Abstract
The aims of the study were to assess the changes in 19‐years use of antibiotics (overall, by age, sex and geographical area) and of those classes deemed to be quality indicators for their consumption and to evaluate factors associated to antibiotic use. We analyzed drug prescription data collected in the administrative database of the Lombardy Region (Northern Italy) for outpatients aged 40+ years from 2000 to 2019. Logistic regression analyses were performed to evaluate the association between receiving at least one antibiotic prescription and year of observation, gender, age groups, area of residence, polypharmacy and hospitalizations in the index year. The prevalence of patients prescribed with antibiotics remained high from 2000 (33.8%) to 2019 (32.6%). Prevalence of use of second‐line choice antibiotics (penicillin combinations with beta‐lactamase inhibitors, third and fourth generation cephalosporins, macrolides) continued to increase, only fluoroquinolones decreased in 2019 (19%) comparing to 2018 (26%), at the time when the Italian Medicines Agency promulgated safety warnings. Females (OR 1.28, 95%CI 1.27–1.28), people living in Brescia (OR 1.24, 95%CI 1.24–1.25), those exposed to polypharmacy (OR 2.57, 95%CI 2.56–2.57) and those hospitalized 1 to 3 (OR 1.86, 95%CI 1.85–1.86) or more than 3 (OR 2.02, 95%CI 2.01–2.03) times a year had a statistically significant higher risk of receiving antibiotics. The high use of antibiotics over the study period further reinforces the need of impactful interventions, in order to improve the rational use of antibiotics and to reduce the risks of antimicrobial resistance. The differences outlined should be considered when monitoring and planning these interventions.
Keywords: antibiotic, antimicrobial resistance, changes in drug use, inappropriate prescription, quality indicators
We analyzed drug prescription data collected in the administrative database of the Lombardy Region (Northern Italy) for outpatients aged 40+ years from 2000 to 2019, in order to assess the changes in the use of antibiotics and of those classes deemed to be quality indicators for their consumption and to evaluate factors associated to antibiotic use. The prevalence of patients prescribed with antibiotics remained high from 2000 (33.8%) to 2019 (32.6%). Prevalence of use of second‐line choice antibiotics (penicillin combinations with beta‐lactamase inhibitors, third and fourth generation cephalosporins, macrolides) continued to increase, only fluoroquinolones decreased in 2019 (19%) comparing to 2018 (26%), at the time when the Italian Medicines Agency promulgated safety warnings.

Abbreviations
- ATC
Anatomical Therapeutic Classification
- ATS
Agenzia per la Tutela della Salute
- DDD
defined daily doses
- DID
Defined Daily Doses (DDD) per 1000 inhabitants per day
- EMA
European Medicines Agency
- GPs
general practitioners
- NHS
National Health Service
- WHO
World Health Organization
1. INTRODUCTION
Antibiotics have drastically changed modern medicine and extended the average human lifespan but, more than 100 years from the first antibiotic deployment, antibiotic bacteria resistance is now one of the biggest threats for human health. 1 , 2 Recent estimates show that each year more than 670 000 infections occur in European countries due to antibiotic resistance, and as consequence nearly 33 000 people die for these infections, with Italy at the first and worst place accounting for almost 11 000 deaths. 3 Antimicrobial resistance has been increasing across European countries overtime and currently close to one in five infections is due to antibiotic‐resistant bacteria. 4 In general, lower percentages of resistance are reported by countries in the north of Europe and higher percentages by countries in the south and east of Europe, being Escherichia coli (44.2%) the most commonly resistant bacterial species, followed by Staphylococcus aureus (20.6%) and Klebsiella pneumoniae (11.3%). 2 In 2019, 57% of the Escherichia coli isolates were resistant to at least one antibiotic or even to multiple antimicrobial groups, in particular aminopenicillins (57%), fluoroquinolones (24%) and third generation cephalosporins (15%), with a significantly increasing 2015–2019 trend for resistance to third generation cephalosporins. 2 The management of antimicrobial resistance dramatically impacts on costs for European countries health services, which are expected to spend up to EUR 1.1 billion between 2005 to 2015. 2 , 4 For this reason, in 2017, the European Commission and the States decided to adopt a One Health Action Plan to fight antimicrobial resistance. 2 , 3 , 4 , 5
In addition to this concern, the use of antibiotics is responsible for the occurrence of several adverse events. 6 In USA it has been estimated that at least 30% of antibiotics prescribed in physicians’ offices and emergency departments were unnecessary. 7 Several reports demonstrated the inappropriate use of antibiotics in outpatient settings, 8 , 9 where most of the antibiotics are prescribed, 10 mainly due to inadequate knowledge by general practitioners and patients, the too easy access to antibiotics without prescription and the lack of rapid diagnostics tests. 11 Older people, often prescribed with multiple drugs, are particularly vulnerable to the deleterious effects of inappropriate drug use, so that particular attention must be paid when they are prescribed with antibiotics, for example with fluoroquinolones which are associated to increased risk of tendinitis. 12 , 13 Some studies have highlighted different prescribing patterns of antibiotic use by gender and geographical area, 14 , 15 , 16 but scanty data are available on antibiotic use over a large time span in Italy. 17
Based on this background, the primary aim of this study was to assess the changes in antibiotic use over 19 years in adult outpatients living in Lombardy Region, by considering gender, age, living area and antibiotic class. The secondary aim was to assess the changes in the use of specific classes of antibiotics, and possible associations with other drugs, deemed as quality indicators for antibiotic consumption. Finally, we aimed to evaluate the factors possibly associated to the antibiotic use.
2. MATERIALS AND METHODS
2.1. Data source
Data on drug prescriptions were obtained from the Drug Administrative Database of the Lombardy Region, Northern Italy, i.e. the most populous Italian region, accounting for nearly ten million of individuals (around 16% of the overall Italian population). Since 2015 the Lombardy region was divided in eight local health units, called Agency for the Protection of Health (Agenzia per la Tutela della Salute—ATS), distributed throughout the territory of the region (Figure 1). 18 These units deal with the implementation of the regional socio‐health program and the provision of health services through public and private facilities, as well as health control and prevention in public and work environments. Thus, in this context they are involved in the epidemiological surveillance of antibiotic use and antimicrobial resistance. 18
FIGURE 1.
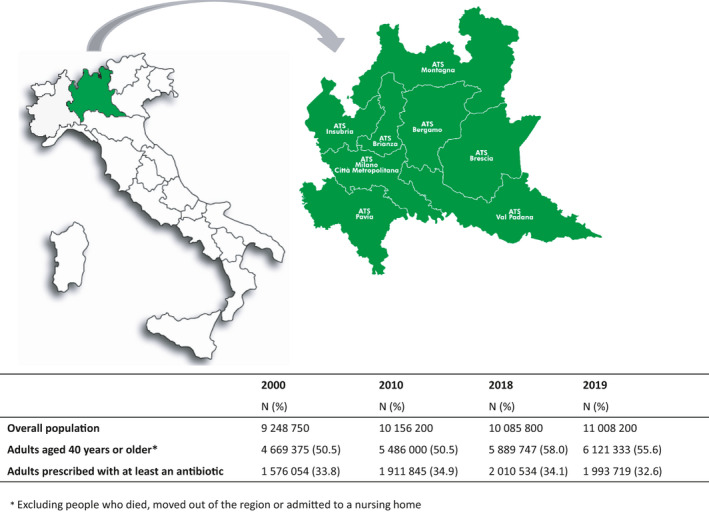
Location of the Lombardy Region in Italy and its health units (Aziende della Tutela della Salute—ATS)
The structure of the Lombardy region database, routinely updated for administrative and reimbursement purposes, has been described in detail elsewhere. 19 , 20 , 21 , 22 , 23 Briefly, it stores all drug prescriptions made by General Practitioners (GPs) to outpatients living in the region, provided free of charge by the Italian National Health Service (NHS) and finally distributed through the pharmacies of the Italian territory. So that, each prescription record contains information on the drug prescribed and then dispensed to the individuals. All data used in this study were managed according to current Italian laws on privacy, and each person was identified by an anonymous code.
2.2. Study population
The population available for this analysis included all residents of the Lombardy Region, aged 40 years or older in each study year, between 1 January 2000 and 31 December 2019. We excluded individuals who died, moved out of the region or institutionalized in each index year.
2.3. Antibiotic use
Antibiotics were defined as all substances belonging to the J01 main therapeutic group of the Anatomical Therapeutic Classification (ATC) system. The use of antibiotic drugs in adult population was evaluated using the prevalence as measure. Prevalence was calculated by dividing the numbers of subjects with at least one antibiotic prescription in the index year by the total number of subjects 40 years or older living in the Region. Prevalence was stratified by age groups, sex and local health units (ATS). In order to point out the main differences over time in the prevalence use, we chose to show results at the beginning, in the middle and at the end (i.e. in 2000, 2010, 2018 and 2019) of the study period. We decided to display also 2018 because in November the European Medicines Agency (EMA) endorsed restrictions on the use of quinolone and fluoroquinolone antibiotics, 24 probably shaping the pattern of use.
The extent of antibiotic drug consumption in the adult population was estimated using the Defined Daily Doses (DDD) per 1000 inhabitants per day (DID), calculated as: [active substance (expressed in g)]/[365 × DDD] divided by the number of inhabitants/1000. The DID was then stratified by age groups, sex and ATS.
Prevalence of antibiotic use was calculated for the overall antibiotics (J01) and for the following sub‐classes: tetracyclines (J01A), amphenicols (J01B), penicillins (J01C), cephalosporins (J01D), sulfonamides and trimethoprim (J01E), macrolides, lincosamides and streptogramins (J01F), aminoglycoside antibacterials (J01G), quinolones (J01M), combinations of antibacterials (J01R), other antibacterials, including glicopeptide, imidazole derivates, and nitrofuran derivates (J01X).
Based on the European Surveillance of Antimicrobial Consumption (ESAC) quality indicators for antibiotic consumption in the community, 25 , 26 we specifically assessed the use of combinations of penicillin with beta‐lactamase inhibitors (J01CR), third‐ and fourth‐generation cephalosporins (J01DD + J01DE), fluoroquinolones (J01MA) and the combination of fluoroquinolones and corticosteroids (H02) in the same index year, in order to point out the potential inappropriate use of antibiotics. We also assessed the seasonality of antibiotic prescription rates by stratifying the prevalence of antibiotic users by each month of the years considered.
2.4. Statistical analysis
Characteristics of subjects included in the study was presented as mean and standard deviation for continuous variables and percentage of the total for categorical variables.
The geographical distribution of antibiotics consumptions was plotted on a choropleth map. The values of DID were categorized into tertiles based on mean and standard deviation.
Univariable and multivariable logistic regression analyses were performed to evaluate the association between receiving at least one antibiotic prescription and year of observation, gender, age groups (categorized as 40–49, 50–59, 60–69, 70–79, 80–89 and ≥90 years old), local health unit of residence, polypharmacy (exposure to 5+ different drugs, excluding antibiotics), number of hospitalizations in the index year (categorized as 0, 1–3, >3). Given that subjects included in the study could be repeatedly present in the different observation years, a cluster correction on standard errors was introduced in the model. Statistical analysis was performed using JMP Pro 12 and Stata IC 15 software. A p‐value <.05 was considered to be statistically significant.
2.5. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY, 27 and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20. 28
3. RESULTS
3.1. Overall antibiotic use
During the study period, nearly 5 million of adult outpatients (about 50% of the overall Lombardy population) were included in the analysis. Out of them, 33.8% received at least an antibiotic in 2000 (17.6 DID), 34.9% in 2010 (18.2 DID), 34.1% in 2018 (18.2 DID) and 32.6% in 2019 (17.0 DID) (Figure 1). Demographic characteristics of antibiotic users were similar over the years of the study period (data not shown). Each treated patient received in 2000–2019 an average of 1.5 ± 0.9–1.4 ± 0.8 antibiotics (median 1; interquartile range 1–2), an average of 1.9 ± 1.8–1.9 ± 1.7 antibiotic prescriptions (median 1; interquartile range 1–2) and of 3.4 ± 5.3–3.0 ± 4.0 antibiotic boxes (median 2; interquartile range 1–4). The overall study population was mainly composed by females (54%–52.5% in 2000–2019, respectively). The mean age ranged from 59.3 ± 12.8 in 2000 up to 60.7 ± 13.7 in 2019.
Figure 2 shows the prevalence of antibiotic use over time according to the main antibiotic classes (ATC 3d level). Prevalence of subjects with at least a J01 prescription in the year remained stable over the all‐study period not showing a specific trend.
FIGURE 2.
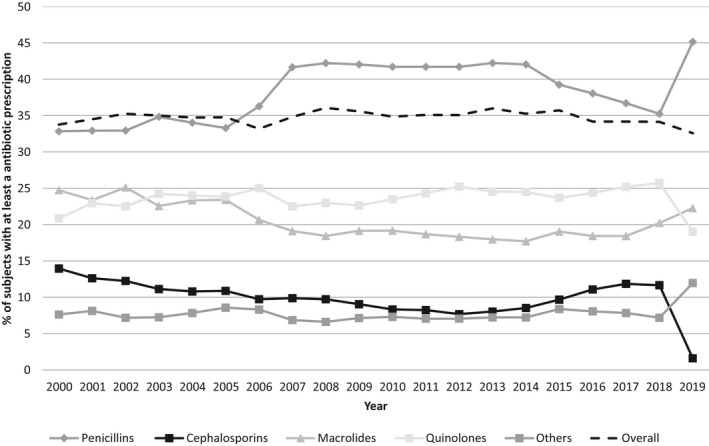
Prevalence (%) of people receiving at least an antibiotic by main antibiotic classes from 2000 to 2019
Penicillins were always the most prescribed class (2000–2019: 32.8%–45.1%), followed by macrolides (24.7% in 2000), which from 2003 were exceeded by quinolones until 2018, when they decreased again (19.0%) and macrolides increased (22.3%). In 2000, amoxicillin with beta‐lactamase inhibitors was the most prescribed antibiotic (20.2%), followed by amoxicillin (19.9%) and clarithromycin (14%). In 2019, amoxicillin with beta‐lactamase inhibitors remained the most prescribed with a higher prevalence of use (43.1%), followed by azithromycin (15.4%) and levofloxacin (13.0%).
3.2. Antibiotic use for gender and age
Figure S1 shows that females were constantly more prescribed than males over time (females vs. males: 35.9% vs. 31.2% in 2000 and 35.5% vs. 29.3% in 2019, p‐value <.0001) with about 18 DID for females and 16 DID for males. In particular, 40–69 years old females were more prescribed over time compared to males (females vs. males: 34.5% vs. 29.1% in 2000 and 33.7% vs. 26.6% in 2019, p‐value <.0001), but 80+ old males exceeded females (females vs. males: 42.2% vs. 46.1% in 2000 and 41.8% vs. 42.8% in 2019, p‐value <.0001). Older people were always more prescribed than the youngest, ranging from 31.6 to 24.9 DID in 2000–2019 for the 90+ class of age and 13.7–13.8 DID for the 40–49 age groups, with a parallel pattern of prevalence overtime (Figure S2).
In 2000, females were less prescribed than males with penicillins (31.8 vs. 34.1%, p‐value <.0001), but from 2010 the pattern of prescription has reversed, having 54.5% of females prescribed vs 32.2% males, and 50% females vs. 31.4% males in 2019 (p‐value <.0001). Even quinolones started in 2000 with a similar pattern of use in both sexes, but from 2010 females were more prescribed than males (29 vs. 19%, p‐value <.0001) (Figure 3A–D). Penicillins were always the most prescribed antibiotic class over time in the younger groups of age, with a linear decreasing trend at advanced age (in 2000, 40–49 years: 38.2% vs. 90+ years: 21.5%; in 2010, 40–49: 47% vs. 90+: 30%; in 2019, 40–49: 45% vs. 90+: 29%, p‐value <.0001). On the contrary quinolones were always the most prescribed class of antibiotic over time in the oldest population (in 2000, 40–49 years: 14.6% vs. 90+ years: 27.5%; in 2010, 40–49: 16.7% vs. 90+: 29.6%; in 2019, 40–49: 12.3% vs. 90+: 18.6%, p‐value <.0001) (Figure 4A–D).
FIGURE 3.
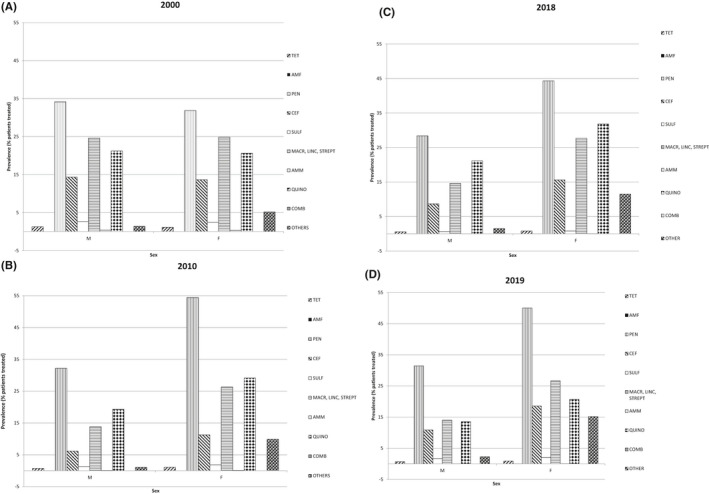
Prevalence (%) of people receiving at least an antibiotic according to antibiotic classes by sex in 2000 (A), 2010 (B), 2018 (C) and 2019 (D). TET‐Tetraciclines (J01A); AMF‐Amfenicoles (J01B); PEN‐Penicillins (J01C); CEF‐Cefalosporins and carbapenems (J01D); SULF‐Sulfonamides and trimetoprim (J01E); MACR, LINC, STREPT‐ Macrolides, lincosamides e streptogrammines (J01F); AMM‐ amminoglucosides (J 01G); QUINO‐Quinolones (J01M); Combinations (J01R); OTHER including Glycopeptide, Imidazole derivates, Nitrofuran derivates (J01X)
FIGURE 4.
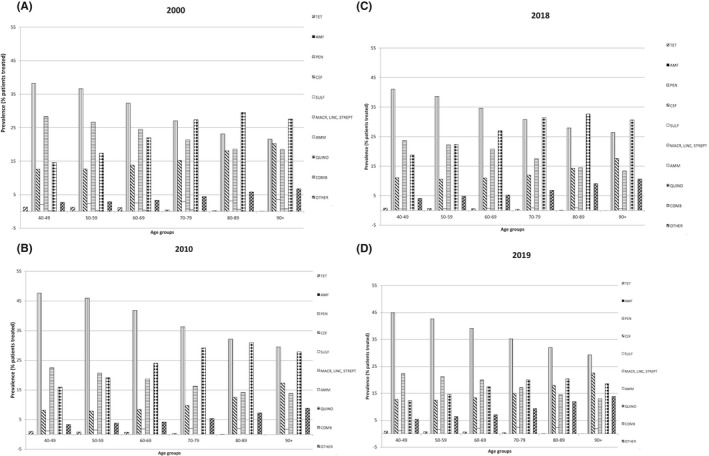
Prevalence (%) of people receiving at least an antibiotic according to antibiotic classes by age groups in 2000 (A), 2010 (B), 2018 (C), and 2019 (D). TET‐Tetraciclines (J01A); AMF‐Amfenicoles (J01B); PEN‐Penicillins (J01C); CEF‐Cefalosporins and carbapenems (J01D); SULF‐Sulfonamides and trimetoprim (J01E); MACR, LINC, STREPT‐ Macrolides, lincosamides e streptogrammines (J01F); AMM‐ amminoglucosides (J 01G); QUINO‐Quinolones (J01M); Combinations (J01R); OTHER including Glycopeptide, Imidazole derivates, Nitrofuran derivates (J01X)
3.3. Antibiotic use for health units (Agenzia per la Tutela della Salute—ATS)
Differences were found in the prevalence of patients prescribed with antibiotics between health units (ATS) over time: in 2000 the area with the lowest prevalence of patients prescribed (31.5%–15.5 DID) was the ATS Montagna (corresponding to the mountainous and hilly area in the North of the region) and that with the highest prevalence (39.5%—21.3 DID) was Brescia ATS (an industrialized area in the East of the region) (p‐value <.0001) (Figure 5A). During the study period the geographical distribution of antibiotic use changed among areas, but Brescia ATS always had the highest prevalence of antibiotic use, with a slight decline over time (38.7%—21.4 DID in 2010, 36.9%—20.2 DID in 2018, 35.6%—19 DID in 2019) (Figure 5B–D). The area of Montagna ATS of was at lowest prevalence of use also in 2010 (33.1%—17.2 DID), but Milan city ATS (the most populous city of the region) took its place in the ranking in 2018 (32.8%—17.7 DID) and in 2019 (31.4%—16.7 DID) (Figure 5B–D). Penicillins were the most used class of antibiotics over time in all the ATS, having the highest prevalence of use in Brescia ATS (2000: 40%, 2010: 45%, 2018: 39%, 2019: 42%).
FIGURE 5.
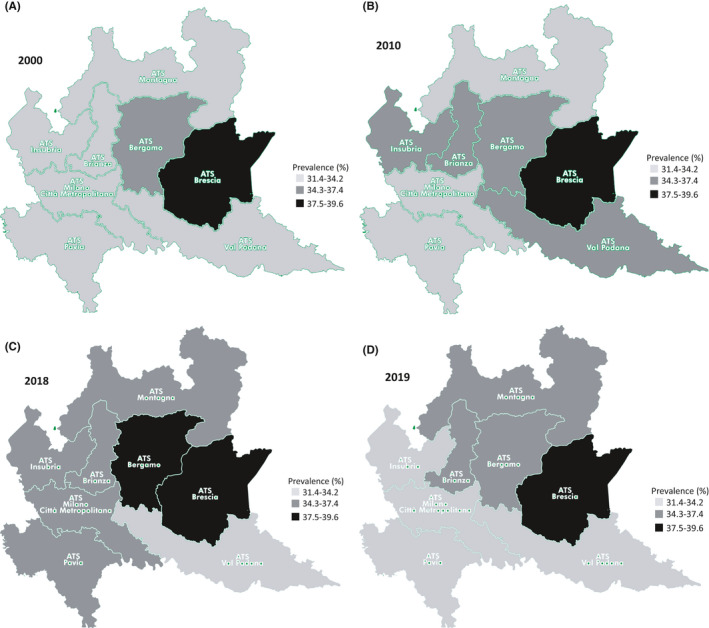
Prevalence (%) of people receiving at least an antibiotic by health units (called ATS) in 2000 (A), 2010 (B), 2018 (C), and 2019 (D)
3.4. Quality indicators of antibiotic use
Figure 6 shows the prevalence of people receiving at least a prescription over time of penicillin combinations with beta‐lactamase inhibitors, of third and fourth generation cephalosporins and of fluoroquinolones.
FIGURE 6.
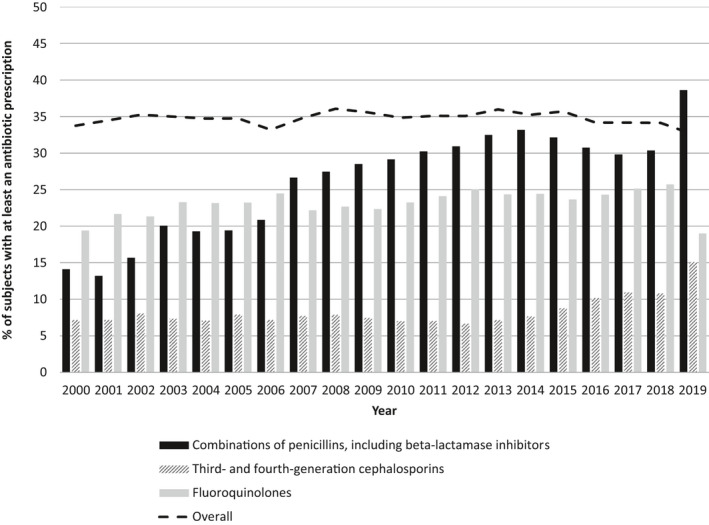
Prevalence (%) of people receiving at least a prescription of combinations of penicillins, or third‐ and fourth‐generation cephalosporins and fluoroquinolones over time
Prevalence of patients prescribed with penicillin combinations with beta‐lactamase inhibitors increased from 14.1% in 2000 up to 32.2% in 2015. After this time, it constantly decreased but in 2019 dramatically increased (38.6%). The prevalence of use of cephalosporins of third and fourth generation gradually doubled from 7.2% in 2000 to 15% in 2019. The overall fluoroquinolone prescription rate was slightly increasing over time but fastly decreased in 2019 (19%) in comparison with 2018 (25.7%) (Figure 6). Among fluoroquinolone users, females and 60+ years old were in general less prescribed than males and the youngest, respectively, but the proportion of people aged 60+ increased over time (Figure S3A–C). Furthermore, in females aged 40–59 years old and in people 70 years or older the prevalence of use of fluoroquinolones remained stable from 2018 to 2019 (36.0%–35.9% and 42.1%–42.5%, respectively). Corticosteroids has been prescribed in 12% and 18% of fluoroquinolones users in 2000 and in 2019, respectively.
Along the study period, the prevalence of use of antibiotics increased during the winter respect to summer months (about 93% vs. 66% in 2000 and 91.5% vs. 47.7% in 2019) (Figure S4).
3.5. Factors associated to antibiotic use
At univariable analysis all the variables considered were statistically significant associated with the risk of being prescribed with at least an antibiotic, but results from the multivariable logistic regression model showed that gender, local health unit, polypharmacy and number of hospital admissions were the main determinants (Table 1). In particular, females (OR 1.28, 95%CI 1.27–1.28), people living in Brescia (OR 1.24, 95%CI 1.24–1.25) and in particular those exposed to polypharmacy (OR 2.57, 95%CI 2.56–2.57) and hospitalized 1 to 3 (OR 1.86, 95%CI 1.85–1.86) or more than 3 (OR 2.02, 95%CI 2.01–2.03) times a year had a higher likelihood of receiving antibiotics.
TABLE 1.
Results from the multivariable regression model to assess factors associated to antibiotic use
| Variables | OR (95% CI) |
|---|---|
| Year of observation | |
| 2000 | Reference |
| 2010 | 1.02 (1.02–1.03) |
| 2018 | 0.99 (0.98–0.99) |
| 2019 | 0.91 (0.91–0.92) |
| Gender | |
| Male | Reference |
| Female | 1.28 (1.27–1.28) |
| Age groups (years) | |
| 40–49 | Reference |
| 50–59 | 1.00 (0.99–1.00) |
| 60–69 | 1.08 (1.07–1.08) |
| 70–79 | 1.02 (1.01–1.02) |
| 80–89 | 0.93 (0.93–0.94) |
| 90+ | 1.08 (1.07–1.09) |
| Local health units (Agenzie per la Tutela della Salute—ATS) | |
| ATS Milano City | Reference |
| ATS Insubria | 1.04 (1.04–1.05) |
| ATS Montagna | 1.00 (0.99–1.01) |
| ATS Brianza | 1.08 (1.07–1.08) |
| ATS Bergamo | 1.10 (1.10–1.11) |
| ATS Brescia | 1.24 (1.24–1.25) |
| ATS Val Padana | 1.02 (1.02–1.03) |
| ATS Pavia | 1.00 (0.99–1.00) |
| Polypharmacy (≥5 drugs) | 2.57 (2.56–2.57) |
| Number of hospitalizations in the index year | |
| 0 | Reference |
| 1–3 | 1.86 (1.85–1.86) |
| >3 | 2.02 (2.01–2.03) |
Abbreviations: R, odds ratio; CI, confidence interval.
4. DISCUSSION
This study showed a high prevalence of antibiotic use from 2000 to 2019 in Northern Italy, with a slight decrease only in the last period. Differences in the patterns of prescribed antibiotic classes were found over time, with penicillins always the most prescribed. Females and the older age groups remained always the most prescribed population, with oldest males more prescribed than oldest females. Even intraregional variations were outlined, and Brescia always remained with the highest prevalence of people receiving at least one antibiotic prescription. The use of penicillin combinations with beta‐lactamase inhibitors and that of cephalosporins (third and fourth generation) drastically increased at the end of study period, whereas quinolones decreased in 2019. Corticosteroid use doubled in quinolones users in 2019. The major use of antibiotics is noted during winter months, with a regular pattern over study years. Female gender, living in Brescia, being exposed to polypharmacy and hospital admissions were the main factors associated to an increased likelihood to receive an antibiotic prescription.
In the European Union community‐dwelling people the mean consumption of antibiotics in 2019 was 18.0 DDD per 1000 inhabitants per day. 29 In the same year in Italy, antibiotics represented one of the most prescribed drug class, with an overall higher consumption (21.7 DDD/1000 inhabitants/day), even if the trend of use was statistically decreasing. 17 , 29 Our results in the Lombardy region showed an overall use somewhat lower (18.2 DID in 2018) than those of the mean of Italy and of the other European countries, with a slight decreasing trend in last years. Despite this, the overall prevalence of antibiotic use is still far away from the goal prefixed by the Italian Ministry of Health to reduce the systemic use of antibiotics at local level by at least of 10%. 5 This notwithstanding the increasing focus on the importance of antimicrobial overuse worldwide. Indeed, from 2017 Italy joined the European Commission and the Member States in order to adopt a One Health Action Plan to fight against antimicrobial resistance by identifying in‐hospital and community interventions and stewardship programs, promoting a more prudent use of antibiotics, improving hygiene and diagnostic testing and the implementation of educational campaigns. 5
National differences showed the highest consumption of antibiotics in the Southern regions with respect to Northern ones, 17 but our results also point out that intraregional differences exist, being Brescia at the top of antibiotic prescribing, confirming our previous analyses. 14 We also confirmed the highest use of antibiotics in females and in the extreme age groups, especially in younger females and older males, being probably due to disease epidemiology. 14 , 17 Penicillins were the most prescribed drug class, among which amoxicillin/clavulanic acid had the highest prevalence. This could indicate a potential inappropriate use of these combination, given that amoxicillin alone is considered to be the first‐choice antibiotic for the most common respiratory infections, the broad‐spectrum antibiotics causing the risk to prompt the development of resistance in a broader group of bacteria. 2 On the other hand, we have to consider the increased resistance to the first‐choice antibiotic therapies. For example, Escherichia coli, mainly responsible for the urinary tract infections (especially in the older population) shows a high and increasing rate of aminopenicillin resistance (68% in Italy in 2019), posing the need for different choices. 2 In light of this, our results showed that the use of other antibiotics (not including penicillins, cephalosporins, macrolides and quinolones) trends upwards in recent years, possibly due to the increasing antimicrobial resistance to the first line choice therapies.
Unfortunately, the most worrying scenario occurring worldwide is the growing resistance to second‐ and third‐line antibiotics, that represents our last line of defense to treat bacterial infections. 2 For this reason, the World Health Organization (WHO) grouped antibiotics into three categories, "Access", "Watch" and "Reserve", in order to guide their prescribing and reduce the risk of adverse reactions and the development of bacterial resistance. 30 The “Access” antibiotics (i.e. penicillins and nitrofurantoin) should always be used as first‐choice treatment for many infections. The “Watch” group includes antibiotics (e.g. third generation cephalosporins, macrolides and fluoroquinolones) with a greater risk of inducing resistance and therefore generally recommended as second choice treatments, or to be preferred for specific cases only. The "Reserve" group includes antibiotics (e.g. fourth generation cephalosporins) of last choice and used only in the most severe cases, when all other alternatives have not been successful, such as for multi‐resistant infections. 30 This notwithstanding, the ratio of consumption of broad‐spectrum penicillins, cephalosporins, macrolides and fluoroquinolones to the consumption of narrow‐spectrum penicillins, cephalosporins and erythromycin increased for overall countries of Europe from 2010 to 2019 and in particular in Italy ranged from 4.9 DDD/1000 inhabitants/day up to 7.5. 29 Even in our study the prescription rate of the third and fourth generation cephalosporins dramatically doubled from 7.2% in 2000 to 15% in 2019. Even macrolides, the second most prescribed class in our study, had an increment of use, in 2019 azithromycin reaching 15% of prevalence. Compared to 2019, in the first months of 2020 the use of azithromycin is further increased of +160%. 17 Indeed, in this period, it has been proposed (alone or in combination with hydroxychloroquine) as potential treatment of coronavirus disease‐19 (COVID‐19), despite the lack of strong clinical evidence for their effectiveness and the risk of QT prolongation occurrences. 31 This will imply an overall further risk of antimicrobial resistance due to the inappropriate use of antibiotics during the COVID‐19 pandemia. 17 , 32 Even fluoroquinolones, which are among the antibiotics most commonly and inappropriately used worldwide, 33 , 34 have been watched by regulatory agencies. Indeed in 2016 the US Food and Drug Administration 35 and then in 2018 the European Medicines Agency 36 discouraged their use for acute uncomplicated urinary tract infections (UTI) or sinusitis, pushing physicians to have particular caution in older population, where concerns of the risks outweigh the benefits. 37 , 38 As happened in the USA after the FDA’ actions, 39 even in our study after the 2018 EMA’s recommendation the fluoroquinolone use declined from 26% in 2018 to 19% in 2019. Unfortunately, we did not find the same trend in the younger women aged 40–59 years (where fluoroquinolones were commonly used for UTI) and in the older population 70+ years (where the risks for tendon injury is known to be higher), with a 2018–2019 use prevalence that remained flat. Furthermore, in our study in 2019 about 20% of fluoroquinolones users were co‐prescribed with corticosteroids, with again a resulting potential increased risk of tendon injury occurrences. 36 , 38
The marked seasonal variation in the consumption of antibiotics during winter months in our study could again indicate a possible poor practice of these drugs for the treatment of respiratory infections of viral etiology. Indeed, it has been shown in the same years in Italy a correlation between the peaks in the incidence of the flu infections and the increase in the consumption of antibiotics not always associated with secondary bacterial infections, as confirmed by analyzing data of General Practitioners. 17
Lack of knowledge and awareness in prescribers and patients, pharmaceutical promotion, lack of rapid diagnostic tests, patient‐doctor interaction and time pressure have been shown to be major drivers of irrational use of antibiotics. 11 , 40 , 41 In our previous study conducted in 2005 in the Lombardy region we already found that gender, age and local health unit were the main determinants of antibiotic prescription. 14 We now confirmed these associations, but found that polypharmacy and hospital admissions were the strongest determinants, probably due to the fact that these variables explained a worse health state of patient.
4.1. Limitations
Some limitations deserve to be cited. Firstly, the administrative database does not collect the indications for the antibiotic use, that would allow a more precise evaluation of the prevalence of their inappropriate use, estimated to be around 25% in Italy. 17 Secondly, the exposure to antibiotics could be underestimated, because drugs sold out without medical prescriptions were not collected in this database. Then, patients’ features such as socio—cultural variables, living arrangement and income were not available, thus being information, which could be relevant in determining different pattern of use of antibiotics and assessing the appropriateness of drug prescription. Finally, there are no antibiogram results that could allow the assessment of possible association with the different patterns of antibiotic use and the antimicrobial resistance in sexes, age groups and geographical areas.
5. CONCLUSIONS
To our knowledge this is the first study which assessed changes in patterns of antibiotic use and in associated factors over about 20‐year time span in Italy. Given that antimicrobial resistance is a huge matter of concern worldwide and is related to an inappropriate use of antibiotic drugs, the differences we found in the prevalence of antibiotic use and in the patterns of poor prescribing, over than the factors associated to the risks of their use, should be taken into account when monitoring and planning interventions for patients and healthcare professionals, in order to improve the rational use of these drugs and reduce risk of antimicrobial resistance. Indeed, the relatively stable use of antibiotics over the study period, despite an increasing focus on the importance of antimicrobial overuse, further reinforces that there is much work to be done to implement impactful interventions.
DISCLOSURE
None to disclose.
AUTHORS’ CONTRIBUTIONS
CF conceived and designed the study and wrote the paper, SM performed statistical analyses, IF and AN critically review and approval of the final manuscript.
ETHICS APPROVAL
In Italy, studies using retrospective aggregated‐anonymous data from administrative databases do not require Ethics Committee/IRB approval. 42
Supporting information
Supplementary Material
Franchi C, Mandelli S, Fortino I, Nobili A. Antibiotic use and associated factors in adult outpatients from 2000 to 2019. Pharmacol Res Perspect. 2021;9:e00878. doi: 10.1002/prp2.878
Funding information
This work was supported by grants from the Regional Health Ministry of the Lombardy Region (Progetto Epidemiologia dei farmaci‐EPIFARM).
DATA AVAILABILITY STATEMENT
The data used in this study are property of Lombardy region and stored by ARIA S.p.A (Healthcare utilization databases). It is only possible to have access to the data but they cannot be shared. The data access procedure implies the submission of a study protocol to the data owner and the protocol evaluation from a qualified committee. If the research question is of interest for the data owner and the study is well designed, the permission for data access is provided.
REFERENCES
- 1. Hutchings MI, Truman AW, Wilkinson B. Antibiotics: past, present and future. Curr Opin Microbiol. 2019;51:72‐80. doi: 10.1016/j.mib.2019.10.008. Epub 2019 Nov 13 PMID: 31733401. [DOI] [PubMed] [Google Scholar]
- 2. European Centre for Disease Prevention and Control . Antimicrobial resistance in the EU/EEA (EARS‐Net) ‐Annual Epidemiological Report 2019. ECDC; 2020. https://www.ecdc.europa.eu/en/publications‐data/surveillance‐antimicrobial‐resistance‐europe‐2019 [Google Scholar]
- 3. Cassini A, Högberg LD, Plachouras D, et al. Attributable deaths and disability‐adjusted life‐years caused by infections with antibiotic‐resistant bacteria in the EU and the European Economic Area in 2015: a population‐level modelling analysis. Lancet Infect Dis. 2019;19(1):56‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Organisation for Economic Co‐operation and Development (OECD) and European Centre for Disease Prevention and Control (ECDC) . Antimicrobial Resistance. Tackling the Burden in the European Union. Briefing note for EU/EEA countries. OECDC; 2019. https://www.oecd.org/health/health‐systems/AMR‐Tackling‐the‐Burden‐in‐the‐EU‐OECD‐ECDC‐Briefing‐Note‐2019.pdf [Google Scholar]
- 5. Italian Ministry of Health . National Action Plan on Antimicrobial Resistance 2017‐2020. http://www.salute.gov.it/portale/documentazione/p6_2_5_1.jsp?lingua=italiano&id=362
- 6. Shehab N, Lovegrove MC, Geller AI, Rose KO, Weidle NJ, Budnitz DS. US emergency department visits for outpatient adverse drug events, 2013–2014. JAMA. 2016;316(20):2115‐2125. doi: 10.1001/jama.2016.16201. PMID: 27893129; PMCID: PMC6490178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hersh AL, King LM, Shapiro DJ, Hicks LA, Fleming‐Dutra KE. Unnecessary antibiotic prescribing in US ambulatory care settings, 2010–2015. Clin Infect Dis. 2021;72(1):133‐137. doi: 10.1093/cid/ciaa667. PMID: 32484505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dekker AR, Verheij TJ, van der Velden AW. Inappropriate antibiotic prescription for respiratory tract indications: most prominent in adult patients. Fam Pract. 2015;32(4):401‐407. doi: 10.1093/fampra/cmv019. Epub 2015 Apr 24 PMID: 25911505. [DOI] [PubMed] [Google Scholar]
- 9. Fleming‐Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA. 2016;315(17):1864‐1873. doi: 10.1001/jama.2016.4151. PMID: 27139059. [DOI] [PubMed] [Google Scholar]
- 10. Duffy E, Ritchie S, Metcalfe S, Van Bakel B, Thomas MG. Antibacterials dispensed in the community comprise 85%‐95% of total human antibacterial consumption. J Clin Pharm Ther. 2018;43:59‐64. doi: 10.1111/jcpt.12610. pmid:28833324 [DOI] [PubMed] [Google Scholar]
- 11. Machowska A, Stålsby LC. Drivers of irrational use of antibiotics in Europe. Int J Environ Res Public Health. 2018;16(1):27. doi: 10.3390/ijerph16010027. PMID: 30583571; PMCID: PMC6338985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Beckett CL, Harbarth S, Huttner B. Special considerations of antibiotic prescription in the geriatric population. Clin Microbiol Infect. 2015;21(1):3‐9. doi: 10.1016/j.cmi.2014.08.018. Epub 2014 Oct 13 PMID: 25636920. [DOI] [PubMed] [Google Scholar]
- 13. Alves C, Mendes D, Marques FB. Fluoroquinolones and the risk of tendon injury: a systematic review and meta‐analysis. Eur J Clin Pharmacol. 2019;75(10):1431‐1443. doi: 10.1007/s00228-019-02713-1. Epub 2019 Jul 4 PMID: 31270563. [DOI] [PubMed] [Google Scholar]
- 14. Franchi C, Sequi M, Bonati M, et al. Differences in outpatient antibiotic prescription in Italy's Lombardy region. Infection. 2011;39(4):299‐308. doi: 10.1007/s15010-011-0129-1. Epub 2011 Jun 25 PMID: 21706227. [DOI] [PubMed] [Google Scholar]
- 15. Schröder W, Sommer H, Gladstone BP, et al. Gender differences in antibiotic prescribing in the community: a systematic review and meta‐analysis. J Antimicrob Chemother. 2016;71(7):1800‐1806. doi: 10.1093/jac/dkw054. Epub 2016 Apr 3 PMID: 27040304. [DOI] [PubMed] [Google Scholar]
- 16. Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis. 2015;60(9):1308‐1316. doi: 10.1093/cid/civ076. Epub 2015 Mar 5 PMID: 25747410. [DOI] [PubMed] [Google Scholar]
- 17. The Medicines Utilisation Monitoring Centre . National Report on Medicines use in Italy. Year 2019. Italian Medicines Agency; 2020. https://www.aifa.gov.it/‐/rapporto‐osmed‐2019 [Google Scholar]
- 18. Lombardy Region. Agenzie di Tutela della Salute (ATS). https://www.regione.lombardia.it/wps/portal/istituzionale/HP/DettaglioServizio/servizi‐e‐informazioni/Cittadini/salute‐e‐prevenzione/agenzie‐di‐tutela‐della‐salute/ser‐aziende‐sanitarie‐locali‐sal/agenzie‐tutela‐salute
- 19. Franchi C, Marcucci M, Mannucci PM, et al. Changes in clinical outcomes for community‐dwelling older people exposed to incident chronic polypharmacy: a comparison between 2001 and 2009. Pharmacoepidemiol Drug Saf. 2016;25(2):204‐211. doi: 10.1002/pds.3938. Epub 2015 Dec 21 PMID: 26687829. [DOI] [PubMed] [Google Scholar]
- 20. Franchi C, Tettamanti M, Pasina L, et al. Changes in drug prescribing to Italian community‐dwelling elderly people: the EPIFARM‐Elderly Project 2000–2010. Eur J Clin Pharmacol. 2014;70(4):437‐443. doi: 10.1007/s00228-013-1621-6. Epub 2014 Jan 8 PMID: 24398968. [DOI] [PubMed] [Google Scholar]
- 21. Franchi C, Giussani G, Messina P, et al.; EPIRES Group . Validation of healthcare administrative data for the diagnosis of epilepsy. J Epidemiol Community Health. 2013;67(12):1019‐1024. doi: 10.1136/jech-2013-202528. Epub 2013 Sep 10. PMID: 24022813. [DOI] [PubMed] [Google Scholar]
- 22. Franchi C, Lucca U, Tettamanti M, et al. Cholinesterase inhibitor use in Alzheimer's disease: the EPIFARM‐Elderly Project. Pharmacoepidemiol Drug Saf. 2011;20(5):497‐505. doi: 10.1002/pds.2124. PMID: 21432941. [DOI] [PubMed] [Google Scholar]
- 23. Franchi C, Cartabia M, Risso P, et al. Geographical differences in the prevalence of chronic polypharmacy in older people: eleven years of the EPIFARM‐Elderly Project. Eur J Clin Pharmacol. 2013;69(7):1477‐1483. doi: 10.1007/s00228-013-1495-7. Epub 2013 Mar 28 PMID: 23535883. [DOI] [PubMed] [Google Scholar]
- 24. European Medicines Agency . Disabling and potentially permanent side effects lead to suspension or restrictions of quinolone and fluoroquinolone antibiotics. 2019. https://www.ema.europa.eu/en/documents/referral/quinolone‐fluoroquinolone‐article‐31‐referral‐disabling‐potentially‐permanent‐side‐effects‐lead_en.pdf
- 25. European Centre for Disease Prevention and Control . Quality indicators for antibiotic. Consumption in the community. https://www.ecdc.europa.eu/en/antimicrobial‐consumption/database/quality‐indicators
- 26. Coenen S, Ferech M, Haaijer‐Ruskamp FM, et al.; ESAC Project Group . European Surveillance of Antimicrobial Consumption (ESAC): quality indicators for outpatient antibiotic use in Europe. Qual Saf Health Care. 2007;16(6):440‐445. doi: 10.1136/qshc.2006.021121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harding SD, Sharman JL, Faccenda E, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2019: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res. 2018;46:D1091‐D1106. doi: 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alexander SP, Kelly E, Mathie A, et al. THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: introduction and other protein targets. Br J Pharmacol. 2021;178(Suppl 1):S1‐S26. doi: 10.1111/bph.15537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. European Centre for Disease Prevention and Control . Antimicrobial consumption ‐ Annual Epidemiological Report for 2019. https://www.ecdc.europa.eu/en/publications‐data/surveillance‐antimicrobial‐consumption‐europe‐2019
- 30. World Health Organization . The 2019 WHO AWaRe classification of antibiotics for evaluation and monitoring of use. World Health Organization; 2019. Licence: CC BY‐NC‐SA 3.0 IGO. https://apps.who.int/iris/handle/10665/327957 [Google Scholar]
- 31. Italian Agency of Medicine . Azithromycinin the treatmentof adult patients with COVID‐19. 2020. https://www.aifa.gov.it/documents/20142/1267737/Azithromycin_EN_05.05.2020.pdf
- 32. Hsu J. How Covid‐19 is accelerating the threat of antimicrobial resistance. BMJ. 2020;369:m1983. doi: 10.1136/bmj.m1983 [DOI] [PubMed] [Google Scholar]
- 33. Centers for disease Control and Prevention . Fluoroquinolones. https://arpsp.cdc.gov/profile/antibiotic‐use/214#comparing‐antibiotics‐classes
- 34. Centers for disease Control and Prevention . Inappropriate prescribing of antibiotics: the example of fluoroquinolones. 2018. https://arpsp.cdc.gov/story/inappropriate‐prescribing‐antibiotics‐fluoroquinolones
- 35. US Food and Drug Administration . FDA Drug Safety Communication: FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects. 2016. https://www.fda.gov/drugs/drug‐safety‐and‐availability/fda‐drug‐safety‐communication‐fda‐updates‐warnings‐oral‐and‐injectable‐fluoroquinolone‐antibiotics
- 36. European Medicines Agency . Disabling and potentially permanent side effects lead to suspension or restrictions of quinolone and fluoroquinolone antibiotics. 2018. https://www.ema.europa.eu/en/news/disabling‐potentially‐permanent‐side‐effects‐lead‐suspension‐restrictions‐quinolone‐fluoroquinolone
- 37. Stephenson AL, Wu W, Cortes D, Rochon PA. Tendon injury and fluoroquinolone use: a systematic review. Drug Saf. 2013;36(9):709‐721. doi: 10.1007/s40264-013-0089-8. PMID: 23888427. [DOI] [PubMed] [Google Scholar]
- 38. Gorelik E, Masarwa R, Perlman A, et al. Fluoroquinolones and cardiovascular risk: a systematic review, meta‐analysis and network meta‐analysis. Drug Saf. 2019;42(4):529‐538. doi: 10.1007/s40264-018-0751-2. PMID: 30368737. [DOI] [PubMed] [Google Scholar]
- 39. Tran PT, Antonelli PJ, Hincapie‐Castillo JM, Winterstein AG. Association of US food and drug administration removal of indications for use of oral quinolones with prescribing trends. JAMA Intern Med. 2021;181(6):808. doi: 10.1001/jamainternmed.2021.1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gualano MR, Gili R, Scaioli G, Bert F, Siliquini R. General population's knowledge and attitudes about antibiotics: a systematic review and meta‐analysis. Pharmacoepidemiol Drug Saf. 2015;24(1):2‐10. doi: 10.1002/pds.3716. Epub 2014 Sep 24 PMID: 25251203. [DOI] [PubMed] [Google Scholar]
- 41. Teixeira Rodrigues A, Roque F, Falcão A, Figueiras A, Herdeiro MT. Understanding physician antibiotic prescribing behaviour: a systematic review of qualitative studies. Int J Antimicrob Agents. 2013;41(3):203‐212. doi: 10.1016/j.ijantimicag.2012.09.003. Epub 2012 Nov 3 PMID: 23127482. [DOI] [PubMed] [Google Scholar]
- 42. Italian Data Protection Authority . General authorisation to process personal data for scientific research purposes—1 March 2012 [1884019]. doi: 10.1094/PDIS-11-11-0999-PDN [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data used in this study are property of Lombardy region and stored by ARIA S.p.A (Healthcare utilization databases). It is only possible to have access to the data but they cannot be shared. The data access procedure implies the submission of a study protocol to the data owner and the protocol evaluation from a qualified committee. If the research question is of interest for the data owner and the study is well designed, the permission for data access is provided.


