Abstract
Purpose
Capsaicin (8-methyl-N-geranyl-6-nonamide; CAP) is an alkaloid isolated from chili peppers, which has complex pharmacological properties, including beneficial effects against various diseases. The aim of this study was to investigate the role of CAP in lipopolysaccharide (LPS)-induced acute lung injury (ALI), and the possible underlying mechanisms.
Materials and Methods
ALI was induced by intranasal administration of LPS (0.5 mg/kg), and CAP (1 mg/kg) injected intraperitoneally 3 days before exposure to LPS. Then, the histopathological changes were evaluated by hematoxylin and eosin staining. Enzyme-linked immunosorbent assay and qPCR were used to detect pro-inflammatory cytokines in serum and lung tissue. The expressions of HMGB1/NF-κB, PI3K/AKT/mTOR signaling pathways and apoptosis-associated molecules were determined by Western blot and/or qPCR. In addition, the lung cell apoptosis was analyzed by TUNEL staining, and the expression and location of cleaved caspase-3 were detected by immunofluorescence analysis.
Results
CAP pretreatment significantly protected mice from LPS-induced ALI, with reduced lung wet/dry weight ratio, lung histological damage, myeloperoxidase (MPO) activity, malondialdehyde (MDA) content and pro-inflammatory cytokine levels, and significant increased superoxide dismutase (SOD) activity. In addition, CAP pretreatment significantly inhibited the high-mobility group protein B1 (HMGB1) expression, nuclear factor-kappa B (NF-κB) activation, and the PI3K/AKT/mTOR signaling pathway. Furthermore, mice pre-treated with CAP exhibited reduced apoptosis of lung tissues, with associated down-regulation of caspase-3, cleaved caspase-3, and BAX expression, and up-regulation of BCL-2.
Conclusion
Our data demonstrate that CAP can protect against LPS-induced ALI by inhibiting oxidative stress, inflammatory responses and apoptosis through down-regulation of the HMGB1/NF-κB and PI3K/AKT/mTOR pathways.
Keywords: capsaicin, acute lung injury, HMGB1/NF-κB, PI3K/AKT/mTOR, apoptosis, inflammation
Introduction
Acute lung injury (ALI) is a series of clinical syndromes caused by damage to alveolar epithelial cells and capillary endothelial cells, which mainly manifest as diffuse alveolar parenchymal injury and refractory hypoxemia, while some severe cases may develop into acute respiratory distress syndrome (ARDS).1–3 ALI is typically characterized by increased respiratory rate, pulmonary edema, progressive hypoxemia, and respiratory distress, and is associated with high mortality rates.4 ALI has been reported in clinical cases with severe acute respiratory syndrome (SARS),5 which is prevalent in 2003, and in the current global outbreak of coronavirus disease (COVID-19),6 manifesting as diffuse alveolar damage, hyaline membrane formation, and interstitial thickening, with severe cases resulting in pulmonary fibrosis and ARDS. Effective strategies for the treatment of ALI have been lacking to date. Therefore, it is necessary to develop new effective drugs to improve the pathological features in patients with ALI. ALI pathogenesis is strongly associated with excessive oxidative stress, inflammation, apoptosis, and autophagy.7 Lipopolysaccharide (LPS) is the main component of the cell wall of Gram-negative bacteria, can cause immune and inflammatory disorders, and is widely used to induce ALI in animal models.8 LPS stimulation can activate neutrophils and macrophages, as well as the secretion of numerous pro-inflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β).9
Capsaicin (8-methyl-N-geranyl-6-nonamide, CAP) is an alkaloid isolated from chili peppers that has a variety of biological effects.10 CAP can selectively activate transient receptor potential vanilloid receptor 1 (TRPV1), thereby exerting its biological effects.11 CAP has protective roles in various conditions, including cardiovascular disease,12 arthritis,13 obesity,14 and cancer.15 In addition, the efficacy of CAP in the treatment of neuropathic pain and autoimmune skin diseases has been verified.16,17 Several other studies have reported that CAP can protect mice from acute liver injury by down-regulating the expression of pro-inflammatory cytokines.18 Furthermore, a recent study found that CAP exerts a protective effect on acetaminophen-induced acute liver injury by inhibiting the HMGB1/TLR4/NF-κB signaling pathway and hepatocyte apoptosis.19 Lin et al reported that CAP can inhibit PI3K/AKT/mTOR signaling in human nasopharyngeal carcinoma cells, thereby inducing autophagy and promoting caspase-3 activation and apoptosis.20
Hence, CAP can inhibit inflammation by regulating cell signaling, and the pathogenesis of ALI is associated with uncontrolled inflammatory responses. The effects of CAP on ALI and its underlying mechanism have yet to be reported. In this study, we aimed to investigate whether CAP has protective effects against LPS-induced ALI. Further, the expressions of HMGB1/NF-κB, PI3K/AKT/mTOR, caspase-3/cleaved caspase-3, and BAX/BCL-2 were assessed to elucidate the possible mechanisms involved.
Materials and Methods
Animals
Male Balb/c mice (8 weeks; 20–22 g) were provided by the Center for Animal Experiment of China Three Gorge University (Yichang, China). Mice were housed for at least 1 week in specific pathogen-free conditions, with a 12 h light/dark cycle prior to the experiment. All experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD, USA). The study protocol was also approved by the Animal Ethics Committee of Yangtze University (Jingzhou, China).
LPS-Induced ALI and Experimental Design
Mice were randomly divided into four groups: vehicle, CAP, LPS, and CAP/LPS. LPS-induced ALI was induced using a slightly modified version of a previously described method.21 Briefly, mice were anesthetized by isoflurane inhalation, and 10 µg LPS (Escherichia coli 055:B5; Sigma, St. Louis, USA) in 50 µL phosphate buffered saline (PBS) administered intratracheally to induce ALI.22,23 CAP (MedChem Express, Monmouth Junction, NJ, USA) was dissolved in a 10% Tween 80/10% Ethanol/80% PBS mixture. Before LPS administration, all mice were injected intraperitoneally for 3 days with 10% Tween 80/10% Ethanol/80% PBS in the vehicle and LPS groups, or CAP (1 mg/kg) dissolved in 10% Tween 80/10% Ethanol/80% PBS in the CAP and CAP/LPS groups. Thirty minutes later, the vehicle and CAP groups were administered PBS intranasally, and the LPS and CAP/LPS group received intranasal administration of LPS. Mice were sacrificed at 6h and 24 h after LPS injection.
Determination of Lung Wet/Dry Weight Ratio
To assess LPS-induced pulmonary edema, lung wet/dry weight ratio was determined. Briefly, six mice in each group were sacrificed 24 h after LPS injection, their right lungs excised, fluid and blood on the lung surface quickly removed using filter paper, weighed, and the wet weight recorded. Then, the lungs were placed in an incubator at 80°C for 48 h to determine the dry weight. The wet/dry weight ratio was determined by dividing the dry weight by the wet weight.
Analysis of Cell Counts and Protein Content in Bronchoalveolar Lavage Fluid (BALF)
BALF was collected after the lungs were lavaged with 1 mL PBS for three times as described previously.24 Collected BALF was centrifuged at 1000 g for 10 min at 4°C, and the supernatant frozen at −80°C for subsequent measurements. The sediment cells were resuspended in 1% BSA, and total cells and neutrophils were counted. Total protein concentrations in BALF were determined using a BCA protein assay kit (MultiSciences, Hangzhou, China).
Determination of Oxidative Stress and Myeloperoxidase (MPO) Activity
Malondialdehyde (MDA) and superoxide dismutase (SOD) are the major indices used to evaluate the lipid oxidation levels in acute lung injury. MPO activity can be used to assess the parenchymal infiltration of neutrophils. The levels of MDA, SOD and MPO measured using commercial test kits (Jiancheng Institute of Biotechnology, Nanjing, China). All steps were performed in accordance with the manufacturer’s procedures.
ELISA to Assess Cytokine Levels
The amounts of TNF-α, IL-1β and IL-6 in BALF were determined using commercial kits (MultiSciences, Hangzhou, China), according to the manufacturer’s instructions.
Histology
To observe the histological modifications induced by LPS, left lung upper lobes were fixed in 4% neutral formaldehyde for 24 h. Then, lung specimens were dehydrated in a series of gradient ethanol concentrations, cleared in xylene, embedded in paraffin, and sliced into 4-μm-thick sections. After deparaffinization, sections were subjected to standard hematoxylin and eosin staining to assess the histopathologic changes in lung tissue under a light microscope. The histopathologic scores were calculated to evaluate the hyperaemia, hemorrhage, edema of the alveolar wall and inflammatory cell infiltration for each tissue using a semi-quantitative scoring system. The severity of lung injury is in grade 0–5 (0 normal, 1 mild or very small amount, 2 mild or small amount, 3 moderate or more amount, 4 severe or large amount, 5 very severe or extremely large amount).25 To evaluate lung tissue apoptosis, paraffin-embedded lung tissue samples were stained using a TUNEL Apoptosis Detection Kit (Roche, Basel, Switzerland). Lung apoptotic features were observed and photographed under an inverted fluorescence microscope.
qPCR
Total RNA was extracted from 50 mg lung tissue using TRIzol reagent (Ambion Life, Technologies, Carlsbad, CA, USA). Reverse transcription was performed from 250 ng of total RNA using a PrimeScript™ RT reagent Kit with gDNA Eraser (TaKaRa, Shiga, Japan). qPCR was undertaken with an Ambion 7500 Real-Time PCR System using the TB Green® Premix Ex Taq™ I Kit (TaKaRa, Shiga, Japan). The primers sequences used for mRNA expression analysis are as follows:
Bax forward 5ʹ-GCG ATG AAC TGG ACA ACA ACA-3ʹ, reverse 5ʹ-CTG CCA CAC GGA AGA AGA C-3ʹ
Bcl-2 forward 5ʹ-GGG CTA CGA GTG GGA TAC TGG AG-3ʹ, reverse 5ʹ-CGG GCG TTC GGT TGC TCT-3ʹ
Hmgb1 forward 5ʹ-GAT GGG CAA AGG AGA TCC TAA G-3ʹ, reverse 5ʹ-TCA CTT TTT GTC TCC CCT TTG GG-3ʹ
Il-6 forward 5ʹ-GTT GCC TTC TTG GGA CTG ATG-3ʹ, reverse 5ʹ-ACT CTT TTC TCA TTT CCA CGA TTT-3ʹ
Tnf-α forward 5ʹ-CTT GCC CTC TAC AAC CAA CA-3ʹ, reverse 5ʹ-ACT TGC GAC CCA CGT AGT AGA-3ʹ
Il-1β forward 5ʹ-CAT CCA GCT TCA AAT CTC GCA G-3ʹ, reverse 5ʹ-CAC ACA CCA GCA GGT TAT CAT C-3ʹ
Gapdh forward 5ʹ-GGT TAT CTC CTG CGA CTT CA-3ʹ, reverse 5ʹ-TGG TCC AGG GTT TCT TAC TCC-3ʹ
Western Blot
Lung tissues were homogenized in RIPA lysis buffer (MultiSciences, Hangzhou, China) containing protease inhibitor cocktail (Servicebio, Wuhan, China). The BCA protein assay kit (MultiSciences, Hangzhou, China) was used for protein quantification. Samples were separated by 10% SDS-PAGE and then electro-transferred onto polyvinylidene difluoride membranes. After blocking for 1 h with 5% nonfat dry milk at room temperature, blots were incubated at 4°C with the following specific antibodies: anti-p-PI3K (4228S), anti-PI3K (4292S), anti-p-AKT (4060S), anti-AKT (9272S), anti-p-mTOR (2971S), anti-mTOR (2972S), anti-caspase-3 (9662S), anti-cleaved caspase-3 (9664S), anti-p-IκBα (2859S), anti-IκBα (4812S), anti-p-p65 (3033S), anti-p65 (8242S), and anti-HMGB1 (6893S) (all from Cell Signaling Technology); anti-BAX (ab32503, Abcam) and anti-BCL-2 (ab182858, Abcam); and anti-GAPDH (GB11002, Servicebio). After incubating with primary antibody overnight, and washing three times with tris-buffered saline containing Tween 20, blots were incubated with the corresponding HRP-conjugated secondary antibody for 1 h at room temperature. Immunoreactivity was determined using an enhanced chemiluminescence reagent ECL kit (MultiSciences, Hangzhou, China).
Immunofluorescence Analysis
The fresh left lung tissues were washed with PBS and then embedded in O.C.T. and snapped frozen in liquid nitrogen. Frozen sections (6 μm) were then cut, mounted on slides, treated with 0.1% Triton X-100 in PBS for 5 min, and blocked with 5% donkey serum albumin. Washing the slides for three times with PBS, the sections were exposed to primary antibodies overnight. After incubation with Alexa Fluor 594 conjugated secondary antibodies (8889S, Cell Signaling Technology) for 1 h at room temperature and counterstained with DAPI for 15 min. Images were observed using a confocal laser-scanning microscope (Leica, Germany).
Statistical Analysis
Data are presented as mean ± standard deviation (SD). Graphs were generated and statistical analysis conducted using GraphPad Prism 6 Software (San Diego, CA). Results were analyzed using the Student’s t-test (two groups) or one-way analysis of variance (ANOVA; multiple groups). p < 0.05 was considered statistically significant.
Results
CAP Pretreatment Alleviates LPS-Induced ALI
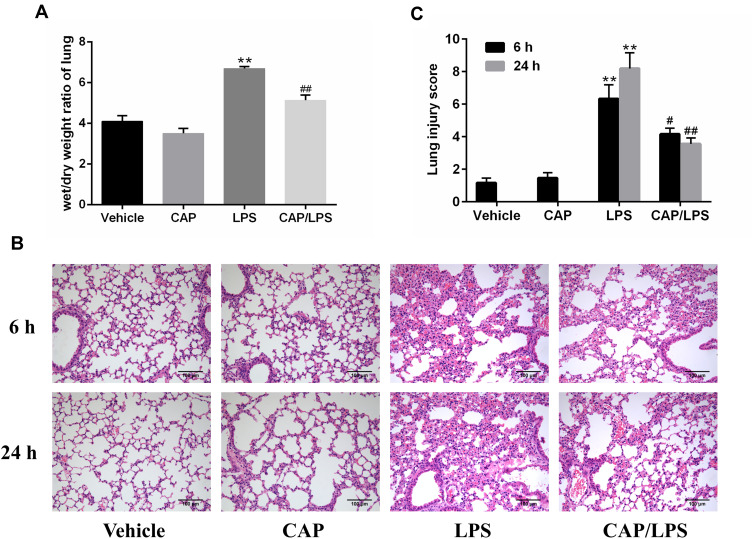
We wished to investigate the effects of CAP on LPS-induced ALI. Therefore, mice were injected intraperitoneally with CAP for 3 days before LPS stimulation. Pulmonary edema is a dominant pathological change observed in LPS-induced ALI; therefore, lung wet/dry weight ratio was used to evaluate the effect of CAP on pulmonary edema 24 h after LPS stimulation. As shown in Figure 1A, the lung wet/dry weight ratio increased significantly after LPS stimulation relative to the vehicle group; however, this increased ratio was suppressed by CAP pretreatment. In addition, the ratio in the group treated with CAP alone did not differ significantly from that of the vehicle group. These results provided preliminary evidence that CAP has protective effects against LPS-induced ALI while having no effect on healthy control mice.
Figure 1.
Capsaicin (CAP) pretreatment ameliorates lipopolysaccharide (LPS)-induced pathological changes of acute lung injury. (A) Lung wet/dry weight ratios were determined 24 h after LPS stimulation. (B) Hematoxylin and eosin staining of lung specimens 6 h and 24 h after LPS stimulation (original magnification ×200). (C) The histological changes were scored at the 6 h and 24 h time points. Data are presented as means ± SD (n = 6–8 for each group). **p < 0.01 versus the vehicle group; #p < 0.05 and ##p < 0.01 versus the LPS group. Three independent experiments were performed.
Next, lung sections stained with hematoxylin and eosin were used to assess pathological changes 6 h and 24 h after LPS stimulation. As shown in Figure 1B and C, no significant destructive changes were observed in lung tissue samples from the vehicle and CAP groups; however, lung tissues from mice exposed to LPS showed severe inflammatory cell infiltration, edema, enhanced interstitial congestion, and thickened alveolar walls, and these pathologic changes were significantly ameliorated by CAP pretreatment.
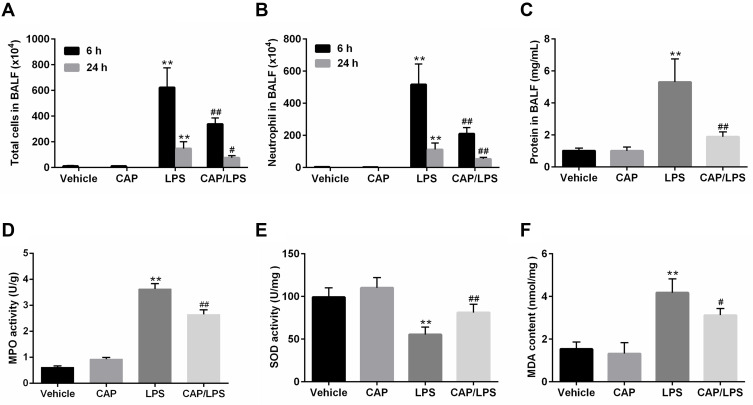
To further identify the anti-inflammatory property of CAP, cell counts and total protein concentrations in BALF and the MPO activity in the lung tissues were examined. As shown in Figure 2A and B, 6 h and 24 h after LPS stimulation, the number of total cells and neutrophils significantly increased in BALF. Nevertheless, CAP pretreatment significantly reduced the numbers of total cells and neutrophils in BALF. However, the number of total cells and neutrophils at 6 h after the early LPS stimulation was evidently higher than 24 h. Similarly, after 24 h of LPS stimulation, the total protein concentration in the BALF was significantly increased compared to the vehicle group, whereas its level was significantly reduced by CAP pretreatment (Figure 2C). In addition, MPO activities in lung tissues were assessed to determine neutrophil infiltration in LPS-induced ALI. MPO activity did not differ significantly between the vehicle and CAP alone groups; however, it increased markedly in the LPS group (Figure 2D), while CAP pretreatment significantly reduced the activity of MPO in lung tissue. Oxidative stress is one of the main features during sepsis-induced ALI.26 Therefore, we next determined the activity of anti-oxidase SOD and the content of peroxidation product (MDA) in lung tissues. As expected, LPS gave rise to significant decrease in SOD activity and significant increase of MDA content, which were almost reversed by CAP pretreatment (Figure 2E and F). Taken together, these results suggested that CAP pretreatment can alleviate LPS-induced ALI.
Figure 2.
Capsaicin (CAP) pretreatment attenuates lipopolysaccharide (LPS)-induced inflammatory reactions and oxidative stress. The counts of total cells (A) and neutrophils (B) in BALF at 6 h and 24 h after LPS stimulation. (C) BALF protein concentrations were assessed 24 h after LPS stimulation. MPO activity (D), SOD activity (E) and MDA content (F) in lung tissue samples 24 h after LPS stimulation. Data are presented as mean ± SD (n = 6–8 for each group). **p < 0.01 versus the vehicle group; #p < 0.05 and ##p < 0.01 versus the LPS group. Three independent experiments were performed.
CAP Pretreatment Inhibits Pro-Inflammatory Cytokine Expression in LPS-Induced ALI
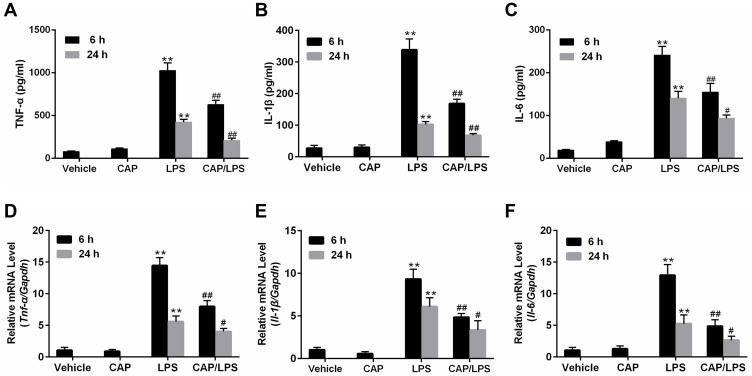
Inflammatory mediators, particularly TNF-α, IL-1β, and IL-6, play critical roles in the pathogenesis of LPS-induced ALI.27,28 Hence, we next evaluated the effect of CAP on the production of the pro-inflammatory cytokines, TNF-α, IL-1β, and IL-6. ELISA results showed that TNF-α, IL-1β, and IL-6 levels in BALF increased significantly as early as 6 h after LPS stimulation and this persisted until 24 h, whereas CAP pretreatment significantly inhibited the increases in TNF-α, IL-1β, and IL-6 levels in LPS-induced ALI in mice (Figure 3A–C). Next, mRNA levels of pro-inflammatory cytokines in lung tissue were measured by qPCR. As expected, the levels of Tnf-α, Il-1β, and Il-6 mRNA increased significantly as early as 6 h after LPS stimulation and continued to 24 h, while the level in CAP/LPS group was significantly lower than that of the LPS group (Figure 3D–F); however, CAP had no effect on the expression of pro-inflammatory cytokines in healthy control mice.
Figure 3.
Capsaicin (CAP) pretreatment inhibits pro-inflammatory cytokine expression in lipopolysaccharide (LPS)-induced acute lung injury. Bronchoalveolar lavage fluid (BALF) and lung tissues samples were collected 6 h and 24 h after LPS stimulation. The level of TNF-α (A) and IL-1β (B), IL-6 (C) in BALF were measured by ELISA. Tnf-α (D), Il-1β (E), and Il-6 (F) mRNA levels in lung tissues were determined by qPCR. Data are presented as mean ± SD (n = 6–8 for each group). **p < 0.01 versus the vehicle group; #p < 0.05 and ##p < 0.01 versus the LPS group. Three independent experiments were performed.
CAP Pretreatment Inhibits HMGB1 Release and NF-κB Activation in LPS-Induced ALI
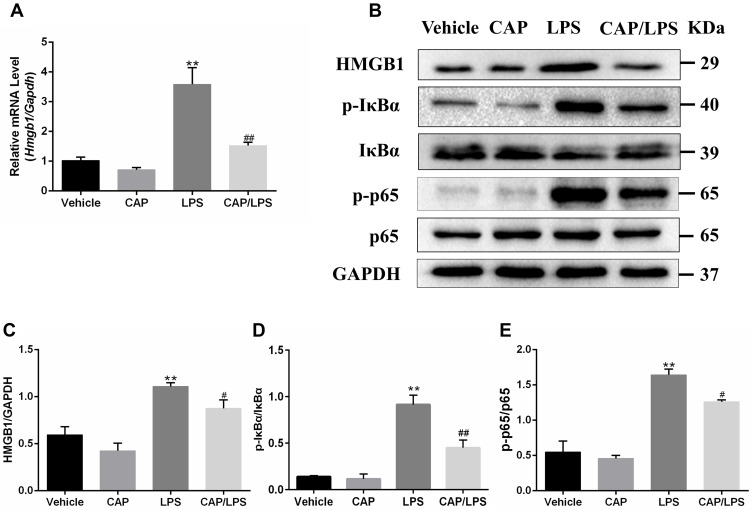
High-mobility group box 1 (HMGB1) is an important indicator of inflammation and tissue damage,29,30 which is secreted from activated immune cells stimulated with LPS, IL-1β, or TNF-α, and plays a key role in ALI pathogenesis through the NF-κB pathway.31 Therefore, we explored whether the protective effect of CAP on LPS-induced ALI in mice is attributable to inhibition of the HMGB1/NF-κB pathway. As shown in Figure 4A, Hmgb1 mRNA levels in the LPS group were significantly increased compared with the vehicle group and significantly decreased by CAP pretreatment.
Figure 4.
Capsaicin (CAP) pretreatment down-regulates lipopolysaccharide (LPS)-induced HMGB1 expression and NF-κB activation. (A) Relative mRNA expression of Hmgb1 in lung tissue was determined by qPCR. (B) Western blot analysis of HMGB1, p-IκBα, IκBα, p-p65, and p65 expression in lung tissue. GAPDH was used as the loading control. (C–E) Quantitative analysis of (B). Results are representative of three independent experiments and data are presented as mean ± SD (n = 6–8 for each group). **p < 0.01 versus the vehicle group; #p < 0.05 and ##p < 0.01 versus the LPS group.
In addition, the HMGB1/NF-κB signaling pathway protein expression levels were determined by Western blot (Figure 4B–E). HMGB1, p-IκBα and p-p65 were significantly increased after LPS stimulation compared with the vehicle group; however, CAP pretreatment led to significant decreases in levels of these proteins on LPS stimulation, while it had no effect on HMGB1, p-IκBα and p-p65 expression in healthy control mice. These findings suggest that the protective effects of CAP on LPS-induced ALI are closely associated with inhibition of HMGB1 release and NF-κB activation.
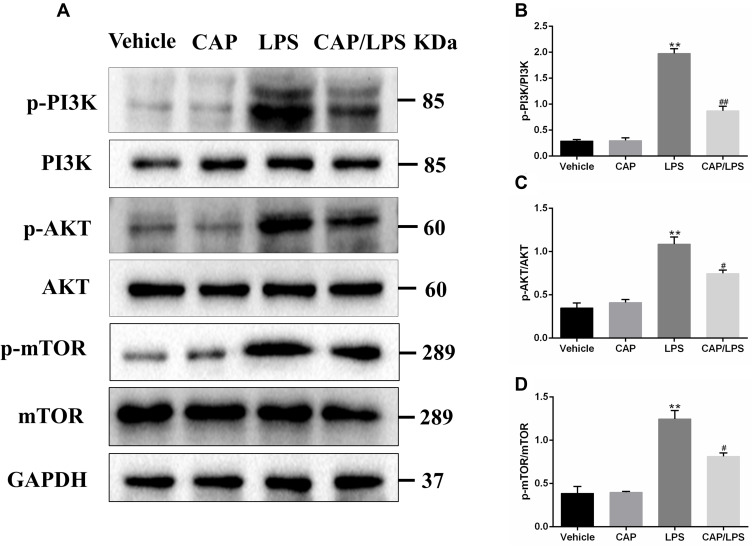
CAP Pretreatment Protects Against LPS-Induced ALI by Inhibiting Activation of the PI3K/AKT/mTOR Signaling Pathway
PI3K/AKT/mTOR signaling is an important functional regulator of NF-κB activation,32 and can promote cell division and proliferation, as well as regulating apoptosis, cell autophagy, and anti-inflammatory cytokine release.33 Therefore, we assessed the activation of PI3K, AKT, and mTOR by Western blot assays. The results showed that LPS administration led to increased expression of p-PI3K, p-AKT, and p-mTOR (Figure 5), while CAP pretreatment decreased their expression significantly. These results suggest that CAP can effectively attenuate LPS-induced ALI by inhibiting the PI3K/AKT/mTOR signaling pathway.
Figure 5.
Capsaicin (CAP) pretreatment inhibits the activation of PI3K/AKT/mTOR signaling in lipopolysaccharide (LPS)-induced acute lung injury. (A) Western blot analysis of p-PI3K, PI3K, p-AKT, AKT, p-mTOR, and mTOR expression in lung tissue. GAPDH was used as the loading control. (B–D) Quantitative analysis of (A). Results are representative of three independent experiments and data are presented as mean ± SD (n = 6–8 for each group). **p < 0.01 versus the vehicle group; #p < 0.05 and ##p < 0.01 versus the LPS group.
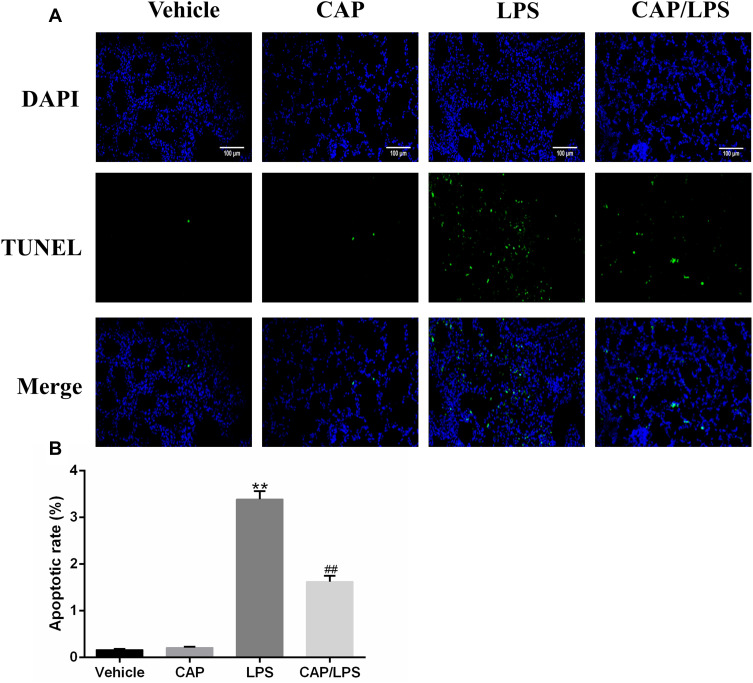
CAP Pretreatment Decreased LPS-Induced Apoptosis
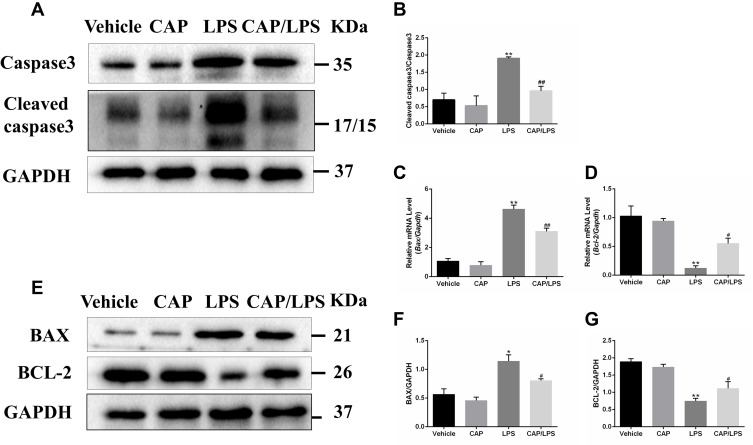
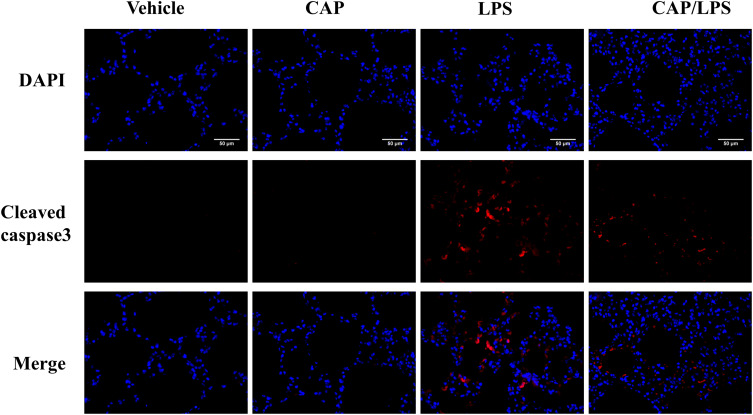
To determine the effects of CAP on apoptosis in LPS-induced ALI, lung tissues were evaluated using the TUNEL assay. As shown in Figure 6A and B, increased proportions of TUNEL-positive cells were observed in the LPS group compared with the vehicle group, while CAP pretreatment significantly decreased the percentage of TUNEL-positive cells. Meanwhile, we evaluated the expression of the apoptosis-associated proteins, BAX, BCL-2, caspase-3 and cleaved caspase-3. As shown in Figure 7A and B, the expression levels of caspase-3, cleaved caspase-3 and the ratio of cleaved caspase-3/caspase-3 were significantly higher after LPS treatment compared with the vehicle group, while CAP pretreatment significantly reduced their expression, indicating that CAP can protect lung tissue cell apoptosis induced by LPS. Next, we detected the cleaved caspase-3 location in LPS-induced ALI by employing the immunofluorescence analysis. Immunoreactivity was weakened in the vehicle group, whereas the high expressions of cleaved caspase-3 were observed in the LPS group. However, its expression was significantly inhibited by CAP pretreatment (Figure 8).
Figure 6.
Capsaicin (CAP) pretreatment inhibits lipopolysaccharide (LPS)-induced apoptosis. (A) Mouse pulmonary cell apoptosis was analyzed by TUNEL staining (original magnification, ×200). (B) Quantitative data are presented as the ratio of TUNEL-positive cells detected within the examined area. Data are presented as mean ± SD (n = 6–8 for each group). **p < 0.01 versus the vehicle group; ##p < 0.01 versus the LPS group. Three independent experiments were performed.
Figure 7.
The effects of capsaicin (CAP) on apoptosis indices in lipopolysaccharide (LPS)-induced acute lung injury. (A) Western blot analysis of the expression of caspase-3 and cleaved caspase-3 in lung tissue. GAPDH was used as the loading control. (B) Quantitative analysis of (A). (C and D) mRNA expression of Bax and Bcl-2 were determined by qPCR. (E) Western blot analysis of protein expression of BAX and BCL-2 in lung tissue. GAPDH was used as the loading control. (F and G) Quantitative analysis of (E). Results are representative of three independent experiments and data are presented as mean ± SD (n = 6–8 for each group). *p < 0.05 and **p < 0.01 versus the vehicle group; #p < 0.05 and ##p < 0.01 versus the LPS group.
Figure 8.
The effect of capsaicin (CAP) on the expression of cleaved caspase3 in lipopolysaccharide (LPS)-induced acute lung injury by immunofluorescence analysis (original magnification, ×400). Three independent experiments were performed.
In addition, to further explore the anti-apoptotic effect of CAP in ALI, the expressions of BCL-2 family proteins were assessed by Western blot and qPCR. As shown in Figure 7C–G, lower levels of BCL-2 and higher levels of BAX were detected in the LPS group than the vehicle group, whereas CAP pretreatment significantly increased BCL-2 and decreased BAX expression levels, thereby modulating the BCL-2/BAX ratio. These results confirm the anti-apoptotic effects of CAP in LPS-induced ALI.
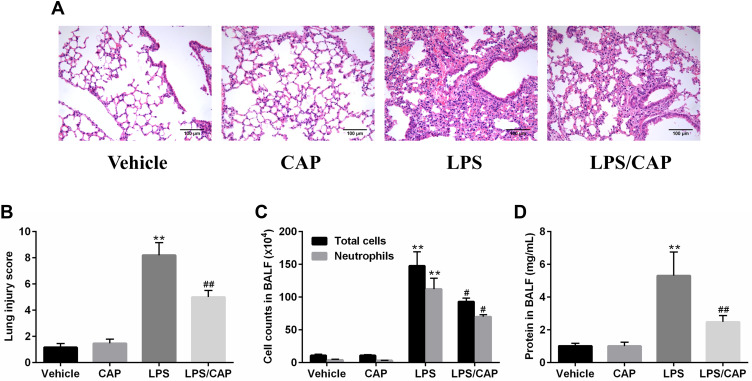
CAP Treatment Reduce LPS-Induced Acute Lung Injury in Mice
Previous results showed us the preventive effect of CAP on LPS-induced ALI, which prompted us to investigate the possible therapeutic effect of CAP. Generally, mice were intraperitoneally injected with CAP 30 min after LPS exposure as treatment group. As shown in Figure 9A and B, no pathological changes were observed in lung tissues from the vehicle group and CAP group. However, the lung tissues from LPS group displayed notable levels of lung edema, alveolar hemorrhage, thickened alveolar septum, and severe inflammatory cells infiltration. In contrast, CAP treatment significantly decreased the pathological changes that interstitial edema, inflammatory cell infiltration, and pulmonary hemorrhage were improved. Meanwhile, the total cells, neutrophils and total protein concentration increased in BALF of LPS-treated mice. Nevertheless, these indicators were reduced by CAP treatment (Figure 9C and D). Taken together, our results demonstrate that CAP has similar therapeutic effects on acute lung injury compared with CAP pretreatment group.
Figure 9.
Capsaicin (CAP) treatment attenuates lipopolysaccharide (LPS)-induced acute lung injury. (A) Hematoxylin and eosin staining of lung specimens 24 h after LPS stimulation (original magnification ×200). (B)The histological changes were scored at the 24 h time points. (C) The cell counts in BALF 24 h after LPS stimulation. (D) BALF protein concentrations were assessed 24 h after LPS stimulation. Data are presented as mean ± SD (n = 6–8 for each group). **p < 0.01 versus the vehicle group; #p < 0.05 and ##p < 0.01 versus the LPS group. Three independent experiments were performed.
Discussion
The high incidence and mortality rates of ALI represent a serious threat to the lives of individuals. In recent years, drugs with potential for use in the treatment of ALI have been the subject of increasing research. Our previous studies demonstrated that sodium butyrate and hesperidin can alleviate LPS-induced ALI.23,34 CAP, isolated from chili peppers, is an active ingredient with anti-inflammatory effects that has been widely applied in the treatment of various inflammatory disease models;35 however, the effects of CAP in pneumonia models have rarely been studied. The aims of this investigation were to assess the effects of CAP on LPS-induced ALI and to explore the possible underlying mechanisms.
The main pathological changes in ALI include alveolar wall damage, as well as infiltration of neutrophils, macrophages, and other inflammatory cells.36 In our study, we found that CAP pretreatment could reduce LPS-induced lung injury, manifested as decreased pulmonary edema and inflammatory cell infiltration, as well as significantly reducing alveolar oxidative stress caused by LPS. In addition, pro-inflammatory cytokines, including TNF-α, IL-6, and IL-1β, are important for the initiation and development of ALI, especially in the early stages of the inflammatory response, and a continuous increase in pro-inflammatory cytokines often indicates poor prognosis for patients with ALI.27 Here, we found that the expression levels of TNF-α, IL-6, and IL-1β in a mouse model of LPS-induced ALI were increased, while CAP exerted a protective role by inhibiting their expression. A similar protective effect of CAP was also observed in a concanavalin A-induced model of acute liver injury.37
HMGB1 is an important inflammatory response alarmin involved in the pathogenesis of various inflammatory diseases, including sepsis, acute liver injury, and ALI.25,38,39 In addition, recombinant HMGB1 can aggravate LPS-induced ALI, while anti-HMGB1 can significantly reduce the inflammatory response in this model.40 Our data also demonstrate that the expression of HMGB1 is significantly up-regulated after LPS stimulation, whereas this elevation is inhibited by CAP, indicating that CAP may function as an HMGB1 inhibitor. Consistent with current results, our previous study also found CAP can protect against acetaminophen-induced acute liver injury by inhibiting HMGB1 expression.19 NF-κB is a transcription factor important for HMGB1 synthesis; when activated, it translocates to the nucleus, binds to multiple target genes involved in injury and inflammation, and promotes their expression.41 Our results show that the expression of NF-κB and HMGB1 increases in the LPS-induced ALI, while CAP pretreatment significantly inhibits the activation of NF-κB and HMGB1 release. The expression and release of HMGB1 in ALI are positively correlated with NF-κB and toll-like receptor-4 (TLR4) expression,25 and the HMGB1/TLR4 axis is a key pro-inflammatory signaling pathway that can activate NF-κB, causing transcription and release of pro-inflammatory cytokines (such as TNF-α, IL-1β and IL-6);25 hence, CAP participation in protection against LPS-induced ALI may be related to inhibition of the inflammatory HMGB1/NF-κB signaling pathway.
To further investigate the mechanism involved in CAP protection from LPS-induced ALI, we studied PI3K/AKT/mTOR signaling. The PI3K/AKT/mTOR pathway is ubiquitous in cells and participates in regulation of cell growth, differentiation, apoptosis, and autophagy.42 The PI3K/AKT/mTOR and TLR4/NF-κB signaling pathways are coordinated during inflammatory responses,33 and NF-κB is a downstream target of AKT. Phosphorylated AKT can activate NF-κB, leading to p65 translocation to the nucleus and expression of inflammatory genes.43 In our study, phosphorylation levels of PI3K, AKT, and mTOR were increased, while their activation was inhibited by CAP. These data are consistent with previous findings that CAP induces autophagy and apoptosis in human nasopharyngeal carcinoma cells by down-regulating the PI3K/AKT/mTOR pathway.20
Apoptosis of lung epithelial cells is important in ALI pathogenesis; hence, reducing excessive activation of apoptosis could be an important therapeutic strategy.44 When mitochondria are damaged, mitochondrial permeability transition pores (mPTP) are opened, and cytochrome C and second mitochondria-derived activator of caspase (SMAC), as well as other pro-apoptotic small-molecule proteins, are released into the cytoplasm. Subsequently, they form a multimeric complex with apoptotic protease activating factor-1, which recruits caspsase-9 precursors into the cytoplasm and initiates the caspase cascade through self-cleavage, thereby activating downstream caspase-3 and resulting in apoptosis.45 BCL-2 and BAX are representative BCL-2 family anti-apoptotic and pro-apoptotic molecules, respectively, which can regulate apoptosis by controlling mPTP.46 In our study, TUNEL-positive cells in lung tissue were significantly increased in the LPS-induced ALI model, and the expression levels of BAX, caspase-3, and cleaved caspase-3 were also increased in lung tissues; however, CAP pretreatment significantly ameliorated lung cell apoptosis by inhibiting the expression of BAX, caspase-3, and cleaved caspase-3, and promoting the expression of BCL-2, suggesting that CAP may protect lung cells from apoptosis by regulating mitochondrial signals in the LPS-induced ALI. Similarly, another study demonstrated that CAP can protect against high temperature-induced spermatogenic cell death in the scrotum, through its anti-apoptotic effects.47
In conclusion, our data suggest that CAP has a protective effect against LPS-induced ALI, which may be attributed to CAP-mediated reduction of pro-inflammatory cytokine release and apoptosis through inhibition of the HMGB1/NF-κB and PI3K/AKT/mTOR pathways. These findings lay the foundation for further clinical research on the pathological mechanisms involved in and intervention strategies to treat lung injury.
Acknowledgments
We gratefully thank Dr. Daping Zhang for the critical reading of the manuscript. This work was supported by the Joint Foundation of the Health Commission of Hubei Province (Grant no. WJ2018H173), Central Funds Guiding the Local Science and Technology Development of Hubei Province (Grant no. 2019ZYYD066), and the National Natural Science Foundation of China (Grant no. 81271872). The authors are grateful to the research group members of the Department of Immunology, School of Medicine, Yangtze University.
Abbreviations
ALI, acute lung injury; ARDS, acute respiratory distress syndrome; BALF, bronchoalveolar lavage fluid; CAP, capsaicin; HMGB1, high-mobility group box 1; IL-1β, interleukin-1β; IL-6, interleukin-6; LPS, lipopolysaccharide; MDA, malondialdehyde; MPO, myeloperoxidase; NF-κB, nuclear factor-kappa B; PBS, phosphate balanced solution; SD, standard deviation; SOD, superoxide dismutase; TLR4, toll-like receptor-4; TNF-α, tumor necrosis factor-α.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval for the version to be published; and agreed to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest.
References
- 1.Wang T, Yegambaram M, Gross C, et al. RAC1 nitration at Y(32) IS involved in the endothelial barrier disruption associated with lipopolysaccharide-mediated acute lung injury. Redox Biol. 2020;38:101794. doi: 10.1016/j.redox.2020.101794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li X, Huang R, Liu K, et al. Fucoxanthin attenuates LPS-induced acute lung injury via inhibition of the TLR4/MYD88 signaling axis. Aging. 2020;12:2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y, Xiang D, Zhang H, Yao H, Wang Y. Hypoxia-inducible Factor-1: a potential target to treat acute lung injury. Oxid Med Cell Longev. 2020;2020:8871476. doi: 10.1155/2020/8871476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butt Y, Kurdowska A, Allen TC. Acute lung injury: a clinical and molecular review. Arch Pathol Lab Med. 2016;140(4):345–350. doi: 10.5858/arpa.2015-0519-RA [DOI] [PubMed] [Google Scholar]
- 5.Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39(5):529–539. doi: 10.1007/s00281-017-0629-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X, Ma X. Acute respiratory failure in COVID-19: is it “typical” ARDS? Crit Care. 2020;24(1):198. doi: 10.1186/s13054-020-02911-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Z, Wang X, Ma C, et al. Genipin protects rats against lipopolysaccharide-induced acute lung injury by reinforcing autophagy. Int Immunopharmacol. 2019;72:21–30. doi: 10.1016/j.intimp.2019.03.052 [DOI] [PubMed] [Google Scholar]
- 8.Klein G, Raina S. Regulated assembly of LPS, its structural alterations and cellular response to LPS defects. Int J Mol Sci. 2019;20(2):356. doi: 10.3390/ijms20020356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi J, Zhao Y, Wang Y, et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514(7521):187–192. doi: 10.1038/nature13683 [DOI] [PubMed] [Google Scholar]
- 10.Basith S, Cui M, Hong S, Choi S. Harnessing the therapeutic potential of capsaicin and its analogues in pain and other diseases. Molecules. 2016;21(8):966. doi: 10.3390/molecules21080966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Srinivasan K. Biological activities of red pepper (Capsicum annuum) and its pungent principle capsaicin: a review. Crit Rev Food Sci Nutr. 2016;56(9):1488–1500. doi: 10.1080/10408398.2013.772090 [DOI] [PubMed] [Google Scholar]
- 12.He H, Zhou Y, Huang J, et al. Capsaicin protects cardiomyocytes against anoxia/reoxygenation injury via preventing mitochondrial dysfunction mediated by SIRT1. Oxid Med Cell Longev. 2017;2017:1035702. doi: 10.1155/2017/1035702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horváth Á, Borbély É, Bölcskei K, et al. Regulatory role of capsaicin-sensitive peptidergic sensory nerves in the proteoglycan-induced autoimmune arthritis model of the mouse. J Neuroinflammation. 2018;15(1):335. doi: 10.1186/s12974-018-1364-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang C, Wang B, Kaliannan K, et al. Gut microbiota mediates the protective effects of dietary capsaicin against chronic low-grade inflammation and associated obesity induced by high-fat diet. mBio. 2017;8:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chapa-Oliver AM, Mejía-Teniente L. Capsaicin: from plants to a cancer-suppressing agent. Molecules. 2016;21(8):931. doi: 10.3390/molecules21080931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blair HA. Capsaicin 8% dermal patch: a review in peripheral neuropathic pain. Drugs. 2018;78(14):1489–1500. doi: 10.1007/s40265-018-0982-7 [DOI] [PubMed] [Google Scholar]
- 17.Deng Y, Huang X, Wu H, et al. Some like it hot: the emerging role of spicy food (capsaicin) in autoimmune diseases. Autoimmun Rev. 2016;15(5):451–456. doi: 10.1016/j.autrev.2016.01.009 [DOI] [PubMed] [Google Scholar]
- 18.Fernandes ES, Cerqueira AR, Soares AG, Costa SK. Capsaicin and its role in chronic diseases. Adv Exp Med Biol. 2016;929:91–125. [DOI] [PubMed] [Google Scholar]
- 19.Zhan X, Zhang J, Chen H, et al. Capsaicin alleviates Acetaminophen-induced acute liver injury in mice. Clin Immunol. 2020;220:108578. doi: 10.1016/j.clim.2020.108578 [DOI] [PubMed] [Google Scholar]
- 20.Lin YT, Wang HC, Hsu YC, Cho CL, Yang MY, Chien CY. Capsaicin induces autophagy and apoptosis in human nasopharyngeal carcinoma cells by downregulating the PI3K/AKT/mTOR pathway. Int J Mol Sci. 2017;18(7):1343. doi: 10.3390/ijms18071343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J Immunol. 2000;165(6):2950–2954. doi: 10.4049/jimmunol.165.6.2950 [DOI] [PubMed] [Google Scholar]
- 22.Gong Q, Yin H, Fang M, et al. Heme oxygenase-1 upregulation significantly inhibits TNF-alpha and Hmgb1 releasing and attenuates lipopolysaccharide-induced acute lung injury in mice. Int Immunopharmacol. 2008;8(6):792–798. doi: 10.1016/j.intimp.2008.01.026 [DOI] [PubMed] [Google Scholar]
- 23.Liu XX, Yu DD, Chen MJ, et al. Hesperidin ameliorates lipopolysaccharide-induced acute lung injury in mice by inhibiting HMGB1 release. Int Immunopharmacol. 2015;25(2):370–376. doi: 10.1016/j.intimp.2015.02.022 [DOI] [PubMed] [Google Scholar]
- 24.Ning L, Wei W, Wenyang J, Rui X, Qing G. Cytosolic DNA-STING-NLRP3 axis is involved in murine acute lung injury induced by lipopolysaccharide. Clin Transl Med. 2020;10(7):e228. doi: 10.1002/ctm2.228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meng L, Li L, Lu S, et al. The protective effect of dexmedetomidine on LPS-induced acute lung injury through the HMGB1-mediated TLR4/NF-κB and PI3K/Akt/mTOR pathways. Mol Immunol. 2018;94:7–17. doi: 10.1016/j.molimm.2017.12.008 [DOI] [PubMed] [Google Scholar]
- 26.Xia W, Pan Z, Zhang H, Zhou Q, Liu Y. Inhibition of ERRα aggravates sepsis-induced acute lung injury in rats via provoking inflammation and oxidative stress. Oxid Med Cell Longev. 2020;2020:2048632. doi: 10.1155/2020/2048632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Zheng Y, Li MX, Yang CW, Liu YF. Tanshinone IIA alleviates lipopolysaccharide-induced acute lung injury by downregulating TRPM7 and pro-inflammatory factors. J Cell Mol Med. 2018;22(1):646–654. doi: 10.1111/jcmm.13350 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Ito K, Mizutani A, Kira S, Mori M, Iwasaka H, Noguchi T. Effect of Ulinastatin, a human urinary trypsin inhibitor, on the oleic acid-induced acute lung injury in rats via the inhibition of activated leukocytes. Injury. 2005;36(3):387–394. doi: 10.1016/j.injury.2004.06.018 [DOI] [PubMed] [Google Scholar]
- 29.Qu L, Chen C, Chen Y, et al. High-Mobility Group Box 1 (HMGB1) and Autophagy in Acute Lung Injury (ALI): a review. Med Sci Monit. 2019;25:1828–1837. doi: 10.12659/MSM.912867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Entezari M, Javdan M, Antoine DJ, et al. Inhibition of extracellular HMGB1 attenuates hyperoxia-induced inflammatory acute lung injury. Redox Biol. 2014;2:314–322. doi: 10.1016/j.redox.2014.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu X, Xu Q, Mei L, et al. Paeonol attenuates acute lung injury by inhibiting HMGB1 in lipopolysaccharide-induced shock rats. Int Immunopharmacol. 2018;61:169–177. doi: 10.1016/j.intimp.2018.05.032 [DOI] [PubMed] [Google Scholar]
- 32.Keppler-Noreuil KM, Parker VE, Darling TN, Martinez-Agosto JA. Somatic overgrowth disorders of the PI3K/AKT/mTOR pathway & therapeutic strategies. Am J Med Genet C Semin Med Genet. 2016;172(4):402–421. doi: 10.1002/ajmg.c.31531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang CY, Deng JS, Huang WC, Jiang WP, Huang GJ. Attenuation of lipopolysaccharide-induced acute lung injury by hispolon in mice, through regulating the TLR4/PI3K/Akt/mTOR and Keap1/Nrf2/HO-1 pathways, and suppressing oxidative stress-mediated ER stress-induced apoptosis and autophagy. Nutrients. 2020;12(6):1742. doi: 10.3390/nu12061742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li N, Liu XX, Hong M, et al. Sodium butyrate alleviates LPS-induced acute lung injury in mice via inhibiting HMGB1 release. Int Immunopharmacol. 2018;56:242–248. doi: 10.1016/j.intimp.2018.01.017 [DOI] [PubMed] [Google Scholar]
- 35.Lu M, Chen C, Lan Y, et al. Capsaicin-the major bioactive ingredient of chili peppers: bio-efficacy and delivery systems. Food Funct. 2020;11(4):2848–2860. doi: 10.1039/D0FO00351D [DOI] [PubMed] [Google Scholar]
- 36.Kumar V. Pulmonary innate immune response determines the outcome of inflammation during pneumonia and sepsis-associated acute lung injury. Front Immunol. 2020;11:1722. doi: 10.3389/fimmu.2020.01722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang H, Bai Y, Gao M, et al. Hepatoprotective effect of capsaicin against concanavalin A-induced hepatic injury via inhibiting oxidative stress and inflammation. Am J Transl Res. 2019;11(5):3029–3038. [PMC free article] [PubMed] [Google Scholar]
- 38.Lee W, Yuseok O, Yang S, et al. JH-4 reduces HMGB1-mediated septic responses and improves survival rate in septic mice. J Cell Biochem. 2019;120(4):6277–6289. doi: 10.1002/jcb.27914 [DOI] [PubMed] [Google Scholar]
- 39.Yang G, Zhang L, Ma L, et al. Glycyrrhetinic acid prevents Acetaminophen-induced acute liver injury via the inhibition of CYP2E1 expression and HMGB1-TLR4 signal activation in mice. Int Immunopharmacol. 2017;50:186–193. doi: 10.1016/j.intimp.2017.06.027 [DOI] [PubMed] [Google Scholar]
- 40.Li R, Zhang J, Pan S, et al. HMGB1 aggravates lipopolysaccharide-induced acute lung injury through suppressing the activity and function of Tregs. Cell Immunol. 2020;356:104192. doi: 10.1016/j.cellimm.2020.104192 [DOI] [PubMed] [Google Scholar]
- 41.Rahman A, Fazal F. Blocking NF-κB: an inflammatory issue. Proc Am Thorac Soc. 2011;8(6):497–503. doi: 10.1513/pats.201101-009MW [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liby KT, Sporn MB. Synthetic oleanane triterpenoids: multifunctional drugs with a broad range of applications for prevention and treatment of chronic disease. Pharmacol Rev. 2012;64(4):972–1003. doi: 10.1124/pr.111.004846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu J, Sun C, Wang R, et al. Cardioprotective effect of paeonol against epirubicin-induced heart injury via regulating miR-1 and PI3K/AKT pathway. Chem Biol Interact. 2018;286:17–25. doi: 10.1016/j.cbi.2018.02.035 [DOI] [PubMed] [Google Scholar]
- 44.Matute-Bello G, Martin TR. Science review: apoptosis in acute lung injury. Crit Care. 2003;7(5):355–358. doi: 10.1186/cc1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin TR, Nakamura M, Matute-Bello G. The role of apoptosis in acute lung injury. Crit Care Med. 2003;31(4 Suppl):S184–188. doi: 10.1097/01.CCM.0000057841.33876.B1 [DOI] [PubMed] [Google Scholar]
- 46.Li J, Chen Q, He X, et al. Dexmedetomidine attenuates lung apoptosis induced by renal ischemia-reperfusion injury through α(2)AR/PI3K/Akt pathway. J Transl Med. 2018;16(1):78. doi: 10.1186/s12967-018-1455-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park SG, Yon JM, Lin C, et al. Capsaicin attenuates spermatogenic cell death induced by scrotal hyperthermia through its antioxidative and anti-apoptotic activities. Andrologia. 2017;49(5):e12656. doi: 10.1111/and.12656 [DOI] [PubMed] [Google Scholar]