Abstract
Background
Lewy bodies have been described in the locus coeruleus of some patients with essential tremor (ET), and this brainstem nucleus plays an important role in sleep cycle regulation. Despite this, no studies have investigated the relationship between daily sleep duration and the risk of ET. We determined whether baseline daily sleep duration was associated with an increased risk of incident ET.
Methods
In this prospective, population-based study of people >65 years of age (NEDICES), participants were evaluated at baseline and 3 years later. At baseline, participants indicated their daily sleep duration as the sum of nighttime sleep and daytime napping. The average daily total sleep duration was grouped into four categories: ≤ 5 (short sleepers), 6, 7 to 8 (reference), and ≥ 9 (long sleepers) hours.
Results
3,303 participants had a median duration of follow-up of 3.3 years. There were 76 incident ET cases at follow-up. The relative risks (RRs) for short sleepers and for long sleepers were 2.25 (95% Confidence Interval [CI], 1.21 – 4.16, p = 0.01) and 0.74 (95% CI, 0.41 – 1.32, p = 0.31), respectively. After adjustment for potential confounders, including age, gender, educational level, current smoker, current drinker, and depressive symptoms or antidepressant use, the risk remained significantly increased for short sleepers (1.95 [95% CI, 1.03 – 3.70, p = 0.04]).
Conclusions
Short daily sleep duration may be a pre-motor marker for ET. Additional prospective studies are needed to confirm these results, and the biological basis for this association merits additional investigation.
Keywords: Elderly, epidemiology, incident essential tremor, sleep duration
Introduction
Essential tremor (ET) is a neurological disorder with a particularly high incidence and prevalence.1–4 In addition, the disease poses public health challenges, as it impacts negatively on several areas of function, health-related quality of life, and morale and, it may increase short-term mortality in the elderly.5–7
In the last decade, there have been many advances in our understanding of this disease,8–10 including its pathogenesis and the presence of nonmotor symptoms.10 Recent neuropathogical and imaging findings demonstrate abnormalities in the cerebellum in many cases,11–13 whereas other ET cases have variably been reported to have brainstem Lewy bodies, particularly in the locus coeruleus.12, 14, 15 The locus coeruleus has a role in maintaining normal sleep patterns.16,17 Dysregulation of locus coeruleus-noradrenergic neurotransmission is known to contribute to cognitive and/or arousal dysfunction associated with a variety of psychiatric disorders, including affective disorders, attention-deficit hyperactivity disorder, and sleep and arousal disorders.18 We are aware of three case-control studies that have examined the association between sleep dysregulation and ET.19–21 We know of no previous study that has prospectively investigated the relation between baseline daily sleep duration and risk of incident ET.
We hypothesized that short or long sleep duration could predate the development of ET and serve as a marker of increased risk. To address this issue, we assessed the relationship between baseline self-reported daily sleep duration and incident ET after 3 years of follow-up among participants enrolled in the Neurological Disorders in Central Spain (NEDICES) cohort, a prospective, population-based study.1,2
Methods
Study population
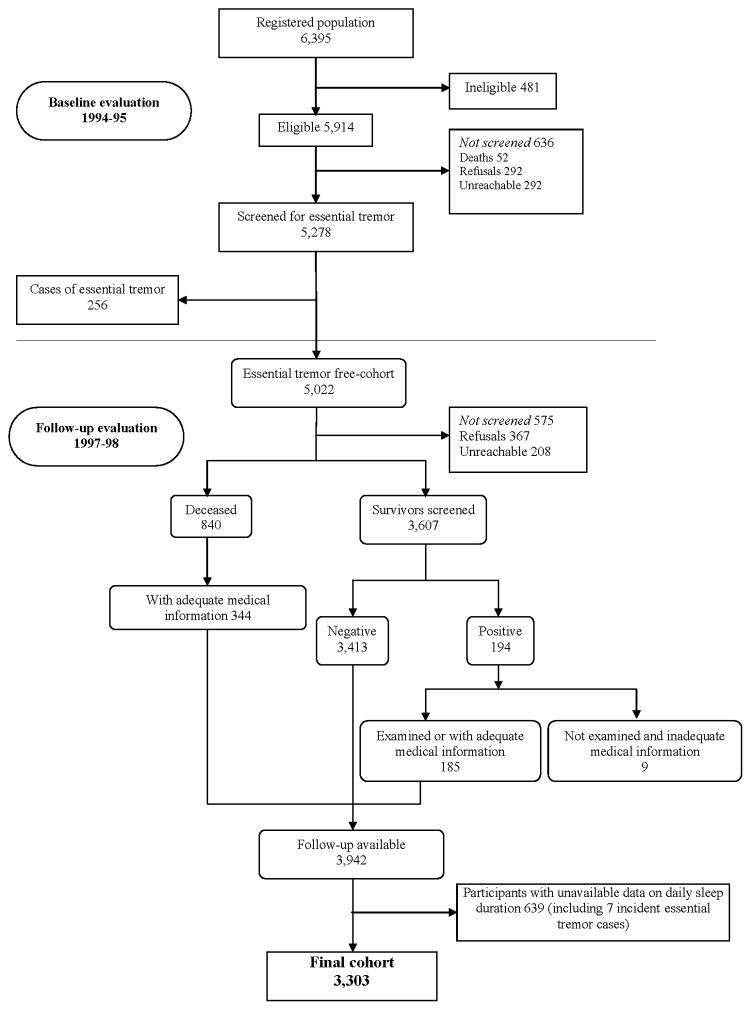
This investigation was part of NEDICES, a longitudinal, population-based survey of major age-associated conditions of persons age 65 years and older. As described previously,22–25 the study sample was taken from the census of three communities in central Spain: Las Margaritas, Lista, and Arévalo. The three communities represent a broad spectrum of current social and economic conditions in Spain. The registered study population consisted of 6,395 individuals, but 481 were ineligible (e.g., census issues, incorrect address, death), leaving 5,914 eligible subjects, of whom 5,278 (89.2%) were evaluated at baseline (1994 to 1995).22–25 All procedures were approved by the ethical standards committees on human experimentation at the University Hospitals “12 de Octubre” (Madrid) and “La Princesa” (Madrid). Written (signed) informed consent was obtained from all enrollees.
Screening Questionnaires
Detailed accounts of the study population, sampling methods, and study assessments have been published.1, 2, 22–25 Each participant received either a face-to-face evaluation (at baseline, 1994–1995 and at follow-up, 1997– 1998) or a questionnaire (mailed to participants who were unavailable for face-to-face interviews). During the face-to-face interview, data were collected on demographics, current medications, medical conditions, depressive symptoms (the question, “do you suffer from depression?”), and life style questions. One screening question for ET was included (“have you ever suffered from tremor of the head, hands, or legs that has lasted longer than several days?”).1, 2 This question was a Spanish adaptation of that used by the Italian Longitudinal Study on Aging (ILSA) Working Group.26 To assess the performance of this screening question, a random sample of approximately 4% of those who had screened negative was selected and contacted.25 Of 205 subjects who were contacted, 183 were successfully scheduled for a neurological examination by a senior neurologist who routinely evaluates patients with movement disorders (J.O. [see http://www.ciberned.es/estudio-nedices]).25 During the neurological examination, participants were asked to perform three manual tasks to assess postural and kinetic tremors, including sustained bilateral arm extension, bilateral finger-nose-finger maneuver (>6 repetitions with each arm), and an Archimedes spiral drawn with the dominant arm.1, 2 The diagnostic criteria for ET were similar to those used in the Sicilian Study27 (see below) and none (0%) of the 183 subjects were diagnosed as ET.25
To assess sleep duration, each participant was asked to indicate their “total hours of actual sleep in a 24-hour period”. Average sleep duration was grouped into the following five categories: ≤ 5, 6, 7, 8, and > 8 hours. These categories have been used in other sleep studies of older adult populations in Spain and elsewhere.28–30
Diagnostic criteria for essential tremor (ET)
Diagnostic criteria for ET were similar those used in the Sicilian Study.27 First, action tremor of the head, limbs, or voice without any other recognizable cause, and second the tremor had to be of gradual onset (i.e., slow and progressive) and (1) present for at least 1 year or (2) accompanied by a family history of the same disorder (at least one reportedly-affected first-degree relative). Third, on an Archimedes spiral, tremor severity had to be moderate or greater (rating ≥ 2 according to the Washington Heights-Inwood Genetic Study of ET Rating Scale).31 Based on their medical and medication history, participants with tremor related to alcohol withdrawal, hyperthyroidism, anxiety, medications (including an extensive list of medications that result in dopamine receptor blockade and an extensive list of medications [e.g., lithium, theophylline, β-adrenergic agonists, valproate, selective serotonin reuptake inhibitors, cyclosporine] that induce tremor) were not considered to have ET. Similarly, based on their neurologic examination, participants with action tremor related to PD, dystonia, orthostatic tremor, or other movement disorders were not considered to have ET. Because a mild intentional component may accompany the kinetic tremor during the finger-nose-finger maneuver in patients with ET, the presence of an intentional component to the kinetic tremor did not exclude the diagnosis of ET. During this examination, participants were also asked to perform three manual tasks to assess postural and kinetic tremors, as noted above. Participants with ET identified by one of the eight NEDICES neurologists (see http://www.ciberned.es/estudio-nedices) were subsequently examined independently by two additional neurologists (who were chosen from among the eight), who examined the participant together. Participants were classified as having ET only when the three neurologists agreed. Their agreement was high (>90%). PD and other forms of parkinsonism were diagnosed when at least two cardinal signs were present on UPDRS.32–34 For participants who could not be examined, medical records were obtained from general practitioners, from in-patient hospitalizations, and from neurological specialists (if they had visited one). In addition, death certificate diagnoses were reviewed for each screened participant who had died prior to neurological examination. We applied the same diagnostic criteria for ET (see above)27 in those cases whose diagnosis was based on review of their medical records, except signatures rather than Archimedes spirals were sometimes evaluated in those cases.
Follow-up evaluation
Of the 5,278 participants who were evaluated at baseline (1994 – 1995), 256 prevalent ET cases (4.8%) were detected, and these were excluded from the current analyses, leaving 5,022 who were free of ET at baseline.
As described,1, 2 there were 5,022 participants without ET at baseline (see figure, ET free cohort). At the time of the follow-up evaluation (1997 to 1998), 575 (11.4%) of 5,022 were lost to the follow-up because they either declined to participate (N = 367) or were unreachable (N = 208). In addition, 840 (16.7%) had died before they were contacted for the follow-up assessment. Therefore, 3,607 (71.8%) participants completed the follow-up evaluation that included questions about health status and our screening question for ET (i.e., precisely the same screening question for ET used at the baseline evaluation). Of these 3,607 participants, 3,413 (94.6%) screened negative and 194 (5.4%) screened positive for ET. Nine (4.6%) of the 194 participants who screened positive for ET could not be examined because they declined to participate and were not included in the final cohort (see figure); adequate medical records (i.e., medical records from their general practitioner, hospitalizations, and neurologist [if applicable]) could not be obtained from these nine participants. Of the 185 remaining participants who screened positive during the follow-up assessment, in-person neurologic examinations were administered by neurologists to 138 (see previous description of neurologic examination performed by one of eight NEDICES neurologists) and medical records (without the neurologic examination) were reviewed in 47. In the final cohort, we also included 344 of the 840 deceased participants who had adequate medical records (i.e., medical records from their general practitioner, hospitalizations, and neurologist [if applicable]), so that the final cohort of 3,942 participants consisted of 3,413 participants who screened negative for ET, 185 who screened positive who were either examined or had adequate medical records, and 344 deceased participants with adequate medical records (see figure). The median duration of follow-up was 3.3 ± 0.9 years (range 0.08 to 6.58). In our paper on the incidence of ET,2 we reported that we were able to assess 185/194 (95.4%) participants who screened positive for ET. Of these 185, 83 were diagnosed with ET (44.9%).2
Figure 1.
Flow chart of the study.
The diagnostic approach to ET cases was identical to that performed during the baseline evaluation.1, 2
Final sample
Follow-up data were available on 3,942 of the remaining 5,022 participants (including 83 incident ET cases and 3,859 participants without incident ET). We further excluded 639 participants (including 7 [8.4%] with incident ET and 632 {16.4%] without incident ET) without available data on daily sleep duration, which left 3,303 participants for our analyses (Figure). We compared the final sample of 3,303 participants to the 639 participants without data on daily sleep duration, and they were similar in age (73.5 ± 6.6 vs. 73.6 ± 6.8 years), and gender (1,878 [56.9%] women vs. 395 [61.8%] women).
Statistical Analyses
Analyses were performed in SPSS (version 20.0). All tests were two sided, and significance was accepted at the 5% level (α = 0.05). Using a one-sample Kolmogorov–Smirnov test, we determined that age and sleep duration were not normally distributed. Therefore, although mean and median values were reported, differences were compared using a nonparametric (Mann–Whitney U and Kruskal Wallis tests). The χ2 test was used to analyze categorical variables. We used the Cox proportional hazards models to estimate the relative risks (RR) of developing incident ET in participants who slept ≤ 5 hours (short sleepers), 6 hours, 7 to 8 hours (reference) and ≥ 9 hours (long sleepers) per 24 hours. The reference category was 7 to 8 hours of sleep. We chose this reference category because the mean hours of sleep in adults in Madrid are 7.3 hours (http://www.madridsalud.es/publicaciones/OtrasPublicaciones/EstudioSaludCiudadMadrid.pdf).8 We began with an unadjusted model. Then, in adjusted models, we first considered baseline variables that were associated with both the exposure (sleep duration) and the outcome (incident ET) (“Model 1” [more restrictive criteria for confounding]) and then considered baseline variables that were associated with either sleep duration or incident ET (“Model 2” [less restrictive criteria for confounding]). Variables assessed at baseline that we considered included age in years, gender, educational level (illiterate, can read and write, primary studies, secondary and higher studies), hypertension, diabetes mellitus, heart diseases, current smoker, body mass index, current drinker, and depressive symptoms (“do you suffer from depression?”) or antidepressant use.
In participants who developed incident ET, person-years were the time between the baseline evaluation and the reported date of ET onset. When the date of onset of ET was unknown, person-years were calculated as the midpoint between the baseline evaluation and the follow-up evaluation. For participants who did not develop incident ET, person-years was the time between the baseline evaluation and (1) the follow up evaluation or (2) death in the participants who died prior to follow-up evaluation yet who had adequate medical information.
Results
The 3,303 participants (1,425 men [43.1%] and 1,878 [56.9%] women) had a mean duration of follow-up of 3.4 years (median 3.3 years; range 1.5 – 6.6 years). Seventy-six (2.3%) of 3,303 participants had developed incident ET by their follow-up evaluation. No ET cases were excluded because tremor duration was less than one year. These 76 incident ET cases included 39 who had in-person baseline examinations and did not have ET on those examinations, and 37 who did not have in-person baseline examinations. To assess whether these 37 cases had ET at baseline, each of the 37 was re-interviewed during the follow-up evaluation to establish that the onset of their tremor had been after the baseline assessment. More important than self-reported information, however, was that baseline handwriting samples from the 37 incident ET cases and 31 age-matched controls were blindly reviewed by one of the authors (E.D.L.) and rated using Bain and Findley’s ten-point scale. None of the cases and controls had tremor that was in the ET range (all had Bain and Findley35 handwriting tremor scores ≤ 1, which are within the range of normal),35 hence it is unlikely that these cases had ET at baseline.
Participants with incident ET were similar to those without incident ET in terms of age, gender, education and most other factors; they differed from those without incident ET in terms of depressive symptoms or antidepressants use and sleep duration ET (Table 1). Sixty (85.7%) of the 70 participants who were taking an antidepressant were taking a tricyclic antidepressant, 7 (10.0%) were taking a selective serotonin reuptake inhibitor, two (2.9%) were taking both, and one (1.4%) was taking a monoamine oxidase inhibitor.
Table 1.
Baseline demographic and clinical characteristics of participants with incident essential tremor vs. participants without incident essential tremor
| Participants with Incident Essential Tremor (N = 76) | Participants without Incident Essential Tremor (N = 3,227) | p value | |
|---|---|---|---|
|
| |||
| Age in years (hours) | 73.8 ± 6.4 (73) | 73.5 ± 6.6 (72) | 0.595 a |
|
| |||
| Gender (women) | 45 (59.2%) | 1,833 (56.8%) | 0.675 b |
|
| |||
| Educational level | |||
| Illiterate | 13 (17.1%) | 415 (12.9%) | 0.560 b |
| Can read and write | 34 (44.7%) | 1,362 (42.2%) | |
| Primary studies | 18 (23.7%) | 955 (29.6%) | |
| Secondary and higher studies | 11 (14.5%) | 495 (15.3%) | |
|
| |||
| Hypertension * c | 46 (61.3%) | 1,634 (50.7%) | 0.07 b |
|
| |||
| Diabetes mellitus * c | 14 (18.4%) | 545 (17.0%) | 0.748 b |
|
| |||
| Heart diseases * c | 7 (9.3%) | 303 (9.4%) | 0.982 b |
|
| |||
| Current smoker | 5 (6.6%) | 384 (11.9%) | 0.154 b |
|
| |||
| Current drinker | 27 (35.5%) | 1,119 (34.7%) | 0.887 b |
|
| |||
| Body mass index (kg/m2) * | 28.2 ± 4.3 (28.4) | 27.6 ± 5.0 (27.0) | 0.111 a |
|
| |||
| Depressive symptoms or antidepressant use * | 27 (36.5%) | 789 (24.6%) | 0.020 b |
|
| |||
| Depressive symptoms * | 27 (36.5%) | 789 (24.6%) | 0.020 b |
|
| |||
| Antidepressant use | 3 (3.9%) | 67 (2.1%) | 0.217 b |
|
| |||
| Sleep duration (hours) | 7.2 ± 1.9 (7) | 8.1 ± 2.1 (8) | < 0.001 a |
Mean ± SD (median) and frequency (%) are reported.
Mann–Whitney U test
chi-square test or Fisher p test for categorical variables.
by self-report.
Data on some participants were missing.
Baseline characteristics of the participants in the four sleep duration categories are shown in Table 2. At baseline, short sleepers were more frequently women, older and less educated. In addition, they were more likely to have depressive symptoms or use antidepressants and consumed less alcohol and smoked less (Table 2).
Table 2.
Baseline characteristics of the study participants, according to habitual sleep duration in 1994–1995
| Sleep duration (hours per day) | |||||
|---|---|---|---|---|---|
|
| |||||
| ≤5 (n = 355) | 6 (n = 416) | 7–8 (n = 1,168) | ≥9 (n = 1,364) | p value | |
|
| |||||
| Age in years | 74.6 ± 6.8 (74) | 73.6 ± 6.2 (72) | 72.6 ± 6.1 (71) | 74.1 ± 6.9 (72) | 0.001 a |
|
| |||||
| Gender (women) | 246 (69.3%) | 258 (62.0%) | 660 (56.5%) | 714 (52.3%) | < 0.001 b |
|
| |||||
| Educational level | |||||
| Illiterate | 60 (16.9%) | 60 (14.4%) | 125 (10.7%) | 183 (13.4%) | < 0.001 b |
| Can read and write | 150 (42.3%) | 174 (41.8%) | 470 (40.2%) | 602 (44.1%) | |
| Primary studies | 108 (30.4%) | 129 (31.0%) | 354 (30.3%) | 382 (28.0%) | |
| Secondary and higher studies | 37 (10.4%) | 53 (12.7%) | 219 (18.8%) | 197 (14.4%) | |
|
| |||||
| Hypertension * c | 189 (53.2%) | 214 (51.6%) | 599 (51.3%) | 678 (49.8%) | 0.658 b |
|
| |||||
| Diabetes mellitus * c | 73 (20.6%) | 74 (18.0%) | 177 (15.3%) | 235 (17.4%) | 0.106 b |
|
| |||||
| Heart diseases * c | 30 (8.5%) | 46 (11.1%) | 105 (9.0%) | 129 (9.5%) | 0.562 b |
|
| |||||
| Current smoker | 25 (7.0%) | 41 (9.9%) | 146 (12.5%) | 177 (13.0%) | 0.009 b |
|
| |||||
| Current drinker | 92 (25.9%) | 127 (30.7%) | 449 (38.4%) | 478 (35.1%) | < 0.001 b |
|
| |||||
| Body mass index (kg/m2) * | 27.7 ± 5.0 (27.3) | 27.7 ± 4.1 (27.5) | 27.5 ± 5.2 (26.9) | 27.5 ± 5.0 (27.0) | 0.235 a |
|
| |||||
| Depressive symptoms or antidepressant use * | 131 (37.3%) | 110 (26.7%) | 282 (24.3%) | 302 (22.3%) | < 0.001 b |
|
| |||||
| Depressive symptoms * | 130 (37.0%) | 110 (26.7%) | 280 (24.1%) | 296 (21.9%) | < 0.001 b |
|
| |||||
| Antidepressant use | 9 (2.5%) | 5 (1.2%) | 21 (1.8%) | 35 (2.6%) | 0.275 b |
|
| |||||
| Incident essential tremor | 17 (4.8%) | 13 (3.1%) | 25 (2.1%) | 21 (1.5%) | 0.002 b |
Mean ± SD (median) and frequency (%) are reported.
Kruskal Wallis test and
chi-square test.
by self-report.
Data on some participants were missing.
In unadjusted analyses, short sleep duration was associated with a significantly increased risk of incident ET. For short sleepers (≤ 5 hours), the unadjusted RR was 2.25, 95% CI = 1.21 – 4.16, p = 0.01 (Table 3). After adjusting for depressive symptoms or antidepressants use (variable associated with both sleep duration and ET) (Model 1), the risk of ET was increased for short sleepers (1.99 [95% CI = 1.06 – 3.74], p = 0.03) (Table 3). In addition, after adjusting for age, gender, educational level, current smoker, current drinker, and depressive symptoms or antidepressants use (variables associated with either sleep duration or ET) (Model 2), the risk of ET still remained significantly increased for short sleepers (1.95 [95% CI = 1.03 – 3.70], p = 0.04) (Table 3).
Table 3.
Relative risks of incident essential tremor according to habitual sleep duration at baseline
| Sleep duration (hours per day)
| ||||
|---|---|---|---|---|
| ≤5 (n = 355) | 6 (n = 416) | 7–8 (n = 1,168) | ≥9 (n = 1,364) | |
|
| ||||
| Unadjusted | ||||
| Relative risk | 2.25 | 1.46 | 1 | 0.74 |
| 95% CI | 1.21–4.16 | 0.75–2.85 | 0.41–1.32 | |
| p value | 0.01 | 0.27 | 0.31 | |
|
| ||||
| Adjusted (Model 1) * | ||||
| Relative risk | 1.99 | 1.46 | 1 | 0.71 |
| 95% CI | 1.06–3.74 | 0.74–2.85 | 0.40–1.28 | |
| p value | 0.03 | 0.27 | 0.26 | |
|
| ||||
| Adjusted (Model 2) ** | ||||
| Relative risk | 1.95 | 1.45 | 1 | 0.69 |
| 95% CI | 1.03–3.70 | 0.74–2.84 | 0.38–1.26 | |
| p value | 0.04 | 0.28 | 0.23 | |
Adjusted for depressive symptoms or antidepressant use (variable associated both with sleep duration categories and essential tremor).
Adjusted for age, gender, educational level, current smoker, current drinker, and depressive symptoms or antidepressant use (variables associated with either sleep duration categories or essential tremor).
We also conducted a sensitivity analysis in which we excluded the 37 ET cases who did not have an in-person baseline examination. In unadjusted analyses, short sleep duration was associated with a significantly increased risk of incident ET. For short sleepers, the unadjusted RR was 3.62, 95% CI = 1.60 – 8.21, p = 0.002. The adjusted RRs were 3.02 (95% CI = 1.30 – 7.01, p = 0.010, Model 1) and 2.66 (95% CI = 1.13 – 6.24, p = 0.025, Model 2).
Discussion
In this prospective, population-based study, we observed a significant positive association between self-reported short sleep duration and incident ET. This difference persisted even after controlling for potential confounding factors.
Short sleep duration is associated with cardiovascular disease36, 376 and other health outcomes such as obesity,38 raised levels of inflammatory markers,39 and depressive symptoms.40 To date, the association between sleep dysregulation and ET has only been assessed in three previous studies.19–21 Adler et al.19 compared Epworth Sleepiness Scale (ESS) scores in 53 ET cases to 49 PD cases and 175 normal controls, and noted no ET case–control difference; in that study ESS scores in ET were intermediate between normal controls and PD but far more similar to those of normal controls. Chandran et al.20 found a marked difference in Pittsburgh Sleep Quality Index (PASQI) scores in 50 ET cases as compared with 50 controls, yet no difference in ESS scores. Finally, Gerbin et al.21 compared the ESS and the PSQI scores in 120 ET cases, 120 normal controls, and 40 PD cases. The ET case-control difference was not significant, yet in a test for trend, PD cases had the highest PSQI score, followed by ET (intermediate), and lowest scores in controls.21 The current study is the first prospective, population-based study, and the first that demonstrates that short sleep duration is associated with an increased risk of ET.
The mechanisms through which short sleep might be related to incident ET are yet to be established. We may only speculate on possible explanations for this association. One possibility is that short sleep duration may be an early symptom of ET, possibly predating its official diagnosis as a motor disorder. Second, scarce sleep per se could directly lead to an increased risk of ET. Currently, however, we know of no plausible physiologic explanation for such a cause-and-effect relationship.
This study had a number of potential limitations. First, we adjusted the risk for a number of important confounders but our evaluation of depression was limited. Depression may be associated with short sleep duration 40 and depression in the elderly is also associated with an increased risk of ET.41 We assessed depression by self-report and, although our screening question was modeled on a question that correctly diagnosed depression in 85.4% of participants,41, 42 we may have under-ascertained depression. However, based on a validation study in which we showed a high level of agreement between the data generated from this screening question and a more detailed in-person psychiatric assessment,40 we think that such misclassification errors were likely to be low. Second, information on sleep duration was captured through self-report and is therefore subject to error, including the potential existence of a change in the usual duration of sleep predating the appearance of ET. Such errors, though, are equally likely for all study groups and thus, given the size of our sample, may have become negligible by chance. It would be important to compare our results with those of future studies that use polysomnographic measures of sleep disturbance. Third, we did not differentiate between nighttime sleep and daytime napping. There are somewhat separate literatures on each of these aspects of sleep and their associations with different adverse health outcomes, and another literature regarding the impact of daytime napping on nighttime sleep.43 Fourth, the mean baseline age of our ET cases was 73.8 ± 6.4 years and the follow-up interval was relatively brief; further studies are needed to determine the extent to which our findings may be generalized from this highly selected age and time window. Finally, collection of similar information on sleep at follow-up was not available, but such data would have added to the utility of our analyses.
Strengths of this study include the prospective, population-based, cohort design and the standardized assessments of ET at the baseline and follow-up evaluations.
In summary, short sleep duration was a significant predictor of incident ET even after controlling for numerous potential confounders. Clinicians should be aware that short sleep duration is likely to be a marker of poor physical and mental health status, including ET. Further studies are needed to better elucidate the biological mechanisms underlying this association.
Acknowledgments
Acknowledgments and Funding
Additional information about collaborators and detailed funding of the NEDICES Study can be found on the web (http://www.ciberned.es/estudio-nedices). The Spanish Health Research Agency and the Spanish Office of Science and Technology supported NEDICES. Drs. Benito-León and Bermejo-Pareja are supported by NIH R01 NS039422 from the National Institutes of Health, Bethesda, MD, USA. Dr. Elan D. Louis has received research support from the National Institutes of Health, Bethesda, MD, USA: NINDS #R01 NS042859 (principal investigator), NINDS #R01 NS39422 (principal investigator), NINDS #T32 NS07153-24 (principal investigator), NINDS #R01 NS073872 (principal investigator), NINDS #R21 NS077094 (co-Investigator), and NINDS #R01 NS36630 (co-Investigator), as well as the Parkinson’s disease Foundation (principal investigator).
Footnotes
Disclosure: The authors report no conflicts of interest.
Disclosures:
Dr. Benito-León reports no disclosures.
Dr. Louis reports no disclosures.
Dr. Bermejo-Pareja reports no disclosures.
Authors Roles
Dr. Benito-León (jbenitol@meditex.es) collaborated in: 1) the conception, organization and execution of the research project; 2) the statistical analysis design, and; 3) the writing of the manuscript first draft and the review and critique of the manuscript.
Dr. Louis (EDL2@columbia.edu) collaborated in: 1) the conception, organization and execution of the research project; 2) the statistical analysis design, and; 3) the writing of the manuscript first draft and the review and critique of the manuscript.
Dr. Bermejo-Pareja (fbermejop2004@yahoo.es) collaborated in: 1) the conception, organization and execution of the research project, and; 2) the review and critique of the manuscript.
References
- 1.Benito-Leon J, Bermejo-Pareja F, Morales JM, Vega S, Molina JA. Prevalence of essential tremor in three elderly populations of central Spain. Mov Disord. 2003;18(4):389–394. doi: 10.1002/mds.10376. [DOI] [PubMed] [Google Scholar]
- 2.Benito-Leon J, Bermejo-Pareja F, Louis ED Neurological Disorders in Central Spain Study G. Incidence of essential tremor in three elderly populations of central Spain. Neurology. 2005;64(10):1721–1725. doi: 10.1212/01.WNL.0000161852.70374.01. [DOI] [PubMed] [Google Scholar]
- 3.Benito-Leon J. How common is essential tremor? Neuroepidemiology. 2009;32(3):215–216. doi: 10.1159/000195692. [DOI] [PubMed] [Google Scholar]
- 4.Benito-Leon J. Essential tremor: one of the most common neurodegenerative diseases? Neuroepidemiology. 2011;36(2):77–78. doi: 10.1159/000323572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez-Martin P, Jimenez-Jimenez FJ, Carroza Garcia E, et al. Most of the Quality of Life in Essential Tremor Questionnaire (QUEST) psychometric properties resulted in satisfactory values. Journal of clinical epidemiology. 2010;63(7):767–773. doi: 10.1016/j.jclinepi.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Louis ED, Benito-Leon J, Bermejo-Pareja F Neurological Disorders in Central Spain Study G. Philadelphia Geriatric Morale Scale in essential tremor: a population-based study in three Spanish communities. Movement disorders: official journal of the Movement Disorder Society. 2008;23(10):1435–1440. doi: 10.1002/mds.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Louis ED, Benito-Leon J, Ottman R, Bermejo-Pareja F Neurological Disorders in Central Spain Study G. A population-based study of mortality in essential tremor. Neurology. 2007;69(21):1982–1989. doi: 10.1212/01.wnl.0000279339.87987.d7. [DOI] [PubMed] [Google Scholar]
- 8.Benito-Leon J, Louis ED. Clinical update: diagnosis and treatment of essential tremor. Lancet. 2007;369(9568):1152–1154. doi: 10.1016/S0140-6736(07)60544-3. [DOI] [PubMed] [Google Scholar]
- 9.Benito-Leon J, Louis ED. Update on essential tremor. Minerva medica. 2011;102(6):417–439. [PubMed] [Google Scholar]
- 10.Tan EK, Fook-Chong S, Lum SY, et al. Non-motor manifestations in essential tremor: use of a validated instrument to evaluate a wide spectrum of symptoms. Parkinsonism & related disorders. 2005;11(6):375–380. doi: 10.1016/j.parkreldis.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Louis ED, Faust PL, Vonsattel JP. Purkinje cell loss is a characteristic of essential tremor. Parkinsonism & related disorders. 2011;17(6):406–409. doi: 10.1016/j.parkreldis.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Louis ED, Faust PL, Vonsattel JP, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain: a journal of neurology. 2007;130(Pt 12):3297–3307. doi: 10.1093/brain/awm266. [DOI] [PubMed] [Google Scholar]
- 13.Benito-Leon J, Alvarez-Linera J, Hernandez-Tamames JA, Alonso-Navarro H, Jimenez-Jimenez FJ, Louis ED. Brain structural changes in essential tremor: voxel-based morphometry at 3-Tesla. Journal of the neurological sciences. 2009;287(1–2):138–142. doi: 10.1016/j.jns.2009.08.037. [DOI] [PubMed] [Google Scholar]
- 14.Louis ED, Honig LS, Vonsattel JP, Maraganore DM, Borden S, Moskowitz CB. Essential tremor associated with focal nonnigral Lewy bodies: a clinicopathologic study. Archives of neurology. 2005;62(6):1004–1007. doi: 10.1001/archneur.62.6.1004. [DOI] [PubMed] [Google Scholar]
- 15.Ross GW, Dickson D, Cersosimo M. Pathological investigation of essential tremor. Neurology. 2004;62(7):S5, A537–A538. [Google Scholar]
- 16.Nitz D, Siegel JM. GABA release in the locus coeruleus as a function of sleep/wake state. Neuroscience. 1997;78(3):795–801. doi: 10.1016/s0306-4522(96)00549-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi K, Kayama Y, Lin JS, Sakai K. Locus coeruleus neuronal activity during the sleep-waking cycle in mice. Neuroscience. 2010;169(3):1115–26. doi: 10.1016/j.neuroscience.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain research Brain research reviews. 2003;42(1):33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- 19.Adler CH, Hentz JG, Shill HA, et al. Probable RBD is increased in Parkinson’s disease but not in essential tremor or restless legs syndrome. Parkinsonism & related disorders. 2011;17(6):456–458. doi: 10.1016/j.parkreldis.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chandran V, Pal PK, Reddy JY, Thennarasu K, Yadav R, Shivashankar N. Non-motor features in essential tremor. Acta neurologica Scandinavica. 2012;125(5):332–337. doi: 10.1111/j.1600-0404.2011.01573.x. [DOI] [PubMed] [Google Scholar]
- 21.Gerbin M, Viner AS, Louis ED. Sleep in essential tremor: a comparison with normal controls and Parkinson’s disease patients. Parkinsonism & related disorders. 2012;18(3):279–284. doi: 10.1016/j.parkreldis.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morales JM, Bermejo FP, Benito-Leon J, et al. Methods and demographic findings of the baseline survey of the NEDICES cohort: a door-to-door survey of neurological disorders in three communities from Central Spain. Public health. 2004;118(6):426–433. doi: 10.1016/j.puhe.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Bermejo-Pareja F, Benito-Leon J, Vega QS, et al. The NEDICES cohort of the elderly. Methodology and main neurological findings. Revista de neurologia. 2008;46(7):416–423. [PubMed] [Google Scholar]
- 24.Vega S, Benito-Leon J, Bermejo-Pareja F, et al. Several factors influenced attrition in a population-based elderly cohort: neurological disorders in Central Spain Study. Journal of clinical epidemiology. 2010;63(2):215–222. doi: 10.1016/j.jclinepi.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Bermejo F, Gabriel R, Vega S, et al. Problems and issues with door-to-door, two-phase surveys: an illustration from central Spain. Neuroepidemiology. 2001;20(4):225–231. doi: 10.1159/000054794. [DOI] [PubMed] [Google Scholar]
- 26.Maggi S, Zucchetto M, Grigoletto F, et al. The Italian Longitudinal Study on Aging (ILSA): design and methods. Aging (Milano) 1994;6(6):464–473. doi: 10.1007/BF03324279. [DOI] [PubMed] [Google Scholar]
- 27.Salemi G, Savettieri G, Rocca WA, et al. Prevalence of essential tremor: a door-to-door survey in Terrasini, Sicily. Sicilian Neuro-Epidemiologic Study Group. Neurology. 1994;44(1):61–64. doi: 10.1212/wnl.44.1.61. [DOI] [PubMed] [Google Scholar]
- 28.Patel SR, Malhotra A, White DP, Gottlieb DJ, Hu FB. Association between reduced sleep and weight gain in women. American journal of epidemiology. 2006;164(10):947–954. doi: 10.1093/aje/kwj280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopez-Garcia E, Faubel R, Leon-Munoz L, Zuluaga MC, Banegas JR, Rodriguez-Artalejo F. Sleep duration, general and abdominal obesity, and weight change among the older adult population of Spain. The American journal of clinical nutrition. 2008;87(2):310–316. doi: 10.1093/ajcn/87.2.310. [DOI] [PubMed] [Google Scholar]
- 30.Benito-Leon J, Bermejo-Pareja F, Vega S, Louis ED. Total daily sleep duration and the risk of dementia: a prospective population-based study. European journal of neurology: the official journal of the European Federation of Neurological Societies. 2009;16(9):990–997. doi: 10.1111/j.1468-1331.2009.02618.x. [DOI] [PubMed] [Google Scholar]
- 31.Louis ED, Barnes L, Wendt KJ, et al. A teaching videotape for the assessment of essential tremor. Movement disorders: official journal of the Movement Disorder Society. 2001;16(1):89–93. doi: 10.1002/1531-8257(200101)16:1<89::aid-mds1001>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 32.Martinez-Martin P, Gil-Nagel A, Gracia LM, Gomez JB, Martinez-Sarries J, Bermejo F. Unified Parkinson’s Disease Rating Scale characteristics and structure. The Cooperative Multicentric Group. Movement disorders: official journal of the Movement Disorder Society. 1994;9(1):76–83. doi: 10.1002/mds.870090112. [DOI] [PubMed] [Google Scholar]
- 33.Benito-Leon J, Bermejo-Pareja F, Rodriguez J, et al. Prevalence of PD and other types of parkinsonism in three elderly populations of central Spain. Movement disorders: official journal of the Movement Disorder Society. 2003;18(3):267–274. doi: 10.1002/mds.10362. [DOI] [PubMed] [Google Scholar]
- 34.Benito-Leon J, Bermejo-Pareja F, Morales-Gonzalez JM, et al. Incidence of Parkinson disease and parkinsonism in three elderly populations of central Spain. Neurology. 2004;62(5):734–741. doi: 10.1212/01.wnl.0000113727.73153.68. [DOI] [PubMed] [Google Scholar]
- 35.Bain PG, Findley LJ, Atchison P, et al. Assessing tremor severity. Journal of neurology, neurosurgery, and psychiatry. 1993;56(8):868–873. doi: 10.1136/jnnp.56.8.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. European heart journal. 2011;32(12):1484–1492. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- 37.Hoevenaar-Blom MP, Spijkerman AM, Kromhout D, van den Berg JF, Verschuren WM. Sleep duration and sleep quality in relation to 12-year cardiovascular disease incidence: the MORGEN study. Sleep. 2011;34(11):1487–1492. doi: 10.5665/sleep.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity. 2008;16(3):643–653. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller MA, Kandala NB, Kivimaki M, et al. Gender differences in the cross-sectional relationships between sleep duration and markers of inflammation: Whitehall II study. Sleep. 2009;32(7):857–864. [PMC free article] [PubMed] [Google Scholar]
- 40.Krueger PM, Friedman EM. Sleep duration in the United States: a cross-sectional population-based study. American journal of epidemiology. 2009;169(9):1052–1063. doi: 10.1093/aje/kwp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Louis ED, Benito-Leon J, Bermejo-Pareja F Neurological Disorders in Central Spain Study G. Self-reported depression and anti-depressant medication use in essential tremor: cross-sectional and prospective analyses in a population-based study. European journal of neurology: the official journal of the European Federation of Neurological Societies. 2007;14(10):1138–1146. doi: 10.1111/j.1468-1331.2007.01923.x. [DOI] [PubMed] [Google Scholar]
- 42.Mahoney J, Drinka TJ, Abler R, et al. Screening for depression: single question versus GDS. Journal of the American Geriatrics Society. 1994;42(9):1006–1008. doi: 10.1111/j.1532-5415.1994.tb06597.x. [DOI] [PubMed] [Google Scholar]
- 43.Ancoli-Israel S, Martin JL. Insomnia and daytime napping in older adults. Journal of clinical sleep medicine: JCSM: official publication of the American Academy of Sleep Medicine. 2006;2(3):333–342. [PubMed] [Google Scholar]