoV-2 vaccinations.2 VST can manifest with headache, nuchal rigidity, and with ischemic stroke, all being present in the index case. We should know if the patient was a smoker and if the D-dimer was elevated on admission. Additionally, we should know the results of magnetic resonance venography (MRV).
Missing is the presentation of cerebral arteries on magnetic resonance angiography (MRA). Despite the young age the patient can theoretically have macro- or micro-angiopathy, which could be responsible for the ischemic callosal lesion. We should know if the family history was positive for stroke and if the index patient carried any classical cardiovascular risk factors (smoking, hyperlipidemia, diabetes, arterial hypertension, atrial fibrillation, coagulation disorder).
Since SARS-CoV-2 vaccinations can be also complicated by myocarditis3 and since myocarditis can be complicated by cardio-embolism, we should know the results of echocardiography or cardiac MRI with contrast medium. Did the patient ever complain about anginal chest pain and was the troponin level ever elevated?
To exclude acute, disseminated encephalomyelitis (ADEM) as a differential of the callosal lesion, application of contrast medium is missing. Usually, ADEM lesions show up with gadolinium enhancement.4 Additionally, MRI of the spinal cord should have been carried out to confirm or exclude a spinal lesion. Furthermore, follow-up MRI after treatment with steroids, is required to document the resolution of the abnormality.
Missing are the serum and cerebro-spinal fluid (CSF) levels of cytokines, such as IL-8, IL-6, IL-1A, and TNF-alpha.
Overall, the elegant study has some limitations which challenge the results and their interpretation. These limitations should be addressed to further strengthen the conclusions.
Footnotes
Disclosure: The authors declare no conflicts of interest.
- Conceptualization: Finsterer J, Scorza FA.
- Methodology: Finsterer J, Scorza FA.
- Project administration: Finsterer J, Scorza FA.
- Writing - original draft: Finsterer J.
- Writing - review & editing: Finsterer J, Scorza FA.
References
- 1.Youn T, Yang H. Cytotoxic lesion of the corpus callosum (CLOCCs) after SARS-CoV-2 mRNA vaccination. J Korean Med Sci. 2021;36(31):e228. doi: 10.3346/jkms.2021.36.e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shazley O, Alshazley M. A COVID-Positive 52-year-old man presented with venous thromboembolism and disseminated intravascular coagulation following Johnson & Johnson vaccination: a case-study. Cureus. 2021;13(7):e16383. doi: 10.7759/cureus.16383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khogali F, Abdelrahman R. Unusual presentation of acute perimyocarditis following SARS-COV-2 mRNA-1237 Moderna vaccination. Cureus. 2021;13(7):e16590. doi: 10.7759/cureus.16590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu JG, Qiao WY, Dong QW, Zhang HL, Zheng KH, Qian HR, et al. [Clinical features and neuroimaging findings of 12 patients with acute disseminated encephalomyelitis involved in corpus callosum] Zhonghua Yi Xue Za Zhi. 2012;92(43):3036–3041. [PubMed] [Google Scholar]