Abstract
Safranal (SFR), an active ingredient extracted from saffron, exhibits a protective effect on the cardiovascular system. However, the mechanism of SFR against hypoxia/reoxygenation (H/R)-induced cardiomyocyte injury has previously not been investigated in vitro. The aim of the present study was therefore to observe the protective effects of SFR on H/R-induced cardiomyocyte injury and to explore its mechanisms. A H/R injury model of H9c2 cardiac myoblasts was established by administering 800 µmol/l CoCl2 to H9c2 cells for 24 h and reoxygenating the cells for 4 h to induce hypoxia. H9c2 cardiac myoblasts were pretreated with SFR for 12 h to evaluate the associated protective effects. A Cell Counting Kit-8 assay was used for cell viability detection, and the expression levels of lactate dehydrogenase (LDH), creatine kinase-MB (CK-MB), glutathione peroxidase (GSH-px), catalase (CAT), superoxide dismutase (SOD), malondialdehyde (MDA) and caspase-3, and the intracellular Ca2+ concentration were measured using the corresponding commercial kits. Levels of reactive oxygen species (ROS) in the cells were detected using 2,7-dichlorodihydrofluorescein diacetate. Flow cytometry was used to determine the degree of apoptosis and the level of mitochondrial membrane potential (MMP). Moreover, the expression levels of phosphorylated (p-)PI3K, AKT, p-AKT, glycogen synthase kinase 3β (GSK3β), p-GSK3β, Bcl-2, Bax, caspase-3 and cleaved caspase-3 were measured using western blot analysis. Results of the present study demonstrated that the H9c2 cardiac myoblasts treated with SFR exhibited significantly improved levels of viability and significantly reduced levels of ROS, compared with the H/R group. Furthermore, compared with the H/R group, SFR treatment significantly increased the MMP levels and antioxidant enzyme levels, including CAT, SOD and GSH-px; whereas the levels of CK-MB, LDH, MDA and intracellular Ca2+ concentration were significantly decreased. Moreover, the results of the present study demonstrated that SFR significantly reduced caspase-3, cleaved caspase-3 and Bax protein expression levels, but upregulated the Bcl-2 protein expression levels. SFR also increased the protein expressions of PI3K/AKT/GSK3β. In summary, the results suggested that SFR may exert a protective effect against H/R-induced cardiomyocyte injury, which occurs in connection with the inhibition of oxidative stress and apoptosis via regulation of the PI3K/AKT/GSK3β signaling pathway.
Keywords: safranal, H9c2 cardiac myoblasts, anti-oxidant stress, hypoxia/reoxygenation, anti-apoptosis, PI3K/AKT/GSK3β signaling pathway
Introduction
Since 1990, the global cardiovascular disease (CVD) mortality rate has increased annually, with CVD being the leading cause of death worldwide, especially in developing countries (1-3). Acute myocardial infarction (AMI) is a significant contributor towards high mortality in CVD (4). For patients with AMI, rapid and effective opening of the blocked vessel rescues ischemic myocardium, reduces infarct size and improves the effectiveness of treatment. Reperfusion therapies, such as thrombolysis and percutaneous coronary intervention, are the most effective treatments for AMI (5). However, reperfusion of blood flow brought about by the opening of the culprit vessel causes damage to cardiac myoblasts, which is known as myocardial ischemia/reperfusion injury (MIRI) (6). A previous study demonstrated that the area of myocardial infarction caused by MIRI was 50% of the total area of myocardial infarction (7). Therefore, the attenuation of MIRI and improvements to the efficacy of reperfusion therapies in AMI are major focus areas of research.
The concept of MIRI was first proposed by Jennings (8) in 1960; however, the pathophysiological mechanisms of MIRI are still not completely understood. Currently, the accepted mechanisms for MIRI include oxygen radical generation, intracellular Ca2+ ([Ca2+]i) overload and apoptosis (9,10). Moreover, previous studies have confirmed that cardiomyocyte apoptosis is an important pathway and characteristic in MIRI (11,12). During MIRI, mechanisms such as ([Ca2+]i) overload, increases in mitochondrial permeability and excessive accumulation of reactive oxygen species (ROS) can induce cardiomyocyte apoptosis, thereby exacerbating MIRI (13). It is therefore important to identify drugs that can effectively alleviate oxidative stress, reduce ([Ca2+]i) concentration and improve mitochondrial function in MIRI.
During MIRI, the reperfusion injury salvage kinase (RISK) signaling pathway is activated, which includes protein kinase B (AKT) and phosphatidylinositol 3 kinase (PI3K) (14). The PI3K/AKT/glycogen synthase kinase 3β (GSK3β) axis is an intracellular signaling transduction pathway that serves important biological functions in cellular activities, including apoptosis, survival and proliferation (15). PI3K can be phosphorylation-activated by extracellular signals such as growth factors. Activated PI3K further catalyzes the phosphorylation of the 3'-OH position on the inositol ring of phosphatidylinositol 4,5-bisphosphate at the cell membrane surface to form the 3,4,5-triphosphate phosphatidylinositol (PIP3), and PIP3 activates the phosphorylation of AKT as a secondary messenger (16). Phosphorylated AKT can then further phosphorylate GSK3β initiating a cardio-protective mechanism (16). It has been reported that drug pretreatment can activate the PI3K/AKT/GSK3β signaling pathway in cardiomyocytes in vivo and in vitro, which serves an important role in combating MIRI (17,18). Furthermore, a previous study has demonstrated that ischemic preconditioning can reduce myocardial infarct size following ischemia/reperfusion in mice, during which the level of phosphorylated AKT in vivo is significantly increased (19). GSK3β can open the mitochondrial permeability transition pore and promote apoptosis following its activation, whereas inactive phosphorylated GSK3β can shield cardiomyocytes and mitochondria from damage, serving a myocardial protective role (20).
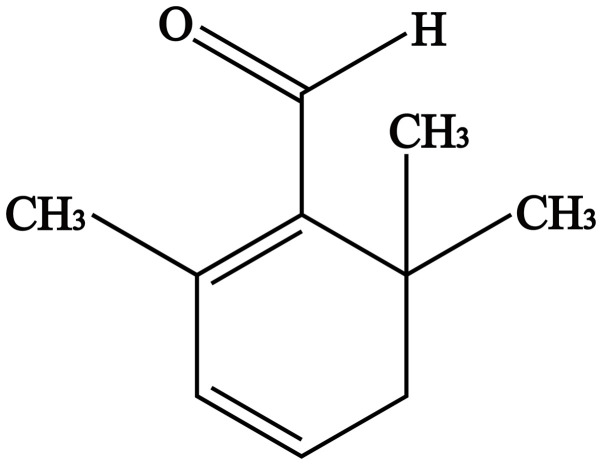
Saffron, which is derived from the dried stigmas of the saffron plant of the Iridaceae family, is native to Europe and is expensive due to its low yield and manual plucking methods (21). Saffron has been used for thousands of years as a dye and as a spice for cooking (22). It is also commonly used in the clinic to treat cardio and cerebrovascular diseases (23,24). Safranal (SFR; C10H14O; Fig. 1) is a major aromatic factor in saffron, accounting for 60-70% of the volatile constituents (25). Studies have previously demonstrated that SFR has numerous pharmacological properties, including as an antioxidant (26), antiapoptotic (27), anticancer (28,29) and cardioprotective factor (30-32). Furthermore, in our previous study, Xue et al (33) determined that SFR could inhibit the L-type Ca2+ current and cell contractility and improve ischemic myocardial tissue. Results of a previous study demonstrated that the protective effects of SFR against MIRI are associated with the PI3K/AKT/endothelial nitric oxide synthase signaling pathway in vivo (32), cardiac function is regulated by a series of factors, including the nervous system and hormones. Therefore, the mechanism of SFR in MIRI needs further investigation at the cellular level.
Figure 1.
The chemical structure of safranal.
In the present study, a hypoxia/reoxygenation (H/R) injury model was established by subjecting H9c2 cardiac myoblasts to hypoxia with cobalt chloride (CoCl2) containing medium followed by reoxygenation with normal medium. The protective effects of SFR were evaluated by exploring the H9c2 cardiac myoblast viability, as well as the oxidative stress and apoptotic levels of the cells in the H/R model. Furthermore, the protein expression levels of the PI3K/AKT/GSK3β signaling pathway were explored to elucidate the potential mechanism of SFR. The effect of SFR on intracellular ([Ca2+]i) overload and ROS production was also observed. The present study builds upon the findings of our previous study (33) and provides an experimental and theoretical basis for the clinical application of SFR in the treatment of MIRI.
Materials and methods
Reagents
The SFR (cat. no. S081900) and CoCl2 (cat. no. C633133) used in the present study was purchased from Toronto Research Chemicals, Inc. The rat cardiac myoblast H9c2 cell line was obtained from Bluefbio Biotechnology Development, Inc (cat. no. BFN60804388). High glucose Dulbecco's modified eagle medium (DMEM; cat. no. 12430047) and fetal bovine serum (FBS; cat. no. 10093188) were purchased from Gibco (Thermo Fisher Scientific, Inc.). The Cell Counting Kit (CCK)-8 assay (cat. no. G021-1-1), lactate dehydrogenase (LDH) assay (cat. no. A020-2-1), creatine kinase-MB (CK-MB) assay (cat. no. H197-1-1), glutathione peroxidase (GSH-Px) assay (cat. no. A005-1-2), catalase (CAT) assay (cat. no. A007-1-1), superoxide dismutase (SOD) assay (cat. no. A001-3-2), malondialdehyde (MDA) assay (cat. no. A003-1-2), caspase-3 assay (cat. no. G015-1-3), BCA protein assay (cat. no. A045-3-2) and Ca2+ assay (cat. no. C004-1-1) were obtained from Nanjing Jiancheng Bioengineering Inc. LY294002, a specific antagonist of PI3K/AKT (cat. no. HY-10108), was purchased from MedChemExpress, Inc. All other reagents used in the present study were purchased from Sigma-Aldrich (Merck KGaA) unless otherwise stated.
Cell culture
H9c2 cells were cultured in the high-glucose DMEM containing 10% FBS and 100 U/ml penicillin/streptomycin. The rat cardiac myoblast H9c2 cell line was initially cultured from rat embryo tissue. The cells grew in a 5% CO2 incubator at 37˚C and medium was replaced every 2 days. When cells were ~80% confluent, cells were trypsinized, passaged and seeded into a 96-well or six-well plate. Cells in the logarithmic growth phase were used for further experiments.
H/R model establishment
H9c2 cardiac myoblasts were prepared into 5x104/ml cell suspension and inoculated in a 96-well plate at 100 µl/well. When the cells covered 80% of the wells, different treatments were performed applied according to the needs of the experiment. In the preliminary experiment, 200, 400, 600, 800, 1,000 and 1,200 µmol/l CoCl2 were applied to H9c2 cells and incubated at 37˚C for 24 h. Therefore, the optimal concentration of CoCl2 for hypoxic conditions was determined to be 800 µmol/l. Following hypoxic treatment, high glucose DMEM was used to reoxygenate the cells at 37˚C for 2, 4, 6 and 8 h to establish the optimal reoxygenation time. In summary, the H/R injury model of H9c2 cardiac myoblasts was established by administering 800 µmol/l CoCl2 for 24 h to establish hypoxia prior to reoxygenation for 4 h.
Treatment
All manipulations involving incubation of cells were performed at 37˚C. H9c2 cardiac myoblasts were inoculated in 96-well plates at 5,000 cells/well. H9c2 cardiac myoblasts were randomly divided into the following six groups: i) Control group (CONT), cultured in high-glucose DMEM; ii) H/R injury group (H/R), hypoxia induced cells using 800 µmol/l CoCl2-containing medium for 24 h, followed by reoxygenation with high-glucose DMEM for 4 h; iii) low dose SFR group (SFRL + H/R), the cells were pretreated with 10 µmol/l of SFR for 12 h, followed by hypoxia induction with 800 µmol/l CoCl2-containing medium for 24 h and reoxygenation with high-glucose DMEM for 4 h; iv) high dose SFR group (SFRH + H/R), the cells were pretreated with 30 µmol/l of SFR for 12 h, followed by hypoxia induction using 800 µmol/l CoCl2-containing medium for 24 h and reoxygenation with high-glucose DMEM for 4 h; v) high dose SFR alone group (SFRA), the cells were treated with 30 µmol/l of SFR for 12 h and then cultured in high-glucose DMEM; and vi) antagonist LY294002 group (LY294002 + SFRH + H/R), the cells were pre-incubated with 20 µmol/l LY294002 for 1 h prior to treatment with 30 µmol/l SFR for 12 h, followed by hypoxia induction using 800 µmol/l CoCl2-containing medium for 24 h and reoxygenation with high-glucose DMEM for 4 h. Furthermore, a preliminary study on the therapeutic effects of post-treatment with SFR was also performed. H9c2 cardiac myoblasts were inoculated into 96-well plates at 5,000 cells/well. H9c2 cardiac myoblasts were randomly divided into the following five groups: i) Control group, high-glucose DMEM culture; ii) H/R injury group, hypoxia was induced using CoCl2 containing medium for 24 h, followed by reoxygenation with high-glucose DMEM for 4 h; iii) low dose SFR group, following H/R treatment, the cells were treated with 10 µmol/l of SFR for 12 h; iv) high dose SFR group, following H/R treatment, the cells were treated with 30 µmol/l of SFR for 12 h; and v) high dose SFR only group, the cells were treated with 30 µmol/l of SFR for 12 h and then cultured in high-glucose DMEM.
Cell viability assay
H9c2 cardiac myoblasts in the logarithmic growth phase were seeded into 96-well plates (5,000 cells/well) and incubated for 24 h at 37˚C. Subsequently, 1, 3, 10, 30, 100 and 300 µmol/l of SFR were added to the 96-well plates. After following incubation for 12 h at 37˚C, 10 µl of CCK-8 reagent was added to the cells in each well and incubated for 2 h at 37˚C. The absorbance of each well was measured using a microplate reader at a wavelength of 450 nm. The viability of H9c2 cardiac myoblasts was calculated according to the manufacturer's protocol.
Evaluation of biochemical indexes
H9c2 cardiac myoblasts were prepared into 5x104/ml cell suspension and inoculated in 96-well plates at 100 µl/well. Following treatment, LDH and CK-MB levels in cell supernatants were quantified using a microplate reader. The cell supernatants were collected and analyzed according to the manufacturer's protocol. Cells from each group were also digested with 0.25% trypsin, washed with PBS twice and ultrasonically pulverized to obtain a cell homogenate. This was then used to detect intracellular levels of GSH-Px, CAT, SOD, MDA and caspase-3 according to the corresponding manufacturer's protocol.
Detection of ROS levels
The common dichlorofluorescein, 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA; cat. no. 85155: Cayman Chemical Company) was used to detect the levels of intracellular ROS in the present study. DCFH-DA can cross the cell membrane and enter the cell where it is hydrolyzed into DCFH by esterases. DCFH cannot cross the cell membrane, but non fluorescent DCFH can be oxidized by intracellular ROS to fluorescent DCF and, therefore, the intracellular ROS level can be quantified via fluorescence intensity (34). Following treatment, DCFH-DA (10 µmol/l) dye was added to each group and incubated for 20 min in the dark at 37˚C. Cells were imaged using a fluorescence microscope (magnification, x200; Nikon Eclipse C1; Nikon Corp). Image-Pro Plus 6.0 software (Media Cybernetics, Inc.) was used for quantification.
Analysis of ([Ca2+]i) concentration
H9c2 cardiac myoblasts were prepared into a 5x104/ml cell suspension and inoculated in 96-well plates at 100 µl/well. Following treatment, the medium was replaced with PBS and H9c2 cardiac myoblasts were frozen at -20˚C, thawed and disrupted with an ultrasonic cell disruptor. H9c2 cardiac myoblasts were then centrifuged at 10,195 x g for 10 min at 4˚C and the supernatant was collected. The ([Ca2+]i) concentration in the supernatant was detected at a wavelength of 610 nm using a colorimetric method as stated in the Ca2+ assay kit, according to the manufacturer's protocol.
Detection of apoptosis and mitochondrial membrane potential (MMP)
For quantification of apoptosis, flow cytometry was performed (FC500; Beckman Coulter, Inc.) using an Apoptosis Detection kit (cat. no. CA1050; Beijing Solarbio Science & Technology Co., Ltd.). Briefly, cells were suspended in 1X binding buffer at a cell density of 1x106 cells/ml. To a centrifuge tube 100 µl of the cell suspension was added, followed by 5 µl of Annexin V-FITC and 5 µl PI. After mixing cells were incubated at 10˚C for 25 min before 500 µl PBS was added and cells were detected via flow cytometry (this step was carried out within 1 h). FlowJo 10 software (v10.6.2; BD Biosciences) was used to analyze the obtained flow cytometry data.
To detect the effects of SFR on MMP, the JC-1 MMP Detection kit (cat. no. M8650; Beijing Solarbio Science & Technology Co., Ltd.) was used. Following treatment, H9c2 cardiac myoblasts were incubated with JC-1 reagent (10 µg/ml) in the dark at 37˚C for 20 min, washed with PBS twice and detected via flow cytometry. FlowJo 10 software was used to analyze the obtained flow cytometry data.
Western blotting
The protein expression levels of PI3K, phosphorylated (p)-PI3K, AKT, p-AKT, GSK3β, p-GSK3β, B-cell lymphoma-2 (Bcl-2), Bcl-2 associated X protein (Bax), caspase-3 and cleaved caspase-3 were detected via western blotting. Following treatment, the H9c2 cardiac myoblasts were rinsed with precooled PBS buffer and RIPA buffer (cat. no. G2002; Wuhan Servicebio Technology, Co., Ltd.) was used to extract total protein. Total protein concentration was determined using the BCA protein assay kit and 30 µg protein/lane was separated by SDS-PAGE on a 10% gel. The separated proteins were transferred onto a PVDF membrane and subsequently blocked with 5% skimmed milk for 30 min at 37˚C. Membranes were subsequently incubated with the following primary antibodies against: Caspase-3 (1:1,000; cat. no. GB11767C), cleaved caspase-3 (1:1,000; cat. no. GB11532) (both from Wuhan Servicebio Technology Co., Ltd.), Bax (1:1,000; cat. no. 50599-2-Ig; ProteinTech Group, Inc.), Bcl-2 (1:500; cat. no. BM4985; Wuhan Boster Biological Technology, Ltd.), p-PI3K (1:600; cat. no. AF4372; Affinity Biosciences), PI3K (1:1,000; cat. no. ab151549; Abcam), p-GSK3β (1:1000; cat. no. AF2016; Affinity Biosciences), GSK3β (1:1,000; cat. no. AF7814; Affinity Biosciences), p-AKT (1:1,000; cat. no. 9611S; Cell Signaling Technology, Inc.), AKT (1:600; cat. no. 60203-2-Ig; ProteinTech Group, Inc.) and β-actin (1:1,000; cat. no. GB12001; Wuhan Servicebio Technology Co., Ltd.), overnight at 4˚C. After washing with PBS containing 0.05% Tween-20, the membranes were incubated with horseradish peroxidase-labelled secondary antibodies Goat anti-rabbit; (1:3,000; cat. no. ZB2301; Origene Technologies, Inc.) and Goat anti-mouse (1:3,000; cat. no. ZB2305; Origene Technologies, Inc.) at room temperature for 90 min. The gray values were semi-quantified using Tanon GIS software (version, Tanon GIS 2010; Tanon Science and Technology Co., Ltd.) after the film was imaged. β-actin was used as the internal reference gene.
Statistical analysis
Data analysis was performed using Origin version 9.1 software (OriginLab) and data are presented as the mean ± standard error of the mean (SEM). The statistical differences among three or more groups were analyzed using one-way analysis of variance (ANOVA) followed by Tukey's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of SFR on cell viability in H/R-induced H9c2 cardiac myoblasts
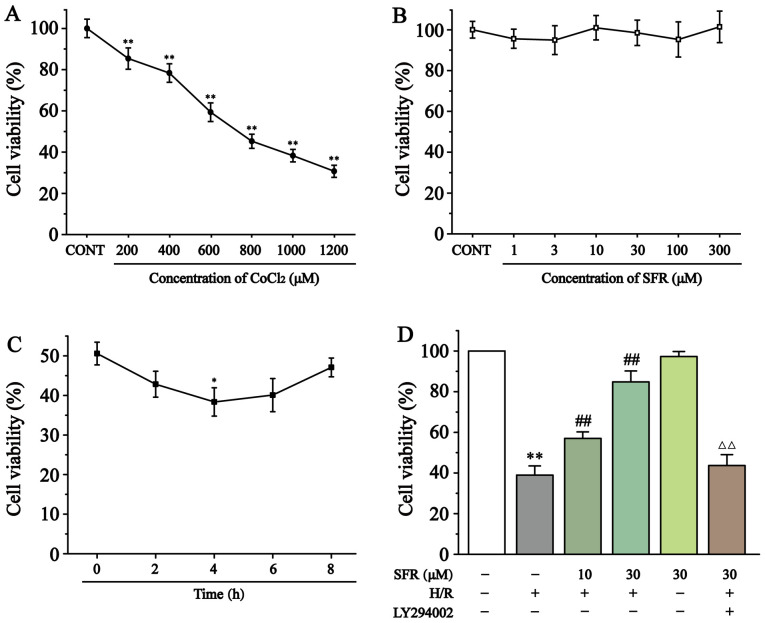
As demonstrated in Fig. 2A and B, cytotoxicity experiments in H9c2 cardiac myoblasts determined that CoCl2 significantly inhibited cell viability in a dose-dependent manner compared with the CONT group. However, SFR treatment had no significant effect on the H9c2 cardiac myoblast viability. Therefore, 800 µmol/l CoCl2 was chosen to induce hypoxia and 10 and 30 µmol/l SFR were used as low and high doses, respectively, to pre-protect H9c2 cardiac myoblasts. Fig. 2C demonstrates that maximum amount of H9c2 cell damage was significantly generated after 4 h of reoxygenation (P<0.05). Fig. 2D indicates that cell viability was significantly reduced in the H/R group compared with the CONT group (P<0.01). However, SFR treatment significantly increased the cell viability of H9c2 cardiac myoblasts in a dose-dependent manner compared with the H/R group (P<0.01). Furthermore, LY294002 significantly inhibited the protective effects of SFR on H/R-induced cardiomyoblast damage compared with the SFRH + H/R group (P<0.01). The effects of SFRA on H9c2 cardiac myoblasts were not significantly different compared with the CONT group. These results demonstrated that SFR could increase the viability of H9c2 cardiac myoblasts damaged by H/R, which may be related to the activation of PI3K/AKT/GSK3β signaling pathway.
Figure 2.
Effect of CoCl2 and SFR on the vitality of H9c2 cardiac myoblasts was detected using a CCK-8 assay. (A) H9c2 cardiac myoblasts were cultured with different concentrations of CoCl2 for 24 h. **P<0.01 vs. CONT. (B) H9c2 cardiac myoblasts were cultured with different concentrations of SFR for 12 h. (C) Effect of reoxygenation time on the vitality of H9c2 cardiac myoblasts. *P<0.05 vs. Reoxygenation 0 h group. (D) The vitality of H9c2 cardiac myoblasts in CONT, H/R, SFRL, SFRH, SFRA and LY294002 + SFRH + H/R groups was detected using a CCK-8 assay. Data are presented as the mean ± SEM. n=6. **P<0.01 vs. CONT; ##P<0.01 vs. H/R; and ΔΔP<0.01 vs. SFRL, low dose SFR; SFRH, high dose SFR; SFRA, SFR alone. SFRH + H/R. SFR, safranal; H/R, hypoxia/reoxygenation; CONT, control; CCK-8, Cell Counting Kit-8.
Effects of SFR on LDH and CK-MB levels
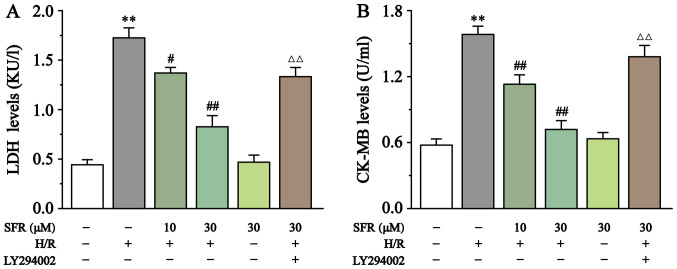
As displayed in Fig. 3, LDH and CK-MB levels were detected to assess H/R-induced H9c2 cardiac myoblast injury. Compared with the CONT group, CK-MB and LDH levels in the H/R group were significantly increased (P<0.01). However, SFR treatment significantly decreased the CK-MB and LDH levels compared with the H/R group (P<0.05 or P<0.01). Furthermore, LY294002 significantly inhibited the protective effect of SFR on H/R-induced cardiomyoblast damage compared with the SFRH + H/R group (P<0.01). The effects of SFRA on H9c2 cardiac myoblasts were not significantly different compared with the CONT group. These results indicated that SFR alleviated H/R-induced H9c2 cardiac myoblast injury, which may be associated with the activation of the PI3K/AKT/GSK3β signaling pathway.
Figure 3.
Effect of SFR on LDH and CK-MB levels. Effects of SFR treatment on (A) LDH and (B) CK-MB levels in H/R-induced H9c2 cardiac myoblasts. Data are presented as the mean ± SEM. n=6. **P<0.01 vs. CONT; #P<0.05, ##P<0.01 vs. H/R; and ΔΔP<0.01 vs. SFRH + H/R. SFR, safranal; LDH, lactate dehydrogenase; CK-MB, creatine kinase-MB; CONT, control; H/R, hypoxia/reoxygenation; SFRH, high dose SFR.
Effect of SFR on oxidative stress
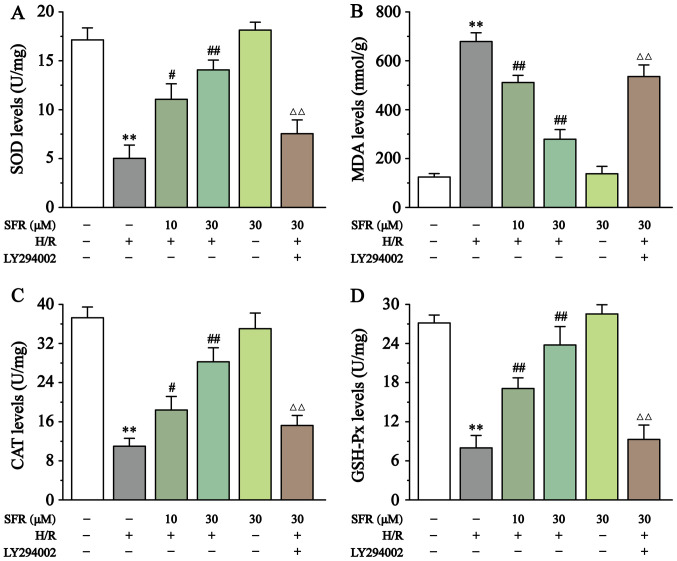
As displayed in Fig. 4, compared with the CONT group, SOD, CAT and GSH-Px levels were significantly decreased in the H/R group (P<0.01), whereas MDA levels were significantly increased (P<0.01). However, SFR treatment reversed these effects. SOD, CAT and GSH-Px levels were significantly increased, whereas MDA levels were significantly decreased in the SFR treated groups compared with the H/R group (P<0.05 or P<0.01). Furthermore, LY294002 significantly inhibited the protective effect of SFR against H/R-induced oxidative stress damage (P<0.01) compared with the SFRH + H/R group. The effects of SFRA on H9c2 cardiac myoblasts were not significantly different compared with the CONT groups. These results indicated that SFR protected against H/R-induced oxidative stress injury, and this protective effect may be associated with the activation of the PI3K/AKT/GSK3β signaling pathway.
Figure 4.
Effect of SFR on oxidative stress. Effect of SFR on (A) SOD, (B) MDA, (C) CAT and (D) GSH-Px. Data are presented as the mean ± SEM. n=6. **P<0.01 vs. CONT; #P<0.05, ##P<0.01 vs. H/R; and ΔΔP<0.01 vs. SFRH + H/R. SFR, safranal; SOD, superoxide dismutase; MDA, malondialdehyde; CAT, catalase; GSH-Px, glutathione peroxidase; CONT, control; H/R, hypoxia/reoxygenation; SFRH, high dose SFR.
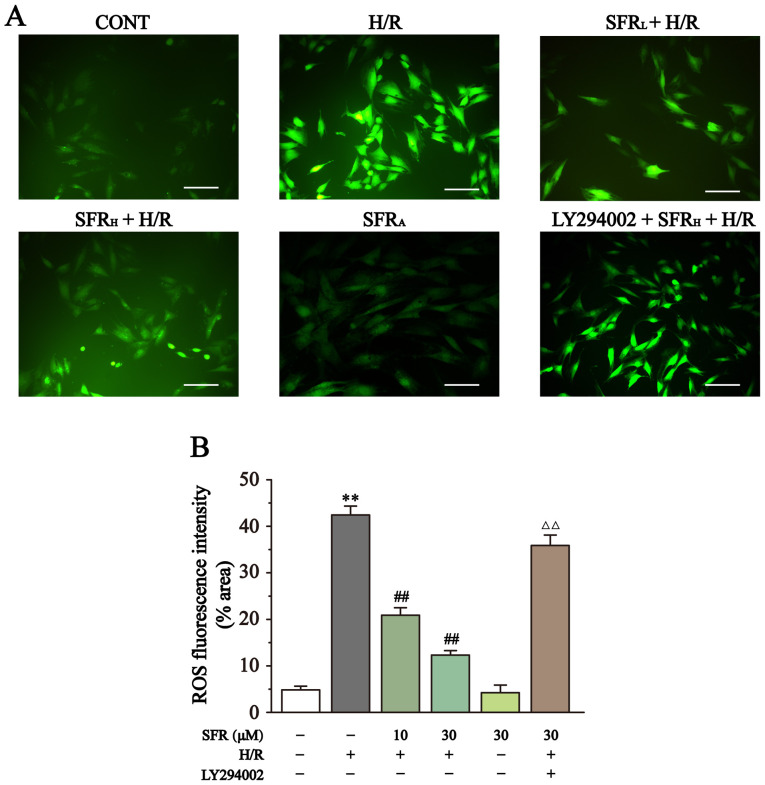
Effect of SFR on ROS levels
As displayed in Fig. 5, the ROS levels were detected by DCFH-DA. DCFH-DA is commonly used to detect ROS levels and when the intracellular ROS level increases a strong green fluorescence is exhibited. Compared with the CONT group, the H9c2 cardiac myoblasts in the H/R group exhibited significantly increased fluorescence and therefore ROS levels (P<0.01). However, SFR treatment reversed this effect and significantly decreased the fluorescence intensity and ROS levels compared with the H/R group (P<0.01). Furthermore, LY294002 significantly inhibited the effect of SFR on the H/R-induced increase of ROS levels compared with the SFRH + H/R group (P<0.01). The effects of SFRA on H9c2 cardiac myoblasts were not significantly different compared with the CONT group. These results suggested that SFR could decrease the ROS levels induced by H/R, which may be related to the activation of PI3K/AKT/GSK3β signaling pathway.
Figure 5.
Effect of SFR on ROS levels. (A) Representative ROS fluorescence images (magnification, x200; scale bar, 100 µm) from CONT, H/R, SFRL + H/R, SFRH + H/R, SFRA and LY294002 + SFRH + H/R groups. (B) ROS fluorescence intensity. Data are presented as the mean ± SEM. n=6. **P<0.01 vs. CONT; ##P<0.01 vs. H/R; ΔΔP<0.01 vs. SFRH + H/R. SFR, safranal; H/R, hypoxia/reoxygenation; CONT, control; ROS, reactive oxygen species; SFRL, low dose SFR; SFRH, high dose SFR; SFRA, SFR alone.
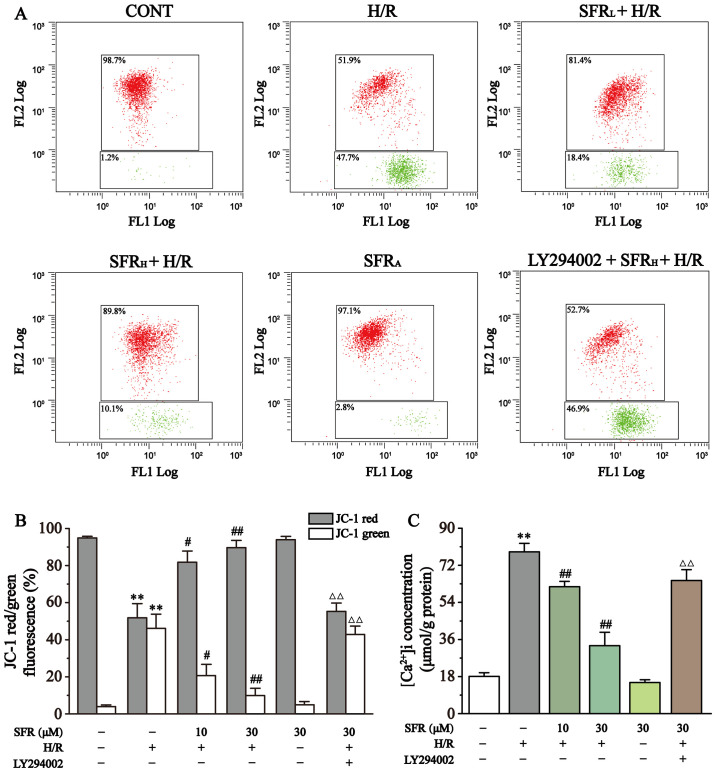
Effect of SFR on MMP levels
As displayed in Fig. 6A and B, MMP levels in each group were quantified using flow cytometry. The change of MMP is an important parameter that reflects mitochondrial viability and can be detected by JC-1, a lipophilic fluorescent dye (35). At high levels of MMP, JC-1 converges to form polymers in the mitochondrial matrix and produces red fluorescence. At low levels of MMP, JC-1 cannot aggregate in the mitochondrial matrix and only produces green fluorescence as a monomer. The subsequent change in levels of MMP are detected by the relative proportion of red:green fluorescence. The levels of MMP were significantly decreased by H/R in H9c2 cardiac myoblasts compared with the CONT group (P<0.01). However, SFR treatment significantly increased the level of MMP compared with the H/R group (P<0.05 or P<0.01). Furthermore, LY294002 significantly inhibited the effect of SFR on the H/R-induced decrease of MMP compared with the SFRH + H/R group (P<0.01). The effects of SFRA on H9c2 cardiac myoblasts were not significantly different compared with the CONT group. These results indicated that SFR protected against H/R-induced reduction of MMP, and this protective effect may be associated with the activation of the PI3K/AKT/GSK3β signaling pathway.
Figure 6.
Effect of SFR on MMP and [Ca2+]i concentration. (A) Flow cytometry analysis of the CONT, H/R, SFRL + H/R, SFRH + H/R, SFRA and LY294002 + SFRH + H/R groups. (B) Data summary of MMP flow cytometry results. n=3. (C) [Ca2+]i concentration. n=6. Data are represented as the mean ± SEM. **P<0.01 vs. CONT; #P<0.05, ##P<0.01 vs. H/R group; and ΔΔP<0.01 vs. SFRH + H/R. SFR, safranal; H/R, hypoxia/reoxygenation; CONT, control; SFRL, low dose SFR; SFRH, high dose SFR; SFRA, SFR alone; [Ca2+]i, intracellular Ca2+.
Effect of SFR on ([Ca2+]i) concentration
As displayed in Fig. 6C, the effect of SFR on ([Ca2+]i) concentration was detected. The results demonstrated that ([Ca2+]i) concentration was significantly increased in the H/R group compared with the CONT group (P<0.01). However, compared with the H/R group, SFR treatment significantly decreased the ([Ca2+]i) concentration (P<0.01). Furthermore, LY294002 significantly inhibited the protective effects of SFR on H/R-induced ([Ca2+]i) overload compared with the SFRH + H/R group (P<0.01). The effects of SFRA on H9c2 cardiac myoblasts were not significantly different compared with the CONT group. These results suggested that SFR alleviated H/R-induced ([Ca2+]i) overload, which may be associated with the activation of the PI3K/AKT/GSK3β signaling pathway.
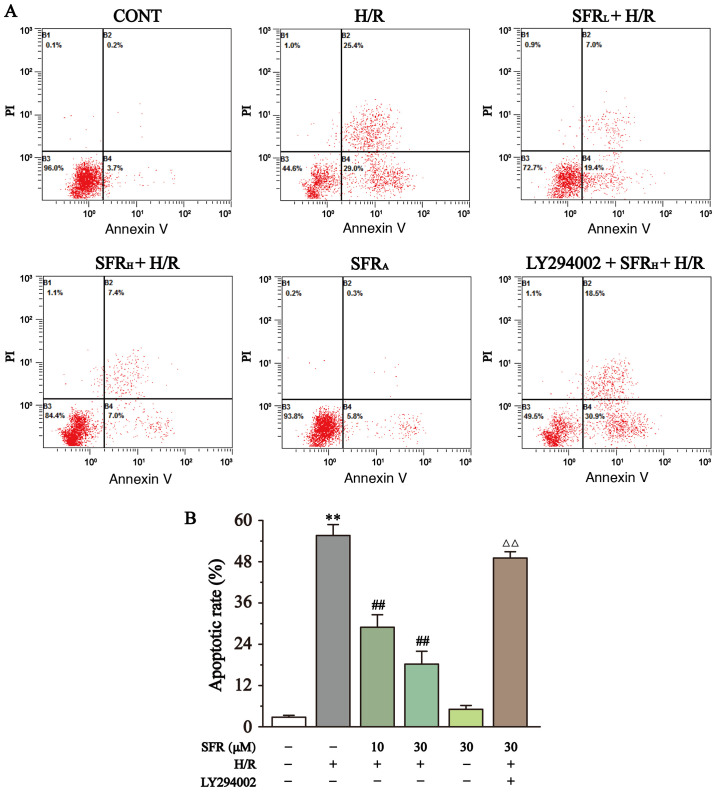
Effect of SFR on apoptotic rate
The effect of SFR on apoptotic rate was determined using flow cytometry as displayed in Fig. 7. The results demonstrated that the apoptotic rate in the H/R group was significantly upregulated compared with the CONT group (P<0.01), whereas SFR treatment significantly decreased the H/R-induced increase apoptotic rate compared with the H/R group (P<0.01).
Figure 7.
Effects of SFR on cell apoptosis. (A) Flow cytometry analysis of CONT, H/R, SFRL + H/R, SFRH + H/R, SFRA and LY294002 + SFRH + H/R groups. (B) Data summary of apoptotic rate flow cytometry analysis. Data are represented as the mean ± SEM. n=3. **P<0.01 vs. CONT; ##P<0.01 vs. H/R; and ΔΔP<0.01 vs. SFRH + H/R. SFR, safranal; H/R, hypoxia/reoxygenation; CONT, control; SFRL, low dose SFR; SFRH, high dose SFR; SFRA, SFR alone.
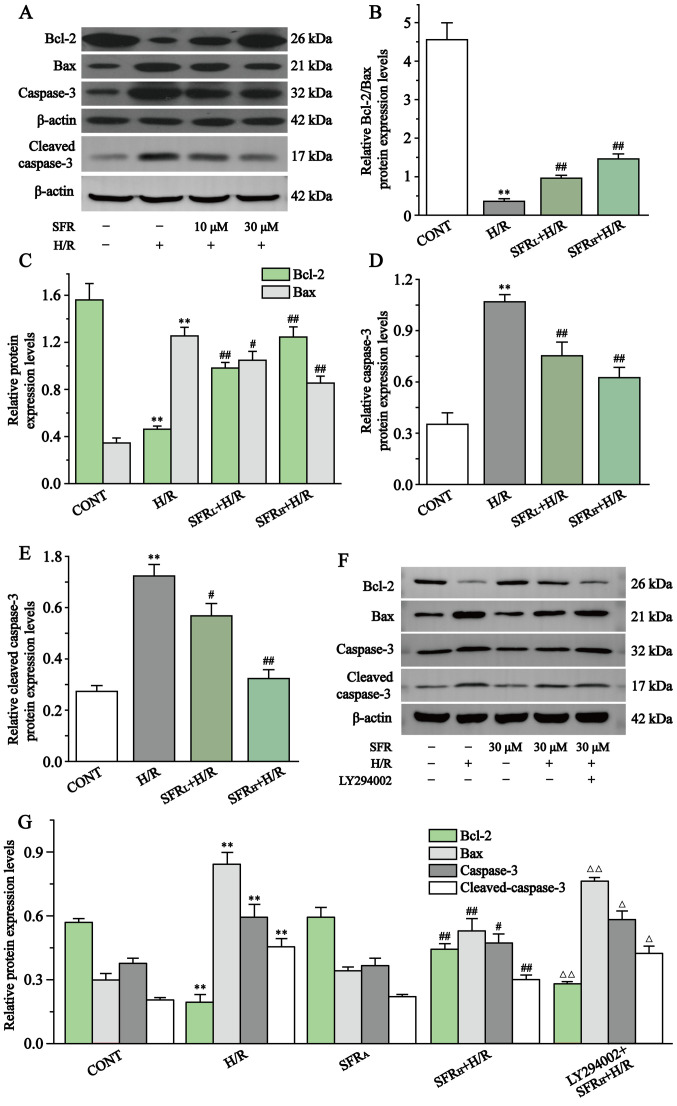
Fig. 8 displays the effect of SFR on caspase-3, cleaved caspase-3, Bcl-2 and Bax protein expression levels in H/R-induced H9c2 cardiac myoblast injury. Compared with the CONT group, Bcl-2 protein expression levels were significantly decreased in the H/R group, whereas caspase-3, cleaved caspase-3 and Bax protein expression levels were significantly upregulated (P<0.01). However, SFR treatment reversed this effect. SFR treatment could significantly decrease caspase-3, cleaved caspase-3 and Bax protein expression levels and significantly increased the protein expression levels of Bcl-2 compared with the H/R group (P<0.05 or P<0.01). These results indicated that SFR alleviated H/R-induced apoptotic damage. Furthermore, as displayed in Fig. 8F and G, the results of caspase-3, cleaved caspase-3, Bax and Bcl-2 protein expression levels demonstrated that LY294002 significantly inhibited the protective effects of SFR on H/R-induced cardiomyocyte apoptosis compared with the SFRH + H/R group (P<0.01). The effects of SFRA on H9c2 cardiac myoblasts were not significantly different compared with the CONT group. These results indicated that SFR alleviated H/R-induced apoptotic damage, which may be associated with the activation of the PI3K/AKT/GSK3β signaling pathway.
Figure 8.
Effect of SFR on the protein expression levels of Bax, Bcl-2, caspase-3 and cleaved caspase-3. (A) Western blot analysis of the Bcl-2, Bax, capase-3 and cleaved caspase-3 expression levels in CONT, H/R, SFRL + H/R and SFRH + H/R groups. (B) The ratio of Bcl-2 to Bax expression levels normalized to β-actin. (C) Quantification of the Bcl-2 and Bax protein expression levels normalized to β-actin. Quantification of the (D) capase-3 and (E) cleaved caspase-3 protein expression levels normalized to β-actin. (F) Western blot analysis of Bax, Bcl-2, capase-3 and cleaved caspase-3 in CONT, H/R, SFRL + H/R, SFRH + H/R, SFRA and LY294002 + SFRH + H/R groups. (G) Quantification of the Bcl-2, Bax, capase-3 and cleaved caspase-3 protein expression levels normalized to β-actin. Data are presented as the mean ± SEM. n=3 **P<0.01 vs. CONT; #P<0.05, ##P<0.01 vs. H/R; and ΔP<0.05, ΔΔP<0.01 vs. SFRH + H/R. SFRL, low dose SFR; SFRH, high dose SFR; SFRA, SFR alone. SFR, safranal; H/R, hypoxia/reoxygenation; CONT, control.
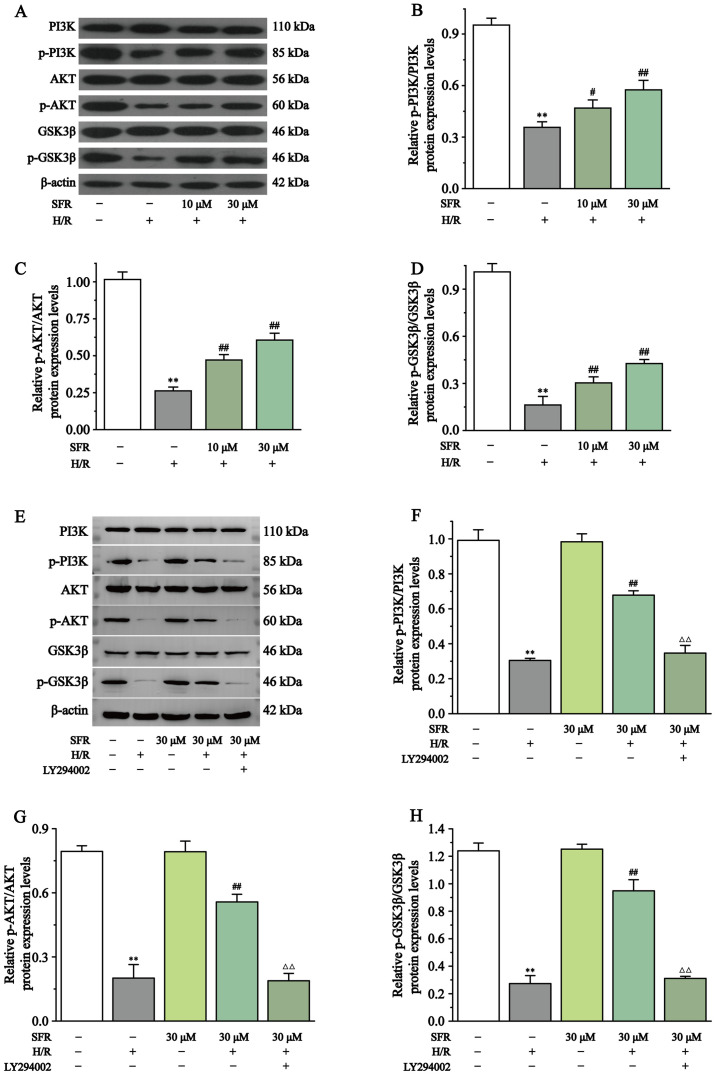
Effect of SFR on the protein expression levels of AKT, p-AKT, PI3K, p-PI3K, GSK3β and p-GSK3β
Fig. 9 displays the effects of SFR on the PI3K/AKT/GSK3β signaling pathway in H/R-induced H9c2 cardiac myoblasts. The results demonstrated that p-AKT/AKT, p-PI3K/PI3K and p-GSK3β/GSK3β protein expression levels in the H/R group were significantly decreased compared with the CONT group (P<0.01). Compared with the H/R group, the protein expression levels of p-AKT/AKT, p-PI3K/PI3K and p-GSK3β/GSK3β were significantly increased when treated with SFR (P<0.05 or P<0.01). Furthermore, LY294002 significantly inhibited the effect of SFR on the protein expression levels of p-AKT/AKT, p-PI3K/PI3K and p-GSK3β/GSK3β in H9c2 cardiac myoblasts exposed to CoCl2, compared with the SFRH + H/R group (P<0.01). No significant difference was observed in the protein expression levels of p-AKT/AKT, p-PI3K/PI3K and p-GSK3β/GSK3β between the CONT group and the SFRA group. These results suggested that SFR alleviated H9c2 cardiac myoblast injury induced by H/R via regulation of the PI3K/AKT/GSK3β pathway.
Figure 9.
Effect of SFR on the PI3K/AKT/GSK3β signaling pathway protein expression levels. (A) Western blot analysis of the PI3K, p-PI3K, AKT, p-AKT, GSK3β and p-GSK3β expression levels in CONT, H/R, SFRL + H/R and SFRH + H/R groups. The ratio of (B) p-PI3K to PI3K, (C) p-AKT to AKT and (D) p-GSK3β to GSK3β normalized to β-actin. (E) Western blot analysis of the PI3K, p-PI3K, AKT, p-AKT, GSK3β and p-GSK3β expression levels in CONT, H/R, SFRH + H/R, SFRA and LY294002 + SFRH + H/R groups. The ratio of (F) p-PI3K to PI3K, (G) p-AKT to AKT and (H) p-GSK3β to GSK3β normalized to β-actin. Data are presented as the mean ± SEM. n=3. **P<0.01 vs. CONT; #P<0.05, ## P<0.01 vs. H/R; and ΔΔP<0.01 vs. SFRH + H/R. SFRL, low dose SFR; SFRH, high dose SFR; SFRA, SFR alone. SFR, safranal; CONT, control; H/R, hypoxia/reoxygenation; p, phosphorylated.
Effect of SFR post-treatment on the viability of H9c2 cardiac myoblasts and LDH, CK-MB, SOD, MDA and caspase-3 levels
Fig. S1 displays the protective effects of SFR post-treatment on H/R-induced H9c2 cardiac myoblast injury. The results demonstrated that compared with the control group, the H9c2 cardiac myoblast viability and SOD levels in the H/R group were significantly reduced, whereas LDH, CK-MB, MDA and caspase-3 levels were significantly increased (P<0.01). However, SFR post-treatment reversed this effect. Compared with the H/R group, SFR post-treatment significantly increased H9c2 cardiac myoblast viability and SOD levels, whereas LDH, CK-MB, MDA and caspase-3 levels were significantly decreased (P<0.05 or P<0.01). These results indicated that SFR post-treatment also has a certain protective effect against H/R-induced H9c2 cardiac myoblast injury. Furthermore, the effects of SFR only on H9c2 cardiac myoblasts were not significantly different compared with the control group. These results suggested that SFR alleviated H9c2 cardiac myoblast injury induced by H/R.
Discussion
CVDs are the main causes of mortality worldwide and are currently difficult to treat (36). CVD was the primary cause of 17.7 million deaths worldwide in 2015, which is predicted to increase to 23.6 million by 2030 (37,38). SFR, the main active substance extracted from saffron, has been proven to have cardio-protective properties (30-32). However, to the best of our knowledge, there are no published studies investigating the protective effect of SFR against H/R-induced myocardial injury in vitro.
Therefore, the present study investigated the effect of SFR on MIRI in vitro, which excluded disturbances caused by neural and humoral reflex regulation in vivo, more clearly demonstrating the therapeutic effect of SFR. The results demonstrated that SFR can protect against MIRI by acting directly on cardiomyoblasts. Moreover, previous studies have reported that CoCl2 can inhibit H9c2 cell viability and induce apoptosis in a dose-dependent manner (39-41). Apoptotic death of cardiomyocytes is an important pathological feature observed in MIRI (9). Therefore, using CoCl2 to establish a hypoxia model was beneficial for exploring the regulatory mechanism of SFR on hypoxia- induced apoptosis and for demonstrating the myocardial protective effect of SFR. The results of the present study demonstrated that both SFR pre-treatment and post-treatment significantly increased H9c2 cell viability in the H/R group. Furthermore, the protective effects of SFR on H/R-induced damage were demonstrated via quantification of oxidative stress, apoptosis, ([Ca2+]i) overload, MMP and the associated signaling pathway.
CoCl2 is commonly used for chemical hypoxia simulation during in vitro experiments (42). Ca2+ in CoCl2 can replace the Fe2+ in the hemoglobin porphyrin ring, so that hemoglobin cannot bind oxygen and remains in a reduced state, thereby imitating an hypoxic environment and producing a series of reactions similar to those in hypoxic conditions (43). Moreover, under normoxic conditions, CoCl2 can strongly stabilize hypoxia-inducible factor (HIF)-1α and HIF-2α, which are heterodimeric transcription factors composed of an oxygen-regulated α subunit (42). Compared with low oxygen-induced hypoxia and the use of other hypoxia mimics, the stabilization of HIF-1α and HIF-2α is sustained for several hours (42). Therefore, this model in normoxic conditions provides more time in which samples can be analyzed (42). The cardiac myoblast H9c2 cell line is an immortalized cell line isolated from rat embryonic heart tissue (44). H9c2 cells show morphological characteristics similar to those of immature embryonic cardiocytes but have preserved several elements of the electrical and hormonal signal pathway found in adult cardiac cells (44). H9c2 cardiac myoblasts have been commonly used for experimental studies of cardiomyocyte physiology and pathology because of their similar physiological characteristics to adult rat cardiomyocytes in terms of electrophysiology and signal transduction (44). The predictors of cardiac pathological changes are CK-MB and LDH, which are stable cytosolic enzymes in cardiomyocytes (45-47). When the myocardial cell membrane is damaged, CK-MB and LDH are rapidly released from the cells into the culture medium (47). In the present study, the results demonstrated that both SFR pre-treatment and post-treatment significantly reduced the LDH and CK-MB levels, indicating that SFR may attenuate H/R-induced cardiomyoblast injury.
MIRI is a process that causes oxidative stress, whereby a large number of ROS are generated via multiple pathways, endogenous free radical scavenging systems dysfunction and ROS aggregation occurs within the body (48). ROS accumulation results in cardiomyocyte dysfunction, which further causes intracellular protein denaturation and degradation, loss of cellular function and results in the cytochrome oxidase system delivering significantly fewer electrons, leading to energy metabolism disorders (49). MIRI is often accompanied by intense lipid peroxidation that dysregulates the unsaturated fatty acid/protein ratio of the cell membrane. This results in enhanced cell membrane permeability and destruction of the antioxidant enzyme activity within the cell (48). MDA is an oxidative end product of oxygen radicals acting on lipids; thus, levels of MDA reflect the extent of oxygen free radical damage to cells (50). The antioxidant enzyme activities of GSH-Px, CAT and SOD can reflect the body's ability to scavenge oxygen free radicals (51).
A previous study has reported that spices containing phenolic and flavonoid compounds have antioxidant properties (52). The antioxidant capacities of saffron are mainly attributed to its active constituents, including SFR, crocin and crocetin, which have been determined to have antioxidant effects (53). The antioxidant effects of SFR may be associated with the aldehyde group in its structure, which has strong reducing properties. Furthermore, in a previous study on the oxygen radical scavenging ability of SFR, Assimopoulou et al (54) attributed the antioxidant effect of SFR to its ability to donate hydrogen atoms to 2,2-diphenyl-1-picrylhydrazyl radicals (54). In the present study, the results demonstrated that SFR significantly reduced ROS and MDA levels but significantly enhanced SOD, CAT and GSH-Px levels. These results indicated that SFR may exert cardiomyoblast protective effects by enhancing the body's scavenging ability against oxygen free radicals and alleviating lipid peroxidation injury in the cell membrane. However, the inhibitor LY294002 reversed the aforementioned beneficial effects of SFR, demonstrating that the protective effects of SFR against H/R-induced injury are associated with the activation of the PI3K/AKT/GSK3β signaling pathway. Furthermore, SFR post-treatment significantly increased SOD levels but decreased MDA levels, indicating that SFR post-treatment may also have a protective effect against H/R-induced oxidative stress injury.
Mitochondria, the major site of cellular oxidative phosphorylation, serve an important role in the process of cell necrosis and apoptosis induced by MIRI. In normal physiological conditions, the mitochondrial permeability transition pore (mPTP) is in the closed state. When MIRI occurs, factors such as ([Ca2+]i) overload and oxidative stress can stimulate mPTP opening (55,56). As a result, the homeostasis of ([Ca2+]i) is destroyed, leading to increased Ca2+ influx, unbalanced free Ca2+ reuptake and Ca2+ accumulation in the cytoplasm (57). The opening of mPTP can further lead to mitochondrial swelling, mitochondrial destruction, cytochrome c release from mitochondria into the cytosol and activation of the caspase family, which induces apoptosis (58).
Apoptosis is one of the major forms of cell death during MIRI (59), which is a pathological process that is controlled by a variety of genes and mediated via signaling pathways, such as PI3K/AKT (60), Wnt (61) and JNK (62) signaling pathways. Apoptosis is therefore controlled by complex regulatory mechanisms (60). Both Bax and Bcl-2 belong to the Bcl-2 family, in which Bcl-2 is an apoptosis suppressor gene and Bax is a proapoptotic gene, with the two interacting together to regulate apoptosis (63). Jayanthi et al (64) reported that the regulation of apoptosis is dependent on the Bcl-2/Bax ratio, and that the lower the Bcl-2/Bax ratio, the higher the degree of apoptosis. The caspase protein family are important cell regulatory genes, which are involved in the initiation and regulation of apoptosis. Caspase-3 is considered to be a key protease activated by various apoptotic stimuli (65). In the present study, the results demonstrated that SFR significantly elevated MMP levels, while it decreased ([Ca2+]i) concentration and the apoptotic rate of H/R-induced H9c2 cardiac myoblasts, improving cell survival. Furthermore, western blotting demonstrated that SFR significantly inhibited the caspase-3, cleaved caspase-3 and Bax protein expression levels and significantly upregulated Bcl-2 protein expression levels. However, LY294002 reversed the aforementioned beneficial effects of SFR and therefore demonstrated that the protective effects of SFR against H/R-induced injury may be associated with the activation of the PI3K/AKT/GSK3β signaling pathway. Furthermore, SFR post-treatment significantly decreased caspase-3 levels, suggesting that SFR post-treatment also has a protective effect against H/R-induced apoptosis.
The intracellular PI3K/AKT/GSK3β signal transduction pathway serves important biological roles in cellular mechanisms, such as apoptosis, cell survival and proliferation (66). During MIRI, the RISK signaling pathway is activated and the expression levels of numerous types of proteins with endogenous protective effects increase (14). PI3K, which serves a key role in the RISK signaling pathway, is activated, causing downstream AKT to transfer from the cytosol to the cell membrane (67). AKT translocates from the cytosol to the cell membrane and undergoes a conformational change, which is subsequently activated by phosphorylation at the Ser473 site. Activated AKT exhibits anti-myocardial ischemia/hypoxia and anti-myocardial apoptosis effects (68). p-AKT further promotes the phosphorylation of downstream GSK3β proteins, thereby reducing the activation of caspase-3 and closing the mPTP to exert cell survival, antioxidant and antiapoptotic effects (69,70). p-AKT can also activate Bcl-2, reducing the activation of Bax and stabilizing the inner and outer mitochondrial membranes to further exert antiapoptotic effects (71). The results of the present study demonstrated that SFR significantly increased the phosphorylation levels of PI3K, AKT and GSK3β. However, the protein expression levels of apoptosis-related proteins were significantly decreased when cells were treated with SFR. These results demonstrated that the activation of caspase-3 may be inhibited by SFR, which may be associated with the activation of the PI3K/AKT/GSK3β signaling pathway. In order to further confirm that the myocardial protective effect of SFR was associated with the activation of the PI3K/AKT/GSK3β signaling pathway, the PI3K/AKT specific inhibitor LY294002 was used for further verification. The results demonstrated that LY294002 significantly inhibited the effects of SFR on PI3K/AKT/GSK3β signaling pathway apoptosis-related protein expression levels, demonstrating the important role of the PI3K/AKT/GSK3β signaling pathway in combating H/R injury.
In conclusion, SFR has been demonstrated to alleviate H/R-induced cardiomyocyte injury and reduce oxidative stress, ([Ca2+]i) overload and apoptotic rate, which are associated with the involvement of SFR in the regulation of the PI3K/AKT/GSK3β signaling pathway. The present study provides a theoretical basis for investigating the use of SFR as a therapeutic for the treatment of MIRI. However, the present study has certain limitations. We used H9c2 cardiac myoblasts to study the protective effect of SFR in vitro, although with the advantages of easy operation and the ability to intuitively reflect the effects of the studied drugs, compared with in vivo experiments, certain processes cannot be simulated in vitro. Therefore, further exploration is still needed.
Supplementary Material
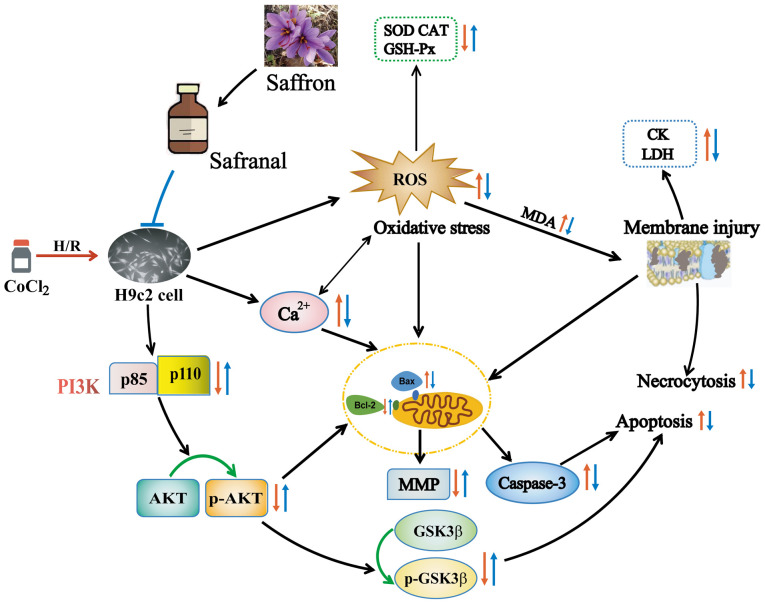
Figure 10.
Mechanism for the protective effects of safranal. Red arrow, damage caused by H/R. The direction of the red arrow represents the trend of oxidative stress- and apoptosis-related indicators, and PI3K/AKT/GSK3β pathway protein expression caused by H/R. Blue arrow, protective effect of SFR. The direction of the blue arrow represents the effect of SFR on indicators associated with oxidative stress and apoptosis, and PI3K/AKT/GSK3β pathway protein expression. ROS, reactive oxygen species; H/R, hypoxia/reoxygenation; SOD, superoxide dismutase; MDA, malondialdehyde; CAT, catalase; GSH-Px, glutathione peroxidase; LDH, lactate dehydrogenase; CK-MB, creatine kinase-MB.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Research Fund of Administration of Traditional Chinese Medicine of Hebei Province (grant no. 2019081) and the Research Foundation of Hebei University of Chinese Medicine (grant nos. YTZ2019003 and KTZ2019009).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JS, YL and SS designed and planned the study. HeW, BZ, KC and XH carried out the experiments. BZ, HοW and LL performed the data analysis. HeW wrote the original draft. BZ, KC, XH, LL, JS and YL reviewed and edited the manuscript. JS and YL supervised the project. SS was involved in the identification of resources. HeW and SS confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Sanderson JE, Mayosi B, Yusuf S, Reddy S, Hu S, Chen Z, Timmis A. Global burden of cardiovascular disease. Heart. 2007;93(1175) doi: 10.1136/hrt.2007.131060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahmood SS, Levy D, Vasan RS, Wang TJ. The Framingham Heart Study and the epidemiology of cardiovascular disease: A historical perspective. Lancet. 2014;383:999–1008. doi: 10.1016/S0140-6736(13)61752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang G, Gao S, Li X, Zhang L, Tan H, Xu L, Chen Y, Geng Y, Lin Y, Aertker B, et al. Pharmacological postconditioning with lactic acid and hydrogen rich saline alleviates myocardial reperfusion injury in rats. Sci Rep. 2015;5(9858) doi: 10.1038/srep09858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Najib K, Boateng S, Sangodkar S, Mahmood S, Whitney H, Wang CE, Racsa P, Sanborn TA. Incidence and characteristics of patients presenting with acute myocardial infarction and non-obstructive coronary artery disease. Catheter Cardiovasc Interv. 2015;86 (Suppl 1):S23–S27. doi: 10.1002/ccd.26043. [DOI] [PubMed] [Google Scholar]
- 5.O'Neill WW, Topol EJ, Pitt B. Reperfusion therapy of acute myocardial infarction. Prog Cardiovasc Dis. 1988;30:235–266. doi: 10.1016/0033-0620(88)90018-7. [DOI] [PubMed] [Google Scholar]
- 6.Braunwald E, Kloner RA. Myocardial reperfusion: A double-edged sword? J Clin Invest. 1985;76:1713–1719. doi: 10.1172/JCI112160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hausenloy DJ, Yellon DM. Targeting myocardial reperfusion injury-the search continues. N Engl J Med. 2015;373:1073–1075. doi: 10.1056/NEJMe1509718. [DOI] [PubMed] [Google Scholar]
- 8.Jennings RB. Historical perspective on the pathology of myocardial ischemia/reperfusion injury. Circ Res. 2013;113:428–438. doi: 10.1161/CIRCRESAHA.113.300987. [DOI] [PubMed] [Google Scholar]
- 9.Turer AT, Hill JA. Pathogenesis of myocardial ischemia-reperfusion injury and rationale for therapy. Am J Cardiol. 2010;106:360–368. doi: 10.1016/j.amjcard.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moens AL, Claeys MJ, Timmermans JP, Vrints CJ. Myocardial ischemia/reperfusion-injury, a clinical view on a complex pathophysiological process. Int J Cardiol. 2005;100:179–190. doi: 10.1016/j.ijcard.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 11.Cheng BC, Huang HS, Chao CM, Hsu CC, Chen CY, Chang CP. Hypothermia may attenuate ischemia/reperfusion-induced cardiomyocyte death by reducing autophagy. Int J Cardiol. 2013;168:2064–2069. doi: 10.1016/j.ijcard.2013.01.162. [DOI] [PubMed] [Google Scholar]
- 12.Reeve JL, Duffy AM, O'Brien T, Samali A. Don't lose heart - therapeutic value of apoptosis prevention in the treatment of cardiovascular disease. J Cell Mol Med. 2005;9:609–622. doi: 10.1111/j.1582-4934.2005.tb00492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blebea J, Kerr JC, Padberg FT Jr, Hobson RW II. Triphenyl tetrazolium chloride as a histochemical marker of skeletal muscle ischemia and reperfusion injury. Curr Surg. 1987;44:134–136. [PubMed] [Google Scholar]
- 14.Heusch G. Molecular basis of cardioprotection: Signal transduction in ischemic pre-, post-, and remote conditioning. Circ Res. 2015;116:674–699. doi: 10.1161/CIRCRESAHA.116.305348. [DOI] [PubMed] [Google Scholar]
- 15.Hausenloy DJ, Yellon DM. Survival kinases in ischemic preconditioning and postconditioning. Cardiovasc Res. 2006;70:240–253. doi: 10.1016/j.cardiores.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 16.Ban K, Cooper AJ, Samuel S, Bhatti A, Patel M, Izumo S, Penninger JM, Backx PH, Oudit GY, Tsushima RG. Phosphatidylinositol 3-kinase gamma is a critical mediator of myocardial ischemic and adenosine-mediated preconditioning. Circ Res. 2008;103:643–653. doi: 10.1161/CIRCRESAHA.108.175018. [DOI] [PubMed] [Google Scholar]
- 17.Zhang CM, Gao L, Zheng YJ, Yang HT. Berbamine protects the heart from ischemia/reperfusion injury by maintaining cytosolic Ca(2+) homeostasis and preventing calpain activation. Circ J. 2012;76:1993–2002. doi: 10.1253/circj.cj-11-1431. [DOI] [PubMed] [Google Scholar]
- 18.Wu QL, Shen T, Ma H, Wang JK. Sufentanil postconditioning protects the myocardium from ischemia-reperfusion via PI3K/Akt-GSK-3β pathway. J Surg Res. 2012;178:563–570. doi: 10.1016/j.jss.2012.05.081. [DOI] [PubMed] [Google Scholar]
- 19.Quintieri AM, Baldino N, Filice E, Seta L, Vitetti A, Tota B, De Cindio B, Cerra MC, Angelone T. Malvidin, a red wine polyphenol, modulates mammalian myocardial and coronary performance and protects the heart against ischemia/reperfusion injury. J Nutr Biochem. 2013;24:1221–1231. doi: 10.1016/j.jnutbio.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Miura T, Miki T. GSK-3beta, a therapeutic target for cardiomyocyte protection. Circ J. 2009;73:1184–1192. doi: 10.1253/circj.cj-09-0284. [DOI] [PubMed] [Google Scholar]
- 21.Abu-Izneid T, Rauf A, Khalil AA, Olatunde A, Khalid A, Alhumaydhi FA, Aljohani AS, Sahab Uddin M, Heydari M, Khayrullin M, et al. Nutritional and health beneficial properties of saffron (Crocus sativus L): A comprehensive review. Crit Rev Food Sci Nutr. 2020;17:1–24. doi: 10.1080/10408398.2020.1857682. [DOI] [PubMed] [Google Scholar]
- 22.Arzi L, Hoshyar R. Saffron anti-metastatic properties, ancient spice novel application. Crit Rev Food Sci Nutr. 2021;3:1–12. doi: 10.1080/10408398.2020.1871320. [DOI] [PubMed] [Google Scholar]
- 23.Maggi MA, Bisti S, Picco C. Saffron: Chemical composition and neuroprotective activity. Molecules. 2020;25(5618) doi: 10.3390/molecules25235618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su X, Yuan C, Wang L, Chen R, Li X, Zhang Y, Liu C, Liu X, Liang W, Xing Y. The beneficial effects of saffron extract on potential oxidative stress in cardiovascular diseases. Oxid Med Cell Longev. 2021;2021(6699821) doi: 10.1155/2021/6699821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rameshrad M, Razavi BM, Hosseinzadeh H. Saffron and its derivatives, crocin, crocetin and safranal: A patent review. Expert Opin Ther Pat. 2018;28:147–165. doi: 10.1080/13543776.2017.1355909. [DOI] [PubMed] [Google Scholar]
- 26.Cerdá-Bernad D, Valero-Cases E, Pastor JJ, Frutos MJ. Saffron bioactives crocin, crocetin and safranal: Effect on oxidative stress and mechanisms of action. Crit Rev Food Sci Nutr. 2020;24:1–18. doi: 10.1080/10408398.2020.1864279. [DOI] [PubMed] [Google Scholar]
- 27.Forouzanfar F, Asadpour E, Hosseinzadeh H, Boroushaki MT, Adab A, Dastpeiman SH, Sadeghnia HR. Safranal protects against ischemia-induced PC12 cell injury through inhibiting oxidative stress and apoptosis. Naunyn Schmiedebergs Arch Pharmacol. 2021;394:707–716. doi: 10.1007/s00210-020-01999-8. [DOI] [PubMed] [Google Scholar]
- 28.Lambrianidou A, Koutsougianni F, Papapostolou I, Dimas K. Recent advances on the anticancer properties of saffron (Crocus sativus L.) and its major constituents. Molecules. 2020;26(86) doi: 10.3390/molecules26010086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang X, Li Y, Feng JL, Nik Nabil WN, Wu R, Lu Y, Liu H, Xi ZC, Xu HX. Safrana l prevents prostate cancer recurrence by blocking the re-activation of quiescent cancer cells via downregulation of S-Phase kinase-associated protein 2. Front Cell Dev Biol. 2020;8(598620) doi: 10.3389/fcell.2020.598620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin W, Xue Y, Xue Y, Liang Y, Zhang Y, Zhang J, Chu X, Wang H, Guan S. Inhibitory effects of four active components in saffron on human ether-a-go-go-related gene (hERG) K+ currents. Gen Physiol Biophys. 2020;39:491–498. doi: 10.4149/gpb_2020025. [DOI] [PubMed] [Google Scholar]
- 31.Mehdizadeh R, Parizadeh MR, Khooei AR, Mehri S, Hosseinzadeh H. Cardioprotective effect of saffron extract and safranal in isoproterenol-induced myocardial infarction in wistar rats. Iran J Basic Med Sci. 2013;16:56–63. [PMC free article] [PubMed] [Google Scholar]
- 32.Bharti S, Golechha M, Kumari S, Siddiqui KM, Arya DS. Akt/GSK-3β/eNOS phosphorylation arbitrates safranal-induced myocardial protection against ischemia-reperfusion injury in rats. Eur J Nutr. 2012;51:719–727. doi: 10.1007/s00394-011-0251-y. [DOI] [PubMed] [Google Scholar]
- 33.Xue Y, Jin W, Xue Y, Zhang Y, Wang H, Zhang Y, Guan S, Chu X, Zhang J. Safranal, an active constituent of saffron, ameliorates myocardial ischemia via reduction of oxidative stress and regulation of Ca2+ homeostasis. J Pharmacol Sci. 2020;143:156–164. doi: 10.1016/j.jphs.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 34.Kooy NW, Royall JA, Ischiropoulos H. Oxidation of 2',7'-dichlorofluorescin by peroxynitrite. Free Radic Res. 1997;27:245–254. doi: 10.3109/10715769709065763. [DOI] [PubMed] [Google Scholar]
- 35.Sivandzade F, Bhalerao A, Cucullo L. Analysis of the mitochondrial membrane potential using the cationic JC-1 dye as a sensitive fluorescent probe. Bio Protoc. 2019;9(e3128) doi: 10.21769/BioProtoc.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cui H, Miao S, Esworthy T, Zhou X, Lee SJ, Liu C, Yu ZX, Fisher JP, Mohiuddin M, Zhang LG. 3D bioprinting for cardiovascular regeneration and pharmacology. Adv Drug Deliv Rev. 2018;132:252–269. doi: 10.1016/j.addr.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee: Heart disease and stroke statistics-2020 Update: A report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 38.Simon-Yarza T, Bataille I, Letourneur D. Cardiovascular Bio-Engineering: Current State of the Art. J Cardiovasc Transl Res. 2017;10:180–193. doi: 10.1007/s12265-017-9740-6. [DOI] [PubMed] [Google Scholar]
- 39.Pang Z, Wang T, Li Y, Wang L, Yang J, Dong H, Li S. Liraglutide ameliorates COCl2-induced oxidative stress and apoptosis in H9C2 cells via regulating cell autophagy. Exp Ther Med. 2020;19:3716–3722. doi: 10.3892/etm.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fang Z, Luo W, Luo Y. Protective effect of α-mangostin against CoCl2-induced apoptosis by suppressing oxidative stress in H9C2 rat cardiomyoblasts. Mol Med Rep. 2018;17:6697–6704. doi: 10.3892/mmr.2018.8680. [DOI] [PubMed] [Google Scholar]
- 41.Yang Z, Yang C, Xiao L, Liao X, Lan A, Wang X, Guo R, Chen P, Hu C, Feng J. Novel insights into the role of HSP90 in cytoprotection of H2S against chemical hypoxia-induced injury in H9c2 cardiac myocytes. Int J Mol Med. 2011;28:397–403. doi: 10.3892/ijmm.2011.682. [DOI] [PubMed] [Google Scholar]
- 42.Muñoz-Sánchez J, Chánez-Cárdenas ME. The use of cobalt chloride as a chemical hypoxia model. J Appl Toxicol. 2019;39:556–570. doi: 10.1002/jat.3749. [DOI] [PubMed] [Google Scholar]
- 43.Xiao L, Lan A, Mo L, Xu W, Jiang N, Hu F, Feng J, Zhang C. Hydrogen sulfide protects PC12 cells against reactive oxygen species and extracellular signal-regulated kinase 1/2-mediated downregulation of glutamate transporter-1 expression induced by chemical hypoxia. Int J Mol Med. 2012;30:1126–1132. doi: 10.3892/ijmm.2012.1090. [DOI] [PubMed] [Google Scholar]
- 44.Hescheler J, Meyer R, Plant S, Krautwurst D, Rosenthal W, Schultz G. Morphological, biochemical, and electrophysiological characterization of a clonal cell (H9c2) line from rat heart. Circ Res. 1991;69:1476–1486. doi: 10.1161/01.res.69.6.1476. [DOI] [PubMed] [Google Scholar]
- 45.Goyal SN, Arora S, Sharma AK, Joshi S, Ray R, Bhatia J, Kumari S, Arya DS. Preventive effect of crocin of Crocus sativus on hemodynamic, biochemical, histopathological and ultrastuctural alterations in isoproterenol-induced cardiotoxicity in rats. Phytomedicine. 2010;17:227–232. doi: 10.1016/j.phymed.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 46.Muders F, Neubauer S, Luchner A, Fredersdorf S, Ickenstein G, Riegger GA, Horn M, Elsner D. Alterations in myocardial creatinine kinase (CK) and lactate dehydrogenase (LDH) isoenzyme-distribution in a model of left ventricular dysfunction. Eur J Heart Fail. 2001;3:1–5. doi: 10.1016/s1388-9842(00)00085-4. [DOI] [PubMed] [Google Scholar]
- 47.Amani M, Jeddi S, Ahmadiasl N, Usefzade N, Zaman J. Effect of HEMADO on level of CK-MB and LDH enzymes after ischemia/reperfusion injury in isolated rat heart. Bioimpacts. 2013;3:101–104. doi: 10.5681/bi.2013.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ding M, Li M, Zhang EM, Yang HL. FULLEROL alleviates myocardial ischemia-reperfusion injury by reducing inflammation and oxidative stress in cardiomyocytes via activating the Nrf2/HO-1 signaling pathway. Eur Rev Med Pharmacol Sci. 2020;24:9665–9674. doi: 10.26355/eurrev_202009_23056. [DOI] [PubMed] [Google Scholar]
- 49.Madungwe NB, Zilberstein NF, Feng Y, Bopassa JC. Critical role of mitochondrial ROS is dependent on their site of production on the electron transport chain in ischemic heart. Am J Cardiovasc Dis. 2016;6:93–108. [PMC free article] [PubMed] [Google Scholar]
- 50.Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005;15:316–328. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 51.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;115:500–508. doi: 10.1172/JCI24408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghadrdoost B, Vafaei AA, Rashidy-Pour A, Hajisoltani R, Bandegi AR, Motamedi F, Haghighi S, Sameni HR, Pahlvan S. Protective effects of saffron extract and its active constituent crocin against oxidative stress and spatial learning and memory deficits induced by chronic stress in rats. Eur J Pharmacol. 2011;667:222–229. doi: 10.1016/j.ejphar.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 53.Rahaiee S, Moini S, Hashemi M, Shojaosadati SA. Evaluation of antioxidant activities of bioactive compounds and various extracts obtained from saffron (Crocus sativus L.): A review. J Food Sci Technol. 2015;52:1881–1888. doi: 10.1007/s13197-013-1238-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Assimopoulou AN, Sinakos Z, Papageorgiou VP. Radical scavenging activity of Crocus sativus L. extract and its bioactive constituents. Phytother Res. 2005;19:997–1000. doi: 10.1002/ptr.1749. [DOI] [PubMed] [Google Scholar]
- 55.Ong SB, Samangouei P, Kalkhoran SB, Hausenloy DJ. The mitochondrial permeability transition pore and its role in myocardial ischemia reperfusion injury. J Mol Cell Cardiol. 2015;78:23–34. doi: 10.1016/j.yjmcc.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 56.Ertracht O, Malka A, Atar S, Binah O. The mitochondria as a target for cardioprotection in acute myocardial ischemia. Pharmacol Ther. 2014;142:33–40. doi: 10.1016/j.pharmthera.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 57.Weiss JN, Korge P, Honda HM, Ping P. Role of the mitochondrial permeability transition in myocardial disease. Circ Res. 2003;93:292–301. doi: 10.1161/01.RES.0000087542.26971.D4. [DOI] [PubMed] [Google Scholar]
- 58.Park SS, Zhao H, Mueller RA, Xu Z. Bradykinin prevents reperfusion injury by targeting mitochondrial permeability transition pore through glycogen synthase kinase 3beta. J Mol Cell Cardiol. 2006;40:708–716. doi: 10.1016/j.yjmcc.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 59.McCully JD, Wakiyama H, Hsieh YJ, Jones M, Levitsky S. Differential contribution of necrosis and apoptosis in myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2004;286:H1923–H1935. doi: 10.1152/ajpheart.00935.2003. [DOI] [PubMed] [Google Scholar]
- 60.Yu P, Ma S, Dai X, Cao F. Elabela alleviates myocardial ischemia reperfusion-induced apoptosis, fibrosis and mitochondrial dysfunction through PI3K/AKT signaling. Am J Transl Res. 2020;12:4467–4477. [PMC free article] [PubMed] [Google Scholar]
- 61.Tao J, Abudoukelimu M, Ma YT, Yang YN, Li XM, Chen BD, Liu F, He CH, Li HY. Secreted frizzled related protein 1 protects H9C2 cells from hypoxia/re-oxygenation injury by blocking the Wnt signaling pathway. Lipids Health Dis. 2016;15(72) doi: 10.1186/s12944-016-0240-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li HW, Xiao FY. Effect of hydrogen sulfide on cardiomyocyte apoptosis in rats with myocardial ischemia-reperfusion injury via the JNK signaling pathway. Eur Rev Med Pharmacol Sci. 2020;24:2054–2061. doi: 10.26355/eurrev_202002_20383. [DOI] [PubMed] [Google Scholar]
- 63.Dong JW, Zhu HF, Zhu WZ, Ding HL, Ma TM, Zhou ZN. Intermittent hypoxia attenuates ischemia/reperfusion induced apoptosis in cardiac myocytes via regulating Bcl-2/Bax expression. Cell Res. 2003;13:385–391. doi: 10.1038/sj.cr.7290184. [DOI] [PubMed] [Google Scholar]
- 64.Jayanthi S, Deng X, Bordelon M, McCoy MT, Cadet JL. Methamphetamine causes differential regulation of pro-death and anti-death Bcl-2 genes in the mouse neocortex. FASEB J. 2001;15:1745–1752. doi: 10.1096/fj.01-0025com. [DOI] [PubMed] [Google Scholar]
- 65.Zhou T, Guo S, Wang S, Li Q, Zhang M. Protective effect of sevoflurane on myocardial ischemia-reperfusion injury in rat hearts and its impact on HIF-1α and caspase-3 expression. Exp Ther Med. 2017;14:4307–4311. doi: 10.3892/etm.2017.5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang J, Liu J, Xie L, Cai X, Ma X, Gong J. Bisoprolol, a β1 antagonist, protects myocardial cells from ischemia-reperfusion injury via PI3K/AKT/GSK3β pathway. Fundam Clin Pharmacol. 2020;34:708–720. doi: 10.1111/fcp.12562. [DOI] [PubMed] [Google Scholar]
- 67.Maulik A, Davidson SM, Piotrowska I, Walker M, Yellon DM. Ischaemic preconditioning protects cardiomyocytes from anthracycline-induced toxicity via the PI3K pathway. Cardiovasc Drugs Ther. 2018;32:245–253. doi: 10.1007/s10557-018-6793-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu X, Kihara T, Akaike A, Niidome T, Sugimoto H. PI3K/Akt/mTOR signaling regulates glutamate transporter 1 in astrocytes. Biochem Biophys Res Commun. 2010;393:514–518. doi: 10.1016/j.bbrc.2010.02.038. [DOI] [PubMed] [Google Scholar]
- 69.Wang MY, Meng M, Yang CC, Zhang L, Li YL, Zhang L, Li L. Cornel iridoid glycoside improves cognitive impairment induced by chronic cerebral hypoperfusion via activating PI3K/Akt/GSK-3β/CREB pathway in rats. Behav Brain Res. 2020;379(112319) doi: 10.1016/j.bbr.2019.112319. [DOI] [PubMed] [Google Scholar]
- 70.Thirunavukkarasu M, Selvaraju V, Tapias L, Sanchez JA, Palesty JA, Maulik N. Protective effects of Phyllanthus emblica against myocardial ischemia-reperfusion injury: The role of PI3-kinase/glycogen synthase kinase 3β/β-catenin pathway. J Physiol Biochem. 2015;71:623–633. doi: 10.1007/s13105-015-0426-8. [DOI] [PubMed] [Google Scholar]
- 71.Wang D, Zhang X, Li D, Hao W, Meng F, Wang B, Han J, Zheng Q. Kaempferide protects against myocardial ischemia/reperfusion injury through activation of the PI3K/Akt/GSK-3beta pathway. Mediators Inflamm. 2017;2017(5278218) doi: 10.1155/2017/5278218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.