Abstract
Fusarium species are opportunistic nosocomial pathogens that often cause fatal invasive mycoses. We designed a primer pair that amplifies by PCR a fragment of a gene coding for the rRNA of Fusarium species. The DNAs of the main Fusarium species and Neocosmospora vasinfecta but not the DNAs from 11 medically important fungi were amplified by these primers. The lower limit of detection of the PCR system was 10 fg of Fusarium solani DNA by ethidium bromide staining. To test the ability of this PCR system to detect Fusarium DNA in tissues, we developed a mouse model of disseminated fusariosis. Using the PCR, we detected Fusarium DNA in mouse tissues and in spiked human blood. Furthermore, F. solani, Fusarium moniliforme, and Fusarium oxysporum were testing by random amplified polymorphic DNA (RAPD) analysis. The bands produced by RAPD analysis were purified, cloned, and sequenced. The information was used to design primer pairs that selectively amplified one or several Fusarium species. The method developed may be useful for the rapid detection and identification of Fusarium species both from culture and from clinical samples.
Disseminated fungal infections constitute one of the most difficult challenges for clinicians caring for immunocompromised patients (3). Candidiasis and aspergillosis remain the most common mycoses in neutropenic patients. However, other life-threatening infections caused by new opportunistic pathogens also occur. One of the most frequently occurring of these pathogens is Fusarium (15, 19).
Members of the genus Fusarium are ubiquitous fungi commonly found in soils and plants (22). Fusarium species have long been recognized as a cause of localized infections (11). Because of bone marrow grafts and immunosuppressive therapy, invasive Fusarium infections have increased during the last decade. The immunologic status of the host and the extent of the infection are the most important factors for the clinical outcome of Fusarium infections (13).
Because an invasive Fusarium infection may mimic aspergillosis, patients are usually treated with amphotericin B, an antifungal agent with poor activity against fusariosis (9). Hence, early identification is an important factor for a successful outcome. Furthermore, diagnosis requires the demonstration of hyphae in pathological samples; however, hyphae of Aspergillus, Scedosporium, and Fusarium are difficult to discriminate. Positive culture is thus needed for the identification of a Fusarium sp. Currently, the identification of members of the genus Fusarium is based on the characteristic colony morphology and the microscopic characters, which include the production of multiseptated sickle-shaped conidia called macroconidia; however, recognition may be difficult when the macroconidia are not produced in culture (11). This usually happens with strains isolated from clinical samples which have been developed in unfavorable conditions. In this case, the isolates can be confused with other genera such as Acremonium and Verticilium. Furthermore, precise determination of Fusarium species remains a prerequisite for studying the spread, host infections, and treatment.
The PCR technique is extremely sensitive and has been used successfully for the specific detection of several fungi (20). We report here on the use of a competitive PCR technique for the detection of Fusarium spp. in blood and tissues and PCRs for the identification of the Fusarium species. In order to test the PCR system, we also developed a mouse model of fusariosis.
MATERIALS AND METHODS
Culture conditions.
DNA was isolated from several Fusarium species and a range of medically important fungi. The collection used is listed in Table 1. The Fusarium isolates were maintained on potato dextrose agar at 25°C. The other fungi were maintained on Sabouraud-chloramphenicol (SC) at 30°C. Malassezia furfur was cultured on Dixon agar at 37°C.
TABLE 1.
Yeast and filamentous fungi screened by PCR
| Species | Strains/Sources |
|---|---|
| Fusarium moniliforme | IPa 1090.74, IP 1550.84, IP 1579.85, IP 1685.87, IP 2333.95 |
| Fusarium solani | IP 1195.79, IP 1272.81, IP 1684.87, IP 2330.95, IP 2447.97, IP 2451.98 |
| Fusarium oxysporum | IP 625.72, IP 95.82, 9026 |
| Fusarium proliferatum | IP 2240.94, IP 2290.94, IP 2295.95, 68G |
| Fusarium dimerum | IP 1516.83, IP 2356.96 |
| Fusarium semitectum | 2239 |
| Fusarium subglutinans | 2241, 2378 |
| Fusarium chlamydosporum | IP 1542.84 |
| Fusarium nivale | 2238 |
| Fusarium equiseti | IP 1707.87 |
| Fusarium anthophilum | 2367.96 |
| Acremonium strictum | IP 2334.936 |
| Alternaria alternata | CBS 1545-31 |
| Aspergillus flavus | IP 2452.98 |
| Aspergillus fumigatus | |
| Aspergillus niger | IP 1431.83 (ATCC 16404) |
| Candida albicans | IP 48.72 (ATCC 10231) |
| Cryptococcus neoformans | IP 960.67 (CDC B 236) |
| Exophiala jeansemei | IP 69.52 |
| Malassezia furfur | IP 2386.96 |
| Neocosmospora vasinfecta | IP 2450.97 |
| Paecilomyces variotii | IP 1024.70 |
| Penicillium purpurogenum | 9701 |
| Pseudallescheria boydii | IP 1448.83 |
| Trichosporon cutaneum | IP 2217.94 |
IP, Collection of Fungi, Institut Pasteur, Paris, France.
DNA preparation from fungal cells.
The total cell DNA was extracted from mycelium or yeast grown in YPG liquid medium by the method described by Dellaporta et al. (8). Briefly, the mycelium and the yeast were mechanically disrupted within liquid nitrogen and were mixed with 15 ml of fungal extraction buffer (Tris-HCl, 10 mM; EDTA, 50 mM; NaCl, 500 mM), 1 ml of sodium dodecyl sulfate (10%) was added, and the mixture was incubated for 20 min at 65°C. Then, 5 ml of potassium acetate (3 M) was added, and the homogenate was incubated for 30 min on ice. After centrifugation, the DNA was precipitated with 0.7 volume of isopropanol, and the samples were centrifuged at 9,000 × g for 20 min at 4°C. The pellet was dissolved in 700 μl of Tris (Tris-HCl, 10 mM [pH 8]) and incubated at 65°C, and the DNA was precipitated with 0.7 volume of isopropanol and 0.1 volume of sodium acetate (3 M). The DNA was washed with 70% ethanol, dried, and resuspended in 200 μl of Tris (10 mM; pH 8).
Oligonucleotide design, internal control, PCR amplification, and detection of PCR products.
The design of oligonucleotides P28SL and P58SL was based on comparison of the sequences of the ribosomal genes (rDNAs) from a large number of isolates belonging to the genus Fusarium found in the EMBL/GenBank database (Table 2). The sequences were analyzed with the PILEUP program of the Genetics Computer Group software package as reported earlier (9, 10). Primers P28SL and P58SL (Oligoexpress, Paris, France) amplified a fragment of 329 bp containing ITS2 and a portion of 5.8S and 28S rDNA.
TABLE 2.
GenBank accession numbers for the Fusarium DNAs used
To avoid false-negative results, a positive internal control was made from a part of λ phage DNA (4). We designed two primers, primers C1 and C2 (Table 3), whose 3′ ends correspond to the λ DNA and whose 5′ ends correspond to the primers used in the PCR amplification. The first PCR generated a fragment of 517 bp which contains the Fusarium sequence at the ends. This fragment was amplified with the Fusarium primers and was purified with the Qiaquick PCR amplification kit (Qiagen, Courtaboeuf, France). After dilution of the fragments, PCRs were performed. The highest dilution that gave a positive result after electrophoresis was chosen as the internal control. One microliter of internal control was added to each PCR mixture.
TABLE 3.
Nucleotide sequences of the primers
| System and primer | Sequence |
|---|---|
| Fusarium PCR | |
| P58SL | 5′-AGT ATT CTG GCG GGC ATG CCT GT-3′ |
| P28SL | 5′-ACA AAT TAC AAC TCG GGC CCG AGA-3′ |
| Internal control PCR | |
| C1 | 5′-ACA AAT TAC AAC TCG GGC CCG AGA CCA CAG CGC-3′ |
| C2 | 5′-AGT ATT CTG GCG GGC ATG CCT GTG TAC AAC TGG-3′ |
The PCRs were performed according to the manufacturer’s instructions (Ready to go kit; Pharmacia Biotech, Uppsala, Sweden), with the following temperatures cycles: initial denaturation at 94°C for 5 min, followed by 40 cycles of denaturation at 94°C for 1 min, annealing at 68°C for 1 min, and extension at 72°C for 1 min. The thermal cycles were terminated by a final extension of 10 min at 72°C. One nanogram of template DNA was used per reaction mixture.
To ensure that no contaminating DNA could give a positive result, one sample without DNA was included in each series of reactions. To examine the specificity of the P58SL and P28SL primer pair, the samples of genomic DNA extracted from fungi and from human blood were tested to verify whether the primer pair selectively amplified the DNA from Fusarium (Table 1). To determine the lower limit of detection, PCRs were performed with water samples containing 1 ng, 100 pg, 10 pg, 1 pg, 100 fg, 10 fg, 1 fg, and 0.1 fg of genomic Fusarium solani DNA.
Aliquots (10 μl) of the PCR products were analyzed on a 2% agarose gel after electrophoresis in Tris-acetate-EDTA buffer. A 100-bp DNA Ladder (Boehringer Mannheim GmbH, Mannheim, Germany) was used as a molecular size marker. The gels were stained with ethidium bromide (1 μg/ml), visualized under UV light, and photographed with Polaroid 667 film.
Preparation of PCR template from F. solani-containing blood.
Human blood (obtained from healthy volunteers after they provided appropriate written consent) was collected in tubes containing EDTA as an anticoagulant and was inoculated with 10-fold dilutions of Fusarium mycelium in sterile saline. The cells in the Fusarium-spiked blood samples were lysed (GFX genomic blood DNA purification kit; Pharmacia Biotech) and mixed with 200 μl of enzyme buffer (sorbitol, 0.9 M; Tris, 0.1 M; EDTA, 0.1 M) and 20 μl of Lyticase (Sigma, St. Louis, Mo.). Following incubation at 37°C for 90 min, proteinase K (10 μl, 20 mg/ml; Sigma) was added, and each sample was incubated at 55°C for 30 min, followed by the addition of 5 μl of RNase (Boehringer Mannheim GmbH). After incubation at 37°C for 30 min, the DNA was extracted by the use of the GFX genomic blood DNA purification kit, according to the manufacturer’s instructions. PCR was performed by using these extracts as templates (2 μl). Aliquots (10 μl) of the PCR products were analyzed by electrophoresis on a 2% agarose gel as described above.
Mouse model of invasive fusariosis.
Four male BALB/c mice (Charles River, Saint Aubin Les Elbeuf, France) weighing between 20 g and 25 g were immunosuppressed with three intraperitoneal injections of triamcinolone (Kenacort; Bristol-Myers Squibb, Paris, France) at a dose of 2 mg/kg of body weight on days −2, 0, and +3. On day 0, they were also inoculated in the lateral tail veins with 106 conidia of F. solani IP 1684.87 in a 200-μl volume of sterile saline. One control mouse was treated in the same way and received 200 μl of sterile saline on day 0. When the mice died, portions of organs and blood were removed. A total of 50 μl of each blood sample was cultured on SC at 25°C. Various organs (spleen, lung, heart, kidney, and liver) were cultured on SC at 25°C. A small piece of each organ was retained for histology. These sections were stained with hematoxylin-eosin-safran and Gomori methenamine silver stains. The remainder of each organ was ground in a 1.5-ml microcentrifuge tube and was stored at −20°C. DNA from both blood and tissues was extracted by the protocol described above.
Differentiation of Fusarium species.
For random amplified polymorphic DNA (RAPD) analysis, DNA fragments were amplified with primers F 24 and A8 (12), as follows: one cycle of 5 min at 92°C, followed by 36 cycles of denaturation at 92°C for 30 s, annealing at 36°C for 1 min, and extension at 72°C for 2 min, with a final cycle of 2 min at 72°C. The DNAs of the three main Fusarium species (F. solani, Fusarium moniliforme, and Fusarium oxysporum) were tested. Three high-intensity bands produced by RAPD analysis for each species were purified from agarose and were cloned into the vector pUC 18. The ends of the cloned fragments were sequenced according to the manufacturer’s instructions (Thermosequenase Cycle Sequencing; Amersham Life Science, Buckinghamshire, United Kingdom), and the sequences obtained were used to design the primer pairs. The PCR amplifications and the analysis of the DNA were performed as described above.
RESULTS
Oligonucleotide design.
After alignment and visual assessment of the Fusarium and non-Fusarium sequences, primers pair P58SL and P28SL, which amplified a 329-bp fragment, was selected (Table 3).
Specificity and sensitivity studies.
On the basis of the sequences of the 5.8S and 28S regions of the rDNA complex, a product of 329 bp was amplified by PCR with primer pair P58SL and P28SL from all 27 strains of 11 medically important Fusarium species and from Neocosmospora vasinfecta but not from any of the other fungi tested (Fig. 1). The specificity of the 329-bp fragment was verified with the restriction enzymes HincII and XbaI (Gibco Life Technologies, Cergy-Pontoise, France); as expected, the 329-bp fragment was not digested by XbaI and gave two fragments of 200 and 129 bp upon HincII digestion. No amplification was observed with human DNA.
FIG. 1.
Specificity of PCR with primer pair P58SL and P28SL. An ethidium bromide-stained agarose gel of PCR products amplified from 1 ng of genomic DNA templates from various fungi is shown. (A) Lanes: 1, F. moniliforme IP 1579.85; 2, F. moniliforme IP 233.95; 3, F. solani IP 2330.95; 4, F. solani IP 2447.97; 5, F. solani IP 2451.98; 6, F. oxysporum 9582; 7, F. oxysporum IP 625.72; 8, F. oxysporum 9026; 9, F. proliferatum 68G; 10, F. dimerum IP 1516.83; 11, F. semitectum 2239; 12, F. subglutinans 2241; 13, F. nivale 2238. (B) Lanes: 1, Candida albicans; 2, Cryptococcus neoformans; 3, Penicillium purpurogenum; 4, Aspergillus fumigatus; 5, Acremonium strictum; 6, Trischoporon cutaneum; 7, Malassezia furfur; 8, Exophiala jeanselmei; 9, Trichophyton rubrum; 10, Alternaria alternata; 11, Aspergillus flavus; 12, Neocosmospora vasinfecta; 13, F. solani IP 1681.87; lane T−, negative control. Lanes M, 100-bp DNA ladder.
The sensitivity allowed detection of as little as 10 fg of F. solani DNA by visualization under UV light. There was no amplified product from the negative control (sample without Fusarium DNA).
PCR template from Fusarium-containing blood.
As shown in Fig. 2, with primer pair P58SL and P28SL the PCR system was able to amplify the Fusarium DNA from blood artificially inoculated with mycelium. Amplified products were not detected from control samples that were devoid of Fusarium DNA.
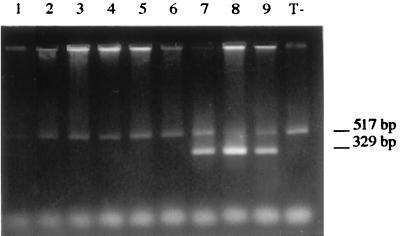
FIG. 2.
Ethidium bromide-stained agarose gel of products from F. solani IP 1684.87-spiked blood samples in serial 10-fold dilutions. The fragment of 517 bp corresponds to the internal control. The fragment of 329 bp corresponds to amplified F. solani DNA. Lane M, 100-bp DNA ladder.
Mouse model.
A summary of mouse survival and the culture, histology, and PCR results is given in Table 4. Fifteen samples had positive results by culture. Seven culture-positive samples were also positive by PCR (with primer pair P58SL and P28SL), and 3 of 13 culture-negative samples also had positive PCR results. The result for sample 24 was nonconclusive: the fragment corresponding to the internal control was not observed (Fig. 3). Despite the use of proteinase K and a column extraction, amplification inhibitor factors may not have been eliminated. All PCR products led to the amplification of a single fragment. The result of PCR, culture, and histology were all negative for uninfected mice.
TABLE 4.
Murine model of F. solani infection
| Mouse no. (day of death) | Sample no. | Sample | Culture result | Histology result | PCR result |
|---|---|---|---|---|---|
| 1 (+9) | 1 | Brain | + | − | − |
| 2 | Heart | + | − | − | |
| 3 | Liver | − | − | + | |
| 4 | Lung | + | − | NDa | |
| 5 | Spleen | + | − | − | |
| 6 | Kidney | + | + | + | |
| 7 | Blood | − | − | ||
| 2 (+10) | 8 | Brain | + | − | + |
| 9 | Heart | + | − | ND | |
| 10 | Liver | − | − | − | |
| 11 | Lung | − | − | − | |
| 12 | Spleen | − | − | − | |
| 13 | Kidney | + | + | + | |
| 14 | Blood | − | − | ||
| 3 (+10) | 15 | Brain | − | − | + |
| 16 | Heart | + | − | ND | |
| 17 | Liver | − | − | + | |
| 18 | Lung | + | − | − | |
| 19 | Spleen | + | − | − | |
| 20 | Kidney | + | + | + | |
| 21 | Blood | − | − | ||
| 4 (+10) | 22 | Heart | − | − | ND |
| 23 | Liver | + | − | + | |
| 24 | Lung | − | − | NCb | |
| 25 | Spleen | + | − | + | |
| 26 | Kidney | + | + | + | |
| 27 | Blood | − | − |
ND, not done.
NC, nonconclusive.
FIG. 3.
Three possible results expected by competitive PCR with primers P28SL and P58SL. The results of gel electrophoresis of PCR products amplified from genomic DNA extracted from mouse organs are shown. The fragment of 517 bp corresponds to the internal control. The fragment of 329 bp corresponds to amplified F. solani DNA. Lane 1, inconclusive PCR result; lanes 2, 3, 4, 5, and 6, PCR-negative results; lanes 7, 8, and 9, PCR-positive results; lane T−, negative control.
Differentiation of the Fusarium species.
Table 5 shows the primer pairs that were selected and their characteristics. These primer pairs were used to perform new PCR assays. The use in the same PCR mixture of the two primer pairs OX 31–OX 32 and OX 41–OX 42 allowed the amplification of the F. oxysporum DNAs tested (Fig. 4). Although primer pair SOL 31–SOL 32 was specific for F. solani, only four of five strains tested were amplified (Table 5).
TABLE 5.
Specificity and characteristics of species-specific primer pairs
| Primer | Sequence (5′-3′) | Specificity
|
Annealing temp (°C) | |
|---|---|---|---|---|
| Positive PCR result | Negative PCR result | |||
| OX 31 | TGA CTT GGA TGA GAC CTT GGC G | F. oxysporum 95, 82, and 9026 | F. moniliforme, F. solani, F. oxysporum IP 625.72, F. proliferatum, F. dimerum, F. subglutinans, F. semitectum, and F. chlamydosporum | 66 |
| OX 32 | CAG GAT TTA CCG ACA CAG CTT TTG | |||
| OX 41 | GTA GGA AAA ACA ATT GCT CAG TCG | F. oxysporum 9582 and IP 625.72 | F. moniliforme, F. solani, F. oxysporum 9026, F. proliferatum, F. dimerum, F. subglutinans, F. semitectum, and F. chlamydosporum | 66 |
| OX 42 | AAG AGA GTG TGT AGT GGT GTG G | |||
| OX 1 | GTC ACG ACA TTT TCA CAA GCT G | F. moniliforme, F. oxysporum, F. proliferatum IP 2240.94, and F. subglutinans | F. solani, F. proliferatum IP 2290.94, IP 2296.95, and 68G, F. dimerum, F. chlamydosporum, and F. semitectum | 63 |
| OX 2 | GGT CTC TCG ATA ACT TTG ACA G | |||
| SOL 31 | GCT ACC GAG GCC ATC AAT TCA TG | F. solani IP 2451.98, IP 1195.79, and IP 2330.95 | F. moniliforme, F. solani IP 1984.87, F. oxysporum, F. proliferatum, F. subglutinans, F. semitectum, F. dimerum, and F. chlamydosporum | 62 |
| SOL 32 | TGA TGT TGT ACT TCT CCT TGC CC | |||
| MON 1 | GAG AGC TGG ATG TAC GAA TG | F. moniliforme, F. oxysporum, F. solani, F. subglutinans, and F. proliferatum | F. semitectum, F. dimerum, and F. chlamydosporum | 60 |
| MON 2 | CAC AGA GAT GGT TCA CTG AG | |||
FIG. 4.
Specificity of the primer pairs OX 31–OX 32 and OX 41–OX 42. An ethidium bromide-stained agarose gel of PCR products amplified from 1 ng of genomic templates from various fungi is shown. Lane 1, F. oxysporum IP 625.72; lane 2, F. oxysporum 9582; lane 3, F. oxysporum 9026; lane 4, F. moniliforme IP 1579.85; lane 5, F. solani IP 2330.95; lane 6, F. proliferatum IP 2240.94; lane 7, F. dimerum IP 1516.83; lane 8, F. semitectum 2239; lane 9, F. subglutinans 2241; lane 10, F. chlamydosporum IP 1542.84; lane 11, Penicillium purpurogenum 9701; lane 12, Aspergillus fumigatus; lane 13, Acremonium strictum IP 2234.96; lane 14, Candida albicans IP 48.72; lane T−, negative control. Lane M, 100-bp DNA ladder. pb, base pairs.
DISCUSSION
The results obtained in the present study demonstrate that the rDNA-based PCR method has high degrees of sensitivity and specificity for the detection of a wide range of medically important Fusarium species from spiked blood and from tissue from an animal model.
Several previous studies have used PCR technology to detect fungi (20). The primers used target three kinds of sequences: (i) sequences coding for an antifungal target, (ii) fungus-specific regions of conserved proteins such as actin, and (iii) repeated sequences such as those of rDNA and mitochondrial DNA. For diagnostic purposes, it is essential that repeated sequences be used as targets to ensure good sensitivity, which justifies our choice of primers. We reported that the binding sites for primer pair P58SL and P28SL, within the 5.8S and the 28S regions, were conserved among Fusarium species. They amplified DNAs from all 11 different Fusarium species tested and also from N. vasinfecta, which is a sexual form of an Acremonium sp. whose species name is not yet defined. Only two cases of infection with the latter species have been reported in humans (7).
One of the limitations of the molecular methods of diagnosis of microbial infections, especially PCR amplification, are the false positivity and the false negativity of the test results. These issues are being addressed in several ways: standardization of the technical procedure (one-step procedure) and the use of an internal control (2). Impurities in nucleic acid preparations (e.g., ethanol and isopropanol) or in biological samples (e.g., hemoglobin, EDTA, and heparin) can inhibit or reduce the sensitivity of PCR amplification (16). Thus, the internal control is necessary both for clinical diagnosis and for the development of a DNA extraction procedure.
Although many studies regarding the PCR detection of yeast (5, 6, 17, 18, 21, 24) and Aspergillus fumigatus (25) have been reported, few PCR systems for the detection of other fungi have been developed (1, 26). Moreover, the PCRs described for the latter case have not been tested with blood or tissues. For this reason, we thought it desirable to test the PCR with primers P28SL and P58SL for the detection of Fusarium both in spiked blood and in tissues and blood from experimentally infected mice. In the mouse model, the correlation between PCR results and culture results was not high (46%); however, 23% of the samples with culture-negative results were positive by PCR amplification. One explanation is the poor efficiency of the extraction protocol. Another explanation is the existence of a localized necrotic abscess. Indeed, before extraction histology and culture, each sample was divided into three portions, but not all portions may have been infected.
Different molecular methods are used to differentiate species of fungi: restriction fragment length polymorphism (RFLP) analysis (14, 16) or RAPD analysis (23). RFLP analysis is long and laborious and often requires the use of radioactive probes. RAPD analysis is poorly reproducible, and results are difficult to interpret. Furthermore, RAPD and RFLP analyses require axenic cultures of the fungus, whereas PCR can be performed with tissue samples. PCR should be considered as complementary method for the identification of Fusarium species when microscopic characterization cannot distinguish between two species.
Further experiments will be necessary to determine whether specificity and sensitivity similar to those obtained in vitro and with spiked blood samples can be obtained with clinical samples. Larger prospectives studies that focus on immunocompromised patients at high risk of invasive fusariosis should evaluate both the utility and the sensitivity of this PCR assay in a routine setup in comparison to those of other available methods (culture and histology).
ACKNOWLEDGMENTS
We are grateful to Nalin Rastogi and Christophe Sola for helpful discussions.
REFERENCES
- 1.Alexandrakis G, Sears M, Glorr P. Postmortem diagnosis of Fusarium panophthalmitis by the polymerase chain reaction. Am J Ophthalmol. 1996;121:221–222. doi: 10.1016/s0002-9394(14)70594-x. [DOI] [PubMed] [Google Scholar]
- 2.Blum H. Molecular diagnosis of microbiol infections. Biologicals. 1996;24:193–195. doi: 10.1006/biol.1996.0025. [DOI] [PubMed] [Google Scholar]
- 3.Boutati E I, Anaissie E J. Fusarium: a significant emerging pathogen in patients with hematologic malignancy: ten year’s experience at a cancer center and implication for management. Blood. 1997;90:999–1008. [PubMed] [Google Scholar]
- 4.Bretagne S, Costa J M, Marmorat-Khuong A, Poron F. Detection of Aspergillus species DNA in bronchoalveolar lavage samples by competitive PCR. J Clin Microbiol. 1995;33:1164–1168. doi: 10.1128/jcm.33.5.1164-1168.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burgener-Kairuz P, Zuber J P, Jaunin P, Buchman T G. Rapid detection and identification of Candida albicans and Torulopsis (Candida) glabrata in clinical specimens by species-specific nested PCR amplification of a cytochrome P-450 lanosterol-α-demethylase (L1A1) gene fragment. J Clin Microbiol. 1994;32:1902–1907. doi: 10.1128/jcm.32.8.1902-1907.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlotti A, Chaib F, Couble A, Bourgeois N, Blanchard V, Villard J. Rapid identification and fingerprinting of Candida krusei by PCR-based amplification of the species-specific repetitive polymorphic sequence CKRS-1. J Clin Microbiol. 1997;35:1337–1343. doi: 10.1128/jcm.35.6.1337-1343.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chandenier J, Hayette M P, de Bièvre C, Weestel P F, Petit J. Tuméfaction de la jambe àNeocosmospora vasinfecta chez un transplanté rénal. J Mycol Med. 1993;3:165–168. [Google Scholar]
- 8.Dellaporta S L, Wood J, Hicles J B. A plant DNA minipreparation. Plant Mol Biol Rep. 1983;1:19–21. [Google Scholar]
- 9.Deveureux J, Haeberli P, Smith O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng D, Dolittle R F. Progressive sequence alignment as a prerequisite to phylogenetics trees. J Mol Evol. 1987;25:351–360. doi: 10.1007/BF02603120. [DOI] [PubMed] [Google Scholar]
- 11.Guarro J, Gene J. Opportunistic fusarial infections in humans. Eur J Clin Microbiol Infect Dis. 1995;14:747–754. doi: 10.1007/BF01690988. [DOI] [PubMed] [Google Scholar]
- 12.Hening O, Niremberg H I. Differencition of Fusarium fuckel sensu lato and related species by RAPD PCR. Mycopathologia. 1995;129:159–164. doi: 10.1007/BF01103341. [DOI] [PubMed] [Google Scholar]
- 13.Hennequin C, Lavarde V, Poirot J L, Rabodonirina M. Invasive Fusarium infections: a retrospective survey of 31 cases. J Med Vet Mycol. 1997;35:107–114. [PubMed] [Google Scholar]
- 14.Hopfer R L, Walden P, Setterquist S, Highsmith W E. Detection and differentiation of fungi in clinical specimens using polymerase chain reaction (PCR) amplification and restriction enzyme analysis. J Med Vet Mycol. 1993;31:65–75. doi: 10.1080/02681219380000071. [DOI] [PubMed] [Google Scholar]
- 15.Krcmery V, Jesenska, Spaniks S, Gyarfas J. Fungemia due to Fusarium spp. in cancer patients. J Hosp Infect. 1997;36:223–228. doi: 10.1016/s0195-6701(97)90197-3. [DOI] [PubMed] [Google Scholar]
- 16.Löffert D, Stump S, Schaffrarh N. PCR: effect to template quality. Qiagen News. 1997;1:8–9. [Google Scholar]
- 17.Maiwald M, Murayama S Y, Yamaguchi H. Rapid presumptive identification of medically relevent yeast to the species level by polymerase chain reaction and restriction enzyme analysis. J Med Vet Mycol. 1994;32:115–122. doi: 10.1080/02681219480000161. [DOI] [PubMed] [Google Scholar]
- 18.Makimura K, Murayama S Y, Yamaguchi H. Detection of a wide range of medically important fungi by the polymerase chain reaction. J Med Microbiol. 1994;40:358–364. doi: 10.1099/00222615-40-5-358. [DOI] [PubMed] [Google Scholar]
- 19.Martino P, Gastaldi R, Raccah R, Girenmenia C. Clinical patterns of Fusarium infections in immunocompromised patients. J Infect. 1994;28:7–15. doi: 10.1016/s0163-4453(94)95911-0. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell T G, Sandin R L, Bowman B H, Meyer W, Merz W G. Molecular mycology: DNA probes and applications of PCR technology. J Med Vet Mycol. 1994;32:351–366. doi: 10.1080/02681219480000961. [DOI] [PubMed] [Google Scholar]
- 21.Miyakawa Y, Mabuchi T, Fukazawa Y. New method for detection of Candida albicans in human blood by polymerase chain reaction. J Clin Microbiol. 1993;31:3344–3347. doi: 10.1128/jcm.31.12.3344-3347.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nelson P E, Dignani M C, Anaissie E J. Taxonomy, biology, and clinical aspects of Fusarium species. Clin Microbiol Rev. 1994;7:479–504. doi: 10.1128/cmr.7.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ouellet T, Seifert K A. Genetic characterisation of Fusarium graminearum strains using RAPD and PCR amplification. Phytopathologia. 1993;8:1003–1007. [Google Scholar]
- 24.Polanco A M, Rodriguez-Tudela J L, Martnies-Suares J V. Detection of pathogenic fungi in human blood by the polymerase chain reaction. Eur J Clin Microbiol Infect Dis. 1995;14:618–621. doi: 10.1007/BF01690738. [DOI] [PubMed] [Google Scholar]
- 25.Spreadbury C, Hoden D, Aufrauve-Brauwn A, Baimbridge B, Cohen J. Detection of Aspergillus fumigatus by polymerase chain reaction. J Clin Microbiol. 1993;31:615–621. doi: 10.1128/jcm.31.3.615-621.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanittanakom N, Merz W G, Sittisombut N, Khmawan C, Nelson E K. Specific identification of Penicillium marneffei by polymerase chain reaction/hybridization technique. Med Mycol. 1998;36:169–175. [PubMed] [Google Scholar]