Abstract
Background
Globally, lifestyles have changed to prevent the spread of coronavirus disease 2019 (COVID-19). Therefore, we aimed to understand health and lifestyle conditions associated with frailty transition over 6 months and devise a method for identifying frailty among community-dwelling older people during the COVID-19 pandemic.
Method
This community-based prospective cohort study was conducted from May to July 2020 (baseline) and November 2020 to January 2021 (follow-up) in Japan, with 1,953 community-dwelling older people (≥65 years) at baseline. To identify transition from non-frailty at baseline to frailty at follow-up, the Frailty Screening Index was used. For predicting frailty transition, two self-reported questionnaires assessing health and lifestyle conditions were employed.
Results
Overall, 706 individuals returned the baseline and follow-up questionnaires. Among the 492 non-frail older people at baseline, there was a 9.8% increase in frailty transition. The adjusted model for frailty transition by age, sex, multimorbidity, and living arrangements indicated that forgetfulness (odds ratio [OR] 2.74, 95% confidence interval [CI]: 1.00 to 7.51), falls in the past year (OR 2.26, 95% CI: 1.08 to 4.74), and subjective leg muscle weakness (OR 1.83, 95% CI: 1.05 to 3.21) were predictors of frailty transition. The combination of age ≥75 years and subjective leg muscle weakness showed moderate sensitivity, specificity, and % accuracy (0.688, 0.696, and 69.5%, respectively).
Conclusions
Approximately 10% of older people showed new transitions to frailty over 6 months during the COVID-19 pandemic. A combination of age and subjective leg muscle weakness is a feasible measure to optimally identify frailty transition.
Keywords: frailty, frailty transition, community-dwelling, COVID-19
1. INTRODUCTION
On April 7, 2020, the Japanese government issued a state of emergency to prevent the spread of coronavirus disease 2019 (COVID-19) (Looi, 2020a). This was the first state of emergency declaration in Japan's history. It was extended to all 47 prefectures on April 16, 2020 (Looi, 2020b). The Japanese government requested the public to avoid mass gatherings and implement social distancing. As a result, gatherings to implement communication among older people were cancelled.
Japan has a hyper-aged society. Thus, there is a need to facilitate healthy aging and maintain functional capacity (Muramatsu and Akiyama, 2011). A community-based Integrated Care System has, therefore, been built to support community-dwelling older people (Ministry of Health & Labour, 2016). Gatherings for group exercise, implementing communication, or for regular visits to prevent anxiety and isolation are recommended for older people. These activities are not public services; they are undertaken by local volunteers (Tsutsui, 2014) and are important for enhancing the quality of lives of older people and preventing frailty among them. These local activities including gatherings and regular visits delay frailty onset among older people (Okura et al, 2018). Frailty is a condition wherein a person has increased vulnerability to stress due to decline in physiological reserves, which ultimately results in poor health (Fried et al, 2001).
However, lifestyle and local activities have changed dramatically because of countermeasures imposed to prevent the spread of COVID-19. It is hypothesized that these countermeasures could result in “Corona-Frailty” (Shinohara et al, 2020a). In fact, after the declaration of emergency in Japan, the prevalence of frailty and prefrailty has increased substantially. According to a meta-analysis in Japan (Kojima et al, 2017), the prevalence rates of frailty and prefrailty were 7.5% and 48.1%, respectively, whereas 1 month after the declaration of emergency, these were 8.8% and 52.1%, respectively (Shinohara et al, 2021a). Previous studies have reported several transition rates from non-frailty to frailty based on longitudinal investigations (Ye et al, 2020; Rabassa et al, 2015; Chan et al, 2015; Ramsay et al, 2018; Semba et al, 2006; Tanaka et al, 2018; Iwasaki et al, 2018; Abe et al, 2020). In a systematic review, multiple factors including sociodemographic, physical, biological, lifestyle, and psychological factors showed longitudinal association with frailty (Feng et al, 2017). However, the impact of COVID-19 countermeasures on frailty among older people has not been investigated hitherto. To meet people's unique needs, it was suggested that the health system pertaining to aging and frailty should be improved (Sturmberg, 2020). Although many studies have assessed the characteristics of patients with COVID-19, studies reporting on the impact of COVID-19 on frailty among community-dwelling older people are scarce. Furthermore, it is important to identify older people with or transitioning to frailty and to implement beneficial health systems and policies during the pandemic. Identifying the risk factors for frailty among non-frail older people can help health professionals and policy makers to delay frailty transition.
This cohort study aimed to assess the impact of COVID-19 on the development of frailty among community-dwelling older people and the factors associated with frailty (Shinohara et al, 2020b). We determined the transition rate from non-frailty to frailty during the COVID-19 pandemic and compared it with other studies’ transition rates during the non-pandemic period because these data were not available from before the pandemic period for our study participants. Moreover, we aimed to understand health and lifestyle conditions associated with frailty transition over 6 months among community-dwelling older people living in local homes and not requiring treatment during the COVID-19 countermeasure period in Japan and to devise a method to assess frailty transition using self-administered questionnaires.
2. METHODS
2.1. Participants and procedure
A prospective cohort study was conducted in Takasaki City, Gunma Prefecture, Japan. Approximately 1,953 community-dwelling older people (≥65 years) who lived in local homes and received regular help from local volunteers were included. Care home residents and those who had stroke or cancer were excluded. The selected candidates received survey forms and instructions for this study. Those who agreed to participate were asked to mention their names on the survey forms and return it by post.
Participants underwent frailty status, health condition, and lifestyle assessments at baseline and follow-up at 6 months. Assessments were conducted using a self-reported questionnaire to comply with COVID-19 countermeasures. The purpose was to enable the older people to answer without any support and interference from local volunteers. The survey form included questions on age, sex, morbidity, and living arrangements (with a cohabitant or alone). Morbidity was selected from the list provided in the survey form: hypertension, diabetes mellitus, dyslipidemia, osteoporosis, heart disease, pulmonary emphysema, cancer, stroke, knee osteoarthritis, arthritis, bone fracture, and others (free records). The baseline assessment was conducted from May 11 to July 10, 2020, and follow-up assessment was conducted from November 11, 2020, to January 10, 2021.
This study was approved by the Research Ethics Committee of the Takasaki University of Health and Welfare (approval number 2009).
2.2. Measurements
The study outcome was frailty during follow-up. Frailty was assessed using the Frailty Screening Index (FSI) (Yamada et al, 2015). Based on the FSI, pre-frail older and frail older people had significantly elevated risks of care insurance use after 2 years (adjusted hazard ratio: 8.4 and 22.7, respectively) (Yamada et al, 2015). Frailty status based on the FSI is significantly associated with social frailty status (Yamada et al, 2018). The FSI has predictive validity for disability and concurrent validity for social frailty among older Japanese people. The FSI is a questionnaire comprising five items: “Have you lost 2 kg or more in the past 6 months?”, “Do you think you walk slower than before?”, “Do you go for a walk for your health at least once a week?”, “Can you recall what happened 5 minutes ago?”, and “In the past 2 weeks, have you felt tired without reason?”. These questions had a simple Yes/No answer, and 1 point was scored if an answer indicated frail condition. The total score of FSI ranges from 0 to 5. Actual measurement such as grip strength or walking speed is not required. Frailty status was based on the participant's score; a score of ≥3 was defined as frail, 1–2 as pre-frail, and 0 as robust (Yamada et al, 2015).
For defining the predictors of frailty, we used two self-reported questionnaires. To assess health conditions and lifestyle, we used a Questionnaire for Older Senior Citizens (QO) (Ministry of Health & Labour, 2019). The QO was prepared by the Japanese Ministry of Health, Labour and Welfare. It aims to comprehensively evaluate health conditions and lifestyle based on the characteristics of older people and has construct validity and frailty criterion-related validity (Shinohara et al, 2021c). Considering the burden on older people, only 15 items were included in the questionnaire.
The QO is useful for assessing the current lifestyle and health status, but recent changes due to COVID-19 countermeasures have not been clarified. Therefore, another questionnaire, the Questionnaire for Change for Life (QCL) was developed to evaluate the impact of COVID-19 countermeasures on changes related to lifestyle and physical or psychological conditions. The QCL comprised five items related to the three dimensions of frailty. Physical frailty relates to the amount of daily movement (Okura et al, 2018), leg muscle strength (Fried et al, 2001), and meal size (O'Connell et al, 2020); cognitive frailty relates to the amount of daily movement (Shimada et al, 2016), including worry or anxiety (Makizako et al, 2015); and social frailty relates to opportunities to talk to people (Kojima et al, 2020). The response options used a 5-point Likert scale to make it easier for older people to answer. The participants were asked about subjective changes in the past month to evaluate changes due to implementation of countermeasures. Each item was scored on the following scale: increased or stronger = 1, slightly increased or stronger = 2, unchanged = 3, slightly decreased or weaker = 4, decreased or weaker = 5. Items about worry or anxiety were scored as decreased = 1, slightly decreased = 2, unchanged = 3, slightly increased = 4, increased = 5. Almost all items of QCL related to frailty status have been validated previously (Shinohara et al, 2021a; Shinohara et al, 2021b).
2.3. Statistical analyses
Descriptive statistics for demographic variables, the QO, and the QCL at baseline were presented as frequency and percentage. Non-frailty was considered when the participant was pre-frail or robust based on the FSI score (Yamada et al, 2015). To indicate predictors of transition from non-frailty to frailty, the non-frail participants at baseline were analyzed. We compared each item on the QO and QCL at baseline using the chi-square test or Fisher's exact test. Univariate logistic regression analysis was used to indicate items contributing to frailty transition, and multivariate logistic regression analysis was used with the following adjusted coefficients: age, sex, multimorbidity, and living arrangements. Multimorbidity was considered when a participant had more than one chronic disease (World Health Organization, 2016). Three items of the QO (items 6, 7, and 9) were also the items of the FSI; therefore, these items were excluded from logistic regression analysis. Moreover, the items that had significant associations with frailty transition were considered predictors for the method to extract frailty transition. Receiver operating characteristic (ROC) curve analysis was used to elucidate the relationship between the number of applicable predictors and frailty transition to indicate the usefulness of this method based on the number of predictors to identify frailty transition. Accuracy was assessed using the area under the curve (AUC), which indicated the probability of correctly classifying participants based on frailty. The AUC is interpreted as follows: a test with an area of >0.9 has high accuracy, 0.7–0.9 indicates moderate accuracy, 0.5–0.7 indicates low accuracy, and 0.5 indicates a chance result (Fischer, 2003). The sensitivity, specificity, positive likelihood ratio (LR+), negative likelihood ratio (LR−), % accuracy, Youden index were calculated for all predictors and number of applicable predictors. LR+ and LR− were calculated for each predictor. Next, we calculated these based on the combinations of age range (≥70, ≥75, and ≥80 years) and predictors (e.g., age ≥75 years and falls) to improve prediction abilities. Statistical analyses were performed using IBM SPSS Statistics for Windows, version 26 (IBM Corp., Armonk, N.Y., USA), with a p value of <0.05 indicating statistical significance.
3. RESULTS
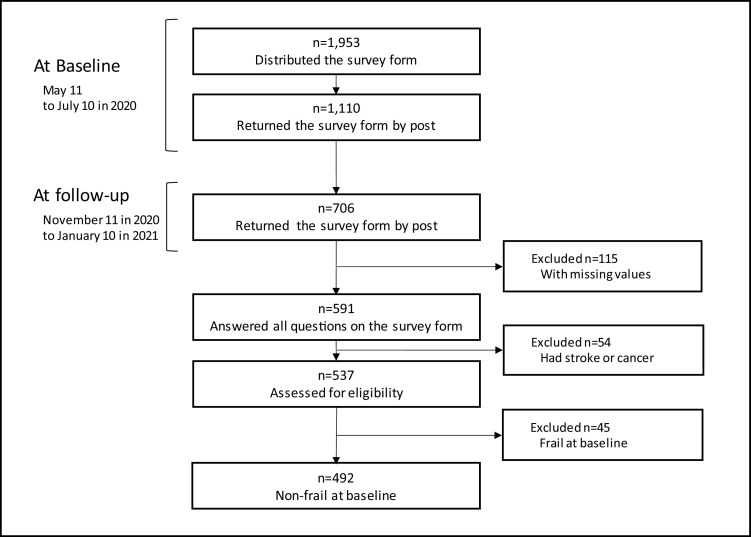
The flow diagram is presented in Fig. 1 . Considering both baseline and follow-up, 706 older people returned the survey forms with written consent, and the total response rate was 36.1%. Approximately 537 older people could answer all questions of the survey form and met the inclusion criteria. In total, 492 older people who were non-frail at baseline were included for analysis.
Fig. 1.
Flow diagram of the participants enrolled in the study.
Participants’ mean age was 78.6 ± 6.2 years, and 77.2% were women. The mean interval between baseline and follow-up was 186.5 ± 17.0 days (Table 1 ). Forty-eight participants became frail, and the frailty transition rate was 9.8%.
Table 1.
Participants' characteristics.
| Characteristics | |||
|---|---|---|---|
| Age, mean ± SD (years) | 78.6±6.2 | ||
| Time interval, mean ± SD (days) | 186.5±17.0 | ||
| Female, n (%) | 380 | (77.2) | |
| Morbidity, n (%) | |||
| Hypertension | 204 | (41.5) | |
| Osteoporosis | 77 | (15.7) | |
| Dyslipidemia | 73 | (14.8) | |
| Heart disease | 62 | (12.6) | |
| Diabetes mellitus | 58 | (11.8) | |
| Multimorbidity, n (%) | 153 | (31.1) | |
| Living arrangements, n (%) | |||
| With cohabitant | 148 | (30.1) | |
| Alone | 344 | (69.9) | |
There were significant differences in seven items on the QO (p < 0.01) and one item on the QCL at baseline (p < 0.001) between the non-frailty and frailty groups (Tables 2 , 3 ). Table 4 shows the results of the univariate (unadjusted model) and multivariate (adjusted model) logistic regression analyses including items for prediction of frailty: 12 items of the QO (except number 6, 7, and 9) and 5 items of the QCL and frailty transition. In the unadjusted model, the following five items of the QO—subjective health, chewing food, falls, forgetfulness, and discernment—and two items of the QCL—leg muscle strength and meal size—were associated with frailty transition. After entering all items into the model and adjusting for age, sex, multimorbidity, and living arrangements, three predictors had significant associations with frailty transition. In the QO, participants who experienced falls in the past year (odds ratio [OR] 2.26, 95% confidence interval [CI]: 1.08 to 4.74) and experienced forgetfulness (OR 2.74, 95% CI: 1.00 to 7.51) tended to experience frailty transition. In the QCL, participants with change in subjective leg muscle weakness in the past month (OR 1.83, 95% CI: 1.05 to 3.21) experienced frailty transition.
Table 2.
Association between frailty transition and the Questionnaire for Older Senior Citizens.
| No | Item | Response | Total | Non-frailty | Frailty | p-value | |||
|---|---|---|---|---|---|---|---|---|---|
| n = 492 | n = 444 | n = 48 | |||||||
| 1 | How is your health? | 1. Good | 111 | (22.6) | 106 | (22.6) | 5 | (10.4) | .000 |
| 2. Fairly good | 121 | (24.6) | 111 | (24.6) | 10 | (20.8) | |||
| 3. Normal | 235 | (47.8) | 210 | (47.8) | 25 | (52.1) | |||
| 4. Not very good | 23 | (4.7) | 17 | (4.7) | 6 | (12.5) | |||
| 5. Bad | 2 | (0.4) | 0 | (0.4) | 2 | (4.2) | |||
| 2 | Are you satisfied with your daily life? | 1. Satisfied | 185 | (37.6) | 168 | (37.6) | 17 | (35.4) | .671 |
| 2. Somewhat satisfied | 254 | (51.6) | 231 | (51.6) | 23 | (47.9) | |||
| 3. Somewhat dissatisfied | 46 | (9.3) | 40 | (9.3) | 6 | (12.5) | |||
| 4. Dissatisfied | 7 | (1.4) | 5 | (1.4) | 2 | (4.2) | |||
| 3 | Do you consistently eat 3 meals a day? | 1. Yes | 468 | (95.1) | 422 | (95.1) | 46 | (95.8) | 1.000 |
| 2. No | 24 | (4.9) | 22 | (4.9) | 2 | (4.2) | |||
| 4 | Has it become more difficult to eat hard food than it was 6 months ago? | 1. No | 367 | (74.6) | 340 | (74.6) | 27 | (56.3) | .002 |
| 2. Yes | 125 | (25.4) | 104 | (25.4) | 21 | (43.8) | |||
| 5 | Do you sometimes choke on tea or soup? | 1. No | 380 | (77.2) | 346 | (77.2) | 34 | (70.8) | .265 |
| 2. Yes | 112 | (22.8) | 98 | (22.8) | 14 | (29.2) | |||
| 6 | Have you lost 2-3 kg or more in the past 6 months? | 1. No | 462 | (93.9) | 415 | (93.9) | 47 | (97.9) | .343 |
| 2. Yes | 30 | (6.1) | 29 | (6.1) | 1 | (2.1) | |||
| 7 | Do you think you walk slower than before? | 1. No | 288 | (58.5) | 278 | (58.5) | 10 | (20.8) | .000 |
| 2. Yes | 204 | (41.5) | 166 | (41.5) | 38 | (79.2) | |||
| 8 | Have you fallen in the past year? | 1. No | 408 | (82.9) | 378 | (82.9) | 30 | (62.5) | .000 |
| 2. Yes | 84 | (17.1) | 66 | (17.1) | 18 | (37.5) | |||
| 9 | Do you take a walk to exercise at least once a week? | 1. Yes | 387 | (78.7) | 362 | (78.7) | 25 | (52.1) | .000 |
| 2. No | 105 | (21.3) | 82 | (21.3) | 23 | (47.9) | |||
| 10 | Are you told that you are forgetful, with comments such as "you are always telling me the same thing"? | 1. No | 460 | (93.5) | 421 | (93.5) | 39 | (81.3) | .002 |
| 2. Yes | 32 | (6.5) | 23 | (6.5) | 9 | (18.8) | |||
| 11 | Do you sometimes forget what day and month it is that day? | 1. No | 413 | (83.9) | 379 | (83.9) | 34 | (70.8) | .009 |
| 2. Yes | 79 | (16.1) | 65 | (16.1) | 14 | (29.2) | |||
| 12 | Do you smoke cigarettes? | 1. No, I do not smoke | 414 | (84.1) | 375 | (84.1) | 39 | (81.3) | .832 |
| 2. I quit smoking | 50 | (10.2) | 44 | (10.2) | 6 | (12.5) | |||
| 3. Yes, I smoke | 28 | (5.7) | 25 | (5.7) | 3 | (6.3) | |||
| 13 | Do you go out at least once a week? | 1. Yes | 459 | (93.3) | 417 | (93.3) | 42 | (87.5) | .120 |
| 2. No | 33 | (6.7) | 27 | (6.7) | 6 | (12.5) | |||
| 14 | Are you normally in close contact with family and friends? | 1. Yes | 468 | (95.1) | 423 | (95.1) | 45 | (93.8) | .720 |
| 2. No | 24 | (4.9) | 21 | (4.9) | 3 | (6.3) | |||
| 15 | Do you have anyone to talk to if you feel unwell? | 1. Yes | 466 | (94.7) | 421 | (94.7) | 45 | (93.8) | .732 |
| 2. No | 26 | (5.3) | 23 | (5.3) | 3 | (6.3) | |||
Table 3.
Association between frailty transition and the Questionnaire for Change of Life.
| No | Item | Response | Total | Non-frailty | Frailty | p-value | |||
|---|---|---|---|---|---|---|---|---|---|
| n = 492 | n = 444 | n = 48 | |||||||
| (1) | Amount of daily movement | 1. Increased | 3 | (0.6) | 3 | (0.7) | 0 | (0.0) | .933 |
| 2. Slightly increased | 14 | (2.9) | 14 | (3.2) | 0 | (0.0) | |||
| 3. Unchanged | 278 | (56.5) | 250 | (56.3) | 28 | (58.3) | |||
| 4. Slightly decreased | 133 | (27) | 122 | (27.5) | 11 | (22.9) | |||
| 5. Decreased | 64 | (13) | 55 | (12.4) | 9 | (18.8) | |||
| (2) | Leg muscle strength | 1. Stronger | 0 | (0) | 0 | (0.0) | 0 | (0.0) | .000 |
| 2. Slightly stronger | 4 | (0.8) | 4 | (0.9) | 0 | (0.0) | |||
| 3. Unchanged | 275 | (55.9) | 263 | (59.2) | 12 | (25) | |||
| 4. Slightly weaker | 173 | (35.2) | 147 | (33.1) | 26 | (54.2) | |||
| 5. Weaker | 40 | (8.1) | 30 | (6.8) | 10 | (20.8) | |||
| (3) | Meal size | 1. Increased | 2 | (0.4) | 2 | (0.5) | 0 | (0.0) | .177 |
| 2. Slightly increased | 28 | (5.7) | 26 | (5.9) | 2 | (4.2) | |||
| 3. Unchanged | 396 | (80.5) | 364 | (82) | 32 | (66.7) | |||
| 4. Slightly decreased | 59 | (12) | 46 | (10.4) | 13 | (27.1) | |||
| 5. Decreased | 7 | (1.4) | 6 | (1.4) | 1 | (2.1) | |||
| (4) | Worry or anxiety | 1. Decreased | 2 | (0.4) | 1 | (0.2) | 1 | (2.1) | .404 |
| 2. Slightly decreased | 8 | (1.6) | 8 | (1.8) | 0 | (0.0) | |||
| 3. Unchanged | 276 | (56.1) | 248 | (55.9) | 28 | (58.3) | |||
| 4. Slightly increased | 175 | (35.6) | 162 | (36.5) | 13 | (27.1) | |||
| 5. Increased | 31 | (6.3) | 25 | (5.6) | 6 | (12.5) | |||
| (5) | Opportunities of talking to people | 1. Increased | 2 | (0.4) | 2 | (0.5) | 0 | (0.0) | .994 |
| 2. Slightly increased | 4 | (0.8) | 4 | (0.9) | 0 | (0.0) | |||
| 3. Unchanged | 195 | (39.6) | 173 | (39) | 22 | (45.8) | |||
| 4. Slightly decreased | 157 | (31.9) | 144 | (32.4) | 13 | (27.1) | |||
| 5. Decreased | 134 | (27.2) | 121 | (27.3) | 13 | (27.1) | |||
Table 4.
Multivariate regression for transition from non-frailty to frailty.
| Item | Odds Ratio (95%CI) | ||||
|---|---|---|---|---|---|
| Unadjusted modela | Adjusted model b | ||||
| The Questionnaire for Older Senior Citizens | |||||
| 1 | How is your health? | 1.953 | (1.335-2.858) | 1.246 | (0.805-1.928) |
| 2 | Are you satisfied with your daily life? | 1.283 | (0.842-1.957) | 1.228 | (0.685-2.201) |
| 3 | Do you consistently eat 3 meals a day? | 0.834 | (0.190-3.661) | 0.486 | (0.093-2.532) |
| 4 | Has it become more difficult to eat hard food than it was 6 months ago? | 2.543 | (1.380-4.685) | 1.492 | (0.706-3.157) |
| 5 | Do you sometimes choke on tea or soup? | 1.454 | (0.750-2.817) | 0.698 | (0.319-1.530) |
| 8 | Have you fallen in the past year? | 3.436 | (1.812-6.518) | 2.258 | (1.075-4.741) |
| 10 | Are you told that you are forgetful, with comments such as "you are always telling me the same thing"? | 4.224 | (1.828-9.760) | 2.744 | (1.003-7.510) |
| 11 | Do you sometimes forget what day and month it is that day? | 2.401 | (1.222-4.719) | 1.979 | (0.890-4.400) |
| 12 | Do you smoke cigarettes? | 1.135 | (0.671-1.920) | 1.570 | (0.848-2.906) |
| 13 | Do you go out at least once a week? | 2.206 | (0.862-5.647) | 0.939 | (0.306-2.879) |
| 14 | Are you normally in close contact with family and friends? | 1.343 | (0.385-4.678) | 1.212 | (0.227-6.462) |
| 15 | Do you have anyone to talk to if you feel unwell? | 1.220 | (0.353-4.224) | 1.210 | (0.240-6.109) |
| The Questionnaire for Change of Life | |||||
| (1) | Amount of daily movement | 1.226 | (0.844-1.781) | 0.925 | (0.557-1.535) |
| (2) | Leg muscle strength | 2.833 | (1.848-4.342) | 1.834 | (1.048-3.209) |
| (3) | Meal size | 2.137 | (1.246-3.667) | 1.615 | (0.853-3.057) |
| (4) | Worry or anxiety | 1.057 | (0.674-1.659) | 0.839 | (0.493-1.427) |
| (5) | Opportunities of talking to people | 0.947 | (0.665-1.348) | 0.900 | (0.575-1.407) |
CI, confidence interval
model for univariate analysis;
model for multivariate analysis and adjustment for age, gender, multimorbidity, and living arrangements.
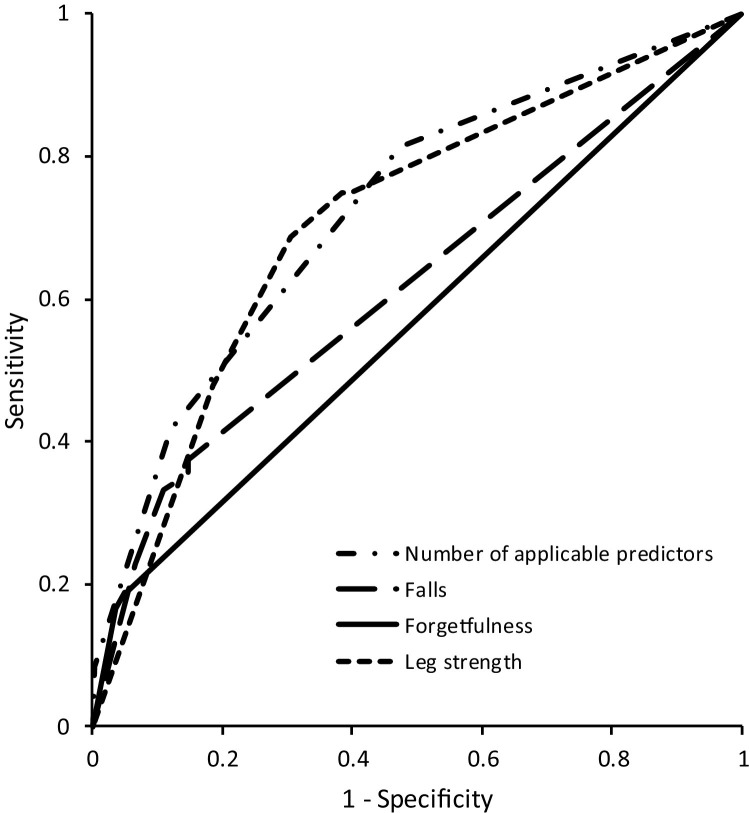
As per the ROC curve analysis to elucidate the relationship between the number of applicable predictors and frailty transition, the AUC was 0.723 (95% CI: 0.645 to 0.801, Fig. 2 ). Table 5 shows abilities to discriminate frailty transition using the following predictors: falls, forgetfulness, and subjective leg muscle weakness. The presence of one of the three predictors indicated high sensitivity (0.813) but low specificity (0.527) and low % accuracy (55.5%). Among the combinations of age and predictors, age of ≥75 years and leg muscle weakness showed moderate sensitivity, specificity, % accuracy, and Youden index (0.688, 0.696, 69.5%, and 0.383, respectively).
Fig. 2.
Receiver operating characteristic curves for the number of applicable predictors, and the combinations of age and each predictor for elucidating the association with frailty transition. Each predictor, falls, forgetfulness, and leg strength weakness, is shown in combination with each age (>65, 70, 75, and 80).
Table 5.
Discriminating transition from non-frailty to frailty using predictors.
| Sensitivity | Specificity | LR+ | LR- | % Accuracy | Youden Index | |||
|---|---|---|---|---|---|---|---|---|
| One predictor | 0.813 | 0.527 | 1.72 | 0.356 | 55.5 | 0.340 | ||
| Two predictors | 0.417 | 0.878 | 3.43 | 0.664 | 83.3 | 0.295 | ||
| Three predictors | 0.083 | 0.971 | 2.85 | 0.944 | 90.7 | 0.079 | ||
| One predictor | ||||||||
| Falls | 0.375 | 0.851 | 2.52 | 0.734 | 80.5 | 0.226 | ||
| Forgetfulness | 0.188 | 0.948 | 3.62 | 0.857 | 87.4 | 0.136 | ||
| Leg strength | 0.750 | 0.601 | 1.88 | 0.416 | 61.6 | 0.351 | ||
| Two predictors | ||||||||
| Falls | 0.571 | 0.909 | 6.27 | 0.472 | 90.4 | 0.480 | ||
| Forgetfulness | 0.288 | 0.928 | 4.00 | 0.767 | 85.2 | 0.216 | ||
| Leg strength | 0.350 | 0.913 | 4.02 | 0.712 | 89.0 | 0.263 | ||
| Combinations | ||||||||
| Over 70 years old and | ||||||||
| Falls | 0.354 | 0.854 | 2.42 | 0.757 | 83.5 | 0.208 | ||
| Forgetfulness | 0.188 | 0.948 | 3.62 | 0.857 | 87.4 | 0.136 | ||
| Leg strength | 0.750 | 0.615 | 1.95 | 0.407 | 62.8 | 0.365 | ||
| Over 75 years old and | ||||||||
| Falls | 0.333 | 0.890 | 3.02 | 0.749 | 83.5 | 0.223 | ||
| Forgetfulness | 0.188 | 0.950 | 3.78 | 0.855 | 88.0 | 0.138 | ||
| Leg strength | 0.688 | 0.696 | 2.26 | 0.449 | 69.5 | 0.383 | ||
| Over 80 years old and | ||||||||
| Falls | 0.229 | 0.935 | 3.51 | 0.825 | 86.6 | 0.164 | ||
| Forgetfulness | 0.167 | 0.964 | 4.63 | 0.864 | 89.0 | 0.131 | ||
| Leg strength | 0.479 | 0.813 | 2.56 | 0.641 | 78.0 | 0.292 | ||
LR+, positive likelihood ratio; LR−, negative likelihood ratio
4. DISCUSSION
The transition rate from non-frailty to frailty was 9.8% among the non-frail community-dwelling older people. In a review of longitudinal studies related to frailty transition rate, Ye et al, 2020 reported that 6.9% older people became frail over the 2-year follow-up of the community residents who were aged ≥60 years. Rabassa et al, 2015 indicated that 4.4% of older people whose mean age was 72.7 years were frail over the 3-year follow-up, and Chan et al, 2015 reported that 1.1% of older people whose mean age was 71.8 years were affected with frailty over the 4-year follow-up period. Semba et al, 2006 suggested that frailty was 19.1 per 100 person-years among older women. Iwasaki et al, 2018 showed that 15.2% older people whose mean age was 75.0 years were affected by frailty during the average follow-up period of 4.2 years. In a longer-term study, Abe et al, 2020 suggested that 16.8% of older people whose mean age was 74.7 years were affected by frailty over the 5-year follow-up period. However, the region, age, and follow-up period of the target population differed depending on the survey. Moreover, the frailty assessment also differed. If we assume transition rates of 4.4% (Rabassa et al, 2015) to 10% (Ramsay et al, 2018) as per the 2- to 3-year follow-up surveys for people in their 70s, the transition rate of 9.8% over 6 months in this study may be high. Due to the COVID-19 pandemic, physical activity significantly decreased compared with that before the pandemic in Japan (Yamada et al, 2020). Approximately ≥40% non-frail community-dwelling older people in Japan experienced decrease in physical activity, leg muscle strength, and opportunity to socialize during the COVID-19 pandemic (Shinohara et al, 2021b). The frailty transition rate in this study could be affected by the implementation of the COVID-19 countermeasures among community-dwelling older people; further, it indicates corona-frailty (Shinohara et al, 2020a).
The three predictors, i.e., falls in the past year, forgetfulness, and subjective leg muscle weakness, had significant associations with frailty transition. Cognitive impairment was independently associated with increased risk of frailty over a 10-year period (Raji et al, 2010). Among four domains of cognitive ability (visuospatial ability, memory, speed, and crystallized ability), speed may be an important indicator of frailty risk over a 6-year period (Gale, 2017). From a 1-year study, Chong et al., 2015 suggested that mild to moderate Alzheimer's disease influenced physical frailty progression among community-dwelling older people. These studies indicate that cognitive impairment was useful for predicting frailty transition among older people. Although forgetfulness was assessed using only one item of the QO, the results were supported by those of previous studies.
The next strongest association with frailty transition was “falls in the past year.” According to meta-analyses (Cheng et al, 2017; Jehu et al, 2021), frailty assessments are recommended because frail older people are likely to experience recurrent falls. However, we showed the reverse causality of previous studies and that experiencing falls was likely to lead to frailty. Kamide et al, 2020 indicated that fall-related efficacy could predict frailty progression. It was suggested that various factors contribute to falls, such as muscle weakness, balance deficits, or depression (Guideline for the prevention of falls in older persons, 2001). We believe that falls in the past year could cause a mild decline in physical or psychological function and could lead to frailty.
Similarly, subjective leg muscle weakness was associated with frailty transition. Subjective weakness implies physical impairment including muscle atrophy and psychological problems such as self-efficacy. We could not assess both problems, but one factor that contributes to physical impairment is decreased physical activity. Sedentary behavior was associated with frailty among middle to older aged adults (Blodgett et al, 2015), and farming, exercise, intellectual activity, and social participation were associated with lower odds of becoming frail (Ye et al, 2020; Abe et al, 2020). Moreover, physical function and psychological factors show longitudinal association with frailty (Feng et al, 2017). Therefore, subjective leg muscle weakness among older people may involve physical and/or psychological problems and should be assessed to predict frailty transition.
The AUC was 0.723; thus, evaluating the number of applicable predictors to identify frailty transition was valid. By assessing the combination of age and one of the three predictors, we were able to increase the predictability. The combination of age ≥75 years and subjective leg muscle weakness had the most optimal and acceptable predictability. Pegorari et al, 2019 suggested that age ≥80 years was predictive of prefrailty and frailty. Age played a role in determining whether the frailty status improved or worsened (Ye et al, 2020). In a systematic review of longitudinal studies, aging was a typical sociodemographic factor associated with frailty (Feng et al, 2017). The health heterogeneity increased with age and peak heterogeneity was observed at approximately 70 years (Nguyen et al, 2020); thus, health condition and frailty should be analyzed with aging. Therefore, the combination with age was appropriate to extract frailty transition. However, an LR+ >5 and LR− <0.2 indicate the usefulness of predictors due to their high probability of correctly identifying frailty transition (McGee, 2002). We believe the reasons for insufficient predictive ability were that actual measurements could not be obtained and only minimal information on sociodemographic, physical, biological, lifestyle-related, and psychological factors was collected due to the COVID-19 countermeasures. Although the predictive abilities for frailty were moderate, the strength of the current study was that we were able to make predictions using a simple questionnaire without face-to-face interviews.
In total, 706 participants returned both the baseline and follow-up questionnaires, and 591 participants (83.7%) answered all questions in the survey form. This indicated that most older people could answer without assistance. In cases when community-dwelling older people cannot be assessed using actual measurements such as during the COVID19 countermeasures, this method is feasible and useful for determining frailty risk among older people, and the data would aid medical professionals, community supporters, and policy makers. This questionnaire could also be used even when COVID-19 countermeasures are imposed.
This study had several limitations. First, this study was not a random sampling survey because participants included those to whom local volunteers could distribute the survey forms. Second, we could not assess participants who dropped out. The survey forms were collected only by mail, without telephone confirmation. Those participants with frailty risk may not have sent the survey form, and we may have underestimated the frailty transition rate. Third, we used the FSI questionnaire, which had predictive validity for disability; however, we could not collect data requiring actual measurements to assess frailty because of COVID-19 countermeasures. The FSI should be validated by comparing it with other testing methods involving face-to-face measurements in future studies. Forgetfulness was one of the risks for frailty transition. We may have underestimated the true value of memory impairment for predicting frailty among those who failed to return the survey form. Moreover, there were limitations in collecting detailed participant information from older people who could not answer the questionnaire without any support. For adjusting coefficients and improving prediction ability, other aspects, e.g., socio-economic, literacy, and body mass index, should be considered. Finally, we used the predictors to extract information about frailty transition in a specific cohort population within a limited duration of 6 months. The study findings must be validated in future prospective studies based on other cohort populations followed up for a longer period.
5. CONCLUSION
This cohort study was conducted following the government's declaration of a state of emergency to prevent the spread of COVID-19 in Japan. The community-dwelling older people were requested to avoid mass gatherings and implement social distancing. Thus, we used a survey form for this study. Among 492 community-dwelling older people, the frailty transition rate was 9.8%. The adjusted model by age, sex, multimorbidity, and living arrangements indicated the following three predictors, i.e., falls in the past year, forgetfulness, and subjective leg muscle weakness, to identify frailty transition. We believe that the combination of age ≥75 years and subjective leg muscle weakness assessed using an easy questionnaire is feasible and useful to assess frailty risk when requiring social distancing.
Author contributions
Tomoyuki Shinohara: study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript. Kosuke Saida: acquisition of data, analysis, and interpretation of data. Shigeya Tanaka and Akihiko Murayama: Critical revision of the manuscript for important intellectual content. Daisuke Higuchi: analysis and interpretation of data, critical revision of the manuscript for important intellectual content.
Funding
The parts of this work are supported by the Nippon Life Insurance Foundation (Grant 2020-0203-04) and the Japan Society for the Promotion of Science KAKENHI (Grant 19K19712).
Declaration of Competing Interest
The authors have no declarations of interest.
Acknowledgements
We would like to express our sincere gratitude to Satoshi Tanaka, Atsushi Kuwabara, Munehisa Suto, Kenichi Suto, Chieko Mesaki, Junko Ishii, Kumi Aoki, Norie Torizuka, Miyuki Ogawa, Yumi Ino, Izumi Tsutsumi, Susumu Shimomura, Nobuko Kaseda, Kazuaki Kuwabara, Yuriko Yoshiara, Ryo Koike, Masaaki Arai, Shizuko Nakajima, Noriko Takizawa, Misao Shiraishi, Tomiko Takeuchi, Masae Ozawa, Tamotsu Nakamura, Tadaaki Nakazawa, Yasuo Nagasaka, Toshiko Nakazawa, Setsuko Okada, Noriko Umeki, and Kakeko Kosone, and Ayako Yamazaki. The parts of this work are supported by the Nippon Life Insurance Foundation (Grant 2020-0203-04) and the Japanese Society for the Promotion of Science KAKENHI (Grant 19K19712). We would like to thank Editage (www.editage.com) for English language editing for supporting COVID-19 research.
References
- Abe T., Nofuji Y., Seino S., Murayama H., Yoshida Y., Tanigaki T., et al. Healthy lifestyle behaviors and transitions in frailty status among independent community-dwelling older adults: The Yabu cohort study. Maturitas. 2020;136:54–59. doi: 10.1016/j.maturitas.2020.04.007. [DOI] [PubMed] [Google Scholar]
- Blodgett J., Theou O., Kirkland S., Andreou P., Rockwood K. The association between sedentary behaviour, moderate-vigorous physical activity and frailty in NHANES cohorts. Maturitas. 2015;80:187–191. doi: 10.1016/j.maturitas.2014.11.010. [DOI] [PubMed] [Google Scholar]
- Chan R., Leung J., Woo J. Dietary Patterns and Risk of Frailty in Chinese Community-Dwelling Older People in Hong Kong: A Prospective Cohort Study. Nutrients. 2015;7:7070–7084. doi: 10.3390/nu7085326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng M.H., Chang S.F. Frailty as a Risk Factor for Falls Among Community Dwelling People: Evidence From a Meta-Analysis. J Nurs Scholarsh. 2017;49:529–536. doi: 10.1111/jnu.12322. [DOI] [PubMed] [Google Scholar]
- Chong M.S., Tay L., Chan M., Lim W.S., Ye R., Tan E.K., Ding Y.Y. Prospective longitudinal study of frailty transitions in a community-dwelling cohort of older adults with cognitive impairment. BMC Geriatr. 2015;15:175. doi: 10.1186/s12877-015-0174-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z., Lugtenberg M., Franse C., Fang X., Hu S., Jin C., Raat H. Risk factors and protective factors associated with incident or increase of frailty among community-dwelling older adults: A systematic review of longitudinal studies. PLoS One. 2017;12 doi: 10.1371/journal.pone.0178383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer J.E., Bachmann L.M., Jaeschke R. A readers' guide to the interpretation of diagnostic test properties: clinical example of sepsis. Intensive Care Med. 2003;29:1043–1051. doi: 10.1007/s00134-003-1761-8. [DOI] [PubMed] [Google Scholar]
- Fried L.P., Tangen C.M., Walston J., Newman A.B., Hirsch C., Gottdiener J., et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- Gale C.R., Ritchie S.J., Cooper C., Starr J.M., Deary I.J. Cognitive Ability in Late Life and Onset of Physical Frailty: The Lothian Birth Cohort 1936. J Am Geriatr Soc. 2017;65:1289–1295. doi: 10.1111/jgs.14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guideline for the prevention of falls in older persons American Geriatrics Society, British Geriatrics Society, and American Academy of Orthopaedic Surgeons Panel on Falls Prevention. J Am Geriatr Soc. 2001;49:664–672. [PubMed] [Google Scholar]
- Iwasaki M., Yoshihara A., Sato N., Sato M., Minagawa K., Shimada M., et al. A 5-year longitudinal study of association of maximum bite force with development of frailty in community-dwelling older adults. J Oral Rehabil. 2018;45:17–24. doi: 10.1111/joor.12578. [DOI] [PubMed] [Google Scholar]
- Jehu D.A., Davis J.C., Falck R.S., Bennett K.J., Tai D., Souza M.F., et al. Risk factors for recurrent falls in older adults: A systematic review with meta-analysis. Maturitas. 2021;144:23–28. doi: 10.1016/j.maturitas.2020.10.021. [DOI] [PubMed] [Google Scholar]
- Kamide N., Inoue N., Sakamoto M., Sato H., Shiba Y. Fall-related efficacy is associated with the progression of frailty in community-dwelling older people. Nihon Ronen Igakkai Zasshi. 2020;57:308–315. doi: 10.3143/geriatrics.57.308. [DOI] [PubMed] [Google Scholar]
- Kojima G., Iliffe S., Tanigschi Y., Shimada H., Rakugi H., Walters K. Prevalence of frailty in Japan: a systematic review and meta-analysis. J Epidemiol. 2017;27:347–353. doi: 10.1016/j.je.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima G., Taniguchi Y., Kitamura A., Fujiwara Y. Is living alone a risk factor of frailty? A systematic review and meta-analysis. Ageing Res Rev. 2020;59 doi: 10.1016/j.arr.2020.101048. [DOI] [PubMed] [Google Scholar]
- Looi M.K. Covid-19: Japan declares state of emergency as Tokyo cases soar. BMJ. 2020;369:m1447. doi: 10.1136/bmj.m1447. [DOI] [PubMed] [Google Scholar]
- Looi M.K. Covid-19: Japan prepares to extend state of emergency nationwide as "untraceable" cases soar. BMJ. 2020;369:m1543. doi: 10.1136/bmj.m1543. [DOI] [PubMed] [Google Scholar]
- Makizako H., Shimada H., Doi T., Yoshida D., Anan Y., Tsutsumimoto K., et al. Physical frailty predicts incident depressive symptoms in elderly people: prospective findings from the Obu Study of Health Promotion for the Elderly. J Am Med Dir Assoc. 2015;16:194–199. doi: 10.1016/j.jamda.2014.08.017. [DOI] [PubMed] [Google Scholar]
- McGee S. Simplifying likelihood ratios. J Gen Intern Med. 2002;17:646–649. doi: 10.1046/j.1525-1497.2002.10750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health, Labour and Welfare. (2019). The Questionnaire for the elderly. https://www.mhlw.go.jp/content/12401000/000557576.pdf. Accessed August 7, 2021.
- Ministry of Health, Labour and Welfare. (2016) Establishing ‘the Community-based Integrated Care System’ https://www.mhlw.go.jp/english/policy/care-welfare/care-welfare-elderly/dl/establish_e.pdf. Accessed August 7, 2021.
- Muramatsu N., Akiyama H. Japan: super-aging society preparing for the future. Gerontologist. 2011;51(4):425–432. doi: 10.1093/geront/gnr067. [DOI] [PubMed] [Google Scholar]
- Nguyen Q.D., Moodie E.M., Forget M.F., Desmarais P., Keezer M.R., Wolfson C. Health Heterogeneity in Older Adults: Exploration in the Canadian Longitudinal Study on Aging. J Am Geriatr Soc. 2020;69:678–687. doi: 10.1111/jgs.16919. [DOI] [PubMed] [Google Scholar]
- O'Connell M.L., Coppinger T., McCarthy A.L. The role of nutrition and physical activity in frailty: A review. Clin Nutr ESPEN. 2020;35:1–11. doi: 10.1016/j.clnesp.2019.11.003. [DOI] [PubMed] [Google Scholar]
- Okura M., Ogita M., Yamamoto M., Nakai T., Numata T., Arai H. Community activities predict disability and mortality in community-dwelling older adults. Geriatr Gerontol Int. 2018;18:1114–1124. doi: 10.1111/ggi.13315. [DOI] [PubMed] [Google Scholar]
- Pegorari M.S., Tavares D. Frailty-associated factors among Brazilian community-dwelling elderly people: longitudinal study. Sao Paulo Med J. 2019;137:463–470. doi: 10.1590/1516-3180.2019.0179160919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabassa M., Zamora-Ros R., Urpi-Sarda M., Bandinelli S., Ferrucci L., Andres-Lacueva C., Cherubini A. Association of habitual dietary resveratrol exposure with the development of frailty in older age: the Invecchiare in Chianti study. Am J Clin Nutr. 2015;102:1534–1542. doi: 10.3945/ajcn.115.118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raji M.A., Snih S.A., Ostir G.V., Markides K.S., Ottenbacher K.J. Cognitive status and future risk of frailty in older Mexican Americans. J Gerontol A Biol Sci Med Sci. 2010;65:1228–1234. doi: 10.1093/gerona/glq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay S.E., Papachristou E., Watt R.G., Tsakos G., Lennon L.T., Papacosta A.O., et al. Influence of Poor Oral Health on Physical Frailty: A Population-Based Cohort Study of Older British Men. J Am Geriatr Soc. 2018;66:473–479. doi: 10.1111/jgs.15175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba R.D., Bartali B., Zhou J., Blaum C., Ko C.W., Fried L.P. Low serum micronutrient concentrations predict frailty among older women living in the community. J Gerontol A Biol Sci Med Sci. 2006;61:594–599. doi: 10.1093/gerona/61.6.594. [DOI] [PubMed] [Google Scholar]
- Shimada H., Makizako H., Lee S., Doi T., Lee S., Tsutsumimoto K., et al. Impact of Cognitive Frailty on Daily Activities in Older Persons. J Nutr Health Aging. 2016;20:729–735. doi: 10.1007/s12603-016-0685-2. [DOI] [PubMed] [Google Scholar]
- Shinohara T., Saida K., Tanaka S., Murayama A. Rapid Response: Impact of the COVID-19 pandemic on frailty in the elderly citizen; corona-frailty. BMJ. 2020;369:m1543. [Google Scholar]
- Shinohara T., Saida K., Tanaka S., Murayama A. Do lifestyle measures to counter COVID-19 affect frailty rates in elderly community dwelling? Protocol for cross-sectional and cohort study. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-040341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara T., Saida K., Tanaka S., Murayama A. Actual frailty conditions and lifestyle changes in community-dwelling older adults affected by coronavirus disease 2019 countermeasures in Japan: a cross-sectional study. SAGE Open Nursing. 2021;7:1–8. doi: 10.1177/23779608211025117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara T., Saida K., Tanaka S., Murayama A. Association between frailty and changes in lifestyle and physical or psychological conditions among older adults affected by the coronavirus disease 2019 countermeasures in Japan. Geriatr Gerontol Int. 2021;21:39–42. doi: 10.1111/ggi.14092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturmberg JP. Covid-19 highlights the failings of the health system as a whole. BMJ. 2020;370:m3329. doi: 10.1136/bmj.m3329. [DOI] [PubMed] [Google Scholar]
- Shinohara T., Saida K., Tanaka S., Murayama A. Construct validity of the Questionnaire for Older Senior Citizens based on a confirmatory factor analysis: A study during the period of self-restraint to prevent the spread of coronavirus disease 2019. Geriatr Gerontol Int. 2021 doi: 10.1111/ggi.14285. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Takahashi K., Hirano H., Kitsutani T., Watanabe Y., Ohara Y., et al. Oral Frailty as a Risk Factor for Physical Frailty and Mortality in Community-Dwelling Elderly. J Gerontol A Biol Sci Med Sci. 2018;73:1661–1667. doi: 10.1093/gerona/glx225. [DOI] [PubMed] [Google Scholar]
- Tsutsui T. Implementation process and challenges for the community-based integrated care system in Japan. Int J Integr Care. 2014;14:e002. doi: 10.5334/ijic.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M., Arai H. Predictive Value of Frailty Scores for Healthy Life Expectancy in Community-Dwelling Older Japanese Adults. J Am Med Dir Assoc. 2015;16(1002):e7–11. doi: 10.1016/j.jamda.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Yamada M., Arai H. Social Frailty Predicts Incident Disability and Mortality Among Community-Dwelling Japanese Older Adults. J Am Med Dir Assoc. 2018;19:1099–1103. doi: 10.1016/j.jamda.2018.09.013. [DOI] [PubMed] [Google Scholar]
- Yamada M., Kimura Y., Ishiyama D., Otobe Y., Suzuki M., Koyama S., et al. Effect of the COVID-19 epidemic on physical activity in community-dwelling older adults in Japan: A cross-sectional online survey. J Nutr Health Aging. 2020;24:948–950. doi: 10.1007/s12603-020-1501-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye B., Chen H., Huang L., Ruan Y., Qi S., Guo Y., et al. Changes in frailty among community-dwelling Chinese older adults and its predictors: evidence from a two-year longitudinal study. BMC Geriatr. 2020;20:130. doi: 10.1186/s12877-020-01530-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2016). Multimorbidity, Technical Series on Safer Primary Care. https://www.who.int/publications/i/item/multimorbidity. Accessed October 2, 2021.