Abstract
γ-aminobutyric acid or GABA is an amino acid that functionally acts as a neurotransmitter and is critical to neurotransmission. GABA is also a metabolite in the Krebs cycle. It is therefore unsurprising that GABA and its receptors are also present outside of the central nervous system, including in immune cells. This observation suggests that GABAergic signaling impacts events beyond brain function and possibly human health beyond neurological disorders. Indeed, GABA receptor subunits are expressed in pathological disease states, including in disparate cancers. The role that GABA and its receptors may play in cancer development and progression remains unclear. If, however, those cancers have functional GABA receptors that participate in GABAergic signaling, it raises an important question whether these signaling pathways might be targetable for therapeutic benefit. Herein we summarize the effects of modulating Type-A GABA receptor signaling in various cancers and highlight how Type-A GABA receptors could emerge as a novel therapeutic target in cancer.
Keywords: GABA, GABAA receptors, ion channels, cancer, benzodiazepines
Impact statement
GABA is a neurotransmitter and an amino acid with critical roles in neurotransmission and cell signaling. As a ligand for several receptors, it exerts effects that contribute to cellular differentiation, stem cell and organ development, and neural firing. This mini-review focuses on what is known concerning the presence and function of GABA with respect to Type-A GABA receptors in disparate cancers and the potential of this receptor to be leveraged therapeutically in cancer.
Introduction
GABA was first described in 1950 as an amine present in high concentration in the brain that is synthesized from glutamic acid and biochemically differs from other amines. 1 This discovery was soon followed by the observation that GABA could inhibit the firing of neuronal action potentials. Subsequently, GABA was recognized to be a ligand that exerts its effects by binding to its cognate receptors, which were also identified. 2 We now recognize that GABA functions as a ligand that modulates the activity of at least two distinct classes of receptors: the Type-A GABA receptors (GABAARs), which are chloride anion channels; and the Type-B GABA receptors, which are metabotropic G-protein-coupled receptors. Recently, GABA has also been reported to modulate a voltage-gated potassium channel. 3
GABAergic signaling (i.e. GABA and its interplay with its receptors) contributes to the development of the central nervous system (CNS). A nerve cell synapse is either inhibitory or excitatory, as a consequence of the neurotransmitter type that is released. The neurotransmitter glutamate, for example, functions as the principal excitatory neurotransmitter and exerts its function as a ligand that modulates at least three types of glutamate receptors (NMDA, kainate, and AMPA). The neurotransmitter GABA, on the other hand, is the primary inhibitory neurotransmitter, at least in adults. The balance between GABA’s GABAergic inhibitory activity and glutamate’s glutamatergic excitatory activity regulates the excitability of a nerve cell and its output. Homeostasis is necessary to prevent neuronal dysfunction and disease. Such dysfunction has been implicated in varied CNS disease states, including pathogenesis of anoxic-ischemic injury and epilepsy. 4 GABA signaling is also associated with the inflammatory response following traumatic brain injury and contributes to the neuronal effects of stroke. 4
Importantly, GABA and its varied receptors are found not only in the CNS but throughout the body, including immune cells. Figure 1 illustrates the presence of GABAARs in many human cell types. This ubiquity suggests that the role of GABA and its varied receptors may be far greater than the regulation of firing of action potentials in neurons. Indeed, GABA receptor function has been linked to several critical roles outside the CNS. One of the more studied roles is its contribution to pancreatic function. 5 GABA is synthesized with insulin in the beta-cells of pancreatic islets, while the glucagon-producing alpha-cells contain GABAARs. The fascinating interplay that has evolved between GABA secretion from beta-cells and its binding to GABAAR in alpha-cells acts to regulate glucagon levels. In addition, GABA signaling may contribute to tumorigenesis within the CNS and in systemic organs, potentially mediating crosstalk between normal and cancer tissue to create an ameliorated microenvironment for the tumor and/or drive metabolic processes resulting in growth of cancer.6–9 The expression of GABA and its receptors combined with their possible functions in disparate cancers suggest that they might be therapeutically beneficial targets for the treatment of cancers.
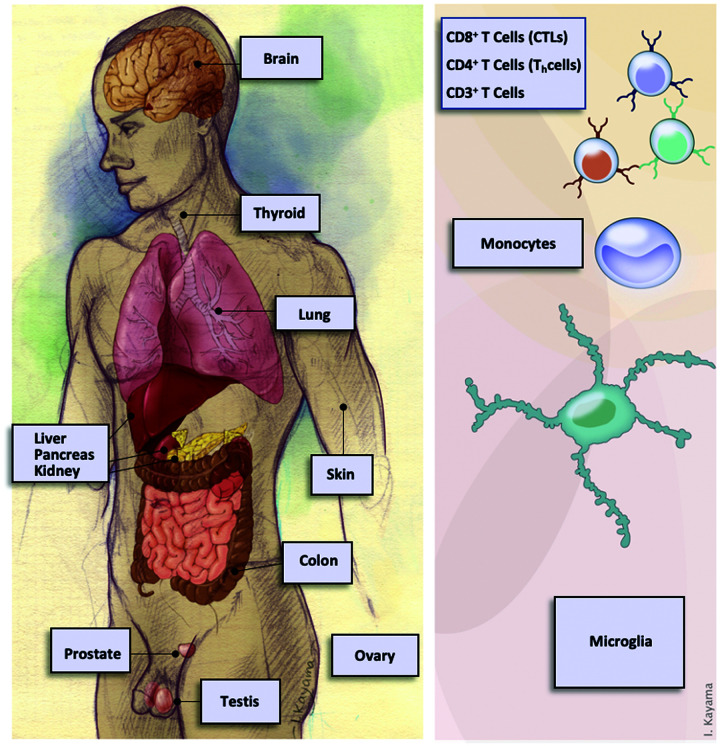
Figure 1.
Expression of Type-A GABA receptor subunit genes in normal human tissues. Genes coding for subunits of Type-A GABA receptor, GABR genes, has been observed in the CNS and from many systemic sources (left). Expression of GABR genes has also been reported in various cells of the immune system (right). In many of these tissues and cells, Type-A GABA receptor activity has been reported and connected to function.
GABAAR structure
At least 19 genes code for different GABAAR subunits.10,11 The complexity of possible GABAARs that may be formed from such a repertoire of genes is further expanded by alternative splicing, use of alternative promoters, and post-translational modification of subunits. At baseline, GABR genes contribute to the assembly of a ligand-gated pentameric chloride anion channel, the GABAAR. Most commonly, GABAARs are composed of two α, two β, and a γ-subunit encoded by GABR genes GABRA (1 to 6), GABRB (1 to 3), and GABRG (1 to 3), respectively. 12 Its five subunits assemble around a central transmembrane hole that forms the chloride anion conduction pore (Figure 2(a)). Structurally, all GABAAR subunits form a similar topology. 12 The two binding sites for the receptor’s endogenous ligand GABA are created at the interface between the α- and β-subunits.
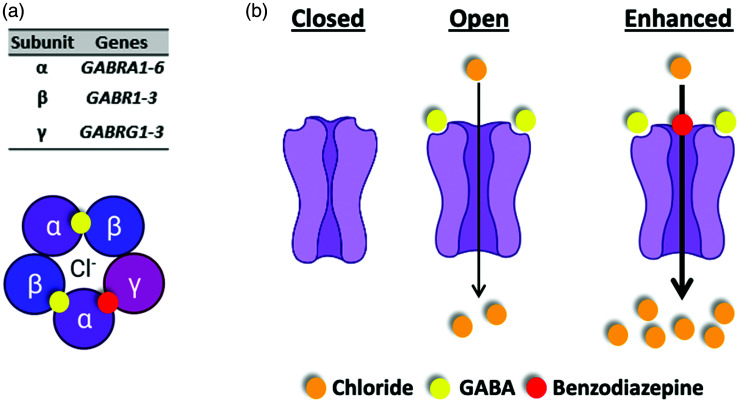
Figure 2.
Type-A GABA receptor function-structure. (a) Type-A GABA receptor is a pentameric assembly with five transmembrane subunits that form a ligand-gated ion channel. Shown are binding sites for two GABA ligands (yellow spheres) and benzodiazepine (red sphere). (b) Type-A GABA receptors function to move chloride anions across the cell membrane in response to the binding of its ligand (agonist) GABA. Benzodiazepines are positive allosteric modulators of the receptors and so act to enhance movement of chloride anions when GABA is bound to the receptor.
GABAAR function in the developing and mature central nervous system
GABA itself is released from pre-synaptic neurons and binds to GABAARs, which are primarily embedded in the post-synaptic neuronal membrane. The binding of GABA to GABAAR acts to promote a chloride anion flux into postsynaptic cells leading to hyperpolarization (Figure 2(b)). This well-studied phenomenon has been a major pharmacologic target since the introduction of the benzodiazepines diazepam (Valium) and chlordiazepoxide (Librium) nearly 70 years ago, which function as positive allosteric modulators, acting to enhance the chloride anion flux through GABAARs in the presence of GABA 12 (Figure 2(b)). What remains less well understood is the role of GABA and GABAAR in the developing neuron and brain structures, their role(s) outside of the central nervous system, and their contribution to brain and systemic pathologies, including cancers.
In the developing brain, the effect of GABA is depolarizing, not hyperpolarizing, and it acts as a principal excitatory, not inhibitory, neurotransmitter. 13 GABA stimulation in immature neurons functions to trigger an efflux of chloride anions, mediated by the triggering of sodium spikes and activating voltage-gated Ca2+ channels. Through this process, GABA acts as a trophic factor during neuronal development and influences cellular events including proliferation, migration, differentiation, synapse maturation, and cell death.14,15 Shortly after birth, a chemical change occurs, historically referred to as the “GABA switch”, which is crucial for neuronal development and activation of neuronal cells. In pre-natal neuronal development, chloride levels are strictly regulated within a range of 15–20 mM, whereas within the first week after birth, the resting chloride concentration drops to 4 mM. 16 The GABA response shifts from excitatory to inhibitory in neurons driven by these changes in intracellular chloride concentrations. Functionally, the equilibrium potential [Cl−]i in neurons shifts from −46 mV at birth to −82 mV at postnatal day 10, which is very close to the depolarization threshold. 17 Ca2+ imaging studies in rodents show that commensurate with this change, activation by GABA results in the accumulation of an intracellular concentration of Ca2+ that is required for downstream signaling, resulting in increased expression of membrane transporters. 13 In immature neurons, the Na+-K+-2Cl− (NKCC1) co-transporter is expressed in the early stages of development and is responsible for the elevated [Cl−]i concentration. The chloride exporter K+-Cl− cotransporter (KCC2), which maintains lower [Cl]i, has delayed expression 17 and thus a major role in the GABA switch from excitatory to inhibitory. 16 The effects of postsynaptic currents are mediated by Ca2+ influx, which leads to an increased KCC2 signal. This signal is described as a new form of GABAAR feedback, resulting in the GABA switch.18–20
GABA and GABAAR function beyond the central nervous system
GABA is present throughout the body and expression of GABAAR subunits has been observed in diverse tissues, including lung, 21 pancreas,5,22,23 kidney, 24 intestine, 25 prostate, 26 testis,10,27 ovary,10,28 liver, 29 thyroid, 30 and skin (melanocytes) 31 (Figure 1). Several studies have highlighted the importance of GABA signaling to cell proliferation, migration, and differentiation.32–34 Still, the importance of GABA and GABAAR to cell signaling beyond synapses remains largely unexplored. Two potentially important roles of GABA and GABAAR that may be linked to their importance in cancer are within the immune system and stem cell development.
GABA and GABAAR appear to contribute to the development and functioning of the mammalian immune system. Nucleated immune cells that express subunits of GABAARs include all the white blood cell types, lymphocytes of the CD4+ and CD8+ T cell lineages, neutrophils, and macrophages.35–37 Antigen-presenting cells (APCs) and T cells both secrete endogenous GABA and possess functional GABAARs (e.g. the receptors exhibit a current in response to GABA). 38 It is important to note that GABA has been reported to function as an immuno-modulator with an ability to either activate or suppress cytokine secretion, modulate T cell proliferation, and alter the migration of T cells. 39 GABAAR in CD3+ T cells, for example, can regulate T cell responses in inflammation. Moreover, pharmacologic agents that modulate GABAergic signaling can stimulate GABAARs present on APCs and macrophages.40,41 For example, blocking GABAAR function prevents pressure-induced macrophage phagocytosis, suggesting that GABAAR plays a role in this process. 40 Studies have also shown that functional GABAARs are present on monocytes, and anesthetics like propofol and thiopental impair monocyte function by directly acting on GABAAR. 40 Blocking GABAAR reverses the inhibition of monocyte migration and phagocytosis induced by anesthetics. 42
Stem cell renewal requires proliferation under sustained maintenance of multipotency. GABA signaling (autocrine or paracrine) through GABAAR inhibits proliferation of embryonic stem (ES) cells and peripheral neural crest stem (NCS) cells and attenuates pre-implantation embryonic growth and proliferation in the stem cell niche. 43 Activation of GABAAR triggers accumulation of stem cells in the S phase, leading to a rapid reduction in cell proliferation. Inhibition of endogenous signaling by the GABAAR antagonist bicuculline or siRNA-mediated knockdown of GABAAR subunits in high-density ES cell cultures significantly increases proliferation of NCS cells. 43 In addition, the GABAAR agonist muscimol rapidly increases phosphorylated histone H2AX (γ-H2AX) levels in the nuclear foci of ES cells. 44 Further, Wang et al. reported that propofol, a GABAAR positive allosteric modulator, inhibits proliferation of rat embryonic NCS cells. 45 These observations position GABAAR as a key regulator during development via control of chromatin structure-function.
Ion channels in cancer
Ion channels, including GABAAR, regulate important physiological functions such as cellular excitability, ion homeostasis, and cell migration. And as noted for GABAARs, ion channel dysfunction contributes to various disorders or channelopathies. Ion channels may also contribute to invasive tumor metastasis and tumor development and progression. 46 Rapidly proliferating cancer cells have a depolarized membrane potential as compared to non-proliferating cells, which can contribute to driving cell proliferation. 47 This was discussed further in Sengupta et al., 48 whereby cancer cells expressing GABAARs were similar electrophysiologically to GABAARs during embryonal development. Further, ion channels orchestrate intracellular signaling such as a sustained influx of Ca2+ ions that trigger downstream signals essential for various intracellular processes, such as activation of transcription factors, release of cytokines, and cell proliferation.49,50 For example, ion channels such as KCa3.2 and Orai1 can enhance the migratory ability of cancer cells, contributing to metastasis in breast and colon cancers.46,51,52 Ion channels can also generate aberrant bioelectric signals that can initiate oncogenic processes. 53
Tumors have developed multiple ways to escape immune surveillance. Tumor microenvironments are highly acidic in nature. Moreover, ion channels (e.g. the P2X family) in cancer cells assist in the production of chemicals such as chemokines and cellular metabolites such as adenosine that help tumors metastasize and evade immune cells. 54 The adenosine pathway is one of the most well-characterized extrinsic mechanisms of resistance to immunotherapy. 55 Adenosine is involved in reducing the function of KCa3.1 channels through the A2A receptor on peripheral blood and tumor-infiltrating CD8+ T lymphocytes (TILs), thereby disabling their migratory abilities during tumor infiltration of head and neck cancer patients. 56 Hypoxic tumor microenvironments downregulate the expression of Kv1.3 channels and reduce their function on TILs in head and neck cancer. Tumor microenvironments have an elevated K+ concentration, which suppresses T cell function. Overexpression of the voltage-gated Kv1.3 channel and Ca2+-dependent KCa3.1 channels in T cells of a mouse melanoma model has been shown to reset the ionic checkpoint by lowering the concentration of K+ ions inside the cells and counteracting T cell suppression by elevated K+ ions. 55
Ion channels and their association with membrane receptors such as integrins have an intricate relationship in cancer development that has been shown to increase tumor malignancy. Several studies in neuronal and leukemic cells show that integrins are involved in differentiation, migration, and neurite extension, and this activity is mediated through ion channel activation. 57 Voltage-gated sodium channels have accessory beta subunits that are altered and detected in the early onset of tumor metastasis, highlighting the critical role of accessory subunits of ion channels in cancer development.58,59
Like other ion channels, GABAARs may contribute to the development and maintenance of cancers. Genes coding for subunits of GABAAR have been reported to have roles in cancers of the central nervous system (gliomas,60–62 medulloblastoma,48,63–65 and neuroblastoma66,67) as well as systemic cancers, including of the lung,9,68,69 breast,70,71 pancreas, 72 liver, 73 colon, 74 prostate, 75 thyroid, 76 ovaries, 30 and skin (melanoma). 31 In many of these cancers, GABRA3, which codes for the α-3 subunit of GABAAR, appears critical. For example, there is enhanced expression of GABRA3 in breast cancer cells and it appears to contribute to its migration and invasive properties.70,71 Specifically, in these cells, GABAAR containing α-3 contributes to the activation of the serine/threonine-specific protein Akt, which has a prominent role in regulating cell proliferation and migration in cancer cells. This may explain the high metastatic propensity of breast cancer cells with enhanced GABRA3 expression.
Pomeroy and his co-workers reported in their analysis of genomic sequencing of medulloblastoma tumors from patients, an enhanced expression of GABRA5, which codes for the α-5 subunit of GABAAR. 63 Interestingly, a more extensive analysis of GABR expression in the four subgroups of medulloblastoma revealed enhanced GABRA3 in another subgroup, and within a subset of patients within another subgroup, a different, unique set of GABR genes were expressed 65 (Figure 3). In a study exploring the importance of GABRA5 in medulloblastoma patients, it was found that knock-down of GABRA5 and ostensibly GABAAR function, significantly reduced the growth of patient-derived medulloblastoma cells. This suggested that GABAAR contributed to the growth of this cancer. 48 It remains to be determined whether the other subgroup-specific set of GABR genes are important in the development of these different medulloblastoma subgroups.
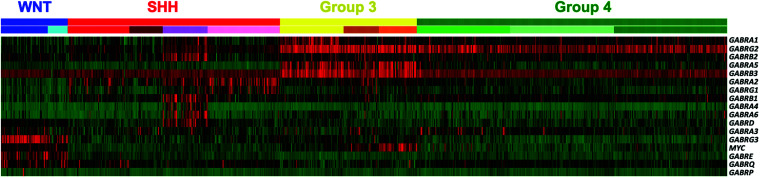
Figure 3.
Expression of Type-A GABA receptor subunit genes in the pediatric brain cancer medulloblastoma. Shown is a heatmap across four molecular subgroups of medulloblastoma (Top row: WNT, SHH, Group 3, and Group 4) and subtypes within each subgroup (lower row), where color scaling indicates low (green) to high (red) expression. In Group 3 patient tumors (yellow), there is enhanced expression of GABRA5, which codes for the α-5 subunit. In contrast, in WNT patient tumors (blue), there is enhanced expression of a different set of GABR genes. While in SHH patient tumors (red) there is a subset of patients (purple) that share an enhanced expression of yet a different set of GABR genes. Figure adapted from Kallay, et al. Modulating native GABAA receptors in medulloblastoma with positive allosteric benzodiazepine-derivatives induces cell death. J Neurooncol 2019;142:411–422.
In addition to GABRA3 and GABRA5, enhanced expression in breast cancer cells of the GABAAR subunit pi, encoded for by GABRP, has been reported. This subunit is incorporated in place of the gamma to form a pentameric channel with an alpha2-beta2-pi1 stoichiometry. In basal-like breast cancer (or BLB-C subtype), GABRP expression is enhanced, 70 appears associated with metastases to the brain, and correlated with poorer prognosis in patients. Mechanistically, GABAAR containing the subunit pi appears to also play a role in maintaining basal-like cytokeratin expression and ERK1/2 phosphorylation and activation, both of which sustain the pro-migratory phenotype of BLB-C subtype cells. This may explain the intrinsic aggressive behavior of BLB-C and the enhanced propensity of visceral metastasis, including to the CNS. Enhanced expression of GABRP has also been noted in pancreatic ductal adenocarcinoma and GABA is reported to stimulate cell proliferation. 72 This phenomenon appears mechanistically to be triggered by activation of the MAPK/ERK pathway, which also contributes to the maintenance of the tumor phenotype and possibly metastasis to the CNS. 72
GABAAR as a therapeutic target in cancer
If GABAergic signaling contributes to the development and/or growth of cancer cells, might it be possible to perturb tumor formation and/or cancer proliferation by disrupting GABAergic signaling? A clinical report from over 35 years ago suggests it may. Kleinerman et al. conducted a retrospective analysis of breast cancer patients and benzodiazepine usage, reporting that use of the benzodiazepine diazepam correlated with reduced primary tumor size and less incidence of lymph node involvement. 77 A possible explanation is that patients taking diazepam fared better because these patients sought and received an anxiolytic (e.g. a benzodiazepine) that helped them in dealing with the understandable anxiety that they were experiencing. Alternatively, the breast cancer tumor cells and/or cells in the tumor microenvironment may have been responsive to diazepam in a way that contributed to an anti-cancer effect.
Recent studies in the pediatric brain cancer medulloblastoma and in melanoma indicate that benzodiazepines have an anti-cancer effect, but how could this be? Interestingly, it was reported in a study of medulloblastoma that a series of benzodiazepine analogs that had a preference to bind to GABRA5 containing GABAAR impaired viability of cells in culture (IC50 1–0.1 micromolar) and induced apoptotic responses in vivo.48,64 Strikingly, the effect in vivo was more significant and specific than standard-of-care chemotherapeutic. 64 Mechanistically, this phenomenon was dependent upon TP53 expression as well as homeobox transcription factor HOXA5, which regulates p53 expression. 48 These results on face value seem counterintuitive, how could a pharmacologic that enhances GABAAR function impair cancer cell viability, while knock-down of GABAAR function elicit the opposite effect? In a follow-up study, exploring details of how benzodiazepines may impair medulloblastoma cells, it was shown by single-cell electrophysiology that the benzodiazepine analogs tested induced a chloride anion efflux from the medulloblastoma cells, which depolarized their mitochondria as well as induced fission 65 (Figure 4). Given that during the development of cells GABAAR is also depolarizing and NKCC1 expression is observed, a contributing role to the development of medulloblastoma may be that these cells have not undergone a “GABA-switch”, which is reflective of the embryonal origin of medulloblastomas. In conclusion, GABAAR may indeed contribute to cancer development, but when chloride anion efflux is significant as a consequence of benzodiazepine binding to GABAAR, then a stress response is elicited that drives an apoptotic response via the intrinsic (mitochondrial) pathway involving the activation of the pro-apoptotic protein BAD, BCL2 associated agonist of death48, 65 (Figure 4).
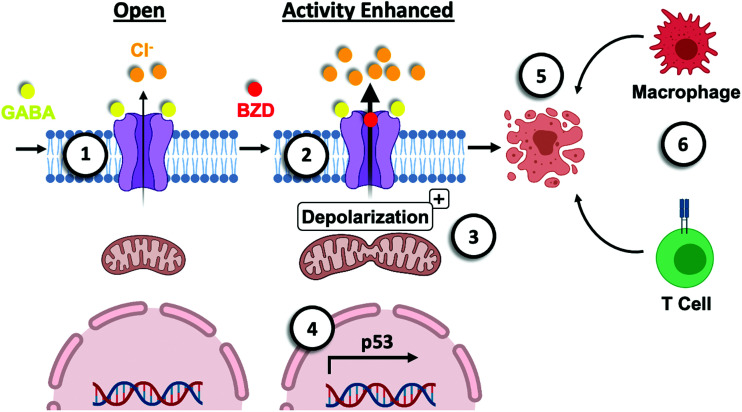
Figure 4.
Model of the mechanism of benzodiazepine-mediated cell death. (1) Binding of GABA (agonist) to a Type-A GABA receptor (GABAAR) “opens” the channel to allow flow of chloride anions out of a cancer cell. This efflux of chloride anions is reflective of the depolarizing nature of the GABAAR in embryonal cells. (2) Benzodiazepines (positive allosteric modulators of the receptor) enhance the chloride efflux. (3) The significant movement of chloride anions contributes to depolarizing of the mitochondria in the cancer cell and induces mitochondrial fission. This may contribute to mitochondrial dysfunction such as release of reactive oxygen and/or nitrogen, as well as impact ATP production. (4) The p53 signaling pathway is activated in these cancer cells in response to perturbation in ion homeostasis. (5) In addition, the intrinsic (mitochondrial) apoptotic pathway is triggered with an associated role for the pro-apoptotic protein BAD, BCL2 associated agonist of death. (6) In addition to binding to resident GABAAR on cancer cells, benzodiazepine binds to the GABAAR on immune cells. This event may contribute to enhanced infiltration of polyfunctional CD8+ T cells and macrophage phagocytosis.
These observations in medulloblastoma are seen in other CNS and systemic cancers. In gliomas, for example, there is a correlation between the expression of certain GABR genes and poor prognosis. 61 While Blanchart et al. found that muscimol, a competitive agonist of GABA derived from the mushroom Amanita muscaria, can regress glioblastoma tumor growth and increase overall survival in a mouse model. 62
Turning to systemic cancer, it was recently reported that melanoma cells possess GABA-responsive GABAARs and benzodiazepines enhance chloride anion transport through the receptors. Importantly, the effect of the benzodiazepines on melanoma cells was similar to that on medulloblastoma cancer cells, benzodiazepines elicited a chloride efflux, which depolarized their mitochondria and induced apoptosis. 31 Interestingly, while the IC50 in culture was not significant (∼1–5 micromolar), the effect in vivo was pronounced. The benzodiazepines mediated a significant regression in tumor size, even at a concentration equivalent to what an adult would take as an anxiolytic. The melanoma mouse model used in these studies was syngeneic and so the role of the immune system in this phenomenon could be analyzed. Interestingly, immuno-profiling of the melanoma tumors revealed enhanced infiltration of immune cells in the tumors of benzodiazepine-treated mice. This may indicate that while benzodiazepines were capable of eliciting apoptotic responses in tumor cells, also contributing to regression of the tumors were immune cells such as macrophages responding to benzodiazepines enhancing their GABAAR function (Figure 4).
Is it reasonable to consider benzodiazepines as an anti-cancer therapeutic in cancers that have functional GABAARs? Clinically, treating a patient with a singular drug approach does not show durable responses in many cancers. Where benzodiazepines may have the greatest impact is as a “sensitizer” of cancer cells. In medulloblastoma, benzodiazepines were capable of sensitizing cancer cells to chemotherapy or radiation. 48 This is critically important as the doses required of both treatment modalities cause cognitive deficits in children. Thus, if it were possible to both increase chemotherapeutic and radiation efficacy while reducing their toxic side-effects, this would be a victory for an “add-on” therapeutic. In the case of metastatic melanoma patients, ∼50% have brain metastases and the median survival of metastatic melanoma is just seven to eight months. Treatment with radiation and immune checkpoint inhibitors is completely inadequate for metastatic melanoma, even though we are seeing positive outcomes for non-metastatic melanoma. 78 Benzodiazepines were shown in a melanoma mouse model to enhance the effectiveness of radiation, even at a sub-lethal dose, and immune checkpoint inhibitors. 31 The data appear promising for benzodiazepines as a potential sensitizer of standard-of-care for melanoma. When melanoma mice were treated with benzodiazepines in combination with radiation and an immune checkpoint inhibitor, there was a complete loss of tumors in most mice.
Conclusions
GABAergic signaling has evolved to serve multiple specialized functions; for example, nociception in the gastric tract, pancreatic beta-cell insulin secretion, and enhancing proliferation of stem cells. Interestingly, the role(s) of GABAergic signaling differ during and post-development. Unfortunately, GABAergic signaling may also contribute to the pathobiology of a wide array of disorders, including CNS and systemic cancers. We have only scratched the surface at understanding how GABAergic signaling may contribute to the development of cancers as well as contribution to the maintenance of a tumor microenvironment in the context of normal tissue. There remain many questions, one being how GABAAR may mediate crosstalk in the tumor microenvironment between non-cancer cells including immune cells and the tumor cells. We have detailed studies that show that by enhancing GABAergic signaling by employing GABAAR positive allosteric modulators such as benzodiazepines, tumor invasiveness, and proliferation of CNS and systemic cancers can be inhibited. In addition, activation or enhancement of GABAAR activity can sensitize cancer cells to radiation, chemotherapeutic, and immune checkpoint inhibitors. A clinically available brain-penetrant anxiolytic that can function to fight cancer should be a welcomed addition to the anti-cancer arsenal.
Footnotes
AUTHORS’ CONTRIBUTIONS: All authors contributed to the writing of the manuscript.
DECLARATION OF CONFLICTING INTERESTS: All author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Thomas E. and Pamela M. Mischell Family Foundation and the Harold C. Schott Foundation funding of the Harold C. Schott Endowed Chair, UC College of Medicine, to S.S.
ORCID iDs: Abigail Koehler https://orcid.org/0000-0002-2961-0836
Daniel Pomeranz Krummel https://orcid.org/0000-0003-3928-0786
References
- 1.Roberts E, Frankel S. Gamma-aminobutyric acid in brain: its formation from glutamic acid. J Biol Chem 1950; 187:55–63 [PubMed] [Google Scholar]
- 2.Bowery N, Smart T. GABA and glycine as neurotransmitters: a brief history. Br J Pharmacol 2006; 147:S109–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manville R, Papanikolaou M, Abbott G. Direct neurotransmitter activation of voltage-gated potassium channels. Nat Commun 2018; 9:1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu C, Sun D. GABA receptors in brain development, function, and injury. Metab Brain Dis 2015; 30:367–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang W, Reyes A, Lan N. Identification of the GABAA receptor subtype mRNA in human pancreatic tissue. FEBS Lett 1994; 346:257–62 [DOI] [PubMed] [Google Scholar]
- 6.Wang T, Huang W, Chen F. Baclofen, a GABAB receptor agonist, inhibits human hepatocellular carcinoma cell growth in vitro and in vivo. Life Sci 2008; 82:536–41 [DOI] [PubMed] [Google Scholar]
- 7.Jiang X, Su L, Zhang Q, He C, Zhang Z, Yi P, Liu J. GABAB receptor complex as a potential target for tumor therapy. J Histochem Cytochem 2012; 60:269–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young S, Bordey A. GABA's control of stem and cancer cell proliferation in adult neural and peripheral niches. Physiology 2009; 24:171–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X, Zhang R, Zheng Y, Shen J, Xiao D, Li J, Shi X, Huang L, Tang H, Liu J, He J, Zhang H. Expression of gamma-aminobutyric acid receptors on neoplastic growth and prediction of prognosis in non-small cell lung cancer. J Transl Med 2012; 11:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hedblom E, Kirkness E. A novel class of GABAA receptor subunit in tissues of the reproductive system. J Biol Chem 1997; 272:15346–50 [DOI] [PubMed] [Google Scholar]
- 11.Everington E, Gibbard A, Swinny J, Seifi M. Molecular characterization of GABA-A receptor subunit diversity within major peripheral organs and their plasticity in response to early life psychosocial stress. Front Mol Neurosci 2018; 11:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olsen R. GABAA receptor: positive and negative allosteric modulators. Neuropharmacology 2018; 136:10–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci 2002; 3:728–39 [DOI] [PubMed] [Google Scholar]
- 14.Owens D, Kriegstein A. Is there more to GABA than synaptic inhibition? Nat Rev Neurosci 2002; 3:715–27 [DOI] [PubMed] [Google Scholar]
- 15.Represa A, Ben-Ari Y. Trophic actions of GABA on neuronal development. Trends Neurosci 2005; 28:278–83 [DOI] [PubMed] [Google Scholar]
- 16.Zamponi G. Tuning the regulator: phosphorylation of KCC2 at two specific sites is critical for neurodevelopment. Sci Signal 2019; 12:eaay8960. [DOI] [PubMed] [Google Scholar]
- 17.Kandler K, Friauf E. Development of glycinergic and glutamatergic synaptic transmission in the auditory brainstem of perinatal rats. J Neurosci 1995; 15:6890–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganguly K, Schinder A, Wong S, Poo M. GABA itself promotes the developmental switch of neuronal GABAergic responses from excitation to inhibition. Cell 2001; 105:521–32 [DOI] [PubMed] [Google Scholar]
- 19.Rivera C, Voipio J, Payne Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl- co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 1999; 397:251–5 [DOI] [PubMed] [Google Scholar]
- 20.Staley K, Smith R. A new form of feedback at the GABA(A) receptor. Nat Neurosci 2001; 4:674–6 [DOI] [PubMed] [Google Scholar]
- 21.Jin N, Guo Y, Sun P, Bell A, Chintagari N, Bhaskaran M, Rains K, Baviskar P, Chen Z, Weng T, Liu L. Ionotropic GABA receptor expression in the lung during development. Gene Expr Patterns 2008; 8:397–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilon P, Bertrand G, Loubatieres-Mariani M, Remacle C, Henquin J. The influence of gamma-aminobutyric acid on hormone release by the mouse and rat endocrine pancreas. Endocrinology 1991; 129:2521–9 [DOI] [PubMed] [Google Scholar]
- 23.Borboni P, Porzio O, Fusco A, Sesti G, Lauro R, Marlier L. Molecular and cellular characterization of the GABAA receptor in the rat pancreas. Mol Cell Endocrinol 1994; 103:157–63 [DOI] [PubMed] [Google Scholar]
- 24.Takano K, Yatabe M, Abe A, Suzuki Y, Sanada H, Watanabe T, Kimura J, Yatabe J. Characteristic expressions of GABA receptors and GABA producing/transporting molecules in rat kidney. PLoS One 2014; 9:e105835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Auteri M, Zizzo M, Serio R. GABA and GABA receptors in the gastrointestinal tract: from motility to inflammation. Pharmacol Res 2015; 93:11–21 [DOI] [PubMed] [Google Scholar]
- 26.Napoleone P, Bronzetti E, Cavallotti C, Amenta F. Predominant epithelial localization of type a gamma-aminobutyric acid receptor sites within rat seminal vesicles and prostate glands. Pharmacology 1990; 41:49–56 [DOI] [PubMed] [Google Scholar]
- 27.Geigerseder C, Doepner R, Thalhammer A, Frungieri M, Gamel-Didelon K, Calandra R, Köhn F, Mayerhofer A. Evidence for a GABAergic system in rodent and human testis: local GABA production and GABA receptors. Neuroendocrinology 2003; 77:314–23 [DOI] [PubMed] [Google Scholar]
- 28.MacKenzie G, Maguire J. The role of ovarian hormone-derived neurosteroids on the regulation of GABAA receptors in affective disorders. Psychopharmacology 2014; 231:3333–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biju M, Pyroja S, Rajeshkumar N, Paulose C. Hepatic GABAA receptor functional regulation during rat liver cell proliferation. Hepatol Res 2001; 21:136–46 [DOI] [PubMed] [Google Scholar]
- 30.Roberts S, Mendonça-Torres M, Jensen K, Francis G, Vasko V. GABA receptor expression in benign and malignant thyroid tumors. Pathol Oncol Res 2009; 15:645–50 [DOI] [PubMed] [Google Scholar]
- 31.Pomeranz Krummel D, Nasti T, Kaluzova M, Kallay L, Bhattacharya D, Melms J, Izar B, Xu M, Burnham A, Ahmed T, Li G, Lawson D, Kowalski J, Cao Y, Switchenko J, Ionascu D, Cook J, Medvedovic M, Jenkins A, Khan M, Sengupta S. Melanoma cell intrinsic GABAA receptor enhancement potentiates radiation and immune checkpoint inhibitor response by promoting direct and T cell-mediated antitumor activity. Int J Radiat Oncol Biol Phys 2021; 109:1040–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leonzino M, Busnelli M, Antonucci F, Verderio C, Mazzanti M, Chini B. The timing of the excitatory-to-inhibitory GABA switch is regulated by the oxytocin receptor via KCC2. Cell Rep 2016; 15:96–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ben-Ari Y, Gaiarsa J, Tyzio R, Khazipov R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev 2007; 87:1215–84 [DOI] [PubMed] [Google Scholar]
- 34.Avoli M, Krnjevic K. The long and winding road to gamma-amino-butyric acid as neurotransmitter. Can J Neurol Sci 2016; 43:219–26 [DOI] [PubMed] [Google Scholar]
- 35.Mendu S, Bhandage A, Jin Z, Birnir B. Different subtypes of GABA-A receptors are expressed in human, mouse and rat T lymphocytes. PLoS One 2012; 7:e42959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bjurstöm H, Wang J, Ericsson I, Bengtsson M, Liu Y, Kumar-Mendu S, Issazadeh-Navikas S, Birnir B. GABA, a natural immunomodulator of T lymphocytes. J Neuroimmunol 2008; 205:44–50 [DOI] [PubMed] [Google Scholar]
- 37.Bhat R, Axtell R, Mitra A, Miranda M, Lock C, Tsien R, Steinman L. Inhibitory role for GABA in autoimmune inflammation. Proc Natl Acad Sci U S A 2010; 107:2580–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tian J, Chau C, Hales T, Kaufman D. GABA(A) receptors mediate inhibition of T cell responses. J Neuroimmunol 1999; 96:21–8 [DOI] [PubMed] [Google Scholar]
- 39.Jin Z, Mendu S, Birnir B. GABA is an effective immunomodulatory molecule. Amino Acids 2013; 45:87–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shiratsuchi H, Kouatli Y, Yu G, Marsh H, Basson M. Propofol inhibits pressure-stimulated macrophage phagocytosis via the GABAA receptor and dysregulation of p130cas phosphorylation. Am J Physiol Cell Physiol 2009; 296:C1400–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim J, Kim Y, Lee H, Jin H, Neupane C, Kim S, Lee S, Min J, Sasai M, Jeong J, Choe S, Kim J, Yamamoto M, Choy H, Park J, Jo E. GABAergic signaling linked to autophagy enhances host protection against intracellular bacterial infections. Nat Commun 2018; 9:4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wheeler D, Thompson A, Corletto F, Reckless J, Loke J, Lapaque N, Grant A, Mastroeni P, Grainger D, Padgett C, O'Brien J, Miller N, Trowsdale J, Lummis S, Menon D, Beech J. Anaesthetic impairment of immune function is mediated via GABA(A) receptors. PLoS One 2011; 6:e17152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andäng M, Hjerling-Leffler J, Moliner A, Lundgren T, Castelo-Branco G, Nanou E, Pozas E, Bryja V, Halliez S, Nishimaru H, Wilbertz J, Arenas E, Koltzenburg M, Charnay P, El Manira A, Ibañez C, Ernfors P. Histone H2AX-dependent GABA(A) receptor regulation of stem cell proliferation. Nature 2008; 451:460–4 [DOI] [PubMed] [Google Scholar]
- 44.Fernando R, Eleuteri B, Abdelhady S, Nussenzweig A, Andäng M, Ernfors P. Cell cycle restriction by histone H2AX limits proliferation of adult neural stem cells. Proc Natl Acad Sci U S A 2011; 108:5837–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang J, Cheng W, Xu T, Yang Z. Propofol induces apoptosis and inhibits the proliferation of rat embryonic neural stem cells via gamma-aminobutyric acid type a receptor. Genet Mol Res 2015; 14:14920–8 [DOI] [PubMed] [Google Scholar]
- 46.Prevarskaya N, Skryma R, Shuba Y. Ion channels in cancer: are cancer hallmarks oncochannelopathies? Physiol Rev 2018; 98:559–621 [DOI] [PubMed] [Google Scholar]
- 47.Yang M, Brackenbury W. Membrane potential and cancer progression. Front Physiol 2013; 4:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sengupta S, Weeraratne S, Sun H, Phallen J, Rallapalli S, Teider N, Kosaras B, Amani V, Pierre-Francois J, Tang Y, Nguyen B, Yu F, Schubert S, Balansay B, Mathios D, Lechpammer M, Archer T, Tran P, Reimer R, Cook J, Lim M, Jensen F, Pomeroy S, Cho Y. α5-GABAA receptors negatively regulate MYC-amplified medulloblastoma growth. Acta Neuropathol 2014; 127:593–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Choi J, Chiang A, Taulier N, Gros R, Pirani A, Husain M. A calmodulin-binding site on cyclin E mediates Ca2+-sensitive G1/s transitions in vascular smooth muscle cells. Circ Res 2006; 98:1273–81 [DOI] [PubMed] [Google Scholar]
- 50.Ouadid-Ahidouch H, Ahidouch A. K+ channel expression in human breast cancer cells: involvement in cell cycle regulation and carcinogenesis. J Membr Biol 2008; 221:1–6 [DOI] [PubMed] [Google Scholar]
- 51.Chantôme A, Potier-Cartereau M, Clarysse L, Fromont G, Marionneau-Lambot S, Guéguinou M, Pagès J, Collin C, Oullier T, Girault A, Arbion F, Haelters J, Jaffrès P, Pinault M, Besson P, Joulin V, Bougnoux P, Vandier C. Pivotal role of the lipid raft SK3-Orai1 complex in human cancer cell migration and bone metastases. Cancer Res 2013; 73:4852–61 [DOI] [PubMed] [Google Scholar]
- 52.Guéguinou M, Harnois T, Crottes D, Uguen A, Deliot N, Gambade A, Chantôme A, Haelters J, Jaffrès P, Jourdan M, Weber G, Soriani O, Bougnoux P, Mignen O, Bourmeyster N, Constantin B, Lecomte T, Vandier C, Potier-Cartereau M. SK3/TRPC1/Orai1 complex regulates SOCE-dependent colon cancer cell migration: a novel opportunity to modulate anti-EGFR mAb action by the alkyl-lipid ohmline. Oncotarget 2016; 7:36168–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tuszynski J, Tilli T, Levin M. Ion channel and neurotransmitter modulators as electroceutical approaches to the control of cancer. Curr Pharm Des 2017; 23:4827–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Di Virgilio F, Adinolfi E. Extracellular purines, purinergic receptors and tumor growth. Oncogene 2017; 36:293–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharma P, Hu-Lieskovan S, Wargo J, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 2017; 168:707–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chimote A, Balajthy A, Arnold M, Newton H, Hajdu P, Qualtieri J, Wise-Draper T, Conforti L. A defect in KCa3.1 channel activity limits the ability of CD8+ T cells from cancer patients to infiltrate an adenosine-rich microenvironment. Sci Signal 2018; 11:eaaq1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arcangeli A. Ion channels and transporters in cancer. 3. Ion channels in the tumor cell-microenvironment cross talk. Am J Physiol Cell Physiol 2011; 301:C762–771 [DOI] [PubMed] [Google Scholar]
- 58.Brackenbury W, Calhoun J, Chen C, Miyazaki H, Nukina N, Oyama F, Ranscht B, Isom L. Functional reciprocity between Na+ channel Nav1.6 and beta1 subunits in the coordinated regulation of excitability and neurite outgrowth. Proc Natl Acad Sci U S A 2010; 107:2283–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brackenbury W, Djamgoz M, Isom L. An emerging role for voltage-gated Na+ channels in cellular migration: regulation of central nervous system development and potentiation of invasive cancers. Neuroscientist 2008; 14:571–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Synowitz M, Ahmann P, Matyash M, Kuhn S, Hofmann B, Zimmer C, Kirchhoff F, Kiwit J, Kettenmann H. GABA(a)-receptor expression in glioma cells is triggered by contact with neuronal cells. Eur J Neurosci 2001; 14:1294–302 [DOI] [PubMed] [Google Scholar]
- 61.Smits A, Jin Z, Elsir T, Pedder H, Nistér M, Alafuzoff I, Dimberg A, Edqvist P, Pontén F, Aronica E, Birnir B. GABA-A channel subunit expression in human glioma correlates with tumor histology and clinical outcome. PLoS One 2012; 7:e37041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blanchart A, Fernando R, Häring M, Assaife-Lopes N, Romanov R, Andäng M, Harkany T, Ernfors P. Endogenous GABAA receptor activity suppresses glioma growth. Oncogene 2017; 36:777–86 [DOI] [PubMed] [Google Scholar]
- 63.Cho Y, Tsherniak A, Tamayo P, Santagata S, Ligon A, Greulich H, Berhoukim R, Amani V, Goumnerova L, Eberhart C, Lau C, Olson J, Bilbertson R, Gajjar A, Delattre O, Kool M, Ligon K, Meyerson M, Mesirov J, Pomeroy S. Integrative genomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. J Clin Oncol 2011; 29:1424–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jonas O, Calligaris D, Methuku K, Poe M, Francois J, Tranghese F, Changelian A, Sieghart W, Ernst M, Krummel D, Cook J, Pomeroy S, Cima M, Agar N, Langer R, Sengupta S. First in vivo testing of compounds targeting group 3 medulloblastomas using an implantable microdevice as a new paradigm for drug development. J Biomed Nanotechnol 2016; 12:1297–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kallay L, Keskin H, Ross A, Rupji M, Moody O, Wang X, Li G, Ahmed T, Rashid F, Stephen M, Cottrill K, Nuckols T, Xu M, Martinson D, Tranghese F, Pei Y, Cook J, Kowalski J, Taylor M, Jenkins A, Pomeranz Krummel D, Sengupta S. Modulating native GABAA receptors in medulloblastoma with positive allosteric benzodiazepine-derivatives induces cell death. J Neurooncol 2019; 142:411–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roberts S, Mori M, Pattee P, Lapidus J, Mathews R, O'Malley J, Hsieh Y, Turner M, Wang Z, Tian Q, Rodland M, Reynolds C, Seeger R, Nagalla S. GABAergic system gene expression predicts clinical outcome in patients with neuroblastoma. J Clin Oncol 2004; 2:4127–34 [DOI] [PubMed] [Google Scholar]
- 67.Hackett C, Quigley D, Wong R, Chen J, Cheng C, Song Y, Wei J, Pawlikowska L, Bao Y, Goldenberg D, Nguyen K, Gustafson W, Rallapalli S, Cho Y, Cook J, Kozlov S, Mao J, Van Dyke T, Kwok P, Khan J, Balmain A, Fan Q, Weiss W. Expression quantitative trait loci and receptor pharmacology implicate Arg1 and the GABA-A receptor as therapeutic targets in neuroblastoma. Cell Rep 2014; 9:1034–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu Y, Guo F, Dai M, Wang D, Tong Y, Huang J, Hu J, Li G. Gammaaminobutyric acid a receptor alpha 3 subunit is overexpressed in lung cancer. Pathol Oncol Res 2009; 15:351–8 [DOI] [PubMed] [Google Scholar]
- 69.Liu L, Yang C, Shen J, Huang L, Lin W, Tan H, Liang W, Shao W, Zhang H, He J. GABRA3 promotes lymphatic metastasis in lung adenocarcinoma by mediating upregulation of matrix metalloproteinases. Oncotarget 2016; 7:32341–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sizemore G, Sizemore S, Seachrist D, Keri R. GABA(A) receptor pi (GABRP) stimulates basal-like breast cancer cell migration through activation of extracellular-regulated kinase 1/2 (ERK1/2). J Biol Chem 2014; 289:24102–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gumireddy K, Li A, Kossenkov A, Sakurai M, Yan J, Li Y, Xu H, Wang J, Zhang P, Zhang L, Showe L, Nishikura K, Huang Q. The mRNA-edited form of GABRA3 suppresses GABRA3-mediated akt activation and breast cancer metastasis. Nat Commun 2016; 7:10715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takehara A, Hosokawa M, Eguchi H, Ohigashi H, Ishikawa O, Nakamura Y, Nakagawa H. Gamma-aminobutyric acid (GABA) stimulates pancreatic cancer growth through overexpressing GABAA receptor pi subunit. Cancer Res 2007; 67:9704–12 [DOI] [PubMed] [Google Scholar]
- 73.Liu Y, Li Y, Guo F, Wang J, Sun R, Hu J, Li G. Gamma-aminobutyric acid promotes human hepatocellular carcinoma growth through overexpressed gamma-aminobutyric acid a receptor alpha 3 subunit. World J Gastroenterol 2008; 14:7175–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thaker P, Yokoi K, Jennings N, Li Y, Rebhun R, Rousseau D, Jr, Fan D, Sood A. Inhibition of experimental Colon cancer metastasis by the GABA-receptor agonist nembutal. Cancer Biol Ther 2005; 4:753–8 [DOI] [PubMed] [Google Scholar]
- 75.Wu W, Yang Q, Fung K, Humphreys M, Brame L, Cao A, Fang Y, Shih P, Kropp B, Lin H. Linking gamma-aminobutyric acid a receptor to epidermal growth factor receptor pathways activation in human prostate cancer. Mol Cell Endocrinol 2014; 383:69–79 [DOI] [PubMed] [Google Scholar]
- 76.Sung H, Yang S, Ju W, Ahn J. Aberrant epigenetic regulation of GABRP associates with aggressive phenotype of ovarian cancer. Exp Mol Med 2017; 49:e335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kleinerman R, Brinton L, Hoover R, Fraumeni JF., Jr. Diazepam use and progression of breast cancer. Cancer Res 1984; 44:1223–5 [PubMed] [Google Scholar]
- 78.Wolchok J, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob J, Cowey C, Lao C, Wagstaff J, Schadendorf D, Ferrucci P, Smylie M, Dummer R, Hill A, Hogg D, Haanen J, Carlino M, Bechter O, Maio M, Marquez-Rodas I, Guidoboni M, McArthur G, Lebbe C, Ascierto P, Long G, Cebon J, Sosman J, Postow M, Callahan M, Walker D, Rollin L, Bhore R, Hodi F, Larkin J. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017; 377:1345–56 [DOI] [PMC free article] [PubMed] [Google Scholar]