Key Points
Question
What proportion of US residents with employer-sponsored insurance who were dispensed new cancer drugs received drugs without documented overall survival benefit, and what were the costs between 2011 and 2018?
Findings
In this cross-sectional study among 37 348 patients who received 1 or more of 44 new oral targeted cancer drugs, the proportion of patients receiving drugs without documented overall survival benefit increased from 13% in 2011 to 59% in 2018, accounting for 52% of the $3.5 billion estimated cumulative spending on the new oral targeted cancer drugs by the end of 2018.
Meaning
Results of this study suggest that the estimated use of new cancer drugs without documented clinical benefit has substantially increased over the past decade, with major cost implications.
Abstract
Importance
Launch prices of new cancer drugs in the US have substantially increased in recent years despite growing concerns about the quantity and quality of evidence supporting their approval by the US Food and Drug Administration (FDA).
Objective
To assess the use of and spending on new oral targeted cancer drugs among US residents with employer-sponsored insurance between 2011 and 2018, stratified by the strength of available evidence of benefit.
Design, Setting, and Participants
In this cross-sectional study, dispensing claims for oral targeted cancer drugs first approved by the FDA between January 1, 2011, and December 31, 2018, were analyzed. The number of patients with drugs dispensed and the total payment for all claims were aggregated by calendar year, and these outcomes were arrayed according to evidence underlying FDA approvals, including pivotal study design (availability of randomized clinical trials) and overall survival (OS) benefit, as documented in drug labels. This study was conducted from July 17, 2019, to July 23, 2021.
Main Outcomes and Measures
Annual and cumulative numbers of patients who had dispensing events, and annual and cumulative sums of payment for eligible drugs.
Results
Of 37 348 patients who had at least 1 of the 44 new oral targeted drugs dispensed between 2011 and 2018, 21 324 were men (57.1%); mean (SD) age was 64.1 (13.1) years. Most individuals (36 246 [97.0%]) received drugs for which evidence from randomized clinical trials existed; however, a growing share of patients received drugs without documented OS benefit during the study period: from 12.7% in 2011 to 58.8% in 2018. Cumulative spending on all sample drugs totaled $3.5 billion by the end of 2018, of which 96.8% was spent on drugs that were approved based on a pivotal randomized clinical trial. Cumulative spending on drugs without documented OS benefit ($1.8 billion [51.6%]) surpassed that on drugs with documented OS benefit ($1.7 billion [48.4%]) by the end of 2018.
Conclusions and Relevance
The findings of this cross-sectional study suggest that drugs used for treatment of cancer without documented OS benefits are adopted in the health system and account for substantial spending.
This cross-sectional examines the use of and spending on oral cancer drugs by the level of benefit shown in randomized clinical trials.
Introduction
In recent years, US Food and Drug Administration (FDA) approvals of drugs used in the treatment of cancer have been based on less complete data than traditionally required.1 An increasing proportion of new cancer drugs have been evaluated in single-arm studies without control groups, which can lead to overestimation of benefit.2 Also, pivotal clinical studies of new cancer drugs are more likely to measure surrogate end points, such as response rate and progression-free survival alone, rather than clinical outcomes, such as overall survival (OS) benefit.3,4 Although surrogate end points can shorten the duration of clinical development,5 they are not reliable predictors of prolonged survival or better quality of life in most settings.6,7 Therefore, FDA approvals based on studies that lack randomization or use surrogate end points alone are associated with substantial uncertainty about whether a new cancer drug extends the duration or improves the quality of life of patients.5,8,9
Despite this uncertainty of clinical benefits, cancer drug prices in the US have increased substantially, with mean monthly point-of-sale prices for oral targeted cancer drugs reaching nearly $14 000 per fill in 2018.10 Total cancer drug expenditures grew from $26.8 billion in 2011 to $42.1 billion in 2016, representing an estimated 9.4% of total US drug expenditures in 2016.11 According to earlier studies, there is no clear association between drug prices and benefits.12,13
Previous studies have evaluated the use of and spending on drugs for specific cancers (eg, breast, lung)14,15,16 and for individual drugs or classes of drugs.10,11,17,18,19 To our knowledge, a systematic analysis documenting the use of and spending on new cancer drugs among patients with employer-sponsored insurance is lacking. Employer-sponsored plans account for almost half of the insured population in the US.20 Our objective was to assess the use of and spending on new oral targeted cancer drugs among US residents with employer-sponsored health insurance between 2011 and 2018. We conducted the study from July 17, 2019, to July 23, 2021. We characterized whether use and associated spending varied by the strength of available evidence underlying FDA approvals.
Methods
Data Source
To estimate real-world uptake of and spending on oral targeted cancer medications in the US, we used one of the largest proprietary sources of data on health services (MarketScan; IBM Corp), including drug use by privately and publicly insured individuals in the US. We obtained data from January 1, 2011, to December 31, 2018. The databases cover a nationally representative (by age, sex, geography, and type of coverage) sample of US residents with employer-provided health insurance.21 Although decreasing over time (eTable 6 in the Supplement), in each study year, our version of the database comprised a median of 44.2 million commercially insured members and 3.9 million members with a supplementary Medicare benefit, representing members from all 50 states in each year. For each member, the database contains dispensing claims for medications identified by the National Drug Code with total gross payments attached to each dispensing claim. We identified dispensing events of interest based on National Drug Codes for the drugs of interest, using the First Databank reference database.22 The databases are deidentified and fully compliant with the Health Insurance Portability and Accountability Act of 1996. The study was determined to not constitute human participants research by the Harvard Pilgrim Health Care Institutional Review Board and therefore exempt from review, approval, and need for informed patient consent. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Sample Description
This study focused on oral targeted cancer drugs. Targeted cancer drugs “block the growth and spread of cancer by interfering with specific molecules (‘molecular targets’) that are involved in the growth, progression, and spread of cancer.”23 By our estimate for this study, oral targeted cancer drugs dispensed by pharmacies and taken by patients, as opposed to clinician-administered injectable drugs, account for almost 60% of 83 targeted cancer drug indication approvals from 2011 through 2018. We identified dispensing events for cancer medications of interest and estimated spending according to the strength of evidence underlying regulatory approvals in a large, nationally representative population. Claims data allow comprehensive assessment of drugs dispensed in a retail setting and billed to insurers under the National Drug Code.
We identified all oral targeted cancer drugs first approved by the FDA from January 1, 2011, to December 31, 2018, using the publicly available FDA database.24 Both original indications and supplementary indications of cancer drugs that received their first approval between 2011 and 2018 were extracted from the indications and usage section of their FDA-approved labels. We included all oral targeted cancer drugs that had only cancer indications.
Patients were eligible for inclusion if they received at least 1 dispensing event of at least 1 of the oral targeted cancer drugs from 2011 to 2018. We also identified patients who started the study drugs for the first time in each year (eg, had no prior dispensing claim of a particular study drug). One patient could have received more than 1 of the study drugs.
Key Variables
To examine evidence of the drug’s benefit, we reviewed the clinical studies sections of the FDA-approved labels to identify the pivotal studies that contributed to the original and supplementary indication approvals of the drugs of interest. We first determined whether drugs in our sample had at least 1 randomized clinical trial (RCT) supporting approval for at least 1 indication. In RCTs, participants were randomly allocated to receive either the study drug or another active comparator, standard of therapy, or placebo. Similar to other studies,25,26 we did not consider as RCTs noncomparative randomized trials, which evaluated different doses of the same treatment without a control group.
We then reviewed drug labeling available in the FDA database to systematically identify whether drugs had documented evidence of statistically significant OS benefit demonstrated in RCTs or statistically significant interim results on OS in RCTs in their latest available FDA-approved labels by December 31, 2018 (P < .05 in a drug label was considered documented evidence of statistical significance) (eTable 1 in the Supplement). Drugs were classified as not having documented OS benefit if survival data were obtained from a trial with a noninferiority design, labels documented a lack of a statistically significant OS benefit, OS was not mentioned in the label, or OS results were not reported or not reported in a way that could support inference.27 Our classification was consistent with availability of OS benefit as characterized in earlier studies.25,28,29 We also assessed whether the drugs had evidence of quality-of-life benefits reported in their latest available FDA-approved labels by the end of 2018.
We used publicly available lists24 compiled by the FDA’s Center for Drug Evaluation and Research30 to determine whether a cancer drug’s first indication was approved under one of the FDA’s expedited development and approval programs: the fast-track designation, priority review, accelerated approval pathway, or breakthrough therapy designation. We also determined whether drugs received an orphan drug designation for their first approved indications using the FDA’s orphan drug product designation database.31
Statistical Analysis
We analyzed all dispensing claims for the sample drugs in the study time frame. We aggregated by calendar year the numbers of patients with dispensing claims according to evidence underlying drug approvals (pivotal study design and survival benefit evidence). We calculated the number of unique patients exposed to each drug in each year and the cumulative numbers of patients exposed to drugs of interest from 2011 through 2018.
Because actual drug prices paid are confidential and not recorded on insurance claims, we summed payments associated with each dispensing event and calculated annual and cumulative sums of payment for all dispensing events to estimate an upper bound of spending on sample drugs from 2011 through 2018. Payment constitutes total gross payment to a provider for each claim, including insurer and patient contributions.32 Payments do not account for confidential rebates. We present unadjusted annual payments herein. Consumer price index adjustment did not change the results and is presented in eFigure 2 in the Supplement.
Patient demographics included age, sex, region of residence (Northeast, North Central, South, West, unknown), urbanicity (metropolitan statistical area or nonmetropolitan statistical area), and health plan type (commercial or supplementary Medicare). Descriptive statistics were used for patient characteristics with means (SD) reported for continuous variables and counts (proportions) reported for categorical variables. We conducted analyses using Stata, version 14 (StataCorp), and created plots using Microsoft Excel, version 16 (Microsoft Corporation). This descriptive study did not involve statistical tests.
Results
Study Sample and Characteristics
Between January 1, 2011, and December 31, 2018, 47 brand-name oral targeted cancer drugs that had only cancer indications were approved by the FDA. Of these, 44 drugs had at least 1 dispensing claim in the database; the 3 drugs without dispensing claims were approved late in 2018. Indications approved by the FDA of 44 sample drugs are listed in eTable 2 in the Supplement. Approvals of 34 drugs (77.3%) were supported by at least 1 RCT for at least 1 indication; 10 drugs (22.7%) were approved on the basis of single-arm studies or noncomparative trials alone (Table 1). Only 11 drugs (25.0%) had documented, statistically significant OS benefit for at least 1 indication in FDA-approved labels by the end of 2018, 4 of which had OS as primary end point in pivotal RCTs. The median extension of OS benefit of 11 drugs was 4.6 (range, 1.1 [afatinib] to 6.4 [trametinib and dabrafenib]) months against the comparator. eTable 3 in the Supplement provides detailed trial characteristics for drugs with information on OS benefit in FDA-approved labels, and eTable 4 in the Supplement provides the magnitude of OS extension. By the end of 2018, none of the labels for the 44 drugs documented a statistically significant quality-of-life benefit. Among the sample drugs, 32 (72.7%) had an orphan drug designation, 21 drugs (47.7%) had first indications approved under the fast track pathway, 34 drugs (77.3%) were approved under the priority review pathway, 16 drugs (36.4%) were approved under the accelerated approval pathway, and 15 drugs (34.1%) had a breakthrough therapy designation. Only 4 of the 44 drugs were approved without an expedited designation.
Table 1. Characteristics of 44 Oral Targeted Cancer Drugs Approved From 2011 to 2018a.
| Drug characteristic | No. (%) |
|---|---|
| First approval year | |
| 2011 | 4 (9.1) |
| 2012 | 7 (15.9) |
| 2013 | 4 (9.1) |
| 2014 | 3 (6.8) |
| 2015 | 8 (18.2) |
| 2016 | 2 (4.5) |
| 2017 | 8 (18.2) |
| 2018 | 8 (18.2) |
| RCT | |
| With RCT | 34 (77.3) |
| Without RCT | 10 (22.7) |
| OS benefit | |
| With documented OS benefitb | 11 (25.0) |
| Without documented OS benefit | 33 (75.0) |
| FDA approvalc | |
| Orphan drug designation | 32 (72.7) |
| Fast track review | 21 (47.7) |
| Priority review | 34 (77.3) |
| Accelerated approval | 16 (36.4) |
| Breakthrough therapy designation | 15 (34.1) |
Abbreviations: FDA, US Food and Drug Administration; OS, overall survival; RCT, randomized clinical trial.
Brand-name oral targeted cancer drugs with only cancer indications.
Cabozantinib has 2 brand names: Cometriq (without documented OS benefit) and Cabometyx (documented OS benefit postmarketing). We defined cabozantinib as “with OS benefit” in this study.
Start dates of FDA special review and approval programs: orphan drug designation, 1984; fast track review, 1988; priority review, 1992; accelerated approval, 1992; and breakthrough therapy designation, 2012.
A total of 37 348 patients had a dispensing claim for 1 or more study drugs from 2011 to 2018. Mean (SD) age of the patients was 64.1 (13.1) years, 21 324 were men (57.1%), 16 024 were women (42.9%), and 15 420 patients (41.3%) had supplemental Medicare insurance. The most prevalent region of residence was the South (14 103 [37.8%]) and 28 683 patients (76.8%) lived in a metropolitan area.
Use and Costs by Trial Design and Evidence of Survival Benefit
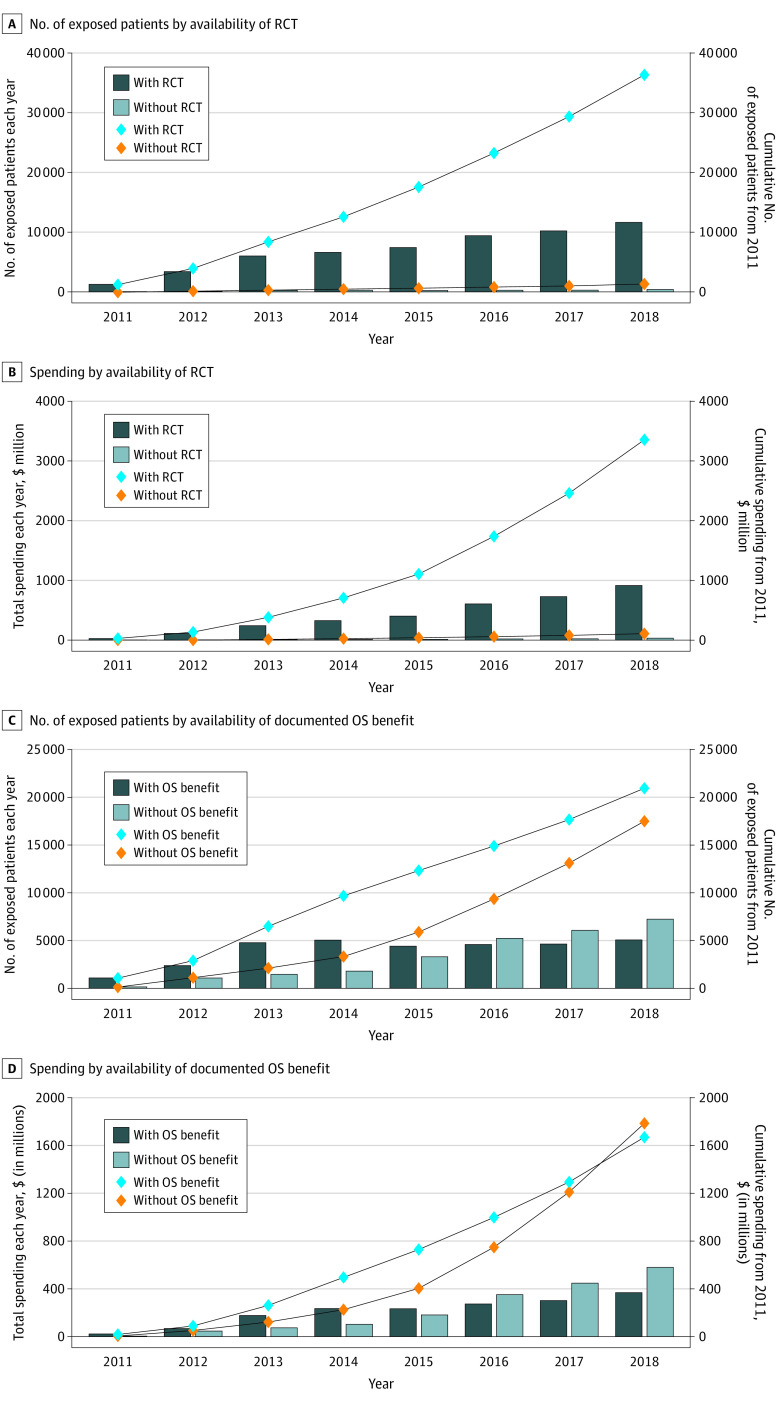
The number of patients starting at least 1 of the 44 oral targeted cancer drugs increased from 1257 in 2011 to 7154 in 2018, as presented in Table 2 and the Figure. By the end of 2018, 36 246 patients (97.0%) used drugs that had at least 1 pivotal RCT. Cumulative spending on all sample drugs totaled $3.5 billion by the end of 2018, of which 96.8% was spent on drugs that were approved based on a pivotal RCT.
Table 2. Characteristics of Patients Exposed to 44 Oral Targeted Cancer Drugs, 2011-2018a,b.
| Patient characteristics | No. (%) |
|---|---|
| New startersc | |
| 2011 | 1257 (3.4) |
| 2012 | 2804 (7.5) |
| 2013 | 4613 (12.4) |
| 2014 | 4351 (11.7) |
| 2015 | 5125 (13.7) |
| 2016 | 5788 (15.5) |
| 2017 | 6256 (16.8) |
| 2018 | 7154 (19.2) |
| Health plan | |
| Commercial | 21 928 (58.7) |
| Supplementary Medicare | 15 420 (41.3) |
| Gender | |
| Female | 16 024 (42.9) |
| Male | 21 324 (57.1) |
| Age at first dispensing, y | |
| 0-14 (children) | 106 (0.3) |
| 15-24 (youth) | 159 (0.4) |
| 25-64 (adults) | 22 088 (59.1) |
| ≥65 (seniors) | 14 995 (40.2) |
| Region of residence | |
| Northeast | 8094 (21.7) |
| North Central | 8873 (23.8) |
| South | 14 103 (37.8) |
| West | 6057 (16.2) |
| Unknown | 221 (0.6) |
| Urbanicity | |
| MSA | 28 683 (76.8) |
| Non-MSA | 4740 (12.7) |
| Unknown | 3925 (10.5) |
| Total | 37 348 (100) |
Abbreviation: MSA, metropolitan statistical area.
One patient could have received more than 1 study drug.
Brand-name oral targeted cancer drugs with only cancer indications.
Patients without dispensing events for each study drug in prior years.
Figure. Annual and Cumulative Numbers of Patients Exposed to and Spending on 44 Oral Targeted Cancer Drugs by Evidence Underlying Approvals, 2011-2018.
A, Annual and cumulative numbers of patients by availability of randomized clinical trial (RCT) evidence (at least 1 RCT supporting approval for at least 1 indication by December 31, 2018). B, Annual and cumulative spending by availability of RCT evidence. C, Annual and cumulative numbers of patients by availability of documented overall survival (OS) benefit evidence (evidence of OS benefit by December 31, 2018; 5 of 6 regulatory programs to speed review and approval of new drugs were implemented before 2000; breakthrough therapy designation began in 2012). D, Annual and cumulative spending by availability of documented OS benefit evidence.
The cumulative number of patients with a dispensing claim for at least 1 drug with documented OS benefit by the end of 2018 was 20 976, which accounted for 56.2% of all patients. Although fewer patients received drugs without documented OS benefit, the gap increasingly narrowed from 2014 onward. By 2018, 58.8% of patients received drugs without OS benefit vs 12.7% in 2011. Cumulative spending on drugs without documented OS benefit ($1.8 billion [51.6%]) exceeded cumulative spending on drugs with documented OS benefit ($1.7 billion [48.4%]) by the end of 2018. Annual and cumulative numbers of patients exposed to and spending on 44 oral targeted cancer drugs by FDA orphan drug designation and the accelerated approval pathway are shown in eFigure 1 in the Supplement.
Total Spending on Top 20 Oral Targeted Cancer Drugs in 2018
The top 20 study drugs by spending amounts in 2018 are listed in Table 3; spending on all 44 drugs is reported in eTable 5 in the Supplement. Total spending on these drugs was $861.7 million, which accounted for 90.9% of the total spending on all study drugs in 2018. Among the top 20 drugs, only 1 drug was without a pivotal RCT, but 13 drugs had no documented OS benefit at the time (3 lacked statistically significant evidence of OS benefit in interim analysis, OS data for 1 were obtained from a noninferiority design trial, OS was not mentioned for 1, evidence for 1 was supported only by a single-arm study, and OS data were not reported for 7; eTable 3b in the Supplement); 15 drugs had orphan drug designations, 6 drugs were granted breakthrough therapy designation, and 10 drugs were approved under fast track, 15 drugs under priority review, and 8 drugs under accelerated approval pathways. Estimated spending according to drugs’ magnitude of OS benefit is reported in eTable 4 in the Supplement. Palbociclib was the drug with the highest spending amount ($209.0 million, $82 301 per patient), followed by abiraterone acetate ($95.8 million, $63 962 per patient) and ruxolitinib phosphate ($75.8 million, $92 290 per patient). Brief summaries of evidence documented in FDA labels for the top 3 drugs by 2018 are shown in the eBox in the Supplement.
Table 3. Top 20 Oral Targeted Cancer Drugs by Spending in 2018a.
| Rank | Generic name | Spending, $ millionb | No. of exposed patients | Spending per patient, $ | First approval time | Cancer type | RCTc | OS benefitd | OS primary end pointe | Orphan drug designation | FDA approval typef | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fast track | Priority review | Breakthrough therapy | Accelerated approval | |||||||||||
| 1 | Palbociclib | 209.0 | 2539 | 82 301 | 2015 | Breast cancer | Yes | No | No | No | No | Yes | Yes | Yes |
| 2 | Abiraterone acetate | 95.8 | 1497 | 63 962 | 2011 | Prostate cancer | Yes | Yes | Yes | No | No | Yes | NA | No |
| 3 | Ruxolitinib phosphate | 75.8 | 821 | 92 290 | 2011 | Myelofibrosis/polycythemia vera | Yes | No | No | Yes | No | Yes | NA | No |
| 4 | Enzalutamide | 74.3 | 1156 | 64 234 | 2012 | Prostate cancer | Yes | Yes | Yes | No | Yes | Yes | No | No |
| 5 | Pomalidomide | 69.3 | 734 | 94 452 | 2013 | Myeloma | Yes | Yes | No | Yes | Yes | No | No | Yes |
| 6 | Osimertinib mesylate | 61.9 | 650 | 95 176 | 2015 | Lung cancer | Yes | No | No | Yes | Yes | Yes | Yes | Yes |
| 7 | Cabozantinib s-malate | 38.1 | 485 | 78 455 | 2012 | Thyroid cancer/renal cell carcinoma | Yes | Yes | No | Yes | Yes | Yes | No | No |
| 8 | Olaparib | 28.2 | 440 | 63 983 | 2014 | Ovarian/fallopian tube/peritoneal/breast cancer | Yes | Yes | No | Yes | No | Yes | No | Yes |
| 9 | Ixazomib citrate | 24.5 | 404 | 60 644 | 2015 | Myeloma | Yes | No | No | Yes | No | Yes | No | No |
| 10 | Alectinib hydrochloride | 23.8 | 222 | 106 999 | 2015 | Lung cancer | Yes | No | No | Yes | No | Yes | Yes | Yes |
| 11 | Neratinib maleate | 20.2 | 351 | 57 443 | 2017 | Breast cancer | Yes | No | No | No | No | No | No | No |
| 12 | Abemaciclib | 19.7 | 377 | 52 126 | 2017 | Breast cancer | Yes | No | No | No | Yes | Yes | Yes | No |
| 13 | Trametinib dimethyl sulfoxide | 19.0 | 378 | 50 259 | 2013 | Melanoma/lung cancer/thyroid cancer | Yes | Yes | No | Yes | Yes | No | No | No |
| 14 | Bosutinib monohydrate | 16.4 | 170 | 96 592 | 2012 | Leukemia | Yes | No | No | Yes | No | No | No | No |
| 15 | Dabrafenib mesylate | 16.3 | 346 | 47 184 | 2013 | Melanoma/lung cancer/thyroid cancer | Yes | Yes | No | Yes | Yes | No | No | No |
| 16 | Lenvatinib mesylate | 16.1 | 215 | 74 758 | 2015 | Thyroid cancer/renal cell carcinoma/hepatocellular carcinoma | Yes | No | No | Yes | No | Yes | No | No |
| 17 | Niraparib tosylate | 14.4 | 254 | 56 743 | 2017 | Ovarian/fallopian tube/peritoneal cancer | Yes | No | No | Yes | Yes | Yes | Yes | No |
| 18 | Crizotinib | 13.0 | 143 | 91 250 | 2011 | Lung cancer | Yes | No | No | Yes | Yes | Yes | NA | Yes |
| 19 | Ponatinib hydrochloride | 13.0 | 87 | 149 362 | 2012 | Leukemia | No | No | No | Yes | Yes | Yes | No | Yes |
| 20 | Venetoclax | 12.9 | 318 | 40 588 | 2016 | Leukemia | Yes | No | No | Yes | No | Yes | Yes | Yes |
Abbreviations: FDA, US Food and Drug Administration; NA, not applicable; OS, overall survival; RCT, randomized clinical trial.
Oral targeted cancer drugs with only cancer indications.
Sum of total gross payments associated with dispensing events.
At least 1 RCT supporting approval for at least 1 indication by December 31, 2018.
Documented overall survival benefit by December 31, 2018.
The primary end point in label-reported pivotal RCTs by December 31, 2018.
Data NA when the drug was approved before breakthrough therapy designation option (2012).
Discussion
In this study, we examined the uptake and cost implications of new oral targeted cancer drugs among a nationally representative population of US residents with private, employer-sponsored insurance. Between 2011 and 2018, 37 348 patients in the MarketScan Database had a dispensing claim for at least 1 of the newly approved oral targeted cancer drugs. Most patients receiving newly approved oral targeted cancer drugs received drugs for which pivotal RCTs exist; however, a growing share of patients received drugs without documented OS evidence during the study period: from 12.7% in 2011 to 58.8% in 2018. Although 96.8% of the $3.5 billion estimated spending on new cancer drugs from 2011 through 2018 was for drugs with RCTs, more than half ($1.8 billion) was on drugs without documented OS benefit. Spending on drugs without documented OS benefit increased markedly and surpassed spending on drugs with documented OS benefit by 2018. None of the drugs had documented quality-of-life benefits.
The goal of any medical intervention should be to help patients live longer and have better lives. Randomized clinical trials that document OS and quality-of-life benefits are the standard for establishing new cancer therapies that meet these goals.33 Despite these fundamental tenets, an increasing number of cancer drugs are approved based on surrogate end points; even for drugs whose approval is based on a pivotal RCT, substantial methodologic problems may compromise the extent to which clinical trials identify efficacious new treatment options for patients.34 Use of suboptimal control arms and inappropriate use of crossover are common, which increase the likelihood of obtaining favorable results.29,35 Our findings suggest that cancer drugs with major shortcomings in their evidence base are adopted in the health system and account for substantial spending.
Cancer drug spending growth is accelerating, yet the US lags behind other countries in health gains obtained per dollar spent on cancer drugs.36 During our study period, an estimated $3.5 billion was spent by private insurers on oral targeted cancer drugs shortly after their approval. Thirty-three of the drugs contributing to this spending lacked evidence of statistically significant OS benefit. Estimated palbociclib spending in 2018 was the highest. Initially approved in 2015, palbociclib is a kinase inhibitor indicated for the treatment of advanced hormone receptor–positive, human epidermal receptor 2–negative breast cancer. Adding palbociclib to usual care in clinical trials resulted in significantly longer median progression-free survival time (6-10 months) over letrozole alone.37 However, studies determined that palbociclib is not cost-effective in the US at a price of $10 000 per month.38,39 In an open-label study, OS data on palbociclib were not mature at the time of the final 2020 progression-free survival analysis.37 Given the high annual expenditure, patients and prescribers would benefit from additional evaluations to confirm that the statistically significant progression-free survival extension translates into meaningful OS benefit.
Value-based pricing might reduce the cost of some drugs with high spending amounts. For example, the approval of neratinib, ranked 11th in spending in our study, was controversial owing to its minimal gains in disease-free survival and the severity of adverse effects.40,41 According to one study, neratinib’s price would need to be reduced by 85% for it to be cost-effective at a willingness-to-pay threshold of $150 000 per quality-adjusted life-year gained for cancer treatments in the US.41 Regorafenib, ranked 21st in spending in our study, was also not cost-effective either as a second-line agent in the treatment of hepatocellular carcinoma or as third-line treatment of metastatic colorectal cancer.42,43,44,45
Our findings reflect the combination of regulatory and economic characteristics of the US cancer drug ecosystem. Increasingly lenient FDA approvals, expectations for and use of drugs advertised directly to consumers, no price regulation, fragmented purchasing and reimbursement systems, coverage mandates, and limited ability by payers to negotiate prices have facilitated increasing numbers of patients with cancer receiving drugs with unknown clinical benefit with substantial economic consequences. “Onco-exceptionalism”46 in drug development, approval, pricing, and coverage raises questions at the individual patient level of care quality and at the societal level of allocation of resources.47 In addition, because it incentivizes development of new drugs similarly lacking evidence of benefit in the largest pharmaceutical market, onco-exceptionalism may negatively affect cancer patients worldwide.48
Effective policy approaches would likely need to target approval regulations, pricing, and coverage mandates for cancer drugs. On the regulatory side, the FDA accelerated approval pathway, which allows cancer drugs to be approved based on surrogate measures, could be used more effectively by enforcing timely, well-designed confirmatory trials with OS and/or quality-of-life end points and accelerated withdrawal of approvals when confirmatory trials do not demonstrate clinical benefits in time.4 Postmarketing surveillance of use, adverse events, and, to some degree, OS, of cancer drugs using national databases, such as the FDA Sentinel system, could address some uncertainties in the evidence base over time.49 Policy makers and purchasers should also seek to link prices for cancer drugs to demonstrated benefits. The US can learn important lessons from other countries.48 For example, fewer cancer drugs approved based on surrogate end points in the US are approved and covered by insurance systems in England50 and Canada,51 and drug prices are better aligned with their clinical benefits.52 Patients and clinicians need to be able to discern easily which benefits a new drug can be expected to have and which have not been demonstrated. Drug labeling should therefore routinely inform patients and clinicians in nontechnical language about whether OS and/or quality of life benefits have been shown at the time of market entry and in the postmarketing period.27,53
Limitations
The study has limitations. First, the MarketScan Databases contain dispensing events for a sample of US residents with employer-sponsored insurance. Data are obtained from private payers across the US, some of which have more members in the South. Although the data are considered representative of US prescribing for privately insured members,21 they represent varying proportions of privately insured members across years and do not capture publicly insured and uninsured individuals. We therefore underestimated overall use and spending associated with new oral targeted cancer drugs. The observed use and spending trajectories may not generalize to other private and public payers. It is therefore difficult to extrapolate drug spending according to evidence underlying FDA approvals to the entire system. Second, although total gross drug payment amounts per claim in the MarketScan Databases constitute the sum of actual member and gross insurer payments and reflect pricing guidelines, such as fee schedules and discounts, payments do not reflect confidential discounts negotiated between payers and pharmaceutical companies. Third, this was an aggregated drug-level analysis. We did not assess proportions of patients with cancer over time who use the sample drugs vs other drugs. Patient-level analyses are needed to assess patterns of drug use among patients with different cancer diagnoses. Fourth, we conservatively attributed RCT and OS benefit evidence to a drug when at least 1 of the drug’s cancer indications is supported by a pivotal RCT or documented evidence of OS benefit. Fifth, drugs without documented statistically significant results on OS may have other benefits, such as improvements in quality of life, although this was not the case in our study sample. There are also methodologic reasons for not observing statistically significant OS results in RCTs, for example, when patients in the control arm switch to receive active treatment following cancer progression. Sixth, a small number of drugs for highly prevalent conditions (eg, palbociclib) may have affected our estimates. Seventh, we only assessed oral targeted cancer drugs and did not consider injectable cancer drugs.
Conclusions
This cross-sectional study assesses the trajectory of real-world use of and spending on oral targeted cancer drugs among US residents with employer-sponsored health insurance. Almost all patients receiving newly approved oral targeted cancer drugs received drugs for which data from pivotal RCTs exist, and the percentage of patients receiving drugs without documented OS benefit increased substantially from 2011 to 2018. In 2018, cumulative spending on drugs without documented OS benefit exceeded that of drugs with documented OS benefit.
eTable 1. Definitions of Evidence of OS Benefit in FDA-Approved Labels
eTable 2. Indications Approved by the FDA for 44 Oral Targeted Cancer Drugs, 2011-2018
eTable 3. Trials of Drugs With Information on OS Benefit Reported in FDA-Approved Labels, 2011-2018
eTable 4. Magnitude of OS Benefit and Spending Associated With Oral Targeted Cancer Drugs With Documented OS Benefit, as Reported in FDA-Approved Labels in 2018
eTable 5. Spending Associated With Dispensing Events for 44 Oral Targeted Cancer Drugs in 2018
eTable 6. Number of Members per Year in the IBM MarketScan Research Databases, 2011-2018
eFigure 1. Annual and Cumulative Numbers of Patients Exposed to and Spending on 44 Oral Targeted Cancer Drugs, by FDA Orphan Designation and Accelerated Approval Pathway
eFigure 2. Annual and Cumulative Inflation-Adjusted Spending on 44 Oral Targeted Cancer Drugs
eBox. Brief Summary of Evidence Documented in FDA Labels for the Top 3 Drugs by Spending in 2018
References
- 1.Darrow JJ, Avorn J, Kesselheim AS. Approval and regulation of pharmaceuticals, 1983-2018. JAMA. 2020;323(2):164-176. doi: 10.1001/jama.2019.20288 [DOI] [PubMed] [Google Scholar]
- 2.Del Paggio JC, Berry JS, Hopman WM, et al. Evolution of the onco-exceptionalism. JAMA Oncol. 2021;7(5):728-734. doi: 10.1001/jamaoncol.2021.0379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DiMagno SSP, Glickman A, Emanuel EJ. Accelerated approval of cancer drugs—righting the ship of the US Food and Drug Administration. JAMA Intern Med. 2019;179(7):922-923. doi: 10.1001/jamainternmed.2019.0584 [DOI] [PubMed] [Google Scholar]
- 4.Gyawali B, Hey SP, Kesselheim AS. Assessment of the clinical benefit of cancer drugs receiving accelerated approval. JAMA Intern Med. 2019;179(7):906-913. doi: 10.1001/jamainternmed.2019.0462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen EY, Joshi SK, Tran A, Prasad V. Estimation of study time reduction using surrogate end points rather than overall survival in oncology clinical trials. JAMA Intern Med. 2019;179(5):642-647. doi: 10.1001/jamainternmed.2018.8351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prasad V, Kim C, Burotto M, Vandross A. The strength of association between surrogate end points and survival in oncology: a systematic review of trial-level meta-analyses. JAMA Intern Med. 2015;175(8):1389-1398. doi: 10.1001/jamainternmed.2015.2829 [DOI] [PubMed] [Google Scholar]
- 7.Haslam A, Hey SP, Gill J, Prasad V. A systematic review of trial-level meta-analyses measuring the strength of association between surrogate end-points and overall survival in oncology. Eur J Cancer. 2019;106:196-211. doi: 10.1016/j.ejca.2018.11.012 [DOI] [PubMed] [Google Scholar]
- 8.Naci H, Davis C. Inappropriate use of progression-free survival in cancer drug approvals. BMJ. 2020;368:m770. doi: 10.1136/bmj.m770 [DOI] [PubMed] [Google Scholar]
- 9.Chen EY, Raghunathan V, Prasad V. An overview of cancer drugs approved by the US Food and Drug Administration based on the surrogate end point of response rate. JAMA Intern Med. 2019;179(7):915-921. doi: 10.1001/jamainternmed.2019.0583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dusetzina SB, Huskamp HA, Keating NL. Specialty drug pricing and out-of-pocket spending on orally administered anticancer drugs in Medicare Part D, 2010 to 2019. JAMA. 2019;321(20):2025-2027. doi: 10.1001/jama.2019.4492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong SJ, Li EC, Matusiak LM, Schumock GT. Spending on antineoplastic agents in the United States, 2011 to 2016. J Oncol Pract. 2018;14(11):JOP1800069. doi: 10.1200/JOP.18.00069 [DOI] [PubMed] [Google Scholar]
- 12.Mailankody S, Prasad V. Five years of cancer drug approvals: innovation, efficacy, and costs. JAMA Oncol. 2015;1(4):539-540. doi: 10.1001/jamaoncol.2015.0373 [DOI] [PubMed] [Google Scholar]
- 13.Trotta F, Mayer F, Barone-Adesi F, et al. Anticancer drug prices and clinical outcomes: a cross-sectional study in Italy. BMJ Open. 2019;9(12):e033728. doi: 10.1136/bmjopen-2019-033728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balkhi B, Alqahtani S, Altayyar W, et al. Drug utilization and expenditure of anticancer drugs for breast cancer. Saudi Pharm J. 2020;28(6):669-674. doi: 10.1016/j.jsps.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah C, Hong Y-R, Bishnoi R, Jones D, Huo J. Utilization of antineoplastic agents and Medicare spending in elderly patients with extensive-stage small-cell lung cancer between 2001 and 2013. JCO Oncol Pract. 2020;16(7):e610-e621. doi: 10.1200/JOP.19.00559 [DOI] [PubMed] [Google Scholar]
- 16.Bradley CJ, Yabroff KR, Mariotto AB, Zeruto C, Tran Q, Warren JL. Antineoplastic treatment of advanced-stage non–small-cell lung cancer: treatment, survival, and spending (2000 to 2011). J Clin Oncol. 2017;35(5):529-535. doi: 10.1200/JCO.2016.69.4166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seiger K, Mostaghimi A, Silk AW, Schmults CD, Ruiz ES. Association of rising cost and use of oral anticancer drugs with Medicare Part D spending from 2013 through 2017. JAMA Oncol. 2020;6(1):154-156. doi: 10.1001/jamaoncol.2019.4906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tichy EM, Schumock GT, Hoffman JM, et al. National trends in prescription drug expenditures and projections for 2020. Am J Health Syst Pharm. 2020;77(15):1213-1230. doi: 10.1093/ajhp/zxaa116 [DOI] [PubMed] [Google Scholar]
- 19.Shih YT, Xu Y, Liu L, Smieliauskas F. Rising prices of targeted oral anticancer medications and associated financial burden on Medicare beneficiaries. J Clin Oncol. 2017;35(22):2482-2489. doi: 10.1200/JCO.2017.72.3742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.International Agency for Research on Cancer . Estimated number of new cases in 2020, all cancers, both sexes, all ages. Accessed July 8, 2021. https://gco.iarc.fr/today/online-analysis-table?v=2020&mode=population&mode_population=countries&population=900&populations=900&key=asr&sex=0&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&group_cancer=1&include_nmsc=1&include_nmsc_other=1
- 21.Watson Health . IBM MarketScan Research Databases for life sciences researchers. Accessed October 21, 2020. https://www.ibm.com/downloads/cas/OWZWJ0QO
- 22.First Databank . First Databank databases. Accessed May 25, 2021. https://www.fdbhealth.com/
- 23.National Cancer Institute . Targeted cancer therapies. Accessed August 9, 2021. https://www.cancer.gov/about-cancer/treatment/types/targeted-therapies/targeted-therapies-fact-sheet
- 24.US Food and Drug Administration . Drugs@FDA: FDA-approved drugs. Accessed April 21, 2020. https://www.accessdata.fda.gov/scripts/cder/daf/
- 25.Davis C, Naci H, Gurpinar E, Poplavska E, Pinto A, Aggarwal A. Availability of evidence of benefits on overall survival and quality of life of cancer drugs approved by European Medicines Agency: retrospective cohort study of drug approvals 2009-13. BMJ. 2017;359:j4530. doi: 10.1136/bmj.j4530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naci H, Davis C, Savović J, et al. Design characteristics, risk of bias, and reporting of randomised controlled trials supporting approvals of cancer drugs by European Medicines Agency, 2014-16: cross sectional analysis. BMJ. 2019;366:l5221. doi: 10.1136/bmj.l5221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naci H, Guan X, Woloshin S, Xu Z, Wagner AK. Communication of survival data in US Food and Drug Administration–approved labeling of cancer drugs. JAMA Intern Med. 2021;e213505. doi: 10.1001/jamainternmed.2021.3505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim C, Prasad V. Cancer drugs approved on the basis of a surrogate end point and subsequent overall survival: an analysis of 5 years of US Food and Drug Administration approvals. JAMA Intern Med. 2015;175(12):1992-1994. doi: 10.1001/jamainternmed.2015.5868 [DOI] [PubMed] [Google Scholar]
- 29.Hilal T, Gonzalez-Velez M, Prasad V. Limitations in clinical trials leading to anticancer drug approvals by the US Food and Drug Administration. JAMA Intern Med. 2020;180(8):1108-1115. doi: 10.1001/jamainternmed.2020.2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.US Food and Drug Administration The Center for Biologics Evaluation and Research (CBER). Biological approvals by year. Accessed January 18, 2021. https://www.fda.gov/vaccines-blood-biologics/development-approval-process-cber/biological-approvals-year
- 31.US Food and Drug Administration . Search orphan drug designations and approvals. Accessed April 21, 2020. https://www.accessdata.fda.gov/scripts/opdlisting/oopd/
- 32.Truven Health Analytics Inc . 2016. Truven Health Marketscan commercial claims and encounters Medicare supplemental and coordination of benefits data dictionary. 2017. Accessed July 18, 2021. https://theclearcenter.org/wp-content/uploads/2020/01/IBM-MarketScan-Data-Dictionary.pdf
- 33.Tannock IF, Amir E, Booth CM, et al. Relevance of randomised controlled trials in oncology. Lancet Oncol. 2016;17(12):e560-e567. doi: 10.1016/S1470-2045(16)30572-1 [DOI] [PubMed] [Google Scholar]
- 34.Del Paggio JC, Tannock IF. The fragility of phase 3 trials supporting FDA-approved anticancer medicines: a retrospective analysis. Lancet Oncol. 2019;20(8):1065-1069. doi: 10.1016/S1470-2045(19)30338-9 [DOI] [PubMed] [Google Scholar]
- 35.Hilal T, Sonbol MB, Prasad V. Analysis of control arm quality in randomized clinical trials leading to anticancer drug approval by the US Food and Drug Administration. JAMA Oncol. 2019;5(6):887-892. doi: 10.1001/jamaoncol.2019.0167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salas-Vega S, Mossialos E. Cancer drugs provide positive value in nine countries, but the United States lags in health gains per dollar spent. Health Aff (Millwood). 2016;35(5):813-823. doi: 10.1377/hlthaff.2015.1453 [DOI] [PubMed] [Google Scholar]
- 37.US Food and Drug Administration . Ibrance (palbociclib) capsules, for oral use. September 2019. Accessed December 21, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/207103Orig1s012lbl.pdf
- 38.Mamiya H, Tahara RK, Tolaney SM, Choudhry NK, Najafzadeh M. Cost-effectiveness of palbociclib in hormone receptor–positive advanced breast cancer. Ann Oncol. 2017;28(8):1825-1831. doi: 10.1093/annonc/mdx201 [DOI] [PubMed] [Google Scholar]
- 39.Zhang B, Long EF. Cost-effectiveness analysis of palbociclib or ribociclib in the treatment of advanced hormone receptor-positive, HER2-negative breast cancer. Breast Cancer Res Treat. 2019;175(3):775-779. doi: 10.1007/s10549-019-05190-3 [DOI] [PubMed] [Google Scholar]
- 40.Martin M, Holmes FA, Ejlertsen B, et al. ; ExteNET Study Group . Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(12):1688-1700. doi: 10.1016/S1470-2045(17)30717-9 [DOI] [PubMed] [Google Scholar]
- 41.Schwartz NRM, Flanagan MR, Babigumira JB, Steuten LM, Roth JA. Cost-effectiveness analysis of adjuvant neratinib following trastuzumab in early-stage HER2-positive breast cancer. J Manag Care Spec Pharm. 2019;25(10):1133-1139. doi: 10.18553/jmcp.2019.25.10.1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parikh ND, Singal AG, Hutton DW. Cost effectiveness of regorafenib as second-line therapy for patients with advanced hepatocellular carcinoma. Cancer. 2017;123(19):3725-3731. doi: 10.1002/cncr.30863 [DOI] [PubMed] [Google Scholar]
- 43.Kashiwa M, Matsushita R. Comparative cost-utility analysis of regorafenib and trifluridine/tipiracil in the treatment of metastatic colorectal cancer in Japan. Clin Ther. 2020;42(7):1376-1387. doi: 10.1016/j.clinthera.2020.05.014 [DOI] [PubMed] [Google Scholar]
- 44.Cho SK, Hay JW, Barzi A. Cost-effectiveness analysis of regorafenib and TAS-102 in refractory metastatic colorectal cancer in the United States. Clin Colorectal Cancer. 2018;17(4):e751-e761. doi: 10.1016/j.clcc.2018.08.003 [DOI] [PubMed] [Google Scholar]
- 45.Goldstein DA, Ahmad BB, Chen Q, et al. Cost-effectiveness analysis of regorafenib for metastatic colorectal cancer. J Clin Oncol. 2015;33(32):3727-3732. doi: 10.1200/JCO.2015.61.9569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salcher-Konrad M, Naci H. Unintended consequences of coverage laws targeting cancer drugs. J Law Med Ethics. 2020;48(3):552-554. doi: 10.1177/1073110520958880 [DOI] [PubMed] [Google Scholar]
- 47.Wagner AK, Ubel PA, Wharam JF. Financial pollution in the US health care system. JAMA Health Forum. 2021;2(3):e210195. doi: 10.1001/jamahealthforum.2021.0195 [DOI] [PubMed] [Google Scholar]
- 48.Prasad V, Kim MS. Approval and coverage of cancer drugs in England, Canada, and the US. JAMA Intern Med. 2021;181(4):509-510. doi: 10.1001/jamainternmed.2020.8587 [DOI] [PubMed] [Google Scholar]
- 49.US Food and Drug Administration . FDA’s Sentinel Initiative. October 18, 2019. Accessed January 18, 2021. https://www.fda.gov/safety/fdas-sentinel-initiative
- 50.Cherla A, Naci H, Kesselheim AS, Gyawali B, Mossialos E. Assessment of coverage in England of cancer drugs qualifying for US Food and Drug Administration accelerated approval. JAMA Intern Med. 2021;181(4):490-498. doi: 10.1001/jamainternmed.2020.8441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meyers DE, Jenei K, Chisamore TM, Gyawali B. Evaluation of the clinical benefit of cancer drugs submitted for reimbursement recommendation decisions in Canada. JAMA Intern Med. 2021;181(4):499-508. doi: 10.1001/jamainternmed.2020.8588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Emanuel EJ, Zhang C, Glickman A, Gudbranson E, DiMagno SSP, Urwin JW. Drug reimbursement regulation in 6 peer countries. JAMA Intern Med. 2020;180(11):1510-1517. doi: 10.1001/jamainternmed.2020.4793 [DOI] [PubMed] [Google Scholar]
- 53.Naci H, Salcher-Konrad M, Kesselheim AS, et al. Generating comparative evidence on new drugs and devices before approval. Lancet. 2020;395(10228):986-997. doi: 10.1016/S0140-6736(19)33178-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Definitions of Evidence of OS Benefit in FDA-Approved Labels
eTable 2. Indications Approved by the FDA for 44 Oral Targeted Cancer Drugs, 2011-2018
eTable 3. Trials of Drugs With Information on OS Benefit Reported in FDA-Approved Labels, 2011-2018
eTable 4. Magnitude of OS Benefit and Spending Associated With Oral Targeted Cancer Drugs With Documented OS Benefit, as Reported in FDA-Approved Labels in 2018
eTable 5. Spending Associated With Dispensing Events for 44 Oral Targeted Cancer Drugs in 2018
eTable 6. Number of Members per Year in the IBM MarketScan Research Databases, 2011-2018
eFigure 1. Annual and Cumulative Numbers of Patients Exposed to and Spending on 44 Oral Targeted Cancer Drugs, by FDA Orphan Designation and Accelerated Approval Pathway
eFigure 2. Annual and Cumulative Inflation-Adjusted Spending on 44 Oral Targeted Cancer Drugs
eBox. Brief Summary of Evidence Documented in FDA Labels for the Top 3 Drugs by Spending in 2018