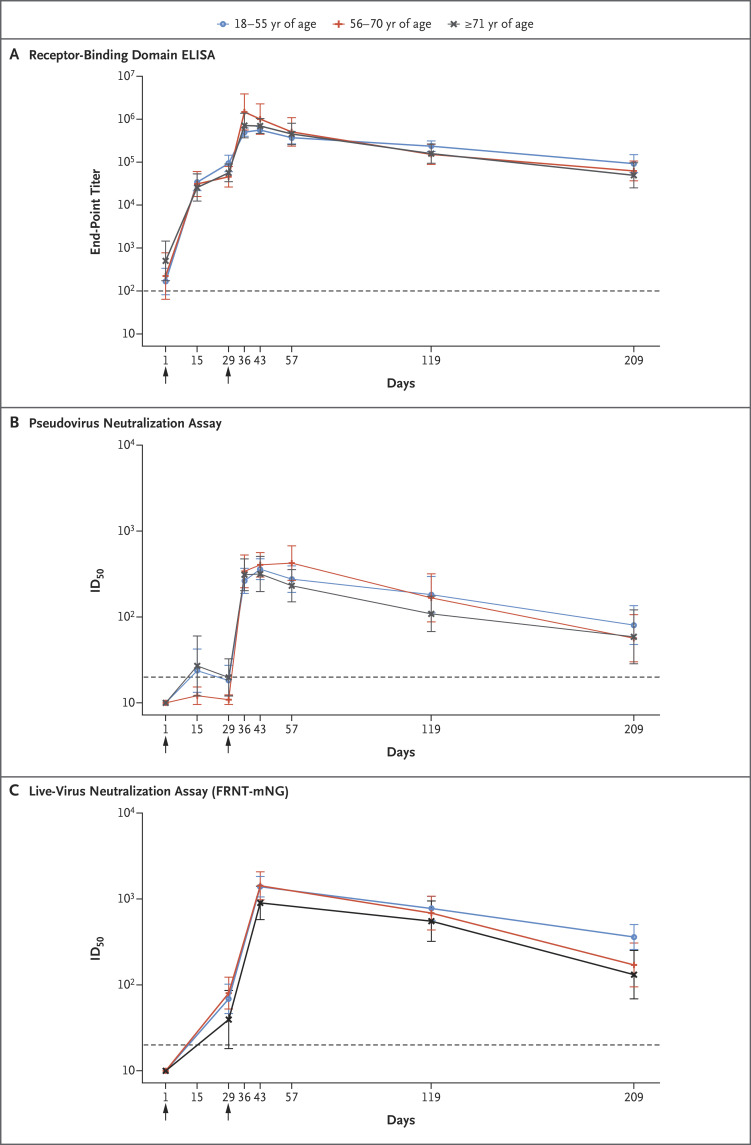
To the Editor: Interim results from a phase 3 trial of the Moderna mRNA-1273 severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine indicated 94% efficacy in preventing coronavirus disease 2019 (Covid-19).1 The durability of protection is currently unknown. We describe mRNA1273-elicited binding and neutralizing antibodies in 33 healthy adult participants in an ongoing phase 1 trial,2-4 stratified according to age, at 180 days after the second dose of 100 μg (day 209).
Antibody activity remained high in all age groups at day 209. Binding antibodies, measured by means of an enzyme-linked immunosorbent assay against SARS-CoV-2 spike receptor–binding domain,2 had geometric mean end-point titers (GMTs) of 92,451 (95% confidence interval [CI], 57,148 to 149,562) in participants 18 to 55 years of age, 62,424 (95% CI, 36,765 to 105,990) in those 56 to 70 years of age, and 49,373 (95% CI, 25,171 to 96,849) in those 71 years of age or older. Nearly all participants had detectable activity in a pseudovirus neutralization assay,2 with 50% inhibitory dilution (ID50) GMTs of 80 (95% CI, 40 to 135), 57 (95% CI, 30 to 106), and 59 (95% CI, 29 to 121), respectively. On the more sensitive live-virus focus-reduction neutralization mNeonGreen test,4 all the participants had detectable activity, with ID50 GMTs of 361 (95% CI, 258 to 504), 171 (95% CI, 95 to 307), and 131 (95% CI, 69 to 251), respectively; these GMTs were lower in participants 56 to 70 years of age (P=0.03) and in those 71 years of age or older (P=0.005) than in those 18 to 55 years of age (Figure 1; also see the Supplementary Appendix, available with the full text of this letter at NEJM.org).
Figure 1. Time Course of SARS-CoV-2 Antibody Binding and Neutralization Responses after mRNA-1273 Vaccination.
All the participants received 100 μg of mRNA-1273 on days 1 and 29, indicated by arrows. The numbers of participants in each age group with data available at day 209 are as follows: 18 to 55 years, 15 participants; 56 to 70 years, 9 participants; and 71 years or older, 9 participants. The titers shown are the binding to spike receptor–binding domain protein (the end-point dilution titer) assessed on enzyme-linked immunosorbent assay (ELISA) on days 1, 15, 29, 36, 43, 57, 119, and 209 (Panel A); the 50% inhibitory dilution (ID50) titer on pseudovirus neutralization assay on days 1, 15, 29, 36, 43, 57, 119, and 209 (Panel B); and the ID50 titer on the live-virus focus-reduction neutralization mNeonGreen test (FRNT-mNG) on days 1, 29, 43, 119, and 209 (Panel C). Lines show geometric mean titers for each age group; 𝙸 bars indicate 95% confidence intervals. The dashed line indicates the limit of detection for each assay.
The estimated half-life of binding antibodies after day 43 for all the participants was 52 days (95% CI, 46 to 58) calculated with the use of an exponential decay model, which assumes a steady decay rate over time, and 109 days (95% CI, 92 to 136) calculated with the use of a power-law model (at day 119), which assumes that decay rates decrease over time. The neutralizing antibody half-life estimates in the two models were 69 days (95% CI, 61 to 76) and 173 days (95% CI, 144 to 225) for pseudovirus neutralization and 66 days (95% CI, 59 to 72) and 182 days (95% CI, 153 to 254) for live-virus neutralization. As measured by ΔAICc (change in Akaike information criterion, corrected for small sample size), the best fit for binding and neutralization were the power-law and exponential decay models, respectively (see the Supplementary Appendix). These results are consistent with published observations of convalescent patients with Covid-19 through 8 months after symptom onset.5
Although the antibody titers and assays that best correlate with vaccine efficacy are not currently known, antibodies that were elicited by mRNA-1273 persisted through 6 months after the second dose, as detected by three distinct serologic assays. Ongoing studies are monitoring immune responses beyond 6 months as well as determining the effect of a booster dose to extend the duration and breadth of activity against emerging viral variants. Our data show antibody persistence and thus support the use of this vaccine in addressing the Covid-19 pandemic.
Supplementary Appendix
Disclosure Forms
This letter was published on April 6, 2021, and last updated on January 19, 2022, at NEJM.org.
Footnotes
Supported by grants (UM1AI148373, to Kaiser Washington; UM1AI148576 and UM1AI148684, to Emory University; NIH AID AI149644, to the University of North Carolina; UM1Al148684-01S1, to Vanderbilt University Medical Center; and HHSN272201500002C, to Emmes) from the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH); by a grant (UL1 TR002243, to Vanderbilt University Medical Center) from the National Center for Advancing Translational Sciences, NIH; and by the Dolly Parton Covid-19 Research Fund (to Vanderbilt University Medical Center). Laboratory efforts were in part supported by the Emory Executive Vice President for Health Affairs Synergy Fund award, the Center for Childhood Infections and Vaccines, Children’s Healthcare of Atlanta, a Woodruff Health Sciences Center 2020 COVID-19 CURE Award, the Georgia Research Alliance, and North Carolina Policy Collaboratory at the University of North Carolina at Chapel Hill, with funding from the North Carolina Coronavirus Relief Fund established and appropriated by the North Carolina General Assembly. Additional support was provided by the Intramural Research Program of the Vaccine Research Center, NIAID, NIH. Funding for the manufacture of mRNA-1273 phase 1 material was provided by the Coalition for Epidemic Preparedness Innovation.
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021;384:403-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2 — preliminary report. N Engl J Med 2020;383:1920-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Widge AT, Rouphael NG, Jackson LA, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med 2021;384:80-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med 2020;383:2427-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021;371(6529):eabf4063-eabf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.