Abstract
Background
Atopic dermatitis (AD) is a chronic and relapsing inflammatory skin disease mediated by T helper type 2 (Th2) cells in acute phase. Group 2 innate lymphoid cells (ILCs) play a role in the initiation of the Th2 response. Although mold exposure is associated with the development of AD, studies on the underlying mechanisms are lacking. This study investigated whether group 2 ILCs are involved in inflammation in AD-like skin induced by Aspergillus fumigatus (Af).
Methods
We investigated changes of group 2 ILCs population in Af-induced AD-like skin lesions. To induce AD-like skin lesions, Af extracts were applied to the dorsal skin of BALB/c and Rag1−/− mice five times per week, with repeat exposures at 2-week intervals.
Results
The clinical parameters were higher in the Af-treated group than in the control group. Histologic findings revealed epiderrmal and dermal thickening as well as eosinophil and mast cell infiltration into the skin of Af-treated mice. Populations of group 2 ILCs in the skin were also significantly higher in the Af-treated group. In addition, interleukin-33 mRNA expression was significantly higher in the skin lesions of the Af-treated mice. In the Rag1−/− mice lacking mature lymphocytes, AD-like skin lesions were still induced by Af and ILCs depletion using an anti-CD90.2 mAb lowered the Af-induced inflammatory response.
Conclusions
Group 2 ILCs may play a role in a murine model of Af-induced AD-like skin lesions.
Keywords: Atopic Dermatitis, Aspergillus fumigatus, Group 2 Innate Lymphoid Cells, T Helper 2 Response
INTRODUCTION
Atopic dermatitis (AD) is a chronic inflammatory skin disease that affects 10–20% of children worldwide and characterized by pruritus and relapsing clinical course.1 Due to the increasing prevalence of AD, its earlier onset than other allergic diseases, and its progression to other allergic diseases in later life, there has been increasing interest in identifying the pathophysiology and preventing the development of this disorder.2
Studies on the innate cell sources of type 2 cytokines, which are responsible for allergic diseases and protection against helminth infection, have identified the group 2 innate lymphoid cells (ILCs) as critical sources of innate effector cells in type 2 immunity.3,4 Group 2 ILCs produce type 2 cytokines, including interleukin (IL)-5, IL-9, and IL-13 in response to IL-25 and IL-33, thereby promoting allergic inflammation.4 These group 2 ILCs have been shown to be involved in the development of AD.5 Although the production of ILCs may be influenced by the type of allergic disease and exposure to specific allergens and/or environmental factors,6 studies on these pathways are lacking.
The involvement of group 2 ILCs in allergic diseases was first demonstrated in animal studies.4 The intranasal administration of IL-25 was found to induce the production of T helper type 2 (Th2)-type cytokines, such as IL-4, IL-5, and IL-13, by non-T/non-B cells and enhance airway inflammation.7 More recently, a number of studies have assessed the role of group 2 ILCs in the pathogenesis of asthma,8,9 but fewer reports have evaluated the involvement of group 2 ILCs in AD.5,10,11 In a previous study using lymphocyte-deficient Rag1−/− mice, group 2 ILCs were identified as a source of type 2 cytokines in the skin in a murine model of AD,5 and IL-33 was found to induce group 2 ILCs.11 Even in human skin, group 2 ILCs play a pivotal role in skin inflammation in AD through IL-33 dependent and/or IL-33 independent thymic stromal lymphopoietin (TSLP) pathways.5,10
Although previous studies have identified mold exposure as a factor associated with the development of AD, studies on the mechanisms underlying this relationship are lacking.12,13 Alternaria extracts have been shown to induce group 2 ILCs in mouse lungs but there have been no prior studies on epicutaneous mold-induced group 2 ILCs in a murine model of AD. In our current study therefore, we assessed whether group 2 ILCs are involved in the skin inflammation induced by epicutaneous exposure to mold, specifically Aspergillus fumigatus (Af).
METHODS
Murine model of AD
Female 8-week-old BALB/c mice were purchased from Orient Bio Inc. (Seongnam, Korea). Female 8-week-old Rag1−/− and C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). Af antigen was purchased from Greer (Lenoir, NC, USA). All animal experiments were performed in the specific pathogen-free (SPF) facility. To induce AD-like skin inflammation 40 µg of Af extract was epicutaneously applied daily to a 1 cm2 area on the shaved dorsal surface for five consecutive days (days 0–4). This procedure was repeated twice with 2-week intervals. Control group mice were treated with normal saline.
Antibody treatment
The isotype control (LTF-2) and anti-CD90.2 mAb (30H12) were purchased from Bio X Cell (West Lebanon, NH, USA). Rag1−/− mice were administrated intraperitoneally (i.p.) every 2 days at a dose of 30 µg/mouse starting in the 2nd period of Af application.
Clinical parameters
The clinical scores of the skin lesions were assessed by a single investigator on days 0, 5, and 24. Dryness, scaling, erosion, excoriation, and hemorrhage were scored as 0 (absent), 1 (mild), 2 (moderate), and 3 (severe) with the sums of these items defined as the clinical scores (maximum score, 15). Epidermal permeability barrier function was evaluated by measuring transepidermal water loss (TEWL) using a Vapometer® SWL-3 (Delfin Technologies Ltd., Kuopio, Finland).
Cell preparation and culture
Skin lymph nodes (LNs) were dissected immediately after sacrifice and kept on ice in RPMI-1640 media (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS; HyClone, Logan, UT, USA) and 1% penicillin/streptomycin (Gibco). Cell suspensions were obtained by pressing the LNs through a cell strainer (40 µm) (SPL Life Science, Pocheon, Korea) and counted using a hemocytometer at 4 × 106 cells/mL. The LN cells were then cultured in the presence of Af (50 µg/mL) at 37°C for 3 days and their supernatants were stored at −80°C.
Histological examination of the skin
Skin samples were fixed with 10% formalin, embedded in paraffin and cut into 5 µm thick microsections for staining with hematoxylin–eosin or toluidine blue. Cell counts were calculated as the mean of eight random fields on each slide (magnification, ×400).
Measurement of cytokines and immunoglobulins
Cell suspensions were obtained by pressing the LNs through a 40 µm cell strainer and then cultured with Af (50 µg/mL) in RPMI-1640 media supplemented with 10% FBS and 1% penicillin/streptomycin (Gibco) for 3 days. The IL-13, interferon (IFN)-γ, IL-17A levels in the LNs cell culture supernatants were measured using ELISA Ready-SET-Go!® (eBioscience, San Diego, CA, USA) according to the manufacturer's instruction. Total serum immunoglobulin E (IgE) concentrations were measured using the BD OptEIA ELISA set (BD Biosciences, San Jose, CA, USA) according to the manufacturer's instruction. Af-specific IgE was measured as described previously.14
Flow cytometry
Skin tissues were chopped into small pieces and incubated in RPMI-1640 media (Gibco) containing 2.5 mg/mL collagenase IV (Worthington Biochemical, Lakewood, NJ, USA) and 0.2 mg/mL DNase 1 (Roche Molecular Systems, Somerville, NJ, USA) at 37°C for 60 minutes. For FACS surface staining, single cells were stained with a combination of the following antibodies: allophycocyanin (APC)-conjugated anti-CD25, PerCP-eFlour-710-conjugated anti-ST2 (IL-33R), APC-Cy7-conjugated anti-CD45, FITC-conjugated anti-CD3, CD45R, CD49b, CD11b, CD11c, and FcεR1. All antibodies were purchased from eBioscience except for anti-CD45 which was obtained from BD Biosciences. Samples were assayed using a FACS Canto flow cytometer (BD Biosciences) and FlowJo software (v10). Group 2 ILCs were identified after gating on live CD45+ cells and defined as cells negative for CD3, CD45R, CD49b, CD11b, CD11c, and FcεR1 and positive for CD25 and IL-33R.
Quantitative real-time polymerase chain reaction (PCR)
Total RNA from mouse skin was prepared using Genomic RNA Mini Kits (ALPHαGENE, Seongnam, Korea) and cDNA was synthesized using a WizScript™ cDNA Synthesis Kit (Wizbio Solutions, Seongnam, Korea). TaqMan primers and probes were obtained from Applied Biosystems (Foster City, CA, USA). Real-time PCR was performed using the ABI PRISM 7900HT Sequence Detection System (Applied Biosystems). The target gene expression levels were normalized using 18S expression. The product numbers of the probes for IL-33 and 18S were Mm00505403_m1 and Mm03928990_g1, respectively.
Immunohistochemistry
Immunohistochemistry was performed on paraformaldehyde-fixed and paraffin-embedded mouse skin. Sections of skin tissue (5 μm) were deparaffinized and rehydrated. Antigen retrieval was performed by autoclaving samples in 0.01 M citrate buffer (pH 6.0) for 10 minutes. The sections were incubated with the primary antibody goat anti-mouse IL-33 polyclonal antibody (2.5 μg/mL dilution; R&D Systems, Inc., Minneapolis, MN, USA) overnight at 4°C. Tissues were subsequently labeled with biotinylated goat anti-mouse IgG antibody (Vector Laboratories, Burlingame, CA, USA) at room temperature for 1 hour, following streptavidin-HRP (Dako, Glostrup, Denmark) and DAB Chromogen (Dako) incubation.
Statistical analysis
The study groups were compared statistically using the t-test (SAS software version 9.4; SAS Institute Inc., Cary, NC, USA). Significance for all statistical tests is shown in the figures for P < 0.05, P < 0.01, and P < 0.001.
Ethics statement
All animal procedures were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) of Asan Medical Center and Ulsan University College of Medicine. The IACUC abides by the Institute of Laboratory Animal Resources (ILAR) guide (Permit number: 2014-12-064).
RESULTS
Epicutaneous application of Af extract induced AD-like skin inflammation
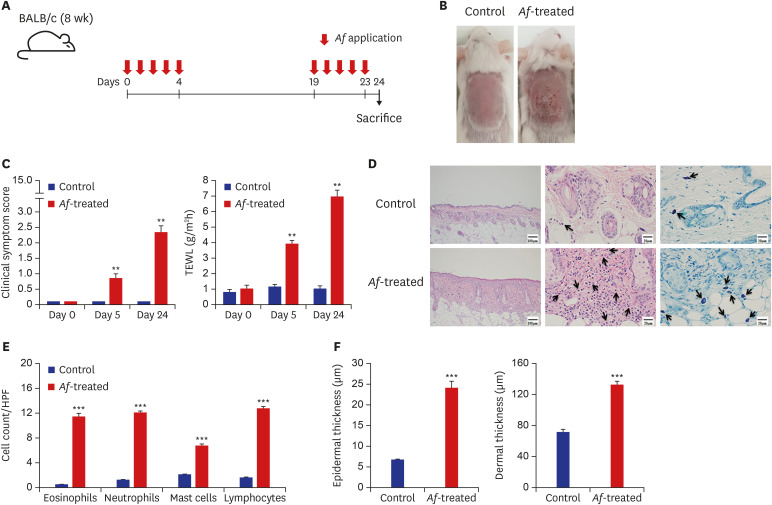
Epicutaneous exposure to Af extract was shown to induce AD-like skin lesions in mice, with patches containing Af extract.14 To mimic exposure to mold in daily life, an Af extract, one of the most commonly encountered species of mold,15 was applied to the dorsal skin of mouse without patches (Fig. 1A).
Fig. 1. BALB/c mouse model of Af-induced AD. (A) Schematic of the protocol used in this study. Af extract (40 µg) was epicutaneously applied to the dorsal skin of the mice for five consecutive days, with a 2-week interval before a second series of applications for 5 days. (B) Macroscopic cutaneous manifestations in the normal saline-control and Af-induced groups. Skin lesions such as erythema, erosions, crusts, and scale were more remarkable in the Af-treated group than in the control group on day 24. (C) Clinical symptom scores and transepidermal water loss levels were significantly increased in the Af-treated AD group, compared to the control group on days 5 and 24. (D) Histopathological analysis of Af-induced AD skin. Skin lesions from the Af-treated group showed increased thickening of the epidermal and dermal layers (left), infiltration of eosinophils (middle, arrows; hematoxylineosin staining; original magnification, × 400) and toluidine blue-positive mast cells (right, arrows). (E) The numbers of eosinophils, neutrophils, mast cells, and lymphocytes in the skin were significantly higher in the Af-treated group. (F) Epidermal and dermal thickness were significantly greater in the skin from Af- treated mice than control mice.
Data are expressed as a mean ± SEM (n = 6 per group).
Af = Aspergillus fumigatus, AD = atopic dermatitis, HPF = high power field, SEM = standard error of the mean.
*P < 0.05, **P < 0.01, and ***P < 0.001 compared with the control group.
The Af-treated BALB/c mice developed erythematous and edematous skin changes with excoriation and greater dryness, whereas no changes were observed in the skin of saline-treated mice (Fig. 1B). The clinical scores and TEWL were significantly increased in the Af-treated group compared to the controls (Fig. 1C). In addition, the clinical scores and TEWL significantly differed between Af-treated and control groups on days 0, 5, and 24 (P < 0.01, respectively).
Histopathologic examination of the lesional skin of the Af-treated mice on day 24 revealed marked hyperplasia of the epidermis and dermis, and infiltration into the dermis of many inflammatory cells, including eosinophils and mast cells (Fig. 1D). In addition, the numbers of eosinophils, neutrophils, mast cells, and lymphocytes on day 24 were significantly higher in the lesional skin from Af-treated mice than control mice (Fig. 1E). In addition, the application of Af extracts to the mouse skins increased both their epidermal and dermal thickness (Fig. 1F).
The epicutaneous application of Af extracts causes a Th2-type dominant systemic immune response
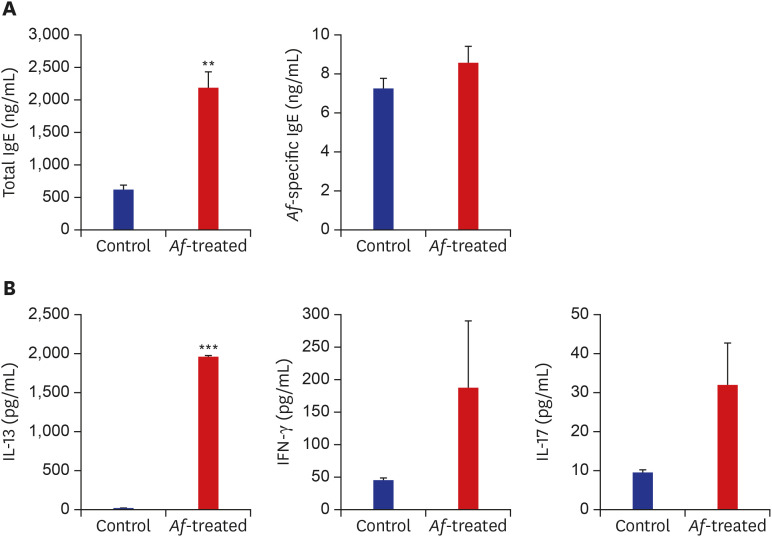
The serum concentrations of total IgE were significantly higher in the Af group than in the control group. The levels of Af-specific IgE were also higher in the Af group but without statistical significance (Fig. 2A). Moreover, the IL-13 levels in the supernatants of the Af-extract-stimulated skin-draining LNs were higher in the Af-treated group than from the control group. The IL-17 and IFN-γ concentrations were higher in cultures from the Af-treated group, but this was not statistically significant (Fig. 2B).
Fig. 2. The immune responses in Af-induced AD mouse model. (A) Total serum IgE and Af-specific Ig-E levels in the control and Af-treated group. (B) The IL-13, IFN-γ and IL-17A responses on day 24 after epicutaneous Af application.
Data are expressed as a mean ± SEM (n = 6 per group).
Af = Aspergillus fumigatus, AD = atopic dermatitis, IgE = immunoglobulin E, IL = interleukin, SEM = standard error of the mean.
**P < 0.01 and ***P < 0.001 compared with the control group.
The epicutaneous application of Af extracts increases the levels of Lin−CD25+IL-33R+ cells and IL-33 mRNA expression in the skin
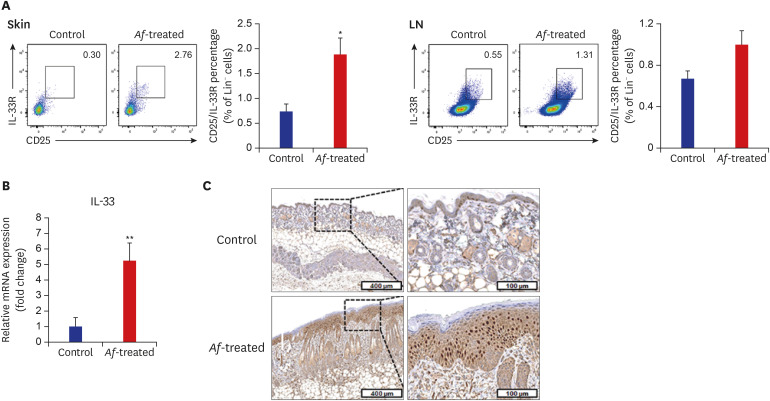
The populations of Lin−CD25+IL-33R+ cells were significantly higher in the skin lesions of the Af-treated BALB/c mice group compared to the control mice, whereas there were no significant differences in the percentage of Lin−CD25+IL-33R+ cells in the skin draining LN between the control and Af-treated groups (Fig. 3A). To identify the possible causes of the Lin−CD25+IL-33R+ cell induction, we measured the expression levels of IL-33 mRNA in the skin, which were significantly increased in the lesional skin of the Af-treated group compared to the control group (Fig. 3B). Moreover, IHC showed IL-33 expression was increased in Af-treated mouse skin compared to the control group (Fig. 3C).
Fig. 3. Levels of Lin−CD25+IL-33R+ group 2 ILCs in the skin lesions and skin-drained LNs in BALB/c mouse model. (A) FACS analysis showing that the percentage of Lin−CD25+IL-33R+ group 2 ILCs was significantly higher in the skin and LNs of Af-treated group than control group. (B) The IL-33 mRNA level was significantly increased in the lesional skin of the Af-treated group compared with the control group. Gene expression levels were normalized to 18S rRNA. (C) Immunohistochemistry showing that IL-33 expression was increased in Af-treated mouse skin.
Data are expressed as a mean ± SEM (n = 6 per group).
IL = interleukin, ILC = innate lymphoid cell, LN = lymph node, Af = Aspergillus fumigatus, SEM = standard error of the mean.
*P < 0.05 and **P < 0.01 compared with the control group.
The epicutaneous application of Af extracts causes AD-like skin inflammation and increases the levels of group 2 ILCs, even in Rag1−/− mice
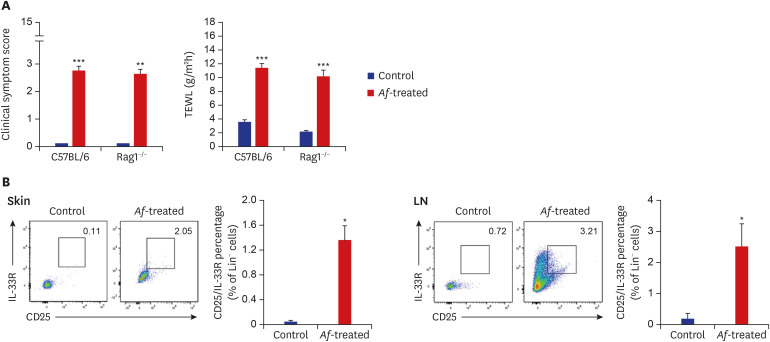
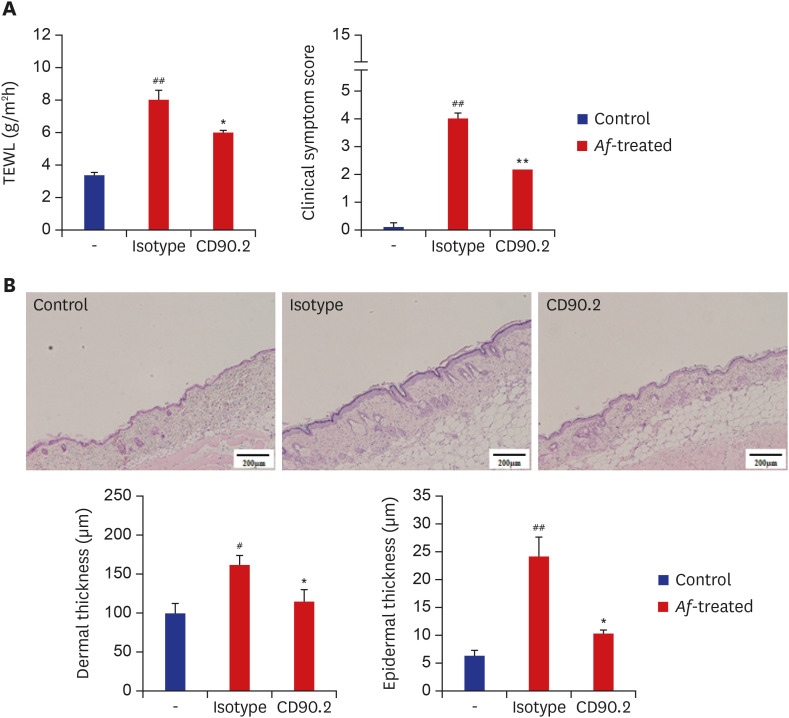
To elucidate the role of group 2 ILCs in exposure to Af extract in the skin, we used Rag1−/− mice that lack mature lymphocytes. The clinical symptom score and TEWL were significantly increased in the Af-treated C57BL/6 mice and Rag1−/− mice compared to the control group (Fig. 4A). The Lin−CD25+IL-33R+ cell populations were also significantly higher in the skin lesions of Af-treated C57BL/6 mice compared to the control group, whereas there was no significant difference in the LN (Supplementary Fig. 1). In the Rag1−/− mice, the Lin−CD25+IL-33R+ cell populations were significantly higher in the skin lesions and LNs of the Af-treated Rag1−/− mice compared to the control mice (Fig. 4B). To further investigate the role of the group 2 ILCs in the generation of AD-like skin inflammation, we used an anti-CD90.2 mAb to deplete ILCs in Rag1−/− mice.16 The clinical symptom score and TEWL were significantly decreased in anti-CD90.2-treated Rag1−/− mice compared to isotype-control mAb-treated mice (Fig. 5A). Moreover, the depletion of group 2 ILCs in the anti-CD90.2 mAb-treated group was accompanied by significantly reduced immune cell infiltration and both epidermal and dermal thickness (Fig. 5B). These data suggest that group 2 ILCs play a critical role in Af-induced AD skin inflammation.
Fig. 4. Increase of Lin−CD25+IL-33R+ group 2 ILCs in Rag1−/− mouse model. (A) Af induces increases in clinical symptom scores and TEWL in both C57BL/6 and Rag1−/− mice. (B) FACS analysis of Lin−CD25+IL-33R+ group 2 ILCs in the skin and LNs of Rag1−/− mice. Af exposure increased the percentage of Lin−CD25+IL-33R+ group 2 ILCs in this Rag1−/− mouse model.
IL = interleukin, ILC = innate lymphoid cell, Af = Aspergillus fumigatus, TEWL = transepidermal water loss, LN = lymph node, SEM = standard error of the mean.
Data are expressed as a mean ± SEM (n = 6 per group).
*P < 0.05 and ***P < 0.001 compared with the control group.
Fig. 5. Effects of anti-CD90.2 mAb treatment in Rag1−/− AD mouse model. (A) Clinical symptom scores and transepidermal water loss levels were significantly decreased in the anti-CD90.2 mAb-treated mouse group, compared to isotype control mAb-treated group. (B) Skin lesions from the anti-CD90.2 mAb-treated group showed decreased thickening of the epidermal and dermal layers (scale bars = 200 µm).
Data are expressed as a mean ± SEM (n = 6 per group).
AD = atopic dermatitis, Af = Aspergillus fumigatus, TEWL = transepidermal water loss.
*P < 0.05 and **P < 0.01, compared with the isotype-treated group; #P < 0.05 and ##P < 0.01, compared with the control group.
DISCUSSION
The results of our present study have revealed that epicutaneous exposure to Af extracts induces AD-like skin inflammation in mice with a Th2-dominant immune response. Infiltration of eosinophils and mast cells into the lesional skin and increases in Th2 cytokines in the supernatants of skin-draining LNs in the Af-treated mice support the hypothesis that epicutaneous exposure to molds in the environment induces the development of AD through deviation to Th2 immune responses. Furthermore, enrichment of group 2 ILCs in Af-treated mice including Rag1−/− mice means group 2 ILCs play an essential role in the development of AD related to mold exposure.
Epidemiologic evidence has suggested that mold exposure is associated with the development of asthma in children.17 On the other hand, in cases of patients with AD, there are only a few epidemiologic studies with insufficient supporting experimental data on its underlying mechanisms.13,18,19 In a study regarding the effect of yeast Malassezia furfur on the maturation of human dendritic cells, the immature monocyte-derived dendritic cells from human internalize this opportunistic yeast and produce pro-inflammatory and immune-regulatory cytokines.20 These cytokines favor the induction of a Th2-type immune response and contribute to the inflammatory reaction in patients with AD.20,21 However, these previous studies did not evaluate the involvement of group 2 ILCs in the development of AD in exposure to mold.
According to the Environment Relative Moldiness Index (ERMI), which is a DNA-based test to identify mold species found in the home, 36 different mold species are present in the indoor environment, among which exposure to Aspergillus ochraceus, Aspergillus unguis, and Penicillium variabile during infancy has been significantly associated with the development of childhood asthma.22 In addition, epicutaneous sensitization with Af was found to induce an AD-like skin inflammation in a murine model.14 Based on these previous findings, we here evaluated Af as a representative mold species associated with the development of AD in daily life. Our results in the AD mouse model suggest that epicutaneous exposure to mold can elicit AD-like skin inflammation via group 2 ILC-mediated allergic inflammation, in combination with Th2 immune responses. Our current findings in the mouse also indicate that epicutaneous exposure to Af increases the percentage of group 2 ILCs in the lesional skin. These results are supported by the findings of other studies which showed that group 2 ILCs are enriched in the skin of AD patients and are associated with the elevated production of type 2 cytokines and AD development in humans.5,10,23 Given the lack of data on the mechanisms underlying AD development following exposure to mold, our current results have provided important new insights by demonstrating the involvement of group 2 ILCs and skewed Th2 immune responses in this process following exposure to the ubiquitous mold Af.
Interestingly, group 2 ILCs were increased in the Af-induced skin lesions but not in their skin-draining LN, while IL-13 producing CD4+ T cell populations were increased in these LN (Supplementary Fig. 2). Upon exposure to environmental factors such as allergens, the levels of group 2 ILC are increased in the first contact organs of the external stimuli.24 Study on group 2 ILCs in allergic diseases in a mouse model have reported decreased levels of group 2 ILCs in the LN compared to skin or lungs,25 although the underlying mechanisms have not been identified. The Th2-dominant immune responses we here identified, including increases in skin eosinophils and total serum IgE levels, are likely due in part to the increases of group 2 ILCs in the skin, which promote the production of type 2 cytokines during allergic inflammation.
The IL-33 mRNA level was found to be higher in the skin lesions of the Af-treated group than of the control group. IL-33 has been shown previously to elicit group 2 ILCs and enhance the release of Th2 cytokines.6 A previous study has also reported that group 2 ILCs in the human skin are highly receptive to IL-33 and TSLP, resulting in an inflammatory phenotype with increased type 2 cytokine production.10 Also, although human IL-25 and/or TSLP can induce the production of type 2 cytokines, IL-33 is more chemotactic for group 2 ILCs than IL-25 or TSLP.10,26
TSLP can trigger group 2 ILCs in the skin of AD patients.10 The expression of TSLP mRNA in skin lesions was higher in the Af-treated mice than the control group, without statistically significant in the present study (data not shown). Human TSLP expression can be increased not only by viruses but by various environmental factors, including microbes and allergens, which induce intracellular signals through toll-like receptors.27,28 Moreover, mold proteases can induce TSLP in human airway epithelial cell lines through protease-activated receptor-2.29 Thus, specific components of mold, including Af extracts, may combine with their receptors to shape subsequent dominant action mechanisms through their compatible immune-regulatory responses. We did not evaluate the main components of Af extracts and their associated main receptors in our present analyses because our investigation was focused on the mechanisms underlying the development of AD in response to mold exposure itself. Further studies are thus needed to elucidate the underlying mechanisms of mold-associated AD according to the components of the mold and its associated receptor-pathophysiologic axis.
To further identify the role of group 2 ILCs in Af-treated AD skin lesions, we also treated Af extract in the skin of the Rag1−/− mice. In response to Af treatment in the skin of Rag1−/− mice, AD-like skin lesions were induced. The Lin−CD25+IL-33R+ group 2 ILCs were significantly increased in the skins of Af-treated C57BL/6 and Rag1−/− mice compared to the control mice. Moreover, depletion of ILCs in Rag1−/− mice abrogated the Af-induced AD-like skin inflammation. Taken together therefore, the results of our present study suggest that group 2 ILCs play an important role in the development of AD in response to mold exposure and that this occurs in combination with Th2 immune responses.
Several murine models of AD have been developed to study the mechanisms of AD and evaluate the effects of novel therapeutic agents.30 These animal models have included transgenic mice that over- or under-express selective cytokines such as TSLP and IL-18, and a hapten-induced mouse model. Nevertheless, the pathophysiology of these models is limited in representing AD-like skin inflammation induced by specific environmental factors. Models of epicutaneous sensitization with specific allergens have been developed using patches for allergen delivery,14 but also have limitations including difficulties in maintaining these patches in situ for the required period of time leading to individual variations in the duration of allergen exposure. The epicutaneous Af-induced AD model we used in our current analyses may better represent actual exposure to mold than those that use patches. Our experimental approach enabled us to better approximate the pathophysiological mechanisms associated with mold-induced AD in humans. However, our model still cannot fully reflect actual fungal skin exposure in a normal human environment in terms of viability, amount, and life cycle of Af on the exposure.
In conclusion, our present findings suggest that epicutaneous exposure to mold induces AD-like skin inflammation through the induction of group 2 ILCs in the skin in concert with Th2 immune responses. This epicutaneous Af-induced AD mouse model may better reflect the actual pathophysiologic mechanisms of mold induced-AD in humans. Our results also provide valuable new insights into the mechanisms underlying the effect of mold exposure on the development of AD, and may therefore help to develop new therapeutic strategies for the treatment of AD.
Footnotes
Funding: This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2018R1D1A1B07049168).
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Yoon J, Yu J.
- Methodology: Yoon J, Yu J.
- Formal analysis: Lee E, Park AR, Suh NY, Yoon J, Yu J.
- Investigation: Park AR, Park HJ, Park MN, Lee E, Song KB, Lee JH, Yoon J, Jung SS, Yoon JS.
- Writing - original draft: Lee E, Park AR.
- Writing - review & editing: Yoon J, Yu J.
SUPPLEMENTARY MATERIALS
FACS analysis of Lin−CD25+IL33R+ group 2 ILCs in the skin and lymph node of C57BL/6 mice. Mouse ILC2 were gated on CD45+ Lin−(CD3−CD45R−CD49b−CD11b−CD11c−and FcεR1−) CD25+IL-33R+ expression.
FACS analysis of CD4+IL13+ cells in the lymph node of C57BL/6 mice.
References
- 1.Leung DY, Bieber T. Atopic dermatitis. Lancet. 2003;361(9352):151–160. doi: 10.1016/S0140-6736(03)12193-9. [DOI] [PubMed] [Google Scholar]
- 2.Williams HC. Clinical practice. Atopic dermatitis. N Engl J Med. 2005;352(22):2314–2324. doi: 10.1056/NEJMcp042803. [DOI] [PubMed] [Google Scholar]
- 3.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature. 2010;463(7280):540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 4.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464(7293):1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med. 2013;5(170):170ra16. doi: 10.1126/scitranslmed.3005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barlow JL, McKenzie AN. Type-2 innate lymphoid cells in human allergic disease. Curr Opin Allergy Clin Immunol. 2014;14(5):397–403. doi: 10.1097/ACI.0000000000000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15(6):985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 8.Oboki K, Ohno T, Kajiwara N, Arae K, Morita H, Ishii A, et al. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc Natl Acad Sci U S A. 2010;107(43):18581–18586. doi: 10.1073/pnas.1003059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Licona-Limón P, Kim LK, Palm NW, Flavell RA. TH2, allergy and group 2 innate lymphoid cells. Nat Immunol. 2013;14(6):536–542. doi: 10.1038/ni.2617. [DOI] [PubMed] [Google Scholar]
- 10.Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska-Owsiak D, Wang X, et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med. 2013;210(13):2939–2950. doi: 10.1084/jem.20130351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imai Y, Yasuda K, Sakaguchi Y, Haneda T, Mizutani H, Yoshimoto T, et al. Skin-specific expression of IL-33 activates group 2 innate lymphoid cells and elicits atopic dermatitis-like inflammation in mice. Proc Natl Acad Sci U S A. 2013;110(34):13921–13926. doi: 10.1073/pnas.1307321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Platt SD, Martin CJ, Hunt SM, Lewis CW. Damp housing, mould growth, and symptomatic health state. BMJ. 1989;298(6689):1673–1678. doi: 10.1136/bmj.298.6689.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JY, Seo JH, Kwon JW, Yu J, Kim BJ, Lee SY, et al. Exposure to gene-environment interactions before 1 year of age may favor the development of atopic dermatitis. Int Arch Allergy Immunol. 2012;157(4):363–371. doi: 10.1159/000328778. [DOI] [PubMed] [Google Scholar]
- 14.Akei HS, Brandt EB, Mishra A, Strait RT, Finkelman FD, Warrier MR, et al. Epicutaneous aeroallergen exposure induces systemic TH2 immunity that predisposes to allergic nasal responses. J Allergy Clin Immunol. 2006;118(1):62–69. doi: 10.1016/j.jaci.2006.04.046. [DOI] [PubMed] [Google Scholar]
- 15.Agarwal R, Gupta D. Severe asthma and fungi: current evidence. Med Mycol. 2011;49(Suppl 1):S150–S157. doi: 10.3109/13693786.2010.504752. [DOI] [PubMed] [Google Scholar]
- 16.Sonnenberg GF, Monticelli LA, Alenghat T, Fung TC, Hutnick NA, Kunisawa J, et al. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science. 2012;336(6086):1321–1325. doi: 10.1126/science.1222551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karvonen AM, Hyvärinen A, Korppi M, Haverinen-Shaughnessy U, Renz H, Pfefferle PI, et al. Moisture damage and asthma: a birth cohort study. Pediatrics. 2015;135(3):e598–e606. doi: 10.1542/peds.2014-1239. [DOI] [PubMed] [Google Scholar]
- 18.Sharpe RA, Thornton CR, Tyrrell J, Nikolaou V, Osborne NJ. Variable risk of atopic disease due to indoor fungal exposure in NHANES 2005–2006. Clin Exp Allergy. 2015;45(10):1566–1578. doi: 10.1111/cea.12549. [DOI] [PubMed] [Google Scholar]
- 19.Nissen D, Petersen LJ, Esch R, Svejgaard E, Skov PS, Poulsen LK, et al. IgE-sensitization to cellular and culture filtrates of fungal extracts in patients with atopic dermatitis. Ann Allergy Asthma Immunol. 1998;81(3):247–255. doi: 10.1016/S1081-1206(10)62821-9. [DOI] [PubMed] [Google Scholar]
- 20.Buentke E, Heffler LC, Wallin RP, Löfman C, Ljunggren HG, Scheynius A. The allergenic yeast Malassezia furfur induces maturation of human dendritic cells. Clin Exp Allergy. 2001;31(10):1583–1593. doi: 10.1046/j.1365-2222.2001.01199.x. [DOI] [PubMed] [Google Scholar]
- 21.Johansson C, Eshaghi H, Linder MT, Jakobson E, Scheynius A. Positive atopy patch test reaction to Malassezia furfur in atopic dermatitis correlates with a T helper 2-like peripheral blood mononuclear cells response. J Invest Dermatol. 2002;118(6):1044–1051. doi: 10.1046/j.1523-1747.2002.01758.x. [DOI] [PubMed] [Google Scholar]
- 22.Reponen T, Lockey J, Bernstein DI, Vesper SJ, Levin L, Khurana Hershey GK, et al. Infant origins of childhood asthma associated with specific molds. J Allergy Clin Immunol. 2012;130(3):639–644.e5. doi: 10.1016/j.jaci.2012.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mashiko S, Mehta H, Bissonnette R, Sarfati M. Increased frequencies of basophils, type 2 innate lymphoid cells and Th2 cells in skin of patients with atopic dermatitis but not psoriasis. J Dermatol Sci. 2017;88(2):167–174. doi: 10.1016/j.jdermsci.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Klose CS, Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol. 2016;17(7):765–774. doi: 10.1038/ni.3489. [DOI] [PubMed] [Google Scholar]
- 25.Roediger B, Kyle R, Yip KH, Sumaria N, Guy TV, Kim BS, et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat Immunol. 2013;14(6):564–573. doi: 10.1038/ni.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mjösberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12(11):1055–1062. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 27.Lee EB, Kim KW, Hong JY, Jee HM, Sohn MH, Kim KE. Increased serum thymic stromal lymphopoietin in children with atopic dermatitis. Pediatr Allergy Immunol. 2010;21(2 Pt 2):e457–e460. doi: 10.1111/j.1399-3038.2009.00919.x. [DOI] [PubMed] [Google Scholar]
- 28.Lee HC, Headley MB, Loo YM, Berlin A, Gale M, Jr, Debley JS, et al. Thymic stromal lymphopoietin is induced by respiratory syncytial virus-infected airway epithelial cells and promotes a type 2 response to infection. J Allergy Clin Immunol. 2012;130(5):1187–1196.e5. doi: 10.1016/j.jaci.2012.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kouzaki H, O'Grady SM, Lawrence CB, Kita H. Proteases induce production of thymic stromal lymphopoietin by airway epithelial cells through protease-activated receptor-2. J Immunol. 2009;183(2):1427–1434. doi: 10.4049/jimmunol.0900904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin H, He R, Oyoshi M, Geha RS. Animal models of atopic dermatitis. J Invest Dermatol. 2009;129(1):31–40. doi: 10.1038/jid.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FACS analysis of Lin−CD25+IL33R+ group 2 ILCs in the skin and lymph node of C57BL/6 mice. Mouse ILC2 were gated on CD45+ Lin−(CD3−CD45R−CD49b−CD11b−CD11c−and FcεR1−) CD25+IL-33R+ expression.
FACS analysis of CD4+IL13+ cells in the lymph node of C57BL/6 mice.