Graphical abstract
Keywords: Covid-19, SARS-CoV-2, RdRp, Chain terminator
Abstract
Remdesivir (GS-5734, Veklury®) has remained the only antiviral drug formally approved by the US FDA for the treatment of Covid-19 (SARS-CoV-2 infection). Its key structural features are the fact that it is a C-nucleoside (adenosine) analogue, contains a 1′-cyano function, and could be considered as a ProTide based on the presence of a phosphoramidate group. Its antiviral spectrum and activity in animal models have been well established and so has been its molecular mode of action as a delayed chain terminator of the viral RdRp (RNA-dependent RNA polymerase). Its clinical efficacy has been evaluated, but needs to be optimized with regard to timing, dosage and duration of treatment, and route of administration. Safety, toxicity and pharmacokinetics need to be further addressed, and so are its potential combinations with other drugs such as corticosteroids (i.e. dexamethasone) and ribavirin.
1. Introduction
More than a year after the disease had been originally recognized, on May 1, 2020, Remdesivir got its Emergency Use Authorization (EUA) from the US Food and Drug Administration (FDA), to allow its use for the treatment of Covid-19 (SARS-CoV-2 infection) in adult and pediatric patients hospitalized with severe disease [1], the clinical use of the drug (marketed as Veklury®) has followed the path of a roller coaster. The scope of the EUA was expanded on August 28, 2020 and, again, on October 22, 2020 to approve its use in adults and pediatric patients (12 years of age or older) [1]. Yet, the enthusiasm in using Remdesivir was dampened by the World Health Organization (WHO) Solidarity Trial [2], as echoed in the editorial of Science [3].
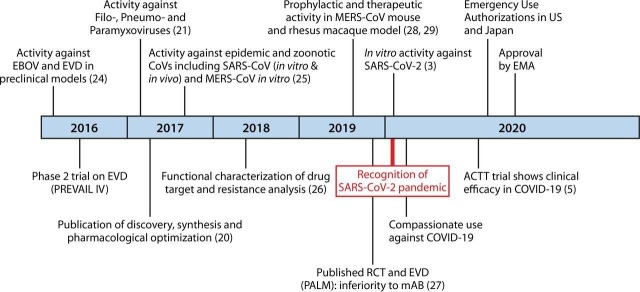
Milestones in the discovery of Remdesivir (GS-5734) leading to its use in the treatment of Covid-19 are depicted in Fig. 1 [4].
Fig. 1.
Abbreviations EBOV, Ebolavirus; EMA, European Medicines Agency; EVD, Ebolavirus disease; MERS-CoV, Middle East Respiratory Syndrome coronavirus; SARS-CoV-2, severe acute respiratory distress syndrome coronavirus; RCT, randomized controlled clinical trial; mAB, monoclonal antibody. Figure taken from Malin et al. [4].
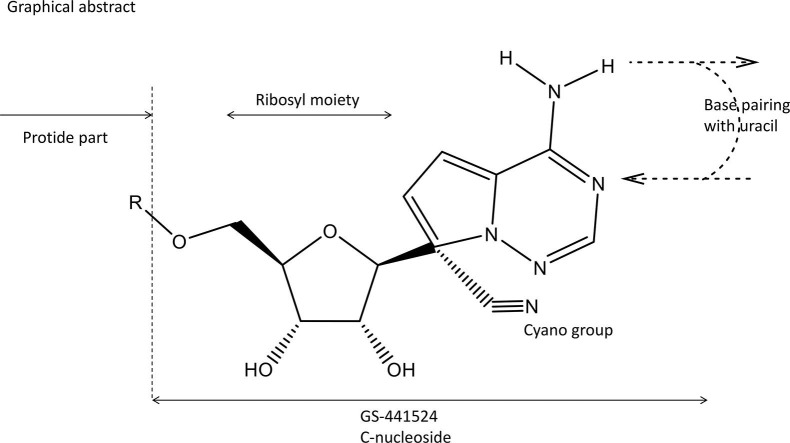
2. Chemical structure
In reviewing the milestones (Fig. 1) in the discovery of Remdesivir, Malin et al. [4] did not mention the paper of Cho et al. [5]. Yet, this was the first paper where the parent compound (GS-441524) of Remdesivir was mentioned. GS-441524 was even found superior to Remdesivir for Covid-19 treatment [6].
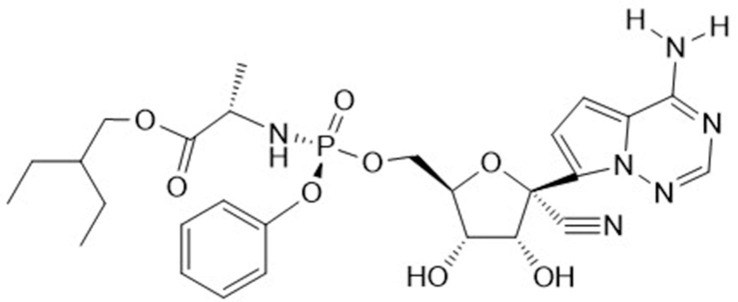
When analyzing the chemical structure of Remdesivir (GS-5734, Veklury®) (Fig. 2 ) the following structural determinants could be considered as essential: (1) the heterocyclic part analogous to adenine, allowing hydrogen bonding [i.e. base pairing with uracil in its inhibitory effect on the RdRp (RNA-dependent RNA polymerase level)]; (2) the fact that GS-5734 is a C-nucleoside, thus imparting protection from phosphorylating cleavage at the HN-C linkage; (3) the presence of a 1′-CN group; (4) the ribosyl moiety ensuing inhibition of RNA (instead of DNA) synthesis, and (5) the presence of a phosphoramidate group, contributing to its tissue targeting (i.e. lung epithelial cells). The 1′-CN group is the most intriguing structural determinant. It was already highlighted in the original paper [4], and again emphasized in later studies on the mechanism of action of Remdesivir, i.e. its interaction with the SARS-CoV-2 RdRp [7], [8].
Fig. 2.
Chemical structure of Remdesivir (GS-5734, Veklury®).
3. Chemical synthesis
Like other ProTide prodrugs, Remdesivir contains a chiral phosphorus center; therefore, a novel chemoenzymatic strategy has been developed to enable the synthesis of the pure (Rp)-diastereomer of Remdesivir [9]. Other attempts towards a practical approach of the chemical synthesis of Remdesivir have been published by Xie et al. [10] and Wang et al. [11]. A facile, one-pot chemical synthesis that offers an excellent opportunity for industrial production of Remdesivir, stems from Gannedi et al. [12].
4. Antiviral activity spectrum
Remdesivir owes its activity against SARS-CoV-2 to its interaction with the viral RNA-dependent RNA polymerase (RdRp) (see below). This also explains why Remdesivir is not only active against SARS-CoV-2 but also zoonotic coronaviruses with a highly divergent RdRp [13], [14].
Remdesivir can efficiently inhibit RdRps from various flaviviruses, such as Yellow fever, West Nile fever, Japanese encephalitis, Tick-borne encephalitis, Zika and Dengue [15], and, besides Ebola, novel filoviruses (i.e. Lloviu and Bombali) [16]. Most importantly, the antiviral activity of Remdesivir was found to extend to Respiratory Syncytial Virus (RSV), even in vivo (African Green Monkey model) and, again the 1′-CN group was found to be essential for this antiviral effect [17].
5. Mechanism of action
Several studies have pointed to the RdRp as the molecular target of action of Remdesivir [18]. The importance of the 1′-CN group in delayed chain termination was highlighted by Shannon et al. [19]. Remdesivir would be a better substrate than ATP for the viral RdRp [20]. It would be covalently incorporated into the primer strand “at the first replicated base pair” and thereby terminate chain elongation [21].
That Remdesivir might be a delayed chain terminator was again hypothesized by Wang et al. [22]: primer extension would be paused when the added Remdesivir monophosphate (RMP) is translocated at the i + 3 position (with i being the nascent base pair at the initial insertion site of RMP). Delayed RNA chain termination was again suggested by Götte and co-workers [23]: once incorporated at position i, the inhibitor caused RNA synthesis arrest at position i + 3. This mechanism of chain termination originally proposed for the MERS viral RdRp was then reiterated for SARS-CoV-2 RdRp [24]. A second mechanism of action was postulated, whereby the efficiency of incorporation of the complimentary UTP opposite template RMP would be compromised [25].
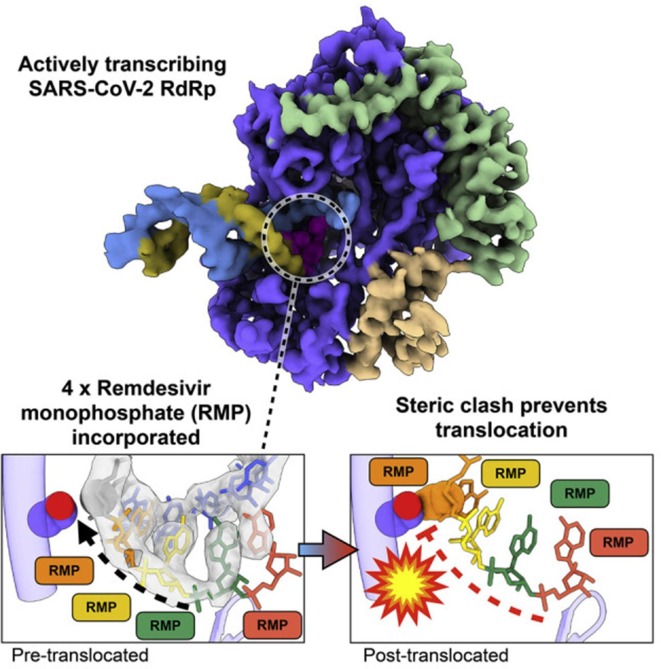
Remdesivir could be viewed as a delayed translocation inhibitor of the SARS-CoV-2 RdRp (Fig. 3 ). Blocking of the translocation occurs after the incorporation of the fourth RMP. This leads to a stalling of the polymerization process and is due to a steric clash between the 1′-cyano group of RMP and the protein (RdRp) [26]. That the SARS-CoV-2 RdRp is stalled by Remdesivir after the addition of three more nucleotides (which means that it is blocked only by the addition of the fourth RMP) has also been suggested by Kokic et al. [27]. The SARS-CoV-2 RdRp Nsp12 has also a unique nidovirus RdRp-associated nucleotidyl transferase (NiRAN) domain that transfers nucleoside monophosphates to the Nsp9 protein and the nascent RNA. Remdesivir may also interfere with the allosteric activation of NiRAN [28].
Fig. 3.
Chain termination at RdRp level by Remdesivir. Figure taken from Bravo et al. [26].
6. Metabolism
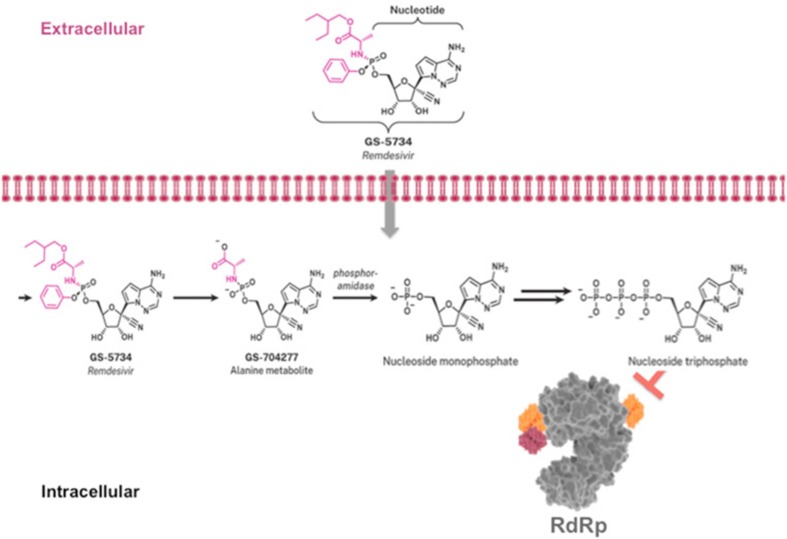
Remdesivir (GS-5734) is taken up by the target cells (Fig. 4 ) and first hydrolyzed by hydrolases [Cathepsin A (CatA), carboxylesterase 1 (CES1)] to its alanine intermediate Met X (GS-704277), which is further hydrolyzed to the monophosphate form by histidine triad nucleotide-binding protein 1 (HINT1). The monophosphate is then consecutively phosphorylated to the di- and triphosphate by cellular phosphotransferases. The picture for the intracellular processing of Remdesivir (GS-5734) (modified) is taken from Santoro and Carafoli [29]. Li and co-workers [30] assumed that Remdesivir is sufficiently taken up by the lung cells to enable efficient intracellular metabolism of Remdesivir and Met X to its monophosphate and successive phosphorylation to its active triphosphate metabolite GS-443902.
Fig. 4.
Intracellular processing of Remdesivir. Figure taken from Santoro and Carafoli [29].
7. Animal model infections
Prophylactic Remdesivir administration initiated 24 h prior to MERS-CoV infection in rhesus macaques completely prevented the disease, and even when treatment was initiated 12 h post-inoculation it provided a clear clinical benefit accompanied by a reduction of virus replication in the lungs [31]. This benefit was confirmed with Remdesivir treatment initiated early after infection of rhesus macaques with SARS-CoV-2, suggesting that clinical benefit with Remdesivir treatment may be expected if started (as) early (as possible) after Covid-19 infection in human patients [32]. Similarly, Remdesivir has proved efficacious in cynomolgus macaques infected with the filovirus Marburg when treatment was initiated 5 days post virus inoculation [33].
GS-441524, the active metabolite of Remdesivir has been found to inhibit SARS-CoV-2 infection in murine models [34], without notable toxicity. Remarkably, GS-441524 was accredited as a promising inexpensive drug candidate for treating Covid-19 and other CoV diseases. It is anticipated that following its uptake by the cells it is readily converted to its monophosphate (indicated by nucleoside monophosphate in Fig. 4) and subsequently to its triphosphate before targeting the RdRp.
8. Clinical efficacy
That Remdesivir would be efficacious in the treatment of Covid-19 was first reported by Grein et al. [35] on 10 April 2020. They observed clinical improvement in 36 of 53 patients (68%). Then, on 29 April 2020, a randomized, double-blind, placebo-controlled, multi-centered trial revealed that Remdesivir was not associated with statistically significant clinical benefits [36]. Then, on 22 May 2020, Beigel et al. [37] stated that Remdesivir would be superior to placebo in shortening the time to recovery in adults hospitalized with Covid-19.
Among hospitalized adults with severe Covid-19, Remdesivir may shorten the time to clinical improvement [38], and this faster time to recovery and survival may be observed particularly following early treatment [39].
Goldman et al. [40] in their article published on May 27, 2020, did not observe a significant difference between a 5-day course and a 10-day course of Remdesivir in patients with severe Covid-19. Jiang et al. [41] reported a significantly higher rate of clinical improvement with a 5-day course of Remdesivir compared to the 10-day course. However, it was not translated into 14-day mortality benefit [42].
According to Kaka et al. [43], in patients not receiving ventilation, a 5-day course may provide greater benefits (with lower drug costs) than a 10-day Remdesivir course. Overall, in their final report, where Remdesivir was administered at a 200 mg loading dose on day 1, followed by 100 mg daily for up to 9 additional days, or placebo, for up to 10 days, Beigel et al. [37] on 8 October 2020 concluded that Remdesivir was superior to placebo in shortening the time to recovery in adults who were hospitalized with Covid-19 and had evidence of lower respiratory tract infection.
Among the factors that may influence the clinical efficacy of Remdesivir, timing, dosage, duration of treatment and route of administration may all seem primordial. There is little doubt that Remdesivir treatment should be started as soon as possible [44]. The dosage is more critical as the currently recommended dosage [37] is assumed to be safe and efficacious [45]. Yet, Yan and Muller [46] suggested that the leading dose of 200 mg could be increased to 300 mg, followed with a maintenance dose of 200 mg (instead of 100 mg) for 5 days. But, a 10-day course of Remdesivir may lead to a significantly higher rate of serious adverse effects than a 5-day course [47].
Remdesivir is invariably administered by the intravenous (iv) route. Why have other routes [subcutaneous (sc), peroral (po), and intranasal (in)] not been explored? Especially the latter route, for treating a respiratory infection, would seem attractive, if not logical. Aerosolized Remdesivir has been recommended [48] and the necessary technology for Remdesivir inhalation has been developed [49].
9. Safety
Xu et al. [50] reported low potential for Remdesivir to elicit off-target toxicity including mitochondria-specific toxicity. In one randomized controlled trial (RCT), there were more adverse events (12.5%) in the Remdesivir treatment group than in the placebo group (5%) [36]. Safety assessment of Remdesivir for Covid-19 patients, therefore, needs to be evaluated [51]. The compassionate use of Remdesivir in women with severe Covid-19 led to high recovery rates with a low rate of serious adverse events [52]. The compassionate use of Remdesivir in a few pregnant patients with Covid-19 was reported by Igbinosa et al. [53]. Further safety and efficacy data are directly needed to support Remdesivir’s use during pregnancy.
The use of Remdesivir would not seem to be contra-indicated in patients with impaired renal function [54], and Remdesivir was even reported to alleviate acute kidney injury by inhibiting the activation of the NOD-, LPR- and pyrin domain-containing protein 3 inflammasome in macrophages [55].
The most characteristic side effect linked to the clinical use of Remdesivir and perhaps not unexpected for an adenosine analogue is sinus bradycardia [56], heralded by an electrocardiographic QT prolongation [57]. This bradycardia was confirmed in several other reports [58], [59], [60], [61], [62]. It was transient and subsided after discontinuation of Remdesivir treatment.
10. Resistance
The recently emerged variants of SARS-CoV-2 from humans suggests minimal pre-existing resistance to Remdesivir [63]. Remdesivir failure has been noted in a B-cell immunodeficient patient with persistent SARS-CoV-2 viremia who had a mutation (D484Y) in the RdRp. Such a mutation may arise in vivo following a 5-day course of Remdesivir and lead to treatment failure. Remdesivir targets a structurally analogous region of the Ebolavirus and SARS-CoV-2 RNA polymerases: a single mutation (F548S) in this region conferred low-level reduced susceptibility to Remdesivir. Molecular surveillance of this region of the polymerase may seem warranted in Covid-19 patients treated with Remdesivir [64].
11. Combinations
Combinations of Remdesivir with several entities have been initiated. Combination of Remdesivir with convalescent plasma has proven to be beneficial in a patient with X-linked agammaglobulinemia [65] and did not lead to any adverse effects in a newborn with vertically acquired SARS-CoV-2 who developed acute respiratory failure [66].
Drug synergy of Remdesivir with the antifungal itraconazole and the antidepressant fluoxetine was reported [67], and synergistic antiviral effects were also reported for the combinations of Remdesivir with the folate antagonist methotrexate [68] and the anticancer agent gemcitabine [69]. Likewise, synergistic inhibition of SARS-CoV-2 replication was noted with the combination of Remdesivir and the Zn-ejector drugs disulfiram or ebselen [70].
Strong additive effects at subtoxic concentrations were reported for the combination of Remdesivir with the human soluble ACE2 (Angiotensin-converting enzyme 2) [71]; these data may lay the groundwork for the study of such regimens in future Covid-19 clinical trials. Such clinical trials with Remdesivir and baricitinib, a Janus kinase inhibitor, have already proven useful in reducing the recovery time and accelerating clinical improvement among the patients with Covid-19, notably among those receiving high-flow oxygen or noninvasive ventilation [72].
The most widely acclaimed combination is that of Remdesivir with corticosteroids, i.e. dexamethasone [73]. Such combination has proven to be beneficial in a hamster model of SARS-CoV-2 infection [74] and was also found to reduce the 30-day mortality rate from 19.7% to 12.6% in severe Covid-19 [75].
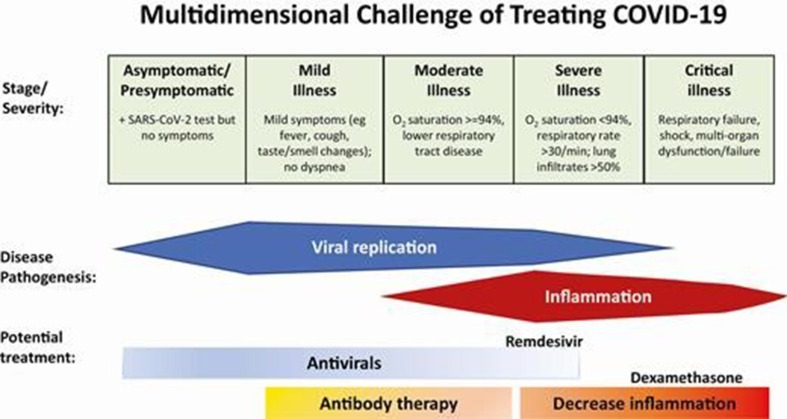
When Remdesivir is combined with corticosteroids such as dexamethasone in the treatment of Covid-19, the crucial question is the timing for the administration of both components. For Remdesivir, as an antiviral it should be administered as early as possible, but for dexamethasone its administration should depend on the inflammation reaction (“cytokine storm”) and thus, in principle, fall later than the height of virus replication (Fig. 5 ) [76]. Along these lines, it is surprising that Garibaldi et al. [77] did not observe that Remdesivir plus corticosteroid administration did not reduce the time to death compared with Remdesivir administered alone.
Fig. 5.
Abbreviations: COVID-19, coronavirus disease 2019; SARS CoV-2, severe acute respiratory syndrome coronavirus 2. Figure taken from Gandhi [76].
As a possible drug combination with Remdesivir, I would recommend ribavirin. Ribavirin is widely considered as a broad-spectrum antiviral agent, but it is largely ignored as an immunosuppressant [78]. In this sense, it could suppress the “cytokine storm” accompanying Covid-19. But, unlike corticosteroids which, as their side reaction stimulate virus replication, ribavirin may be expected to inhibit virus replication and thus, as an immunosuppressive agent, it could be safely combined with Remdesivir.
12. Perspectives
Jeremy Hsu [79] wondered about the future for Remdesivir. Among the issues questioned by Hsu were the costs for Remdesivir. Why wasn’t it given in conjunction with corticosteroids? Why wasn’t it replaced by GS-441524 that would be easier to synthesize and manufacture and could be administered orally (instead of intravenously)? It is obvious that a combination of Remdesivir (or GS-441524) with corticosteroids (i.e. dexamethasone) and a comparative evaluation of GS-441524 vs Remdesivir belong to the highest priorities in their further development as antiviral drugs for SARS-CoV-2.
But then there are several other issues, i.e. the efficacy, safety, tolerability and pharmacokinetics of Remdesivir (and/or GS-441524) in children [80]. And there are a variety of questions with regard to the timing and duration of treatment, the route of administration (perorally, subcutaneously or simply by inhalation) and the numerous possible drugs (repurposed or not), in addition to the corticosteroids with which Remdesivir (or GS-441524) could be combined. If this again would be multiplied by the different possibilities regarding timing, duration, and dosage, an astronomical number of combinations could be envisaged, eventually matching the number of victims suffering from SARS-CoV-2 infection.
13. Further information
For further information on Remdesivir, the reader is referred to ref. [81] (probable molecular mechanism), ref. [82] (critical clinical appraisal) and ref. [83] (pharmacology, pre-clinical data, and emerging clinical experience). In addition, a robust SARS-CoV-2 replication model in primary human epithelial cells has been described that verified the effectiveness of Remdesivir and GS-441524 [84].
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
I thank Mrs. Myriam Cornelis for proficient editorial assistance.
Footnotes
Presented at XXVI. Annual Congress of Czech and Slovak Societies for Biochemistry and Molecular Biology with cooperation of Austrian and German Biochemical Sections, České Budějovice, Czech Republic.
References
- 1.Rubin D., Chan-Tack K., Farley J., Sherwat A. FDA approval of Remdesivir - a step in the right direction. N. Engl. J. Med. 2020;383(27):2598–2600. doi: 10.1056/NEJMp2032369. [DOI] [PubMed] [Google Scholar]
- 2.W.S.T. Consortium Repurposed Antiviral Drugs for Covid-19 — Interim WHO Solidarity Trial Results. N. Engl. J. Med. 2020;384(6):497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen J., Kupferschmidt K. 'A very, very bad look' for remdesivir. Science. 2020;370(6517):642–643. doi: 10.1126/science.370.6517.642. [DOI] [PubMed] [Google Scholar]
- 4.Malin J.J., Suárez I., Priesner V., Fätkenheuer G., Rybniker J. Remdesivir against COVID-19 and other viral diseases. Clin. Microbiol. Rev. 2020;34(1) doi: 10.1128/cmr.00162-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho A., Saunders O.L., Butler T., Zhang L., Xu J., Vela J.E., Feng J.Y., Ray A.S., Kim C.U. Synthesis and antiviral activity of a series of 1'-substituted 4-aza-7,9-dideazaadenosine C-nucleosides. Bioorg. Med. Chem. Lett. 2012;22(8):2705–2707. doi: 10.1016/j.bmcl.2012.02.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan V.C., Muller F.L. Advantages of the parent nucleoside GS-441524 over Remdesivir for Covid-19 treatment. ACS Med. Chem. Lett. 2020;11(7):1361–1366. doi: 10.1021/acsmedchemlett.0c00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L., Zhang D., Wang X., Yuan C., Li Y., Jia X., Gao X., Yen H.L., Cheung P.P., Huang X. 1'-Ribose cyano substitution allows Remdesivir to effectively inhibit nucleotide addition and proofreading during SARS-CoV-2 viral RNA replication. PCCP. 2021;23(10):5852–5863. doi: 10.1039/d0cp05948j. [DOI] [PubMed] [Google Scholar]
- 8.Ni X., Schröder M., Olieric V., Sharpe M.E., Hernandez-Olmos V., Proschak E., Merk D., Knapp S., Chaikuad A. Structural insights into plasticity and discovery of Remdesivir metabolite GS-441524 binding in SARS-CoV-2 macrodomain. ACS Med. Chem. Lett. 2021;12(4):603–609. doi: 10.1021/acsmedchemlett.0c0068410.1021/acsmedchemlett.0c00684.s001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bigley A.N., Narindoshvili T., Raushel F.M. A chemoenzymatic synthesis of the (R(P))-isomer of the antiviral prodrug Remdesivir. Biochemistry. 2020;59(33):3038–3043. doi: 10.1021/acs.biochem.0c00591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie Y., Hu T., Zhang Y., Wei D., Zheng W., Zhu F., Tian G., Aisa H.A., Shen J. Weinreb amide approach to the practical synthesis of a key Remdesivir intermediate. J. Org. Chem. 2021;86(7):5065–5072. doi: 10.1021/acs.joc.0c0298610.1021/acs.joc.0c02986.s001. [DOI] [PubMed] [Google Scholar]
- 11.Wang M.o., Zhang L.u., Huo X., Zhang Z., Yuan Q., Li P., Chen J., Zou Y., Wu Z., Zhang W. Catalytic asymmetric synthesis of the anti-COVID-19 drug Remdesivir. Angew. Chem. Int. Ed. Engl. 2020;59(47):20814–20819. doi: 10.1002/anie.v59.4710.1002/anie.202011527. [DOI] [PubMed] [Google Scholar]
- 12.Gannedi V., Villuri B.K., Reddy S.N., Ku C.-C., Wong C.-H., Hung S.-C. Practical Remdesivir synthesis through one-pot organocatalyzed asymmetric (S)-P-phosphoramidation. J. Org. Chem. 2021;86(7):4977–4985. doi: 10.1021/acs.joc.0c02888. [DOI] [PubMed] [Google Scholar]
- 13.Sheahan T.P., Sims A.C., Graham R.L., Menachery V.D., Gralinski L.E., Case J.B., Leist S.R., Pyrc K., Feng J.Y., Trantcheva I., Bannister R., Park Y., Babusis D., Clarke M.O., Mackman R.L., Spahn J.E., Palmiotti C.A., Siegel D., Ray A.S., Cihlar T., Jordan R., Denison M.R., Baric R.S. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017;9(396) doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown A.J., Won J.J., Graham R.L., Dinnon K.H., Sims A.C., Feng J.Y., Cihlar T., Denison M.R., Baric R.S., Sheahan T.P. Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antiviral Res. 2019;169:104541. doi: 10.1016/j.antiviral.2019.104541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Konkolova E., Dejmek M., Hřebabecký H., Šála M., Böserle J., Nencka R., Boura E. Remdesivir triphosphate can efficiently inhibit the RNA-dependent RNA polymerase from various flaviviruses. Antiviral Res. 2020;182:104899. doi: 10.1016/j.antiviral.2020.104899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bodmer B.S., Zierke L., Wendt L., Greßler J., Groseth A., Hoenen T. Remdesivir inhibits the polymerases of the novel filoviruses Lloviu and Bombali virus. Antiviral Res. 2021;192:105120. doi: 10.1016/j.antiviral.2021.105120. [DOI] [PubMed] [Google Scholar]
- 17.Mackman R.L., Hui H.C., Perron M., Murakami E., Palmiotti C., Lee G., Stray K., Zhang L., Goyal B., Chun K., Byun D., Siegel D., Simonovich S., Du Pont V., Pitts J., Babusis D., Vijjapurapu A., Lu X., Kim C., Zhao X., Chan J., Ma B., Lye D., Vandersteen A., Wortman S., Barrett K.T., Toteva M., Jordan R., Subramanian R., Bilello J.P., Cihlar T. Prodrugs of a 1'-CN-4-Aza-7,9-dideazaadenosine C-Nucleoside leading to the discovery of Remdesivir (GS-5734) as a potent inhibitor of respiratory syncytial virus with efficacy in the African green monkey model of RSV. J. Med. Chem. 2021;64(8):5001–5017. doi: 10.1021/acs.jmedchem.1c00071. [DOI] [PubMed] [Google Scholar]
- 18.Gao Y., Yan L., Huang Y., Liu F., Zhao Y., Cao L., Wang T., Sun Q., Ming Z., Zhang L., Ge J., Zheng L., Zhang Y., Wang H., Zhu Y., Zhu C., Hu T., Hua T., Zhang B., Yang X., Li J., Yang H., Liu Z., Xu W., Guddat L.W., Wang Q., Lou Z., Rao Z. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science. 2020;368(6492):779–782. doi: 10.1126/science.abb7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shannon A., Le N.-T., Selisko B., Eydoux C., Alvarez K., Guillemot J.-C., Decroly E., Peersen O., Ferron F., Canard B. Remdesivir and SARS-CoV-2: structural requirements at both nsp12 RdRp and nsp14 exonuclease active-sites. Antiviral Res. 2020;178:104793. doi: 10.1016/j.antiviral.2020.104793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.T.L. Dangerfield, N.Z. Huang, K.A. Johnson, Remdesivir is effective in combating COVID-19 because it is a better substrate than ATP for the viral RNA-dependent RNA polymerase, iScience 23(12) (2020) 101849, doi:10.1016/j.isci.2020.101849. [DOI] [PMC free article] [PubMed]
- 21.Yin W., Mao C., Luan X., Shen D.D., Shen Q., Su H., Wang X., Zhou F., Zhao W., Gao M., Chang S., Xie Y.C., Tian G., Jiang H.W., Tao S.C., Shen J., Jiang Y., Jiang H., Xu Y., Zhang S., Zhang Y., Xu H.E. Structural basis for inhibition of the RNA-dependent RNA polymerase from SARS-CoV-2 by remdesivir. Science. 2020;368(6498):1499–1504. doi: 10.1126/science.abc1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J., Reiss K., Shi Y., Lolis E., Lisi G.P., Batista V.S. Mechanism of inhibition of the reproduction of SARS-CoV-2 and Ebola viruses by Remdesivir. Biochemistry. 2021;60(24):1869–1875. doi: 10.1021/acs.biochem.1c00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon C.J., Tchesnokov E.P., Feng J.Y., Porter D.P., Götte M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J. Biol. Chem. 2020;295(15):4773–4779. doi: 10.1074/jbc.AC120.013056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gordon C.J., Tchesnokov E.P., Woolner E., Perry J.K., Feng J.Y., Porter D.P., Götte M. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J. Biol. Chem. 2020;295(20):6785–6797. doi: 10.1074/jbc.RA120.013679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tchesnokov E.P., Gordon C.J., Woolner E., Kocinkova D., Perry J.K., Feng J.Y., Porter D.P., Götte M. Template-dependent inhibition of coronavirus RNA-dependent RNA polymerase by remdesivir reveals a second mechanism of action. J. Biol. Chem. 2020;295(47):16156–16165. doi: 10.1074/jbc.AC120.015720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bravo J.P.K., Dangerfield T.L., Taylor D.W., Johnson K.A. Remdesivir is a delayed translocation inhibitor of SARS-CoV-2 replication. Mol. Cell. 2021;81(7):1548–1552.e4. doi: 10.1016/j.molcel.2021.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kokic G., Hillen H.S., Tegunov D., Dienemann C., Seitz F., Schmitzova J., Farnung L., Siewert A., Höbartner C., Cramer P. Mechanism of SARS-CoV-2 polymerase stalling by remdesivir. Nat. Commun. 2021;12(1):279. doi: 10.1038/s41467-020-20542-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.B. Wang, V. Svetlov, Y.I. Wolf, E.V. Koonin, E. Nudler, I. Artsimovitch, Allosteric activation of SARS-CoV-2 RNA-dependent RNA polymerase by Remdesivir triphosphate and other phosphorylated nucleotides, mBio 12(3) (2021) e0142321, doi:10.1128/mBio.01423-21. [DOI] [PMC free article] [PubMed]
- 29.Santoro M.G., Carafoli E. Remdesivir: from Ebola to COVID-19. Biochem. Biophys. Res. Commun. 2021;538:145–150. doi: 10.1016/j.bbrc.2020.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.. Li, A. Liclican, Y. Xu, J. Pitts, C. Niu, J. Zhang, C. Kim, X. Zhao, D. Soohoo, D. Babusis, Q. Yue, B. Ma, B.P. Murray, R. Subramanian, X. Xie, J. Zou, J.P. Bilello, L. Li, B.E. Schultz, R. Sakowicz, B.J. Smith, P.Y. Shi, E. Murakami, J.Y. Feng, Key metabolic enzymes involved in Remdesivir activation in human lung cells, Antimicrob. Agents Chemother. (2021) Aac0060221, doi:10.1128/aac.00602-21. [DOI] [PMC free article] [PubMed]
- 31.de Wit E., Feldmann F., Cronin J., Jordan R., Okumura A., Thomas T., Scott D., Cihlar T., Feldmann H. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc. Natl. Acad. Sci. U.S.A. 2020;117(12):6771–6776. doi: 10.1073/pnas.1922083117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williamson B.N., Feldmann F., Schwarz B., Meade-White K., Porter D.P., Schulz J., van Doremalen N., Leighton I., Yinda C.K., Pérez-Pérez L., Okumura A., Lovaglio J., Hanley P.W., Saturday G., Bosio C.M., Anzick S., Barbian K., Cihlar T., Martens C., Scott D.P., Munster V.J., de Wit E. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. Nature. 2020;585(7824):273–276. doi: 10.1038/s41586-020-2423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Porter D.P., Weidner J.M., Gomba L., Bannister R., Blair C., Jordan R., Wells J., Wetzel K., Garza N., Van Tongeren S., Donnelly G., Steffens J., Moreau A., Bearss J., Lee E., Bavari S., Cihlar T., Warren T.K. Remdesivir (GS-5734) is efficacious in cynomolgus macaques infected with Marburg virus. J. Infect. Dis. 2020;222(11):1894–1901. doi: 10.1093/infdis/jiaa290. [DOI] [PubMed] [Google Scholar]
- 34.Li Y., Cao L., Li G., Cong F., Li Y., Sun J., Luo Y., Chen G., Li G., Wang P., Xing F., Ji Y., Zhao J., Zhang Y., Guo D., Zhang X. Remdesivir metabolite GS-441524 effectively inhibits SARS-CoV-2 infection in mouse models. J. Med. Chem. 2021 doi: 10.1021/acs.jmedchem.0c01929. [DOI] [PubMed] [Google Scholar]
- 35.J. Grein, N. Ohmagari, D. Shin, G. Diaz, E. Asperges, A. Castagna, T. Feldt, G. Green, M.L. Green, F.X. Lescure, E. Nicastri, R. Oda, K. Yo, E. Quiros-Roldan, A. Studemeister, J. Redinski, S. Ahmed, J. Bernett, D. Chelliah, D. Chen, S. Chihara, S.H. Cohen, J. Cunningham, A. D'Arminio Monforte, S. Ismail, H. Kato, G. Lapadula, E. L'Her, T. Maeno, S. Majumder, M. Massari, M. Mora-Rillo, Y. Mutoh, D. Nguyen, E. Verweij, A. Zoufaly, A.O. Osinusi, A. DeZure, Y. Zhao, L. Zhong, A. Chokkalingam, E. Elboudwarej, L. Telep, L. Timbs, I. Henne, S. Sellers, H. Cao, S.K. Tan, L. Winterbourne, P. Desai, R. Mera, A. Gaggar, R.P. Myers, D.M. Brainard, R. Childs, T. Flanigan, Compassionate use of Remdesivir for patients with severe Covid-19, N. Engl. J. Med. 382(24) (2020) 2327-2336, doi:10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed]
- 36.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., Fu S., Gao L., Cheng Z., Lu Q., Hu Y., Luo G., Wang K., Lu Y., Li H., Wang S., Ruan S., Yang C., Mei C., Wang Y., Ding D., Wu F., Tang X., Ye X., Ye Y., Liu B., Yang J., Yin W., Wang A., Fan G., Zhou F., Liu Z., Gu X., Xu J., Shang L., Zhang Y., Cao L., Guo T., Wan Y., Qin H., Jiang Y., Jaki T., Hayden F.G., Horby P.W., Cao B., Wang C. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–1578. doi: 10.1016/s0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., Hohmann E., Chu H.Y., Luetkemeyer A., Kline S., Lopez de Castilla D., Finberg R.W., Dierberg K., Tapson V., Hsieh L., Patterson T.F., Paredes R., Sweeney D.A., Short W.R., Touloumi G., Lye D.C., Ohmagari N., Oh M.D., Ruiz-Palacios G.M., Benfield T., Fätkenheuer G., Kortepeter M.G., Atmar R.L., Creech C.B., Lundgren J., Babiker A.G., Pett S., Neaton J.D., Burgess T.H., Bonnett T., Green M., Makowski M., Osinusi A., Nayak S., Lane H.C. Remdesivir for the treatment of Covid-19 - final Report. N. Engl. J. Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pimentel J., Laurie C., Cockcroft A., Andersson N. Clinical studies assessing the efficacy, effectiveness and safety of remdesivir in management of COVID-19: A scoping review. Br. J. Clin. Pharmacol. 2021;87(7):2663–2684. doi: 10.1111/bcp.v87.710.1111/bcp.14677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aleissa M.M., Silverman E.A., Paredes Acosta L.M., Nutt C.T., Richterman A., Marty F.M. New perspectives on antimicrobial agents: Remdesivir treatment for COVID-19. Antimicrob. Agents Chemother. 2020;65(1) doi: 10.1128/aac.01814-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldman J.D., Lye D.C.B., Hui D.S., Marks K.M., Bruno R., Montejano R., Spinner C.D., Galli M., Ahn M.Y., Nahass R.G., Chen Y.S., SenGupta D., Hyland R.H., Osinusi A.O., Cao H., Blair C., Wei X., Gaggar A., Brainard D.M., Towner W.J., Muñoz J., Mullane K.M., Marty F.M., Tashima K.T., Diaz G., Subramanian A. Remdesivir for 5 or 10 days in patients with severe Covid-19. N. Engl. J. Med. 2020;383(19):1827–1837. doi: 10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang Y., Chen D., Cai D., Yi Y., Jiang S. Effectiveness of remdesivir for the treatment of hospitalized COVID-19 persons: A network meta-analysis. J. Med. Virol. 2021;93(2):1171–1174. doi: 10.1002/jmv.v93.210.1002/jmv.26443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kow C.S., Aldeyab M., Hasan S.S. Effect of remdesivir on mortality in patients with COVID-19: A meta-analysis of randomized control trials. J. Med. Virol. 2021;93(4):1860–1861. doi: 10.1002/jmv.v93.410.1002/jmv.26638. [DOI] [PubMed] [Google Scholar]
- 43.Kaka A.S., MacDonald R., Greer N., Vela K., Duan-Porter W., Obley A., Wilt T.J. Major update: Remdesivir for adults with COVID-19: a living systematic review and meta-analysis for the American College of Physicians practice points. Ann. Intern. Med. 2021;174(5):663–672. doi: 10.7326/m20-8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cubeddu L.X., Cubeddu R.J. Early remdesivir treatment in COVID-19: Why wait another day? J. Med. Virol. 2021;93(7):4078–4080. doi: 10.1002/jmv.v93.710.1002/jmv.26792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Juneja K., Humeniuk R., Porter D., Cao H., Feng J. Reply to Yan and Muller, “Remdesivir for COVID-19: why not dose higher?”. Antimicrob. Agents Chemother. 2021;65(4) doi: 10.1128/aac.00085-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yan V.C., Muller F.L. Remdesivir for COVID-19: why not dose higher? Antimicrob. Agents Chemother. 2021;65(4) doi: 10.1128/aac.02713-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shrestha D.B., Budhathoki P., Syed N.-i.-H., Rawal E., Raut S., Khadka S. Remdesivir: A potential game-changer or just a myth? A systematic review and meta-analysis. Life Sci. 2021;264:118663. doi: 10.1016/j.lfs.2020.118663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Contini C., Enrica Gallenga C., Neri G., Maritati M., Conti P. A new pharmacological approach based on remdesivir aerosolized administration on SARS-CoV-2 pulmonary inflammation: A possible and rational therapeutic application. Med Hypotheses. 2020;144:109876. doi: 10.1016/j.mehy.2020.109876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sahakijpijarn S., Moon C., Koleng J.J., Christensen D.J., Williams R.O. Williams Iii, Development of Remdesivir as a dry powder for inhalation by thin film freezing. Pharmaceutics. 2020;12(11):1002. doi: 10.3390/pharmaceutics12111002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu Y., Barauskas O., Kim C., Babusis D., Murakami E., Kornyeyev D., Lee G., Stepan G., Perron M., Bannister R., Schultz B.E., Sakowicz R., Porter D., Cihlar T., Feng J.Y. Off-target in vitro profiling demonstrates that Remdesivir is a highly selective antiviral agent. Antimicrob. Agents Chemother. 2021;65(2) doi: 10.1128/aac.02237-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen H.-L., Chao C.-M., Lai C.-C. The safety of remdesivir for COVID-19 patients. J. Med. Virol. 2021;93(4):1910–1912. doi: 10.1002/jmv.v93.410.1002/jmv.26735. [DOI] [PubMed] [Google Scholar]
- 52.Burwick R.M., Yawetz S., Stephenson K.E., Collier A.Y., Sen P., Blackburn B.G., Kojic E.M., Hirshberg A., Suarez J.F., Sobieszczyk M.E., Marks K.M., Mazur S., Big C., Manuel O., Morlin G., Rose S.J., Naqvi M., Goldfarb I.T., DeZure A., Telep L., Tan S.K., Zhao Y., Hahambis T., Hindman J., Chokkalingam A.P., Carter C., Das M., Osinusi A.O., Brainard D.M., Varughese T.A., Kovalenko O., Sims M.D., Desai S., Swamy G., Sheffield J.S., Zash R., Short W.R. Compassionate use of Remdesivir in pregnant women with severe Covid-19. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Igbinosa I., Miller S., Bianco K., Nelson J., Kappagoda S., Blackburn B.G., Grant P., Subramanian A., Lyell D.J., El-Sayed Y.Y., Aziz N. Use of remdesivir for pregnant patients with severe novel coronavirus disease 2019. Am. J. Obstet. Gynecol. 2020;223(5):768–770. doi: 10.1016/j.ajog.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ackley T.W., McManus D., Topal J.E., Cicali B., Shah S. A valid warning or clinical lore: an evaluation of safety outcomes of Remdesivir in patients with impaired renal function from a multicenter matched cohort. Antimicrob. Agents Chemother. 2021;65(2) doi: 10.1128/aac.02290-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yin L., Zhao H., Zhang H., Li Y., Dong Y., Ju H., Kong F., Zhao S. Remdesivir alleviates acute kidney injury by inhibiting the activation of NLRP3 inflammasome. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.652446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gubitosa J.C., Kakar P., Gerula C., Nossa H., Finkel D., Wong K., Khatri M., Ali H. Marked sinus bradycardia associated with Remdesivir in COVID-19: a case and literature review. JACC Case Rep. 2020;2(14):2260–2264. doi: 10.1016/j.jaccas.2020.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Choi S.W., Shin J.S., Park S.J., Jung E., Park Y.G., Lee J., Kim S.J., Park H.J., Lee J.H., Park S.M., Moon S.H., Ban K., Go Y.Y. Antiviral activity and safety of remdesivir against SARS-CoV-2 infection in human pluripotent stem cell-derived cardiomyocytes. Antiviral Res. 2020;184 doi: 10.1016/j.antiviral.2020.104955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gupta A.K., Parker B.M., Priyadarshi V., Parker J. Cardiac adverse events with Remdesivir in COVID-19 infection. Cureus. 2020;12(10) doi: 10.7759/cureus.11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sanchez-Codez M.I., Rodriguez-Gonzalez M., Gutierrez-Rosa I. Severe sinus bradycardia associated with Remdesivir in a child with severe SARS-CoV-2 infection. Eur. J. Pediatr. 2021;180(5):1627. doi: 10.1007/s00431-021-03940-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.A. Touafchia, H. Bagheri, D. Carrié, G. Durrieu, A. Sommet, L. Chouchana, F. Montastruc, Serious bradycardia and remdesivir for coronavirus 2019 (COVID-19): a new safety concerns, Clin. Microbiol. Infect. 27(5) (2021) 791.e5-8, doi:10.1016/j.cmi.2021.02.013. [DOI] [PMC free article] [PubMed]
- 61.Barkas F., Styla C.P., Bechlioulis A., Milionis H., Liberopoulos E. Sinus bradycardia associated with Remdesivir treatment in COVID-19: a case report and literature review. J. Cardiovasc. Dev. Dis. 2021;8(2) doi: 10.3390/jcdd8020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pallotto C., Suardi L.R., Gabbuti A., Esperti S., Mecocci L., Blanc P. Potential remdesivir-related transient bradycardia in patients with coronavirus disease 2019 (COVID-19) J. Med. Virol. 2021;93(5):2631–2634. doi: 10.1002/jmv.26898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martin R., Li J., Parvangada A., Perry J., Cihlar T., Mo H., Porter D., Svarovskaia E. Genetic conservation of SARS-CoV-2 RNA replication complex in globally circulating isolates and recently emerged variants from humans and minks suggests minimal pre-existing resistance to remdesivir. Antiviral Res. 2021;188 doi: 10.1016/j.antiviral.2021.105033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lo M.K., Albariño C.G., Perry J.K., Chang S., Tchesnokov E.P., Guerrero L., Chakrabarti A., Shrivastava-Ranjan P., Chatterjee P., McMullan L.K., Martin R., Jordan R., Götte M., Montgomery J.M., Nichol S.T., Flint M., Porter D., Spiropoulou C.F. Remdesivir targets a structurally analogous region of the Ebola virus and SARS-CoV-2 polymerases. Proc. Natl. Acad. Sci. U.S.A. 2020;117(43):26946–26954. doi: 10.1073/pnas.2012294117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iaboni A., Wong N., Betschel S.D. A patient with X-linked agammaglobulinemia and COVID-19 infection treated with Remdesivir and convalescent plasma. J. Clin. Immunol. 2021;41(5):923–925. doi: 10.1007/s10875-021-00983-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hopwood A.J., Jordan-Villegas A., Gutierrez L.D., Cowart M.C., Vega-Montalvo W., Cheung W.L., McMahan M.J., Gomez M.R., Laham F.R. Severe acute respiratory syndrome Coronavirus-2 pneumonia in a newborn treated with Remdesivir and coronavirus disease 2019 convalescent plasma. J. Pediatric Infect. Dis. Soc. 2021;10(5):691–694. doi: 10.1093/jpids/piaa165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schloer S., Brunotte L., Mecate-Zambrano A., Zheng S., Tang J., Ludwig S., Rescher U. Drug synergy of combinatory treatment with remdesivir and the repurposed drugs fluoxetine and itraconazole effectively impairs SARS-CoV-2 infection in vitro. Br. J. Pharmacol. 2021;178(11):2339–2350. doi: 10.1111/bph.15418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stegmann K.M., Dickmanns A., Gerber S., Nikolova V., Klemke L., Manzini V., Schlösser D., Bierwirth C., Freund J., Sitte M., Lugert R., Salinas G., Meister T.L., Pfaender S., Görlich D., Wollnik B., Groß U., Dobbelstein M. The folate antagonist methotrexate diminishes replication of the coronavirus SARS-CoV-2 and enhances the antiviral efficacy of remdesivir in cell culture models. Virus Res. 2021;302 doi: 10.1016/j.virusres.2021.198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jang Y., Shin J.S., Lee M.K., Jung E., An T., Kim U.I., Kim K., Kim M. Comparison of antiviral activity of gemcitabine with 2'-fluoro-2'-deoxycytidine and combination therapy with Remdesivir against SARS-CoV-2. Int. J. Mol. Sci. 2021;22(4) doi: 10.3390/ijms22041581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen T., Fei C.Y., Chen Y.P., Sargsyan K., Chang C.P., Yuan H.S., Lim C. Synergistic inhibition of SARS-CoV-2 replication using disulfiram/ebselen and Remdesivir. ACS Pharmacol. Transl. Sci. 2021;4(2):898–907. doi: 10.1021/acsptsci.1c00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Monteil V., Dyczynski M., Lauschke V.M., Kwon H., Wirnsberger G., Youhanna S., Zhang H., Slutsky A.S., Hurtado Del Pozo C., Horn M., Montserrat N., Penninger J.M., Mirazimi A. Human soluble ACE2 improves the effect of remdesivir in SARS-CoV-2 infection. EMBO Mol. Med. 2021;13(1) doi: 10.15252/emmm.202013426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.A.C. Kalil, T.F. Patterson, A.K. Mehta, K.M. Tomashek, C.R. Wolfe, V. Ghazaryan, V.C. Marconi, G.M. Ruiz-Palacios, L. Hsieh, S. Kline, V. Tapson, N.M. Iovine, M.K. Jain, D.A. Sweeney, H.M. El Sahly, A.R. Branche, J. Regalado Pineda, D.C. Lye, U. Sandkovsky, A.F. Luetkemeyer, S.H. Cohen, R.W. Finberg, P.E.H. Jackson, B. Taiwo, C.I. Paules, H. Arguinchona, N. Erdmann, N. Ahuja, M. Frank, M.D. Oh, E.S. Kim, S.Y. Tan, R.A. Mularski, H. Nielsen, P.O. Ponce, B.S. Taylor, L. Larson, N.G. Rouphael, Y. Saklawi, V.D. Cantos, E.R. Ko, J.J. Engemann, A.N. Amin, M. Watanabe, J. Billings, M.C. Elie, R.T. Davey, T.H. Burgess, J. Ferreira, M. Green, M. Makowski, A. Cardoso, S. de Bono, T. Bonnett, M. Proschan, G.A. Deye, W. Dempsey, S.U. Nayak, L.E. Dodd, J.H. Beigel, Baricitinib plus Remdesivir for hospitalized adults with Covid-19, N. Engl. J. Med. 384(9) (2021) 795-807, doi:10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed]
- 73.Lee T.C., McDonald E.G., Butler-Laporte G., Harrison L.B., Cheng M.P., Brophy J.M. Remdesivir and systemic corticosteroids for the treatment of COVID-19: A Bayesian re-analysis. Int. J. Infect. Dis. 2021;104:671–676. doi: 10.1016/j.ijid.2021.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ye Z.W., Yuan S., Chan J.F., Zhang A.J., Yu C.Y., Ong C.P., Yang D., Chan C.C., Tang K., Cao J., Poon V.K., Chan C.C., Cai J.P., Chu H., Yuen K.Y., Jin D.Y. Beneficial effect of combinational methylprednisolone and remdesivir in hamster model of SARS-CoV-2 infection. Emerg. Microbes Infect. 2021;10(1):291–304. doi: 10.1080/22221751.2021.1885998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Benfield T., Bodilsen J., Brieghel C., Harboe Z.B., Helleberg M., Holm C., Israelsen S.B., Jensen J., Jensen T., Johansen I.S., Johnsen S., Madsen B.L., Lundgren J., Meyer C.N., Mohey R., Pedersen L.M., Nielsen H., Nielsen S.L., Obel N., Omland L.H., Podlekareva D., Poulsen B.K., Ravn P., Sandholdt H., Starling J., Storgaard M., Søborg C., Søgaard O.S., Tranborg T., Wiese L., Christensen H.R. Improved survival among hospitalized patients with COVID-19 treated with remdesivir and dexamethasone. A nationwide population-based cohort study. Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciab536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gandhi R.T. The multidimensional challenge of treating COVID-19: Remdesivir is a foot in the door. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Garibaldi B.T., Wang K., Robinson M.L., Zeger S.L., Bandeen-Roche K., Wang M.C., Alexander G.C., Gupta A., Bollinger R., Xu Y. Comparison of time to clinical improvement with vs without Remdesivir treatment in hospitalized patients with COVID-19. JAMA Netw Open. 2021;4(3) doi: 10.1001/jamanetworkopen.2021.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Potter C.W., Phair J.P., Vodinelich L., Fenton R., Jennings R. Antiviral, immunosuppressive and antitumour effects of ribavirin. Nature. 1976;259(5543):496–497. doi: 10.1038/259496a0. [DOI] [PubMed] [Google Scholar]
- 79.Hsu J. Covid-19: What now for remdesivir? BMJ. 2020;371 doi: 10.1136/bmj.m4457. [DOI] [PubMed] [Google Scholar]
- 80.Mendez-Echevarria A., Sándor-Bajusz K.A., Calvo C. Severe sinus bradycardia associated with remdesivir in a child with severe SARS-COV-2 infection-reply. Eur. J. Pediatr. 2021;180(5):1629–1630. doi: 10.1007/s00431-021-03952-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saha A., Sharma A.R., Bhattacharya M., Sharma G., Lee S.-S., Chakraborty C. Probable molecular mechanism of Remdesivir for the treatment of COVID-19: need to know more. Arch. Med. Res. 2020;51(6):585–586. doi: 10.1016/j.arcmed.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chatterjee S. Remdesivir: critical clinical appraisal for COVID 19 treatment. drug_Research. 2021;71(03):138–148. doi: 10.1055/a-1288-4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jorgensen S.C.J., Kebriaei R., Dresser L.D. Remdesivir: review of pharmacology, pre-clinical data, and emerging clinical experience for COVID-19. Pharmacotherapy. 2020;40(7):659–671. doi: 10.1002/phar.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Do T.N.D., Donckers K., Vangeel L., Chatterjee A.K., Gallay P.A., Bobardt M.D., Bilello J.P., Cihlar T., De Jonghe S., Neyts J., Jochmans D. A robust SARS-CoV-2 replication model in primary human epithelial cells at the air liquid interface to assess antiviral agents. Antiviral Res. 2021;192 doi: 10.1016/j.antiviral.2021.105122. [DOI] [PMC free article] [PubMed] [Google Scholar]