Abstract
Background
Healthcare workers (HCWs) have an increased risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection due to occupational exposure. Strict measures generally focus on the patient-to-HCW contacts. However, interactions between the HCWs also pose a high risk for SARS-CoV-2 exposure.
Aims
This study was aimed to investigate the effect of social contacts on the level of SARS-CoV-2 exposure risk among workers by broadening the current risk assessment algorithm.
Methods
Contact tracing records of the workers in a large university hospital between 19th March and 31st December 2020 were analysed. Multivariate conditional logistic regression models were estimated to evaluate factors associated with high-risk exposure for contacts among workers.
Results
Of the 329 exposed clusters, 260 (79%) were HCW-to-HCW contacted clusters. High-risk exposure was higher in the HCW-to-HCW contacts (44%), when compared to the patient-to-HCW contacts (5%) (P < 0.001). A total of 1827 HCWs contacted a laboratory-confirmed COVID-19-positive co-worker. Among the HCW-to-HCW contacts, high-risk exposure was higher in the support staff (49%, P < 0.001), in non-patient care settings (47%, P < 0.001) and in the social contacts (57%, P < 0.001). Social contacts between workers increased the high-risk exposure (adjusted odds ratio: 3.50, 95% confidence interval 2.62–4.69) in multivariate analysis.
Conclusions
A significant association between social contacts among workers and high-risk exposure of SARS-CoV-2 was observed. The results of the study emphasize the need for policies regarding the improved protection of HCWs in social settings in addition to patient care services.
Keywords: Contact tracing, COVID-19, healthcare worker, occupational health, risk assessment, SARS-CoV-2
Key learning points.
What is already known about this subject:
Healthcare workers have an increased risk of severe acute respiratory syndrome coronavirus 2 exposure resulting from providing patient care.
A comprehensive risk assessment is required for protecting the health of healthcare workers and for the prevention of in-hospital outbreaks.
Exposure risk assessment for healthcare workers is well defined in the international and national guidelines when the index case is a known COVID-19 patient.
What this study adds:
This study recommends an algorithm that includes the social interactions between healthcare workers.
The proportion of high-risk exposure was 44% for the contacts between healthcare workers.
Social contact among the healthcare workers significantly increases high-risk exposures of severe acute respiratory syndrome coronavirus 2.
What impact this may have on practice or policy:
Exposure risk assessment should be adapted to incorporate healthcare worker to healthcare worker interactions.
Healthcare workers must be mindful of their social interactions to protect themselves from a high-risk contact with a potentially infected co-worker.
Managerial policies regarding the regulation of containment measures should be implemented in the non-patient areas of hospitals.
Introduction
Healthcare workers (HCWs) have an increased risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection due to occupational exposure [1]. The importance of HCWs in dealing with the pandemic is indisputable. In the early stages of the pandemic, it was reported that HCWs accounted for approximately 10% of the notified cases in many countries including Greece, Italy and the USA [2–4]. However, nationwide data on infected HCWs and deaths in many countries remain publicly unavailable [5]. According to a media briefing released by the Turkish Ministry of Health on coronavirus disease 2019 (COVID-19) response, in the period up to 14th October 2020 more than 40 000 HCWs had been infected, resulting in 107 deaths [6]. Within the healthcare setting, inadequacy of proper personal protective equipment (PPE), work overload and poor infection control measures are all well-known risk factors for SARS-CoV-2 infection [7–9]. For staff working in emergency rooms (ERs), the risk for infection has been shown to be considerably higher [10].
Identifying HCWs who come into contact with COVID-19 patients enables the early diagnosis of secondary cases within a specified quarantine period. It is crucial to perform a fully applicable and precise risk assessment for the SARS-CoV-2-exposed workers prior to introducing work restrictions and initiating quarantine procedures. Within the patient care setting, there are a number of strict measures that focus on this. However, while requiring assessment, the level of SARS-CoV-2 exposure for social interactions among workers, such as during eating, coffee breaks, etc., remains unclear. Furthermore, both national and international guidelines on risk assessment fail to consider the worker interactions [11]. Likewise, national COVID-19 guidelines in Turkey focus primarily on patient-to-HCW contact [12]. Due to this, institutions with the capacity to conduct in-house risk assessment are attempting to adapt their infection prevention and control policies with the integration of protocols for contacts between workers. The SARS-CoV-2 risk assessment algorithm needs to be modified to incorporate social contacts between workers. This study aims to investigate the risk of social contacts on SARS-CoV-2 exposure by introducing a risk assessment protocol for contacts among HCWs.
Methods
This retrospective cohort study included contact tracing data of the exposed workers from 19th March to 31st December 2020 in Dokuz Eylul University, Izmir, Turkey. Dokuz Eylul University is a large university hospital with approximately 4000 staff. Both outpatient and inpatient care for COVID-19 patients continues to be provided at the time of writing. Since the first confirmed patient, reported on 19th March 2020, rigorous contact tracing and risk assessment concerning the level of exposure of workers have been an ongoing practice within the institution. Exposed workers were evaluated by pre-trained public health residents under the supervision of a senior occupational health professional in conjunction with a senior epidemiologist. If the primary case was a worker, the case was interviewed via telephone and a list of potentially exposed co-workers was compiled. When the primary case was not a HCW, exposed worker lists were compiled by supervising nurses. Workers who declared contact with the COVID-19 case, but whose names were missing from the lists, were also evaluated. For symptomatic cases, the period of contact tracing covered the 2 days prior to the onset of symptoms, while for asymptomatic cases, it started 2 days prior to the first positive reverse transcriptase-polymerase chain reaction (RT-PCR) test result.
The national COVID-19 guideline suggests evaluating the exposure risk by two factors: (i) whether the patient was wearing a mask and (ii) the suitability of the worker’s mask and/or other PPE in relation to the procedure performed [12]. The level of exposure is then classified as low, medium or high risk. Exposed workers with low or medium risk were able to continue to work while being monitored for active symptoms for 14 days from the last contact. For a medium-risk-exposed worker, an RT-PCR test was scheduled for the seventh day. For any worker considered high risk, work was restricted and home quarantined implemented until RT-PCR testing on the seventh day.
The modified algorithm has been developed to assess the risk of SARS-CoV-2 transmission for contact among workers in a social setting; i.e. social contacts. Social contact has been defined as any interaction between workers outside of their specific job description. This includes contact in resting areas both in the hospital (drinking coffee/tea, eating, having a break, sharing the same breakroom) and areas out of the hospital (restaurants, house, driving work in the same car).
Three parameters were considered to assess the risks of social contacts:
Use of medical masks indicates whether the worker who tested positive for COVID-19 and his/her contacted colleague were wearing masks.
Distance indicates whether the worker who tested positive for COVID-19 and his/her contacted colleague were less than a 1-m distance apart.
Intensity indicates uncontrolled contact (handshaking etc.) and aerosol-generating situations such as coughing and sneezing. Note chatting, drinking coffee/tea or dining together for more than 15 min was considered as high intensity.
The algorithm for HCW-to-HCW interactions has been summarized in Table 1. HCWs who tested positive for COVID-19 (CP) and the exposed HCWs (P) were classed into four possible categories in terms of mask use, two categories in terms of distance and two categories in terms of exposure intensity. This resulted in 16 possible risk situations. An intensive encounter with a COVID-19 worker who was not wearing a mask within a distance closer than 1 m (conversation, handshaking, etc.) was considered as high risk. HCW-to-HCW contacts were assessed in accordance with the above-mentioned algorithm. The risk assessment was made following national guidelines for patient (non-HCW) to HCW contact. In conjunction with national guidelines, exposed workers were managed through occupational health outpatient clinics. Informative text messages were sent immediately outlining work restrictions and quarantine procedures as well as follow-up RT-PCR dates. With any symptom development, regardless of risk category, the workers were instructed to present immediately to the occupational health outpatient clinic for RT-PCR testing on the same day.
Table 1.
Level of exposure for HCW-to-HCW interactions according to the use of masks, distance and intensity
| Use of masks | >1-m distance | ≤1-m distance | ||
|---|---|---|---|---|
| Low intensity | High intensity | Low intensity | High intensity | |
| CP+ P+ | No risk | Low | Low | Low |
| CP+ P− | Low | Low | Low | Medium |
| CP− P+ | Low | Medium | Medium | High |
| CP− P− | Medium | Medium | High | High |
CP: COVID-19-positive HCW; P: exposed HCW. (+): using the mask; (−): not using the mask.
In this study, the COVID-19 case definition covered SARS-CoV-2 RT-PCR-positive cases. For the patient-to-HCW contacts, work-related contact was defined as the contact with a COVID-19 case while providing healthcare. Following this definition, any patient-to-HCW contact was interpreted as a work-related contact. If the index case was a co-worker, work-related contact included the face-to-face contacts of workers in the same occupational environment but excluded social gatherings. This included such events as clinical meetings, educational meetings, ward rounds and nursing handovers. Working in the same room, a typical issue for office workers, was also interpreted as a work-related contact for any workers not in direct contact with patients.
Professions were identified as physicians (medical doctors, dentists, pharmacists), nurses, allied healthcare personnel (laboratory technicians, dieticians, social workers, physiotherapists, anaesthetic technicians), auxiliary staff (administrative staff, secretaries, office workers) and support staff (cleaning staff, porters, repair workers, garden maintenance staff, kitchen workers, security staff). In addition to COVID-19 ERs/clinics, the place of contact was also classified as general ERs/clinics, i.e. those that were receiving new admissions or providing patient care to those initially believed not to have COVID-19. Non-patient care settings included the units where the non-clinical staff work, administration offices, social areas such as cafeterias and other areas outside of the actual hospital building. We defined a cluster as two or more workers who were exposed to the same COVID-19 case. According to the epidemiological situation of COVID-19 in Turkey, we identified three phases during the study period: a first peak period (March 2020 and April 2020), a post-peak period plus a normalization period (May 2020 to September 2020) and a second peak period (October 2020 to December 2020).
Categorical variables were compared using the chi-squared test and continuous variables were compared using Student’s t-test or one-way analysis of variance (ANOVA). The relative odds of having high-risk exposure for HCW-to-HCW contacts were estimated using multivariate conditional logistic regression, taking account of different numbers of exposed workers in the clusters. Model 1 included work-related contact, social contact, age and gender, while model 2 included model 1 variables plus profession and contact place. In the secondary analysis, we estimated the risk of becoming infected post-exposure stratified by cluster types and the level of exposure. Because unequal probabilities of getting tested lead to selection bias, we did inverse probability weighting, taking into account the difference in testing frequency between the clusters, the difference between the levels of exposure and also the difference between the symptomatic and asymptomatic workers. The secondary attack rates (%) were also calculated with their 95% confidence interval (CI). Statistical analyses and visualizations were performed in R version 4.0.2 [13,14]. A double-sided P value of less than 0.05 was considered as significant. Ethics committee approval was obtained from the Dokuz Eylul University Hospital Ethical Board (No: 2020/17-19).
Results
Between 19th March 2020 and 31st December 2020, a total of 2881 potentially exposed workers originating from 329 clusters were reported to the surveillance team. Of these, 89 failed to respond to consistent calls for status updates. A total of 247 workers declared that they had no contact with the confirmed patient. There was also incomplete data on 17 subjects. Taking this into account, a total of 2528 exposed workers were included in the study. In 260 (79%) clusters, the source was an infected co-worker. The total number of exposed HCWs was 1827 (72%) and 701 (28%) for the HCW-to-HCW clusters and the patient-to-HCW clusters, respectively.
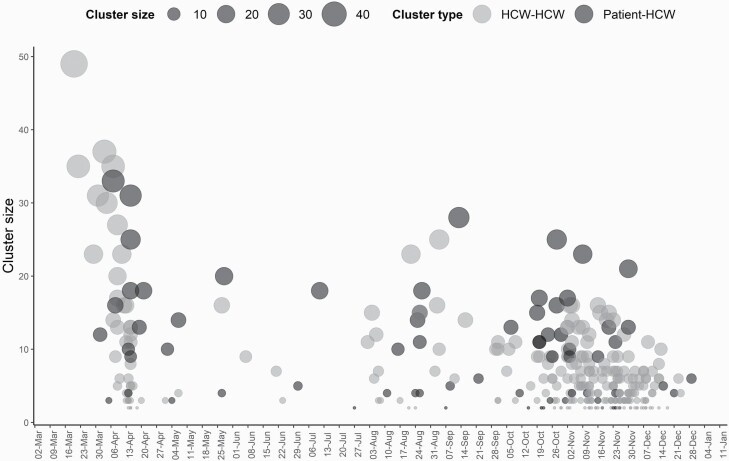
The total number of exposed HCWs within the clusters ranged from 2 to 49 (Figure 1). The median number of exposed workers was 5 (25th to 75th percentile: 3–9) and 10 (25th to 75th percentile: 4–15) for the HCW-to-HCW clusters and the patient-to-HCW clusters (P < 0.001), respectively.
Figure 1.
The number of exposed workers within the clusters by date.
Of the 2528 exposed workers, 850 (34%) were evaluated as low risk, 839 (33%) as medium risk and 839 (33%) as high risk. High-risk exposure was found to be higher in HCW-to-HCW contacts (44%, P < 0.001), in the support staff (42%, P < 0.001), in the non-patient care settings (47%, P < 0.001) as well as in social contacts (57%, P < 0.001) (Table 2).
Table 2.
Characteristics of all contacts by the exposure levels
| Exposure levels | P | |||
|---|---|---|---|---|
| Low risk (n = 850) | Medium risk (n = 839) | High risk (n = 839) | ||
| Contact type, n (%) | *** a | |||
| Patient to HCW | 327 (47) | 339 (48) | 35 (5) | |
| HCW to HCW | 523 (29) | 500 (27) | 804 (44) | |
| The date of the contact, n (%) | *** a | |||
| First peak | 270 (37) | 218 (30) | 237 (33) | |
| Post-peak/normalization | 194 (45) | 137 (32) | 98 (23) | |
| Second peak | 386 (28) | 484 (35) | 504 (37) | |
| Gender, n (%) | NSa | |||
| Female | 522 (35) | 496 (33) | 484 (32) | |
| Male | 328 (32) | 343 (33) | 355 (35) | |
| Age, mean ± SD | 36.3±9.1 | 35.5±8.6 | 34.3±8.5 | *** b |
| Profession, n (%) | *** a | |||
| Physician | 224 (33) | 230 (34) | 218 (33) | |
| Nurse | 277 (37) | 253 (34) | 218 (29) | |
| Allied healthcare personnel | 74 (37) | 76 (37) | 52 (26) | |
| Auxiliary staff | 80 (31) | 98 (37) | 83 (32) | |
| Support staff | 195 (30) | 182 (28) | 268 (42) | |
| Contact place, n (%) | *** a | |||
| COVID-19 ERs/clinics | 33 (27) | 38 (32) | 50 (41) | |
| General ERs/clinics | 580 (41) | 511 (36) | 325 (23) | |
| Non-patient care settings | 237 (24) | 290 (29) | 464 (47) | |
| Work-related contact, n (%) | *** a | |||
| Yes | 731 (43) | 598 (36) | 356 (21) | |
| No | 119 (14) | 241 (29) | 483 (57) | |
| Social contact, n (%) | *** a | |||
| Yes | 161 (13) | 364 (30) | 703 (57) | |
| No | 689 (53) | 475 (37) | 136 (10) | |
NS, not significant.
aChi-squared test.
bOne-way ANOVA.
***P < 0.001.
The characteristics of the HCW-to-HCW contacts via high-risk exposure have been summarized in Table 3. The mean age was found to be younger in high-risk contacts (34.2 ± 8.5 versus 37.3 ± 9.1, P < 0.001). High-risk contacts were higher in the support staff (49%, P < 0.001), in the non-patient care settings (47%, P < 0.001) and also in social contacts (57%, P < 0.001). A total of 1228 workers identified a social contact with an infected co-worker. From these, 839 (68%) occurred while sharing rest areas. In 694 cases (57%), the workers dined together, while 95 (8%) received exposure during a smoking break. Other contacts occurred in households (44/1228, 4%) and during car-pooling (89/1228, 7%).
Table 3.
Characteristics of the HCW-to-HCW contacts by high-risk exposure
| High-risk exposure | P | ||
|---|---|---|---|
| No (n = 1023) | Yes (n = 804) | ||
| The date of the contact, n (%) | ** a | ||
| First peak | 318 (60) | 215 (40) | |
| Post-peak/normalization | 144 (62) | 88 (38) | |
| Second peak | 561 (53) | 501 (47) | |
| Gender, n (%) | NSa | ||
| Female | 598 (57) | 458 (43) | |
| Male | 425 (55) | 346 (45) | |
| Age, mean ± SD | 37.3±9.1 | 34.2±8.5 | *** b |
| Profession, n (%) | *** a | ||
| Physician | 243 (54) | 209 (46) | |
| Nurse | 259 (56) | 201 (44) | |
| Allied healthcare personnel | 80 (63) | 47 (37) | |
| Auxiliary staff | 168 (67) | 83 (33) | |
| Support staff | 273 (51) | 264 (49) | |
| Contact place, n (%) | * a | ||
| COVID-19 ERs/clinics | 69 (58) | 50 (42) | |
| General ERs/clinics | 430 (60) | 291 (40) | |
| Non-patient care settings | 524 (53) | 463 (47) | |
| Work-related contact, n (%) | *** a | ||
| Yes | 663 (67) | 321 (33) | |
| No | 360 (43) | 483 (57) | |
| Social contact, n (%) | *** a | ||
| Yes | 525 (43) | 703 (57) | |
| No | 498 (83) | 101 (17) | |
NS, not significant.
aChi-squared test.
bStudent’s t-test.
*P < 0.05,
**P < 0.01,
***P < 0.001.
Following an adjustment for work-related contacts, age, gender and occupational variables (profession and contact place), social contact between workers was found to significantly increase the high-risk exposure in multivariate analysis (odds ratio [OR]: 3.50, 95% CI 2.62–4.69) (Table 4).
Table 4.
Multivariate conditional logistic regression estimates of ORs for the high-risk exposure within the HCW-to-HCW clusters
| Multivariate | ||
|---|---|---|
| Model 1 | Model 2 | |
| OR (95% CI) | OR (95% CI) | |
| Work-related contact (yes) | 1.10 (0.90–1.35) | 1.13 (0.92–1.39) |
| Social contact (yes) | 3.62 (2.72–4.83) | 3.50 (2.62–4.69) |
| Age (years) | 0.99 (0.98–1.01) | 0.99 (0.98–1.01) |
| Gender (male) | 0.94 (0.80–1.12) | 0.96 (0.80–1.15) |
| Contact place | ||
| General ERs/clinics | Ref. | |
| COVID-19 ERs/clinics | 0.66 (0.35–1.25) | |
| Non-patient care settings | 1.23 (0.93–1.62) | |
| Profession | ||
| Auxiliary staff | Ref. | |
| Physician | 1.20 (0.77–1.86) | |
| Nurse | 1.34 (0.88–2.04) | |
| Allied healthcare personnel | 1.34 (0.79–2.29) | |
| Support staff | 1.19 (0.79–1.80) | |
Model 1 included work-related contact, social contact, age and gender. Model 2 included work-related contact, social contact, age, gender and occupational variables (profession and contact place).
A total of 103 workers tested positive for COVID-19 during the 14-day monitoring which followed the last known exposure. Secondary analysis of the HCW-to-HCW contacts showed the secondary attack rate among high-risk exposures to be 8.5% (95% CI 6.5–10.6) (Table S1, available as Supplementary data at Occupational Medicine Online). When compared with low- to moderate-risk-exposed workers, workers with high-risk exposure were found to have an increased risk for becoming infected (OR: 4.13, 95% CI 1.94–8.79).
Discussion
This study analysed the contact tracing data of workers and the effect of social contacts on the level of SARS-CoV-2 exposure. When assessing the HCW-to-HCW clusters in late March 2020, a broader risk assessment algorithm was necessary for the evaluation of social contacts. More HCW-to-HCW clusters than patient-to-HCW clusters (260 versus 69, respectively) were evaluated during the study period. A new risk assessment algorithm which also considered social contacts facilitated a standard approach for the surveillance team.
The risk factors associated with SARS-CoV-2 infection in HCWs have been well defined in the other studies [1,7,10,15–17]. According to Eyre et al., high COVID-19 rates were observed in specific wards among the staff, even when not facing large numbers of COVID-19 patients [10]. Suárez-García et al. observed that the risk of infection did not differ between occupational exposure risk levels [17]. Both studies indirectly pointed to the role of HCW-to-HCW interactions in SARS-CoV-2 spread. A study from a tertiary care hospital in North Carolina showed unmasked exposure to a co-worker as the cause in most cases (70%) [18]. We observed a higher risk of exposure in HCW-to-HCW contacts, when compared with the patient-to-HCW contacts (44% versus 5%). Using a three-level risk classification, a nationwide study from Greece [4] found a similar high-risk exposure rate when the source of exposure was a co-worker (42%).
Workers pay attention to the appropriate PPE use when giving patient care, but less attention appears to be given to the use of PPE in worker social interactions. Early observations had already indicated the exposure risk in shared breakrooms [19] and in confined workspaces in which maintaining social distancing protocols remained elusive [20]. In this study, social contacts had a 3.50-fold increase for the high-risk exposure. Çelebi et al. stated that failure to keep a social distance from a co-worker and, similarly, consuming food within 1 m of a co-worker increased the risk of SARS-CoV-2 infection [16]. The pandemic has already been active for months, but difficulties with full implementation of protocols which ensure physical distancing among staff while eating continue to contribute to COVID-19 clusters [21]. PPE availability, universal masking, flexible sick leave and quarantining of high-risk-exposed workers have been consistent in our institution since the start of the pandemic. However, we did observe that high-risk contacts among workers were proportionally higher during the second peak when compared to the first peak (47% versus 40%). Given the extended periods of time that workers spend together, it is logical that a sense of trust develops. In conjunction with this, perceptions of risk are reduced and, unfortunately, protection protocols appear to be loosened. A warning system whereby workers are consistently reminded to maintain social distance while eating and drinking in common areas and resting areas within the hospital should be considered.
When HCW-to-HCW contacts were evaluated in terms of profession, support staff were found to have the most high-risk contacts (49%). A network-modelling study by English et al. investigated the contact patterns of the staff in the hospital settings and found that physicians had the lowest rate of contact with other workers. They found that workers, other than physicians or nurses, had the most daily contact with all other workers [22]. Support staff generally work as a group in confined spaces and non-compliance on mask use may result in excessive high-risk contacts. In a tertiary hospital in Taiwan, one infected support staff resulted in 68 exposed workers who shared the same pantry area [23]. In a multicentre study from Ghana, compliance of PPE usage in healthcare settings was found to be lower among support staff than among clinical staff [24]. Our findings confirm the importance of infection prevention and control protocol training for the support staff to increase protection awareness in their interactions with COVID-19 patients as well as their interactions with fellow workers.
High-risk exposures have a large impact on the hospital workforce as they result in, at least, 7-day work restrictions. Thus, prospectively analysis of detection aspects of COVID-19 cases within the exposed clusters was important for the operational implementation of the algorithm. In this study, the risk for becoming infected increased 4.1-fold for workers with a high-risk exposure, and a total of 103 workers were detected as positive for COVID-19 within the active monitoring period. Due to asymptomatic and pre-symptomatic transmission, these findings are confirmation that exposure risk assessments for workers need to be ongoing, and, in conjunction with quarantine and work restriction practice, can play a vital role in reducing further HCW-to-HCW transmission.
This study has some limitations. Firstly, risk assessments were based on personal statements. Differences in personal statements may lead to misclassification in risk categories. More robust contact tracing using CCTV (closed-circuit television) footage may be implemented in institutions with adequate resources. Secondly, infected workers may experience difficulty in terms of recalling the co-workers who they had contacted with. The total number of exposed workers may have actually been higher. Thirdly, we may have not been able to fully differentiate workplace infection from community-based acquisition.
In conclusion, workers must be mindful of their social interactions. High-risk exposure from co-workers, especially in the social areas, presents a threat within the hospital setting. The findings in this study provide a useful insight for hospital policy makers regarding the regulation of containment measures in non-patient areas of hospitals.
Competing interests
None declared.
Data availability
Datasets analysed in this study are not publicly available due to inferences that can be made about the personal data of healthcare workers. Please contact the corresponding author for data request.
Supplementary Material
References
- 1. Nguyen LH, Drew DA, Graham MS et al. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health 2020;5:e475–e483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. CDC COVID-19 Response Team, Burrer SL, de Perio MA, Hughes MM et al. Characteristics of health care personnel with COVID-19—United States, February 12–April 9, 2020. MMWR Morb Mortal Wkly Rep 2020;69:477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Istituto Superiore di Sanità. Sorveglianza Integrata COVID-19 in Italia. https://www.epicentro.iss.it/coronavirus/bollettino/Infografica_7aprile%20ITA.pdf (6 October 2020, date last accessed).
- 4. Maltezou HC, Dedoukou X, Tseroni M et al. SARS-CoV-2 infection in healthcare personnel with high-risk occupational exposure: evaluation of 7-day exclusion from work policy. Clin Infect Dis 2020;71:3182–3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Erdem H, Lucey DR. Healthcare worker infections and deaths due to COVID-19: a survey from 37 nations and a call for WHO to post national data on their website. Int J Infect Dis 2021;102:239–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Anadolu Agency. Türkiye’nin koronavirüsle mücadelesinde son 24 saatte yaşananlar. https://www.aa.com.tr/tr/kategorisayfasi-manset/turkiyenin-koronavirusle-mucadelesindeson-24-saatte-yasananlar/2006751 (6 October 2020, date last accessed).
- 7. Mhango M, Dzobo M, Chitungo I, Dzinamarira T. COVID-19 risk factors among health workers: a rapid review. Saf Health Work 2020;11:262–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chou R, Dana T, Buckley DI, Selph S, Fu R, Totten AM. Epidemiology of and risk factors for coronavirus infection in health care workers: a living rapid review. Ann Intern Med 2020;173:120–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ran L, Chen X, Wang Y, Wu W, Zhang L, Tan X. Risk factors of healthcare workers with coronavirus disease 2019: a retrospective cohort study in a designated hospital of Wuhan in China. Clin Infect Dis 2020;71:2218–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eyre DW, Lumley SF, O’Donnell D et al. Differential occupational risks to healthcare workers from SARS-CoV-2 observed during a prospective observational study. eLife 2020;21:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. World Health Organization. Risk Assessment and Management of Exposure of Health Care Workers in the Context of COVID-19. https://apps.who.int/iris/bitstream/handle/10665/331496/WHO-2019-nCov-HCW_risk_assessment-2020.2-eng.pdf (6 October 2020, date last accessed).
- 12. Turkish Ministry of Health. COVID-19 (SARS-CoV-2 Enfeksiyonu) Rehberi. https://covid19.saglik.gov.tr/TR-66339/temasli-takibi-salgin-yonetimi-evde-hasta-izlemi-ve-filyasyon.html (6 October 2020, date last accessed).
- 13. Subirana I, Sanz H, Vila J. Building bivariate tables: the compareGroups package for R. J Stat Softw 2014;57:1–16.25400517 [Google Scholar]
- 14. Wickham H, Averick M, Bryan J et al. Welcome to the Tidyverse. J Open Source Softw 2019;4:1686. [Google Scholar]
- 15. Lai X, Wang M, Qin C et al. Coronavirus disease 2019 (COVID-2019) infection among health care workers and implications for prevention measures in a tertiary hospital in Wuhan, China. JAMA Netw Open 2020;3:e209666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Çelebi G, Pişkin N, Çelik Bekleviç A et al. Specific risk factors for SARS-CoV-2 transmission among health care workers in a university hospital. Am J Infect Control 2020;48:1225–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Suárez-García I, Martínez de Aramayona López MJ, Sáez Vicente A, Lobo Abascal P. SARS-CoV-2 infection among healthcare workers in a hospital in Madrid, Spain. J Hosp Infect 2020;106:357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Seidelman JL, Lewis SS, Advani SD et al. Universal masking is an effective strategy to flatten the severe acute respiratory coronavirus virus 2 (SARS-CoV-2) healthcare worker epidemiologic curve. Infect Control Hosp Epidemiol 2020;41:1466–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ellsworth M, Chang M, Ostrosky-Zeichner L. Mind the gap: the hospital breakroom. Am J Infect Control 2020;48:1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Belingheri M, Paladino ME, Riva MA. Beyond the assistance: additional exposure situations to COVID-19 for healthcare workers. J Hosp Infect 2020;105:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. BWH Press Release—Brigham and Women’s Hospital. Statement for Media Regarding COVID-19 Cluster. https://www.brighamandwomens.org/about-bwh/newsroom/press-releases-detail?id=3684 (15 May 2021, date last accessed).
- 22. English KM, Langley JM, McGeer A et al. Contact among healthcare workers in the hospital setting: developing the evidence base for innovative approaches to infection control. BMC Infect Dis 2018;18:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wee LE, Sim JXY, Conceicao EP, Aung MK, Tan JY, Venkatachalam I. Containment of COVID-19 among ancillary healthcare workers: an integral component of infection control. J Hosp Infect 2020;106:392–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ashinyo ME, Dubik SD, Duti V et al. Healthcare workers exposure risk assessment: a survey among frontline workers in designated COVID-19 treatment centers in Ghana. J Prim Care Community Health 2020;11:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Datasets analysed in this study are not publicly available due to inferences that can be made about the personal data of healthcare workers. Please contact the corresponding author for data request.