Abstract
Most human and animal disease notification systems are unintegrated and passive, resulting in underreporting. Active surveillance can complement passive efforts, but because they are resource-intensive, their attributes must be evaluated. We assessed the sensitivity and representativeness of One-Health surveillance conducted at health facilities compared to health facilities plus monthly household visits in three rural communities of Guatemala.
From September 2017 to November 2018, we screened humans for acute diarrheal, febrile and respiratory infectious syndromes and canines, swine, equines and bovines for syndromic events or deaths. We estimated the relative sensitivity as the incidence rate ratio of detecting an event in health facility surveillance compared to household surveillance from Poisson models. We used interaction terms between the surveillance method and sociodemographic factors or time trends to assess effect modification as a measure of relative representativeness. We used generalized additive models with smoothing splines to model incidence over time by surveillance method.
We randomized 216 households to health facility surveillance and 198 to health facility surveillance plus monthly household visits. Health facility surveillance alone was less sensitive than when combined with household surveillance by 0.42 (95% CI: 0.34, 0.53), 0.56 (95% CI: 0.39, 0.79), 0.02 (95% CI: 0.00, 0.10), 0.28 (95% CI: 0.15, 0.50) and 0.22 (95% CI: 0.03, 0.92) times for human acute infections, human severe acute infections, and deaths in canines, swine and equines, respectively. Health facility surveillance alone underrepresented Spanish speakers (interaction p-value = 0.0003) and persons in higher economic assets (interaction p-values = 0.0008). The trend in incidence over time was different between the two study groups, with a larger decrease in the group with household surveillance (all interaction p-values <0.10).
Surveillance at health facilities under ascertains syndromes in humans and animals which leads to underestimation of the burden of zoonotic disease. The magnitude of under ascertainment was differentially by sociodemographic factors, yielding an unrepresentative sample of health events. However, it is less time-intensive, thus might be sustained over time longer than household surveillance. The choice between methodologies should be evaluated against surveillance goals and available resources.
Keywords: One-health surveillance, Sensitivity, Representativeness, Health facility surveillance, Household surveillance, Guatemala
Highlights
-
•
We conducted One-Health surveillance for acute infections in Guatemala.
-
•
We contrasted attributes of health facility surveillance against household visits.
-
•
We compared quantitative estimates of attributes between randomly assigned groups.
-
•
Facility surveillance was less sensitive and representative than household visits.
-
•
The representativeness of household surveillance decreased over time.
1. Introduction
Approximately two-thirds of human infectious diseases have a zoonotic origin [[1], [2], [3], [4], [5]]. Strong collaborative efforts between the human, animal and environmental health sectors are increasingly recognized as needed to lessen the adverse impacts of zoonotic diseases on health [6,7]. A One Health approach can be defined as the incremental benefit of a closer cooperation of human and animal health and related sciences, that cannot be achieved if the sectors work along. One-Health surveillance is the systematic collection, validation, analysis, interpretation and dissemination of information collected on humans, animals and the environment [8]. It provides an efficient and cost-effective approach to estimate the burden, identify high-risk populations, and describe the spatial and temporal patterns of disease required to inform health interventions [8,9].
National surveillance for zoonotic diseases in low and middle-income countries is mainly conducted through passive notification of cases and separately within the human and animal health sectors. These systems have the advantage of covering large areas, but reliance on healthcare-seeking and reports from health care providers results in underreporting, inopportune detection of events, or lack of recognition of diseases' presence, leading to delays in responding to infectious disease threats. [4,[10], [11], [12]]. Aside from these limitations related to the low sensitivity of passive surveillance [13,14], another concern is that the cases that are detected at health services are not a random or otherwise representative subset of all cases of a disease, which could introduce selection bias in risk factor effect estimates.
Active surveillance employs staff members to regularly seek information on the population's health conditions, for example, through routine household visits or interviewing persons seeking care at health facilities. Household surveillance conducted at appropriate time intervals allows early detection of nearly every disease event and therefore is appropriate when a prompt response or accurate estimation of disease burden is required. Although household surveillance is expensive and time-consuming, limiting its sustainability and scalability, especially in low-resource settings [15,16], it can be a valuable comparison method to evaluate the properties of passive or health-facility surveillance. Moreover, the cost-effectiveness of household surveillance for zoonotic infections can be improved by using a One Health approach [8]. However, the successful implementation of active One Health surveillance over time requires a balance between information needs and available resources.
Given the role of zoonotic pathogens in the burden of infectious diseases, the potential synergy between human and animal public health sectors, and the need to complement passive national surveillance programs, we implemented active One-Health surveillance in rural Guatemala. In this manuscript, we aimed to evaluate active health facility surveillance by comparing its sensitivity and representativeness against our gold standard composed of active health facility surveillance plus monthly household surveillance implemented in comparable populations. Quantitative estimates of the relative differences in surveillance attributes will contribute to the interpretation of findings from health-facility surveillance.
2. Materials and methods
By using a transdisciplinary approach, based on a consensus-building dialogue with communities, authorities, human and animal health workers and scientists, we implemented One-Health surveillance for zoonotic diseases in three rural communities, named Sabaneta, La Romana, and San Marcos in Poptún, a municipality of the department of Petén in the Northern part of Guatemala, located at a mean elevation of 500 m (1640 ft) above sea level. We surveilled for Leptospirosis, Brucellosis, Bartonellosis, Influenza, Dengue, Zika, Chikungunya, and Malaria in humans and Leptospirosis and Brucellosis in animals. We focus on syndromic surveillance, with the assumption that the results can be generalized to a broad range of diseases with similar presentations. The study was approved by the Ethics Committee Review Board from the Center for Health Studies, Universidad del Valle de Guatemala, under protocol number 154–09-2016 and the Ethik Kommission der Nord und Zentralschweiz (EKNZ) No. 2016–00422.
Baseline survey: We surveyed every household identified that consented to participation. We assessed family size, crowding (>3 persons per bedroom), maternal language, open field defecation, fuel used for cooking, assets, household exposure to flooding, and self-reported observation of rodents in the house during the last year. We used asset (electricity, solar panels, radio, landline, cell phone, television, refrigerator, washer, microwave oven, and computer) to estimate wealth terciles using the first score of principal component analysis. We also inquired about the number of canines, swine, equines and bovines owned. We listed all household members currently living in the house, and for each member, we collected sex, age, education level and occupational exposure to animals or crops.
2.1. One-Health surveillance
Case definitions: We screened humans for signs or symptoms of acute febrile infection (AFI), acute respiratory infection (ARI) and acute diarrheic infection (ADI); and domestic animals for reproductive, gastrointestinal, ocular, muscle-skeletal or respiratory syndromes (Table 1). Syndromes were evaluated independently, allowing individuals to be eligible for more than one syndrome at a time.
Table 1.
Human and animal syndromic case definitions.
| Population | Syndrome | Case definition |
|---|---|---|
| Humans | Acute febrile infection (AFI) | Self-reported fever or measured temperature of ≥38 °C during the last seven days and the absence of surgery or a painful skin lesion. |
| Severe AFI | Acute febrile infection and at least one of the following: unusual bleeding, finger numbness, walking difficulty, muscle weakness, convulsions, lethargy or unconsciousness. | |
| Acute respiratory infection (ARI) | Self-reported cough and difficulty breathing within the preceding seven days, oxygen saturation < 90% or a pneumonia diagnostic given by a medical doctor or nurse. In children <5 years of age: Presence of any danger signs or fast breathing based on caretaker report or study nurse assessment.
|
|
| Severe ARI | Acute respiratory infection and at least one of the following: oxygen saturation < 90%, danger sign, shortness of breath, chest pain, stridor, respiratory groan, respiratory wheezing, nasal flaring, convulsions, lethargy or unconsciousness. | |
| Acute diarrheic infection (ADI) | Self-reported three or more loose or watery stools within 24 h during the previous seven days and with onset during the last 14 days. | |
| Sever ADI | Acute diarrheic infection and at least one of the following: bloody stools, thirstiness, sunken eyes, dry mucosa, skin with poor turgor, 3 or more vomits within a day, convulsions, lethargy or unconsciousness. | |
| Any syndrome | AFI, ARI or ADI. | |
| Animals | Reproductive | Mastitis, hematuria, abortions, dead or weak offspring, orchitis or metritis assessed by field technician/veterinarian or reported by owner. |
| Gastrointestinal | Jaundice, vomits, or diarrhea assessed by field technician/veterinarian or reported by owner. | |
| Ocular | Uveitis, eyelid protrusion, or ocular or nasal secretions assessed by field technician/veterinarian or reported by owner. | |
| Muscle-skeletal | Low back pain, myalgia, arthralgia, lameness, posterior paralysis, or spondylitis assessed by field technician/veterinarian or reported by owner. | |
| Respiratory | Ocular or nasal secretions and cough assessed by field technician/veterinarian or reported by owner. | |
| Any syndrome | Reproductive, gastrointestinal, ocular, muscle-skeletal or respiratory assessed by field technician/veterinarian or reported by owner. |
Health facility surveillance: We conducted health facility surveillance for humans at the only public health clinic in each community. In San Marcos, no clinic existed at the study start, but it was installed as the result of joint actions between community members, the study team, and the Ministry of Health. Clinics were open only on weekdays and in the morning hours. Research nurses screened each person seeking care for acute infections. No public clinics or similar infrastructure for animal health existed in these communities. Screening of animals was triggered by families requesting the study team to visit their households to evaluate their sick animals. Families contacted the study team by phone calls, visits to public health clinics or informal encounters in public areas. A field technician was responsible for visiting the house within 24 h of receiving the request. We conducted health facility surveillance from September 2017 to November 2018.
Monthly household visits: We randomly assigned households to monthly surveillance visits until approximately 50% of the population within each community had agreed to participate in this surveillance group. During the household visits, a research nurse and an animal field technician inquired about human health status during the last week, animal health status and deaths during the previous month and any change in the number of household members. Household surveillance ran from September 2017 to November 2018.
Each surveilled town had its own team comprised of a full-time assistant nurse for human surveillance and one full-time veterinary technician for animal surveillance. Since we surveilled three towns, there were three assistant nurses and three veterinary technicians hired. The work of field technicians was supervised by a physician and a veterinarian, especially concerning clinical evaluation and treatment. Upon identifying a sick human, field technicians offered them acetaminophen and oral rehydration therapy and referred them to a health facility. Owners of animals with clinical manifestations received antibiotics or Ivermectin to treat their animals. The study staff conducted health facility surveillance during morning hours when the public health clinics were opened and monthly household visits during the afternoons. A medical anthropologist was responsible for overviewing fieldwork processes to ensure community acceptance. Resources at the public clinics included a refrigerator with solar panel, basic clinical equipment, a sanitization unit, a small cabinet with basic medicines for alleviating symptoms and tablets to record data. The study staff used the same equipment for the household visits.
2.2. Statistical analysis
Incidence rates and relative sensitivity: To assess the relative sensitivity we compared the incidence detected with each surveillance method. We used null Poisson regression to estimate the incidence rate per 1000 person-years of each acute human syndrome and each acute human severe syndrome; and the incidence rate per 100 household-years of any syndrome and all-cause deaths in animals by species. The population denominator for health facility surveillance assumed a fixed cohort defined at baseline. In contrast, for household surveillance, we adjusted the follow-up time by population dynamics informed by the ongoing censuses. Overdispersion was not detected in the data. The relative sensitivity of health facility surveillance alone compared to health facility plus household surveillance was estimated as the incidence rate ratio obtained from including in the model an indicator variable for the randomized assignment to the household surveillance. The percentage of under ascertainment was defined as 100% – relative sensitivity.
Risk factors and relative representativeness: Represenativity is obtaining comparable sensitivities at all the values of a population characteristic. We defined lack of relative representativeness as a difference between the relative sensitivities by sociodemographic factors. We used multivariable Poisson regression with an indicator variable for the surveillance method, sociodemographic variables, and interaction terms between surveillance method and sociodemographic variable. The interaction terms assessed effect modification as a measure of representativeness. Sociodemographic variables were the community of residence, sex, age group (<5 years, 5 to 19 years, 20 to 49 years and ≥ 50 years), Mayan vs. Spanish language, asset tercile and householder education level (none, less than primary, primary or more). We computed incidence rate ratios with the R package emmeans [17].
Incidence and relative representativeness over time: We used generalized additive models with separate smoothing splines by surveillance method to assess nonlinear time trends in human and animal incidences with the R package mgcv [18]. We used an interaction p-value to evaluate differences in the relative representativeness over time.
We used R: A language and environment for statistical computing, version 4.0.2 released on 2020-06-22 (R Foundation for Statistical Computing, Vienna, Austria) and RStudio: An integrated development environment for R, version 1.3.1073 released on 2020 (RStudio, PBC, Boston, MA, USA) for all analyses.
3. Results
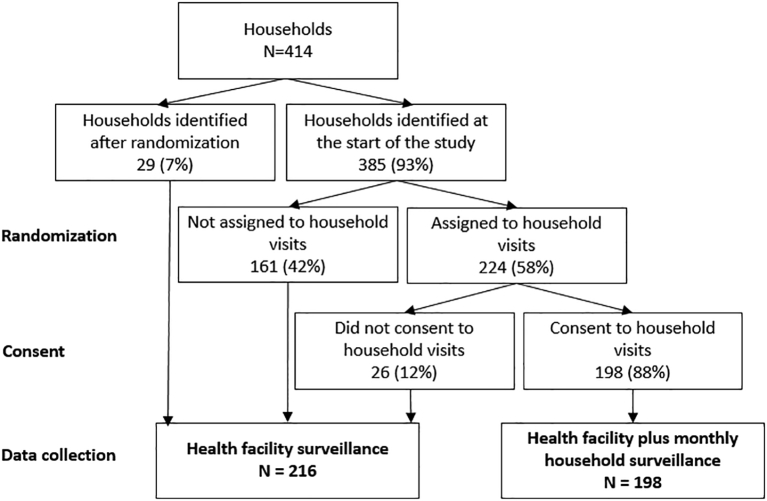
We identified 418 families, of whom 414 (99%) consented to participate in the baseline survey. Of these, 385 (93%) families were randomized to one of the surveillance groups and 29 (7%) were identified after randomization and assigned to the group with only health facility surveillance. Of the 224 (58%) assigned to household surveillance, 198 (88%) accepted monthly household visits (Fig. 1). Household and individual baseline characteristics were not statistically different between surveillance methods (Table 2), except for education level. In the families with household surveillance, 125 (18%) of individuals >7 years had no education in contrast to 167 (23%) in the health facility surveillance method. However, the educational level of householders did not differ among those who accepted or refused participation.
Fig. 1.
Flow chart of the household randomization and assignment to surveillance groups.
Table 2.
Baseline characteristics of households and individuals participating in health facility surveillance or health facility plus monthly household surveillance.
| Baseline characteristics | Health facility Surveillance |
Health facility plus monthly Household surveillance |
p-value |
|---|---|---|---|
| n (%) | n (%) | ||
| Number of households | 216 | 198 | |
| Community | |||
| Sabaneta | 98 (45) | 99 (50) | 0.641 |
| La Romana | 64 (30) | 54 (27) | |
| San Marcos | 54 (25) | 45 (23) | |
| Mayan language | 140 (65) | 136 (69) | 0.465 |
| Family size, median (IQR1) | 4 (3–6) | 4 (3–6) | 0.238 |
| Crowding (>3 persons per bedroom) | 90 (42) | 92 (46) | 0.400 |
| Cooks primarily with wood | 209 (98) | 191 (96) | 0.667 |
| Open field defecation | 95 (44) | 73 (37) | 0.158 |
| Asset tercile | 0.561 | ||
| Low | 77 (39) | 93 (43) | |
| Middle | 64 (32) | 60 (28) | |
| High | 57 (29) | 63 (29) | |
| Exposure to rodents in the last year | 173 (80) | 168 (85) | 0.297 |
| Exposure to flooding in the house | 111 (52) | 108 (55) | 0.621 |
| Ownership of at least one canines | 100 (47) | 108 (55) | 0.125 |
| Number of canines, median (IQR1) | 2 (1–3) | 2 (1–3) | 0.470 |
| Ownership of at least one swine | 74 (34) | 67 (34) | 0.984 |
| Number of swine, median (IQR1) | 2 (1–3) | 2 (1–3) | 0.514 |
| Ownership of at least one equines | 43 (20) | 34 (17) | 0.541 |
| Number of equines, median (IQR1) | 1 (1–2) | 2 (1–2) | 0.623 |
| Ownership of at least one bovines | 14 (7) | 12 (6) | 1.000 |
| Number of bovines, median (IQR1) | 9 (3–17) | 6 (2−22) | 0.624 |
| Number of individuals | 965 | 929 | |
| Male | 466 (48) | 470 (50) | 0.352 |
| Age distribution (years) | |||
| < 5 | 143 (15) | 146 (16) | 0.142 |
| 5 to 19 | 353 (37) | 382 (41) | |
| 20 to 49 | 346 (36) | 295 (32) | |
| ≥ 50 | 118 (12) | 105 (11) | |
| Education2 | |||
| None | 167 (23) | 125 (18) | 0.0434 |
| Less than primary | 353 (48) | 332 (48) | |
| Primary or more than primary | 220 (30) | 240 (34) | |
| Occupational exposure to livestock3 | 115 (12) | 97 (10) | 0.345 |
| Occupational exposure to crops3 | 268 (28) | 253 (27) | 0.779 |
1Interquartile range. 2Includes only individuals >7 years. 3Includes only individuals >15 years. 4Significant difference based on an alpha level of 0.05.
Contribution of monthly household visits: The median proportion of successful monthly screening visits in the group with household and health facility surveillance was 81% (range: 49% in December and 94% in October, November and January) for humans and 79% (range: 55% in December to 95% in November) for animals. The proportion of events detected through household visits in this group was 51% (n = 84) for AFI, 32% (n = 33) for ARI, and 33% (n = 15) for ADI in humans, and 86% (n = 18) of the syndromic events in canines, 85% (n = 23) in swine, 83% (n = 10) in equines, and 80% (n = 4) in bovines, all swine, equine and bovine deaths and 95% (n = 53) of canine deaths.
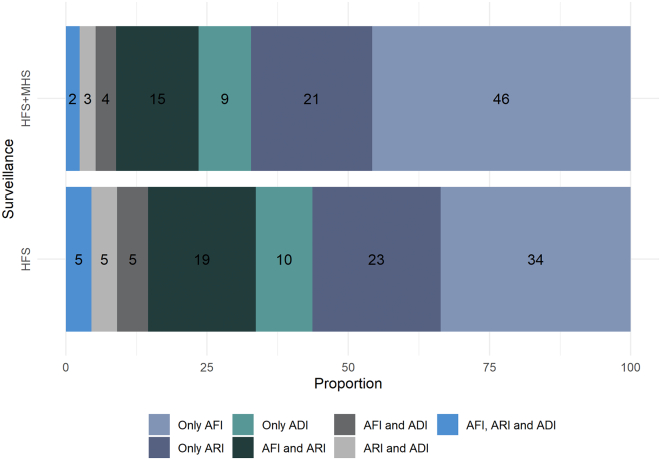
Incidence and relative sensitivity: AFI was the most common syndrome with an incidence per 1000 human-years of 152 (IC95%: 130, 178) with household surveillance, followed by ARI with 94 (95% CI: 78, 115) and ADI with 42 (95% CI: 31, 56). Of all the episodes of acute infections in the household surveillance group, 15% satisfied the case definitions for both AFI and ARI, 4% for AFI and ADI, 3% for ARI and ADI and 2% for all three (Fig. 2). The relative sensitivity of health facility surveillance compared to household surveillance was 0.42 (95% CI: 0.34–0.53) for the detection of all human syndromes and 0.56 (95% CI: 0.39–0.79) for severe human syndromes (Table 3). The relative sensitivity of cases was lower for AFI (0.40, 95% CI: 0.30, 0.53) followed by ARI (0.52, 95% CI: 0.37, 0.72) and ADI (0.57, 95% CI: 0.35, 0.91).
Fig. 2.
Distribution of the proportion of acute human syndromic events detected through health facility surveillance (HFS) compared to health facility plus monthly household surveillance (HFS + MHS).
Table 3.
Human syndromic incidences per 1000 person-years and animal syndromic and mortality incidences per 100 household-years and relative sensitivity of health facility surveillance compared to health facility plus monthly household surveillance.
| Event | Health facility surveillance1 |
Health facility plus monthly household surveillance 2 |
Relative sensitivity |
|||
|---|---|---|---|---|---|---|
| Events / Subject-years |
Incidence (95% CI) | Events / Subject-years |
Incidence (95% CI) |
Incidence rate Ratio (95% CI) |
||
| Humans | ||||||
| AFI | 69/1136 | 61 (48, 77) | 164/1078 | 152 (130, 178) | 0.40 (0.30, 0.53) | |
| Severe AFI | 16/1136 | 14 (9, 23) | 31/1078 | 29 (20, 41) | 0.49 (0.26, 0.88) | |
| ARI | 56/1136 | 49 (38, 64) | 102/1080 | 94 (78, 115) | 0.52 (0.37, 0.72) | |
| Severe ARI | 29/1136 | 26 (18, 37) | 40/1080 | 37 (27, 51) | 0.69 (0.42, 1.11) | |
| ADI | 27/1137 | 24 (16, 35) | 45/1081 | 42 (31, 56) | 0.57 (0.35, 0.91) | |
| Severe ADI | 9/1137 | 8 (4, 15) | 14/1081 | 13 (8, 22) | 0.61 (0.25, 1.39) | |
| Any | 110/1135 | 97 (80, 117) | 246/1077 | 228 (201, 260) | 0.42 (0.34, 0.53) | |
| Any severe | 49/1135 | 43 (32, 57) | 83/1077 | 77 (62, 96) | 0.56 (0.39, 0.79) | |
| Canines | ||||||
| Any syndrome | 13/118 | 11 (6, 19) | 21/140 | 15 (10,23) | 0.74 (0.36, 1.46) | |
| All-cause deaths | 1/118 | 1 (0, 6) | 54/140 | 38 (29, 50) | 0.02 (0.00, 0.10) | |
| Swine | ||||||
| Any syndrome | 14/87 | 16 (9, 28) | 27/95 | 29 (19, 42) | 0.57 (0.29, 1.06) | |
| All-cause deaths | 14/87 | 16 (9, 28) | 54/95 | 57 (43, 75) | 0.28 (0.15, 0.50) | |
| Equines | ||||||
| Any syndrome | 9/51 | 18 (9, 34) | 12/40 | 31 (17, 54) | 0.59 (0.24, 1.39) | |
| All-cause deaths | 2/51 | 4 (1, 16) | 7/40 | 18 (8, 37) | 0.22 (0.03, 0.92) | |
| Bovines | ||||||
| Any syndrome | 1/17 | 6 (1, 44) | 5/14 | 36 (15, 88) | 0.17 (0.01, 1.04) | |
| All-cause deaths | 2/17 | 12 (3, 49) | 5/14 | 36 (15, 88) | 0.34 (0.05, 1.55) | |
1 The denominator for the HFS uses the number of humans and the number of households owning domestic animals at the baseline survey. 2The denominator for HFS + MHS is adjusted for human migration and household domestic animal ownership changes identified at monthly household visits.
Bovines were the animal species with the highest incidence per 100 household-years of a syndromic event in the household surveillance group (36, 95% CI: 15, 88), followed by equines (31, 95% CI: 17, 54), swine (29, 95% CI: 19, 42) and canines (15, 95% CI: 10, 23). However, the highest incidence of all-cause mortality in the household surveillance group occurred for swine (57, 95% CI: 43,75), followed by canines (38, 95% CI: 29, 50), bovines (36, 95% CI: 15, 88) and equines (18, 95% CI: 8, 37). The relative sensitivity of detecting all-cause animal deaths in health facilities compared to households was 0.02 (95% CI: 0.00, 0.10) for canines, 0.28 (95% CI: 0.15, 0.50) for swine and 0.22 (95% CI:0.03, 0.92) for equines (Table 3). Similar associations were found for other events in animals but were not statistically significant.
Risk factors for human syndromes and relative representativeness: Female sex and age < 5 years were significant risk factors for human syndromes in both surveillance groups. Speaking a Mayan language and being in lower asset tercile was associated with higher incidence only in the group with health facility surveillance. We found evidence of differences in the relative sensitivity by sociodemographic variables (i.e. relative representativeness), namely, language spoken and socioeconomic asset terciles. The interaction p-value for language spoken was 0.0003, the relative sensitivity for Mayan speakers was 0.57 (95% CI: 0.43, 0.75) and for Spanish speakers 0.22 (95% CI: 0.15, 0.34). The interaction p-value for socioeconomic asset tercile were 0.0557 and 0.0008, the relative sensitivity for the lowest tertile was 0.61 (95% CI: 0.43, 0.87) and for the highest tercile 0.23 (95% CI: 0.14, 0.36) (Table 4). We obtained similar results in analyses stratified by the specific human syndromes (Supplementary tables).
Table 4.
Sociodemographic factors associated with any acute human syndrome, relative sensitivity, and relative representativeness of health facility surveillance compared to health facility plus monthly household surveillance.
| Sociodemographic characteristic | Health facility surveillance |
Health facility plus monthly household surveillance |
Relative sensitivity |
Relative representativeness |
||
|---|---|---|---|---|---|---|
| Adjusted incidence1 (95% CI) |
Incidence rate ratio (95% CI) | Adjusted incidence1 (95% CI) |
Incidence rate ratio (95% CI) | Incidence rate ratio (95% CI) | Interaction p-value | |
| Community | ||||||
| La Romana | 58 (34, 96) | Reference | 141 (99, 201) | Reference | 0.41 (0.23, 0.74) | Reference |
| Sabaneta | 112 (82, 153) | 1.95 (0.93, 4.09) | 401 (336, 478) | 2.85 (1.74, 4.65) | 0.28 (0.20, 0.39) | 0.2750 |
| San Marcos | 162 (116, 227) | 2.82 (1.42, 5.61) | 212 (153, 295) | 1.51 (0.90, 2.53) | 0.77 (0.51, 1.14) | 0.0852 |
| Sex | ||||||
| Male | 66 (46, 94) | Reference | 199 (159, 250) | Reference | 0.33 (0.22, 0.49) | Reference |
| Female | 137 (106, 175) | 2.07 (1.37, 3.14) | 297 (243, 362) | 1.49 (1.15, 1.93) | 0.46 (0.35, 0.61) | 0.1859 |
| Age group | ||||||
| < 5 years | 251 (182, 346) | Reference | 496 (389, 634) | Reference | 0.51 (0.35, 0.74) | Reference |
| 5 to 19 years | 45 (28, 72) | 0.20 (0.10, 0.40) | 147 (112, 193) | 0.30 (0.20, 0.50) | 0.30 (0.18, 0.51) | 0.1215 |
| 20 to 49 years | 81 (57, 115) | 0.30 (0.20, 0.60) | 185 (141, 243) | 0.40 (0.20, 0.60) | 0.44 (0.29, 0.66) | 0.5975 |
| ≥ 50 years | 85 (48, 149) | 0.30 (0.10, 0.80) | 261 (181, 378) | 0.50 (0.30, 0.90) | 0.32 (0.17, 0.62) | 0.2433 |
| Language spoke | ||||||
| Spanish | 66 (44, 99) | Reference | 297 (228, 388) | Reference | 0.22 (0.15, 0.34) | Reference |
| Mayan | 119 (93, 151) | 1.80 (1.13, 2.88) | 209 (172, 254) | 0.70 (0.51, 0.97) | 0.57 (0.43, 0.75) | 0.0003 |
| Asset tercile | ||||||
| High | 56 (36, 89) | Reference | 250 (189, 332) | Reference | 0.23 (0.14, 0.36) | Reference |
| Middle | 98 (67, 144) | 1.74 (0.88, 3.43) | 234 (177, 311) | 0.94 (0.61, 1.43) | 0.42 (0.27, 0.64) | 0.0557 |
| Low | 142 (105, 190) | 2.51 (1.29, 4.91) | 231 (181, 296) | 0.92 (0.58, 1.47) | 0.61 (0.43, 0.87) | 0.0008 |
| Householder education | ||||||
| None | 102 (72, 145) | Reference | 245 (186, 323) | Reference | 0.42 (0.27, 0.64) | Reference |
| Less than primary | 91 (64, 130) | 0.89 (0.50, 1.58) | 210 (165, 268) | 0.86 (0.57, 1.29) | 0.43 (0.29, 0.64) | 0.8921 |
| Primary or more | 104 (73, 148) | 1.02 (0.57, 1.81) | 272 (213, 347) | 1.11 (0.74, 1.67) | 0.38 (0.26, 0.56) | 0.7673 |
Adjusted incidence per 1000 person-years.
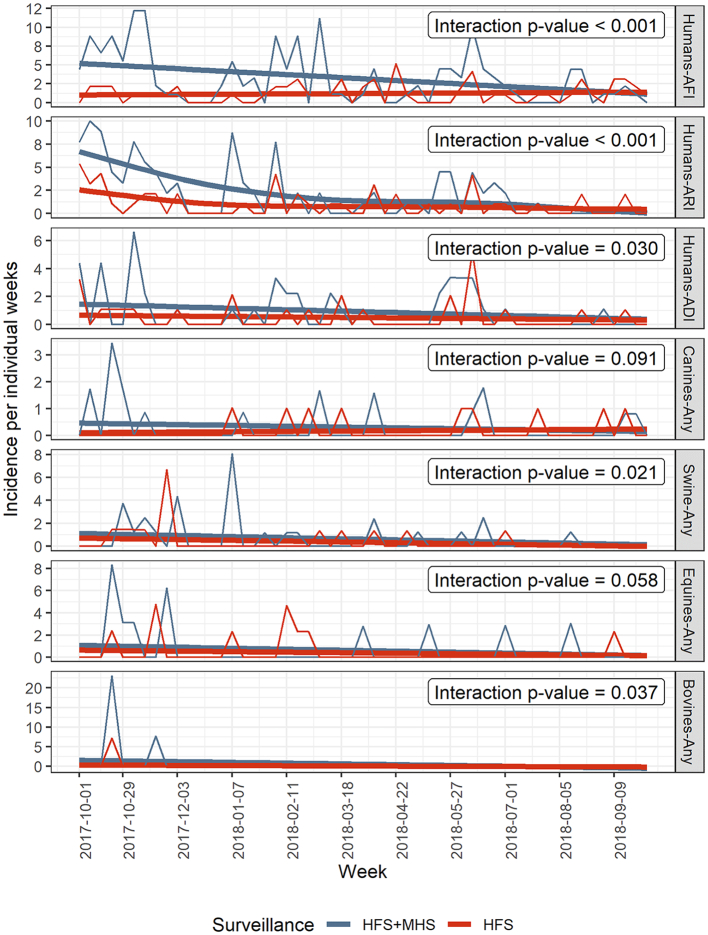
Incidence and relative representativeness over time: Human and animal acute syndromes' incidences decreased over time with both surveillance methods (Fig. 3). The incidences detected by household surveillance trended downward over time, approaching the incidences seen by health facility surveillance. The interaction p-values or relative representativeness over time between surveillance groups were significant at an alpha level of 0.05 for human, swine and bovine syndromes but nearly significant for canines and equines.
Fig. 3.
Temporal trends in human incidences per 1000 person-weeks and animal incidences per 100 household weeks of acute syndromes and relative acceptability (interaction p-value) of health facility surveillance (HFS) compared to health facility plus monthly household visits surveillance (HFS + MHS).
4. Discussion
We contrasted the sensitivity and representativeness of health facility surveillance against health facility surveillance plus monthly household visits. Our goal was to detect acute infections in humans and any syndrome and deaths in animals as screening for potential zoonotic diseases in rural Guatemala using a One-Health approach. Our study contributes uniquely by comparing quantitative estimates of relative differences in surveillance attributes between randomly assigned groups.
The sensitivity of health facility surveillance was lower than for surveillance with household visits. In humans, severe disease events were less under ascertained than when including all disease events, relative sensitivity 0.56 (IC95%: 0.39, 0.79) and 0.42 (IC95%: 0.34, 0.53), respectively. In animals, we found greater under ascertainment for all-cause deaths (relative sensitivity ranging from 0.02 to 0.28) than for syndromic events. Similar results were obtained in a phone-based surveillance system in Kenya, where animal deaths were underreported more frequently than animal illness [19]. Health facility surveillance was less representative for Spanish speakers (0.22 (IC95%: 0.15, 0.34)) than Mayan speakers (0.57 (IC95%: 0.43, 0.75)) and persons in the higher tercile (0.23 (IC95%: 0.14, 0.36)) than the middle (0.42 (IC95%: 0.27, 0.64)) and the lower (0.61 (IC95%: 0.43, 0.87)). Differences in the magnitude of under ascertainment suggest that the sensitivity is influenced by disease severity, species and sociodemographic characteristics. Gaps in sensitivity and representativeness might lead to misleading conclusions about the burden of disease and identification of high-risk groups [20]. Health facility surveillance systems can improve incidence estimates by adjusting them with estimates of the magnitude of under ascertainment obtained from different sociodemographic groups [21,22]. Cross-sectional community surveys of healthcare-seeking behavior provide such estimates, but this design is vulnerable to recall bias and requires a large sample size for rare events. The relative sensitivities estimated in our study can also be used to adjust incidences collected from health facility surveillance. Our design provides a direct estimate of the under ascertainment and is less vulnerable to recall bias as data were collected longitudinally in a randomized field trial.
The relative sensitivity added by household surveillance as compared to health facility surveillance alone decreased over time in all species. The greater sensitivity of household visits occurred mainly in the first months of surveillance, especially for humans. By the last month, the incidence of household surveillance was similar to health facility surveillance. Long-term collaboration with the same population is vulnerable to participation fatigue, which could happen when participants lose motivation to report illnesses. Variable representativeness over time can bias assessment of time trends and associations with time-varying risk factors. as well as reduce sensitivity, sustainability and overall efficiency of surveillance. Thus, surveillance should regularly implement strategies to maintain reporting adherence. Highlighting surveillance benefits, emphasizing the public health importance of the surveilled event, publicizing public health actions derived from surveillance data, promoting community engagement, and keeping easy to complete reports could improve participation [23].
Although health facility surveillance is inherently less sensitive and representative, it has the advantage of requiring fewer resources and being less time-intensive for public health officers and participants. It can be an appropriate design to monitor trends over time, which does not require high sensitivity to detect changes in disease patterns, as long as sensitivity is fairly constant [24]. Health facility surveillance can also be appropriate for studying disease risk factors, as long as any poorly representated characteristic or variables is not strongly associated with the health outcome under surveillance as well as the risk factor of interest [25,26].
Based on both surveillance methodologies, the most common syndrome in humans was AFI (152 per 1000 person-years), followed by ARI (94 per 1000 person-years) and ADI (42 per 1000 person-years) and in children under five years, an incidence per 1000 person-years of 259 for AFI and 151 for ADI. A syndromic surveillance system based on daily reporting through mobile phone, conducted in a cohort of children living in rural communities of the department of Quetzaltenango in Guatemala from April 2015 to June 2016, reported an incidence of AFI of 187 per 1000 person-years, and ADI of 210 per 1000 person-years [27].
Our findings from health facility surveillance are not directly comparable with passive surveillance routinely conducted at health services. In our health facility surveillance, we had study personnel actively screening for disease events, and the presence of our staff in these facilities might have modified or facilitated health-seeking behaviors. In addition, health facility surveillance could have been affected by knowledge of other community members participating in household surveillance. This cross-contamination effect could bias estimation of the effect of surveillance method. A cluster-randomized design could reduce this potential bias in future comparisons of surveillance systems. Participation in household visits could improve if an entire community participates rather than individual households.
This study has some limitations. First, we implemented One-Health surveillance for only 14 months. A longer timeframe is needed to accumulate sufficient data to characterize disease trends better. Second, we did not assess the participants' perceptions on surveillance utility, motivators and barriers to voluntary reporting. Such data could inform strategies to promote surveillance acceptability in the study area. Third, reporting events in health facilities required a public health clinic visit to contact the study field technician. We explored using mobile phones to facilitate reporting events but disregarded this option because of weak cell phone signal reception in the study area. Finally, because households were visited monthly, surveillance in this group could have missed events occurring during weeks without household visits. More frequent visits could have resulted in even lower relative sensitivity of health facility surveillance.
5. Conclusions
Health facility surveillance for zoonotic diseases in rural Guatemala was less sensitive and representative than the combination of health facility plus monthly household surveillance. Health facility surveillance only captured 42% of the acute infections in humans and between 2% to 34% of deaths in different species of animals. It underrepresented Spanish speakers and persons with more economic assets than Mayan speakers and poorer persons. However, the added advantage of household surveillance appeared to decrease over time, perhaps because of participation fatigue. The choice between surveillance methodologies should be evaluated against the surveillance goals and available resources. Household surveillance should be preferred when the goal is to estimate incidence and identify high groups accurately. However, facility surveillance could be used for monitoring trends over time given constant sensitivity. Incidence estimates from health facility surveillance in similar settings could be improved by adjusting for these estimates of relative sensitivity.
Author contributions
Conceptualization: J.P.M., M.B.G., D.A., C.C.R and J.Z. Methodology: J.P.M., M.B.G., D.A., S.M., O.P., C.C.R. and J.Z. Software: L.M.G. Validation: M.B.G., M.R.L. and D.A. Formal analysis: L.M.G. and J.P.M. Investigation: M.B.G., M.R.L., D.A., S.M. and O.P. Data curation: M.R.L., D.A. and L.M.G. Writing—original draft preparation: L.M.G. Writing—review and editing: D.A., S.M., O.P., C.C.R., J.Z. and J.P.M. Visualization: L.M.G. and J.P.M. Supervision: M.B.G, J.P.M., D.A. and C.C.R. Funding acquisition: M.B.G, J.P.M., D.A., C.C.R. and J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Swiss National Science Foundation through a research development project (r4d grant: IZ07Z0_160919 /1), Vontobel Stiftung (27.04.17) and R. Geigy Stiftung (16091–1).
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
We are grateful to all families in Poptún, Petén, Guatemala, who participated in this study. We are also grateful to field workers who closely followed up with participants and their animals.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.onehlt.2021.100336.
Appendix A. Supplementary data
Supplementary material
References
- 1.Taylor L.H., Latham S.M., Woolhouse M.E. Risk factors for human disease emergence. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. Jul 29 2001;356:983–989. doi: 10.1098/rstb.2001.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woolhouse M.E., Haydon D.T., Antia R. Emerging pathogens: the epidemiology and evolution of species jumps. Trends Ecol. Evol. May 2005;20:238–244. doi: 10.1016/j.tree.2005.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones K.E., Patel N.G., Levy M.A., Storeygard A., Balk D., Gittleman J.L., Daszak P. Global trends in emerging infectious diseases. Nature. Feb 21 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatt S., Gething P.W., Brady O.J., Messina J.P., Farlow A.W., Moyes C.L., Drake J.M., Brownstein J.S., Hoen A.G., Sankoh O., Myers M.F., George D.B., Jaenisch T., Wint G.R., Simmons C.P., Scott T.W., Farrar J.J., Hay S.I. The global distribution and burden of dengue. Nature. Apr 25 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ye Z.W., Yuan S., Yuen K.S., Fung S.Y., Chan C.P., Jin D.Y. Zoonotic origins of human coronaviruses. Int. J. Biol. Sci. 2020;16:1686–1697. doi: 10.7150/ijbs.45472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zinsstag J., Schelling E., Waltner-Toews D., Tanner M. From “one medicine” to “one health” and systemic approaches to health and well-being. Prevent. Veterin. Med. Sep 1 2011;101:148–156. doi: 10.1016/j.prevetmed.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeggo M., Mackenzie J.S. Defining the future of One Health. Microbiol. Spectr. Feb 2014;2 doi: 10.1128/microbiolspec.OH-0007-2012. OH-0007-2012. [DOI] [PubMed] [Google Scholar]
- 8.Stark K.D., Arroyo Kuribrena M., Dauphin G., Vokaty S., Ward M.P., Wieland B., Lindberg A. One Health surveillance - more than a buzz word? Prevent. Veterin. Med. Jun 1 2015;120:124–130. doi: 10.1016/j.prevetmed.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 9.Zinsstag J., Crump L., Schelling E., Hattendorf J., Maidane Y.O., Ali K.O., Muhummed A., Umer A.A., Aliyi F., Nooh F., Abdikadir M.I., Ali S.M., Hartinger S., Mausezahl D., de White M.B.G., Cordon-Rosales C., Castillo D.A., McCracken J., Abakar F., Cercamondi C., Emmenegger S., Maier E., Karanja S., Bolon I., de Castaneda R.R., Bonfoh B., Tschopp R., Probst-Hensch N., Cisse G. Climate change and One Health. FEMS Microbiol. Lett. Jun 1 2018;365 doi: 10.1093/femsle/fny085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costa F., Hagan J.E., Calcagno J., Kane M., Torgerson P., Martinez-Silveira M.S., Stein C., Abela-Ridder B., Ko A.I. Global Morbidity and Mortality of Leptospirosis: A Systematic Review. PLoS Negl. Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0003898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dean A.S., Crump L., Greter H., Schelling E., Zinsstag J. Global burden of human brucellosis: a systematic review of disease frequency. PLoS Negl. Trop. Dis. 2012;6:e1865. doi: 10.1371/journal.pntd.0001865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarti E., L’Azou M., Mercado M., Kuri P., Siqueira J.B., Jr., Solis E., Noriega F., Ochiai R.L. A comparative study on active and passive epidemiological surveillance for dengue in five countries of Latin America. Int. J. Infect. Dis. Mar 2016;44:44–49. doi: 10.1016/j.ijid.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 13.Verani J.R., McCracken J., Arvelo W., Estevez A., Lopez M.R., Reyes L., Moir J.C., Bernart C., Moscoso F., Gray J., Olsen S.J., Lindblade K.A. Surveillance for hospitalized acute respiratory infection in Guatemala. PLoS One. 2013;8 doi: 10.1371/journal.pone.0083600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arvelo W., Hall A.J., Henao O., Lopez B., Bernart C., Moir J.C., Reyes L., Montgomery S.P., Morgan O., Estevez A., Parsons M.B., Lopez M.R., Gomez G., Vinje J., Gregoricus N., Parashar U., Mintz E.D., McCracken J., Bryan J.P., Lindblade K.A. Incidence and etiology of infectious diarrhea from a facility-based surveillance system in Guatemala, 2008–2012. BMC Public Health. Oct 22 2019;19:1340. doi: 10.1186/s12889-019-7720-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nsubuga P., White M.E., Thacker S.B., Anderson M.A., Blount S.B., Broome C.V., Chiller T.M., Espitia V., Imtiaz R., Sosin D., Stroup D.F., Tauxe R.V., Vijayaraghavan M., Trostle M. In: Disease Control Priorities in Developing Countries. Jamison D.T., Breman J.G., Measham A.R., Alleyne G., Claeson M., Evans D.B., Jha P., Mills A., Musgrove P., editors. 2006. Public health surveillance: a tool for targeting and monitoring interventions. ed Washington (DC) [Google Scholar]
- 16.Hattendorf J., Bardosh K.L., Zinsstag J. One Health and its practical implications for surveillance of endemic zoonotic diseases in resource limited settings. Acta Trop. Jan 2017;165:268–273. doi: 10.1016/j.actatropica.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Lenth R. 2020. emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.5.0., ed. [Google Scholar]
- 18.Wood S.N. 2nd edition. hapman and Hall/CRC; 2017. Generalized Additive Models: An Introduction with R. [Google Scholar]
- 19.Thumbi S.M., Njenga M.K., Otiang E., Otieno L., Munyua P., Eichler S., Widdowson M.A., McElwain T.F., Palmer G.H. Mobile phone-based surveillance for animal disease in rural communities: implications for detection of zoonoses spillover. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. Sep 30 2019;374:20190020. doi: 10.1098/rstb.2019.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buckeridge D.L. Outbreak detection through automated surveillance: a review of the determinants of detection. J. Biomed. Inform. Aug 2007;40:370–379. doi: 10.1016/j.jbi.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Gibbons C.L., Mangen M.J., Plass D., Havelaar A.H., Brooke R.J., Kramarz P., Peterson K.L., Stuurman A.L., Cassini A., Fevre E.M., Kretzschmar M.E. Measuring underreporting and under-ascertainment in infectious disease datasets: a comparison of methods. BMC Public Health. Feb 11 2014;14:147. doi: 10.1186/1471-2458-14-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deutscher M., Beneden C.V., Burton D., Shultz A., Morgan O.W., Chamany S., Jordan H.T., Zhang X., Flannery B., Feikin D.R., Olack B., Lindblade K.A., Breiman R.F., Olsen S.J. Putting surveillance data into context: the role of health care utilization surveys in understanding population burden of pneumonia in developing countries. J. Epidemiol. Global Health. Jun 2012;2:73–81. doi: 10.1016/j.jegh.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Struchen R., Hadorn D., Wohlfender F., Balmer S., Suptitz S., Zinsstag J., Vial F. Experiences with a voluntary surveillance system for early detection of equine diseases in Switzerland. Epidemiol. Infect. Jul 2016;144:1830–1836. doi: 10.1017/S0950268816000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirch W., editor. Encyclopedia of Public Health. Springer Netherlands; Dordrecht: 2008. Sensitivity of a surveillance systemsensitivity of a surveillance system; p. 1291. [Google Scholar]
- 25.Pini A., Merk H., Carnahan A., Galanis I., VAN Straten E., Danis K., Edelstein M., Wallensten A. High added value of a population-based participatory surveillance system for community acute gastrointestinal, respiratory and influenza-like illnesses in Sweden, 2013–2014 using the web. Epidemiol. Infect. Apr 2017;145:1193–1202. doi: 10.1017/S0950268816003290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Debin M., Turbelin C., Blanchon T., Bonmarin I., Falchi A., Hanslik T., Levy-Bruhl D., Poletto C., Colizza V. Evaluating the feasibility and participants' representativeness of an online nationwide surveillance system for influenza in France. PLoS One. 2013;8:e73675. doi: 10.1371/journal.pone.0073675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olson D., Lamb M., Lopez M.R., Colborn K., Paniagua-Avila A., Zacarias A., Zambrano-Perilla R., Rodriguez-Castro S.R., Cordon-Rosales C., Asturias E.J. Performance of a mobile phone app-based participatory syndromic surveillance system for acute febrile illness and acute gastroenteritis in Rural Guatemala. J. Med. Internet Res. Nov 9 2017;19:e368. doi: 10.2196/jmir.8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material