Abstract
Objective:
Frailty is a critical aging-related syndrome marked by diminished physiologic reserve and heightened vulnerability to stress, predictive of major adverse clinical outcomes in HIV-infected and uninfected adults. Frailty is adynamic state, yet little data exist on predictors and consequences of frailty transitions.
Design/methods:
Frailty was assessed semiannually among HIV-infected and uninfected persons with prior injection drug use using the five Fried phenotype domains. An inflammatory index score was constructed from IL-6 and soluble TNF-α receptor-1 data. Markov transition models assessed determinants of frailty transitions. Cox proportional hazards models estimated mortality risk.
Results:
Among 1353 AIDS Linked to the IntraVenous Experience participants with 9559 frailty transition assessments, 33% were HIV-infected. Younger age, higher education, employment, reduced comorbidity, HIV virologic suppression, elevated CD4+ nadir (>500cells/μl) and absence of a prior AIDS diagnosis were significantly associated with both reduced frailty progression and greater frailty recovery. Each SD decrease in inflammatory index score was associated with decreased frailty progression [odds ratio 0.78; 95% confidence interval (CI), 0.65, 0.92] and increased frailty recovery (odds ratio 1.29; 95% CI, 1.08, 1.53). Being frail at one of two consecutive visits was associated with increased mortality, compared with maintenance of a nonfrail state. Being frail at both of two consecutive visits demonstrated the highest mortality risk (hazard ratio 3.23; 95% CI, 2.1, 4.96).
Conclusion:
Sustained, and to a lesser degree, intermittent frail states are associated with increased mortality. HIV virologic suppression with earlier antiretroviral therapy, reduced comorbidity, and reduced inflammation may prevent frailty progression and promote frailty recovery, consequently improving survival for persons aging with HIV and persons with prior injection drug use.
Keywords: aging, frailty transitions, HIV, inflammation, injection drug use, mortality
Introduction
With combination antiretroviral therapy (ART), HIV-infected persons are living longer [1,2]. With these shifts, older adults account for an increasingly greater proportion of prevalent HIV cases worldwide, and the number of HIV-infected adults 50 years and older is anticipated to triple over the next 2 decades [3,4]. Despite significant survival gains, severe disparities in morbidity and mortality remain among HIV-infected persons, particularly for persons with a history of injection drug use (PWID) [5,6]. Such disparities have been attributed in large part to a rising burden of adverse aging-related conditions in the HIV-infected population [7–10].
Frailty is a critical aging-related phenotype, characterized by decreased physiologic reserve with diminished homeostasis, decreased resilience and heightened vulnerability to stress, associated with major adverse clinical outcomes among older HIV-uninfected adults [11,12]. Recent studies show that frailty burden is heightened in HIV and predictive of key adverse outcomes including increased hospitalization and death, independent of chronic comorbid disease or HIV disease stage [13–19]. In our own prior studies among PWID, being both frail and HIV-infected was associated with an over seven-fold increased risk of death [17]. Frailty is thus a critical target to reduce disparities and to promote healthy aging in HIV-infected and PWID populations.
Prior studies have shown that frailty is a dynamic state with the potential for both frailty progression over time, as well as opportunities for recovery from a frail to a nonfrail state [20–22]. Understanding the factors that precipitate frailty transitions is critical to inform interventions to reduce the marked frailty-associated disparities observed in HIV and among PWID. However, sparse data exist on determinants of frailty transitions in these populations.
In this study, we sought to characterize the relationship of sociodemographic factors, comorbid disease, HIV clinical factors, and inflammation to transitions between frailty states among HIV-infected and uninfected PWID. Then, we evaluated the relationship of frailty transitions to mortality.
Methods
Study participants
The AIDS Linked to the IntraVenous Experience Cohort (ALIVE) cohort has prospectively followed PWID in a community-recruited cohort on a semiannual basis since 1988. Participants aged at least 18 years were recruited through street-based efforts as previously detailed [23]. The ALIVE study has been continually approved by the Johns Hopkins Institutional Review Board.
Frailty assessment
Frailty assessments were incorporated into ALIVE in 2005 and subsequently assessed semiannually as previously described using the five Fried physical frailty phenotype criteria: slow gait, decreased grip strength (weakness), poor endurance (exhaustion), low physical activity, and physical shrinking (weight loss) [17,24].
Other measures/covariates
Additional detailed information obtained at each visit included socioeconomic, behavioral, and clinical parameters for the prior 6-month period as previously described [17,19]. Comorbid conditions ascertained included obesity (BMI≥30) and participant self-report of any provider diagnosis of diabetes, hypertension, or cerebrovascular, cardiovascular, renal, chronic lung, malignant, or liver disease. Depressive symptoms were assessed using the Center for Epidemiological Studies Depression Scale [25]. Hazardous alcohol use was assessed using the Alcohol Use Disorders Identification Test [26]. Participants provided blood specimens for testing, which included antibodies to HIV-1 assayed by ELISA, with Western blot confirmation. CD4+ cell counts were measured on HIV-infected persons at each visit using flow cytometry, and plasma HIV-1 RNA levels determined using reverse-transcriptase PCR methods (Roche Amplicor, Branchburg, New Jersey, USA; limit of detection: 50 copies/ml). Mortality was assessed through linkage to the National Death Index with review of death certificates to confirm correct matches.
Inflammatory index assessment
IL-6 and soluble tumor necrosis factor receptor 1 (sTNFR1) were measured on 800 participants with available specimens at three study visits, each 1 year apart. Testing was performed on serum samples collected and stored at −80°C using ELISA kits (R&D Systems, Minneapolis, Minnesota, USA). IL-6 was measured using a High-Sensitivity Quantikine kit with a detection range of 0.156–10.0 pg/ml and interassay coefficient of variance of 5.7%. sTNFR1 was measured using a DuoSet ELISA kit with a sensitivity of 12.5 pg/ml and an interassay coefficient of variance of 4.9%. Measurements were performed in duplicate and repeated if the measures differed by more than 15% or were out of the measurable range. The average of the two measures was log-transformed to account for nonnormal distribution, as previously performed. The inflammatory index score (IIs) was constructed from the IL-6 and sTNFR1 measurements as previously described and validated (1/3 log IL-6+2/3 log sTNFR1) [19,27].
Statistical analysis
Frailty was considered as both a three-tier categorical variable: robust, 0; prefrail, 1–2; frail, at least three criteria, and as a binary variable: nonfrail, 0–2 and frail, at least three criteria. Markov transition models were employed to evaluate the factors associated with frailty progression: namely, the likelihood of transitioning from a robust to a frail state compared with maintenance of a robust state, as well as the likelihood of transitioning from a nonfrail to a frail state compared with maintenance of a nonfrail state. We also evaluated factors associated with frailty recovery: namely, the likelihood of transitioning from a frail to robust state and from a frail to nonfrail state compared with maintenance of a frail state. Multinomial regression was employed to assess transitions from one frailty state to the next consecutive frailty state as a function of individual covariates prior to each transition, using models in which current frailty status was regressed on prior frailty status, covariates and interactions between prior status and covariates. Only statistically significant interactions were retained. Age, sex, and race were included a priori. We note here that a ‘transition’ could be from a given state to that same state. To additionally evaluate the independent relationships of baseline IIs and 1-year interval change in IIs to frailty status at 1 year, multinomial logistic regression was employed. This score was approximately normally distributed (Supplemental Fig. 1, http://links.lww.com/QAD/B701). To evaluate the relationship between the frailty transition between two adjacent visits and subsequent mortality (time from the latter visit), Kaplan–Meier survival analyses and Cox proportional hazards regression models were performed. First, we performed analyses with observation from the time of first frailty transition assessment (baseline) until date of death or for those remaining alive, 31 December 2014. Then, we performed mortality analyses treating each adjacent-visits frailty transition assessment as a time-varying covariate. Models were adjusted for factors previously found or considered a priori to be associated with mortality [17,19]. The proportional hazards assumption was confirmed using Schoenfeld residuals. Analyses were performed using STATA (version 15; Stata Corp., College Station, Texas, USA).
Results
Study population and frailty transitions
Among 1353 participants, the mean age at initial frailty assessment was 48 years and 33% were HIV-infected (Table 1). Over a median number of eight visits [interquartile range (IQR) 5, 11; N visits = 10 912], there were 9559 frailty transition assessments (Supplemental Table 1, http://links.lww.com/QAD/B701). Of all transitions from a nonfrail state, 8% constituted progression to a frail state; 3.4% of robust transitions constituted progression to a frail state; 68% of frail transitions comprised recovery from a frail to a nonfrail state; 7.5% of frail transitions comprised recovery from a frail to a robust state; 49.6% remained robust; and 32% remained frail. There were 61 deaths before the first transition could be observed.
Table 1.
Baseline characteristics of AIDS Linked to the IntraVenous Experience participants by index frailty transition statea.
| Stable nonfrail N = 1081 | Frail to nonfrail N = 101 | Nonfrail to frail N = 113 | Stable frail N = 58 | |
|---|---|---|---|---|
| All participants | ||||
| Age, mean (SD) (years) | 47.2 (7.8) | 48.8 (9) | 48.5 (7.5) | 50.8 (7.0) |
| Female | 335 (31.0) | 42 (41.6) | 49 (43.4) | 26 (44.8) |
| African American | 955 (88.3) | 93 (92.1) | 99 (87.6) | 53 (91.4) |
| ≥High school education | 462 (42.9) | 46 (45.5) | 44 (38.9) | 14 (24.1) |
| Employed | 276 (25.6) | 21 (20.8) | 14 (12.4) | 7 (12.1) |
| No hazardous alcohol useb | 838 (77.5) | 78 (77.2) | 82 (72.6) | 41 (70.7) |
| Recent injection drug use | 473 (43.8) | 45 (44.6) | 42 (37.2) | 24 (41.4) |
| Recent tobacco use | 884 (82.0) | 90 (89.1) | 95 (84.1) | 50 (86.2) |
| Prescription drug abuse | 107 (9.9) | 15 (14.9) | 19 (16.8) | 11 (19.0) |
| Any noninjection drug use | 489 (45.2) | 51 (50.5) | 55 (48.7) | 29 (50.0) |
| No depressive symptomsc | 868 (80.3) | 64 (63.4) | 82 (72.6) | 30 (51.7) |
| No of comorbid conditionsd | ||||
| ≥3 | 85 (8.0) | 15 (15.2) | 14 (13.0) | 14 (26.9) |
| 2 | 163 (15.4) | 16 (16.2) | 18 (16.7) | 10 (19.2) |
| 1 | 352 (33.2) | 36 (36.4) | 42 (38.9) | 16 (30.8) |
| 0 | 461 (43.5) | 32 (32.3) | 34 (31.5) | 12 (23.1) |
| HIV seropositive | 348 (32.2) | 32 (31.7) | 42 (37.2) | 25 (43.1) |
| HIV-infected participants | ||||
| CD4+ cell count (cells/μl), median (IQR) | 359 (213, 591) | 450 (280, 784) | 371 (191, 691) | 360 (262, 611) |
| HIV RNA, median (IQR), log10 copies/ml | 2.46 (1.60, 4.36) | 2.21 (1.60, 4.47) | 2.02 (1.60, 4.20) | 3.43 (1.76, 4.31) |
| Median CD4+ nadir (IQR) | 143 (57, 260) | 230 (71, 322) | 131 (52, 248) | 226 (87, 280) |
| Prior AIDS diagnosis | 61 (5.6) | 9 (8.9) | 12 (10.6) | 8 (13.8) |
IQR, interquartile range; SD, standard deviation.
Data are number (%) of participants prior to transition, unless otherwise indicated; behavioral variables reflect characteristics within the prior 6 months, unless otherwise indicated; nonfrail participants had a frailty score of 0–2; frail participants had a frailty score of 3–5.
No hazardous alcohol use, score of <8 on the Alcohol Use Disorders Identification Test.
No depressive symptoms, score <21 on the Center for Epidemiological Studies Depression Scale.
Diabetes, hypertension, cerebrovascular accident, cardiovascular disease, renal disease, chronic obstructive pulmonary disease, cancer, obesity, liver disease.
Sociodemographic and comorbid disease factors associated with frailty progression and recovery
In multivariable analysis, younger age, having attained a high school education or greater, being employed, not having depressive symptoms, and the absence of hazardous alcohol use were significantly associated with a reduced likelihood of frailty progression and increased likelihood of recovery to a nonfrail or robust state (Table 2, Model A). Having fewer than three chronic comorbid disease conditions was also significantly associated with reduced frailty progression and increased recovery to a nonfrail or robust state. Of these conditions, diabetes, hypertension, obesity, prior stroke, and kidney disease were all significantly and independently associated with increased frailty progression and reduced frailty recovery (Supplemental Table 2, http://links.lww.com/QAD/B701). Active injection drug use was associated with reduced frailty progression and increased frailty recovery. Adjusting for sociodemographic and behavioral factors and number of comorbid disease conditions, being HIV infected was significantly associated with greater frailty progression and reduced frailty recovery.
Table 2.
Factors associated with frailty progression and recovery in the AIDS Linked to the IntraVenous Experience Cohorta.
| Frailty progression |
Frailty recovery |
|||
|---|---|---|---|---|
| Robust to frailb OR (95% CI) | Nonfrail to frailc OR (95% CI) | Frail to robustd OR (95% CI) | Frail to nonfraile OR (95% CI) | |
| Model Af | ||||
| Age (per 5-year decrease) | 1.01 (0.85, 1.21) | 0.93 (0.87, 0.98) | 1.28 (1.08, 1.52) | 1.08 (1.02, 1.14) |
| Female | 1.17 (0.93, 1.48) | 1.18 (0.95, 1.48) | 0.85 (0.67, 1.07) | 1.27 (0.90, 1.80) |
| African American | 0.81 (0.54, 1.19) | 0.93 (0.67, 1.29) | 1.23 (0.83, 1.82) | 1.07 (0.78, 1.48) |
| ≥High school education | 0.80 (0.65, 0.98) | 0.87 (0.73, 1.04) | 1.25 (1.02, 1.54) | 1.15 (0.96, 1.38) |
| Employed | 0.49 (0.38, 0.64) | 0.56 (0.44, 0.72) | 2.03 (1.56, 2.64) | 1.79 (1.39, 2.30) |
| No hazardous alcohol useg | 0.82 (0.64, 1.02) | 0.82 (0.68, 0.99) | 1.22 (0.97, 1.53) | 1.22 (1.01, 1.47) |
| Recent injection drug use | 1.24 (0.75, 2.04) | 0.83 (0.69, 0.99) | 1.30 (1.05, 1.60) | 1.21 (1.01, 1.46) |
| No depressive symptomsh | 0.47 (0.38, 0.59) | 0.61 (0.51, 0.73) | 2.11 (1.68, 2.63) | 1.64 (1.37, 1.96) |
| No. of comorbid conditionsi | ||||
| ≥3 | Ref | Ref | Ref | Ref |
| 2 | 0.60 (0.44, 0.80) | 0.63 (0.50, 0.80) | 1.70 (1.27, 2.28) | 1.59 (1.26, 2.00) |
| 1 | 0.47 (0.35, 0.63) | 0.55 (0.44, 0.70) | 2.15 (1.60, 2.88) | 1.80 (1.43, 2.27) |
| 0 | 0.34 (0.25, 0.48) | 0.41 (0.32, 0.53) | 2.92 (2.11, 4.04) | 2.44 (1.88, 3.17) |
| HIV positive | 1.37 (1.11, 1.71) | 1.23 (1.03, 1.47) | 0.73 (0.58, 0.90) | 0.81 (0.68, 0.97) |
| Model Bj | ||||
| HIV+, VL+ | Ref | Ref | Ref | Ref |
| HIV+, VL UD | 0.70 (0.50, 0.98) | 0.74 (0.56, 0.99) | 1.43 (1.02, 2.00) | 1.34 (1.01, 1.78) |
| HIV negative | 0.61 (0.47, 0.80) | 0.71 (0.57, 0.88) | 1.63 (1.25, 2.12) | 1.41 (1.14, 1.75) |
| Model Cj | ||||
| HIV+, nadir <200 | Ref | Ref | Ref | Ref |
| HIV+, nadir 200–350 | 0.83 (0.55, 1.27) | 0.89 (0.64, 1.23) | 1.19 (0.79, 1.81) | 1.13 (0.81, 1.56) |
| HIV+, nadir 350–500 | 1.09 (0.52, 2.28) | 1.20 (0.66, 2.16) | 0.92 (0.44, 1.93) | 0.84 (0.46, 1.50) |
| HIV+, nadir >500 | 0.29 (0.10, 0.89) | 0.33 (0.13, 0.89) | 3.35 (1.10, 10.2) | 3.00 (1.13, 7.99) |
| HIV negative | 0.68 (0.50, 0.92) | 0.75 (0.58, 0.96) | 1.47 (1.09, 1.99) | 1.34 (1.04, 1.72) |
| Model Dj | ||||
| HIV+, AIDS | Ref | Ref | Ref | Ref |
| HIV+, no AIDS | 0.60 (0.39, 0.93) | 0.65 (0.47, 0.92) | 1.68 (1.09, 2.61) | 1.53 (1.09, 2.15) |
| HIV negative | 0.49 (0.33, 0.74) | 0.59 (0.43, 0.81) | 2.07 (1.37, 3.11) | 1.70 (1.24, 2.33) |
CI, confidence interval; OR, odds ratio; UD, undetectable, <50 HIV RNA copies/ml; VL, HIV viral load. Bold text indicates P<0.05.
Data presented are adjusted ORs (95% CIs); robust participants had a frailty score of 0; prefrail participants had a frailty score of 1–2; frail participants had a frailty score of 3–5; nonfrail participants had a frailty score of 0–2.
OR for transition to frail state compared with maintaining robust state.
OR for transition to frail state compared with maintaining nonfrail state.
OR for transition to robust state compared with maintaining frail state.
OR for transition to nonfrail state compared with maintaining frail state.
Adjusted for the demographic, behavioral, and clinical factors listed.
No hazardous alcohol use, score <8 on the Alcohol Use Disorders Identification Test.
No depressive symptoms, score <21 on the Center for Epidemiological Studies Depression Scale.
Diabetes, hypertension, cerebrovascular accident, cardiovascular disease, renal disease, chronic obstructive pulmonary disease, cancer, obesity, liver disease.
Adjusted for the demographic, behavioral, and clinical factors listed in Model A.
HIV clinical factors associated with frailty progression and recovery
We next evaluated the relationship of HIV clinical factors to frailty progression and recovery. Compared with HIV-infected patients with detectable viremia, achieving HIV virologic suppression was significantly associated with reduced frailty progression and increased recovery to a nonfrail or robust state, with point estimates approaching that of what was observed for HIV negative individuals (Table 2, Model B). Having a high CD4+ nadir (>500 cells/μl) also was significantly associated with reduced frailty progression and increased frailty recovery (Table 2, Model C). Finally, compared with having a prior AIDS diagnosis, not having had a prior AIDS diagnosis was significantly associated with reduced frailty progression and increased recovery to a nonfrail or robust state (Table 2, Model D). Overall, these results appeared clinically meaningful, ranging from 30 to 70% decreased odds of frailty progression and 34–300% increased odds of frailty recovery for those with less advanced HIV disease, relative to an advanced HIV disease state.
Role of inflammation in frailty progression and recovery
In multivariable analyses, for each SD decrease in the IIs, there was a 22% reduced likelihood of progression from a nonfrail to frail state and 30% reduced likelihood of progression from a robust to frail state (Table 3). Conversely, for each SD decrease in the IIs, there was a 29% increased likelihood of recovery from a frail to nonfrail state and 44% increased likelihood of recovery from a frail to robust state. In further multivariable analysis, a lower IIs at baseline and a 1-year interval decrease in IIs were both independently associated with a lower likelihood of being frail at 1 year (Supplemental Table 3, http://links.lww.com/QAD/B701). The interaction terms between inflammation and HIV status for these models were not statistically significant. Further, when using more refined estimates of HIV clinical status, the risk estimates for the relationships between inflammation and these frailty transitions remained unchanged and were in fact, remarkably stable irrespective of HIV clinical parameters (Supplemental Fig. 2, http://links.lww.com/QAD/B701).
Table 3.
Odds of frailty progression and recovery by level of inflammationa.
| Frailty progression |
Frailty recovery |
|||
|---|---|---|---|---|
| Robust to frailb OR (95% CI) | Nonfrail to frailc OR (95% CI) | Frail to robustd OR (95% CI) | Frail to nonfraile OR (95% CI) | |
| Inflammatory index score (per 1 SD decrease) | 0.70 (0.57, 0.85) | 0.78 (0.65, 0.92) | 1.44 (1.18, 1.75) | 1.29 (1.08, 1.53) |
CI, confidence interval; OR, odds ratio; SD, standard deviation. Bold text indicates P<0.05.
Data are adjusted ORs (95% CIs); robust participants had a frailty score of 0; prefrail participants had a frailty score of 1–2; frail participants had a frailty score of 3–5; nonfrail participants had a frailty score of 0–2; each model was adjusted for age, sex, race, education, employment, hazardous alcohol use, active injection drug use, depressive symptoms, no. of comorbid conditions, and HIV status.
OR for transition to frail state compared with maintaining robust state.
OR for transition to frail state compared with maintaining nonfrail state.
OR for transition to robust state compared with maintaining frail state.
OR for transition to nonfrail state compared with maintaining frail state.
Frailty transitions and mortality
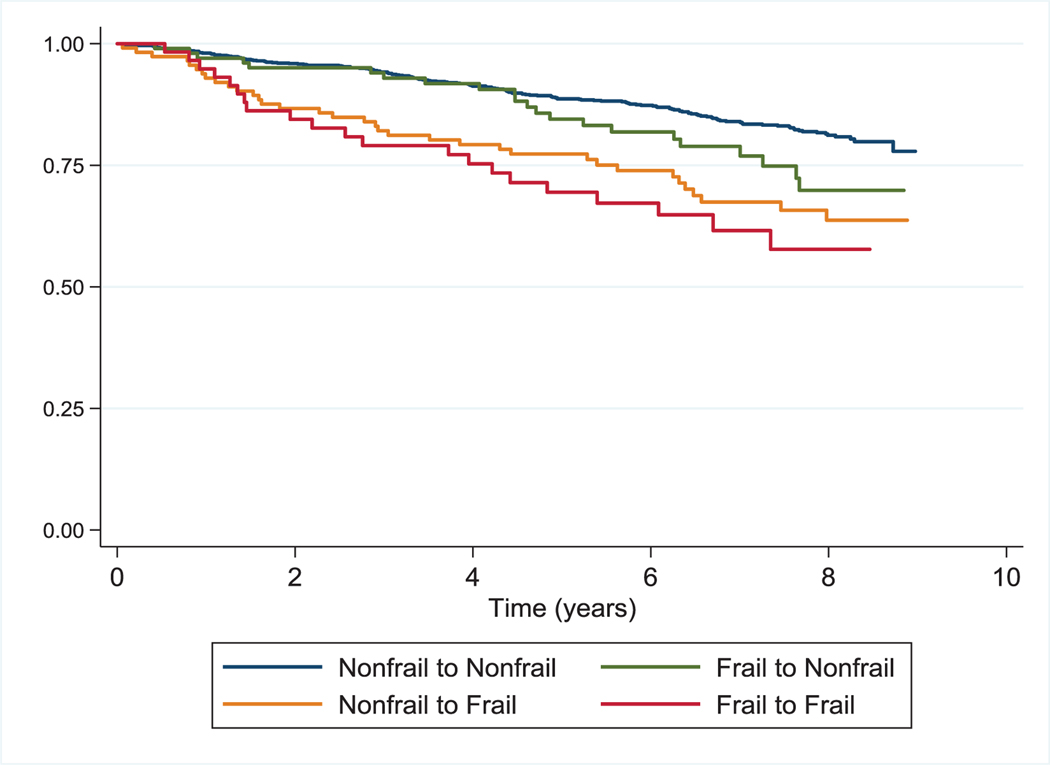
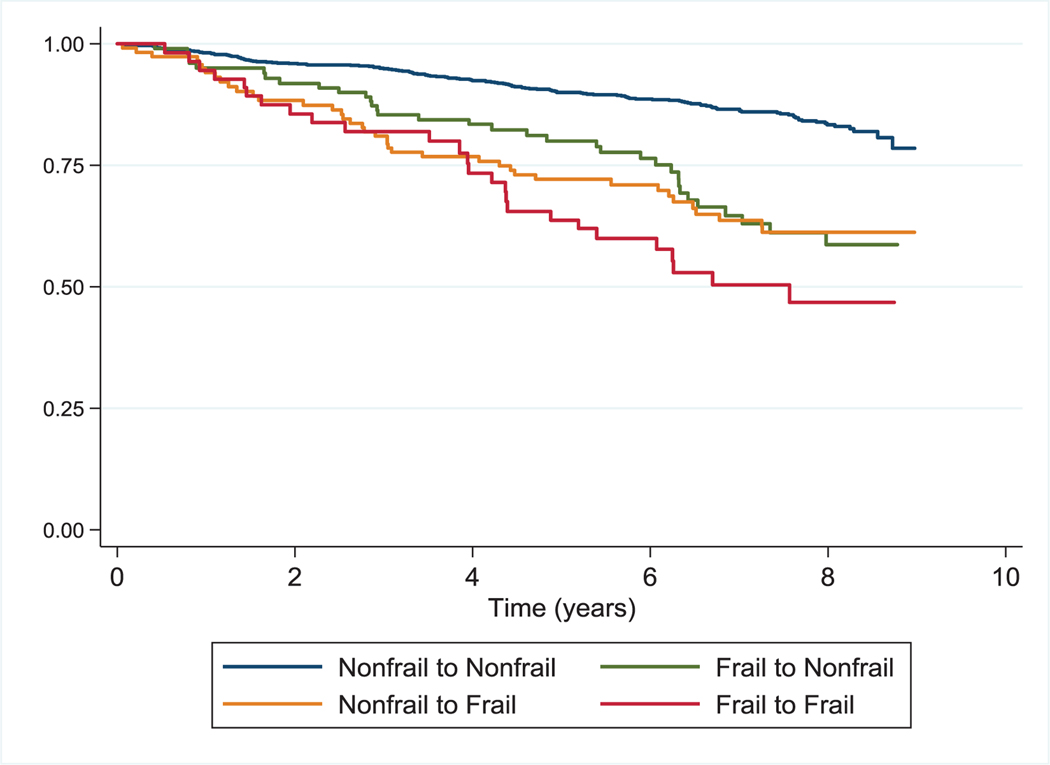
During prospective evaluation of the relationship of frailty transitions with mortality, we observed 248 deaths over 8647 person-years for a mortality rate of 2.9 per 100 person-years. The median follow-up time was 7.8 years (IQR 6.0, 8.2); similar for HIV-infected and uninfected participants. Overall, participants who were frail at one or both of two consecutive visits had a higher mortality risk than those who remained nonfrail (Figs. 1 and 2). Adjusting for sociodemographic factors, number of comorbid disease conditions, and HIV disease status, being frail at one of two consecutive visits was associated with significantly higher mortality risk than those who maintained a nonfrail state in both baseline and time varying analyses (Table 4). In analyses treating each frailty transition as a time varying covariate, the highest mortality risk was observed for those who remained frail at both of two consecutive visits [hazard ratio 3.23; 95% confidence interval (CI), 2.1, 4.96]. In analyses treating frailty as a three-tier variable, similar findings were observed (Supplemental Table 4, http://links.lww.-com/QAD/B701). In these analyses, being frail at both of two consecutive visits was associated with an almost six-fold increased risk of death compared with maintenance of a robust state (hazard ratio 5.91; 95% CI, 3.0, 11.6). The interaction term for HIV status and frailty transition state with mortality was not significant.
Fig. 1. Survival by Frailty Transition State among 1353 Participants at Baseline in the AIDS Linked to the IntraVenous Experience Cohort.
Kaplan–Meier survival curve estimates by baseline (initial) frailty transition state.
Fig. 2. Survival by Frailty Transition State across 9559 AIDS Linked to the IntraVenous Experience Frailty Transition Assessments.
Kaplan–Meier survival curve estimates by frailty transition state across all transition assessments in the AIDS Linked to the IntraVenous Experience Cohort.
Table 4.
Mortality risk associated with frailty transitions in the AIDS Linked to the IntraVenous Experience Cohorta.
| Baselineb | All transitionsc | |
|---|---|---|
| HR (95% CI) | HR (95% CI) | |
| Transition | N = 1353 | N = 9559 |
| Nonfrail to nonfrail | Ref | Ref |
| Frail to nonfrail | 1.55 (1.00, 2.42) | 2.17 (1.48, 3.20) |
| Nonfrail to frail | 2.08 (1.44, 3.01) | 2.32 (1.61, 3.34) |
| Frail to frail | 2.08 (1.30, 3.34) | 3.23 (2.10, 4.96) |
CI, confidence interval; HR, hazard ratio. Bold text indicates P<0.05.
Data are given as adjusted HRs (95% CI); each model was adjusted for age, sex, race, education, no. of comorbid conditions, and HIV status; nonfrail participants had a frailty score of 0–2; frail participants had a frailty score of 3–5.
Multivariable model examining the relationship of initial (baseline) frailty transition with mortality.
Multivariable model examining the relationship of frailty transitions as a time varying covariate with mortality.
Discussion
Frailty remains of major clinical importance for HIV-infected and uninfected populations alike. There have been limited studies on the factors that determine transitions between frailty states and very few longitudinal studies of frailty exist in HIV. This study provides seminal data on the precipitants and consequences of frailty transitions in HIV-infected and PWID populations.
We first show that reduced chronic comorbid disease, improved socioeconomic status, early control of HIV infection, and successful attainment of virologic suppression are strongly associated with both reduced frailty progression and increased frailty recovery. Our findings suggest these factors may be key protective elements, and important intervention targets, in preventing and ameliorating frailty in these populations.
Frailty and HIV both disproportionately burden socioeconomically challenged populations [27,28]. Superimposed on this heightened burden are our findings of the independent relationship of key socioeconomic factors, namely, lower educational attainment and unemployment with greater frailty progression and reduced frailty recovery. Previous studies have shown the association of psychosocial stress with activation of biological stress pathways [29–31]. Whether such pathways mediate the relationship of socioeconomic factors with frailty requires further investigation. Elucidating mediators of this relationship may provide key additional targets for frailty intervention.
Multiple studies have demonstrated the heightened burden of chronic comorbid disease or multimorbidity in the HIV-infected population [2,10]. Comorbid disease burden itself has been predictive of adverse clinical outcomes in HIV, independent of frailty [17]. Our studies suggest that reducing chronic comorbid disease burden or multimorbidity may improve clinical outcomes in HIV through frailty-dependent pathways as well, independent of ART-mediated virologic suppression. While the risk for progression or recovery varied somewhat with specific diseases, our main finding was that each condition is independently associated with the observed transitions, with likely added risk associated with the co-occurrence of multiple comorbidities. Developing effective interventions to reduce chronic comorbid disease burden in HIV remains a key priority that may be critical to improving frailty-related outcomes in this population.
Achieving sustained virologic suppression remains a key challenge for many HIV-infected populations, particularly PWID, exacerbated by behavioral factors and suboptimal care engagement [32,33]. Our findings suggest that achieving not only contemporaneous virologic suppression but also early control of HIV disease, as reflected in the observed CD4+ nadir and AIDS diagnosis frailty relationships, could be protective in preventing frailty progression and promoting frailty recovery. These data suggest that both earlier ART initiation to achieve early control of HIV disease and enhanced efforts to achieve and optimally sustain ART-mediated virologic suppression, particularly for PWID may be critical to reducing adverse frailty-related outcomes. These findings reinforce the importance of improving the HIV care continuum, particularly for HIV-infected persons at higher frailty risk.
To our knowledge, we provide the first longitudinal and temporal assessment of the relationship of inflammation to the frailty phenotype in HIV. We show that not only is a reduced inflammation level at baseline predictive of reduced frailty progression, but also that reductions in inflammation over time may significantly mitigate frailty progression and promote frailty recovery, independent of comorbidity and HIV disease stage.
Understanding the pathophysiologic pathways that precipitate frailty onset and progression is critical to developing interventions to combat frailty in HIV-infected and uninfected populations alike. Dysregulated inflammation is central to HIV pathophysiology. Despite ART-mediated virologic suppression, inflammation persists [34,35]. We and others have previously demonstrated the cross-sectional association of inflammation with frailty in HIV [19,37–39]. Through longitudinal analysis, we now provide novel evidence for a temporal association of inflammation with frailty, highlighting the potential key role of inflammatory pathways as a target to tackle frailty, even beyond ART-mediated virologic suppression. In this study, we found both baseline inflammation and changes in inflammation level to be important and independently associated with transitions in frailty status. Multiple upstream biological drivers of persistent, heightened inflammation in HIV have been proposed [8,34,37]. Specific elucidation of the upstream drivers of frailty-associated inflammation in HIV may be critical to developing effective therapeutic modalities to reduce frailty progression and promote frailty recovery.
Finally, building on our prior work, we demonstrate that both a sustained and intermittent frail state predict mortality risk, supporting the critical importance of preventing and ameliorating frailty to improve clinical outcomes in HIV-infected and PWID populations.
In this study, being frail at just one of two visits carried a higher mortality risk than being not frail at all, emphasizing the importance of preventing frailty progression in the first instance. A sustained frail state over two visits was associated with an even higher mortality risk, suggesting interventions to avoid a sustained frail state once frailty is identified may find additional benefit. Ultimately, the dynamic nature of frailty transitions that we observed and the association of these dynamic changes with heightened mortality risk suggest serial frailty assessments in clinical application may find utility for risk stratification and ultimately targeted frailty intervention.
While our study supports temporal associations of inflammation and the other factors observed with transitions between frailty states, as for any observational study, we are unable to establish definitively a cause–effect relationship with these variables or potential latency of effects. As inflammatory marker testing was performed on available repository specimens, selection effects are also possible. Further studies evaluating inflammatory marker trajectories over longer durations and investigation of the role of inflammation-targeted interventions on frailty could establish biological effect. In addition, frailty transitions in this study were assessed at 6-month intervals. Transitions taking place within the 6-month interval between frailty assessments were not ascertained, but this interval is equivalent to or smaller than reported in other studies of frailty transitions to date. Further, this cohort was a predominantly African American, urban cohort of PWID. Generalizability of our findings to other HIV-infected populations warrants additional study. Nonetheless, this population represents a key subset of the HIV-infected population who suffer the most severe disparities in morbidity and mortality and for whom frailty interventions may find greatest value.
In conclusion, there is increasing evidence that preventing frailty onset and promoting frailty recovery are critical to reducing survival gaps and other adverse frailty-related outcomes in HIV. In this study, we identified key putative targets to reduce frailty onset and support frailty recovery. Our findings suggest that prevention or reduction of chronic comorbid disease, targeted modulation of key socioeconomic factors, sustained efforts to improve the HIV care continuum, pursuit of early and consistent ART, and development of targeted modalities to reduce frailty-associated inflammation may all play a key role in reducing the marked frailty-mediated disparities in clinical outcomes in HIV. These factors should be of key focus in the development and investigation of future interventions to effectively prevent and reverse frailty and its related adverse outcomes in HIV.
Supplementary Material
Acknowledgements
The authors would like to thank the ALIVE study participants and ALIVE study staff for their significant contributions to this work.
The work was supported by the National Institute of Allergy and Infectious Diseases and the National Institute on Drug Abuse at the National Institutes of Health (K23AI108357, RC1AI086053, U01DA023832, RO1DA012568, RO1AG060825, U01DA036297, K24AI118591, K24AI120834), and the Robert Wood Johnson Foundation Harold Amos Program.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.GBD 2015 HIV Collaborators. Estimates of global, regional, and national incidence, prevalence, and mortality of HIV, 1980–2015: the Global Burden of Disease Study 2015. Lancet HIV 2016; 3:e361–e387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deeks SG, Lewin SR, Havlir DV. The end of AIDS:HIV infection as a chronic disease. Lancet 2013; 382:1525–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hontelez JA, de Vlas SJ, Baltussen R, Newell ML, Bakker R, Tanser F, et al. The impact of antiretroviral treatment on the age composition of the HIV epidemic in sub-Saharan Africa. AIDS 2012; 26 (Suppl 1):S19–S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mills EJ, Barnighausen T, Negin J. HIV and aging – preparing for the challenges ahead. N Engl J Med 2012; 366:1270–1273. [DOI] [PubMed] [Google Scholar]
- 5.Marcus JL, Chao CR, Leyden WA, Xu L, Quesenberry CP Jr, Klein DB, et al. Narrowing the gap in life expectancy between HIV-infected and HIV-uninfected individuals with access to care. J Acquir Immune Defic Syndr 2016; 73:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samji H, Cescon A, Hogg RS, Modur SP, Althoff KN, Buchacz K, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013; 8:e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greene M, Covinsky K, Astemborski J, Piggott DA, Brown T, Leng S, et al. The relationship of physical performance with HIV disease and mortality. AIDS 2014; 28:2711–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.High KP, Brennan-Ing M, Clifford DB, Cohen MH, Currier J, Deeks SG, et al. HIV and aging: state of knowledge and areas of critical need for research. A report to the NIH Office of AIDS research by the HIV and Aging Working Group. J Acquir Immune Defic Syndr 2012; 60 (Suppl 1):S1–S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salter ML, Lau B, Go VF, Mehta SH, Kirk GD. HIV infection, immune suppression, and uncontrolled viremia are associated with increased multimorbidity among aging injection drug users. Clin Infect Dis 2011; 53:1256–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smit M, Brinkman K, Geerlings S, Smit C, Thyagarajan K, van Sighem A, et al. Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis 2015; 15:810–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci 2004; 59:255–263. [DOI] [PubMed] [Google Scholar]
- 12.Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med 2011; 27:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Althoff KN, Jacobson LP, Cranston RD, Detels R, Phair JP, Li X, et al. Age, comorbidities, and AIDS predict a frailty phenotype in men who have sex with men. J Gerontol A Biol Sci Med Sci 2014; 69:189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fatukasi TV, Edmonds A, Gustafson DR, Cole SR, Edwards JK, Bolivar H, et al. Prevalence and 1-year incidence of frailty among women with and without HIV in the Women’s Interagency HIV Study. AIDS 2019; 33:357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelly SG, Wu K, Tassiopoulos K, Erlandson KM, Koletar SL, Palella FJ. Frailty is an independent risk factor for mortality, cardiovascular disease, bone disease and diabetes among aging adults with HIV. Clin Infect Dis 2019; 69:1370–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piggott DA, Erlandson KM, Yarasheski KE. Frailty in HIV: epidemiology, biology, measurement, interventions, and research needs. Curr HIV/AIDS Rep 2016; 13:340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piggott DA, Muzaale AD, Mehta SH, Brown TT, Patel KV, Leng SX, Kirk GD. Frailty, HIV Infection, and mortality in an aging cohort of injection drug users. PLoS One 2013; 8:e54910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piggott DA, Muzaale AD, Varadhan R, Mehta SH, Westergaard RP, Brown TT, et al. Frailty and cause-specific hospitalization among persons aging with HIV infection and injection drug use. J Gerontol A Biol Sci Med Sci 2017; 72:389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piggott DA, Varadhan R, Mehta SH, Brown TT, Li H, Walston JD, et al. Frailty, inflammation, and mortality among persons aging with HIV infection and injection drug use. J Gerontol A Biol Sci Med Sci 2015; 70:1542–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gill TM, Gahbauer EA, Han L, Allore HG. The relationship between intervening hospitalizations and transitions between frailty states. J Gerontol A Biol Sci Med Sci 2011; 66:1238–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herr M, Cesari M, Landre B, Ankri J, Vellas B, Andrieu S, MAPT/DSA Study Group. Factors associated with changes of the frailty status after age 70: findings in the MAPT study. Ann Epidemiol 2019; 34:65–70.e1. [DOI] [PubMed] [Google Scholar]
- 22.Kojima G, Taniguchi Y, Iliffe S, Jivraj S, Walters K. Transitions between frailty states among community-dwelling older people: a systematic review and meta-analysis. Ageing Res Rev 2019; 50:81–88. [DOI] [PubMed] [Google Scholar]
- 23.Vlahov D, Anthony JC, Munoz A, Margolick J, Nelson KE, Celentano DD, et al. The ALIVE study, a longitudinal study of HIV-1 infection in intravenous drug users: description of methods and characteristics of participants. NIDA Res Monogr 1991; 109:75–100. [PubMed] [Google Scholar]
- 24.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 25.Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol 1977; 106:203–214. [DOI] [PubMed] [Google Scholar]
- 26.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption – II. Addiction 1993; 88:791–804. [DOI] [PubMed] [Google Scholar]
- 27.Varadhan R, Yao W, Matteini A, Beamer BA, Xue QL, Yang H, et al. Simple biologically informed inflammatory index of two serum cytokines predicts 10 year all-cause mortality in older adults. J Gerontol A Biol Sci Med Sci 2014; 69:165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bandeen-Roche K, Seplaki CL, Huang J, Buta B, Kalyani RR, Varadhan R, et al. Frailty in older adults: a nationally representative profile in the United States. J Gerontol A Biol Sci Med Sci 2015; 70:1427–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Denning P, DiNenno E. Communities in crisis: is there a generalized HIV epidemic in impoverished urban areas of the United States? 2019. https://www.cdc.gov/hiv/group/poverty.html. [Accessed 21 August 2019].
- 30.Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol 2011; 11:625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marsland AL, Walsh C, Lockwood K, John-Henderson NA. The effects of acute psychological stress on circulating and stimulated inflammatory markers: a systematic review and meta-analysis. Brain Behav Immun 2017; 64:208–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matthews KA, Gallo LC. Psychological perspectives on pathways linking socioeconomic status and physical health. Annu Rev Psychol 2011; 62:501–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathers BM, Degenhardt L, Ali H, Wiessing L, Hickman M, Mattick RP, et al. HIV prevention, treatment, and care services for people who inject drugs: a systematic review of global, regional, and national coverage. Lancet 2010; 375:1014–1028. [DOI] [PubMed] [Google Scholar]
- 34.Westergaard RP, Hess T, Astemborski J, Mehta SH, Kirk GD. Longitudinal changes in engagement in care and viral suppression for HIV-infected injection drug users. AIDS 2013; 27:2559–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med 2011; 62:141–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity 2013; 39:633–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Erlandson KM, Ng DK, Jacobson LP, Margolick JB, Dobs AS, Palella FJ Jr, et al. Inflammation, immune activation, immunosenescence, and hormonal biomarkers in the frailty-related phenotype of men with or at risk for HIV infection. J Infect Dis 2017; 215:228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fukui SM, Piggott DA, Erlandson KM. Inflammation strikes again: frailty and HIV. Curr HIV/AIDS Rep 2018; 15:20–29. [DOI] [PubMed] [Google Scholar]
- 39.Margolick JB, Bream JH, Martinez-Maza O, Lopez J, Li X, Phair JP, et al. Frailty and circulating markers of inflammation in HIV+ and HIV− men in the Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr 2017; 74:407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.