Abstract
Background
Multimorbidity measures are useful for resource planning, patient selection and prioritization, and factor adjustment in clinical practice, research, and benchmarking. We aimed to compare the explanatory performance of the adjusted morbidity group (GMA) index in predicting relevant healthcare outcomes with that of other quantitative measures of multimorbidity.
Methods
The performance of multimorbidity measures was retrospectively assessed on anonymized records of the entire adult population of Catalonia (North-East Spain). Five quantitative measures of multimorbidity were added to a baseline model based on age, gender, and socioeconomic status: the Charlson index score, the count of chronic diseases according to three different proposals (i.e., the QOF, HCUP, and Karolinska institute), and the multimorbidity index score of the GMA tool. Outcomes included all-cause death, total and non-scheduled hospitalization, primary care and ER visits, medication use, admission to a skilled nursing facility for intermediate care, and high expenditure (time frame 2017). The analysis was performed on 10 subpopulations: all adults (i.e., aged > 17 years), people aged > 64 years, people aged > 64 years and institutionalized in a nursing home for long-term care, and people with specific diagnoses (e.g., ischemic heart disease, cirrhosis, dementia, diabetes mellitus, heart failure, chronic kidney disease, and chronic obstructive pulmonary disease). The explanatory performance was assessed using the area under the receiving operating curves (AUC-ROC) (main analysis) and three additional statistics (secondary analysis).
Results
The adult population included 6,224,316 individuals. The addition of any of the multimorbidity measures to the baseline model increased the explanatory performance for all outcomes and subpopulations. All measurements performed better in the general adult population. The GMA index had higher performance and consistency across subpopulations than the rest of multimorbidity measures. The Charlson index stood out on explaining mortality, whereas measures based on exhaustive definitions of chronic diagnostic (e.g., HCUP and GMA) performed better than those using predefined lists of diagnostics (e.g., QOF or the Karolinska proposal).
Conclusions
The addition of multimorbidity measures to models for explaining healthcare outcomes increase the performance. The GMA index has high performance in explaining relevant healthcare outcomes and may be useful for clinical practice, resource planning, and public health research.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-021-11922-2.
Keywords: Multimorbidity, Chronic disease, Risk assessment, Health resources, Health planning
Background
Multimorbidity is increasingly common in many countries worldwide, particularly those with higher life expectancy [1–3]. Still, most healthcare systems and therapeutic guidelines rely on disease-centred approaches, losing sight of the complexity of multimorbid patients, and hampering patient-centred approaches in clinical decision-making and healthcare planning [1]. The presence of multiple chronic conditions has been associated with lower quality of life and higher resource utilization and costs [4–7]. Hence, there is growing interest in developing measures of multimorbidity that are useful for resource planning, patient selection and prioritization, and factor adjustment in research and benchmarking [8–10].
The Charlson index, developed in the late ‘80s as a measurement of 1-year mortality risk [11], was among the first tools proposed for quantifying multimorbidity, and it is still broadly used in healthcare and research settings. Since then, various tools for assessing multimorbidity and patient complexity have been proposed, including quantitative measurements based on the count of chronic diseases (e.g., the Quality and Outcome Framework of the NHS [QOF] [8], the proposal of the Karolinska Institute for measuring chronic multimorbidity in older people [12], and the healthcare cost and utilization project [HCUP] of the US Agency for Healthcare Research and Quality [13]), and exhaustive pay tools for stratifying individuals into pre-established categories of multimorbidity (e.g., the Johns Hopkins Adjusted Clinical Groups [ACG®] [14] and the 3 M™ clinical risk groups [CRG] classification system [15]). Aside from marketed and/or nation-wide organizational tools, some authors and healthcare services nearby have explored alternative measures for summarizing the comorbidity burden and/or stratifying the population based on the health risk [16, 17].
Irrespective of the approach used, various factors challenge the development of meaningful indicators of multimorbidity. First, the concept of multimorbidity typically gravitates around chronic diseases, whereas acute conditions (e.g., hip fracture, pancreatitis) may dramatically increase patient risk and complexity [18]. Second, there is a lack of consensus regarding the criteria for identifying chronic diseases among all diagnostics [7, 19]. Finally, some of the proposed indicators (e.g., the QOF, Karolinska measure, and HCUP) are based on unweighted counts of diseases, thus losing sight of the relative contribution of each comorbidity to patient complexity [20]. While the Charlson index does provide a severity-driven weighted measure of chronic diagnostics, it is limited by the short list of diseases and severity categories considered [19].
The implementation of centralized electronic records and administrative databases for billing control in many countries has paved the way for big data strategies that allow developing population-based tools for measuring multimorbidity. The deployment of a Catalan Health Surveillance System (CHSS) in our area in 2012 prompted us to develop a population-based tool for stratifying patients according to their morbidity burden. The tool, named morbidity adjusted groups (GMA, Grupos de Morbilidad Ajustados), is based on the presence of chronic diseases, and it also considers recent acute diagnostic codes [21]. Like other tools, such as the ACG® and CRG® systems, the GMA is a case-mix tool that allows grouping the population according to their comorbidity burden and taking it into account when assessing outcomes of care. However, the GMA provides additional outputs at the individual level, including the number of chronic diseases, the number of organ systems affected by a chronic disease, a clinical summary label, and the multimorbidity index (i.e., a weighted measure of all diagnostics, which allow quantitative health-risk stratification at a population level) [22]. The GMA tool has shown good clinical performance—comparable with the CRGs [23, 24]—, adequate capacity to predict resource utilization in our area [25], and it has been validated in an external population using the ACG® and CRG® systems as a reference [16].
In this analysis, we assessed the performance of the multimorbidity index provided by the GMA tool in explaining health outcomes typically associated with multimorbidity and compared it to that of other quantitative measures of multimorbidity such as the Charlson index and the number of chronic diseases according to the QOF, Karolinska, and HCUP systems.
Methods
Population and data source
This was a retrospective cohort study based on anonymized records of the entire population of Catalonia, a North-East region in Spain with approximately 7.5 million people. The regional Health Department of Catalonia provides universal healthcare to the Catalan population through a network of 64 general hospitals, 27 psychiatry hospitals, 375 primary care centres, 91 skilled nursing facilities for intermediate care, and 130 ambulatory mental health facilities. Data were retrieved from the CHSS, which stores clinical and resource utilization information from various registries, including hospitalization, primary care visits, emergency department visits, skilled nursing facilities, palliative care, and mental health services, information on pharmacy dispensation, out-patient visits to specialists, home hospitalization, medical transportation (urgent and non-urgent), ambulatory rehabilitation, respiratory therapies, and dialysis. The source registries were originally developed for healthcare planning and invoice control to the Catalan Health Service (i.e., the public healthcare insurance in Catalonia). The registries have an automated data validation system aimed to identify inconsistencies between variables (e.g., age-diagnostic correspondence, temporal sequences, among others) and undergo external audits periodically to ensure provider payment accuracy [26]. It is estimated that approximately 20-to-25% of the Catalan population has simultaneous public and private coverage and may, therefore, use resources not reported to the CHSS. However, owing to the pharmaceutical co-payment system, nearly all patients with chronic diseases eventually visit the primary care resources (which records all chronic diagnoses) to get drug prescriptions and benefit from the pharmaceutical co-payment. All data used for the analysis were recorded in the source registries during 2017 and were retrieved in July 2019. The study protocol was approved by the Independent Ethics Committee of the IDIAP Jordi Gol (Spain) (ref. 21/042-P).
The performance of the multimorbidity tools was assessed on 10 subpopulations: all adults (i.e., aged > 17 years), and 9 subpopulations of special interest because of the expected high health risk and/or frequency of resource utilization like people aged > 64 years, people aged > 64 years and institutionalized in a nursing home for long-term care, and people with highly prevalent chronic diseases, including the following diagnose codes of the international classification of diseases (9th and 10th versions, clinical modification; ICD-9-CM and ICD-10-CM; all converted to ICD-9-CM): ischemic heart disease, cirrhosis, dementia, diabetes mellitus, heart failure, chronic kidney disease, and chronic obstructive pulmonary disease. Children and people with chronic mental health diseases were not considered as subpopulations for analysis because current measures of multimorbidity are not suited to capture the complexity of these scenarios.
Tools for multimorbidity assessment
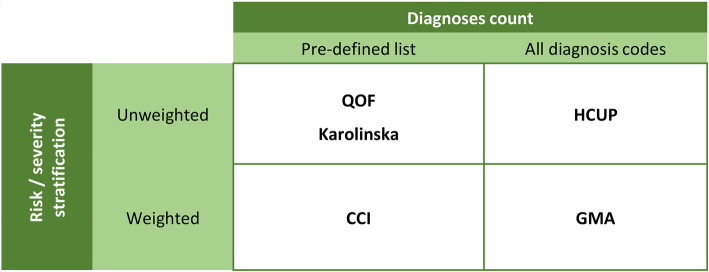
Our analysis aimed to compare tools that allow summarizing the comorbidity burden using a single numeric index. In addition to the GMA tool, we selected indices frequently reported in the literature that cover different approaches regarding two features: (1) the exhaustivity in the list of diagnoses considered and (2) the weight of each diagnosis according to severity (Fig. 1). The analysis included the following five tools: the Charlson index score [11], the count of chronic diseases according to three different proposals (i.e., the QOF [8], HCUP [13], and Karolinska institute [12]), and the multimorbidity index score of the GMA tool. Briefly, the Charlson index was designed as a tool for predicting life expectancy from a list of 17 comorbidities weighted according to their 1-year risk of death. The QOF was intended as a tool for allocating healthcare resources and incentivizing care of patients with chronic diseases, and its predictive capacity for health risk indicators such as mortality has been explored [27]. The QOF tool defines multimorbidity based on the presence of more than one diagnostic from a list of 17 important chronic conditions. The HCUP measures multimorbidity by counting the number of chronic diseases among all conditions codified in the chronic condition indicator (CCI) and grouped with the clinical classification software (CCS) [28]. The HCUP defines a chronic condition based on two criteria: (a) the given disease place limitations on self-care, independent living, and social interactions, and (b) result in the need for ongoing intervention with medical products, services, and special equipment [29]. The Karolinska proposal is a clinically-driven measure of multimorbidity based on the count of chronic diseases from a list of 918 ICD-10 codes (one- to -four-digit level), which are grouped into 60 chronic disease categories to retrieve a summary measure of multimorbidity [12]. In the Karolinska proposal, chronic diseases are selected based on the following criteria, applicable to older populations: prolonged duration and either (a) left residual disability or worsening quality of life or (b) required a long period of care, treatment, or rehabilitation. The GMA tool considers all chronic diagnoses (identified using the CCI of the HCUP) present at a given time and acute diagnoses reported during the study period. The GMA index score is computed by adding the weights of each diagnosis group (defined using the CCS of the HCUP system). For instance, unlike measures based solely on diagnosis counts, the GMA multimorbidity index algorithm gives a different complexity score to patients with hypothyroidism and eczema than those with asthma and diabetes, although accounting for two chronic conditions in both cases. Supplementary file 1 provides further details on the GMA algorithm.
Fig. 1.
Classification of the tools for measuring multimorbidity according to the number of diagnoses included and the accountability for their severity. CCI: Charlson Comorbidity Index. GMA: adjusted morbidity groups (Spanish, Grupos de Morbilidad Ajustada). HCUP: healthcare cost and utilization project. QOF: Quality and Outcome Framework
Study outcomes
We investigated the contribution of each multimorbidity measure to explaining eight outcomes associated with chronic patients: all-cause death, hospitalization, non-scheduled hospitalization, number of primary care visits (including general practitioner, nurse, and social worker, either at the primary care facility, home or via teleconsultation), visits to the emergency room (ER), medication use, admission to a skilled nursing facility for intermediate care, and high expenditure [30]. All outcomes were assessed in a 1-year time frame from January 1 to December 31, 2017.
Statistical analysis
The characteristics of the study population were described as absolute and relative frequencies and rates across all investigated outcomes. To identify individuals with high utilization rate of healthcare resources, we transformed continuous outcomes into to binary based the 95th percentile of the given variable among the overall population as cut-off. The resulting outcome definitions were as follows: admission to a skilled nursing facility for intermediate care (i.e., one or more admissions), admission to hospital (i.e., one or more admissions) and the emergency room (i.e., three or more admissions), visits to primary care services (i.e., more than 21 visits), medication use (i.e., dispensing of more than 13 drugs belonging to a different 5-digit group of the anatomic-therapeutic classification), expenditure (i.e., healthcare cost above € 4315.1). The cut-off value set for the overall study population was used for the sub-analyses on other sub-populations. Supplementary Figs. S2-S5 (Supplementary File 1) show the distribution of each study population for the continuous outcomes transformed based on the 95th percentile. To assess the performance multimorbidity measures, we built six logistic regression models for each of the investigated outcomes adjusted by age, gender, and socioeconomic status: a baseline model (i.e., age, gender, and socioeconomic status as independent variables, and all first-order interactions between them), and five models that added each multimorbidity measure to the baseline model. Multimorbidity measures were introduced into the models as continuous variables, using the indices, which were computed as described by the authors. The socioeconomic status was stratified into four categories of pharmaceutical co-payment: very low (recipient of social rescue aids), low (annual income < € 18,000), moderate (annual income € 18,000 to € 100,000), and high (annual income > € 100,000).
The performance of each model was assessed using four different statistics. For the primary analysis, we chose the area under the curve of the receiving operating characteristics (AUCROC) curve, which assesses the discrimination capacity of the model as the threshold varies and ranges from 0.5 (low discrimination capacity) to 1 (high discrimination capacity). Additionally, we conducted secondary analyses using the Akaike information criterion (AIC), pseudo-R squared (pR2), and the area under the precision-recall (AUC-PR). The AIC estimates the in-sample prediction error by taking into account the trade-off between the goodness of fit (overfitting) and the model simplicity (underfitting); the range of values that may take AIC depend on the study sample, with lower and higher values indicating better and poorer performance, respectively [31]. The pR2 assesses the goodness-of-fit and the variability explained and ranges from 0 (poor fitness of the model) to 100 (very good fitness of the model). The AUC-PR curve shows the trade-off between precision (i.e., low false-positive rate) and recall (i.e., low false-negative rate) and returns a value between 0 and 1, less biased than the ROC curve towards overestimating in outcomes with low frequency [32]. All analyses were performed using the R statistical package (version 3.6.2) [33].
Results
Characteristics of the study population
The analysis included 6,224,316 adult individuals (i.e., the entire adult population of Catalonia by the end of 2017) and the following subpopulations: older than 64 years (n = 1,472,623), older than 64 years institutionalized in a nursing home for long-term care (n = 67,456), ischemic heart disease (n = 244,311), cirrhosis (n = 45,126), dementia (n = 100,786), diabetes mellitus (n = 588,521), heart failure (n = 210,697), chronic kidney disease (n = 284,873), and chronic obstructive pulmonary disease (n = 357,989). Table 1 summarizes the main sociodemographic characteristics and rate of each study outcome for the adult population. The occurrence of most outcomes showed an increasing trend with higher age and lower socioeconomic status. Mortality was similar in the two genders; however, women tended to show higher rates of scheduled and non-scheduled hospitalization, primary care visits, ER admissions, medication use, and admissions to skilled nursing facilities. Men more frequently had expenditure below the threshold of € 4315.1. Tables S1-S9 (Supplementary file 1) summarize the characteristics of individuals included in the subpopulations.
Table 1.
Characteristics of the study population and rate of occurrence of each of the investigated outcomes
| No. (%) | All-cause death | Hospitalization | Non-scheduled hospitalization | Primary care visits | ER utilization | Medication use | Admission to skilled nursing facility | Expenditure | |
|---|---|---|---|---|---|---|---|---|---|
| Gender | |||||||||
| Male | 3,022,978 (48.6) | 1.09 | 8.17 | 4.09 | 4.53 | 3.63 | 4.15 | 0.79 | 6.13 |
| Female | 3,201,338 (51.4) | 1.04 | 9.70 | 4.80 | 5.93 | 4.73 | 6.35 | 1.00 | 5.70 |
| Age group | |||||||||
| 18–44 | 2,654,178 (42.6) | 0.05 | 4.94 | 2.70 | 1.33 | 3.89 | 0.57 | 0.02 | 1.92 |
| 45–64 | 2,097,515 (33.7) | 0.34 | 6.92 | 2.56 | 3.26 | 2.88 | 3.13 | 0.22 | 5.00 |
| 65–74 | 729,565 (11.7) | 1.19 | 14.61 | 5.80 | 8.50 | 4.68 | 11.48 | 1.07 | 10.56 |
| 75–84 | 476,422 (7.7) | 3.64 | 22.49 | 11.89 | 19.19 | 7.84 | 21.32 | 3.82 | 17.28 |
| > 84 | 266,636 (4.3) | 11.90 | 25.34 | 19.89 | 26.17 | 9.73 | 23.50 | 9.27 | 19.64 |
| Socioeconomic status1 | |||||||||
| High | 59,250 (1.0) | 0.54 | 3.31 | 1.55 | 0.96 | 0.93 | 1.66 | 0.12 | 2.62 |
| Moderate | 1,988,779 (32) | 0.65 | 7.01 | 3.05 | 3.10 | 2.50 | 3.30 | 0.42 | 4.21 |
| Low | 3,948,857 (63.4) | 1.27 | 9.87 | 5.10 | 6.23 | 4.85 | 6.08 | 1.13 | 6.55 |
| Very low | 227,430 (3.7) | 1.16 | 11.66 | 6.36 | 8.09 | 8.43 | 9.58 | 1.18 | 10.38 |
1Stratified into four categories of pharmaceutical co-payment: Very low (unemployed or recipient of social rescue aids), low (annual income < 18,000 €), moderate (annual income 18,000 to 100,000 €), and high (annual income > 100,000 €)
Categorical outcomes were transformed to binary variables using the 95th percentile of the given variable among the target population as cut-off: admission to a skilled nursing facility for intermediate care (i.e., one or more admissions), admission to emergency room (i.e., more than two admissions), visit to primary care services (i.e., more than 21 visits), medication use (i.e., dispensation of more than 13 drugs belonging to different 5-digit group of the anatomic-therapeutic classification), expenditure (.i.e., healthcare cost above 4315.1 €)
Measures of multimorbidity
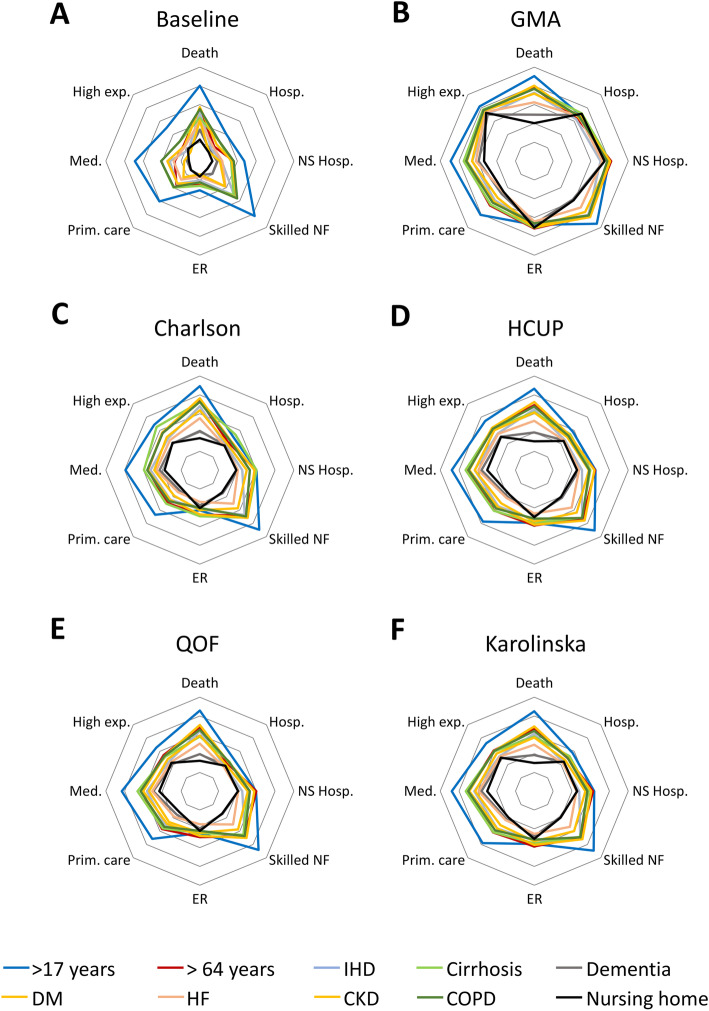
The baseline model based solely on age, gender, and socioeconomic status showed the most deficient performance in explaining the investigated outcomes in all subpopulations according to AUC-ROC estimate (Fig. 2). The poorest performance of the baseline model was consistent across all other statistics (i.e., AIC, pR2, and AUC-PR) (Table S10). Likewise, the addition of any multimorbidity measure to the baseline model improved the performance in explaining all investigated outcomes according to all statistics. In all models, admissions to the ER showed the lowest performance values. The GMA index (added to the baseline model) showed the highest performance in predicting all investigated outcomes. This trend was confirmed in the analyses using other statistics (Table S10). Of the other multimorbidity measures, the Charlson index score showed better performance in explaining mortality than the rest of the explored outcomes. The addition of the Karolinska and HCUP proposals for measuring multimorbidity showed better performance in explaining high use of medicines (i.e., more than 13) and primary care visits (i.e., more than 21).
Fig. 2.
Radar plot of the performance of each multimorbidity measure in explaining health outcomes associated with chronic conditions. The plotted values of the performance of the multimorbidity measures for each outcome correspond to the area under the receiving operating curve (AUC-ROC), which ranges from 0.5 (radar centre) to 1 (external edge). A: reference model including age, gender, and socioeconomic status. B: morbidity adjusted groups (GMA) index. C: Charlson index. D: healthcare cost and utilization project (HCUP) of the US Agency for Healthcare Research and Quality. E: Quality and Outcome Framework of the NHS (QOF). F: proposal of the Karolinska Institute for measuring chronic multimorbidity in older people. For each model, the estimates are shown for ten populations: adults (aged > 17 years), people aged > 64 years, people aged > 64 years and institutionalized in a nursing home for long-term care (nursing home), and people with specific diagnoses: ischemic heart disease (IHD), cirrhosis, dementia, diabetes mellitus (DM), heart failure (HF), chronic kidney disease (CKD), and chronic obstructive pulmonary disease (COPD). The AUC values plotted in this figure are presented in Table S10 (Supplementary file 1). Tables S11-S19 present the corresponding values for the rest of the sub-populations analysed. ER: emergency room. NF: nursing facility. NS: non-scheduled
Regarding the various subpopulations investigated, all models showed a trend to perform better on the general adult population and worse on people > 65 years institutionalized in nursing homes for long-term care. The GMA index showed lower variability across the investigated populations than the rest of the multimorbidity measures. This trend was confirmed when assessing performance with other statistics (Table S11-S19).
Discussion
In this population-based, retrospective analysis of the general adult population and subpopulations of interest regarding chronic conditions, we compared the performance of various multimorbidity measures in explaining relevant healthcare outcomes associated with the management of patients with multiple chronic diseases. The baseline model of age, sex, and socioeconomic status, historically used for predicting healthcare resource utilization [34], showed the lowest performance in all investigated outcomes. Of all composites of the baseline model and multimorbidity measures, the GMA multimorbidity index performed consistently better in all outcomes, across all subpopulations, and according to the various statistical estimates used. The GMA multimorbidity index has three main advantages that may explain these results. First, like the HCUP proposal, it exhaustively considers diagnostic codes potentially associated with chronic conditions; the CCS and CCI—morbidity indicators of the HCUP, also used in the GMA proposal for identifying and classifying chronic conditions—minimizes the likelihood of duplicities. Second, although relying on chronic conditions, the GMA tool also considers recent acute diagnoses (e.g., hip fracture, pancreatitis) that may increase patient complexity and even trigger an increase in resource utilization in the mid-time horizon [18, 35]. Finally, in line with other multimorbidity measures like the Charlson index or ad hoc measures of weighted comorbidity [36], the GMA multimorbidity index rates comorbidities according to the morbidity burden or severity.
Regarding the other measures of multimorbidity investigated, the Charlson index score performed particularly well in explaining mortality. This finding is consistent with the aim of this index, which was initially developed for predicting 1-year mortality in hospitalized patients. Conversely, the two measures of multimorbidity based on diagnostics count from a short pre-selected list (i.e., the Charlson index, and the QOF) were less accurate than measures that identify chronic conditions more exhaustively (e.g., Karolinska, HCUP, GMA) in explaining outcomes associated with healthcare resource utilization such as polypharmacy and ER or primary care visits. This result could be reasonably explained by the tendency of measures based on short lists of chronic conditions towards prioritizing disabling and life-threatening diseases. While these conditions are likely to influence hard endpoints, such as institutionalization or death, they may lose sight of less severe outcomes such as increased medication use or frequency of use of healthcare resources. The definition of a chronic condition has been identified among the most critical challenges of developing multimorbidity measures, and various authors have discussed the adequate trade-off between simplicity (e.g., use of short lists) and exhaustivity of the definition approach [7, 10, 37]. In our experience, measures that consider all possible diagnostic codes (e.g., the HCUP and the GMA, both taking all diagnostic groups of the CCS) tended to perform better than those using predefined lists of diagnostics (e.g., QOF or the Karolinska proposal) in most outcomes.
Our analysis focused on multimorbidity measures that yield a numerical value (i.e., either a composite score or the number of chronic conditions) of comorbidity. While this approach excluded other complex tools such as the ACG [14] or CRG [15] systems, it allowed us quantitative comparisons of performance using statistics like the ROC-AUC. Of note, the multimorbidity index provided by the GMA tool has been previously compared with the ACG and CRG tools, showing better performance for all outcomes, except patients receiving polypharmacy [16, 23, 38]. Taken together, the high performance of the GMA tool to explain differences in a variety of outcomes broadens its applicability, including the clinical sphere (e.g., identification of patients at higher risk through a population-based approach to proactively start a closer follow-up), benchmarking between healthcare centres or areas (e.g., adjusting for multimorbidity when comparing key indicators of healthcare delivery such as re-hospitalization rates), and healthcare resource allocation (e.g., anticipating resource needs or prioritizing in case of resource shortage [39]).
Our analysis was limited by the use of administrative databases, which precluded us from investigating non-recorded outcomes such as quality of life or physical function. In fact, population-based multimorbidity tools, such as those included in our analysis, are typically designed for healthcare planning and resource allocation. Hence, although we have not tested the performance of the GMA for explaining differences in physical or quality of life decline at the individual level, other indexes are expected to perform better on these outcomes [20, 36, 40]. Furthermore, the retrospective design provided an explanatory approach of healthcare outcomes; future studies shall assess the predictive capacity of these measures prospectively. On the other hand, the analysis was strengthened by the consistency of the main results across the various statistical estimates and subpopulations and the population-based approach, which allowed us to test the multimorbidity measures on a study population of over six million people. Of note, although the source datasets collect only resources afforded by the public insurance system, nearly all people with chronic diseases eventually visit the primary care resources to benefit from the pharmaceutical co-payment. Hence, the CHSS is unlikely to miss information on chronic diagnoses. The frequency of visits to the specialist, which is more likely to be biased in people with double healthcare coverage, was not included among study outcomes.
Conclusions
Our results show that the addition of a quantitative measure of multimorbidity to variables considered traditionally explanatory of healthcare outcomes—such as age, gender, and socioeconomic status—increases the performance of the model in explaining these outcomes. In our analysis, the GMA multimorbidity index performed better than other quantitative measures of multimorbidity in explaining relevant outcomes like all-cause death, total and non-scheduled hospitalization, primary care and ER visits, medication use, admission to a skilled nursing facility for intermediate care, and high expenditure. These findings provide policymakers and medical directors with strong evidence on the use of multimorbidity tools for clinical practice, resource planning, and public health researchers with useful insights for health risk stratification.
Supplementary Information
Additional file 1: Supplementary methods (algorithm description) and supplementary results (Tables S1-S19 and Figures S1-S5).
Acknowledgements
Not applicable.
Abbreviations
- ACG
adjusted clinical groups
- AIC
Akaike information criterion
- AUC
area under the curve
- CCI
chronic condition indicator
- CCS
clinical classification software
- CHSS
Catalan health surveillance system
- CRG
clinical risk groups
- ER
emergency room
- GMA
adjusted morbidity groups (from Spanish, Grupos de Morbilidad Ajustada)
- HCUP
healthcare cost and utilization project
- ICD
international classification of diseases
- PR
precision recall
- QOF
quality and outcome framework
- ROC
receiving operating characteristics
Authors’ contributions
EV, MC, and DM were responsible for the study conception and design, and conducted the data analysis. EV, LG-E, and PP-S contributed to data collection; EV, MC, DM, GC-S, MCo, DV, and JP-J contributed to data interpretation. The manuscript was drafted by EV, MC, DM, and GC-S; MCo, DV, JP-J, LG-E, and PP-S revised the manuscript for significant intellectual contribution. All authors have read approved the final version of the manuscript.
Funding
This work received no specific funding. Open Access funding provided by Servei Català de la Salut.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly accessible but are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The study used de-identified, retrospective data, and was conducted according to the World Medical Association Declaration of Helsinki (ethical principles for medical research involving human subjects https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/). The study protocol was approved by the Independent Ethics Committee of the IDIAP Jordi Gol (Spain), which waived the need for written informed consent (Code 00005101).
Consent for publication
Not applicable.
Competing interests
EM, MC, and DM are the developers of the GMA tool. The authors declare no support from any for profit organisation for the submitted work, no financial relationships with any organisations that might have an interest in the submitted work in the previous three years, nor other relationships or activities that could appear to have influenced the submitted work.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380(9836):37–43. doi: 10.1016/S0140-6736(12)60240-2. [DOI] [PubMed] [Google Scholar]
- 2.Fortin M, Bravo G, Hudon C, Vanasse A, Lapointe L. Prevalence of multimorbidity among adults seen in family practice. Ann Fam Med. 2005;3(3):223–228. doi: 10.1370/afm.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tinetti ME, Fried TR, Boyd CM. Designing health care for the most common chronic condition - multimorbidity. JAMA. 2012;307(23):2493–2494. doi: 10.1001/jama.2012.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lehnert T, Heider D, Leicht H, Heinrich S, Corrieri S, Luppa M, Riedel-Heller S, König HH. Review: health care utilization and costs of elderly persons with multiple chronic conditions. Med Care Res Rev. 2011;68(4):387–420. doi: 10.1177/1077558711399580. [DOI] [PubMed] [Google Scholar]
- 5.Jensen NL, Pedersen HS, Vestergaard M, Mercer SW, Glümer C, Prior A. The impact of socioeconomic status and multimorbidity on mortality: a population-based cohort study. Clin Epidemiol. 2017;9:279–289. doi: 10.2147/CLEP.S129415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanesarajah J, Waller M, Whitty JA, Mishra GD. Multimorbidity and quality of life at mid-life: A systematic review of general population studies. Vol. 109, Maturitas. Elsevier Ireland Ltd; 2018. p. 53–62. [DOI] [PubMed]
- 7.Huntley AL, Johnson R, Purdy S, Valderas JM, Salisbury C. Measures of multimorbidity and morbidity burden for use in primary care and community settings: a systematic review and guide. Ann Fam Med. 2012;10(2):134–141. doi: 10.1370/afm.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salisbury C, Johnson L, Purdy S, Valderas JM, Montgomery AA. Epidemiology and impact of multimorbidity in primary care: a retrospective cohort study. Br J Gen Pract. 2011;61(582):e12–e21. doi: 10.3399/bjgp11X548929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.González González AI, Miquel Gómez AM, Rodríguez Morales D, Hernández Pascual M, Sánchez Perruca L, Mediavilla HI. Concordancia y utilidad de un sistema de estratificación para la toma de decisiones clínicas. Aten Primaria. 2017;49(4):240–247. doi: 10.1016/j.aprim.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brilleman SL, Salisbury C. Comparing measures of multimorbidity to predict outcomes in primary care: a cross sectional study. Fam Pract. 2013;30(2):172–178. doi: 10.1093/fampra/cms060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Clin Epidemiol. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 12.Calderón-Larrañaga A, Vetrano DL, Onder G, Gimeno-Feliu LA, Coscollar-Santaliestra C, Carfí A, et al. Assessing and measuring chronic multimorbidity in the older population: a proposal for its operationalization. J Gerontol Ser A Biol Sci Med Sci. 2017;72(10):1417–1423. doi: 10.1093/gerona/glw233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUP) [Internet]. [cited 2020 Dec 3]. Available from: https://www.ahrq.gov/data/hcup/index.html [PubMed]
- 14.Johns Hopkins Bloomberg School of Public Health. The Johns Hopkins ACG® System. Excerpt from Technical Reference Guide Version 9.0 [Internet]. 2009 [cited 2020 Nov 6]. Available from: https://www.healthpartners.com/ucm/groups/public/@hp/@public/documents/documents/dev_057914.pdf
- 15.Hughes JS, Averill RF, Eisenhandler J, Goldfield NI, Muldoon J, Neff JM, Gay JC. Clinical risk groups (CRGs) a classification system for risk-adjusted capitation-based payment and health care management. Med Care. 2004;42(1):81–90. doi: 10.1097/01.mlr.0000102367.93252.70. [DOI] [PubMed] [Google Scholar]
- 16.Arias-López C, Rodrigo Val MP, Casaña Fernández L, Salvador Sánchez L, Dorado Díaz A, Estupiñán Ramírez M. [Validity of predictive power of the Adjusted Morbidity Groups (AMG) with respect to others population stratification tools.]. Rev Esp Salud Publica. 2020;94. https://europepmc.org/article/med/32618288. [PMC free article] [PubMed]
- 17.Orueta JF, Nuño-Solinis R, Mateos M, Vergara I, Grandes G, Esnaola S. Predictive risk modelling in the Spanish population: a cross-sectional study. BMC Health Serv Res. 2013;13(1):269. doi: 10.1186/1472-6963-13-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mercer SW, Smith SM, Wyke S, O’Dowd T, Watt GCM. Multimorbidity in primary care: developing the research agenda. Fam Pract. 2009;26(2):79–80. doi: 10.1093/fampra/cmp020. [DOI] [PubMed] [Google Scholar]
- 19.Gulbech Ording A, Toft SH. Concepts of comorbidities, multiple morbidities, complications, and their clinical epidemiologic analogs. Clin Epidemiol. 2013;5(1):199–203. doi: 10.2147/CLEP.S45305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fortin M, Hudon C, Dubois MF, Almirall J, Lapointe L, Soubhi H. Comparative assessment of three different indices of multimorbidity for studies on health-related quality of life. Health Qual Life Outcomes. 2005;3(1):74. doi: 10.1186/1477-7525-3-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monterde D, Vela E, Clèries M. Grupo colaborativo GMA. [adjusted morbidity groups: a new multiple morbidity measurement of use in primary care] Atención Primaria. 2016;48(10):674–682. doi: 10.1016/j.aprim.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dueñas-Espín I, Vela E, Pauws S, Bescos C, Cano I, Cleries M, Contel JC, de Manuel Keenoy E, Garcia-Aymerich J, Gomez-Cabrero D, Kaye R, Lahr MMH, Lluch-Ariet M, Moharra M, Monterde D, Mora J, Nalin M, Pavlickova A, Piera J, Ponce S, Santaeugenia S, Schonenberg H, Störk S, Tegner J, Velickovski F, Westerteicher C, Roca J. Proposals for enhanced health risk assessment and stratification in an integrated care scenario. BMJ Open. 2016;6(4):e010301. doi: 10.1136/bmjopen-2015-010301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monterde D, Vela E, Clèries M, García Eroles L, Pérez SP. Validity of adjusted morbidity groups with respect to clinical risk groups in the field of primary care. Aten Primaria. 2019;51(3):153–161. doi: 10.1016/j.aprim.2017.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clèries M, Monterde D, Vela E, Guarga À, García Eroles L, Pérez Sust P, Acezat Oliva J, Arevalo Genicio A, Barrot de la Puente J, Burgaña Agoües A, Cárceles Jurado S, Caselles i Rey J, Casanovas Font J, Cruz Cubells L, Cubí i Monfort R, Falcón Vives S, Gallego Laredo X, Garcia Pastor M, Garcia Albas I, Garcia López MC, Gavagnac Bellsolà M, Hidalgo Valls P, Iglesias Martínez M, Jiménez Girado J, Llordes Llordes M, López Lupión X, López i Arpí C, Martin Pascual A, Martínez Ortega M, Martínez Vindel JC, Mercadé Salavert JM, Moleiro Oliva À, Molina Guasch C, Mur Martí T, Narejos Pérez S, Navarrete González L, Panyart Sánchez D, Ribero Genar MD, Ripoll Ramos A, Serrarols Soldevila M, Soler Guerra M, Taberner i Pinsach L, Teixidó Colet M, Vilatimó Pujal R, Villanueva Hernández A, Yuste Marco MC. Clinical validation of 2 morbidity groups in the primary care setting. Aten Primaria. 2020;52(2):96–103. doi: 10.1016/j.aprim.2018.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monterde D, Vela E, Clèries M, Garcia-Eroles L, Roca J, Pérez-Sust P. Multimorbidity as a predictor of health service utilization in primary care: a registry-based study of the Catalan population. BMC Fam Pract. 2020;21(1):39. doi: 10.1186/s12875-020-01104-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vela E. Stratification and morbidity database [Internet]. AQuAS Blog (Agency for Health Quality and Assessement of Catalonia). 2016. Available from: https://blog.aquas.cat/2016/03/31/morbidity-database-2/?lang=en
- 27.Lee ES, Koh HL, Ho EQY, Teo SH, Wong FY, Ryan BL, Fortin M, Stewart M. Systematic review on the instruments used for measuring the association of the level of multimorbidity and clinically important outcomes. BMJ Open. 2021;11(5):1–21. doi: 10.1136/bmjopen-2020-041219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agency for Healthcare Research and Quality. Clinical Classifications Software (CCS) for ICD-9-CM [Internet]. [cited 2020 Oct 29]. Available from: https://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp
- 29.Perrin EC, Newacheck P, Pless IB, Drotar D, Gortmaker SL, Leventhal J, Perrin JM, Stein RE, Walker DK, Weitzman M. Issues involved in the definition and classification of chronic health conditions. Pediatrics. 1993;91(4):787–793. [PubMed] [Google Scholar]
- 30.Vela E, Clèries M, Vella VA, Adroher C, García-Altés A. Population-based analysis of the Healthcare expenditure in Catalonia (Spain): what and who consumes more resources? Gac Sanit. 2019;33(1):24–31. doi: 10.1016/j.gaceta.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 31.Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974;19(6):716–723. doi: 10.1109/TAC.1974.1100705. [DOI] [Google Scholar]
- 32.Saito T, Rehmsmeier M. The precision-recall plot is more informative than the ROC plot when evaluating binary classifiers on imbalanced datasets. PLoS One. 2015;10(3):e0118432. doi: 10.1371/journal.pone.0118432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.R Core Team. R: A language and environment for statistical com-puting [Internet]. R Foundation for Statistical Computing, Vienna, Austria. 2017 [cited 2020 May 25]. Available from: https://www.r-project.org
- 34.Cucciare MA, O’Donohue W. Predicting future healthcare costs: how well does risk-adjustment work? J Health Organ Manag. 2006;20(2):150–162. doi: 10.1108/14777260610661547. [DOI] [PubMed] [Google Scholar]
- 35.Cancio JM, Vela E, Santaeugènia S, Clèries M, Inzitari M, Ruiz D. Long-term impact of hip fracture on the use of healthcare resources: a population-based study. J Am Med Dir Assoc. 2019;20(4):456–461. doi: 10.1016/j.jamda.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Tooth L, Hockey R, Byles J, Dobson A. Weighted multimorbidity indexes predicted mortality, health service use, and health-related quality of life in older women. J Clin Epidemiol. 2008;61(2):151–159. doi: 10.1016/j.jclinepi.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 37.Brilleman SL, Gravelle H, Hollinghurst S, Purdy S, Salisbury C, Windmeijer F. Keep it simple? Predicting primary health care costs with clinical morbidity measures. J Health Econ. 2014;35(1):109–122. doi: 10.1016/j.jhealeco.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Estupiñán-Ramírez M, Tristancho-Ajamil R, Company-Sancho MC, Sánchez-Janáriz H. Comparación de modelos predictivos para la selección de pacientes de alta complejidad. Gac Sanit. 2019;33(1):60–65. doi: 10.1016/j.gaceta.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 39.Vela E, Carot-Sans G, Clèries M, Monterde D, Acebes X, Comella A, et al. Development and performance of a population-based risk stratification model for COVID-19. medRxiv. 2021;2021.05.25.21257783. https://www.medrxiv.org/content/10.1101/2021.05.25.21257783v1. [DOI] [PMC free article] [PubMed]
- 40.Groll DL, To T. Bombardier C, Wright JG. The development of a comorbidity index with physical function as the outcome. J Clin Epidemiol. 2005;58(6):595–602. doi: 10.1016/j.jclinepi.2004.10.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary methods (algorithm description) and supplementary results (Tables S1-S19 and Figures S1-S5).
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly accessible but are available from the corresponding author upon reasonable request.