Abstract
Aims
The severity of myocardial tissue damage following ST-elevation myocardial infarction (STEMI) strongly determines short- and long-term prognosis. This study explored the impact of the coronavirus disease 2019 (COVID-19) pandemic and associated public health restrictions on infarct severity.
Methods and results
STEMI patients treated with primary percutaneous coronary intervention (PCI) and included in the prospective Magnetic Resonance Imaging in Acute ST-Elevation Myocardial Infarction (MARINA-STEMI) cohort study from 2015- 2020 (n = 474) were categorized according to (i) timeframes with and without major public health restrictions in 2020, and (ii) timeframes of major public health restrictions during 2020 and during the corresponding timeframes between 2015-2019. Myocardial damage was evaluated by cardiac magnetic resonance imaging. During major public health restrictions in 2020 (n = 48), there was an increase in infarct size (22 [IQR 12-29] vs. 14 [IQR 6-23]%, P < 0.01), a higher frequency (77% vs. 52%, P < 0.01) and larger extent of microvascular obstruction (1.5 [IQR 0.1-11.4] vs. 0.2 [IQR 0.0-2.6]%, P < 0.01) and a higher rate of intramyocardial haemorrhage (56% vs. 34%, P = 0.02) as compared to the phases without major restrictions in 2020 (n = 101). These findings were confirmed in adjusted analysis and were consistent when comparing patients admitted in 2020 versus patients admitted in the “pre-pandemic” era (2015-2019). Patient characteristics were comparable between groups, except for a significantly longer total ischemia time (P < 0.01) and higher frequency of pre-PCI Thrombolysis in Myocardial Infarction (TIMI) flow 0 during times of major restrictions (P = 0.03).
Conclusion
This study provides novel mechanistic insights demonstrating a significant increase in myocardial damage in STEMI patients admitted during the COVID-19 pandemic with a temporal relation to major public health restrictions.
Keywords: Coronavirus disease 2019, ST-elevation myocardial infarction, cardiac magnetic resonance, collateral damage
Graphical Abstract
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has disrupted usual healthcare around the world. Emerging evidence indicates a substantial increase in mortality during the pandemic that cannot be attributed to COVID-19 deaths alone.1–3 Concerns have therefore arisen about the indirect impact of the pandemic, particularly for those with cardiovascular disease and urgent need for interventions.4,5 In fact, hospitalizations for acute cardiovascular conditions have declined significantly, an observation that was most evident for acute myocardial infarction.6,7 For patients suffering from ST-elevation myocardial infarction (STEMI), current data not only indicate significant reductions in admission rates and catheterization laboratory activation, but also suggest a longer delay to reperfusion, altered reperfusion strategies, as well as increased psychological stress in hospitalized individuals, which may contribute to higher mortality during the pandemic and beyond.8,9 Nevertheless, current existing studies have left several unmet gaps in knowledge, and the true indirect effect of the pandemic on STEMI outcomes is still a matter of controversy.
Cardiac magnetic resonance imaging (MRI) is the non-invasive gold standard for comprehensive myocardial tissue characterization after STEMI.10 Specifically, cardiac MRI is highly accurate in defining the infarct size as well as the severity of microvascular injury11 The latter is the consequence of failed myocardial tissue reperfusion despite a ‘successful’ primary percutaneous coronary intervention (PCI) procedure with restoration of epicardial blood flow12 and manifests clinically as microvascular obstruction (MVO) with or without intramyocardial haemorrhage (IMH). Infarct size, MVO, and IMH, revealed by cardiac MRI during the first week after STEMI, are strongly associated with worse functional recovery, heart failure events, and mortality.13–19
Using data from the ongoing prospective MARINA-STEMI (Magnetic Resonance Imaging in Acute ST-Elevation Myocardial Infarction) cohort study, we aimed to comprehensively investigate myocardial tissue damage characteristics in patients with STEMI in temporal relation to the COVID-19 pandemic and its associated public health restrictions.
Methods
Study design and population
This study analysed data from the ongoing, prospective MARINA-STEMI cohort study (NCT04113356), which includes STEMI patients treated with primary PCI at the Heart Centre of Innsbruck. The Heart Centre of Innsbruck represents the only PCI centre for a regional STEMI network in western Austria. The detailed design was published previously.20 Briefly, patients were eligible for the study if they were ≥18 years old and had a first-ever STEMI defined as having symptoms of ischaemia and ST-segment elevation of at least 0.1 mV in two contiguous extremity leads or at least 0.2 mV in two contiguous precordial leads. Patients with contraindications for cardiac MRI (estimated glomerular filtration rate <30 mL/min/1.73 m2, Killip classification higher than II at the time of cardiac MRI, pacemaker, claustrophobia, orbital foreign body, cerebral aneurysm clip, and known or suggested contrast agent allergy to gadolinium), patients with a history of previous myocardial infarction, and COVID-19-positive STEMI patients were not eligible. For this study, we analysed all STEMI patients that were included between 2015 and 2020. Data were analysed for two pre-specified groups of patients. First, the study population of the entire year 2020 was stratified and compared according to time frames with and without major public health restrictions. In Austria, three periods of major public health restrictions between 10 March and 30 April 2020, between 21 September and 6 December 2020, and between 26 December 2020 and 18 January 2021 were imposed.21 During these time periods, people were encouraged to minimize social interaction and stay at home whenever possible. Furthermore, total self-isolation and quarantining were obligated during certain periods.21 Second, we divided the study cohort into a pandemic and pre-pandemic group, where the pandemic group was defined as patients admitted during major public health restrictions in 2020 and the pre-pandemic group as patients admitted during the exact same time frames between 2015 and 2019.
The primary outcome was defined as infarct size assessed by MRI, and a comparison was made between groups. Further objectives were to analyse differences in other infarct severity characteristics (MVO and IMH), left ventricular (LV) global strain parameters, LV ejection fraction, age, sex, cardiovascular risk factors, as well as treatment and management features. Total ischaemia time was defined as the time from the onset of symptoms consistent with myocardial ischaemia (e.g. persistent chest pain) to reperfusion (wire crossing) during primary PCI. The presence of Q-waves was defined as duration >30 ms and depth >0.1 mV.22
The research ethics committee at the Medical University of Innsbruck approved the study, and the investigation was conducted following the Declaration of Helsinki. Before inclusion, all participants gave written informed consent.
Cardiac magnetic resonance imaging
All cardiac MRI scans were performed on a 1.5 Tesla scanner (Magnetom AVANTO, Siemens, Erlangen, Germany) within 1 week after STEMI. The standardized MRI protocol as well as post-processing have been described previously.20 Briefly, LV ejection fraction was assessed on short-axis (10–12 slices) cine images using breath-hold, retrospective electrocardiogram (ECG)-triggered trueFISP bright blood sequences. Feature tracking analysis was conducted as described previously.20 Based on the 16-segment model, the software algorithm calculated three-dimensional peak strains and subsequently, by averaging the according peak values of the segments, the global strain parameters: global longitudinal strain (GLS), global radial strain, and global circumferential strain. LV ejection fraction and feature tracking analysis was performed using the commercially available LV-specific CVI42 Tissue Tracking software (Circle Cardiovascular Imaging, Inc, Calgary, Canada). ECG-triggered, phase-sensitive inversion recovery sequences were used to obtain late gadolinium enhancement images 15 min after application of a 0.2 mmol/kg bolus of contrast agent (Gadovist®, Bayer, Leverkusen, Germany). Hyperenhancement was defined by a threshold of 5 standard deviations (SD) higher than the signal intensity of remote myocardium in the opposite LV myocardial segment.23 Infarct size was presented as a percentage of the entire LV myocardial mass (LVMM). MVO was defined as an area of ‘hypoenhancement’ within the infarcted territory. IMH was determined by T2* mapping and defined as a region of a hypointense core within the infarcted area with T2* reduction below 20 ms.18 Experienced observers, blinded to all clinical data, conducted the cardiac MRI measurements and analyses.
Statistical analyses
Continuous variables were presented as the median with interquartile range (IQR); categorical variables are shown as frequencies with corresponding percentages. Comparison of continuous variables was made using Mann–Whitney U-test. Categorical variables were compared by using χ2 tests. Multivariable logistic regression analysis was used to determine the independent association of major public health restrictions with infarct size and occurrence of MVO and IMH. The cut-off for large infarct size was defined as 19% according to previous data.16 All baseline characteristics and angiographic parameters shown in Table 1 were entered in univariable regression analysis. Those with a P-value <0.10 in this analysis were further entered in the corresponding multivariable regression model using the forced entry method. In order to provide a more informative summary of measures of association, odds ratios (OR) are presented for a 1 SD increase. All tests were two-sided and P-values <0.05 were considered significant. Statistical analysis was performed using IBM SPSS Statistics 27.0.1 (IBM, Armonk, NY, USA) and MedCalc Version 19.0.7 (Ostend, Belgium). The frequency of infarct size percentiles of the study cohort in relation to the COVID-19 pandemic in 2020 was calculated using Matlab® (The MathWorks, Inc., Natick, MA, USA).
Table 1.
Baseline characteristics and angiographic and magnetic resonance parameters in patients admitted during 2020
| Total population (n = 149) | No major restrictions in 2020 (n = 101) | Major restrictions in 2020 (n = 48) | P-value | |
|---|---|---|---|---|
| Baseline characteristic | ||||
| Age, years | 59 (51–66) | 57 (51–64) | 61 (53–68) | 0.11 |
| Female sex, n (%) | 32 (22) | 18 (18) | 14 (29) | 0.12 |
| Body mass index, kg/m2 | 26 (24–29) | 26 (24–29) | 26 (23–28) | 0.29 |
| Smoking, n (%) | 80 (54) | 56 (55) | 24 (50) | 0.53 |
| Hypertension, n (%) | 62 (42) | 46 (46) | 16 (33) | 0.16 |
| Hyperlipidaemia, n (%) | 75 (50) | 53 (53) | 22 (46) | 0.45 |
| Diabetes mellitus, n (%) | 9 (6) | 6 (6) | 3 (6) | 0.94 |
| Renal insufficiency, n (%) | 12 (8) | 8 (8) | 4 (8) | 0.93 |
| Heart rate, b.p.m. | 77 (67–90) | 78 (68–90) | 73 (63–90) | 0.27 |
| Systolic blood pressure, mmHg | 135 (116–153) | 131 (113–151) | 143 (118–158) | 0.15 |
| Diastolic blood pressure, mmHg | 81 (73–91) | 80 (71–91) | 86 (75–94) | 0.17 |
| TIMI risk score | 3 (1–4) | 3 (1–4) | 3 (1–5) | 0.77 |
| Admission Killip class | 0.46 | |||
| 1 | 100 (67) | 68 (67) | 32 (67) | |
| 2 | 47 (31) | 32 (32) | 15 (31) | |
| 3 | 1 (1) | 0 (0) | 1 (2) | |
| 4 | 1 (1) | 1 (1) | 0 (0) | |
| Total ischaemia time, min | 203 (141–405) | 188 (119–381) | 263 (170–531) | <0.01 |
| Door to reperfusion time, min | 14 (7–43) | 12 (7–41) | 20 (7–45) | 0.51 |
| Periprocedural therapy, n (%) | ||||
| Aspirin | 149 (100) | 101 (100) | 48 (100) | – |
| P2Y12 inhibitors | 149 (100) | 101 (100) | 48 (100) | – |
| Clopidogrel | 18 (12) | 9 (9) | 9 (19) | 0.09 |
| Prasugrel, ticagrelor | 131 (88) | 92 (91) | 39 (81) | 0.09 |
| Heparin | 149 (100) | 101 (100) | 48 (100) | – |
| Glycoprotein IIb/IIIa inhibitors | 10 (7) | 8 (8) | 2 (4) | 0.39 |
| Discharge medication, n (%) | ||||
| Aspirin | 147 (99) | 100 (99) | 47 (98) | 0.59 |
| P2Y12 inhibitors | 147 (99) | 99 (98) | 48 (100) | 0.33 |
| Clopidogrel | 23 (15) | 13 (13) | 10 (21) | 0.21 |
| Prasugrel, ticagrelor | 124 (83) | 86 (85) | 38 (79) | 0.36 |
| Beta-blockers | 137 (92) | 94 (93) | 43 (90) | 0.47 |
| ACE inhibitors/AT-1 antagonists | 142 (95) | 96 (95) | 46 (96) | 0.83 |
| Statins | 149 (100) | 101 (100) | 48 (100) | – |
| Angiographic parameters | ||||
| TIMI flow 0 pre-pPCI, n (%) | 93 (62) | 57 (56) | 36 (75) | 0.03 |
| TIMI flow 3 post-pPCI, n (%) | 133 (89) | 93 (92) | 40 (83) | 0.11 |
| Culprit lesion, n (%) | 0.13 | |||
| RCA | 65 (44) | 47 (46) | 18 (38) | |
| Segment 1 | 27 (18) | 21 (21) | 6 (13) | |
| Segment 2 | 22 (15) | 16 (15) | 6 (13) | |
| Segment 3 | 12 (8) | 7 (7) | 5 (10) | |
| Segment 4 | 4 (3) | 3 (3) | 1 (2) | |
| LAD | 64 (43) | 38 (38) | 26 (54) | |
| Segment 6 | 37 (25) | 23 (23) | 14 (29) | |
| Segment 7 | 22 (15) | 12 (12) | 10 (21) | |
| Segment 8 | 2 (1) | 1 (1) | 1 (2) | |
| Segment 9 | 2 (1) | 1 (1) | 1 (2) | |
| Segment 10 | 1 (1) | 1 (1) | 0 (0) | |
| LCX | 20 (13) | 16 (16) | 4 (8) | |
| Segment 11 | 14 (9) | 12 (12) | 2 (4) | |
| Segment 12 | 2 (1) | 1 (1) | 1 (2) | |
| Segment 13 | 4 (3) | 3 (3) | 1 (2) | |
| Number of affected vessels, n (%) | 0.98 | |||
| 1 | 83 (56) | 56 (55) | 27 (56) | |
| 2 | 43 (29) | 29 (29) | 14 (29) | |
| 3 | 23 (15) | 16 (16) | 7 (15) | |
| Thrombectomy, n (%) | 2 (1) | 2 (2) | 0 (0) | 0.33 |
| Stenting, n (%) | 147 (99) | 99 (98) | 48 (100) | 0.33 |
| Number of stents | 2 (1-3) | 2 (1-3) | 2 (1-3) | 0.46 |
| Multivessel acute PCI, n (%) | 28 (19) | 19 (19) | 9 (19) | 0.99 |
| MRI parameters | ||||
| Time to MRI, days | 4 (3–5) | 4 (3–5) | 4 (3–6) | 0.20 |
| Transmurality, n (%) | 0.01 | |||
| 0–75% | 38 (26) | 32 (32) | 6 (13) | |
| 75–100% | 111 (74) | 69 (68) | 42 (88) | |
| Infarct size, % LVMM | 16 (9–25) | 14 (6–23) | 22 (12–29) | <0.01 |
| MVO, n (%) | 89 (60) | 52 (52) | 37 (77) | <0.01 |
| MVO, % LVMM | 0.6 (0.0–3.5) | 0.2 (0.0–2.6) | 1.5 (0.1–11.4) | <0.01 |
| IMH, n (%) | 56 (41) | 34 (34) | 22 (56) | 0.02 |
| LVEF, % | 49 (41–56) | 50 (45–56) | 46 (35–54) | <0.01 |
| LVEF ≤35%, n (%) | 18 (12) | 6 (6) | 12 (25) | <0.01 |
| LVEF ≤40%, n (%) | 32 (22) | 14 (14) | 18 (38) | <0.01 |
| LV GLS, % | –9 (–11 to –7) | –9 (–11 to –7) | –8 (–10 to –6) | 0.04 |
| LV GRS, % | 24 (19–29) | 23 (20–29) | 21 (16–29) | 0.11 |
| LV GCS, % | –15 (–17 to –12) | –15 (–17 to –13) | –14 (–16 to –10) | 0.03 |
MRI, magnetic resonance imaging; ACE angiotensin-converting enzyme; TIMI, Thrombolysis in Myocardial Infarction; pPCI, primary percutaneous coronary intervention; RCA, right coronary artery; LAD, left anterior descending artery; LCX left circumflex artery; RI ramus intermedius; LVMM, left ventricular myocardial mass; MVO, microvascular obstruction; IMH, intramyocardial haemorrhage; LV, left ventricular; LVEF, left ventricular ejection fraction; GLS, global longitudinal strain; GRS, global radial strain; GCS, global circumferential strain.
Results
Study population and baseline characteristics
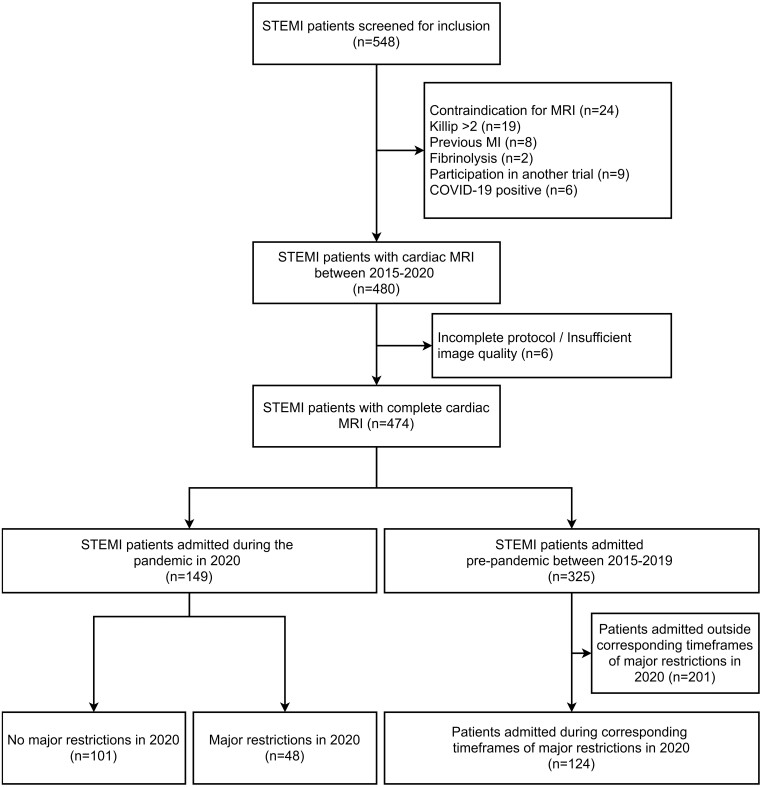
A total of 548 patients were evaluated for MARINA-STEMI between 2015 and 2020. After exclusion of 74 patients (13.5%), a final cohort of 474 patients was analysed. A flow chart of the study population including the number of excluded patients and the reason for exclusion is provided in Figure 1. A comparison of the main patient characteristics between excluded (n = 74) and included (n = 474) patients did not reveal significant differences between groups (data not shown). Of the patients included in 2020 (n = 149), 48 (32%) were admitted during time frames of major public health restrictions and 101 (68%) outside such time frames. A total of 124 patients were included during the corresponding time frames (for major restrictions in 2020) between 2015 and 2019. Characteristics of patients are reported in Tables 1 and 2. The patient profile did not differ significantly between groups in terms of age, sex distribution, and prevalence of cardiovascular risk factors (all P > 0.05). Patients presenting during major public healthcare restrictions had significantly longer total ischaemia times [263 (IQR 170–531) vs. 188 (IQR 119–381) min, P < 0.01] with no differences in door to reperfusion times (P = 0.51). This was also evident when comparing patients admitted in 2020 vs. patients admitted between 2015 and 2019. Furthermore, Q-waves on admission ECG were significantly more frequent in patients presenting during major public health restrictions vs. patients presenting outside such restrictions in 2020 (55% vs. 34%, P = 0.01). Similarly, the frequency of admission Q-waves was significantly higher in patients admitted during major public health restrictions in 2020 as compared with patients admitted during the corresponding time frames in 2015–2019 (55% vs. 34%, P = 0.01).
Figure 1.
Flow diagram of the study cohort. STEMI, ST-elevation myocardial infarction; MRI, magnetic resonance imaging; MI, myocardial infarction; COVID-19, coronavirus disease 2019.
Table 2.
Baseline characteristics and angiographic and magnetic resonance parameters in patients admitted between 2015–2019 and 2020
| Total population (n = 172) | Pre-pandemic 2015–2019 (n = 124) | Pandemic, 2020 (n = 48) | P-value | |
|---|---|---|---|---|
| Baseline characteristic | ||||
| Age, years | 58 (51–68) | 58 (50–68) | 61 (53–68) | 0.26 |
| Female sex, n (%) | 36 (21) | 22 (18) | 14 (29) | 0.10 |
| Body mass index, kg/m2 | 26 (24–28) | 26 (24–28) | 26 (23–28) | 0.57 |
| Smoking, n (%) | 93 (54) | 69 (56) | 24 (50) | 0.51 |
| Hypertension, n (%) | 68 (40) | 52 (42) | 16 (33) | 0.30 |
| Hyperlipidaemia, n (%) | 91 (53) | 69 (56) | 22 (46) | 0.25 |
| Diabetes mellitus, n (%) | 14 (8) | 11 (9) | 3 (6) | 0.57 |
| Renal insufficiency, n (%) | 14 (8) | 10 (8) | 4 (8) | 0.98 |
| Heart rate, b.p.m. | 73 (62–88) | 74 (61–87) | 73 (63–90) | 0.61 |
| Systolic blood pressure, mmHg | 140 (118–160) | 140 (115–160) | 143 (118–158) | 0.90 |
| Diastolic blood pressure, mmHg | 85 (74–100) | 85 (72–100) | 86 (75–94) | 0.91 |
| TIMI risk score | 3 (1–4) | 3 (1–4) | 3 (1–5) | 0.47 |
| Admission Killip class | 0.27 | |||
| 1 | 116 (67) | 84 (68) | 32 (67) | |
| 2 | 55 (32) | 40 (32) | 15 (31) | |
| 3 | 1 (1) | 0 (0) | 1 (2) | |
| 4 | 0 (0) | 0 (0) | 0 (0) | |
| Total ischaemia time, min | 202 (132–354) | 184 (124–314) | 263 (170–531) | <0.01 |
| Door to reperfusion time, min | 21 (7–45) | 22 (7–45) | 20 (7–45) | 0.90 |
| Periprocedural therapy, n (%) | ||||
| Aspirin | 172 (100) | 124 (100) | 48 (100) | – |
| P2Y12 inhibitors | 170 (99) | 122 (98) | 48 (100) | 0.38 |
| Clopidogrel | 37 (22) | 28 (22) | 9 (19) | 0.58 |
| Prasugrel, ticagrelor | 133 (77) | 94 (76) | 39 (81) | 0.44 |
| Heparin | 171 (99) | 123 (99) | 48 (100) | 0.53 |
| Glycoprotein IIb/IIIa inhibitors | 27 (16) | 25 (20) | 2 (4) | 0.01 |
| Discharge medication, n (%) | ||||
| Aspirin | 169 (98) | 122 (98) | 47 (98) | 0.83 |
| P2Y12 inhibitors | 171 (99) | 123 (99) | 48 (100) | 0.53 |
| Clopidogrel | 31 (18) | 21 (17) | 10 (21) | 0.55 |
| Prasugrel, ticagrelor | 140 (81) | 102 (82) | 38 (79) | 0.55 |
| Beta-blockers | 159 (92) | 116 (94) | 43 (90) | 0.38 |
| ACE inhibitors/AT-1 antagonists | 165 (96) | 119 (96) | 46 (96) | 0.97 |
| Statins | 172 (100) | 124 (100) | 48 (100) | – |
| Angiographic parameters | ||||
| TIMI flow 0 pre-pPCI, n (%) | 120 (70) | 84 (68) | 36 (75) | 0.35 |
| TIMI flow 3 post-pPCI, n (%) | 152 (88) | 112 (90) | 40 (83) | 0.20 |
| Culprit lesion, n (%) | 0.35 | |||
| RCA | 58 (34) | 40 (32) | 18 (38) | |
| Segment 1 | 26 (15) | 20 (16) | 6 (13) | |
| Segment 2 | 21 (12) | 15 (12) | 6 (13) | |
| Segment 3 | 10 (6) | 5 (4) | 5 (10) | |
| Segment 4 | 1 (1) | 0 (0) | 1 (2) | |
| LAD | 86 (50) | 60 (48) | 26 (54) | |
| Segment 6 | 53 (30) | 39 (31) | 14 (29) | |
| Segment 7 | 25 (15) | 15 (12) | 10 (21) | |
| Segment 8 | 4 (2) | 3 (2) | 1 (2) | |
| Segment 9 | 3 (2) | 2 (2) | 1 (2) | |
| Segment 10 | 1 (1) | 1 (1) | 0 (0) | |
| LCX | 26 (15) | 22 (18) | 4 (8) | |
| Segment 11 | 14 (8) | 12 (10) | 2 (4) | |
| Segment 12 | 4 (2) | 3 (2) | 1 (2) | |
| Segment 13 | 7 (4) | 6 (5) | 1 (2) | |
| Segment 15 | 1 (1) | 1 (1) | 0 (0) | |
| RI | 2 (1) | 2 (2) | 0 (0) | |
| Segment 16 | 2 (1) | 2 (2) | 0 (0) | |
| Number of affected vessels, n (%) | 0.59 | |||
| 1 | 107 (62) | 80 (64) | 27 (56) | |
| 2 | 42 (25) | 28 (23) | 14 (29) | |
| 3 | 23 (13) | 16 (13) | 7 (15) | |
| Thrombectomy, n (%) | 34 (20) | 34 (27) | 0 (0) | <0.01 |
| Stenting, n (%) | 172 (100) | 124 (100) | 48 (100) | – |
| Number of stents | 1 (1–2) | 1 (1–2) | 2 (1–3) | 0.01 |
| Multivessel acute PCI, n (%) | 23 (13) | 14 (11) | 9 (19) | 0.20 |
| MRI parameters | ||||
| Time to MRI, days | 4 (3–5) | 4 (3–5) | 4 (3–6) | 0.53 |
| Transmurality, n (%) | 0.17 | |||
| 0–75% | 33 (19) | 27 (22) | 6 (13) | |
| 75–100% | 139 (81) | 97 (78) | 42 (88) | |
| Infarct size, % LVMM | 18 (11–27) | 16 (9–24) | 22 (12–29) | 0.02 |
| MVO, n (%) | 103 (60) | 66 (53) | 37 (77) | <0.01 |
| MVO, % LVMM | 0.6 (0.0–2.8) | 0.3 (0.0–1.8) | 1.5 (0.1–11.4) | <0.01 |
| IMH, n (%) | 60 (40) | 38 (35) | 22 (56) | 0.02 |
| LVEF, % | 50 (40–56) | 50 (43–57) | 46 (35–54) | 0.01 |
| LVEF ≤35%, n (%) | 23 (13) | 11 (9) | 12 (25) | <0.01 |
| LVEF ≤40%, n (%) | 41 (24) | 23 (19) | 18 (38) | <0.01 |
| LV GLS, % | –11 (–13 to –8) | –12 (–14 to –10) | –8 (–10 to –6) | <0.01 |
| LV GRS, % | 25 (19–31) | 25 (19–32) | 21 (16–29) | 0.04 |
| LV GCS, % | –14 (–16 to –12) | –14 (–16 to –12) | –14 (–16 to –10) | 0.43 |
MRI, magnetic resonance imaging; ACE, angiotensin-converting enzyme; TIMI, Thrombolysis in Myocardial Infarction; pPCI, primary percutaneous coronary intervention; RCA, right coronary artery; LAD, left anterior descending artery; LCX, left circumflex artery; LVMM, left ventricular myocardial mass; MVO, microvascular obstruction; IMH, intramyocardial haemorrhage; LV, left ventricular; LVEF, left ventricular ejection fraction; GLS, global longitudinal strain; GRS, global radial strain; GCS, global circumferential strain.
Periprocedural therapy and angiographic characteristics
Periprocedural therapy and angiographic characteristics are shown in Tables 1 and 2. Patients who presented during time frames of major public health restrictions had a significantly higher occurrence of pre-PCI TIMI flow 0 [n = 36 (75%) vs. n = 57 (56%), P = 0.03], while this difference was not noted when comparing the pre-pandemic vs. pandemic groups. All other characteristics were comparable between groups.
Cardiac magnetic resonance imaging characteristics
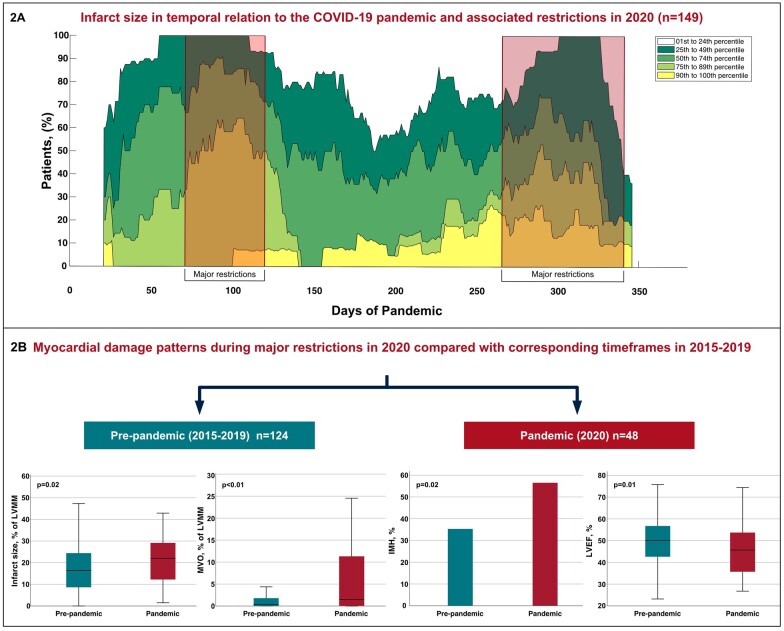
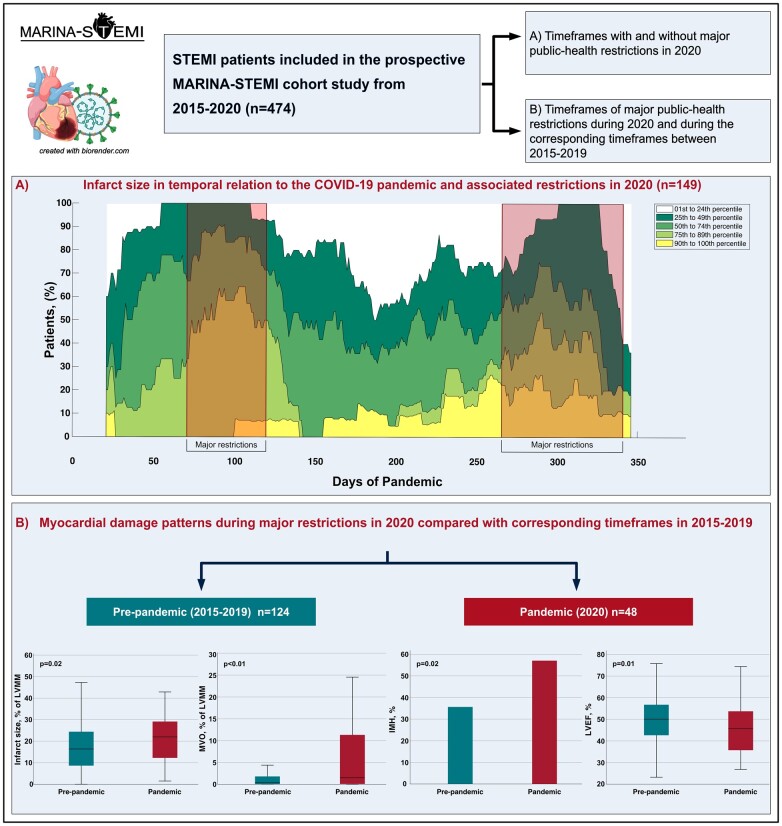
All patients underwent cardiac MRI at 4 (IQR 3–5) days after PCI, with no significant difference between groups (both P > 0.05). Cardiac MRI characteristics are summarized in Tables 1 and 2. Patients admitted during periods of major public health restrictions in 2020 had a significantly larger infarct size [22 (IQR 12–29) vs. 14 (IQR 6–23)% of LVMM, P < 0.01] in comparison with patients admitted outside major restrictions. The temporal association between infarct size and COVID-19 restrictions during the entire year 2020 is further illustrated in Figure 2A, which shows the frequency of infarct size percentiles of the study cohort in temporal relation to the COVID-19 pandemic in 2020. Moreover, a higher frequency [n = 37 (77%) vs. n = 52 (52%), P < 0.01] and larger extent of MVO [1.5 (IQR 0.1–11.4) vs. 0.2 (IQR 0.0–2.6)% of LVMM, P < 0.01] was observed. IMH was also more commonly detected in these patients [n = 22 (56%) and n = 34 (34%), respectively; P = 0.02] (Supplementary Material Online, Figure S3). In an exploratory analysis, we further compared the infarct size between the three different time periods of major restrictions in 2020, and observed a clear trend of a decline in infarct size as the pandemic progresses (Supplementary Material Online, Figure S4). The association of major public health restrictions with infarct size as well as microvascular injury was confirmed in multivariable analysis after adjustment for confounding factors including patient characteristics and angiographic parameters [infarct size: OR 1.46, 95% confidence interval (CI) 1.02–2.10, P = 0.04; MVO: OR 1.60, 95% CI 1.07–2.38, P = 0.02; IMH: OR 1.48, 95% CI 1.01–2.18, P = 0.04] (Table 3). Also a significantly higher GLS [–8 (IQR –10 to –6) vs. –9 (IQR –11 to –7), P = 0.04] and lower LV ejection fraction [46 (IQR 35–54) vs. 50 (IQR 45–56)%, P < 0.01] was observed.
Figure 2.
Impact of the COVID-19 pandemic on cardiac magnetic resonance features in patients with ST-elevation myocardial infarction. (A) Frequency of infarct size percentiles of the study cohort in relation to the COVID-19 pandemic in 2020. Red boxes indicate time frames of major public health restrictions. To obtain a sufficient signal to noise ratio, a sliding window of 40 days has been applied (i.e. each case is expanded by a 20-day time span on the left and right x-axis). Please note that a sliding window adjustment results in an incomplete presentation of the first and last 20 days of the year. (B) Boxplots and bar chart illustrate cardiac magnetic resonance features in STEMI patients admitted during time frames of major public health restrictions in 2020 compared with patients admitted during corresponding time frames in 2015-2019. Abbreviations: COVID-19, coronavirus-disease 2019; LVMM, left ventricular myocardial mass; MVO, microvascular obstruction; IMH, intramyocardial haemorrhage; LVEF, left ventricular ejection fraction.
Table 3.
Logistic regression analysis for the prediction of infarct size and microvascular injury
| Univariable |
Multivariable |
|||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Model A: large infarct size | ||||
| Admission Killip class | 1.40 (1.02–1.93) | 0.04 | – | – |
| Total ischaemia time | 1.42 (1.04–1.93) | 0.03 | – | – |
| TIMI flow 0 pre-pPCI | 1.60 (1.14–2.26) | <0.01 | – | – |
| Number of affected vessels | 0.72 (0.51–1.02) | 0.06 | – | – |
| Major restrictions | 1.57 (1.12–2.20) | 0.01 | 1.46 (1.02–2.10) | 0.04 |
| Model B: occurrence of MVO | ||||
| Diastolic blood pressure | 1.36 (0.95–1.96) | 0.1 | – | – |
| TIMI flow 0 pre-pPCI | 1.82 (1.31–2.54) | <0.01 | 1.82 (1.28–2.59) | <0.01 |
| Culprit lesion | 1.36 (0.94–1.97) | 0.1 | – | – |
| Major restrictions | 1.75 (1.20–2.55) | <0.01 | 1.60 (1.07–2.38) | 0.02 |
| Model C: occurrence of IMH | ||||
| Admission Killip class | 1.38 (0.99–1.92) | 0.06 | – | – |
| TIMI flow 0 pre-pPCI | 1.86 (1.29–2.67) | <0.01 | 1.77 (1.22–2.56) | <0.01 |
| Major restrictions | 1.16 (1.08–2.24) | 0.02 | 1.48 (1.01–2.18) | 0.04 |
Abbreviations: OR, odds ratio; CI, confidence interval; TIMI, Thrombolysis in Myocardial Infarction; pPCI, primary percutaneous coronary intervention; MVO, microvascular obstruction; IMH, intramyocardial haemorrhage.
A significantly larger infarct size [22 (IQR 12–29) vs. 16 (IQR 9–24]% of LVMM, P = 0.02] and larger extent of MVO [1.5 (IQR 0.1–11.4) vs. 0.3 (IQR 0.0–1.8)% of LVMM, P < 0.01], with a higher frequency of MVO [n = 37 (77%) vs. n = 66 (53%), P < 0.01] and IMH [n = 22 (56%) vs. n = 38 (35%), P = 0.02] was also observed in patients admitted during the pandemic compared with ‘pre-pandemic’ times. A significantly higher GLS [–8 (IQR –10 to –6) vs. –12 (IQR –14 to –10), P < 0.01] and lower LV ejection fraction among patients admitted during the pandemic was found [46 (IQR 35–54) vs. 50 (IQR 43–57)%, P = 0.01] (Figure 2B). MRI parameters in pre-pandemic patients admitted during corresponding timeframes of major restrictions in 2020 and during timeframes of no major restrictions in 2020 did not reveal significant differences between groups (Supplementary Material Online, Table S4). Furthermore, no significant difference in clinical characteristics and primary MRI findings in pre-pandemic patients in relation to times of major restrictions in 2020 was observed (Supplementary Material Online, Table S5).
Discussion
This analysis of STEMI patients enrolled in the prospective MARINA-STEMI cohort study describes novel mechanistic insights regarding the impact of the COVID-19 pandemic and associated restrictions on myocardial tissue damage. We observed a larger infarct size, more extensive MVO, and a higher rate of IMH as well as a lower GLS and LV ejection fraction during times of severe public health restrictions due to COVID-19 waves in 2020. These findings were consistent when comparing STEMI patients admitted during times with major COVID-19 burden in 2020 with patients admitted during the same times between 2015 to 2019 (Graphical Abstract). Given that myocardial infarct severity determined by cardiac MRI strongly determines short- and long-term prognosis after STEMI, these findings may not only explain a part of the rise in cardiovascular deaths4 during the pandemic, but also suggest significant cardiac collateral damage for the future.
Graphical Abstract.
STEMI remains a significant cause of morbidity and mortality worldwide, mandating ongoing efforts to provide prompt recognition, uninterrupted emergency care, as well as structured follow-up and long-term management. While multilevel strategies for pandemic control limited the direct impact of COVID-19, there is increasing concern that the pandemic and associated restrictions have discontinued this continuum of care for patients suffering an acute myocardial infarction, with potentially devastating consequences.4,6–8,24,25 The so-far largest registry evaluating the impact of COVID-19 on the treatment and outcome of patients with STEMI was published by De Luca et al.8 This analysis found a higher mortality rate in STEMI patients admitted during the COVID-19 pandemic, probably reflected by the longer ischaemia time related to treatment, while other patient characteristics were similar to those of the control group in 2019. However, while others described comparable findings.24,26,27 there have also been reports questioning a true indirect impact of COVID-19 on STEMI outcomes.28,29 These studies are, however, limited by small sample sizes for clinical outcome evaluation, outcomes were often not systematically reported, missing data were frequent, and observation times were restricted to a few weeks or months in 2020. Moreover, in some studies, patients admitted during the pandemic were younger, and had fewer risk factors and comorbidities, which may have confounded outcome data.29 The lack of cardiac MRI data for a detailed in vivo infarct severity assessment is another important limitation of the current literature. The present study therefore provides unique mechanistic insights into this issue by reporting for the first time on the impact of COVID-19 and associated restrictions on myocardial damage as determined by state-of-the-art cardiac MRI in STEMI patients treated within a STEMI network with a catchment area of ∼0.8 million people. The main finding is a marked increase in infarct size (∼6–8%) in patients admitted during times of high COVID-19 burden and associated major government restrictions in 2020. The impact of COVID-19 was seen throughout 2020, as the mean extent of myocardial damage tended to be greater in that year than in previous years. Nevertheless, the increase in myocardial tissue damage was mainly driven by those patients presenting during times of severe COVID-19 burden. At the same time, there was a trend for a decreasing effect of restrictions on infarct size during the pandemic. Infarct size as measured by cardiac MRI is known to be a principal determinant for worse functional recovery, and ultimately poor clinical outcome.13,16 Stone et al. demonstrated that every 5% increase in infarct size contributes to a 19% increase in 1-year all-cause mortality.13 The study also showed that the influence of prolonged ischaemia time on mortality30 may be mainly affected through increased infarct size.13 As such, the increase in total ischaemia time during the COVID-19 pandemic observed in previous studies.8,9, and re-confirmed in our investigation, might be the main explanation for the findings of increased infarct size. Moreover, patients presenting during times of major restrictions more often exhibited Q-waves on their baseline ECG. These findings further support the observation of a distinct clinical profile that portends more advanced disease in these patients.31 In contrast, we observed no significant differences in age, sex, cardiovascular risk factors, and periprocedural antithrombotic management strategies between STEMI groups which could also have explained our observations. Another strength of our study is that it evaluates, for the first time, the entire year 2020 and compares these findings with a long pre-pandemic period (2015–2019).
In the present study, the cardiac MRI examination was performed within the first days after STEMI, and therefore provides pathophysiological insights not only into the effect of COVID-19 on infarct size but also on markers of severe reperfusion injury (MVO and IMH). It is well known that successful restoration of epicardial blood flow through PCI does not guarantee adequate myocardial reperfusion. We observed a similar rate of post-PCI TIMI flow 3 between groups, but a higher rate of MVO and IMH in STEMI patients who presented during times of major COVID-19 burden. At the same time, a significantly higher rate of pre-PCI TIMI flow 0 among patients admitted during time frames of major public health restrictions was noted. Pre-PCI TIMI flow 0 strongly correlates with mortality after primary PCI.32 This association is independent of infarct size by cardiac MRI13, but might be explained through higher rates of MVO and IMH. In fact, both tissue markers of severe reperfusion injury are increasingly recognized as key prognostic biomarkers after STEMI, even after accounting for infarct size by cardiac MRI.15,18 While the frequency of MVO in our study during ‘non-COVID’ times (53%) is very comparable with a recent analysis of 1688 post-myocardial infarction patients (measured by cardiac MRI at a median of 3 days post-myocardial infarction) 15, the frequency during the investigated time frames of the COVID pandemic (77%) is much higher than expected. We observed a very similar picture for the incidence of IMH as well. Importantly, the higher rate of IMH observed in our study is also of particular concern since recent data demonstrated that: (i) persistent iron within the infarct core is common (∼3 in 5) in patients with IMH during the early stage after STEMI; (ii) persistent iron is associated with prolonged proinflammatory burden and is predictive of adverse cardiac remodelling and worsening function; and (iii) persistent iron is strongly associated with all-cause death or heart failure and major adverse cardiac events in the longer term.33
Given the steep increase in cardiac MRI features of myocardial tissue damage, these findings suggest the presence of significant cardiac collateral damage caused by the COVID-19 pandemic and associated restrictions. The worse LV function as reflected by lower LV ejection fraction and GLS values further underscores this notion.
Limitations
The present study is observational, and thus we cannot conclude that the reported associations are causative. Moreover, these data derived from a STEMI cohort who survived the acute phase of infarction and were stable enough to undergo cardiac MRI within the first week after infarction. Although this represents the majority of the STEMI population, our findings are not generalizable to unstable patients (e.g. cardiogenic shock) or patients with out-of-hospital cardiac arrests. Based on the study design, a survivor bias cannot be excluded. This study represents a reasonably large STEMI cohort undergoing systematic MRI scans; however, the sample size is still limited and further confirmation would be desirable. Hospitalizations due to STEMI have declined significantly during certain phases of the pandemic.6,7, an observation that is unlikely to reflect a true reduction in the incidence of myocardial infarction. This would be consistent with studies indicating that death at home and out-of-hospital cardiac arrests have increased substantially during the pandemic, particularly in areas most affected by COVID-19.1,2,5,29 Therefore, our analysis could have even underestimated the true impact of COVID-19 on myocardial damage since infarcts may be even larger in those who died before assessment or those who refrained from seeking emergent treatment. Although representing only a minority of STEMI patients,34 COVID-19-positive STEMI patients were not enrolled in this study. Other studies, however, have recently demonstrated an even worse outcome in COVID-19-positive STEMI patients.35 This ‘high risk’ subgroup should be investigated in dedicated studies. Total ischaemia time was longer during the COVID-19 pandemic in our study, with no differences in door to reperfusion times. However, data on more details regarding different components of total ischaemia time (such as patient-related delays or emergency medical system-related delays) were not available in our cohort and warrant further investigation. In the present analysis, we focused on established MRI-based myocardial tissue damage parameters, whereas T2-weighted oedema imaging was not included, mainly due to existing controversies regarding the validity and clinical significance of this sequence. The single-centre design represents another limitation. Although the pandemic had a negative impact on healthcare systems worldwide, it should be noted that they were affected differently (including differences in the timing and extent of restrictions imposed), which may limit the generalizability of our results.
Conclusion
This cardiac MRI study provides novel mechanistic data that show a significant increase in infarct size, MVO and IMH in STEMI patients admitted during the COVID-19 pandemic with a temporal relationship to major public health restrictions. While further investigation is necessary for a better understanding of the full impact of COVID-19 for patients with STEMI, these data suggest worse short- and long-term outcomes in these patients.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the Austrian Science Fund (FWF): KLI 772-B (BM), the Tiroler Wissenschaftsfonds, and by the Austrian Society of Cardiology.
Conflict of interest: The Authors declare that there is no conflict of interest.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Baldi E, Sechi GM, Mare C, Canevari F, Brancaglione A, Primi R, Klersy C, Palo A, Contri E, Ronchi V, Beretta G, Reali F, Parogni P, Facchin F, Rizzi U, Bussi D, Ruggeri S, Oltrona Visconti L, Savastano S, Compagnoni S, Fracchia R, Cuzzoli A, Pagliosa A, Matiz G, Russo A, Vecchi AL, Fantoni C, Fava C, Franzosi C, Vimercati C, Franchi D, Storti E, Taravelli E, Giovenzana F, Buetto G, Garzena G, Iotti GA, Villa GF, Botteri M, Caico SI, Cominesi IR, Carnevale L, Caresani M, Luppi M, Migliori M, Centineo P, Genoni P, Bertona R, De Ponti R, Osti R, Buratti S, Danzi GB, Marioni A, De Pirro A, Molinari S, Sgromo V, Musella V, Paglino M, Mojoli F, Lusona B, Pagani M, Curti M, Compagnoni S, Fracchia R, Cuzzoli A, Pagliosa A, Matiz G, Russo A, Vecchi AL, Fantoni C, Fava C, Franzosi C, Vimercati C, Franchi D, Storti E, Taravelli E, Giovenzana F, Buetto G, Garzena G, Iotti GA, Villa GF, Botteri M, Caico SI, Cominesi IR, Carnevale L, Caresani M, Luppi M, Migliori M, Centineo P, Genoni P, Bertona R, De Ponti R, Osti R, Buratti S, Danzi GB, Marioni A, De Pirro A, Molinari S, Sgromo V, Musella V, Paglino M, Mojoli F, Lusona B, Pagani M, Curti M, Lombardia CARe Researchers. COVID-19 kills at home: the close relationship between the epidemic and the increase of out-of-hospital cardiac arrests. Eur Heart J 2020;41:3045–3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Butt JH, Fosbøl EL, Gerds TA, Andersson C, Kragholm K, Biering-Sørensen T, Andersen J, Phelps M, Andersen MP, Gislason G, Torp-Pedersen C, Køber L, Schou M. All-cause mortality and location of death in patients with established cardiovascular disease before, during, and after the COVID-19 lockdown: a Danish Nationwide Cohort Study. Eur Heart J 2021;42:1516–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Woolf SH, Chapman DA, Sabo RT, Zimmerman EB. Excess deaths from COVID-19 and other causes in the US, March 1, 2020, to January 2, 2021. JAMA 2021;325:1786–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cannata A, Bromage DI, McDonagh TA. The collateral cardiovascular damage of COVID-19: only history will reveal the depth of the iceberg. Eur Heart J 2021;42:1524–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wadhera RK, Shen C, Gondi S, Chen S, Kazi DS, Yeh RW. Cardiovascular deaths during the COVID-19 pandemic in the United States. J Am Coll Cardiol 2021;77:159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garcia S, Albaghdadi MS, Meraj PM, Schmidt C, Garberich R, Jaffer FA, Dixon S, Rade JJ, Tannenbaum M, Chambers J, Huang PP, Henry TD. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic. J Am Coll Cardiol 2020;75:2871–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Metzler B, Siostrzonek P, Binder RK, Bauer A, Reinstadler SJ. Decline of acute coronary syndrome admissions in Austria since the outbreak of COVID-19: the pandemic response causes cardiac collateral damage. Eur Heart J 2020;41:1852–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Luca G, Verdoia M, Cercek M, Jensen LO, Vavlukis M, Calmac L, Johnson T, Ferrer GR, Ganyukov V, Wojakowski W, Kinnaird T, van Birgelen C, Cottin Y, IJsselmuiden A, Tuccillo B, Versaci F, Royaards K-J, Berg JT, Laine M, Dirksen M, Siviglia M, Casella G, Kala P, Díez Gil JL, Banning A, Becerra V, De Simone C, Santucci A, Carrillo X, Scoccia A, Amoroso G, Lux A, Kovarnik T, Davlouros P, Mehilli J, Gabrielli G, Rios XF, Bakraceski N, Levesque S, Cirrincione G, Guiducci V, Kidawa M, Spedicato L, Marinucci L, Ludman P, Zilio F, Galasso G, Fabris E, Menichelli M, Garcia-Touchard A, Manzo S, Caiazzo G, Moreu J, Forés JS, Donazzan L, Vignali L, Teles R, Benit E, Agostoni P, Bosa Ojeda F, Lehtola H, Camacho-Freiere S, Kraaijeveld A, Antti Y, Boccalatte M, Deharo P, Martínez-Luengas IL, Scheller B, Alexopoulos D, Moreno R, Kedhi E, Uccello G, Faurie B, Gutierrez Barrios A, Di Uccio FS, Wilbert B, Smits P, Cortese G, Parodi G, Dudek D. Impact of COVID-19 pandemic on mechanical reperfusion for patients with STEMI. J Am Coll Cardiol 2020;76:2321–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reinstadler SJ, Reindl M, Lechner I, Holzknecht M, Tiller C, Roithinger FX, Frick M, Hoppe UC, Jirak P, Berger R, Delle-Karth G, Lassnig E, Klug G, Bauer A, Binder R, Metzler B. Effect of the COVID-19 pandemic on treatment delays in patients with ST-segment elevation myocardial infarction. J Clin Med 2020;9:2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bulluck H, Dharmakumar R, Arai AE, Berry C, Hausenloy DJ. Cardiovascular magnetic resonance in acute ST-segment-elevation myocardial infarction: recent advances, controversies, and future directions. Circulation 2018;137:1949–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Demirkiran A, Everaars H, Amier RP, Beijnink C, Bom MJ, Gotte MJW, van Loon RB, Selder JL, van Rossum AC, Nijveldt R. Cardiovascular magnetic resonance techniques for tissue characterization after acute myocardial injury. Eur Heart J Cardiovasc Imaging 2019;20:723–734. [DOI] [PubMed] [Google Scholar]
- 12. McCartney PJ, Berry C. Redefining successful primary PCI. Eur Heart J Cardiovasc Imaging 2019;20:133–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stone GW, Selker HP, Thiele H, Patel MR, Udelson JE, Ohman EM, Maehara A, Eitel I, Granger CB, Jenkins PL, Nichols M, Ben-Yehuda O. Relationship between infarct size and outcomes following primary PCI: patient-level analysis from 10 randomized trials. J Am Coll Cardiol 2016;67:1674–1683. [DOI] [PubMed] [Google Scholar]
- 14. Carrick D, Haig C, Ahmed N, McEntegart M, Petrie MC, Eteiba H, Hood S, Watkins S, Lindsay MM, Davie A, Mahrous A, Mordi I, Rauhalammi S, Sattar N, Welsh P, Radjenovic A, Ford I, Oldroyd KG, Berry C. Myocardial hemorrhage after acute reperfused ST-segment-elevation myocardial infarction: relation to microvascular obstruction and prognostic significance. Circ Cardiovasc Imaging 2016;9:e004148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Waha S, Patel MR, Granger CB, Ohman EM, Maehara A, Eitel I, Ben-Yehuda O, Jenkins P, Thiele H, Stone GW. Relationship between microvascular obstruction and adverse events following primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: an individual patient data pooled analysis from seven randomized trials. Eur Heart J 2017;38:3502–3510. [DOI] [PubMed] [Google Scholar]
- 16. Eitel I, de Waha S, Wohrle J, Fuernau G, Lurz P, Pauschinger M, Desch S, Schuler G, Thiele H. Comprehensive prognosis assessment by CMR imaging after ST-segment elevation myocardial infarction. J Am Coll Cardiol 2014;64:1217–1226. [DOI] [PubMed] [Google Scholar]
- 17. Masci PG, Ganame J, Francone M, Desmet W, Lorenzoni V, Iacucci I, Barison A, Carbone I, Lombardi M, Agati L, Janssens S, Bogaert J. Relationship between location and size of myocardial infarction and their reciprocal influences on post-infarction left ventricular remodelling. Eur Heart J 2011;32:1640–1648. [DOI] [PubMed] [Google Scholar]
- 18. Reinstadler SJ, Stiermaier T, Reindl M, Feistritzer HJ, Fuernau G, Eitel C, Desch S, Klug G, Thiele H, Metzler B, Eitel I. Intramyocardial haemorrhage and prognosis after ST-elevation myocardial infarction. Eur Heart J Cardiovasc Imaging 2019;20:138–146. [DOI] [PubMed] [Google Scholar]
- 19. Symons R, Pontone G, Schwitter J, Francone M, Iglesias JF, Barison A, Zalewski J, de Luca L, Degrauwe S, Claus P, Guglielmo M, Nessler J, Carbone I, Ferro G, Durak M, Magistrelli P, Lo Presti A, Aquaro GD, Eeckhout E, Roguelov C, Andreini D, Vogt P, Guaricci AI, Mushtaq S, Lorenzoni V, Muller O, Desmet W, Agati L, Janssens S, Bogaert J, Masci PG. Long-term incremental prognostic value of cardiovascular magnetic resonance after ST-segment elevation myocardial infarction: a study of the collaborative registry on CMR in STEMI. JACC Cardiovasc Imaging 2018;11:813–825. [DOI] [PubMed] [Google Scholar]
- 20. Reindl M, Tiller C, Holzknecht M, Lechner I, Beck A, Plappert D, Gorzala M, Pamminger M, Mayr A, Klug G, Bauer A, Metzler B, Reinstadler SJ. Prognostic implications of global longitudinal strain by feature-tracking cardiac magnetic resonance in ST-elevation myocardial infarction. Circ Cardiovasc Imaging 2019;12:e009404. [DOI] [PubMed] [Google Scholar]
- 21. Federal Minestry for Social Affairs, Health, Care and Consumer Protection. https://www.sozialministerium.at/en/Coronavirus/Coronavirus-Information-available-for-download.html.
- 22. Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, Galvani M, Katus HA, Newby LK, Ravkilde J, Chaitman B, Clemmensen PM, Dellborg M, Hod H, Porela P, Underwood R, Bax JJ, Beller GA, Bonow R, Van der Wall EE, Bassand J-P, Wijns W, Ferguson TB, Steg PG, Uretsky BF, Williams DO, Armstrong PW, Antman EM, Fox KA, Hamm CW, Ohman EM, Simoons ML, Poole-Wilson PA, Gurfinkel EP, Lopez-Sendon J-L, Pais P, Mendis S, Zhu J-R, Wallentin LC, Fernández-Avilés F, Fox KM, Parkhomenko AN, Priori SG, Tendera M, Voipio-Pulkki L-M, Vahanian A, Camm AJ, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Morais J, Brener S, Harrington R, Morrow D, Lim M, Martinez-Rios MA, Steinhubl S, Levine GN, Gibler WB, Goff D, Tubaro M, Dudek D, Al-Attar N, Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. Circulation 2007;116:2634–2653. [DOI] [PubMed] [Google Scholar]
- 23. Bondarenko O, Beek AM, Hofman MB, Kuhl HP, Twisk JW, van Dockum WG, Visser CA, van Rossum AC. Standardizing the definition of hyperenhancement in the quantitative assessment of infarct size and myocardial viability using delayed contrast-enhanced CMR. J Cardiovasc Magn Reson 2005;7:481–485. [DOI] [PubMed] [Google Scholar]
- 24. De Rosa S, Spaccarotella C, Basso C, Calabrò MP, Curcio A, Filardi PP, Mancone M, Mercuro G, Muscoli S, Nodari S, Pedrinelli R, Sinagra G, Indolfi C, Angelini F, Barillà F, Bartorelli A, Benedetto F, Bernabò P, Bolognese L, Briani M, Cacciavillani L, Calabrese A, Calabrò P, Caliendo L, Calò L, Casella G, Casu G, Cavallini C, Ciampi Q, Ciccone M, Comito M, Corrada E, Crea F, D’Andrea A, D’Urbano M, De Caterina R, De Ferrari G, De Ponti R, Della Mattia A, Di Mario C, Donazzan L, Esposito G, Fedele F, Ferraro A, Galasso G, Galiè N, Gnecchi M, Golino P, Golia B, Guarini P, Indolfi C, Leonardi S, Locuratolo N, Luzza F, Manganiello V, Francesca Marchetti M, Marenzi G, Margonato A, Meloni L, Metra M, Milo M, Mongiardo A, Monzo L, Morisco C, Nodari S, Novo G, Pancaldi S, Parollo M, Paternò G, Patti G, Priori S, Ravera A, Giuseppe Rebuzzi A, Rossi M, Scherillo M, Semprini F, Senni M, Sibilio G, Sinagra G, Siviglia M, Tamburino C, Tortorici G, Versace F, Villari B, Volpe M, Società Italiana di Cardiologia and the CCU Academy investigators group. Reduction of hospitalizations for myocardial infarction in Italy in the COVID-19 era. Eur Heart J 2020;41:2083–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mafham MM, Spata E, Goldacre R, Gair D, Curnow P, Bray M, Hollings S, Roebuck C, Gale CP, Mamas MA, Deanfield JE, de Belder MA, Luescher TF, Denwood T, Landray MJ, Emberson JR, Collins R, Morris EJA, Casadei B, Baigent C. COVID-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet 2020;396:381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xiang D, Xiang X, Zhang W, Yi S, Zhang J, Gu X, Xu Y, Huang K, Su X, Yu B, Wang Y, Fang W, Huo Y, Ge J. Management and outcomes of patients with STEMI during the COVID-19 pandemic in China. J Am Coll Cardiol 2020;76:1318–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chew NW, Ow ZGW, Teo VXY, Heng RRY, Ng CH, Lee CH, Low AF, Chan MY, Yeo TC, Tan HC, Loh PH. The global impact of the COVID-19 pandemic on STEMI care: a systematic review and meta-analysis. Can J Cardiol 2021. Apr 10; doi: 10.1016/j.cjca.2021.04.003 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rattka M, Dreyhaupt J, Winsauer C, Stuhler L, Baumhardt M, Thiessen K, Rottbauer W, Imhof A. Effect of the COVID-19 pandemic on mortality of patients with STEMI: a systematic review and meta-analysis. Heart 2021;107:482–487. [DOI] [PubMed] [Google Scholar]
- 29. Campo G, Fortuna D, Berti E, Palma RD, Pasquale GD, Galvani M, Navazio A, Piovaccari G, Rubboli A, Guardigli G, Galiè N, Boriani G, Tondi S, Ardissino D, Piepoli M, Banchelli F, Santarelli A, Casella G, on behalf of the AMI-Co Investigators. In- and out-of-hospital mortality for myocardial infarction during the first wave of the COVID-19 pandemic in Emilia-Romagna, Italy. A population-based observational study. Lancet Regional Health Europe 2021;3:100055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. De Luca G, Suryapranata H, Ottervanger JP, Antman EM. Time delay to treatment and mortality in primary angioplasty for acute myocardial infarction: every minute of delay counts. Circulation 2004;109:1223–1225. [DOI] [PubMed] [Google Scholar]
- 31. Armstrong PW, Fu Y, Westerhout CM, Hudson MP, Mahaffey KW, White HD, Todaro TG, Adams PX, Aylward PE, Granger CB. Baseline Q-wave surpasses time from symptom onset as a prognostic marker in ST-segment elevation myocardial infarction patients treated with primary percutaneous coronary intervention. J Am Coll Cardiol 2009;53:1503–1509. [DOI] [PubMed] [Google Scholar]
- 32. Stone GW, Cox D, Garcia E, Brodie BR, Morice MC, Griffin J, Mattos L, Lansky AJ, O'Neill WW, Grines CL. Normal flow (TIMI-3) before mechanical reperfusion therapy is an independent determinant of survival in acute myocardial infarction: analysis from the primary angioplasty in myocardial infarction trials. Circulation 2001;104:636–641. [DOI] [PubMed] [Google Scholar]
- 33. Carberry J, Carrick D, Haig C, Ahmed N, Mordi I, McEntegart M, Petrie MC, Eteiba H, Hood S, Watkins S, Lindsay M, Davie A, Mahrous A, Ford I, Sattar N, Welsh P, Radjenovic A, Oldroyd KG, Berry C. Persistent iron within the infarct core after ST-segment elevation myocardial infarction: implications for LVR and health outcomes. JACC Cardiovasc Imaging 2018;11:1248–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Garcia S, Dehghani P, Grines C, Davidson L, Nayak KR, Saw J, Waksman R, Blair J, Akshay B, Garberich R, Schmidt C, Ly HQ, Sharkey S, Mercado N, Alfonso CE, Misumida N, Acharya D, Madan M, Hafiz AM, Javed N, Shavadia J, Stone J, Alraies MC, Htun W, Downey W, Bergmark BA, Ebinger J, Alyousef T, Khalili H, Hwang CW, Purow J, Llanos A, McGrath B, Tannenbaum M, Resar J, Bagur R, Cox-Alomar P, Stefanescu Schmidt AC, Cilia LA, Jaffer FA, Gharacholou M, Salinger M, Case B, Kabour A, Dai X, Elkhateeb O, Kobayashi T, Kim HH, Roumia M, Aguirre FV, Rade J, Chong AY, Hall HM, Amlani S, Bagherli A, Patel RAG, Wood DA, Welt FG, Giri J, Mahmud E, Henry TD, Society For Cardiac Angiography and Interventions, the Canadian Association of Interventional Cardiology, and the American College of Cardiology Interventional Council. Initial findings from the North American COVID-19 myocardial infarction registry. J Am Coll Cardiol 2021;77:1994–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rodriguez-Leor O, Cid Alvarez AB, Pérez de Prado A, Rossello X, Ojeda S, Serrador A, López-Palop R, Martin-Moreiras J, Rumoroso JR, Cequier A, Ibáñez B, Cruz-González I, Romaguera R, Moreno R. In-hospital outcomes of COVID-19 ST-elevation myocardial infarction patients. EuroIntervention 2021;16:1426–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.