Abstract
The Therapeutically Applicable Research to Generate Effective Treatments (TARGET) project aims to determine molecular changes that drive childhood cancers, including osteosarcoma. The main purpose of the program is to use the open-source database to develop novel, effective, and less toxic therapies. We downloaded TARGET-OS RNA-Sequencing data through R studio and merged the mRNA expression of genes with clinical information (vital status, survival time and gender). Further, we analyzed differential gene expressions between dead and alive patients based on TARGET-OS project. By this study, we found 5758 differentially expressed genes between deceased and alive patients with a false discovery rate below 0.05; 4469 genes were upregulated in deceased patients compared to alive, whereas 1289 genes were downregulated. The survival-related genes were obtained using Kaplan–Meier survival analysis and Cox univariate regression (KM < 0.05 and Cox P-value < 0.05). Out of 5758 differentially expressed genes, only 217 have been associated with overall survival. Eight survival-related downregulated genes (ERCC4, CLUAP1, CTNNBIP1, GCA, RAB40C, SIRPA, USP11, and TCN2) and four survival-related upregulated genes (MUC1, COL13A1, JAG2 and KAZALD1) were selected for further analysis as potential independent prognostic candidate genes. This study may help to discover novel prognostic markers and potential therapeutic targets for osteosarcoma.
Keywords: Osteosarcoma, sarcoma, TARGET, RNA sequencing, survival analysis, differential gene expression
Impact statement
Osteosarcoma (OS) is the most common primary malignant bone tumor and the third most common cancer among children and young adults. It frequently spreads to the lung, and has a five-year survival rate of 70%. A better understanding of the molecular mechanisms, genetic regulation, and prognostic indicators of OS will support the development of more effective and less toxic therapies.
Introduction
Osteosarcoma (OS) is a primary malignant bone tumor with a high incidence rate in children and adolescents. 1 An initial peak has been observed between the ages of 10 and 19 years during the pubertal growth spurt and a secondary peaks after the age of 65 years associated with Paget’s disease. 2 There is a predilection for the metaphyseal area of long bones with approximately 75% of all cases occurring in distal femur (41.6%), proximal tibia (16.9%), proximal humerus (9.2%), and proximal femur (7.7%). 3
Although osteosarcoma has been associated with p53 and retinoblastoma protein dysfunction respectively, our understanding of the prognostic indicators and the genetic mechanisms of disease progression are incomplete. 4 OS is an aggressive, invasive sarcoma that frequently metastasizes, most commonly to the lung, with a five-year survival rate of 70%. 5 Multi-agent chemotherapy was introduced in the 1970s and improved the prognosis from a dismal 15–20% survival to a 60–70% five-year survival rate, but outcomes have not significantly improved since then. Currently, neo-adjuvant chemotherapy using doxorubicin, methotrexate, cisplatin, or ifosfamide to reduce the size of a tumor and eradicate micro-metastases followed by surgery and further chemotherapy is the standard of care for OS. 6 We appreciate that tumor size, stage at presentation and response to chemotherapy are significant prognostic indicators, but our understanding of the molecular mechanisms of disease progression is poor.7,8
Genome-wide RNA sequencing (RNA-Seq) provides a quantitative resolution of the transcriptome, can discover novel transcripts, identify alternative splicing, and detect gene expression changes between different samples.9,10 This functional analysis of the genome improves our understanding of the underlying mechanisms of complex diseases, especially in cancer.11,12 We have used RNA-Seq data to identify potential novel prognostic markers for OS using survival prediction methods based on differential gene expression profiles.
The Therapeutically Applicable Research to Generate Effective Treatments (TARGET) is an open database provided by the National Cancer Institute (NIH, https://ocg.cancer.gov/programs/target). We have analyzed the molecular changes in OS with respect to clinical outcome to better understand the gene regulatory networks, mechanisms of disease progression, and key survivorship determinants.
Materials and methods
Data information
The results presented here are based upon data generated by the TARGET-OS and the data used for the analysis are available at https://portal.gdc.cancer.gov/projects.
TARGET-OS RNA-Seq data were downloaded by R studio (https://www.r-project.org/) through Bioconductor packages of BiocManager, TCGAbiolinks, TCGAWorkflowData, DT, genomic data commons (GDC), and summarized experiment (Bioconductor version of 3.12, https://www.bioconductor.org/). The data category, data type, and workflow type were determined as transcriptome profiling, gene expression quantification, and HTSeq- counts respectively through the TCGAbiolinks package. The RNA-Seq data were merged with clinical information of the patients’ such as vital status, survival time, and gender. Samples without vital status, survival time, and gender were excluded from the data.
Differential gene expression analysis
The genes with missing expression values were removed from the data. Statistical data and differential expression of digital gene expression analyses were performed through edgeR (Empirical Analysis of Digital Gene Expression Data in R) package for R.13,14 Differential gene expression levels between dead and alive patients’ were obtained by the package through investigating the log-2-change of the genes (logFC, the cut-off value of 0.5). The edgeR package also provides the Benjamini–Hochberg method to control the false discovery rate (FDR, the cut-off value of 0.05). 15 The gene expression levels between dead and alive patients were compared with t-test and visualized using R and GraphPad Prism software version 8 (GraphPad software, San Diego, CA, USA). The significant level was set at both FDR < 0.05 and P < 0.05.
Survival analysis
Patients’ survival time and vital status were merged with differentially expressed genes. R's survival packages; survival, survminer, and survdiff were used for survival analysis. Further, Kaplan–Meier (K–M) survival plots were generated and combined with the Xena Functional Genomics Explorer-GDC TARGET-OS. 16 The cut-off criterion was set to K–M < 0.05 and Cox P-value < 0.05 for screening of the survival-related gene at overall survival.
Pathway enrichment analysis and gene–gene functional interactions
The biological process and pathway enrichment analyses were performed through The gene ontology (GO)17,18 and The NCATS BioPlanet 19 datasets, respectively. Further, gene–gene functional interactions of differentially expressed genes were performed using the Cytoscape software (version of 3.8.2) and GeneMANIA 20 datasets.
Results
Sample characteristics
The RNA-Seq data were downloaded from TARGET-OS project and the data were merged with vital status, survival time, and gender. After removing the samples without vital status, survival time, and gender information, the patient sample number was 86. Age ranged from 3 to 34 years (mean 15 years) and there were 37 (43%) females and 49 (57%) males. Patient demography and tumor characteristics are presented in Tables 1 and 2.
Table 1.
The characteristics of the patients.
| Vital status | Female (n = 37) | Male (n = 49) | Total |
|---|---|---|---|
| Alive | 23 | 34 | 57 |
| Dead | 14 | 15 | 29 |
| 86 | |||
Table 2.
The characteristics of the tumors.
| Site of tumor | Number | Disease at diagnosis and metastasis site |
|---|---|---|
| Femur | 39 | 25 non-metastatic and 14 metastatic (8 lung only, 5 lung and bone, 1 bone only) |
| Tibia | 21 | 17 non-metastatic and 4 metastatic (3 lung only, 1 bone and lung) |
| Fibula | 8 | 7 non-metastatic and 1 metastatic (lung only) |
| Humerus | 4 | 3 non-metastatic and 1 metastatic (lung only) |
| Pelvis | 2 | 1 non-metastatic and 1 metastatic (lung only) |
| Pelvis–ilium | 1 | 1 non-metastatic |
| Pelvis/sacrum | 1 | 1 non-metastatic |
| Leg NOSa | 6 | 5 non-metastatic and 1 metastatic (lung only) |
| Foot NOSa | 1 | 1 non-metastatic |
| Radius | 1 | 1 non-metastatic |
| Ilium | 1 | 1 metastatic (lung only) |
| Arm NOSa | 1 | 1 non-metastatic |
| Total | 86 | 63 non-metastatic and 23 metastatic (16 lung only, 6 lung and bone, 1 bone only) |
| Summary | 15 dead metastatic and 15 dead non-metastatic8 alive metastatic and 48 alive non-metastatic | |
aNot otherwise specified.
Differential expression analysis between dead and alive patients
Differential gene expression between dead and alive patients was compared with the edgeR package using R studio. By this analysis, 5758 genes were differentially expressed between dead and alive patients (both P-value and FDR <0.05) and 50,690 genes were not significant (both P-value and FDR >0.05). Of the 5758 genes, 4469 were upregulated, whereas 1289 genes were downregulated in dead versus living patients.
The survival-related genes in OS patients
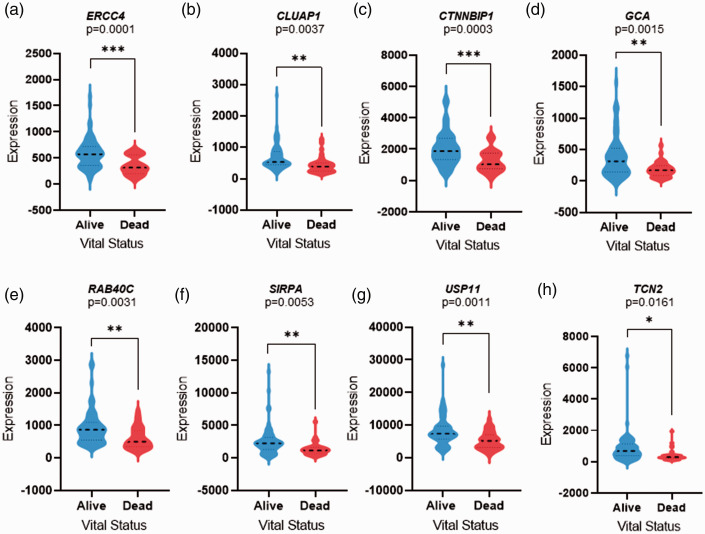
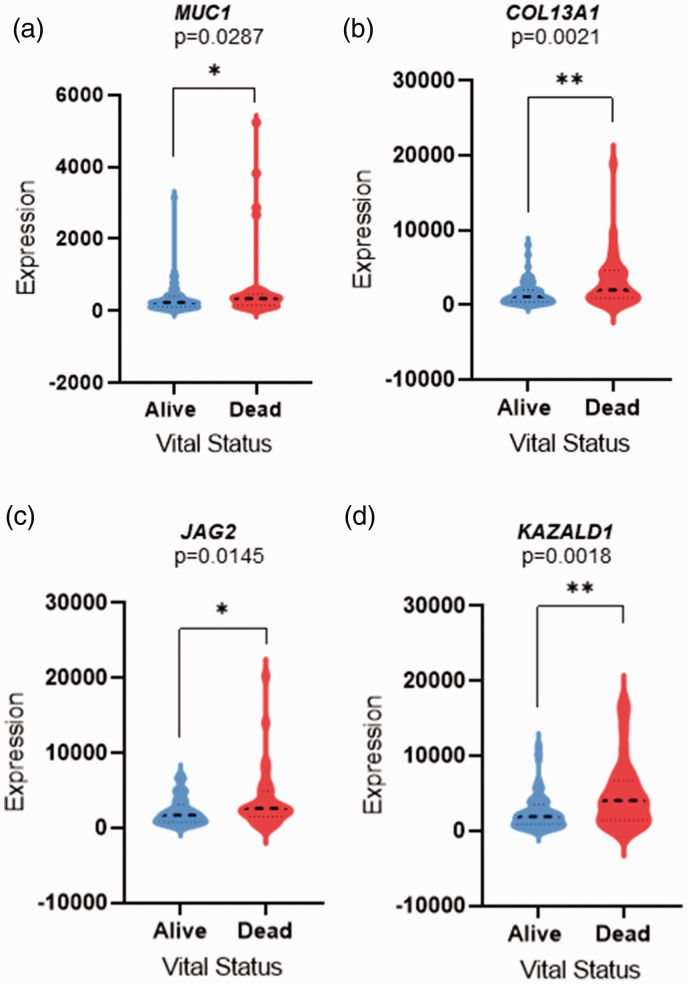
Genes associated with survival were identified using Cox regression. Out of 5758 differentially expressed genes, 217 were found to be associated with overall survival (P < 0.05). Further, the survival-related genes were selected depending on their P-values (preferably lowest). The overall survival-related genes of dead and alive patients were validated and visualized using violin plots (Figures 1 and 2).
Figure 1.
Violin plots displaying the downregulated survival-related genes expression levels; (a) ERCC4, (b) CLUAP1, (c) CTNNBIP1, (d) GCA, (e) RAB40C, (f) SIRPA, (g) USP11, and (h) TCN2. The violin plots are filled in blue (left) and red (right) represent alive and dead patients, respectively. The y-axis highlights the raw expression of the genes, whereas x-axis vital status of the patients. A dashed line in the violin plots represent the median value of the gene expression. P-value was calculated using Student’s t-test, P <0.05 considered statistically significant (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.00001). (A color version of this figure is available in the online journal.)
Figure 2.
Violin plots displaying the upregulated survival-related genes expression levels; (a) MUC1, (b) COL13A1, (c) JAG2, and (d) KAZALD1. The violin plots are filled in blue (left) and red (right) represent alive and dead patients, respectively. The y-axis highlights the raw expression of the genes, whereas x-axis vital status of the patients. A dashed line in the violin plots represent the median value of the gene expression. P-value was calculated using Student’s t-test, P <0.05 considered statistically significant (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.00001). (A color version of this figure is available in the online journal.)
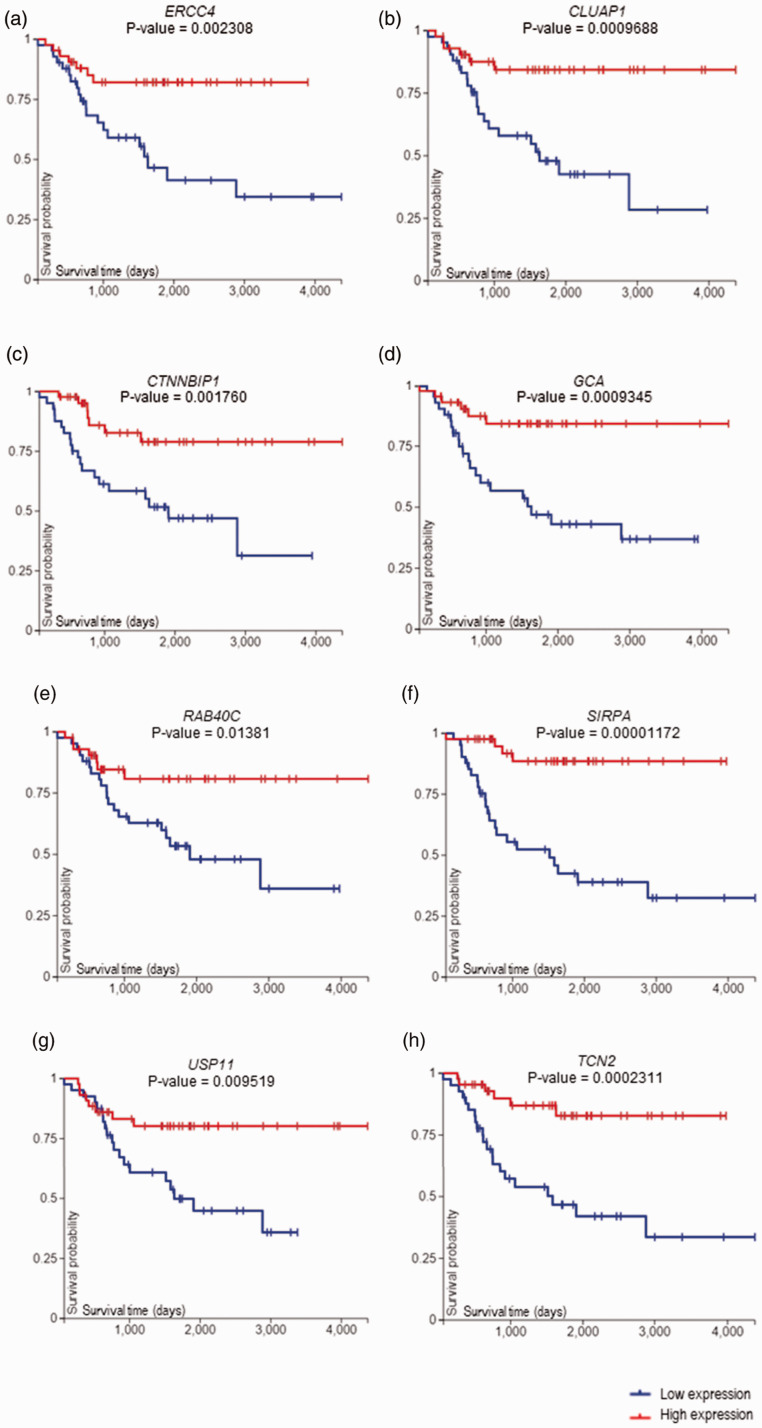
The survival-related genes that were significantly downregulated in the dead compared with living patients include; ERCC excision repair 4 (ERCC4), Clusterin-associated protein 1 (CLUAP1), catenin beta interacting protein 1 (CTNNBIP1), grancalcin (GCA), member RAS oncogene family (RAB40C), signal regulatory protein alpha (SIRPA), ubiquitin-specific peptidase 11 (USP11), and transcobalamin 2 (TCN2) (Table 3). K–M plots of the downregulated genes showed the association between downregulated genes and poor survival outcome of the patients with the significant value of P < 0.05 (Figure 3).
Table 3.
Survival-related downregulated genes in dead patients.
| Symbol | Description | logFC | logCPM | F | P-Value | FDR |
|---|---|---|---|---|---|---|
| ERCC4 | ERCC excision repair 4 | −0.604331065 | 3.6044199 | 21.15321871 | 1.35E−05 | 0.000711 |
| CLUAP1 | Clusterin associated protein 1 | −0.506755578 | 3.835841364 | 15.51321235 | 0.000159425 | 0.004417889 |
| CTNNBIP1 | Catenin beta interacting protein 1 | −0.535497627 | 5.404612024 | 12.20243352 | 0.000736136 | 0.01311661 |
| GCA | Grancalcin | −0.977336188 | 2.918813582 | 13.50950687 | 0.000399133 | 0.008618337 |
| RAB40C | Member RAS oncogene family | −0.526285629 | 4.284398674 | 14.18739992 | 0.000291816 | 0.006857791 |
| SIRPA | Signal regulatory protein alpha | −0.695679783 | 5.630441654 | 10.89208674 | 0.001375861 | 0.020650984 |
| USP11 | Ubiquitin-specific peptidase 11 | −0.526261302 | 7.42223499 | 14.5888379 | 0.000242739 | 0.005965627 |
| TCN2 | Transcobalamin 2 | −0.745119819 | 4.055907039 | 9.088352903 | 0.003325462 | 0.037655142 |
Figure 3.
Kaplan–Meier survival analysis of (a) ERCC4, (b) CLUAP1, (c) CTNNBIP1, (d) GCA, (e) RAB40C, (f) SIRPA, (g) USP11 and (h) TCN2. Blue color represents genes with low expression, whereas red, genes with high expression. P-value was computed by log-rank test, P <0.05 considered statistically significant. (A color version of this figure is available in the online journal.)
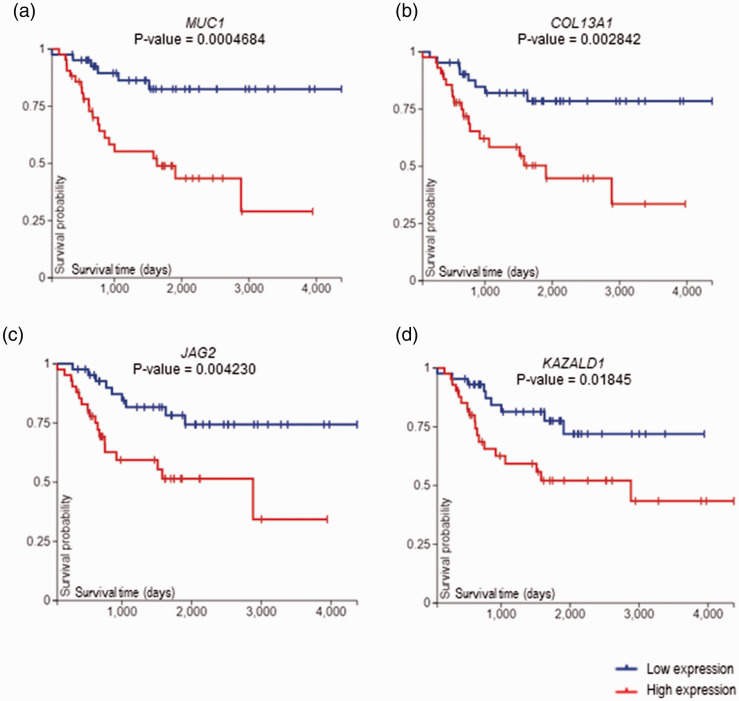
Significantly upregulated genes in the dead patients include; mucin 1-cell surface associated (MUC1), collagen type XIII alpha 1 chain (COL13A1), jagged canonical notch ligand 2 (JAG2), and Kazal type serine peptidase inhibitor domain 1 (KAZALD1) (Table 4). K–M plots highlighted upregulation of these genes and the positive correlation with poor patient survival (Figure 4).
Table 4.
Survival-related upregulated genes in dead patients.
| Symbol | Description | logFC | logCPM | F | P-Value | FDR |
|---|---|---|---|---|---|---|
| MUC1 | Mucin 1, cell surface associated | 1.351910472 | 3.516411994 | 18.43384428 | 4.36E-05 | 0.001702678 |
| COL13A1 | Collagen type XIII alpha 1 chain | 1.1977585 | 5.747573231 | 14.90975716 | 0.000209656 | 0.00541287 |
| JAG2 | Jagged canonical Notch ligand 2 | 0.955088787 | 6.021108153 | 16.09517412 | 0.000122657 | 0.003634496 |
| KAZALD1 | Kazal type serine peptidase inhibitor domain 1 | 1.002413796 | 6.419848871 | 12.11332211 | 0.000767825 | 0.013540203 |
Figure 4.
Kaplan–Meier survival analysis of (a) MUC1, (b) COL12A1, (c) JAG2, and (d) KAZALD1. Blue color represents genes with low expression, whereas red, genes with high expression. P-value was computed by log-rank test, P <0.05 considered statistically significant. (A color version of this figure is available in the online journal.)
GO and BioPlanet pathway enrichment analyses and gene–gene functional interactions of the survival-related genes
To examine the characteristics of the survival-related genes, functional classification and pathway enrichment analyses of downregulated and upregulated genes were performed using the GO tool and BioPlanet, respectively.
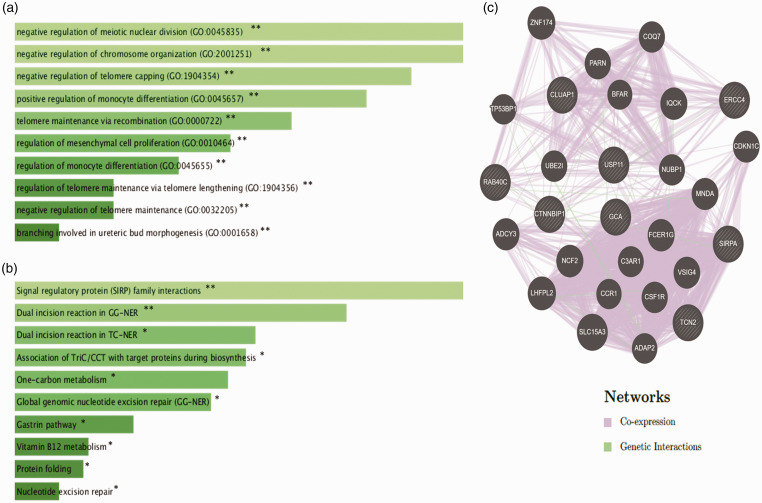
Figure 5(a) highlights the most significantly overrepresented GO terms of biological process of downregulated genes in dead patients. The top six biological process of GO terms are; negative regulation of meiotic nuclear division (GO: 0045835, P-value = 0.002398), negative regulation of chromosome organization (GO: 2001251, P-value = 0.002398), negative regulation of telomere capping (GO: 1904354, P-value = 0.0028), positive regulation of monocyte differentiation (GO: 0045657, P-value = 0.0032), telomere maintenance via recombination (GO: 0000722, P-value = 0.003993), and regulation of mesenchymal cell proliferation (GO: 0010464, P-value = 0.004790). Further, Figure 5(b) shows the pathway enrichment analysis of the downregulated genes, including, signal regulatory protein (SIRP) family interactions (P-value = 0.0051), dual incision reaction in GG-NER (P-value = 0.0079), dual incision reaction in TC-NER (P-value = 0.011), and association of TriC/CCT with target proteins during biosynthesis (P-value = 0.0115). In Figure 5(c), gene–gene interaction of the downregulated genes was shown.
Figure 5.
Enrichment analysis of eight survival-related downregulated genes; functional gene ontology analysis of biological process (a), pathway enrichment analysis (b) and gene–gene interaction network analysis (c). P-values less than 0.05 were considered statistically significant, *P < 0.05, **P < 0.01. (A color version of this figure is available in the online journal.)
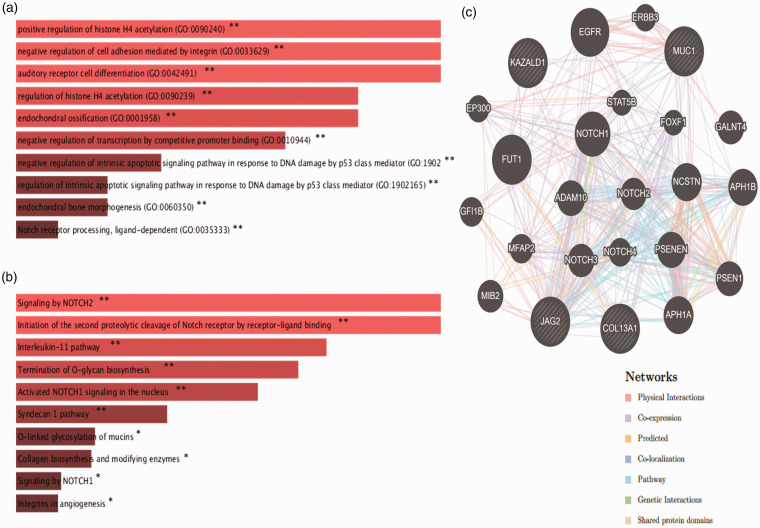
Figure 6(a) highlights the most significantly overrepresented GO terms of biological process of upregulated genes in dead patients such as positive regulation of histone H4 acetylation (GO: 0090240, P-value = 0.0014), negative regulation of cell adhesion mediated by integrin (GO: 0033629, P-value = 0.0014), auditory receptor cell differentiation (GO: 0042491, P-value = 0.0014), regulation of histone H4 acetylation (GO:0090239, P-value = 0.0016), endochondral ossification (GO: 0001958, P-value = 0.0016), and negative regulation of transcription by competitive promoter binding (GO: 0010944, P-value = 0.0018). Figure 6(b) performs the pathway enrichment analysis of the upregulated genes, including, signalling by NOTCH2 (P-value = 0.0027), initiation of the second proteolytic cleavage of Notch receptor by receptor-ligand binding (P-value = 0.0028), interleukin-11 pathway (P-value = 0.0045), termination of O-glycan biosynthesis (P-value = 0.0051), and activated NOTCH1 signalling in the nucleus (P-value = 0.0061). Lastly, Figure 6(c) highlights the gene–gene interaction of the upregulated genes.
Figure 6.
Enrichment analysis of four survival-related upregulated genes; functional gene ontology analysis of biological process (a), pathway enrichment analysis (b) and gene–gene interaction network analysis (c). P-values less than 0.05 were considered statistically significant, *P < 0.05, **P < 0.01. (A color version of this figure is available in the online journal.)
Discussion
Our limited understanding of the genetic regulation and molecular mechanisms of disease progression in osteosarcoma is a barrier to the development of novel precision therapies. Transcriptome analysis is central to the discovery and understanding of prognostic biomarkers, a research priority in OS.
In this study, we analyzed differential gene expression by survival based on 86 TARGET-OS project patients. Of the 5758 differentially expressed genes, 217 were associated with survival (FDR <0.05 and P < 0.05). Based on P values, we selected eight downregulated genes (ERCC4, CLUAP1, CTNNBIP1, GCA, RAB40C, SIRPA, USP11, and TCN2) and four upregulated genes (MUC1, COL13A1, JAG2, and KAZALD1) in patients who died of their disease for further analysis (Tables 3 and 4).
The associations between differentially regulated (both upregulated and downregulated) genes and OS have already been analyzed (except RAB40C, USP11, CTNNBIP1, TNC2, GCA, JAG2, and KAZALD1).21–26 However, in these studies, the prognostic values of the genes have not been investigated.
ERCC4, also known as XPF, gene encodes proteins that can play an important role in DNA repair and chromosome stability. 27 The gene also has been involved in fanconi anemia (FA) which leads to bone marrow failure, congenital malformations, chromosome fragility, and cancer susceptibility including OS.28,29 According to several studies, patients with low expression of ERCC4 were associated with worse overall survival in hepatocellular carcinoma. 30 Our study also highlighted that OS patients with low expression of ERCC4 have displayed worse survival outcome of the patients (Figure 3(a)).
There is an increased expression of CLUAP1 in cell growth and the S phase of cell-cycle progression. 31 Further, over expression of the gene was observed in OS tissue samples and cell lines, including HuO3N1, OS2000, Saos-2, HuO9N2, HOS, and MG63. Noteworthy, over-expression of CLUAP1 has also been observed in ovarian, colon, and lung cancers. 22
This is the first report of an association between CTNNBIP1 and survival in OS, but the gene is known to be a prognostic indicator in lung adenocarcinomas. 32 According to the same study, CTNNBIP1 expression correlated with longer overall survival in lung adenocarcinomas patients. 32
GCA is a protein that has been identified in human neutrophils and the monocytes/macrophage lineage. 33 The function of this protein is not completely understood, but the gene was downregulated in metastatic OS in in vitro studies. 34
Breast cancer patients with high expression CD47 expression (cluster of differentiation 47) and active CD47/SIRPA (SIRPα) pathway have a poor prognosis. 35 Furthermore, one study determined that the CD47/SIRPA pathway is activated in OS. CD47 is known as a “do not eat me” signal that binds to SIRPA in the surface of macrophages which leads to escape from phagocytosis and cell death. 36 Notably, knockout of SIRPA in macrophages enhances phagocytosis of OS cells. 25
Unfortunately, there are no established studies to highlight the direct association between USP11 and OS. However, low expression of USP11 was correlated with better survival outcome in breast cancer patients. 37 Interestingly, a study suggested that over-expression of USP11 promotes growth and metastasis of colorectal cancer. 38
No clear association that has been found yet between TCN2 gene and any cancer type, including OS. Intriguingly, mutation (loss of function) of this gene result in transcobalamin deficiency that leads to an abnormal immunity in the individuals. 39 Our result showed that down regulation of TCN2 is associated with worse survival outcome (Figure 3(h)).
MUC1 has been associated with metastatic progression both in vivo and in vitro in several cancer types through O-glycosylated serine/threonine repeat region (MUC1-ECD), as well as through activities of its intracellular domain (MUC1-CD). 40 It is already established that over-expression of MUC1 predicts poor prognosis in breast cancer patients. 41 Interestingly, high expression level of MUC1 in sarcoma metastasis was correlated with worse overall survival in sarcoma. 26 Our result also correlated a high expression of MUC1 with low survival of OS patients (Figure 4(a)).
Although, no associations have been found regarding to COL13A1 and OS, the gene strongly correlated with poor clinical outcome in bladder cancer. 42 Another study mentioned that over expression of COL13A1 is associated with high risk of disease progression and aggressive invasion in urothelial carcinoma of the bladder. 43 We also found that COL13A1 was upregulated in dead OS patients compared to alive, further high expression of the gene is associated with poor survival outcome (Figure 4(b)). Nevertheless, the functional role of COL13A1 in cancer especially in OS needs to be extensively studied.
Over-expression of JAG2 has been associated with poor survival outcome in oral squamous cell carcinoma. 44 Another study also highlighted that high expression level of JAG2 increases chemo-resistance of colorectal cancer cells. 45 Interestingly, the positive correlation of MYC (MYC proto-oncogene, bHLH transcription factor) and JAG2 are critical pro-survival factors in childhood medulloblastoma.46,47 There are no published studies to highlight the relationship between JAG2 and OS. However, one study suggested that JAG2 may be involved in OS progression and initiation because the gene interacts with both p53 signalling and Notch signalling pathways, which play an essential role in development of bone tumors.48,49
There is no published clear association between KAZALD1 gene and OS. According to one study, high expression of KAZALD1 promotes glioma malignant progression by invasion and high proliferation of tumor cells. The authors also suggested that low expression of KAZALD1 confers better overall survival. 50 Our result also showed that high expression of KAZALD1 is correlated with worse overall survival in OS patients (Figure 4(d)).
In conclusion, in the present study we have identified 5758 differentially expressed genes between dead and alive patients in which 217 of genes showed an association with overall survival. Out of 217 genes, 8 survival-related downregulated (ERCC4, CLUAP1, CTNNBIP1, GCA, RAB40C, SIRPA, USP11, and TCN2) and 4 survival-related upregulated genes (MUC1, COL13A1, JAG2, and KAZALD1) were selected. These genes have not been adequately studied in cancer, especially in OS. Consequently, further research with the candidate genes is required to characterize their roles in OS progression, invasion, and metastasis to provide specific and successful prognostic markers for the disease.
Footnotes
AUTHORS’ CONTRIBUTIONS: All authors made significant contributions to the work presented in this paper. ER contributed to the data collection, data analysis and writing the manuscript. JX and DW both involved in project conception and reviewing the manuscript. SK contributed to the study design, data analysis and reviewing the manuscript.
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING: This study was funded by the Abbie Basson Sarcoma Foundation (Sock it to Sarcoma!).
ORCID iDs: Emel Rothzerg https://orcid.org/0000-0003-1957-930X
Sulev Kõks https://orcid.org/0000-0001-6087-6643
References
- 1.Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the surveillance, epidemiology, and end results program. Cancer 2009; 115:1531–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simpson E, Brown HL. Understanding osteosarcomas. JAAPA 2018; 31:15–9 [DOI] [PubMed] [Google Scholar]
- 3.Sangle NA, Layfield LJ. Telangiectatic osteosarcoma. Arch Pathol Lab Med 2012; 136:572–6 [DOI] [PubMed] [Google Scholar]
- 4.Berman SD, Calo E, Landman AS, Danielian PS, Miller ES, West JC, Fonhoue BD, Caron A, Bronson R, Bouxsein ML, Mukherjee S, Lees JA. Metastatic osteosarcoma induced by inactivation of Rb and p53 in the osteoblast lineage. Proc Natl Acad Sci U S A 2008; 105:11851–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouvier C, Macagno N, Nguyen Q, Loundou A, Jiguet-Jiglaire C, Gentet JC, Jouve JL, Rochwerger A, Mattei JC, Bouvard D, Salas S. Prognostic value of the hippo pathway transcriptional coactivators Yap/TAZ and beta1-integrin in conventional osteosarcoma. Oncotarget 2016; 7:64702–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamplot JD, Denduluri S, Qin J, Li R, Liu X, Zhang H, Chen X, Wang N, Pratt A, Shui W, Luo X, Nan G, Deng ZL, Luo J, Haydon RC, He TC, Luu HH. The current and future therapies for human osteosarcoma. CCTR 2013; 9:55–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng H, Chen L, Zhang Y, Li WF, Mao YP, Liu X, Zhang F, Guo R, Liu LZ, Tian L, Lin AH, Sun Y, Ma J. The tumour response to induction chemotherapy has prognostic value for Long-Term survival outcomes after Intensity-Modulated radiation therapy in nasopharyngeal carcinoma. Sci Rep 2016; 6:24835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donegan WL. Tumor-related prognostic factors for breast cancer. CA Cancer J Clin 1997; 47:28–51 [DOI] [PubMed] [Google Scholar]
- 9.Levin JZ, Yassour M, Adiconis X, Nusbaum C, Thompson DA, Friedman N, Gnirke A, Regev A. Comprehensive comparative analysis of strand-specific RNA sequencing methods. Nat Methods 2010; 7:709–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet 2009; 10:57–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kube DM, Savci-Heijink CD, Lamblin AF, Kosari F, Vasmatzis G, Cheville JC, Connelly DP, Klee GG. Optimization of laser capture microdissection and RNA amplification for gene expression profiling of prostate cancer. BMC Mol Biol 2007; 8:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Milanez-Almeida P, Martins AJ, Germain RN, Tsang JS. Cancer prognosis with shallow tumor RNA sequencing. Nat Med 2020; 26:188–92 [DOI] [PubMed] [Google Scholar]
- 13.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010; 26:139–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCarthy DJ, Chen Y, Smyth GK. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res 2012; 40:4288–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benjamini YH. Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc 1995; 57:289–300 [Google Scholar]
- 16.Goldman MJ, Craft B, Hastie M, Repecka K, McDade F, Kamath A, Banerjee A, Luo Y, Rogers D, Brooks AN, Zhu J, Haussler D. Visualizing and interpreting cancer genomics data via the xena platform. Nat Biotechnol 2020; 38:675–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tulipano PK, Millar WS, Cimino JJ. Linking molecular imaging terminology to the gene ontology (GO). Pac Symp Biocomput 2003;613–23. DOI:10.1142/9789812776303_0057s [DOI] [PubMed] [Google Scholar]
- 18.Gene Ontology C The gene ontology resource: enriching a GOld mine. Nucleic Acids Res 2021; 49:D325–D34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang R, Grishagin I, Wang Y, Zhao T, Greene J, Obenauer JC, Ngan D, Nguyen DT, Guha R, Jadhav A, Southall N, Simeonov A, Austin CP. The NCATS BioPlanet - an integrated platform for exploring the universe of cellular signaling pathways for toxicology. Front Pharmacol 2019; 10:445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franz M, Rodriguez H, Lopes C, Zuberi K, Montojo J, Bader GD, Morris Q. GeneMANIA update 2018. Nucleic Acids Res 2018; 46:W60–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diao C, Xi Y, Xiao T. Identification and analysis of key genes in osteosarcoma using bioinformatics. Oncol Lett 2018; 15:2789–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishikura H, Ikeda H, Abe H, Ohkuri T, Hiraga H, Isu K, Tsukahara T, Sato N, Kitamura H, Iwasaki N, Takeda N, Minami A, Nishimura T. Identification of CLUAP1 as a human osteosarcoma tumor-associated antigen recognized by the humoral immune system. Int J Oncol 2007; 30:461–7 [PubMed] [Google Scholar]
- 23.Fanelli M, Tavanti E, Patrizio MP, Vella S, Fernandez-Ramos A, Magagnoli F, Luppi S, Hattinger CM, Serra M. Cisplatin resistance in osteosarcoma: in vitro validation of candidate DNA Repair-Related therapeutic targets and drugs for tailored treatments. Front Oncol 2020; 10:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li C, Yu X, Guo D, Liu G, Zhang K, Teng Q, Lin H. Association between common polymorphisms in ERCC gene and prognosis of osteosarcoma in patients treated with chemotherapy: a meta-analysis. Onco Targets Ther 2018; 11:3495–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ray M, Lee YW, Hardie J, Mout R, Yesilbag TG, Farkas ME, Rotello VM. CRISPRed macrophages for cell-based cancer immunotherapy. Bioconjug Chem 2018; 29:445–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang L, Tolani B, Yeh CC, Fan Y, Reza JA, Horvai AE, Xia E, Kratz JR, Jablons DM, Mann MJ. Differential gene expression identifies KRT7 and MUC1 as potential metastasis-specific targets in sarcoma. Cancer Manag Res 2019; 11:8209–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manandhar M, Boulware KS, Wood RD. The ERCC1 and ERCC4 (XPF) genes and gene products. Gene 2015; 569:153–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bogliolo M, Schuster B, Stoepker C, Derkunt B, Su Y, Raams A, Trujillo JP, Minguillon J, Ramirez MJ, Pujol R, Casado JA, Banos R, Rio P, Knies K, Zuniga S, Benitez J, Bueren JA, Jaspers NG, Scharer OD, de Winter JP, Schindler D, Surralles J. Mutations in ERCC4, encoding the DNA-repair endonuclease XPF, cause fanconi anemia. Am J Hum Genet 2013; 92:800–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levinson S, Vincent KA. Multifocal osteosarcoma in a patient with fanconi anemia. J Pediatr Hematol Oncol 1997; 19:251–3 [DOI] [PubMed] [Google Scholar]
- 30.Yang L, Xu M, Cui CB, Wei PH, Wu SZ, Cen ZJ, Meng XX, Huang QG, Xie ZC. Diagnostic and prognostic values of the mRNA expression of excision repair cross-complementation enzymes in hepatitis B virus-related hepatocellular carcinoma. Cancer Manag Res 2018; 10:5313–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi M, Lin YM, Nakamura Y, Furukawa Y. Isolation and characterization of a novel gene CLUAP1 whose expression is frequently upregulated in colon cancer. Oncogene 2004; 23:9289–94 [DOI] [PubMed] [Google Scholar]
- 32.Qi W, Chen J, Cheng X, Huang J, Xiang T, Li Q, Long H, Zhu B. Targeting the Wnt-regulatory protein CTNNBIP1 by microRNA-214 enhances the stemness and self-renewal of cancer stem-like cells in lung adenocarcinomas. Stem Cells 2015; 33:3423–36 [DOI] [PubMed] [Google Scholar]
- 33.Xu P, Roes J, Segal AW, Radulovic M. The role of grancalcin in adhesion of neutrophils. Cell Immunol 2006; 240:116–21 [DOI] [PubMed] [Google Scholar]
- 34.Lisle JW, Choi JY, Horton JA, Allen MJ, Damron TA. Metastatic osteosarcoma gene expression differs in vitro and in vivo. Clin Orthop Relat Res 2008; 466:2071–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagahara M, Mimori K, Kataoka A, Ishii H, Tanaka F, Nakagawa T, Sato T, Ono S, Sugihara K, Mori M. Correlated expression of CD47 and SIRPA in bone marrow and in peripheral blood predicts recurrence in breast cancer patients. Clin Cancer Res 2010; 16:4625–35 [DOI] [PubMed] [Google Scholar]
- 36.Luo ZW, Liu PP, Wang ZX, Chen CY, Xie H. Macrophages in osteosarcoma immune microenvironment: implications for immunotherapy. Front Oncol 2020; 10:586580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bayraktar S, Gutierrez Barrera AM, Liu D, Pusztai L, Litton J, Valero V, Hunt K, Hortobagyi GN, Wu Y, Symmans F, Arun B. USP-11 as a predictive and prognostic factor following neoadjuvant therapy in women with breast cancer. Cancer J 2013; 19:10–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun H, Ou B, Zhao S, Liu X, Song L, Liu X, Wang R, Peng Z. USP11 promotes growth and metastasis of colorectal cancer via PPP1CA-mediated activation of ERK/MAPK signaling pathway. EBioMedicine 2019; 48:236–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhan S, Cheng F, He H, Hu S, Feng X. Identification of transcobalamin deficiency with two novel mutations in the TCN2 gene in a Chinese girl with abnormal immunity: a case report. BMC Pediatr 2020; 20:460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horm TM, Schroeder JA. MUC1 and metastatic cancer: expression, function and therapeutic targeting. Cell Adh Migr 2013; 7:187–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jing X, Liang H, Hao C, Yang X, Cui X. Overexpression of MUC1 predicts poor prognosis in patients with breast cancer. Oncol Rep 2019; 41:801–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyake M, Morizawa Y, Hori S, Tatsumi Y, Onishi S, Owari T, Iida K, Onishi K, Gotoh D, Nakai Y, Anai S, Chihara Y, Torimoto K, Aoki K, Tanaka N, Shimada K, Konishi N, Fujimoto K. Diagnostic and prognostic role of urinary collagens in primary human bladder cancer. Cancer Sci 2017; 108:2221–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miyake M, Hori S, Morizawa Y, Tatsumi Y, Toritsuka M, Ohnishi S, Shimada K, Furuya H, Khadka VS, Deng Y, Ohnishi K, Iida K, Gotoh D, Nakai Y, Inoue T, Anai S, Torimoto K, Aoki K, Tanaka N, Konishi N, Fujimoto K. Collagen type IV alpha 1 (COL4A1) and collagen type XIII alpha 1 (COL13A1) produced in cancer cells promote tumor budding at the invasion front in human urothelial carcinoma of the bladder. Oncotarget 2017; 8:36099–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hatano K, Saigo C, Kito Y, Shibata T, Takeuchi T. Overexpression of JAG2 is related to poor outcomes in oral squamous cell carcinoma. Clin Exp Dent Res 2020; 6:174–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vaish V, Kim J, Shim M. Jagged-2 (JAG2) enhances tumorigenicity and chemoresistance of colorectal cancer cells. Oncotarget 2017; 8:53262–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hartl M. The quest for targets executing MYC-dependent cell transformation. Front Oncol 2016; 6:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fiaschetti G, Castelletti D, Zoller S, Schramm A, Schroeder C, Nagaishi M, Stearns D, Mittelbronn M, Eggert A, Westermann F, Ohgaki H, Shalaby T, Pruschy M, Arcaro A, Grotzer MA. Bone morphogenetic protein-7 is a MYC target with prosurvival functions in childhood medulloblastoma. Oncogene 2011; 30:2823–35 [DOI] [PubMed] [Google Scholar]
- 48.Park HS, Kim CG, Hong N, Lee SJ, Seo DH, Rhee Y. Osteosarcoma in a patient with pseudohypoparathyroidism type 1b due to paternal uniparental disomy of chromosome 20q. J Bone Miner Res 2017; 32:770–5 [DOI] [PubMed] [Google Scholar]
- 49.Sasaki Y, Ishida S, Morimoto I, Yamashita T, Kojima T, Kihara C, Tanaka T, Imai K, Nakamura Y, Tokino T. The p53 family member genes are involved in the notch signal pathway. J Biol Chem 2002; 277:719–24 [DOI] [PubMed] [Google Scholar]
- 50.Wang H, Feng Y, Bao Z, Jiang C, Yan W, Wang Y, Zhang C, Liu Y, Zhang Q, Zhang W, Jiang C. Epigenetic silencing of KAZALD1 confers a better prognosis and is associated with malignant transformation/progression in glioma. Oncol Rep 2013; 30:2089–96 [DOI] [PubMed] [Google Scholar]