Abstract
Objective:
To determine the effectiveness of ultraviolet (UV) environmental disinfection system on rates of hospital-acquired vancomycin-resistant enterococcus (VRE) and Clostridium difficile.
Design:
Using active surveillance and an interrupted time-series design, hospital-acquired acquisition of VRE and C. difficile on a bone marrow transplant (BMT) unit were examined before and after implementation of terminal disinfection with UV on all rooms regardless of isolation status of patients. The main outcomes were hospital-based acquisition measured through (1) active surveillance: admission, weekly, and discharge screening for VRE and toxigenic C. difficile (TCD) and (2) clinical surveillance: incidence of VRE and CDI on the unit.
Setting:
Bone marrow transplant unit at a tertiary-care cancer center.
Participants:
Stem cell transplant (SCT) recipients.
Intervention:
Terminal disinfection of all rooms with UV regardless of isolation status of patients.
Results:
During the 20-month study period, 579 patients had 704 admissions to the BMT unit, and 2,160 surveillance tests were performed. No change in level or trend in the incidence of VRE (trend incidence rate ratio [IRR], 0.96; 95% confidence interval [CI], 0.81–1.14; level IRR, 1.34; 95% CI, 0.37–1.18) or C. difficile (trend IRR, 1.08; 95% CI, 0.89–1.31; level IRR, 0.51; 95% CI, 0.13–2.11) was observed after the intervention.
Conclusions:
Utilization of UV disinfection to supplement routine terminal cleaning of rooms was not effective in reducing hospital-acquired VRE and C. difficile among SCT recipients.
Reducing transmission of common environmental pathogens including multidrug-resistant organisms (MDROs) and Clostridium difficile (CD) are important goals in the healthcare setting. Indirect transmission of VRE and C. difficile through a contaminated hospital environment has been demonstrated in several studies.1,2 VRE and C. difficile survive on inanimate surfaces in the patients’ immediate environment including medical equipment,3 and contamination can persist for extended periods of time despite adherence to recommended cleaning practices.
Prior room occupancy by individuals colonized with VRE or C. difficile is associated with a higher risk of acquisition of the same pathogen in subsequent room occupants.2,4 In addition, contamination of healthcare worker hands and gloves can occur during delivery of care, leading to inadvertent onward transmission of pathogens. Reducing the environmental density of pathogens can mitigate the risk of transmission by these routes. To achieve this vital goal for infection prevention in the healthcare environment, there is growing interest in examining the role of augmented cleaning practices including ultraviolet C–emitting devices (UV).
Several studies have examined the effectiveness of UV and have demonstrated a substantial reduction in bioburden of critical pathogens5–14; only a subset of these, however, have measured declines in hospital-acquired infections as the primary outcome.7–14 Furthermore, none of the existing studies have examined the efficacy of UV disinfection among SCT recipients, a highly vulnerable population with exceedingly high rates of VRE and C. difficile, distinctive transmission dynamics,15 and frequent occurrence of asymptomatic carriage.
The primary objective of the study was to determine the effectiveness of environmental UV disinfection on rates of hospital-acquired VRE and C. difficile among SCT recipients on a bone marrow transplant (BMT) unit. The study is uniquely designed to measure hospital-based acquisition through admission and weekly screening for VRE and toxigenic C. difficile (TCD) for all unit occupants.
Methods
Study population
All admissions to the BMT unit between April 2015 and November 2016 were eligible. Data on patient movement, demographic characteristics (age and sex), and laboratory results were obtained from the infection control surveillance system (CKM, Ontario, Canada), Electronic Medical Records (EMR), and the Memorial Sloan Kettering Cancer Center (MSKCC) institutional database (IDB). The MSKCC Institutional Review Board approved this study.
Study design
Study setting.
The study was conducted in a 25-bed BMT unit with single-patient rooms at a large 474-bed tertiary-care cancer center in New York City. Approximately 230 allogeneic and 250 autologous adult transplants are performed annually at MSKCC. The BMT unit has ~440 admissions and ~8,800 patient days annually. The average patient length of stay (LOS) on the unit is 20 days. For patients admitted for transplant, the average LOS is 19 days for autologous SCT recipients, 44 days for cord SCT recipients, and 30 days for T-cell–depleted and conventional SCT recipients. The baseline National Healthcare Safety Network (NHSN)–defined hospital onset CDI rate for the unit is >1.5 per 1,000 patient days. As standard of care, a rectal swab for VRE screening is collected for each patient (1) at the time of admission to the BMT unit, (2) weekly, and (3) upon discharge or transfer to another unit. For this study, rectal swabs were stored and subsequently used for detection of TCD. This testing was performed later, and results were not reported to clinicians. VRE test results were obtained for all surveillance swabs, but for 7.9% of surveillance tests, TCD results could not be obtained due to storage loss.
Study design.
The baseline (preintervention) period extended from April 2015 through October 2015. November 2015 was designated as a washout period to allow environmental service staff (EVS) to fully implement the UV technology. The experimental (postintervention) period extended from December 2015 until November 2016. Baseline daily and terminal room cleaning practices remained unchanged across both periods. Rooms that housed patients diagnosed with CDI were cleaned with hypochlorite solution according to the recommended institutional protocol. A hospital-grade disinfectant (quaternary ammonium compound) was used for other hospital rooms. To assess the evenness of this practice in both study periods, ATP (adenosine triphosphate) measurements of high-touch surfaces (HTSs) were taken at the end of manual cleaning, before UV disinfection. In our facility, a bioluminescent ATP product is applied to designated surfaces by environmental service (EVS) managers prior to manual terminal cleaning of patient rooms. Following cleaning, an emitted bioluminescent signal from the premarked areas is read. Any areas that are above pre-set thresholds require repeat manual cleaning.16 Neither the ATP compliance monitoring system used by EVS or our manager protocol changed across our study period.
Centers for Disease Control and Prevention (CDC) recommendations17 were followed for isolation of patients with CDI, including the use of soap and water for hand hygiene and institution of contact precautions for a period of at least 7 days after treatment was begun and until patient was asymptomatic for at least 48 hours, whichever was longer. Individuals with VRE colonization and infection were placed under contact precautions.
UV Cleaning (Intervention)
Enhanced cleaning was completed with a pulsed-xenon ultraviolet radiation (PX-UV) device (Xenex Healthcare Services, San Antonio, TX). The PX-UV device emits light in high-intensity pulses across a broad wavelength spectrum and includes the peak UV germicidal range of 200 to 320 nm associated with disinfection activity.18 PX-UV disinfection was incorporated into the terminal cleaning of all patient rooms after discharge according to the manufacturer’s protocol. In addition, unit-wide daily PX-UV disinfection of patient bathrooms was performed during the intervention period according to an adapted institutional protocol. For daily bathroom cleanings, the device was operated in one 5-minute cycle, whereas for discharge cleanings, three 5-minute cycles at different positions within the patient room (including the bathroom) were completed according to the manufacturer’s recommended protocol.
An automated data log recorded room number, environmental services operator identification, date, time, number of pulses delivered during device operation time and amount of energy emitted, as well as any error codes. Compliance was measured based on 2 criteria: (1) energy emitted and (2) duration of cleaning episode (minimum, 5 minutes per position). The manufacturer (Xenex) provided all compliance data via its standard restricted-access web-based compliance reporting portal.
Outcomes
The primary outcomes of interest were acquisition of colonization or infection with VRE and TCD. This included (1) incidence of infection (a clinical case in patients with preceding negative surveillance swab) and (2) incidence of colonization (a positive surveillance swab preceded by at least 1 prior negative swab). Patient days at risk were calculated for each outcome separately and were defined as entire LOS for patients who remained negative throughout their hospital stay, zero for prevalent cases, and time from admission to first positive screen or clinical diagnosis for incident cases. Each BMT admission was included in the analysis, and patients were classified negative, prevalent, or incident for each stay (Table 1). If a patient transferred into and out of the BMT unit multiple times in the same hospital admission, each transfer into the BMT visit was catalogued as a new admission to the BMT unit.
Table 1.
Determination of Incidence and Prevalence of VRE and Clostridium difficile Based on Active and Clinical Surveillance
| Scenario | First Swab | Subsequent Swabs | Clinical Infection | Clinical Infection Comes Before First Swab | Classification |
|---|---|---|---|---|---|
| 1 | Negative | All negative | No | N/A | Negative |
| 2 | Negative | All negative | Yes | No | Incident |
| 3 | Negative | All negative | Yes | Yes | Prevalent |
| 4 | Negative | At least 1 positive | No | N/A | Incident |
| 5 | Negative | At least 1 positive | Yes | No | Incident |
| 6 | Negative | At least 1 positive | Yes | Yes | Prevalent |
| 7 | Positive | … | Yes/No | Yes/ No | Prevalent |
Note. N/A, not available.
Heat maps were generated in R for the total number of VRE and CDI cases by room and by month in the pre- and postintervention periods. Color intensity was used to indicate higher number of cases, and circles within each box were used to indicate the number of incident cases.
Laboratory methods
Detection of VRE.
For rectal swabs, Van A testing was performed using the GenXpert (Cepheid, Sunnyvale, CA) according to the manufacturer’s instructions.
Detection of TCD.
The target of detection was the toxin B gene. Rectal swabs were inoculated in pre-reduced selective broth medium for 48 hours. Broth was centrifuged at 10,000–12,000 rpm for 2 minutes. The supernatant was discarded, and the pellet was resuspended in 200 μL InstaGene Matrix (BioRad, Hercules, CA); 1 μL TaqMan Exogenous Internal Positive Control (Life Technologies, Carlsbad, CA) was added to each sample. Sample extraction was performed on a BioRad MyCycler with the following program: 30 minutes at 56°C followed by 8 minutes at 100°C. After extraction was complete, samples were centrifuged, and supernatant was used for the remainder of testing. The 25-μL reaction mixture consisted of 10 μL extracted DNA, 2.5 μL polymerase chain reaction (PCR)–grade water, 2.5 μL 10X EXO IC Mix, 2.5 μL LightCycler-FastStart HybProbe mixture, 3 μL 3mM MgCl, 1.5 μL 10nM probe, and 1.5 μL 50nM forward and reverse prim2ers. The PCR assay was performed with ABI Quant Studio (Life Technologies, Carlsbad, CA) with the following cycling conditions: 10 minutes at 95°C, at 45 cycles of 15 seconds at 95°, and 1 minute at 60°C. Primers: TcdB-F: 5’-CATGC TTTTTTAGTTTCTGGATTGA TcdB-R: 5’-AGCAGTTGAA-TATAGTGGTTTAGTTAGAGTTG Probe: Tcdb-P: FAM-CATCCAGTCTCAATTGTATATGTTTCTCCA-MGB-NFQ.
At MSKCC, clinical diagnostic testing for C. difficile is performed using the Cepheid GeneXpert PCR platform (1 step). A clinical case of CDI was defined by a positive C. difficile GeneXpert on a diarrheal stool specimen.
Statistical analysis
Comparison of unit-level patient characteristics was assessed using the χ2 or Fisher exact test for categorical variables and the t test for continuous variables. The incidence rate was calculated as number of new acquisitions over the total number of patient days at risk. First, mean monthly rates were assessed using a t test. A power analysis was performed to determine the number of time points needed assuming a baseline average incidence of 12 cases per 1,000 patient days (standard deviation [SD], 5) for VRE acquisition and 10 cases per 1,000 patient days (SD, 5) for Clostridium difficile acquisition (based on our analysis of pre- study data on the unit). Because the effectiveness of UV cleaning is not known in this setting, several minimally detectable effect sizes were assessed (a consecutive decrease of 2, 3, and 4 cases per 1,000 days). The number of time points to detect each using a t test was 200, 90, and 52, respectively, at 0.80 power.
The data were also analyzed using an interrupted time series (ITS) analysis with 2 outcomes: incidence of VRE and C. difficile acquisition. The ITS improves upon a before and after design because it accounts for secular trends in outcomes outside of study exposures. It can detect level changes (whether the outcome increased or decreased) as well as trend changes (whether an intervention influenced the slope of the underlying trend). Descriptive statistics were calculated using χ2 and t tests. After assessing overdispersion, the outcome rates were assumed to follow a negative binomial distribution. The regression models included (1) a binary term for the intervention (before and after); (2) secular trends for the periods before and after intervention, and (3) a term for their interaction. Statistical tests revealed no seasonal fluctuations or auto-correlation. We conducted 3 sensitivity analyses: (1) multiple imputation with 5 imputations for missing CDI surveillance data, (2) an analysis using mean weekly rate as the unit of analysis, (3) an analysis adjusting for percent of allogenic patients per month. To assess the effect of possible changes in C. difficile rates by season, each model was adjusted for seasonality. All analyses were completed using SAS version 9.4 software (SAS Institute, Cary, NC).
Results
During the entire study period (excluding washout), 579 patients had 704 admissions to the BMT unit. Patient characteristics for the 2 study periods are presented in Table 2. Patients were more likely to have an allogenic BMT in the period of UV initiation and were also more likely to have slightly longer LOS. Demographic characteristics did not vary between the pre- and postintervention periods (Table 2).
Table 2.
Baseline Demographic and Transplant Characteristics of the Study Population in the Preintervention (April 2015 to October 2015) and Postintervention (December 2015 to November 2016) Study Periodsa
| Variable | Preintervention | Postintervention | P Value |
|---|---|---|---|
| Sex | .8023 | ||
| Male | 93 (42.86) | 159 (43.92) | |
| Female | 124 (57.14) | 203 (56.08) | |
| Age (mean, SD) | 52.77 (13.77) | 54.78 (13.40) | .085 |
| HCT type (no., %) | .0271 | ||
| Allogenic | 109 (50.23) | 199 (54.97) | |
| Autologous | 97 (44.7) | 128 (35.36) | |
| Cord blood | 9 (4.15) | 32 (8.84) | |
| None | 2 (0.92) | 3 (0.83) | |
| Time in days from HCT to before or after admission (median, SD) | 6 (150) | 6 (149) | .2932 |
| LOS (median, SD) | 24.33 (12.59) | 29.48 (16.41) | < .0001 |
Note. LOS, length of hospital stay; HCT, hematopoietic stem cell transplant; SD, standard deviation.
First visit for patients with >1 visit. Does not include patient admissions during washout period of November 2015.
A total of 2,160 surveillance tests were performed during the entire study, not including washout. For a combined total including surveillance and clinical cases, 128 (22.1%) patients tested positive for TCD and 190 patients (32.9%) tested positive for VRE.
In the preintervention period, among 265 admissions, 21 were positive for TCD at the time of admission (5 cases and 16 carriers), for an overall admission prevalence rate of 8%. For VRE, the admission prevalence rate was 17%. In the postintervention period, among 439 unit admissions, 31 had TCD (8 cases and 23 carriers), for an admission prevalence of 7%. For VRE, the admission prevalence was 13% (Figs. 1 and 2).
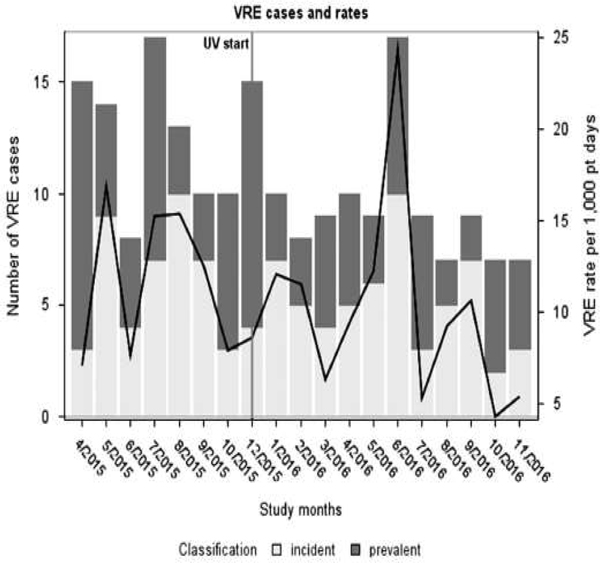
Fig. 1.
Number of incident and prevalent VRE cases (primary axis) and VRE incidence rate per 1,000 patient days (secondary axis) during the study period. Ultraviolet disinfection was implemented in November 2015. Case numbers and rates were derived from patients with colonization and clinical infection.
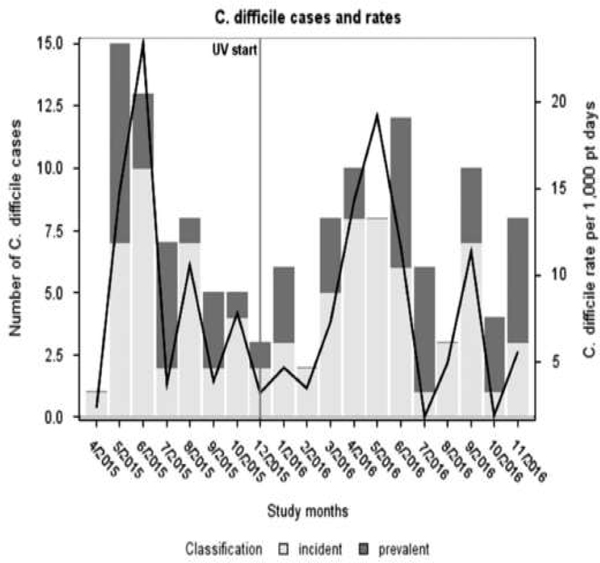
Fig. 2.
Number of incident and prevalent C. difficile cases (primary axis) and C. difficile incidence rate per 1,000 patient days (secondary axis) during the study period. Ultraviolet disinfection was implemented in November 2015. Case numbers and rates were derived from patients with colonization and clinical infection.
In the pre- and postintervention periods, respectively, there were 33 and 49 TCD acquisitions for a mean monthly incidence rate of 9.3 and 7.1 per 1,000 days, respectively (P = .503) (Fig. 2). For VRE, 43 and 61 acquisitions occurred in the 2 study periods, for a mean monthly incidence rate of 12.2 per 1,000 days in the preintervention period and 9.7 per 1,000 days in the postintervention period (P = .4389) (Fig. 1). No significant trend was observed in C. difficile and VRE incidence before or after the intervention. The ITS analysis did not demonstrate any change in level or trend in the incidence of VRE (trend incidence rate ratio [IRR], 0.96; 95% confidence interval [CI], 0.81–1.14; level IRR, 1.34; 95% CI, 0.37–1.18) or C. difficile (trend IRR, 1.08; 95% CI, 0.89–1.31; level IRR, 0.51; 95% CI, 0.13–2.11) after the intervention (Table 3). The hospital-acquired incidence rates for CDI for the 2 study periods were 1.411 and 1.114 per 1,000 patient days, respectively (P = .70), and the hospital-acquired incidence rates for VRE infections for the 2 study periods were 3.0236 and 3.6588 per 1,000 patient days, respectively (P = .60). Heat maps showed no substantial change in the clustering of incident cases across the study rooms in either the pre- or postintervention period (Supplemental Fig. 1A and B).
Table 3.
Interrupted Time Series Analysis With Primary Outcome Measures of VRE and C. difficile Acquisition
| IRR | (95% CI) | P Value | |
|---|---|---|---|
| VRE, full segmented regression model | |||
| Baseline trend | 1.01 | (0.86–1.18) | .911 |
| Level change after UV cleaning | 1.34 | (0.37–4.80) | .652 |
| Trend change after UV cleaning | 0.96 | (0.81–1.14) | .625 |
| Clostridium difficile , full segmented regression model | |||
| Baseline trend | 0.93 | (0.78–1.11) | .415 |
| Level change after UV cleaning | 0.51 | (0.13–2.11) | .356 |
| Trend change after UV cleaning | 1.08 | (0.89–1.31) | .413 |
Note. IRR, incidence rate ratio; UV, ultraviolet; VRE, vancomycin-resistant enterococci.
Overall, 3 sensitivity analyses were conducted. First, multiple imputation was used to predict missing values for C. difficile surveillance swabs (see the Methods section; Supplemental Table 2A). Second, ITS analysis was repeated using week as the unit of analysis rather than month to determine whether using a more granular measure of time would improve the ability to detect differences (Supplemental Table 2 B). Finally, although the pre- and post-UV intervention study populations were not expected to differ, the percentage of allogenic BMT patients increased after the intervention. Therefore, all models were adjusted for monthly rate of allogenic patients. No change in effect was found when models were adjusted for seasonality. All analyses were qualitatively similar to the study’s main results.
Assessment of baseline practices and compliance with UV disinfection
Differences in environmental cleaning were monitored by ATP measurements at the end of manual cleaning and prior to UV disinfection throughout the study. No discernible difference was detected in manual cleaning during any part of the study period as monitored by routine ATP evaluation of manual cleaning practices by environmental services staff (Supplemental Fig. 3D). Similarly, hand hygiene compliance and antibiotic utilization (defined daily dose per 1,000 patient days) on the unit did not differ (Supplemental Fig. 3B and 3C). The monthly percentage of eligible rooms (including terminal and bathroom cleanings) treated by UV averaged 82.6% (range, 73.5%–89.5%) throughout the study period (Supplemental Fig. 3A). Nursing staff mitigated patient refusals.
DISCUSSION
Our study conducted in a BMT unit at a large tertiary-care cancer center with high rates of C. difficile and VRE did not show a reduction in acquisition rates for these 2 important pathogens when routine terminal cleaning was supplemented with UV disinfection.
Although several studies have examined reduction of pathogens in the environment,5,7,19,20 only a few have considered the impact on acquisition rates as an outcome.7,9–12 The most notable among these is a large multicenter cluster randomized trial that found no reductions of clinical CDI with bleach or UV disinfection but a reduction in VRE with combination cleaning but not with UV alone. Other smaller observational studies have shown mixed results for reduction in MDRO and C. difficile acquisition.8–14 Among hematology-oncology patients, 2 previous prospective studies employing UV technology for terminal disinfection of contact isolation rooms have demonstrated a reduction in CDI.21,22
Most of the previous reports have been conducted in acute-care settings, either community-based hospitals or large multiple-specialty academic centers. In contrast, several unique features of our study population and setting may account for the observed differences in the findings between these studies. First, our study was performed in a hyperendemic setting with high rates of VRE and C. difficile. The exposure times on the unit were long, with hospital stays exceeding 21 days. All transplant recipients are routinely placed in protective isolation with no shared occupancy. The nurse-to-patient ratio is typically greater than on other general units, reducing the likelihood of horizontal transmission across rooms. Due to the compromised immune status of the hosts and routine use of prophylactic antibiotics, it is plausible that the susceptibility to MDRO acquisition is not overcome despite a substantial reduction in the environmental burden after terminal UV disinfection. Finally, the incidence of VRE and CDI in transplant recipients may not always represent recent acquisition. Our study, although designed to differentiate between true nosocomial infection and unmasking of colonization, may be limited by the sensitivity of the rectal swab despite the employment of molecular methods for target pathogen detection.
The present study had several strengths. The most notable strength of our study is that it is the first to incorporate active surveillance and therefore can detect true nosocomial infections in the healthcare setting. The acquisition of colonization and development of clinical infection do not always coincide, studies in settings with shorter hospital stays, and those that examine clinical infections only, will miss silent transmission and post-discharge events. Our study was designed to address this and other limitations of current NHSN surveillance criteria that are based solely on diagnosis of infection from the time of admission. Secondly, ITS design is a quasi-experimental design that both reduces omitted variable bias and addresses secular trends in outcomes measures.23,24
Our study has several limitations. Most importantly, extrapolation of our findings to nontransplant settings should be done with caution. UV disinfection is a promising technology as demonstrated in other settings,7–14 but definitive studies are needed before widespread adoption can be justified among hematology-oncology units for HAI reduction. We acknowledge several other limitations: UV compliance did not reach >90% for any of the study months but remained close to 80%, similar to previous studies. Storage loss accounted for 8% of missing surveillance swabs; this attrition was addressed through a sensitivity analysis using multiple imputations without a substantial change in the study results. Finally, although a power analysis indicated that 52–200 time periods were needed to detect a statistically significant effect, the current analysis was restricted to 21 months due to institutional time and cost restraints. A sensitivity analysis was conducted using weekly rates (84 weeks total) and found similar results. Daily bathroom cleaning was used as a supplemental measure in addition to manufacturer recommended terminal cleaning, but this is not a validated protocol.
In summary, our study has several implications for infection prevention in SCT settings. Although UV cleaning has demonstrated benefit in previous studies, our results suggest that the technology may not be as beneficial in reducing VRE or C. difficile acquisition in SCT recipients. Transmission dynamics and characteristics of patient population may explain the differential effect compared to previous studies. More research is needed to determine whether UV disinfection is associated with reduction of hospital acquired infections in other specialized settings.
Supplementary Material
Acknowledgments.
We thank the MSKCC environmental services team for their assistance.
Financial support. This study was partly funded by the New York State Department of Health, Healthcare-Associated Infection Prevention Project (grant no. 1203311156 to M.M., D.C., and M.K.) and the MSKCC Core Cancer Center (grant no. P30 CA008748 to all MSKCC authors).
Footnotes
Conflicts of interest. All authors report no conflicts of interest relevant to this article.
Supplementary material. To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2018.215
References
- 1.Drees M, Snydman DR, Schmid CH, et al. Prior environmental contamination increases the risk of acquisition of vancomycin-resistant Enterococci. Clin Infect Dis 2008;46:678–685. [DOI] [PubMed] [Google Scholar]
- 2.Shaughnessy MK, Micielli RL, DePestel DD, et al. Evaluation of hospital room assignment and acquisition of Clostridium difficile infection. Infect Control Hosp Epidemiol 2011;32:201–206. [DOI] [PubMed] [Google Scholar]
- 3.Kramer A, Schwebke I, Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis 2006;6:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freedberg DE, Salmasian H, Cohen B, Abrams JA, Larson EL. Receipt of antibiotics in hospitalized patients and risk for Clostridium difficile infection in subsequent patients who occupy the same bed. JAMA Intern Med 2016;176:1801–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jinadatha C, Quezada R, Huber TW, Williams JB, Zeber JE, Copeland LA. Evaluation of a pulsed-xenon ultraviolet room disinfection device for impact on contamination levels of methicillin-resistant Staphylococcus aureus. BMC Infect Dis 2014;14:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nerandzic MM, Cadnum JL, Pultz MJ, Donskey CJ. Evaluation of an automated ultraviolet radiation device for decontamination of Clostridium difficile and other healthcare-associated pathogens in hospital rooms. BMC Infect Dis 2010;10:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson DJ, Chen LF, Weber DJ, et al. Enhanced terminal room disinfection and acquisition and infection caused by multidrug-resistant organisms and Clostridium difficile (the Benefits of Enhanced Terminal Room Disinfection study): a cluster-randomised, multicentre, crossover study. Lancet 2017;389:805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green C, Pamplin JC, Chafin KN, Murray CK, Yun HC. Pulsed-xenon ultraviolet light disinfection in a burn unit: impact on environmental bioburden, multidrug-resistant organism acquisition and healthcare associated infections. Burns 2017;43:388–396. [DOI] [PubMed] [Google Scholar]
- 9.Haas JP, Menz J, Dusza S, Montecalvo MA. Implementation and impact of ultraviolet environmental disinfection in an acute care setting. Am J Infect Control 2014;42:586–590. [DOI] [PubMed] [Google Scholar]
- 10.Levin J, Riley LS, Parrish C, English D, Ahn S. The effect of portable pulsed xenon ultraviolet light after terminal cleaning on hospital-associated Clostridium difficile infection in a community hospital. Am J Infect Control 2013;41:746–748. [DOI] [PubMed] [Google Scholar]
- 11.Miller R, Simmons S, Dale C, Stachowiak J, Stibich M. Utilization and impact of a pulsed-xenon ultraviolet room disinfection system and multidisciplinary care team on Clostridium difficile in a long-term acute care facility. Am J Infect Control 2015;43:1350–1353. [DOI] [PubMed] [Google Scholar]
- 12.Nagaraja A, Visintainer P, Haas JP, Menz J, Wormser GP, Montecalvo MA. Clostridium difficile infections before and during use of ultraviolet disinfection. Am J Infect Control 2015;43:940–945. [DOI] [PubMed] [Google Scholar]
- 13.Simmons S, Morgan M, Hopkins T, Helsabeck K, Stachowiak J, Stibich M. Impact of a multi-hospital intervention utilising screening, hand hygiene education and pulsed xenon ultraviolet (PX-UV) on the rate of hospital associated meticillin resistant Staphylococcus aureus infection. J Infect Prevent 2013;14:172–174. [Google Scholar]
- 14.Vianna PG, Dale CR, Simmons S, Stibich M, Licitra CM. Impact of pulsed xenon ultraviolet light on hospital-acquired infection rates in a community hospital. Am J Infect Control 2016;44(3):299–303. [DOI] [PubMed] [Google Scholar]
- 15.Kamboj M, Sheahan A, Sun J, et al. Transmission of Clostridium difficile during hospitalization for allogeneic stem cell transplant. Infect Control Hosp Epidemiol 2016;37:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang Y-S, Chen Y-C, Chen M-L, et al. Comparing visual inspection, aerobic colony counts, and adenosine triphosphate bioluminescence assay for evaluating surface cleanliness at a medical center. Am J Infect Control 2015;43:882–886. [DOI] [PubMed] [Google Scholar]
- 17.Siegel JD, Rhinehart E, Jackson M, Chiarello L. 2007 guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control 2007;35:S65–S164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nerandzic MM, Thota P, Jencson A, et al. Evaluation of a pulsed xenon ultraviolet disinfection system for reduction of healthcare-associated pathogens in hospital rooms. Infect Control Hosp Epidemiol 2015;36:192–197. [DOI] [PubMed] [Google Scholar]
- 19.Yang J-H, Wu U-I, Tai H-M, Sheng W-H. Effectiveness of an ultraviolet-C disinfection system for reduction of healthcare-associated pathogens. J Microbiol Immunol Infect 2017;S1684–S1182. [DOI] [PubMed] [Google Scholar]
- 20.Ghantoji SS, Stibich M, Stachowiak J, et al. Non-inferiority of pulsed xenon UV light versus bleach for reducing environmental Clostridium difficile contamination on high-touch surfaces in Clostridium difficile infection isolation rooms. J Med Microbiol 2015;64:191–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pegues DA, Han J, Gilmar C, McDonnell B, Gaynes S. Impact of ultraviolet germicidal irradiation for no-touch terminal room disinfection on Clostridium difficile infection incidence among hematology-oncology patients. Infect Control Hosp Epidemiol 2017;38:39–44. [DOI] [PubMed] [Google Scholar]
- 22.Sampathkumar P, Nation L, Folkert C, Wentink JE, Zavaleta KW. A trial of pulsed xenon ultraviolet disinfection to reduce C. difficile infection. Am J Infect Control 2016;44(6):S32–S33. [DOI] [PubMed] [Google Scholar]
- 23.Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. New Engl J Med 2015;372:825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ziakas PD, Thapa R, Rice LB, Mylonakis E. Trends and significance of VRE colonization in the ICU: a meta-analysis of published studies. PLoS One 2013;8:e75658. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.