Abstract
Background
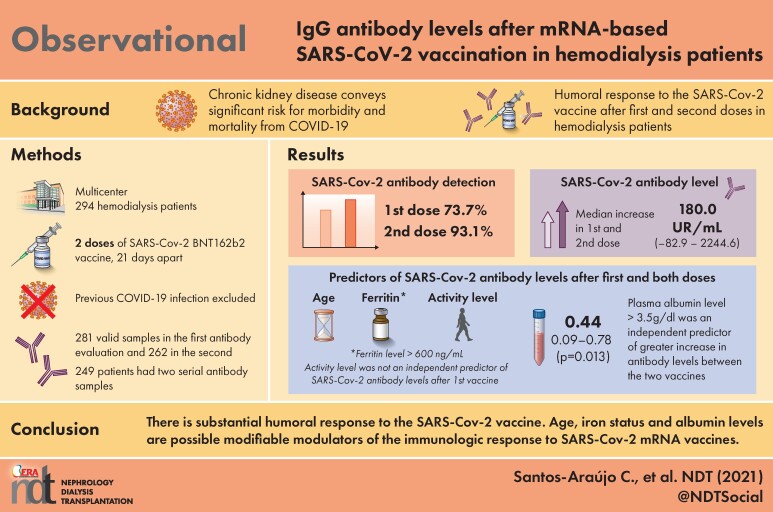
Vaccination programs are essential for the containment of the COVID-19 pandemic, which has affected hemodialysis populations especially hard. Early reports suggest a reduced immunologic response to SARS-Cov-2 vaccines in dialysis patients, in spite of a high degree of seroconversion. We aimed to identify risk factors for a reduced efficacy of an mRNA vaccine in a cohort of hemodialysis patients.
Method
In a multicenter study, including 294 Portuguese hemodialysis patients who had received 2 doses of BNT162b2 with a three week interval, IgG-class antibodies against the SARS-CoV-2 spike protein were determined 3 weeks after the first dose (M1) and 6 weeks after the second dose (M2). The threshold for seroconversion was 10UR/mL. Demographic and clinical data was retrieved from a quality registry. Adverse events were registered using a questionnaire.
Results
At M2, seroconversion was 93.1% with a median antibody level of 197.5U/mL (1.2–3237.0) and a median increase of 180.0U/mL (-82.9–2244.6) from M1. Age (beta -8.9; 95%CI: -12.88 to -4.91; P < 0.0001), ferritin > 600ng/mL (beta 183.93; 95%CI: 74.75 to 293.10; P = 0.001) and physical activity (beta 265.79; 95%CI: 30.7 to 500.88; P = 0.03) were independent predictors of SARS-Cov-2 antibody levels after two vaccine doses. Plasma albumin > 3.5g/dL independently predicted the increase of antibody levels between both doses (OR 14.72; 95%CI: 1.38 to 157.45; P = 0.03). Only mild adverse reactions were observed in 10.9% of patients.
Conclusions
The SARS-Cov-2 vaccine BNT162b2 is safe and effective in hemodialysis patients. Besides age, iron status and nutrition are possible modifiable modulators of the immunologic response to SARS-Cov-2 mRNA vaccines. This data suggests the need for an early identification of populations at higher risk for diminished antibody production and the potential advantage of the implementation of oriented strategies to maximize the immune response to vaccination in these patients.
Keywords: COVID-19, hemodialysis, SARS-Cov-2, SARS-Cov-2 vaccination
Graphical Abstract
Graphical Abstract.
Contributor Information
Carla Santos-Araújo, Diaverum AB, Malmö, Sweden; Cardiovascular Research and Development Unit, Faculty of Medicine, Porto, Portugal.
Pedro Mota Veiga, Polytechnic Institute of Viseu, School of Education, Viseu, Portugal; NECE Research Unit in Business Sciences, University of Beira Interior, Covilhã, Portugal.
Mário João Santos, Unilabs, Portugal.
Lidia Santos, Diaverum, Portugal; Department of Nephrology, Hospital and University Center of Coimbra, Portugal.
Catarina Romãozinho, Nefrovida, Hemodialysis Unit of Coimbra, Diaverum, Portugal; Department of Nephrology, Hospital and University Center of Coimbra, Portugal.
Mónica Silva, Diaverum, Portugal.
Carlos Lucas, Diaverum AB, Malmö, Sweden.
Mary Luz Duarte, Unilabs, Portugal.
Mathias Haarhaus, Diaverum AB, Malmö, Sweden; Division of Renal Medicine, Department of Clinical Sciences, Intervention and Technology, Karolinska Institutet, Karolinska University Hospital, Stockholm, Sweden.
Michael Haase, Diaverum AB, Malmö, Sweden; Medical Faculty, Otto-von-Guericke University Magdeburg, Magdeburg, Germany; Diaverum Renal Care Center, Potsdam, Germany.
Fernando Macário, Diaverum AB, Malmö, Sweden.
Supplementary Material
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.