Abstract
Preterm birth is the leading cause of infant death worldwide, but the causes of preterm birth are largely unknown. During the early COVID-19 lockdowns, dramatic reductions in preterm birth were reported; however, these trends may be offset by increases in stillbirth rates. It is important to study these trends globally as the pandemic continues, and to understand the underlying cause(s). Lockdowns have dramatically impacted maternal workload, access to healthcare, hygiene practices, and air pollution - all of which could impact perinatal outcomes and might affect pregnant women differently in different regions of the world.
In the international Perinatal Outcomes in the Pandemic (iPOP) Study, we will seize the unique opportunity offered by the COVID-19 pandemic to answer urgent questions about perinatal health. In the first two study phases, we will use population-based aggregate data and standardized outcome definitions to: 1) Determine rates of preterm birth, low birth weight, and stillbirth and describe changes during lockdowns; and assess if these changes are consistent globally, or differ by region and income setting, 2) Determine if the magnitude of changes in adverse perinatal outcomes during lockdown are modified by regional differences in COVID-19 infection rates, lockdown stringency, adherence to lockdown measures, air quality, or other social and economic markers, obtained from publicly available datasets. We will undertake an interrupted time series analysis covering births from January 2015 through July 2020.
The iPOP Study will involve at least 121 researchers in 37 countries, including obstetricians, neonatologists, epidemiologists, public health researchers, environmental scientists, and policymakers. We will leverage the most disruptive and widespread “natural experiment” of our lifetime to make rapid discoveries about preterm birth. Whether the COVID-19 pandemic is worsening or unexpectedly improving perinatal outcomes, our research will provide critical new information to shape prenatal care strategies throughout (and well beyond) the pandemic.
Keywords: pandemic lockdowns, COVID-19, preterm birth, stillbirth, low birth weight, perinatal outcomes, global trends
Introduction
The COVID-19 pandemic and response measures taken to mitigate the spread of infection have dramatically impacted health and health systems across the globe. Maternal and child health is at high risk, especially in low- and middle-income countries where resources for health care are already limited 1 . Pandemic response measures may have profound societal impacts owing to the combination of constrained supply, reduced resources, suppressed human interaction, and worsening socio-economic inequality. Projections already suggest about a 45% increase in child deaths and 39% increase in maternal deaths across low- and middle-income countries related to the pandemic 2 .
Unexpectedly, recent evidence from some high-income countries suggests unprecedented reductions in preterm births (up to 90% in Denmark and 23% in the Netherlands) and births classified as very low birth weight (70% in Ireland) following the COVID-19 lockdowns 3– 5 . At the same time, reports from Nepal and India show an alarming increase in stillbirths and preterm births related to changes in maternity care 6, 7 . Increases in stillbirth have also been seen in the UK and Italy (Lazio region) 8, 9 . In California, preterm birth rates seem largely unchanged during the pandemic period, except for a modest increase (11%) in very preterm birth, driven primarily by the Hispanic/Latinx population 10 .
It is critical to evaluate these seemingly contrasting trends and to understand the underlying mechanisms. The pandemic mitigation measures have substantially impacted maternal workload 11 , access to healthcare 12 , hygiene practices 13 , air pollution 14 , nutrition 15– 17 , and non-SARS-CoV-2 infection 18 , each of which may have affected maternal and perinatal outcomes disproportionately in different socio-economic and regional settings. It is plausible that changes in exposures to inflammatory triggers, such as infections 19– 21 and air pollution 22 , may be partly responsible for changes to some perinatal outcomes, such as spontaneous preterm birth. We will therefore seize the unique opportunity resulting from the global COVID-19 pandemic to answer pressing questions on pandemic lockdowns and perinatal health on a global scale.
To address the impact of the pandemic response measures on perinatal health, the international Perinatal Outcomes in the Pandemic (iPOP) study, is working in partnership with the International COVID-19 Data Alliance (ICODA, supported by the COVID-19 Therapeutics Accelerator) to establish an inclusive international research programme that will collaborate to focus on key questions relevant to countries across the globe of all income levels. The initial focus of iPOP will be on the impact of COVID‐19 pandemic lockdowns on perinatal outcomes, including preterm birth, low birth weight, and stillbirth.
Objective
The overall objective of iPOP is to determine the impact of pandemic lockdowns on perinatal outcomes worldwide, and to investigate potential mechanisms underlying these effects.
Protocol
Study goals and conceptual framework
The overarching goal of iPOP is to:
• Investigate the impact of COVID-19 lockdowns on perinatal outcomes (including preterm birth, low birth weight, and stillbirth);
• Compare the impact of COVID-19 lockdown on perinatal outcomes by country income setting: low-income countries (LICs), lower-middle-income countries (LMICs), upper-middle-income countries (UMICs) and high-income countries (HICs);
• Explore the underlying societal and etiological factors that are associated with between-country differences in the impact of COVID-19 lockdown on perinatal outcomes.
The conceptual framework ( Figure 1) for the iPOP project is intended to help build a series of work packages (WPs), each increasing in complexity and building on the previous findings. Within this protocol we address WP1 and WP2.
Figure 1. iPOP Study Conceptual Framework.

OECD, Organisation for Economic Co-operation and Development.
WP1 - Describe global trends and regional differences in adverse perinatal outcomes during COVID-19 pandemic lockdowns, using population-based aggregate data and standardized outcome definitions: Report rates of preterm birth, low birth weight, and stillbirth and describe changes during the pandemic lockdown. Determine if these changes are consistent globally, or if they differ between or within LIC, LMIC, UMIC and HIC settings.
WP2 - Address contextual influences and mechanisms for changes in preterm birth, stillbirth, and low birth weight during the COVID-19 pandemic, using population-based aggregate and publicly available data: determine if the magnitude of regional changes in adverse perinatal outcomes during lockdown are potentially modified by regional differences in COVID-19 infection rates, lockdown stringency, adherence to lockdown measures, air quality, and other social and economic markers available from public datasets.
Possible mechanisms driving the association between pandemic lockdown measures and perinatal outcomes are represented in a directed acyclic graph (DAG) in Figure 2.
Figure 2. Full directed acyclic graph (DAG) of lockdown to perinatal outcomes.

Aims
In WP1, we will estimate the impact of pandemic lockdowns on global incidence of preterm birth, low birth weight, and stillbirth using population-based data. Specifically, we will answer the following questions:
1. Has implementation of COVID-19 pandemic lockdowns been associated with a change in preterm birth rate (<37 weeks gestation)?
2. Does the association vary:
-
a)
When the outcome is restricted to spontaneous preterm birth (preterm birth preceded by spontaneous contractions and/or preterm prelabour rupture of membranes)?
-
b)
When the outcome is restricted to early preterm birth (<32 weeks gestation)?
3. Does any association with preterm birth remain when analyses are restricted to live births only (i.e. exclusion of stillbirths)?
4. Has implementation of COVID-19 pandemic lockdown been associated with a change in low birth weight rate (<2500g)?
5. Has implementation of COVID-19 pandemic lockdown been associated with a change in stillbirth rate?
6. Has implementation of COVID-19 pandemic lockdown been associated with a change in post term birth rate (≥42 weeks gestation)?
7. Do any observed associations with preterm birth, low birth weight, and stillbirth vary by country income setting (LIC, LMIC, UMIC, HIC)?
Our primary hypothesis is that the rate of spontaneous preterm birth, low birth weight and/or stillbirth is changed during pandemic lockdowns worldwide. Our secondary hypothesis is that the magnitude and/or direction of the change in the spontaneous preterm birth, low birth weight, and/or stillbirth varies by country income setting as classified by the World Bank income grouping.
In WP2 we will build directly from WP1 with the addition of national/regional characteristics derived from publicly available datasets to explore the influence of the association between lockdown measures and adverse perinatal outcomes.
Specifically, we will address the following questions:
8. Are the direction and magnitude of any changes in preterm birth, low birth weight and/or stillbirth rates observed in WP1 modified by factors such as:
-
a.
Lockdown stringency index (see section Exposures below)
-
b.
Adherence to lockdown indicated by traffic and social mobility data
-
c.
Ambient air quality
-
d.
COVID-19 rates
-
e.
Parental leave policy
-
f.
Socioeconomic setting
-
g.
Gross domestic product
-
h.
World region (East Asia and Pacific, Europe and Central Asia, Latin America & the Caribbean, Middle East and North Africa, North America, South Asia, Sub-Saharan Africa).
General approach
We will analyse aggregate population-based data provided by collaborators from different national/regional sites. Our primary method of analysis will be an interrupted time series analysis (ITSA) and we will consider alternative quasi-experimental approaches as appropriate.
Results from each contributing site will be meta-analysed, if appropriate. We will classify data into one of three tiers (Standard, Enhanced, or Investigative) based on the nature of the datasets in terms of population coverage, quality and completeness, and availability of required variables ( Table 1). Standard data meet the minimum criteria for inclusion in the main analysis of at least one primary or secondary outcome. Enhanced data meet the minimum criteria for inclusion in the main analysis of a primary or secondary outcome, as well as including additional data allowing inclusion in one or more additional or sensitivity analyses. Investigative data do not meet the minimum criteria to be included in the main analysis of a primary or secondary outcome but can be included in supplementary analyses exploring trends (designed to promote wide geographical coverage).
Table 1. Characteristics of datasets included in the iPOP Study.
| Coverage | |
|---|---|
| Standard dataset | National, subnational, population-based data |
| Enhanced dataset | National, subnational, population-based data |
| Investigative dataset | Institutional level data or other non-population-based data |
| Completeness | |
| Standard dataset | ≥90% births with a meaningful/feasible value for an outcome |
| Enhanced dataset | ≥90% births with a meaningful/feasible value for an outcome |
| Investigative dataset | <90% births with a meaningful/feasible value for an outcome |
| Time period | |
| Standard dataset | 1 Jan 2015 to 31 July 2020 |
| Enhanced dataset | 1 Jan 2015 to most recent data available |
| Investigative dataset | 1 Jan 2018 to 31 July 2020 |
| Breakdown of data | |
| Standard dataset | By consecutive calendar month |
| Enhanced dataset | By consecutive calendar month + by consecutive International
Standard (ISO) week and |
| Investigative dataset | By any other time frame or discontinuous data |
| Birth categories | |
| Standard dataset | All births |
| Enhanced dataset | All births +/-
Live births and stillbirths +/- Spontaneous preterm births |
| Investigative dataset | Live births only, population-based data /
All births, institutional level data or other non-population-based data |
| Gestation | |
| Standard dataset | 28
+0 - 36
+6 weeks
≥37 +0 weeks |
| Enhanced dataset | 22
+0 - 27
+6 weeks
28 +0 - 31 +6 weeks 32 +0 - 36 +6 weeks 37 +0 - 41 +6 weeks ≥42 +0 weeks |
| Investigative dataset | Preterm birth identified by checkbox (without registration of
gestational age) |
| Birth weight | |
| Standard dataset | 1000 – 2499g
≥2500g |
| Enhanced dataset | 500 – 999g (if available)
1000 – 1499g 1500 – 2499g ≥2500g |
| Investigative dataset | Low birth weight identification by checkbox (without registration
of birth weight) |
A single contributing dataset may be categorised in different tiers for different analyses, e.g. a dataset with low completeness on gestational age but high completeness on birth weight might be categorised as investigative for analyses of preterm birth and as enhanced for analyses of low birth weight.
Wherever possible we have aligned our definitions with those of the World Health Organization (WHO) 23 . To allow meaningful comparison of international data, in our main analyses definitions we have used a gestational age threshold of 28 weeks, and birth weight threshold or 1000g for inclusion (identified by the extension “_m”). This is in recognition that inclusion of extreme preterm and extremely low birthweight births can disrupt the validity of such comparisons. However, these births will be included in definitions for the enhanced analyses (identified by the extension “_e”).
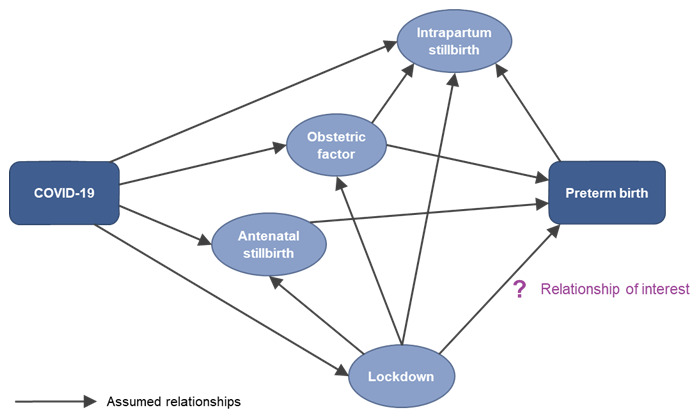
We have chosen to use a denominator of total births for our primary outcome of preterm birth. Figure 3 shows a simplified DAG justifying use of this denominator. COVID-19 infection might increase susceptibility to intrapartum stillbirth, whereas lockdown might reduce susceptibility to intrapartum stillbirth (because of fewer infections/ less pollution) or increase it (due to changes in access to maternity care). Intrapartum stillbirth here is a collider so we should not condition on intrapartum stillbirth. As it is difficult to get good information on whether stillbirths are intrapartum (as opposed to antenatal), total birth is appropriate as the denominator.
Figure 3. Directed acyclic graph (DAG) - Simple version (work package 1).
Methods
Study population
Our aim is to capture, at a minimum, data on all births (live and stillbirth) from 28 +0 to 44 +6 weeks gestation inclusive; or above ≥1000g birth weight. We also aim to capture additional data on all births (live and stillbirth) from 22 +0 to 27 +6 weeks gestation, or between 500g and 999g. These data will be included in enhanced analyses.
Study period
The main analysis study period is January 1, 2015 to July 31, 2020, covering the first lockdown period (in 2020) and the previous five calendar years. We will include data from January 1, 2018 to July 31, 2020 in investigative analysis if earlier data is not available. We will request the most recent data available to allow enhanced analyses covering a wider time period.
Exposures
The primary exposure will be a binary variable for lockdown based on the stringency index. We will use the stringency index from the Oxford COVID-19 Government Response Tracker. The Oxford COVID-19 Government Response Tracker provides a systematic cross-national, cross-temporal measure to understand how government responses have evolved over the full period of the disease’s spread. It collects information on different policies and interventions that governments have instituted in response to the COVID-19 pandemic and using standardized series of indicators creates a suite of composites indices to measure the extent of these responses. The indicators cover information on containment and closure policies (e.g. school closures and restrictions in movement) (C1-C8); economic policies (e.g. income support to citizens or provision of foreign aid) (E1-E4); and record health system policies (e.g. COVID-19 testing regimes or emergency investments into healthcare) (H1-H5). The lockdown stringency index is calculated using only the policy indicators C1-C8 and H1. The value of the index on any given day is the average of nine sub-indices pertaining to the individual policy indicators, each taking a value between 0 and 100. If the most stringent policy is only present in a limited area or region, a binary flag variable denotes limited scope. The codebook for the stringency index is publicly available.
We will define lockdown as a score of ≥50 on the Oxford COVID-19 Government Response Tracker stringency index. The decision on this arbitrary cut off has been influenced by scoping of stringency index data in high income settings and comparison of stringency indexes in settings which have and have not implemented lockdown measures. For example, Sweden (which has not had a ‘lockdown’) never implemented measures during the study period that added up to higher than 50 on the stringency index, compared to neighbouring Denmark, which scored above 50 throughout the study period in 2020. We will record timing of reaching a score ≥50 separately for each country/region. Our primary analysis will focus on the start date of pandemic lockdown defined as the first date when a country/region’s stringency exceeded 49 (i.e. as a stringency score of ≥50).
Subsequent analyses may include the:
• Time period of pandemic lockdown: defined as a continuous calendar period during which a country/region has a stringency score of ≥50
• Total duration of pandemic lockdown: defined as the sum of all calendar periods during which a country/region has a stringency score of ≥50
Note: The beginning and length of lockdown may vary by country/region
Comparator
Births during the 2020 lockdown periods will be compared with births occurring before the first date when a country/region’s stringency exceeded 49 (i.e. as a stringency score of ≥50), defined by lockdown stringency index in each country/region. The exact comparator time period may vary by country/region.
Outcomes
Primary outcome
• Preterm birth rate_m (main analysis: any birth 28 +0- 36 +6 weeks gestation; denominator total births ≥28 +0 weeks).
• Preterm birth rate_e (enhanced analysis: any birth 22 +0- 36 +6 weeks gestation; denominator total births ≥22 +0 weeks).
Secondary outcomes
• Early preterm birth rate_m (main analysis: any birth 28 +0 - 31 +6 weeks gestation; denominator total births ≥28 +0 weeks).
• Early preterm birth rate_e (enhanced analysis: any birth 22 +0 - 31 +6 weeks gestation; denominator total births ≥22 +0 weeks).
• Extreme preterm birth rate_e (enhanced analysis: any birth 22 +0 - 27 +6 weeks gestation; denominator total births ≥22 +0 weeks).
• Spontaneous preterm birth rate_e (enhanced analysis: any birth 28 +0- 36 +6 weeks gestation which is preceded by spontaneous contractions or preterm prelabour rupture of membranes [PPROM]; denominator total births ≥28 +0 weeks).
• Spontaneous preterm birth rate_e (enhanced analysis: any birth 22 +0- 36 +6 weeks gestation which is preceded by spontaneous contractions or preterm prelabour rupture of membranes [PPROM]; denominator total births ≥22 +0 weeks).
• Post term birth rate_m (main analysis: any birth ≥42 +0 weeks gestation; denominator total births ≥28 +0 weeks).
• Stillbirth rate_m (main analysis: any stillbirth ≥28 +0 weeks gestation (or ≥1000g if gestation not available); denominator total births ≥28 +0 weeks (or ≥1000g if gestation not available).
• Stillbirth rate_e (enhanced analysis: any stillbirth ≥22 +0 weeks gestation (or ≥500g if gestation not available); denominator total births ≥22 +0 weeks (or ≥500g if gestation not available).
• Low birth weight rate_m (main analysis: any birth 1000–2500g; denominator live births ≥1000g).
• Low birth weight rate_e (enhanced analysis: any birth 500–2500g; denominator live births ≥500g).
• Very low birth weight rate_m (main analysis: any birth 1000 – 1500g; denominator live births ≥1000g).
• Very low birth weight rate_e (enhanced analysis: any birth 500 – 1500g; denominator live births ≥500g).
• Extremely low birth weight rate_e (enhanced analysis: any birth 500g – 1000g; denominator live births ≥500g).
Potential confounders/effect modifiers
Potential confounders/effect modifiers for the entire iPOP study are represented in a DAG ( Figure 2). We recognise that i) many of the variables in the DAG (e.g. maternal age distribution) are unlikely to have significantly changed within the timeframe of the analysis and thus unlikely to be confounders, and ii) our initial analysis strategy is to compare changes in association with lockdown within datasets; thus these variables are less relevant. To allow expedient provision and analysis of data we propose using aggregate data for WP1 and WP2; with more complex analysis enabled with provision of individual participant data and provider level data in subsequent WPs.
National/regional level societal characteristics that we are interested in exploring include mediating and moderating factors obtained from publicly available datasets as described in the section below. Country classification by income as defined by the World Bank (LIC, LMIC, UMIC, HIC) as a proxy for wider social security and healthcare system.
Data collection and characteristics of datasets
We have extended invitations for national, regional and institutional data custodians of birth data to participate through formal and informal networks, social media, lay and scientific media. Participating countries as of December 1 st 2020 are shown in Figure 4.
Figure 4. Map of iPOP collaborating countries as of Dec 1, 2020.
We will request aggregate data from each data provider using an excel spreadsheet template, which includes details on levels of missing data. We will classify data provided to iPOP as Standard, Enhanced, or Investigative, based on the characteristics described in Table 1.
We will also ask for completion of a questionnaire regarding the source of data including, country of origin, region(s) covered and size of population covered. To assist data providers on which template to use to capture their data, we have constructed a data flow diagram ( Figure 5).
Figure 5. Data request flow diagram.
For WP2 we will use the following publicly available data sources:
• Lockdown stringency: Using the stringency index (see section Exposures) and COVID-19: Containment and Health Index defined as a continuous (0–100) or categorical measures.
• Socioeconomic status: Measured by Organisation for Economic Co-operation and Development (OECD) better life index.
• Ambient air quality: Estimated using the Data Integration Model for Air Quality (DIMAQ) 24 , which uses input data from a variety of public sources including: Open Air Quality, NASA Modern-Era Retrospective analysis for Research and Applications version 2 ( MERRA-2) global modelling initiative, satellite imagery data from the Multiangle Implementation of Atmospheric Correction ( MAIAC), and global population density from the NASA/Columbia University Socioeconomic Data and Applications Center.
• Adherence to lockdown indicated by traffic and movement trends: Obtained from publicly available Google mobility data.
• COVID-19 rates: Nationally available via John Hopkins COVID-19 infection rates
• Parental leave policy: Measured by World Bank Data (yes/no; length of paid maternity leave).
• Other country-level characteristics: Measured by World Bank Data (including variables such as world region, GDP, income expenditure, hospital beds, maternal education), The Global Gender Gap Index, The Global Hunger Index and Political stability index.
Data storage and analysis platform
We will use the Secure Anonymised Information Linkage (SAIL) Databank, Swansea Wales, to store all data provided to iPOP. Upon completion of a Data Contribution Agreement between each iPOP data provider and the SAIL Databank, each data providers will either:
-
i)
upload aggregated data directly to the SAIL central repository, or
-
ii)
transfer their data to the University of Edinburgh (RM), who will upload these to SAIL on their behalf.
Data will be transferred into SAIL using the “Split-file” process with the support of the Informatics Service, National Health Services (NHS) of Wales. Person-level demographics are translated to an Anonymous Linking Field (ALF). Additional information on the SAIL File Structure & Data Transfer processes can be found here.
iPOP Team Members (analysis team) will access data stored within SAIL via a remote access and conduct data analyses remotely on the International COVID-19 Data Alliance (ICODA) Workbench, via a federated approach. ICODA is a new data platform that allows scientists and researchers across the globe to discover, access and analyse multi-dimensional datasets in a confidential and secure environment. More information can be found on the HDR UK website.
To ensure outputs are confidential and safe, all statistical outputs will be checked using Statistical Disclosure Control (SDC) procedures before being exported out of the virtual environment. We will use SDC guiding principles from the Handbook on SDC for Outputs by the UK Data Service. This will prevent the identity of a birth from being revealed or inferred from outputs.
A catalogue on the data variables captured will be recorded alongside relevant metadata. These high-level summaries will be made publicly available.
Data analysis
All analyses will be fully specified in a comprehensive Statistical Analysis Plan. We will adhere to relevant reporting guidance for example the Strengthening the Reporting of Observational studies in Epidemiology ( STROBE).
Descriptive analysis
We will use summary statistics and data visualisations to describe, explore and compare the national/regional data to describe the study outcomes and other perinatal characteristics, including:
• All births
• Live and stillbirths
• Preterm and post term births
• Low birth weight
• Spontaneous preterm births
In WP2 we will use summary statistics and data visualisations (e.g. choropleth maps) to describe, explore and compare the national/regional data.
Statistical modelling
We will undertake population-based ITSA for main analyses of primary and secondary outcomes. We will use time-series techniques to capture any underlying temporal trends and seasonality in the data before the implementation of lockdown measures. We will consider both linear and more flexible trends. We will use these time-series regression models to forecast (or predict) the expected trends and will compare these to the observed trends seen after the lockdown measures. This will capture both immediate (i.e. step) changes and gradual (i.e. slope) changes in the outcome in relation to implementation of lockdown measures in our models. All analyses will be prespecified in a Statistical Analyses Plan before analysis.
Meta-analysis
We will undertake a meta-analysis of national/regional results, on the step-change and the difference between the forecast and observed outcomes at different time points after the implementation of lockdown measures. We will also stratify by country income setting as a dichotomous variable (LIC+LMIC vs UMIC+HIC), since existing data suggests differing effects in these groups. Statistical heterogeneity will be assessed using I 2 test.
For WP2, we will use these pooled estimates from WP1 in meta-regression analyses. These will incorporate the moderator/mediator variables as potential mechanisms at a national/regional level. This will measure the influence of these mechanisms on the association between lockdown measures and adverse perinatal outcomes.
Sensitivity analyses
Where enhanced datasets are available for an outcome, we will perform similar modelling techniques to those described above with these enhanced data as sensitivity analyses to test the robustness of the main analyses in different populations. These analyses will be specified further in a comprehensive Statistical Analysis Plan. Predefined examples include:
• Sensitivity analyses restricting the denominator for our main outcomes of interest (excluding outcomes on spontaneous preterm birth) from all births to only live births. These analyses will be informative for the appropriateness of using datasets which only include information on live births.
• Sensitivity analyses with varying cut-off points for our lockdown definition (i.e. above and below 50) from the stringency index to test the robustness of assigning ≥50 as the primary cut-off point. These analyses will also allow inclusion of countries with less strict lockdown measures, such as Sweden, and inform whether/to what extent the observed associations might vary by lockdown stringency.
We will conduct supplementary analyses in investigative datasets.
Output confidentiality
All outputs will be checked for any potential disclosure and confidentiality breaches, using guidance from the Handbook on SDC for Outputs by the UK Data Service.
Public and patient involvement
Public and patient involvement early in study design and development ensures research studies are responsive to input, guidance and advice, and can help identify and mitigate potential challenges early in the research process 25 . Further, public and patient involvement helps to identify research outcomes that are meaningful and pragmatic to knowledge users.
The iPOP team has engaged parents as patient partners early in the study design and have built a working group to capture and integrate patient involvement in the iPOP study as it moves forward. Meeting monthly, patient partners will be involved in developing effective and meaningful knowledge translation and communication strategies for disseminating iPOP findings. Specific to WP2, patient partners will work with researchers to examine mechanistic effects of the pandemic lockdown on perinatal outcomes. Patient partners will also work with researchers to develop knowledge translation strategies to ensure effective and meaningful dissemination of findings to knowledge users.
Ethical considerations
To ensure transparent, equitable, and meaningful engagement, we have developed Guiding Principles that outline the terms of agreement for study leads and collaborators who are involved in the iPOP Study. Each member of the iPOP Study must read and sign the guiding principles document in order to collaborate on the study. While not legally binding, this document provides guidance and parameters around authorship, roles and responsibilities, research integrity, communication and Team Science guidelines.
The iPOP Study ensures confidentiality and security of the processing of data for electronic files. Data will be safeguarded by an appropriate level of security, technical and organisational measures to prevent unauthorized disclosure or access, accidental or unlawful destruction, accidental loss or alteration, and unlawful forms of processing. WP1 and WP2 will be based on de-identified aggregate data only.
It will be assumed that any Team member sharing data within the iPOP Study does so in accordance with relevant and applicable legal and regulatory standards and obligations including but not limited to, confidentiality, data protection and intellectual property, and access governance agreements. iPOP collaborators must adhere to these policies and processes.
All collaborators must respect the iPOP principles of data protection and processing, which include the following:
All contributed to iPOP data must be
• Processed fairly and lawfully
• Collected for specified and legitimate purposes
• Accurate
• Absent of personal identifiers (names, addresses, etc.)
• Stored not longer than necessary
• Processed under the responsibility and liability of the data Controller for the provided data set
• Handled according to the EU GDPR rules (when hosted in the UK)
Conclusions
Spanning 37 countries ( Figure 4), the iPOP Study brings together expertise in perinatology, epidemiology, environmental science, intersectional feminism, and data science within a collaborative, equitable and interdisciplinary framework. The iPOP Study will leverage the natural experiment arising from the COVID-19 pandemic, to understand possible mechanisms of adverse perinatal outcomes and inform interventions and policy. Further, iPOP will investigate the effects of pandemic lockdowns by country income setting, incorporating data from LICs to HICs across the globe on key perinatal outcomes.
The initial focus of iPOP will be on the impact of COVID‐19 pandemic lockdowns on perinatal outcomes, including preterm birth, low birth weight, and stillbirth. Determining the worldwide extent of changes in perinatal outcomes during the COVID-19 pandemic will advance current understanding of preventable causes of these pervasive perinatal outcomes.
Building on the first two phases of the iPOP Study described in this protocol, iPOP further aims to investigate mechanisms for any observed changes in perinatal outcomes during the COVID-19 pandemic, using individual-level and setting-specific data. In the next study phase (WP3), we aim to examine the impact of maternal comorbidities (e.g. pregnancy complications; pre-existing chronic conditions including mental health), COVID-19 and non-COVID-19 infections, socio-economic factors, prenatal care, and birth practices on any associations between pandemic lockdowns and perinatal outcomes.
Results of the iPOP Study will be rapidly translated through our network of local and international stakeholders to inform further research and testable interventions for improving perinatal healthcare and social support systems during (and well beyond) the COVID-19 pandemic.
Data availability
No data are associated with this article.
Funding Statement
iPOP was established with funding from International COVID Data Alliance (ICODA) and Health Data Research (HDR) UK. This seed funding will be leveraged to secure additional funding to support ongoing operations and expansion of the iPOP Study. Sarah Stock is supported by a Wellcome Trust Clinical Career Development Fellowship (209560/Z/17/Z) and Health Data Research UK, during the conduct of this work. Helga Zoega is supported by a UNSW Scientia Fellowship. Meredith Brockway receives salary funding from the Molly Towell Perinatal Research Foundation. Meghan Azad holds the Canada Research Chair in the Developmental Origins of Chronic Disease and is a Fellow of the CIFAR Humans and the Microbiome Program.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 2 approved]
References
- 1. Phillips DE, Bhutta ZA, Binagwaho A, et al. : Learning from Exemplars in Global Health: a road map for mitigating indirect effects of COVID-19 on maternal and child health. BMJ Glob Health. 2020;5(7): e003430. 10.1136/bmjgh-2020-003430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Johns Hopkins University & Medicine: COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU).Johns Hopkins University & Medicine Coronavirus Resource Center.2020. (accessed July 24 2020). Reference Source [Google Scholar]
- 3. Hedermann G, Hedley PL, Bækvad-Hansen M, et al. : Danish premature birth rates during the COVID-19 lockdown. Arch Dis Child Fetal Neonatal Ed. 2021;106(1):93–95. 10.1136/archdischild-2020-319990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Philip RK, Purtill H, Reidy E, et al. : Reduction in preterm births during the COVID-19 lockdown in Ireland: a natural experiment allowing analysis of data from the prior two decades. medRxiv. 2020. 10.1101/2020.06.03.20121442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Been JV, Ochoa LB, Bertens LCM, et al. : Impact of COVID-19 mitigation measures on the incidence of preterm birth: a national quasi-experimental study. Lancet Public Health. 2020;5(11): e604–e611. 10.1016/S2468-2667(20)30223-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kc A, Gurung R, Kinney MV, et al. : Effect of the COVID-19 pandemic response on intrapartum care, stillbirth, and neonatal mortality outcomes in Nepal: a prospective observational study. Lancet Glob Health. 2020;8(10):e1273–81. 10.1016/S2214-109X(20)30345-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kumari V, Mehta K, Choudhary R: COVID-19 outbreak and decreased hospitalisation of pregnant women in labour. Lancet Glob Health. 2020;8(9):e1116–17. 10.1016/S2214-109X(20)30319-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khalil A, von Dadelszen P, Draycott T, et al. : Change in the Incidence of Stillbirth and Preterm Delivery During the COVID-19 Pandemic. JAMA. 2020;324(7):705–706. 10.1001/jama.2020.12746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. De Curtis M, Villani L, Polo A: Increase of stillbirth and decrease of late preterm infants during the COVID-19 pandemic lockdown. Arch Dis Child Fetal Neonatal Ed. 2020; fetalneonatal-2020-320682. 10.1136/archdischild-2020-320682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Main EK, Chang SC, Carpenter AM, et al. : Singleton preterm birth rates for racial and ethnic groups during the coronavirus disease 2019 pandemic in California. Am J Obstet Gynecol. 2020. 10.1016/j.ajog.2020.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Witteveen D, Velthorst E: Economic hardship and mental health complaints during COVID-19. Proc Natl Acad Sci U S A. 2020;117(44):27277–84. 10.1073/pnas.2009609117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pant S, Koirala S, Subedi M: Access to Maternal Health Services during COVID-19. Europasian Journal of Medical Sciences. 2020;2(2):48–52. 10.46405/ejms.v2i2.110 [DOI] [Google Scholar]
- 13. Rundle CW, Presley CL, Militello M, et al. : Hand hygiene during COVID-19: Recommendations from the American Contact Dermatitis Society. J Am Acad Dermatol. 2020;83(6):1730–1737. 10.1016/j.jaad.2020.07.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Landrigan PJ, Bernstein A, Binagwaho A: COVID-19 and clean air: an opportunity for radical change. Lancet Planet Health. 2020;4(10):e447–9. 10.1016/S2542-5196(20)30201-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pilecco FB, Leite L, Góes EF, et al. : Addressing racial inequalities in a pandemic: data limitations and a call for critical analyses. Lancet Glob Health. 2020;8(12):e1461–e1462. 10.1016/S2214-109X(20)30360-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hamadani JD, Hasan MI, Baldi AJ, et al. : Immediate impact of stay-at-home orders to control COVID-19 transmission on socioeconomic conditions, food insecurity, mental health, and intimate partner violence in Bangladeshi women and their families: an interrupted time series. Lancet Glob Health. 2020;8(11):e1380–9. 10.1016/S2214-109X(20)30366-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roberton T, Carter ED, Chou VB, et al. : Early estimates of the indirect effects of the COVID-19 pandemic on maternal and child mortality in low-income and middle-income countries: a modelling study. Lancet Glob Health. 2020;8(7):e901–8. 10.1016/S2214-109X(20)30229-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yeoh DK, Foley DA, Minney-Smith CA, et al. : The impact of COVID-19 public health measures on detections of influenza and respiratory syncytial virus in children during the 2020 Australian winter. Clin Infect Dis. 2020;ciaa1475. 10.1093/cid/ciaa1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fell DB, Savitz DA, Kramer MS, et al. : Maternal influenza and birth outcomes: systematic review of comparative studies. BJOG. 2017;124(1):48–59. 10.1111/1471-0528.14143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chudnovets A, Liu J, Narasimhan H, et al. : Role of inflammation in virus pathogenesis during pregnancy. J Virol. 2020;JVI.01381–19. 10.1128/JVI.01381-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Regan AK, Feldman BS, Azziz-Baumgartner E, et al. : An international cohort study of birth outcomes associated with hospitalized acute respiratory infection during pregnancy. J Infect. 2020;81(1):48–56. 10.1016/j.jinf.2020.03.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Klepac P, Locatelli I, Korošec S, et al. : Ambient air pollution and pregnancy outcomes: A comprehensive review and identification of environmental public health challenges. Environ Res. 2018;167:144–59. 10.1016/j.envres.2018.07.008 [DOI] [PubMed] [Google Scholar]
- 23. Lander T: Neonatal and perinatal mortality: country, regional and global estimates. Neonatal and perinatal mortality: country, regional and global estimates.2006. Reference Source [Google Scholar]
- 24. Shaddick G, Thomas ML, Green A, et al. : Data integration model for air quality: a hierarchical approach to the global estimation of exposures to ambient air pollution. J R Stat Soc C. 2018;67(1):231–53. 10.1111/rssc.12227 [DOI] [Google Scholar]
- 25. Rayment J, Lanlehin R, McCourt C, et al. : Involving seldom-heard groups in a PPI process to inform the design of a proposed trial on the use of probiotics to prevent preterm birth: a case study. Res Involv Engagem. 2017;3:11. 10.1186/s40900-017-0061-3 [DOI] [PMC free article] [PubMed] [Google Scholar]




