Abstract
Lung cancer represents the world’s leading cause of cancer deaths. Sex differences in the incidence and mortality rates for various types of lung cancers have been identified, but the biological and endocrine mechanisms implicated in these disparities have not yet been determined. While some cancers such as lung adenocarcinoma are more commonly found among women than men, others like squamous cell carcinoma display the opposite pattern or show no sex differences. Associations of tobacco product use rates, susceptibility to carcinogens, occupational exposures, and indoor and outdoor air pollution have also been linked to differential rates of lung cancer occurrence and mortality between sexes. While roles for sex hormones in other types of cancers affecting women or men have been identified and described, little is known about the influence of sex hormones in lung cancer. One potential mechanism identified to date is the synergism between estrogen and some tobacco compounds, and oncogene mutations, in inducing the expression of metabolic enzymes, leading to enhanced formation of reactive oxygen species and DNA adducts, and subsequent lung carcinogenesis. In this review, we present the literature available regarding sex differences in cancer rates, associations of male and female sex hormones with lung cancer, the influence of exogenous hormone therapy in women, and potential mechanisms mediated by male and female sex hormone receptors in lung carcinogenesis. The influence of biological sex on lung disease has recently been established, thus new research incorporating this variable will shed light on the mechanisms behind the observed disparities in lung cancer rates, and potentially lead to the development of new therapeutics to treat this devastating disease.
Keywords: Lung, cancer, sex differences, sex hormones, carcinogenesis, estrogen receptors
Impact statement
Lung cancer is the third most diagnosed cancer in female patients, and the most diagnosed cancer in males. In addition, lung carcinoma ranks as the leading cause of cancer mortality for both men and women worldwide. While tobacco use has been widely associated with its diagnosis in both sexes, epidemiological data indicate that women who do not smoke are more than twice likely than men to develop lung cancer. Hormonal factors, including endogenous and exogenous sex hormones, have been implicated in the disease development, progression, and prognosis. This study provides a summary of the available evidence linking gonadal hormones and lung cancer in women and men. Collectively, the existing literature indicates that tobacco use, as well as hormonal, genetic, environmental, and metabolic factors, contribute to the observed sex and gender differences in lung cancer rates and outcomes, supporting the consideration of sex as a biological variable in lung cancer basic, clinical, and translational research.
Introduction
Lung carcinoma is one of the most-commonly diagnosed cancers worldwide, and the leading cause of cancer deaths across the globe. The most recent epidemiological data indicate that lung cancer is attributed to 1 in 10 (11.4%) cancers diagnosed, and 1 in 5 (18.0%) deaths worldwide. 1 In 2020, there were an estimated 2,206,771 new lung cancer cases and 1,796,144 lung cancer deaths around the world, with 235,760 cases and 131,880 deaths only in the United States.1,2
The main risk factor associated with lung cancer diagnosis is tobacco use, although secondhand smoke exposure, ionizing radiation, air pollution, and several occupational and chemical exposures have also been linked to lung cancer diagnosis.3–7 A role of hormonal factors such as exogenous hormone use in adult women (e.g. oral contraceptives and/or hormone replacement therapy), as well as endogenous circulating levels of sex hormones has also been associated with lung cancer development.8,9 To date, the mechanisms by which reproductive hormones could contribute to lung cancer development, progression, and/or severity have not been fully elucidated.
Sex and gender differences in lung cancer epidemiology
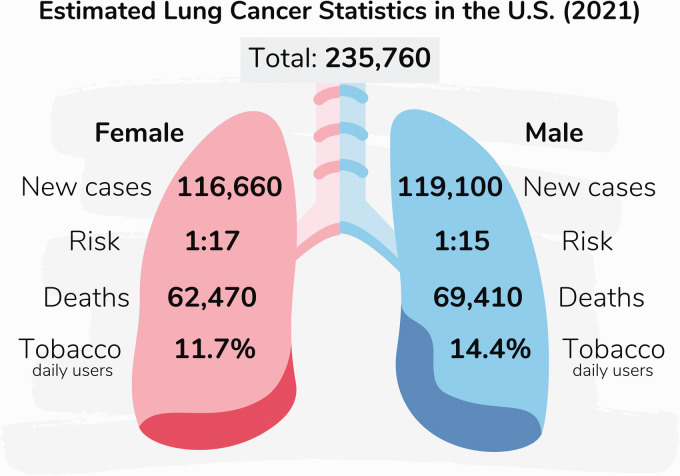
The available data suggest that both biological (sex) and social (gender) influences can impact lung cancer rates. While the incidence of lung carcinoma in both men and women below the age of 40 is relatively low, it increases with age in most populations. 10 According to the most recent report, with 1,435,943 cases reported in males and 770,828 in females worldwide in 2020, the age standardized rate (i.e. the average of age-specific mortality rates/100,000 persons) for lung cancer incidence is 31.5 in males and 14.6 in females, and the cumulative risk for lung cancer incidence by age 74 (the likelihood that a person will develop lung cancer by age 74) is 3.78% in males and 1.77% in females. 1 In the United States, sex stratified data indicate an estimated 119,100 cases and 69,410 deaths in males, and 116,660 cases and 62,470 deaths in females, as illustrated in Figure 1.1,2
Figure 1.
Estimated lung cancer statistics in the U.S. (2021). The estimated rates of lung cancer in the United States for 2021 are: (1) 235,760 new cases, including 119,100 in men and 116,660 in women. (2) The risk to develop lung cancer in one’s lifetime is about 1 in 15 for men and 1 in 17 for women. (3) About 69,410 yearly deaths from lung cancer are men, and 62,470 are women. (4) On average, 14.4% and 11.7% of men and women are daily tobacco users, respectively. (A color version of this figure is available in the online journal.)
While over the past few decades, the overall rates for cancer incidence have decreased in men, they have remained stable in women. On the other hand, all cancer mortality rates have declined in both groups. 11 In the same period, both lung cancer incidence and mortality rates have been reported to display sex differences, with wide variations across countries. 1 The current male to female incidence ratio ranges from 1.2 to 5.6 across nations, but on average, these rates are generally twice as high for men than women. 1 However, variations in this ratio have been reported over time and largely attributed to changes in tobacco use rates.12,13 Regarding global mortality, with 1,188,679 deaths reported in males and 607,465 in females in 2020, the age-standardized rate is 25.9 in males and 11.2 in females, with a cumulative risk for lung cancer mortality by age 74 of 3.08% in males and 1.34% for females. 1 While about 30% of global lung cancer deaths can be attributed to smoking, sex differences in mortality cannot be fully explained by smoking behavior.13,14
In men, lung carcinoma represents the most diagnosed cancer type, and ranks as the leading cause of cancer mortality. In women, lung cancer ranks third for incidence and second for mortality. 1 Interestingly, for women, it represents the leading cause of cancer deaths in both highly industrialized countries, and countries with higher rates of outdoor ambient air pollution and household exposure of burning solid fuels for heating or cooking.15,16 In this regard, the global proportion of cancer deaths attributable to outdoor air pollution exposure ranges from 4.7% to 20.5%, with a global average of 14%. 17
Tobacco smoke and lung cancer rates in men and women
Tobacco use is the most recognized risk factor for lung cancer. In the United States, smoking can be attributed to approximately 80% of lung cancer fatalities. 2 Similarly, more than 85% of all patients diagnosed with lung cancer are current or former smokers, and it is estimated that about 20% of all smokers will develop lung cancer in their lifetime.18,19 On the other hand, about 20% of non-smoker women (and 10% of men) develop lung cancer.3,20 It has been well-recognized that women are more susceptible to the adverse effects of tobacco consumption, although they usually show a better prognosis than men. 21 This sex difference, however, cannot be explained by anatomical differences or smoking history, and it is thought to be associated with a higher susceptibility to tobacco carcinogens in female patients.22,23
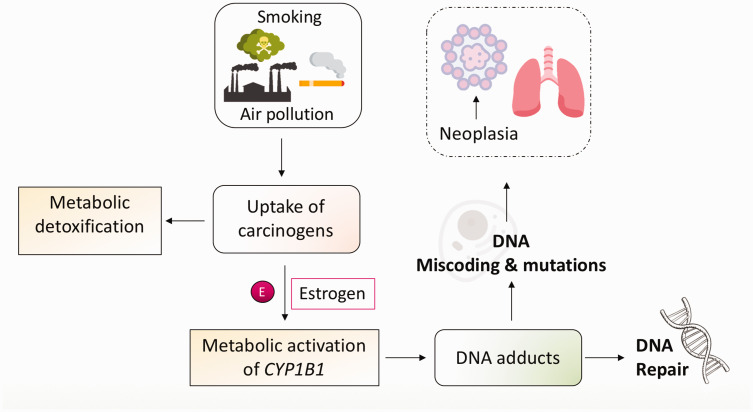
As indicated above, even with lower tobacco use rates, females who smoke are more likely to develop lung cancer than men. One possible factor contributing to this phenomenon is that female sex hormones like estrogens are able to exacerbate the carcinogenic effects of tobacco (Figure 2). 16 The synergism of estrogen with some of the toxic compounds present in tobacco smoke, vis induction of the enzyme CYP1B1 (responsible for estrogenic metabolism), leads to a heightened formation of reactive oxygen species, which in turn promotes carcinogenesis. 24 Epidemiological and experimental evidence also indicates that these mechanisms are also influenced by exposure to air pollutants and other environmental factors such as secondhand/passive smoke. 25 Moreover, female smokers also display greater levels of PAH (polycyclic aromatic hydrocarbon)-derived DNA adducts, as well as a higher frequency of p53 mutations despite lower levels of exposure to these toxins (Figure 2).26–28
Figure 2.
Role of smoking, air pollution, and estrogen in carcinogenesis. Exposure to tobacco smoke and/or environmental toxins leads to the uptake of carcinogens that are metabolized by lung cells. The estrogen metabolizing enzyme CYP1B1 is upregulated by tobacco carcinogens and estrogens, resulting in increased levels of carcinogenic derivatives of estrogen. CYP1B1 also metabolizes polycyclic aromatic hydrocarbons (PAH) resulting in PAH-DNA adduct formation. Unrepaired DNA adducts and inefficient DNA repair can result in DNA miscoding and mutations, leading to uncontrolled cell growth and lung neoplasia. (A color version of this figure is available in the online journal.)
The patterns of lung cancer incidence in many countries also reflect trends in gender-specific smoking behaviors.5,12,29 In the United States, where 20.4 million males and 7.2 million females are current smokers, both lung cancer incidence and death rates among women are on the rise. 30 Interestingly, these rates are declining in men for certain age groups, and increasing in women, reflecting historical patterns of tobacco use in women in some birth cohorts. 31 Globally, there are over 940 million men and 175 million women over the age of 15 who currently smoke. Of these, nearly 75% of male and 50% of female daily smokers live in highly developed countries. Gender influences such as societal acceptance of tobacco use and affordability of use for women could contribute to the differential rates of lung cancer observed in these nations. Regarding cessation, women tend to stop smoking at lower rates than men and are motivated to smoke by a desire to avoid weight gain. 32 Maternal tobacco use during pregnancy still occurs in the United States, where 1 in 14 women who gave birth in 2016 reported smoking during pregnancy, despite this behavior being linked to multiple negative health outcomes. 33
Among non-smokers, females are about 2.5 times more likely to develop lung carcinoma at a younger age than males.32,34,35 These differences have also been attributed to sex steroids, genetic mutations, molecular pathways involving epidermal growth factors and sex hormone receptor signaling, regulation of gene expression by miRNAs, and differences in environmental chemical exposures, such as air pollutants and secondhand smoke toxins.36–45
Sex differences in lung cancer types
About 95% of all lung carcinomas are classified into two main types: (1) non-small cell carcinoma (NSCLC), and (2) small cell carcinoma (SCLC). NSCLC is further classified into two subtypes: squamous cell carcinoma (SCC) and adenocarcinoma (AC).46,47 This distinction is important in the clinic, not only for staging, but also for treatment and prognosis. Sex differences in both AC and SCC have been reported, with AC found more frequently in females, and SCC being more prevalent in males.48–50
Historically, SCC were the most commonly diagnosed lung cancers, accounting for about 50% of all male lung cancers in the 1970s. 51 Over time, the SCC relative rates have decreased and currently account for about 30% of cases. On the other hand, ACs have increased, from representing about 20% of lung cancers in the 1970s, to over 40% of all current cases. The rates for SCLC, on the other hand, have remained constant at about 20%, whereas NSCLC rates have decreased from 10% to < 3%. While SCLC, SCC, and NSCLC rates continue to decrease for both sexes, AC rates are increasing in females and remaining relatively constant in males. 51 As indicated earlier, AC is more represented in female than male patients, accounting for more than 50% of female cases in 2010. 52 In contrast, the SCC rates have decreased from 30% to 20% during the same time period. The trends for SCLC and NSCLC are similar between the sexes. 53
Sex differences in lung cancer treatment
Lung cancer therapy comprises surgery, regular chemotherapy, radiation, and therapy directed against specific tumor genetic and molecular characteristics. Appropriate treatment for lung cancer is based on tumor type and molecular markers, disease staging, and assessing the patient’s clinical condition and comorbidities. 54
NSCLC in the first three stages is routinely treated with a combination of surgery, chemotherapy, radiation therapy, or a combined-modality approach. Immunotherapy may also be part of the management strategy for some unresectable tumors (stage III) and advanced disease, including metastases (stage IV) or recurrence following initial definitive treatment. There are specific targeted therapies for NSCLC patients with mutations in the epidermal growth factor receptor (EGFR) as well as in the B-Raf proto-oncogene (BRAF), those with the echinoderm microtubule-associated protein-like 4 (EML4)-anaplastic lymphoma kinase (ALK) fusion oncogene, and c-ROS oncogene 1 (ROS1) fusions. 55 The current clinical recommendations suggest testing for EGFR mutations and ALK fusion for all patients with advanced staging to guide patient selection for therapy with an EGFR or ALK inhibitor, regardless of sex, race, smoking history, or other risk factors. 55 EGFR inhibitors target cancer cells with superior ability and a better safety profile when compared to conventional chemotherapies. 56
Other approved therapies for metastatic ALK-positive NSCLC tumors include the drugs ceritinib, alectinib, and brigatinib, representing next-generation ALK inhibitors.57–60 Other therapeutics include ALK/ROS1 tyrosine kinase inhibitors (TKIs).61,62 The drug crizotinib is an ALK-TKI indicated for ROS1 positive advanced NSCLC tumors. 60 Immunotherapy (e.g. the anti–PD-L1 antibody atezolizumab) is the first-line treatment for patients without driver mutations and a high level of programmed death-ligand 1 (PD-L1) expression. Combinations of chemotherapy and immunotherapy are available for those with lower PD-L1 expression. In patients with metastatic NSCLC, atezolizumab has provided an overall survival benefit.63,64
In a clinical trial, Socinski et al. found that the combination of atezolizumab to bevacizumab (VEGF-A-targeting monoclonal antibody) plus chemotherapy notably enhanced progression-free survival and overall survival in those with metastatic NSCLC, despite ALK genetic alteration status or EGFR and PD-L1 expression. 65 Overall survival hazard ratios contrasting anti-PD-1/PD-L1 with or without chemotherapy found that anti-PD-1 alone had a considerable effect in men and anti-PD-1/PD-L1 plus chemotherapy had a more significant impact in women. Therefore, these data exhibit the benefit of the addition of chemotherapy to anti-PD-1/PD-L1 in women. 66
The frequency of particular mutations within a tumor is known as tumor mutational burden (TMB). After adjusting for age at the time of surgery, lung cancer stage, and smoking history in a population of 335 patients with lung AC, male tumors showed a statistically more significant burden of genetic alterations when compared to female tumors. 67 Additionally, TMB is a more effective marker for predicting response to immunotherapy in female lung cancer patients than males. 68 Finally, in the modern chemotherapy era, women suffering from advanced NSCLC survive longer than their male counterparts, suggesting that female hormone levels may interact with the efficacy of current chemotherapy prescriptions after adjustment for other prognostic factors.35,69,70
Regarding SCLC, tumors are very responsive to chemotherapy. Limited stage disease, radiation therapy, and—rarely—surgery may be options as well. Cytotoxic agents such as platinum plus etoposide and topotecan remain standard therapy, neither showing survival benefit over the combination of antitumor antibiotics, microtubule inhibitors, or alkylating agents. 71 Despite recent studies that have increased our understanding of genomic alterations and signaling pathways in SCLC, molecularly targeted therapy agents have shown no improvement in overall survival. The two targeted drugs with early promising results are sunitinib (a multitargeted TKI that inhibits tumor cell proliferation and angiogenesis), and alisertib (an inhibitor of aurora A kinase which regulates cell cycle transit from G2 to cytokinesis). Inhibitors of mTOR, including everolimus, also show good efficacy when combined with paclitaxel.72,73 Despite the face of well-known sex differences in innate and adaptive immune responses, neither study assessing the efficacy and safety of these antitumor agents reported sex dissimilarities. 68 Future studies should consider sex as a biological variable, as this variable is crucial to assess the clinical response to these novel treatments and improve their efficacy.68,74
Molecular basis for sex differences in lung cancer
As indicated earlier, several factors have been attributed to the observed differences in lung cancer clinical outcomes between male and female patients.75–77 Mutations in oncogenes, transcription factor genes, and other signaling molecules have also been implicated.78–80 Lung ACs typically present with genetic alterations including mutations in the KRAS (KRAS Proto-Oncogene, GTPase), BRAF, and EGFR genes, and mutations or amplifications of ERBB2 (Erb-B2 Receptor Tyrosine Kinase 2), MET (MET Proto-Oncogene, Receptor Tyrosine Kinase), FGFR1 (Fibroblast Growth Factor Receptor 1), FGFR2, ALK, ROS1, RET (Ret Proto-Oncogene), and NRG1 (Neuregulin1) oncogenes. SCCs, on the other hand, are characterized by mutations in the TP53 (Tumor Protein P53), FGFR1, FGFR2, FGFR3, DDR2 (Discoidin Domain Receptor Tyrosine Kinase 2), PTEN (Phosphatase and Tensin Homolog), and CDKN2A (Cyclin Dependent Kinase Inhibitor 2A), as well as genes involved in the Phosphoinositide 3-kinases (PI3K) pathway. 80
In cancer, Ras proteins are likely to mutate and remain constitutively active.81,82 There are three proteins in the Ras family of small GTPases: N-, H-, and K-Ras. Ras proteins are associated with the activation of surface receptors such as RAF1 (Raf-1 Proto-Oncogene, Serine/Threonine Kinase) and BRAF. Interestingly, estrogen activates human RAF1 in SCLC cells, which inhibits growth by arresting the G1 and G2 phases of the cell cycle. 83 However, sex differences in these mechanisms have not been reported so far.
The aforementioned data indicate that RAF1 triggers signal transduction of the cell cycle and can act as a growth suppressor gene in SCLC. On the other hand, RAF1 association with smokers and female sex in NSCLC is well documented. Therefore, the RAF/MEK/MAP pathways may be considered as potential drug candidates for SCLC and other neuroendocrine tumors, with emphasis on sex differences. Several drugs inhibiting Ras, Raf or MEK, including selumetinib, are currently under clinical investigation.84,85
Several studies have shown the loss of wild-type KRAS alleles in both human and mouse lung SCC and AC. In addition, chemically induced mouse lung ACs have KRAS gene mutations in 67%–100% of cases. In humans, KRAS gene mutations are in 10%–30% of lung carcinomas, indicating robust connotations with smoking. 86 While KRAS mutations have not been extensively reported in SCLC, Kodaz et al. identified 16.2% KRAS mutations in a cohort of 37 SCLC patients, a number much higher than that reported in previous studies for SCLC (1%–3%). 87 On the other hand, EGFR and KRAS mutational analysis in NSCLC patients showed that KRAS mutation (study frequency 28.1%) was more commonly linked with smokers (p < 0.001) and females (p = 0.01). 88
Interestingly, reports have indicated that chemotherapy effects and survival are greater in females than males.89–91 The downside of these studies is that they included almost exclusively postmenopausal women.89–91 New studies have reported an interplay between the EGFR and estrogen signaling mechanisms in lung carcinogenesis. Therefore, a treatment combination based on TKIs and anti-estrogen treatment is being assessed.
Women with a positive EGFR mutation status have earlier onset of lung cancer when compared to men after tobacco exposure.20,91,92 Emerging data also indicate a potential bidirectional crosstalk between EGFR and the estrogen receptors (ERα and ERβ), both of which are present in lung cancer cells.93–97 Immunohistochemical assessment of EGFR and ERα expression in tumor specimens from NSCLC patients after surgery showed the combined overexpression of EGFR and ERα in NSCLC patients is a projection of poor outcome and, thus, represents an important prognostic tool. 98 Drug resistance to EGFR TKIs has also been documented in molecular analysis of tumor samples.99,100 Yu et al. identified the EGFR T790M as the most recurrent pathway of acquired resistance to EGFR-TKI therapy in 63% of patients. 100
Regarding ERs, Kadota et al. also reported that stage I lung AC cells express higher levels of ERα in females (19%) than in males (14%) and that ERα expression associates with smaller tumor size and poor prognostic immune microenvironments. 101 Interestingly, most NSCLC lines express both ERs. 102
In a population based study, estrogen inhibitors in breast cancer patients reduced the risk for lung cancer in older patients (≥50 years), supporting the hypothesis that antiestrogen therapy can modify lung cancer carcinogenesis in older women. 103 At this time, there are clinical trials for lung cancer patients to receive a combined treatment of anti-hormonal drugs such as EGFR TKI. 104 The results from this combination therapy currently show enhanced antiproliferative effects in up to 90% of NSCLC cells. 104
Role of sex hormones in lung carcinogenesis
In the past years, there has been an escalated interest in discovering whether sex hormones such as estrogens, androgens and progesterone can promote lung carcinogenesis.105–107 Epidemiological data suggest that estrogen plays a significant role in lung cancer, as well as in adverse prognosis of female patients with this condition. 108
Estrogens
Regarding circulating estrogen levels, the elevation of estrogen in women together with the reduction of DNA repair capabilities, cause women more vulnerable to the cancer-causing effect of tobacco.109–112 Stabile et al. showed that estrogen signaling is involved in both the lung mesenchyme and the epithelium. 106 The research team further revealed that estrogen could potentially trigger lung cancer via (1) direct or (2) indirect actions, on pre/neoplastic cells and lung fibroblasts, respectively. 106 Meireles et al. reported that tobacco smoke can also induce phase-I cytochrome P450 enzymes such as CYP1B1, which can metabolize endogenous estrogens to potentially carcinogenic catechol and quinine forms. 113 Additionally, estrogen can act as direct or indirect carcinogen, by either altering cell proliferation or regulating cell growth factors. Both ERs are expressed in normal and cancerous lung tissue, and regulate lung development, inflammation, and cancer.47,114–116
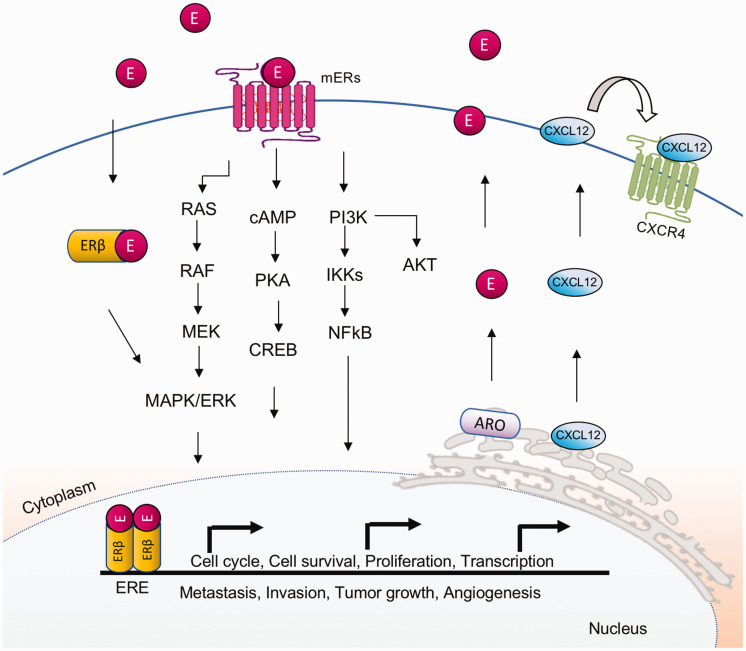
There are several isoforms of the ER receptor (ERα, ERβ, mERs). Of these, ERβ is highly expressed in almost 90% of NSCLC human tumor samples and cell lines derived from both females and males, whereas ERα is commonly low in lung cancer cells. 108 Both ERs can activate carcinogenesis-associated signaling via genomic or non-genomic pathways, although ERβ appears to play a predominant role (Figure 3). 117 A study analyzing histological material from 104 patients (68% men and 32% women) with NSCLC (9% NSCLC, 37% AC, 54% SCC) between 1989 and 1992 indicated that females with ERβ-negative lung cancer had a small reduction in mortality than those with ERβ-positive tumors. On the other hand, a significant decline in mortality rates was observed in males with ERβ positive lung tumors when compared to males with ERβ negative ones, indicating that ERβ could be used as a tool for the prognosis of NSCLC in males. 118 Interestingly, most immune cells that enter the lung express both ERs (ERα and Erβ). Via the genomic pathway, ERβ can displace to the nucleus to modulate transcription. In the non-genomic pathway, ERβ migrates to the cell membrane to regulate ion channels, protein kinases, and secondary messengers (Figure 3).
Figure 3.
Estrogen/estrogen receptor signaling in lung carcinogenesis. Estrogen receptor β is expressed in cytoplasm, nucleus, mitochondria, and plasma membrane of lung cancer cells. ERβ activates the MAPK, cAMP, and AKT signaling mechanisms via the non-canonical (or non-genomic) pathway. Briefly, the E2/ER complexes translocate to the nucleus where they bind to estrogen response elements (ERE) to induce gene expression. Some of the genes regulated by ER and involved in lung cancer can promote apoptosis, metastasis, mitochondrial biogenesis, proliferation aromatase (ARO) mechanism, cell cycle progression (CXCL2), and angiogenesis (VEGF). (A color version of this figure is available in the online journal.)
AKT: AKT serine/threonine kinase 1; cAMP: cellular levels of cyclic AMP; CREB: cAMP-response element binding protein; CXCL2: C-X-C chemokine ligand 2; CXCR4: C-X-C chemokine receptor type 4; E: estrogen; ERβ: estrogen receptor β; ERE: estrogen response elements; IKKs: IκB kinase; MAPK: mitogen-activated protein kinases; MEK: mitogen-activated protein kinase kinase; mERs: membrane estrogen receptors; NFκB: nuclear factor kappa-light-chain-enhancer of activated B cells; PI3K: phosphoinositide-3 kinase; PKA: protein kinase A; RAF: serine/threonine (S/T) kinase; RAS: guanine triphosphatase.
Researchers have shown that estrogen induces propagation of NSCLC cells and tumor growth. 106 Anti-estrogen treatment strategies can decrease tumor size, growth, and cell proliferation which may contribute to improved patient outcomes.119,120 Moreover, cell proliferation and tumor growth are in part due to initiation of the non-genomic mechanism via mitogen-activated protein kinases (MAPK), serine/threonine protein kinase B (or AKT), and cyclic adenosine monophosphate (cAMP) signaling pathways, as well as phosphorylation of the extracellular signal-regulated kinase (ERK) and EGFR. 91 Interestingly, mitochondrial ERβ can modulate apoptosis by physically sequestering the B-cell lymphoma 2 (Bcl-2) associated agonist of cell death (Bad) and prevent its interaction with Bcl-2 and B-cell lymphoma-extra-large (Bcl-XL) proteins in lung cancer cells, suggesting an anti-apoptotic role for estrogens.121–123
A topic that needs further discussion is the effect of estrogens in angiogenesis. Angiogenesis is key in cancer development and metastases since NSCLC is a highly vascularized tumor and its progression depends primarily on vascular supply. 124 It has been shown that estrogen triggers tumor angiogenesis through activation of the vascular endothelial growth factor (VEGF), which causes an increase in proliferation of vascular endothelial cells. 125 Estrogen also promotes the production of the vascular endothelial growth factor A (VEGFA), an essential ligand for the vascular endothelial growth factor receptor-2 (VEGFR-2) expressed in endothelial cells. Remarkably, VEGFA is downstream of EGFR, which is estrogen dependent. Therefore, the combination of fulvestrant and vandetanib, inhibitors of EGFR and ER, respectively, decreased cell proliferation and angiogenesis in NSCLC. 125 In addition, recent studies have shown high expression levels of cytoplasmic and nuclear membrane bound estrogen receptors (mERs or GPER) in NSCLC specimens, and blocking these receptors stops estrogen-induced cell proliferation. 126
Progesterone
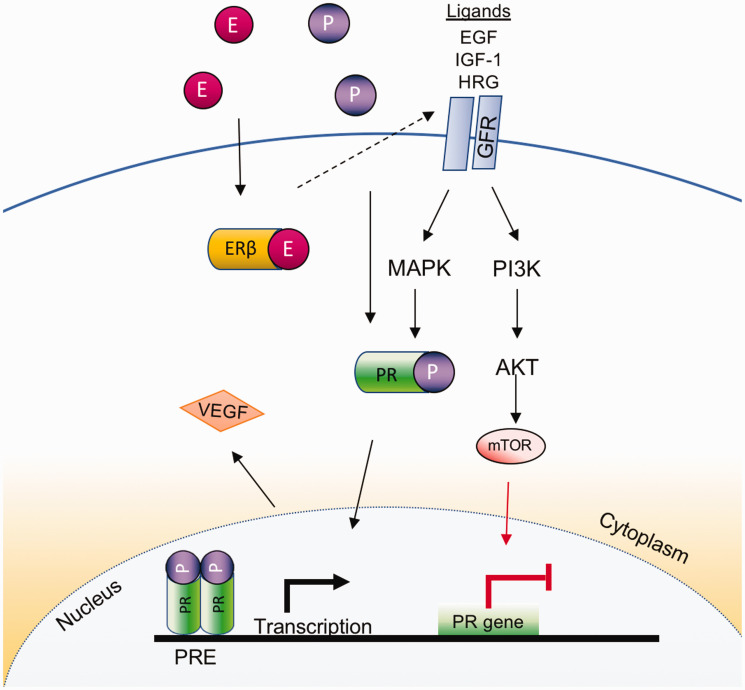
Regarding progesterone, another ovarian hormone involved in cell proliferation and lung disease,40,127 emerging evidence has revealed an active role in lung carcinogenesis. Studies have revealed that progesterone receptors (PRs) are present commonly in non-tumor lung tissues compared with cancer tissue. 129 PRs are localized mostly in both the nucleus and extranuclear areas of the lung.128,129 While researchers have shown that PR expression in tumor-surrounding stromal cells is connected with enhanced survival for both men and women,130,131 it was also revealed that PR expression in tumor epithelial cells is an autonomous, non-favorable predictor for disease-specific survival in women, suggesting that PR expression may be a potential marker for NSCLC. 130 Other studies have proposed that increase in VEGF production and proliferation of endothelial cells are due to a combination of both estrogen and progesterone in NSCLC (Figure 4). 128
Figure 4.
Progesterone/progesterone receptor signaling in lung cancer. The growth factor receptor signaling is stimulated by its ligands (e.g. EGF, IFG-1, HRG), resulting in the activation of MAPK-related pathways and the subsequent stimulation of both (1) the ligand independent receptor activation and (2) the AKT/mTOR pathway, which increases and suppresses PR expression, respectively. In addition, estrogen and progesterone promote VEGF in lung cancer. (A color version of this figure is available in the online journal.)
AKT: AKT Serine/Threonine Kinase 1; E: estrogen; EGF: epidermal growth factor; ERβ: estrogen receptor β; GFR: growth factor receptor; HRG: histidine rich glycoprotein; IGF-1: insulin-like growth factor 1; MAPK: mitogen-activated protein kinases; mTOR: mechanistic target of rapamycin; P: progesterone; PI3K: phosphoinositide-3 kinase; PR: progesterone receptor; PRE: progesterone response elements; VEGF: vascular endothelial growth factor.
Progesterone has also been confirmed to be produced locally in NSCLC surrounding tissues. Expression of progesterone-synthesizing enzymes such as the cholesterol side-chain cleavage enzyme (P450scc), the steroidogenic acute regulatory protein (StAR), and the 3β-hydroxysteroid dehydrogenase (3βHSD) correlates with concentrations of progesterone and PR expression in lung cancer. 131 Further investigation is needed to better understand the multifaceted interaction between these enzymes, progesterone levels and regulatory mechanisms, and lung cancer.
Androgens
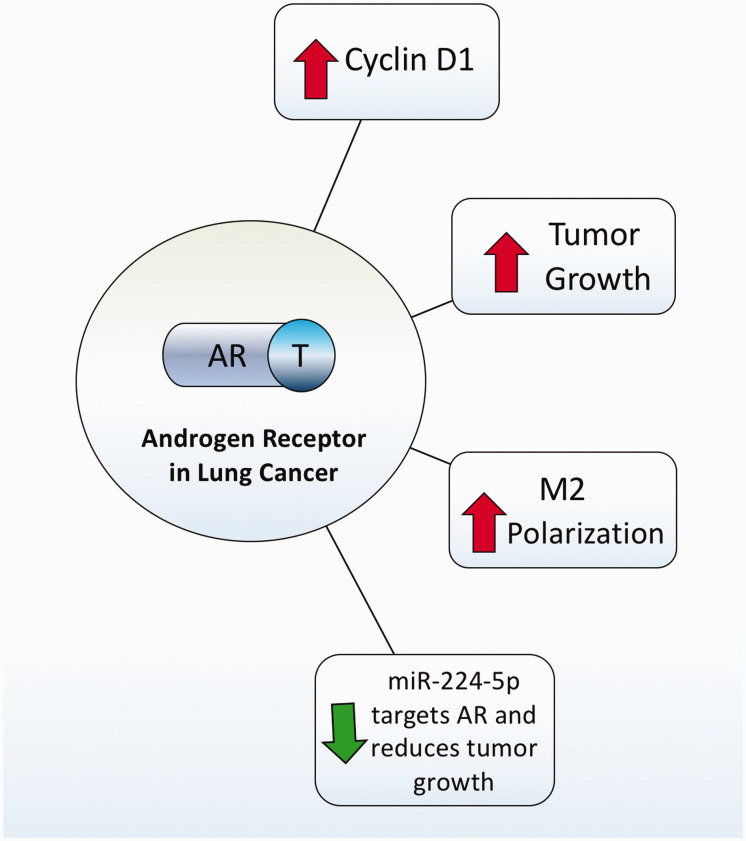
Due to the multiple studies indicating the sex hormones play a role in lung carcinogenesis in female non-smokers, most of the discussion about sex hormones and lung cancer has been centered around estrogens and progesterone. However, the role of male sex hormones in lung cancer has also been reported and studied. In this regard, the androgen receptor (AR), which is expressed mostly in pneumocytes and lung epithelium of male patients, is known to be an active player in lung cancer pathogenesis (Figure 5).132–134 A study using NSCLC cell lines showed that AR inhibition decreases cyclin D1 expression and cell proliferation. 135 Interestingly, researchers created a male inducible AR-KO mice and analyzed tumor formation in the tobacco carcinogens. 136 AR-KO mice reduced tobacco carcinogen-induced tumor size when compared to control suggesting that AR has a central role in the development and progression of lung cancer. Another research group discovered that androgens promote M2 macrophage polarization, which enhances tumor growth and suppresses anti-tumor immune responses.137,138 More recently, it was reported that NSCLC tumor growth was triggered in part by miR-224-5p, which inhibits AR and regulates the epithelial–mesenchymal transition. 139 Overall, more research needs to be done to unveil the cellular and molecular mechanisms of androgens in lung cancer (Figure 5).
Figure 5.
Androgens in lung cancer. Androgen receptor signaling induces cyclin D1 expression, tumor growth, and macrophage M2 polarization. There is also evidence that miR-224-5p negatively targets AR causing a decrease in tumor incidence and growth. (A color version of this figure is available in the online journal.)
AR: androgen receptor; M2: Macrophage M2 polarization; T: testosterone.
Hormone replacement therapy and lung cancer in women
Hormone replacement therapy (HRT) is a common treatment used in postmenopausal women. Due to the effects of female sex hormones on lung cancer tumors discussed above, there are several controversies in the relationship between the HRT and lung cancer.109,110,140 The Women’s Health Initiative trial concluded that treatment with both estrogen and progestin in postmenopausal women did not increase lung cancer incidence. 141 However, HRT was found to increase lung cancer mortality, predominantly, deaths from non-small-cell lung cancer.141,142 These results have led to extensive research on the association of Hormone Therapy (HT) and lung cancer prevalence and outcome.
HRT consists of estrogen alone (estrogen‐only HRT), used in women who have undergone hysterectomy, or estrogen combined with a progestogen (combined HRT) for women who need a progestin to avert endometrial hyperplasia. Taioli et al. observed a 1.7-fold increased risk of lung AC with the use of estrogen replacement therapy, and an even higher risk (OR = 32.4) among women who smoke and receive this type of HRT. 143 In addition, Liu et al. found that induced menopausal women with a history of HRT had a significantly higher risk of lung cancer compared to naturally menopausal women, with a relative risk of 2.40. 144 Other observational studies suggest that estrogen may be related to the cause and outcome of lung cancer, but there has been inconsistency in their findings.142,145–155 We have summarized the most recent studies and their main findings in Table 1. Discussions about risks and benefits considering combined HT are needed for those with a higher risk of developing lung cancer.
Table 1.
Recent clinical studies assessing the role of hormone replacement therapy in lung cancer.
| Study | Study type | Sample size | Conclusions |
|---|---|---|---|
| Brinton et al., 2012 150 | Retrospective cohort study | 118,008 | No evidence that either estrogen therapy-only or estrogen + progestin therapy use is associated with lung cancer risk. |
| Katcoff et al., 2014 148 | Cross-sectional study | 485 | Hormone replacement therapy is associated with improved survival. |
| Clague et al., 2014 149 | Cross-sectional study | 727 | Lung cancer mortality decreased in women who used only estrogen replacement. No association was observed for estrogen + progestin therapy use. |
| Ganti et al. 2016 152 | Retrospective cohort study | 498 | Overall survival was significantly higher in patients with no hormone replacement therapy compared with patients who received it. |
| Chao et al., 2019 153 | Retrospective cohort study | 968,440 | Use of hormone replacement therapy is associated with a decreased risk of lung cancer in women. |
| Titan et al., 2019 154 | Randomized control trial | 75,587 | Hormone replacement therapy has a protective effect on lung cancer development among women. |
| Jeon et al., 2020 155 | Cross-sectional study | 4,775,398 | No statistically significant association was found between reproductive factors and the risk of lung cancer in postmenopausal women. |
| Abdel-Rahman, 2020 151 | Randomized control trial | 77,911 | Prior exposure to hormone replacement therapy is protective against lung cancer development. Similarly, prior exposure to hormone replacement therapy seems to be protective against death from lung cancer. |
Conclusions
The studies summarized in this minireview indicate that there is accruing evidence to support the concept that there are sex differences in the risk of development of lung cancer. The available data support the hypothesis that women are more disposed to the effects of carcinogens in tobacco and tobacco smoke due to hormonal, genetic, and metabolic differences compared to men. Thus, the significance of sex and hormone status as separate contributing factors shall be considered in prognosis and therapeutic management of lung cancers.
Footnotes
AUTHORS’ CONTRIBUTIONS: MSR, NF, and PS conceptualized the review. MSR, NF, and PS reviewed the literature and extracted the data. MSR, NF, and PS wrote and revised the manuscript.
DECLARATION OF CONFLICTING INTERESTS: All authors declare that they have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING: The authors of this study have disclosed receipt of the following financial support for the research, authorship, and publication of this article: Indiana University Research Funds (PS).
ORCID iDs: Nathalie Fuentes https://orcid.org/0000-0002-2075-4219
Patricia Silveyra https://orcid.org/0000-0001-7083-8915
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. Epub ahead of print 4 February 2021. DOI: 10.3322/caac.21660. [DOI] [PubMed]
- 2.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA A Cancer J Clin 2021; 71:7–33 [DOI] [PubMed] [Google Scholar]
- 3.Malhotra J, Malvezzi M, Negri E, La Vecchia C, Boffetta P. Risk factors for lung cancer worldwide. Eur Respir J 2016; 48:889–902 [DOI] [PubMed] [Google Scholar]
- 4.Samet JM, Avila-Tang E, Boffetta P, Hannan LM, Olivo-Marston S, Thun MJ, Rudin CM. Lung cancer in never smokers: clinical epidemiology and environmental risk factors. Clin Cancer Res 2009; 15:5626–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alberg AJ, Brock MV, Ford JG, Samet JM, Spivack SD. Epidemiology of lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143:e1S–e29S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurt OK, Zhang J, Pinkerton KE. Pulmonary health effects of air pollution. Curr Opin Pulm Med 2016; 22:138–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pope CA, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, Thurston GD. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA 2002; 287:1132–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kreuzer M, Gerken M, Heinrich J, Kreienbrock L, Wichmann HE. Hormonal factors and risk of lung cancer among women? Int J Epidemiol 2003; 32:263–71 [DOI] [PubMed] [Google Scholar]
- 9.Hyde Z, Flicker L, McCaul KA, Almeida OP, Hankey GJ, Chubb SA, Yeap BB. Associations between testosterone levels and incident prostate, lung, and colorectal cancer. A population-based study. Cancer Epidemiol Biomarkers Prev 2012; 21:1319–29 [DOI] [PubMed] [Google Scholar]
- 10.Fidler-Benaoudia MM, Torre LA, Bray F, Ferlay J, Jemal A. Lung cancer incidence in young women vs. young men: a systematic analysis in 40 countries. Int J Cancer 2020; 147:811–9 [DOI] [PubMed] [Google Scholar]
- 11.Mattiuzzi C, Lippi G. Current cancer epidemiology. J Epidemiol Glob Health 2019; 9:217–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rivera MP. Lung cancer in women: differences in epidemiology, biology, histology, and treatment outcomes. Semin Respir Crit Care Med 2013; 34:792–801 [DOI] [PubMed] [Google Scholar]
- 13.Stapelfeld C, Dammann C, Maser E. Sex-specificity in lung cancer risk. Int J Cancer 2020; 146:2376–82 [DOI] [PubMed] [Google Scholar]
- 14.Jha P. Avoidable global cancer deaths and total deaths from smoking. Nat Rev Cancer 2009; 9:655–64 [DOI] [PubMed] [Google Scholar]
- 15.Lortet-Tieulent J, Renteria E, Sharp L, Weiderpass E, Comber H, Baas P, Bray F, Coebergh JW, Soerjomataram I. Convergence of decreasing male and increasing female incidence rates in major tobacco-related cancers in Europe in 1988-2010. Eur J Cancer 2015; 51:1144–63 [DOI] [PubMed] [Google Scholar]
- 16.Barta JA, Powell CA, Wisnivesky JP. Global epidemiology of lung cancer. Ann Glob Health 2019; 85:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turner MC, Andersen ZJ, Baccarelli A, Diver Wr Gapstur Sm Pope CA, Prada D, Samet J, Thurston G, Cohen A. Outdoor air pollution and cancer: an overview of the current evidence and public health recommendations. CA Cancer J Clin. Epub ahead of print 25 August 2020. DOI: 103322/caac.21632. [DOI] [PMC free article] [PubMed]
- 18.Krist AH, Davidson KW, Mangione CM, Barry MJ, Cabana M, Caughey AB, Davis EM, Donahue KE, Doubeni CA, Kubik M, Landefeld CS, Li L, Ogedegbe G, Owens DK, Pbert L, Silverstein M, Stevermer J, Tseng CW, Wong JB, Force UPST. Screening for lung cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021; 325:962–70 [DOI] [PubMed] [Google Scholar]
- 19.Ozlü T, Bülbül Y. Smoking and lung cancer. Tuberk Toraks 2005; 53:200–9 [PubMed] [Google Scholar]
- 20.Rivera GA, Wakelee H. Lung cancer in never smokers. Adv Exp Med Biol 2016; 893:43–57 [DOI] [PubMed] [Google Scholar]
- 21.Barrera-Rodriguez R, Morales-Fuentes J. Lung cancer in women. Lung Cancer (Auckl) 2012; 3:79–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ben-Zaken Cohen S, Paré PD, Man SF, Sin DD. The growing burden of chronic obstructive pulmonary disease and lung cancer in women: examining sex differences in cigarette smoke metabolism. Am J Respir Crit Care Med 2007; 176:113–20 [DOI] [PubMed] [Google Scholar]
- 23.Peng J, Meireles SI, Xu X, Smith WE, Slifker MJ, Riel SL, Zhai S, Zhang G, Ma X, Kurzer MS, Ma GX, Clapper ML. Estrogen metabolism in the human lung: impact of tumorigenesis, smoke, sex and race/ethnicity. Oncotarget 2017; 8:106778–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dickinson ME, Flenniken AM, Ji X, Teboul L, Wong MD, White JK, Meehan TF, Weninger WJ, Westerberg H, Adissu H, Baker CN, Bower L, Brown JM, Caddle LB, Chiani F, Clary D, Cleak J, Daly MJ, Denegre JM, Doe B, Dolan ME, Edie Helmut Fuchs SM, Gailus-Durner V, Galli A, Gambadoro A, Gallegos J, Guo S, Horner NR, Hsu CW, Johnson SJ, Kalaga S, Keith LC, Lanoue L, Lawson TN, Lek M, Mark M, Marschall S, Mason J, McElwee ML, Nutter Snlm Peterson KA, Ramirez-Solis R, Rowland DJ, Ryder E, Samocha KE, Seavitt JR, Selloum M, Szoke-Kovacs Z, Tamura M, Trainor AG, Tudose I, Wakana S, Warren J, Wendling O, West DB, Wong L, Yoshiki A, Wurst W, MacArthur DG, Tocchini-Valentini GP, Gao X, Flicek P, Bradley A, Skarnes WC, Justice MJ, Parkinson HE, Moore M, Wells S, Braun RE, Svenson KL, de Angelis MH, Herault Y, Mohun T, Mallon AM, Henkelman RM, Brown SDM, Adams DJ, Lloyd KCK, McKerlie C, Beaudet AL., Murray Mbsa Consortium. Corrigendum: high-throughput discovery of novel developmental phenotypes. Nature 2017; 551:398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Port JL, Yamaguchi K, Du B, De LM, Chang M, Heerdt PM, Kopelovich L, Marcus CB, Altorki NK, Subbaramaiah K, Dannenberg AJ. Tobacco smoke induces CYP1B1 in the aerodigestive tract. Carcinogenesis 2004; 25:2275–81 [DOI] [PubMed] [Google Scholar]
- 26.Mollerup S, Berge G, Baera R, Skaug V, Hewer A, Phillips DH, Stangeland L, Haugen A. Sex differences in risk of lung cancer: expression of genes in the PAH bioactivation pathway in relation to smoking and bulky DNA adducts. Int J Cancer 2006; 119:741–4 [DOI] [PubMed] [Google Scholar]
- 27.Kure EH, Ryberg D, Hewer A, Phillips DH, Skaug V, Baera R, Haugen A. p53 mutations in lung tumours: relationship to gender and lung DNA adduct levels. Carcinogenesis 1996; 17:2201–5 [DOI] [PubMed] [Google Scholar]
- 28.Barta JA, McMahon SB. Lung-enriched mutations in the p53 tumor suppressor: a paradigm for tissue-specific gain of oncogenic function. Mol Cancer Res 2019; 17:3–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang P, Wang Y, Wampfler JA, Xie D, Stoddard SM, She J, Midthun DE. Trends in subpopulations at high risk for lung cancer. J Thorac Oncol 2016; 11:194–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jemal A, Thun MJ, Ries LA, Howe HL, Weir HK, Center MM, Ward E, Wu XC, Eheman C, Anderson R, Ajani UA, Kohler B, Edwards BK. Annual report to the nation on the status of cancer, 1975-2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst 2008; 100:1672–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Escobedo LG, Peddicord JP. Smoking prevalence in US birth cohorts: the influence of gender and education. Am J Public Health 1996; 86:231–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siegfried JM. Women and lung cancer: does oestrogen play a role? Lancet Oncol 2001; 2:506–13 [DOI] [PubMed] [Google Scholar]
- 33.Jamal A, Phillips E, Gentzke AS, Homa DM, Babb SD, King BA, Neff LJ. Current cigarette smoking among adults - United States, 2016. MMWR Morb Mortal Wkly Rep 2018; 67:53–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Townsend EA, Miller VM, Prakash YS. Sex differences and sex steroids in lung health and disease. Endocr Rev 2012; 33:1–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wakelee HA, Wang W, Schiller JH, Langer CJ, Sandler AB, Belani CP, Johnson DH, Group ECO. Survival differences by sex for patients with advanced non-small cell lung cancer on Eastern Cooperative Oncology Group trial 1594. J Thorac Oncol 2006; 1:441–6 [PubMed] [Google Scholar]
- 36.Chen KY, Hsiao CF, Chang GC, Tsai YH, Su WC, Perng RP, Huang MS, Hsiung CA, Chen CJ, Yang PC, Group GS. Hormone replacement therapy and lung cancer risk in Chinese. Cancer 2007; 110:1768–75 [DOI] [PubMed] [Google Scholar]
- 37.Hou L, Wang D, Baccarelli A. Environmental chemicals and microRNAs. Mutat Res 2011; 714:105–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Imkamp K, Bernal V, Grzegorzcyk M, Horvatovich P, Vermeulen CJ, Heijink IH, Guryev V, Kerstjens HAM, van den Berge M, Faiz A. Gene network approach reveals co-expression patterns in nasal and bronchial epithelium. Sci Rep 2019; 9:15835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang X, Sebastiani P, Liu G, Schembri F, Dumas YM, Langer EM, Alekseyev Y, O'Connor GT, Brooks DR, Lenburg ME, Spira A. Similarities and differences between smoking-related gene expression in nasal and bronchial epithelium. Physiol Genomics 2010; 41:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siegfried JM, Stabile LP. Estrongenic steroid hormones in lung cancer. Semin Oncol 2014; 41:5–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siegfried JM. Early changes in pulmonary gene expression following tobacco exposure shed light on the role of estrogen metabolism in lung carcinogenesis. Cancer Prev Res (Phila) ) 2010; 3:692–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cadranel J, Mauguen A, Faller M, Zalcman G, Buisine MP, Westeel V, Longchampt E, Wislez M, Coudert B, Daniel C, Chetaille B, Michiels S, Blons H, Solassol J, De Fraipont F, Foucher P, Urban T, Lacroix L, Poulot V, Quoix E, Antoine M, Danton G, Morin F, Chouaid C, Pignon JP. Impact of systematic EGFR and KRAS mutation evaluation on progression-free survival and overall survival in patients with advanced non-small-cell lung cancer treated by erlotinib in a French prospective cohort (ERMETIC project–part 2). J Thorac Oncol 2012; 7:1490–502 [DOI] [PubMed] [Google Scholar]
- 43.Lei L, Wang WX, Yu ZY, Liang XB, Pan WW, Chen HF, Wang LP, Fang Y, Wang M, Xu CW, Fang MY. A real-world study in advanced non-small cell lung cancer with KRAS mutations. Transl Oncol 2020; 13:329–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beau-Faller M, Texier M, Blons H, Richard N, Escande F, Melaabi S, Lizard S, De Fraipont F, Longchampt E, Morin F, Zalcman G, Pignon JP, Cadranel J. Clinical relevance of EGFR- or KRAS-mutated subclones in patients with advanced non-small-cell lung cancer receiving erlotinib in a French Prospective Cohort (IFCT ERMETIC2 Cohort - Part 2). Clin Lung Cancer 2019; 20:222–30 [DOI] [PubMed] [Google Scholar]
- 45.Dubois C, Rocks N, Blacher S, Primac I, Gallez A, García-Caballero M, Gérard C, Brouchet L, Noël A, Lenfant F, Cataldo D, Pequeux C. Lymph/angiogenesis contributes to sex differences in lung cancer through oestrogen receptor alpha signalling. Endocr Relat Cancer 2019; 26:201–16 [DOI] [PubMed] [Google Scholar]
- 46.Sher T, Dy GK, Adjei AA. Small cell lung cancer. Mayo Clin Proc 2008; 83:355–67 [DOI] [PubMed] [Google Scholar]
- 47.Suster DI, Mino-Kenudson M. Molecular pathology of primary non-small cell lung cancer. Arch Med Res 2020; 51:784–98 [DOI] [PubMed] [Google Scholar]
- 48.Kiyohara C, Ohno Y. Sex differences in lung cancer susceptibility: a review. Gend Med 2010; 7:381–401 [DOI] [PubMed] [Google Scholar]
- 49.North CM, Christiani DC. Women and lung cancer: what is new? Semin Thorac Cardiovasc Surg 2013; 25:87–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schveigert D, Krasauskas A, Didziapetriene J, Kalibatiene D, Cicenas S. Smoking, hormonal factors and molecular markers in female lung cancer. Neoplasma 2016; 63:504–9 [DOI] [PubMed] [Google Scholar]
- 51.Meza R, Meernik C, Jeon J, Cote ML. Lung cancer incidence trends by gender, race and histology in the United States, 1973-2010. PLoS One 2015; 10:e0121323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zang EA, Wynder EL. Differences in lung cancer risk between men and women: examination of the evidence. J Natl Cancer Inst 1996; 88:183–92 [DOI] [PubMed] [Google Scholar]
- 53.Kabir Z, Connolly GN, Clancy L. Sex-differences in lung cancer cell-types? An epidemiologic study in Ireland. Ulster Med J 2008; 77:31–5 [PMC free article] [PubMed] [Google Scholar]
- 54.Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman J, Chirieac LR, D'Amico TA, DeCamp MM, Dilling TJ, Dobelbower M, Doebele RC, Govindan R, Gubens MA, Hennon M, Horn L, Komaki R, Lackner RP, Lanuti M, Leal TA, Leisch LJ, Lilenbaum R, Lin J, Loo BW, Martins R, Otterson GA, Reckamp K, Riely GJ, Schild SE, Shapiro TA, Stevenson J, Swanson SJ, Tauer K, Yang SC, Gregory K, Hughes M. Non-small cell lung cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2017; 15:504–35 [DOI] [PubMed] [Google Scholar]
- 55.Lindeman NI, Cagle PT, Aisner DL, Arcila ME, Beasley MB, Bernicker EH, Colasacco C, Dacic S, Hirsch FR, Kerr K, Kwiatkowski DJ, Ladanyi M, Nowak JA, Sholl L, Temple-Smolkin R, Solomon B, Souter LH, Thunnissen E, Tsao MS, Ventura CB, Wynes MW, Yatabe Y. Updated Molecular Testing Guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: Guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J Mol Diagn 2018; 20:129–59 [DOI] [PubMed] [Google Scholar]
- 56.Chanprapaph K, Vachiramon V, Rattanakaemakorn P. Epidermal growth factor receptor inhibitors: a review of cutaneous adverse events and management. Dermatol Res Pract 2014; 2014:734249–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Landi L, Cappuzzo F. Ceritinib for the treatment of patients with anaplastic lymphoma kinase (ALK)-positive metastatic non-small cell lung cancer. Expert Rev Clin Pharmacol 2016; 9:203–14 [DOI] [PubMed] [Google Scholar]
- 58.Camidge DR, Kim HR, Ahn MJ, Yang JCH, Han JY, Hochmair MJ, Lee KH, Delmonte A, García Campelo MR, Kim DW, Griesinger F, Felip E, Califano R, Spira A, Gettinger SN, Tiseo M, Lin HM, Gupta N, Hanley MJ, Ni Q, Zhang P, Popat S. Brigatinib versus crizotinib in advanced ALK inhibitor-naive ALK-positive non-small cell lung cancer: second interim analysis of the phase III ALTA-1L trial. J Clin Oncol 2020; 38:3592–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tomasini P, Egea J, Souquet-Bressand M, Greillier L, Barlesi F. Alectinib in the treatment of ALK-positive metastatic non-small cell lung cancer: clinical trial evidence and experience with a focus on brain metastases. Ther Adv Respir Dis 2019; 13:1753466619831906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sgambato A, Casaluce F, Maione P, Gridelli C. Targeted therapies in non-small cell lung cancer: a focus on ALK/ROS1 tyrosine kinase inhibitors. Expert Rev Anticancer Ther 2018; 18:71–80 [DOI] [PubMed] [Google Scholar]
- 61.Roskoski R. ROS1 protein-tyrosine kinase inhibitors in the treatment of ROS1 fusion protein-driven non-small cell lung cancers. Pharmacol Res 2017; 121:202–12 [DOI] [PubMed] [Google Scholar]
- 62.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science 2002; 298:1912–34 [DOI] [PubMed] [Google Scholar]
- 63.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC, Cortinovis DL, Leach J, Polikoff J, Barrios C, Kabbinavar F, Frontera OA, De Marinis F, Turna H, Lee JS, Ballinger M, Kowanetz M, He P, Chen DS, Sandler A, Gandara DR, Group OS. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017; 389:255–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mazieres J, Rittmeyer A, Gadgeel S, Hida T, Gandara DR, Cortinovis DL, Barlesi F, Yu W, Matheny C, Ballinger M, Park K. Atezolizumab versus docetaxel in pretreated patients with NSCLC: final results from the randomized phase 2 POPLAR and phase 3 OAK clinical trials. J Thorac Oncol 2021; 16:140–50 [DOI] [PubMed] [Google Scholar]
- 65.Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D, Thomas CA, Barlesi F, Finley G, Kelsch C, Lee A, Coleman S, Deng Y, Shen Y, Kowanetz M, Lopez-Chavez A, Sandler A, Reck M, Group IS. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 2018; 378:2288–301 [DOI] [PubMed] [Google Scholar]
- 66.Conforti F, Pala L, Bagnardi V, Viale G, De Pas T, Pagan E, Pennacchioli E, Cocorocchio E, Ferrucci PF, De Marinis F, Gelber RD, Goldhirsch A. Sex-based heterogeneity in response to lung cancer immunotherapy: a systematic review and meta-analysis. J Natl Cancer Inst 2019; 111:772–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xiao D, Pan H, Li F, Wu K, Zhang X, He J. Analysis of ultra-deep targeted sequencing reveals mutation burden is associated with gender and clinical outcome in lung adenocarcinoma. Oncotarget 2016; 7:22857–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang S, Cowley LA, Liu XS. Sex differences in cancer immunotherapy efficacy, biomarkers, and therapeutic strategy. Molecules 2019; 24:3214–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baik CS, Eaton KD. Estrogen signaling in lung cancer: an opportunity for novel therapy. Cancers (Basel) 2012; 4:969–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, Johnson DH, Group ECO. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002; 346:92–8 [DOI] [PubMed] [Google Scholar]
- 71.Waqar SN, Morgensztern D. Treatment advances in small cell lung cancer (SCLC). Pharmacol Ther 2017; 180:16–23 [DOI] [PubMed] [Google Scholar]
- 72.Tarhini A, Kotsakis A, Gooding W, Shuai Y, Petro D, Friedland D, Belani CP, Dacic S, Argiris A. Phase II study of everolimus (RAD001) in previously treated small cell lung cancer. Clin Cancer Res 2010; 16:5900–7 [DOI] [PubMed] [Google Scholar]
- 73.Sun JM, Kim JR, Do IG, Lee SY, Lee J, Choi YL, Ahn JS, Ahn MJ, Park K. A phase-1b study of everolimus plus paclitaxel in patients with small-cell lung cancer. Br J Cancer 2013; 109:1482–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Botticelli A, Onesti CE, Zizzari I, Cerbelli B, Sciattella P, Occhipinti M, Roberto M, Di Pietro F, Bonifacino A, Ghidini M, Vici P, Pizzuti L, Napoletano C, Strigari L, D'Amati G, Mazzuca F, Nuti M, Marchetti P. The sexist behaviour of immune checkpoint inhibitors in cancer therapy. Oncotarget 2017; 8:99336–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Patel JD. Lung cancer in women. J Clin Oncol 2005; 23:3212–8 [DOI] [PubMed] [Google Scholar]
- 76.Paggi MG, Vona R, Abbruzzese C, Malorni W. Gender-related disparities in non-small cell lung cancer. Cancer Lett 2010; 298:1–8 [DOI] [PubMed] [Google Scholar]
- 77.Harichand-Herdt S, Ramalingam SS. Gender-associated differences in lung cancer: clinical characteristics and treatment outcomes in women. Semin Oncol 2009; 36:572–80 [DOI] [PubMed] [Google Scholar]
- 78.Rosell R, Karachaliou N, Arrieta O. Novel molecular targets for the treatment of lung cancer. Curr Opin Oncol 2020; 32:37–43 [DOI] [PubMed] [Google Scholar]
- 79.Griffin R, Ramirez RA. Molecular targets in non-small cell lung cancer. Ochsner J 2017; 17:388–92 [PMC free article] [PubMed] [Google Scholar]
- 80.Testa U, Castelli G, Pelosi E. Lung cancers: molecular characterization, clonal heterogeneity and evolution, and cancer stem cells. Cancers (Basel) 2018; 10:248–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Crespo P, León J. Ras proteins in the control of the cell cycle and cell differentiation. Cell Mol Life Sci 2000; 57:1613–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ravi RK, Weber E, McMahon M, Williams JR, Baylin S, Mal A, Harter ML, Dillehay LE, Claudio PP, Giordano A, Nelkin BD, Mabry M. Activated raf-1 causes growth arrest in human small cell lung cancer cells. J Clin Invest 1998; 101:153–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Samuels ML, Weber MJ, Bishop JM, McMahon M. Conditional transformation of cells and rapid activation of the mitogen-activated protein kinase cascade by an estradiol-dependent human raf-1 protein kinase. Mol Cell Biol 1993; 13:6241–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jänne PA, Mann H, Ghiorghiu D. Study design and rationale for a randomized, placebo-controlled, double-blind study to assess the efficacy and safety of selumetinib in combination with docetaxel as second-line treatment in patients with KRAS-mutant advanced non-small cell lung cancer (SELECT-1). Clin Lung Cancer 2016; 17:e1–e4 [DOI] [PubMed] [Google Scholar]
- 85.Melosky B, Bradbury P, Tu D, Florescu M, Reiman A, Nicholas G, Basappa N, Rothenstein J, Goffin JR, Laurie SA, Wheatley-Price P, Leighl N, Goss G, Reaume MN, Butts C, Murray N, Card C, Ko J, Blais N, Gray S, Lui H, Brown-Walker P, Kaurah P, Prentice LM, Seymour L. Selumetinib in patients receiving standard pemetrexed and platinum-based chemotherapy for advanced or metastatic KRAS wildtype or unknown non-squamous non-small cell lung cancer: a randomized, multicenter, phase II study. Canadian Cancer Trials Group (CCTG) IND.219. Lung Cancer 2019; 133:48–55 [DOI] [PubMed] [Google Scholar]
- 86.Jancík S, Drábek J, Radzioch D, Hajdúch M. Clinical relevance of KRAS in human cancers. J Biomed Biotechnol 2010; 2010:150960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kodaz H, Taştekin E, Erdoğan B, Hacıbekiroğlu İ, Tozkır H, Gürkan H, Türkmen E, Demirkan B, Uzunoğlu S, Çiçin İ. KRAS mutation in small cell lung carcinoma and extrapulmonary small cell cancer. Balkan Med J 2016; 33:407–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bauml J, Mick R, Zhang Y, Watt CD, Vachani A, Aggarwal C, Evans T, Langer C. Frequency of EGFR and KRAS mutations in patients with non small cell lung cancer by racial background: do disparities exist? Lung Cancer 2013; 81:347–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stabile LP, Dacic S, Land SR, Lenzner DE, Dhir R, Acquafondata M, Landreneau RJ, Grandis JR, Siegfried JM. Combined analysis of estrogen receptor beta-1 and progesterone receptor expression identifies lung cancer patients with poor outcome. Clin Cancer Res 2011; 17:154–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rodriguez-Lara V, Peña-Mirabal E, Baez-Saldaña R, Esparza-Silva AL, García-Zepeda E, Cerbon Cervantes MA, Diaz D, Fortoul TI. Estrogen receptor beta and CXCR4/CXCL12 expression: differences by sex and hormonal status in lung adenocarcinoma. Arch Med Res 2014; 45:158–69 [DOI] [PubMed] [Google Scholar]
- 91.Rodriguez-Lara V, Hernandez-Martinez JM, Arrieta O. Influence of estrogen in non-small cell lung cancer and its clinical implications. J Thorac Dis 2018; 10:482–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, Majem M, Lopez-Vivanco G, Isla D, Provencio M, Insa A, Massuti B, Gonzalez-Larriba JL, Paz-Ares L, Bover I, Garcia-Campelo R, Moreno MA, Catot S, Rolfo C, Reguart N, Palmero R, Sánchez JM, Bastus R, Mayo C, Bertran-Alamillo J, Molina MA, Sanchez JJ, Taron M, Spanning Lung Cancer Group. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 2009; 361:958–67 [DOI] [PubMed] [Google Scholar]
- 93.Pietras RJ, Márquez DC, Chen HW, Tsai E, Weinberg O, Fishbein M. Estrogen and growth factor receptor interactions in human breast and non-small cell lung cancer cells. Steroids 2005; 70:372–81 [DOI] [PubMed] [Google Scholar]
- 94.Levin ER. Bidirectional signaling between the estrogen receptor and the epidermal growth factor receptor. Mol Endocrinol 2003; 17:309–17 [DOI] [PubMed] [Google Scholar]
- 95.Stabile LP, Lyker JS, Gubish CT, Zhang W, Grandis JR, Siegfried JM. Combined targeting of the estrogen receptor and the epidermal growth factor receptor in non-small cell lung cancer shows enhanced antiproliferative effects. Cancer Res 2005; 65:1459–70 [DOI] [PubMed] [Google Scholar]
- 96.Giovannini M, Belli C, Villa E, Gregorc V. Estrogen receptor (ER) and epidermal growth factor receptor (EGFR) as targets for dual lung cancer therapy: not just a case? J Thorac Oncol 2008; 3:684–5 [DOI] [PubMed] [Google Scholar]
- 97.Mollerup S, Jørgensen K, Berge G, Haugen A. Expression of estrogen receptors alpha and beta in human lung tissue and cell lines. Lung Cancer 2002; 37:153–9 [DOI] [PubMed] [Google Scholar]
- 98.Kawai H, Ishii A, Washiya K, Konno T, Kon H, Yamaya C, Ono I, Ogawa J. Combined overexpression of EGFR and estrogen receptor alpha correlates with a poor outcome in lung cancer. Anticancer Res 2005; 25:4693–8 [PubMed] [Google Scholar]
- 99.Jänne PA, Yang JC, Kim DW, Planchard D, Ohe Y, Ramalingam SS, Ahn MJ, Kim SW, Su WC, Horn L, Haggstrom D, Felip E, Kim JH, Frewer P, Cantarini M, Brown KH, Dickinson PA, Ghiorghiu S, Ranson M. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 2015; 372:1689–99 [DOI] [PubMed] [Google Scholar]
- 100.Yu HA, Arcila ME, Rekhtman N, Sima CS, Zakowski MF, Pao W, Kris MG, Miller VA, Ladanyi M, Riely GJ. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res 2013; 19:2240–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kadota K, Eguchi T, Villena-Vargas J, Woo KM, Sima CS, Jones DR, Travis WD, Adusumilli PS. Nuclear estrogen receptor-α expression is an independent predictor of recurrence in male patients with pT1aN0 lung adenocarcinomas, and correlates with regulatory T-cell infiltration. Oncotarget 2015; 6:27505–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Olak J, Colson Y. Gender differences in lung cancer: have we really come a long way, baby? J Thorac Cardiovasc Surg 2004; 128:346–51 [DOI] [PubMed] [Google Scholar]
- 103.Chu SC, Hsieh CJ, Wang TF, Hong MK, Chu TY. Antiestrogen use in breast cancer patients reduces the risk of subsequent lung cancer: a population-based study. Cancer Epidemiol 2017; 48:22–8 [DOI] [PubMed] [Google Scholar]
- 104.Brückl WM, Al-Batran SE, Ficker JH, Wirtz RM, Atmaca A. [ Estrogen receptors and their impact for prognosis and therapy of lung cancer - new insights to an underestimated mechanism. Pneumologie 2015; 69:350–60]. [DOI] [PubMed] [Google Scholar]
- 105.Hsu LH, Liu KJ, Tsai MF, Wu CR, Feng AC, Chu NM, Kao SH. Estrogen adversely affects the prognosis of patients with lung adenocarcinoma. Cancer Sci 2015; 106:51–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stabile LP, Davis AL, Gubish CT, Hopkins TM, Luketich JD, Christie N, Finkelstein S, Siegfried JM. Human non-small cell lung tumors and cells derived from normal lung express both estrogen receptor alpha and beta and show biological responses to estrogen. Cancer Res 2002; 62:2141–50 [PubMed] [Google Scholar]
- 107.Hsu LH, Chu NM, Kao SH. Estrogen, estrogen receptor and lung cancer. Int J Mol Sci 2017; 18:1713–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Smida T, Bruno TC, Stabile LP. Influence of estrogen on the NSCLC microenvironment: a comprehensive picture and clinical implications. Front Oncol 2020; 10:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schabath MB, Wu X, Vassilopoulou-Sellin R, Vaporciyan AA, Spitz MR. Hormone replacement therapy and lung cancer risk: a case-control analysis. Clin Cancer Res 2004; 10:113–23 [DOI] [PubMed] [Google Scholar]
- 110.Schwartz AG, Wenzlaff AS, Prysak GM, Murphy V, Cote ML, Brooks SC, Skafar DF, Lonardo F. Reproductive factors, hormone use, estrogen receptor expression and risk of non small-cell lung cancer in women. J Clin Oncol 2007; 25:5785–92 [DOI] [PubMed] [Google Scholar]
- 111.Dou M, Zhu K, Fan Z, Zhang Y, Chen X, Zhou X, Ding X, Li L, Gu Z, Guo M, Yan M, Deng X, Shen P, Wang S. Reproductive hormones and their receptors may affect lung cancer. Cell Physiol Biochem 2017; 44:1425–34 [DOI] [PubMed] [Google Scholar]
- 112.Musial C, Zaucha R, Kuban-Jankowska A, Konieczna L, Belka M, Marino Gammazza A, Baczek T, Cappello F, Wozniak M, Gorska-Ponikowska M. Plausible role of estrogens in pathogenesis, progression and therapy of lung cancer. Int J Environ Res Public Health 2021; 18:648–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Meireles SI, Esteves GH, Hirata R, Peri S, Devarajan K, Slifker M, Mosier SL, Peng J, Vadhanam MV, Hurst HE, Neves EJ, Reis LF, Gairola CG, Gupta RC, Clapper ML. Early changes in gene expression induced by tobacco smoke: evidence for the importance of estrogen within lung tissue. Cancer Prev Res (Phila ) 2010; 3:707–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Siegfried JM. Smoking out reproductive hormone actions in lung cancer. Mol Cancer Res 2014; 12:24–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fuentes N, Silveyra P. Estrogen receptor signaling mechanisms. Adv Protein Chem Struct Biol 2019; 116:135–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fuentes N, Silveyra P. Endocrine regulation of lung disease and inflammation. Exp Biol Med (Maywood) 2018; 243:1313–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Huang Q, Zhang Z, Liao Y, Liu C, Fan S, Wei X, Ai B, Xiong J. 17β-estradiol upregulates IL6 expression through the ERβ pathway to promote lung adenocarcinoma progression. J Exp Clin Cancer Res 2018; 37:133–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Skov BG, Fischer BM, Pappot H. Oestrogen receptor beta over expression in males with non-small cell lung cancer is associated with better survival. Lung Cancer 2008; 59:88–94 [DOI] [PubMed] [Google Scholar]
- 119.Márquez-Garbán DC, Chen HW, Goodglick L, Fishbein MC, Pietras RJ. Targeting aromatase and estrogen signaling in human non-small cell lung cancer. Ann N Y Acad Sci 2009; 1155:194–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Burns TF, Stabile LP. Targeting the estrogen pathway for the treatment and prevention of lung cancer. Lung Cancer Manag 2014; 3:43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang G, Yanamala N, Lathrop KL, Zhang L, Klein-Seetharaman J, Srinivas H. Ligand-independent antiapoptotic function of estrogen receptor-beta in lung cancer cells. Mol Endocrinol 2010; 24:1737–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liang J, Xie Q, Li P, Zhong X, Chen Y. Mitochondrial estrogen β receptor inhibits cell apoptosis via interaction with bad in a ligand-independent manner. Mol Cell Biochem 2015; 401:71–86 [DOI] [PubMed] [Google Scholar]
- 123.Liao TL, Tzeng CR, Yu CL, Wang YP, Kao SH. Estrogen receptor-β in mitochondria: implications for mitochondrial bioenergetics and tumorigenesis. Ann N Y Acad Sci 2015; 1350:52–60 [DOI] [PubMed] [Google Scholar]
- 124.Daum S, Hagen H, Naismith E, Wolf D, Pircher A. The role of anti-angiogenesis in the treatment landscape of non-small cell lung cancer - new combinational approaches and strategies of neovessel inhibition. Front Cell Dev Biol 2020; 8:610903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Siegfried JM, Gubish CT, Rothstein ME, Henry C, Stabile LP. Combining the multitargeted tyrosine kinase inhibitor vandetanib with the antiestrogen fulvestrant enhances its antitumor effect in non-small cell lung cancer. J Thorac Oncol 2012; 7:485–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu C, Liao Y, Fan S, Fu X, Xiong J, Zhou S, Zou M, Wang J. G-protein-coupled estrogen receptor antagonist G15 decreases estrogen-induced development of non-small cell lung cancer. Oncol Res 2019; 27:283–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sathish V, Martin YN, Prakash YS. Sex steroid signaling: implications for lung diseases. Pharmacol Ther 2015; 150:94–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Marquez-Garban DC, Mah V, Alavi M, Maresh EL, Chen HW, Bagryanova L, Horvath S, Chia D, Garon E, Goodglick L, Pietras RJ. Progesterone and estrogen receptor expression and activity in human non-small cell lung cancer. Steroids 2011; 76:910–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kazmi N, Márquez-Garbán DC, Aivazyan L, Hamilton N, Garon EB, Goodglick L, Pietras RJ. The role of estrogen, progesterone and aromatase in human non-small-cell lung cancer. Lung Cancer Manag 2012; 1:259–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Skjefstad K, Richardsen E, Donnem T, Andersen S, Kiselev Y, Grindstad T, Hald SM, Al-Shibli K, Bremnes RM, Busund LT, Al-Saad ST. Prognostic role of progesterone receptor expression in non-small cell lung cancer patients: gender-related impacts and correlation with disease-specific survival. Steroids 2015; 98:29–36 [DOI] [PubMed] [Google Scholar]
- 131.Ishibashi H, Suzuki T, Suzuki S, Niikawa H, Lu L, Miki Y, Moriya T, Hayashi S, Handa M, Kondo T, Sasano H. Progesterone receptor in non-small cell lung cancer–a potent prognostic factor and possible target for endocrine therapy. Cancer Res 2005; 65:6450–8 [DOI] [PubMed] [Google Scholar]
- 132.Wilson CM, McPhaul MJ. A and B forms of the androgen receptor are expressed in a variety of human tissues. Mol Cell Endocrinol 1996; 120:51–7 [DOI] [PubMed] [Google Scholar]
- 133.Mikkonen L, Pihlajamaa P, Sahu B, Zhang FP, Jänne OA. Androgen receptor and androgen-dependent gene expression in lung. Mol Cell Endocrinol 2010; 317:14–24 [DOI] [PubMed] [Google Scholar]
- 134.Chang C, Lee SO, Yeh S, Chang TM. Androgen receptor (AR) differential roles in hormone-related tumors including prostate, bladder, kidney, lung, breast and liver. Oncogene 2014; 33:3225–34 [DOI] [PubMed] [Google Scholar]
- 135.Lanzino M, Sisci D, Morelli C, Garofalo C, Catalano S, Casaburi I, Capparelli C, Giordano C, Giordano F, Maggiolini M, Andò S. Inhibition of cyclin D1 expression by androgen receptor in breast cancer cells–identification of a novel androgen response element. Nucleic Acids Res 2010; 38:5351–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yeh S-D, Y P-C, Lu H-H, Chang C, Wu C-W. Targeting androgen receptor as a new potential therapeutic approach to battle tobacco carcinogens-induced non-small cell lung cancer. J Transl Med 2012; 10:1–2 22214470 [Google Scholar]
- 137.Becerra-Diaz M, Song M, Heller N. Androgen and androgen receptors as regulators of monocyte and macrophage biology in the healthy and diseased lung. Front Immunol 2020; 11:1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Becerra-Díaz M, Strickland AB, Keselman A, Heller NM. Androgen and androgen receptor as enhancers of M2 macrophage polarization in allergic lung inflammation. J Immunol 2018; 201:2923–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhou J, Wang H, Sun Q, Liu X, Wu Z, Wang X, Fang W, Ma Z. miR-224-5p-enriched exosomes promote tumorigenesis by directly targeting androgen receptor in non-small cell lung cancer. Mol Ther Nucleic Acids 2021; 23:1217–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rodriguez C, Spencer Feigelson H, Deka A, Patel AV, Jacobs EJ, Thun MJ, Calle EE. Postmenopausal hormone therapy and lung cancer risk in the cancer prevention study II nutrition cohort. Cancer Epidemiol Biomarkers Prev 2008; 17:655–60 [DOI] [PubMed] [Google Scholar]
- 141.Chlebowski RT, Schwartz AG, Wakelee H, Anderson GL, Stefanick ML, Manson JE, Rodabough RJ, Chien JW, Wactawski-Wende J, Gass M, Kotchen JM, Johnson KC, O'Sullivan MJ, Ockene JK, Chen C, Hubbell FA, Investigators WHI. Oestrogen plus progestin and lung cancer in postmenopausal women (women's health initiative trial): a post-hoc analysis of a randomised controlled trial. Lancet 2009; 374:1243–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chlebowski RT, Wakelee H, Pettinger M, Rohan T, Liu J, Simon M, Tindle H, Messina C, Johnson K, Schwartz A, Gass M, Wactawski-Wende J. Estrogen plus progestin and lung cancer: follow-up of the Women's Health Initiative Randomized Trial. Clin Lung Cancer 2016; 17:10–7.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Taioli E, Wynder EL. Re: Endocrine factors and adenocarcinoma of the lung in women. J Natl Cancer Inst 1994; 86:869–70 [DOI] [PubMed] [Google Scholar]
- 144.Liu Y, Inoue M, Sobue T, Tsugane S. Reproductive factors, hormone use and the risk of lung cancer among middle-aged never-smoking Japanese women: a large-scale population-based cohort study. Int J Cancer 2005; 117:662–6 [DOI] [PubMed] [Google Scholar]
- 145.Ramnath N, Menezes RJ, Loewen G, Dua P, Eid F, Alkhaddo J, Paganelli G, Natarajan N, Reid ME. Hormone replacement therapy as a risk factor for non-small cell lung cancer: results of a case-control study. Oncology 2007; 73:305–10 [DOI] [PubMed] [Google Scholar]
- 146.Siegfried JM, Hershberger PA, Stabile LP. Estrogen receptor signaling in lung cancer. Semin Oncol 2009; 36:524–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Baik CS, Strauss GM, Speizer FE, Feskanich D. Reproductive factors, hormone use, and risk for lung cancer in postmenopausal women, the Nurses' Health Study. Cancer Epidemiol Biomarkers Prev 2010; 19:2525–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Katcoff H, Wenzlaff AS, Schwartz AG. Survival in women with NSCLC: the role of reproductive history and hormone use. J Thorac Oncol 2014; 9:355–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Clague J, Reynolds P, Henderson KD, Sullivan-Halley J, Ma H, Lacey JV, Chang S, Delclos GL, Du XL, Forman MR, Bernstein L. Menopausal hormone therapy and lung cancer-specific mortality following diagnosis: the California Teachers Study. PLoS One 2014; 9:e103735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Brinton LA, Schwartz L, Spitz MR, Park Y, Hollenbeck AR, Gierach GL. Unopposed estrogen and estrogen plus progestin menopausal hormone therapy and lung cancer risk in the NIH-AARP Diet and Health Study Cohort. Cancer Causes Control 2012; 23:487–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Abdel-Rahman O. Lung cancer incidence and mortality in relationship to hormone replacement therapy use among women participating in the PLCO trial: a post hoc analysis. Int J Clin Oncol 2020; 25:885–91 [DOI] [PubMed] [Google Scholar]
- 152.Ganti AK, Sahmoun AE, Panwalkar AW, Tendulkar KK, Potti A. Hormone replacement therapy is associated with decreased survival in women with lung cancer. J Clin Oncol 2006; 24:59–63 [DOI] [PubMed] [Google Scholar]
- 153.Jin C, Lang B. Hormone replacement therapy and lung cancer risk in women: a meta-analysis of cohort studies: hormone replacement therapy and lung cancer risk. Medicine (Baltimore) 2019; 98:e17532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Titan AL, He H, Lui N, Liou D, Berry M, Shrager JB, Backhus LM. The influence of hormone replacement therapy on lung cancer incidence and mortality. J Thorac Cardiovasc Surg 2020; 159:1546–56.e4 [DOI] [PubMed] [Google Scholar]
- 155.Jeon KH, Shin DW, Han K, Kim D, Yoo JE, Jeong SM, Cho JH. Female reproductive factors and the risk of lung cancer in postmenopausal women: a nationwide cohort study. Br J Cancer 2020; 122:1417–24 [DOI] [PMC free article] [PubMed] [Google Scholar]